Abstract
Purpose
Surgical excision of peripheral iris or ciliary body melanomas can be performed antero-posteriorly (irido-cyclectomy) with mydriasis or postero-anteriorly (cyclo-iridectomy) with miosis. The aim of this study was to evaluate the results of both surgical techniques.
Methods
Patients were enrolled in the study if they underwent irido-cyclectomy or cyclo-iridectomy for iris and/or ciliary body melanoma at the Liverpool Ocular Oncology Centre between 1993 and 2012.
Results
The 24 patients (8 male, 16 female) had a median age of 57 years. The largest median basal tumour diameter and the median tumour thickness were 4.8 and 2.2 mm, respectively. The resection was performed antero-posteriorly in 9 (37%) patients and postero-anteriorly or circumferentially in 15 (63%). Nine tumours contained epithelioid cells. Genetic studies were performed in 10 patients, showing chromosome 3 loss in two. Postoperative complications included hypotony in 9 (37%) patients, cataract in 8 (33%), hyphaema in 8 (33%), cyclodialysis in 1 (4%), wound dehiscence in 1 (4%) and bullous keratopathy in 1 patient (4%). The median follow-up time was 2.4 years. The last known visual acuity was 6/6–6/12 in 20 (91%) patients and 6/18–6/60 in 2 (9%), with 2 (8%) requiring secondary enucleation. Local tumour recurrence developed in 1 patient (4%). Two (8%) patients died of metastatic disease.
Conclusions
Surgical resection of peripheral iris melanomas achieves high rates of visual conservation and local tumour control and may be the preferred option when tissue is required for laboratory studies.
Introduction
Irido-cyclectomy has long been a standard procedure for the treatment of iris melanomas involving the angle and for selected ciliary body melanomas.1, 2, 3 Other forms of treatment of anterior uveal melanomas include brachytherapy and proton beam radiotherapy. These do not provide tissue for diagnosis and/or prognostication unless a biopsy is performed.4 Histological diagnosis is useful because it can be impossible clinically to differentiate melanoma from other pigmented tumours, such as naevus, melanocytoma, and adenocarcinoma.5, 6
The conventional method of performing irido-cyclectomy is to resect the tumour antero-posteriorly, starting the dissection at the pupil margin (ie, ‘irido-cyclectomy').3, 7 Some authors have advised preoperative mydriasis.2, 3 This method results in a large iris coloboma, which is disfiguring and which can cause troublesome photophobia. These problems can be alleviated by prescribing a painted contact lens, inserting an artificial iris, or suturing the iris.
To overcome these difficulties, the senior author (BD) has modified his surgical technique, resecting the tumour in a postero-anterior or circumferential direction, starting the dissection at the iris root (ie, ‘cyclo-iridectomy'), with preoperative pupillary constriction to facilitate conservation of the iris sphincter.8
The aim of the study was to audit the results of irido-cyclectomy and cyclo-iridectomy of iris and ciliary body melanomas at the Liverpool Ocular Oncology Centre (LOOC).
Materials and methods
Patients were included in the study if they underwent primary irido-cyclectomy or cyclo-iridectomy for uveal melanoma at LOOC between 1993 and 2012. They were excluded if the tumour extended posterior to the ora serrata or if it had previously been treated by radiotherapy. Generic consent was prospectively obtained from all patients for their data, tissues, and images to be used for research, teaching, and audit. As this was a service evaluation, ethical committee approval for the audit was not required.
Preoperative assessment
Assessment included the following: systematic history; visual acuity measured with a Snellen chart until 1997 and with a LogMAR chart thereafter; applanation tonometry; slit-lamp examination; gonioscopy; binocular indirect ophthalmoscopy; high-frequency ultrasonography (since 1997); and anterior segment photography. Systemic investigations were performed for anaesthetic reasons and not to screen for metastatic disease.
Surgical procedure
The operation evolved during the course of this audit. Until 1997, all tumour excisions were performed antero-posteriorly, if possible conserving the iris sphincter but always dilating the pupil preoperatively with 1% cyclopentolate and 2.5% phenylephrine drops (ie, ‘irido-cyclectomy'). After 2005, all tumour resections were performed postero-anteriorly or circumferentially, commencing the dissection at the iris root (ie, ‘cyclo-iridectomy'). Preoperative miosis with 2% pilocarpine was commenced in 2007. Between 2000 and 2007, the tumour resection was performed in different ways (ie, antero-posteriorly in some patients and postero-anteriorly or circumferentially in others). Any adjunctive radiotherapy was administered with a ruthenium plaque, delivering a dose of 100 Gy at 1 mm. Initially this radiotherapy was given at the time of the tumour resection, but after 2001 it was administered after a delay of at least 1 month, to prevent hypotony.
The current technique is as follows: the pupil is constricted preoperatively with 2% pilocarpine. The surgery is performed under general anaesthesia, with minimal or no hypotension. A 180° conjunctival peritomy is performed. Two traction sutures are placed, one on either side of the intended resection area. The tumour is localized by trans-pupillary and trans-ocular trans-illumination, using a 20-gauge fibreoptic light, pre-bent by the manufacturer 90°, and the tumour margins are marked on the sclera with a pen. Any tumour visible in the anterior chamber is used as a reference point. Account is taken of the ultrasonographic tumour dimensions. If the tumour extends close to a rectus muscle insertion, the muscle is dis-inserted.
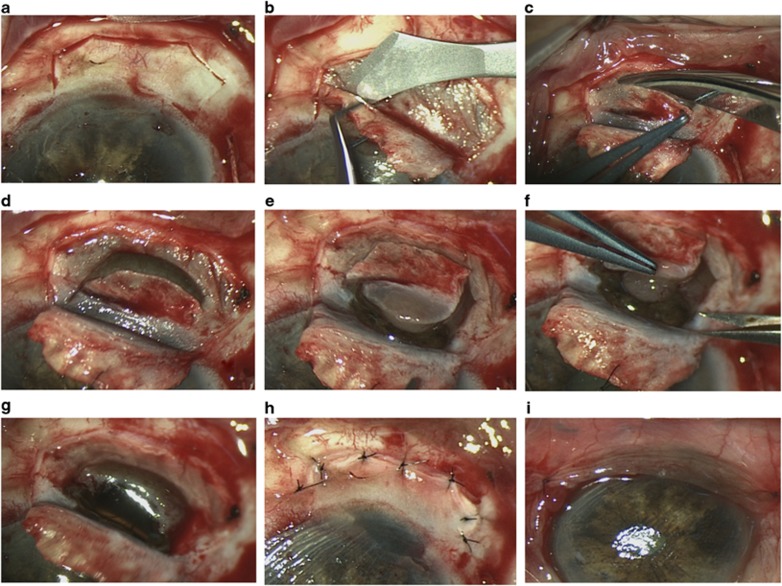
Using a 30° microfeather (Feather Safety Razor Co., Ltd, Osaka, Japan) and then a 5700 scleral blade (Surgistar, Vista, CA, USA) (ie, Desmarres scarifier), a lamellar scleral flap is made, which is as thick as possible, hinged anteriorly, and which extends into the cornea so that the normal iris anterior to the tumour margin is visible through the deep cornea (Figures 1a and b). A ‘bulldog' (A5132, Altomed, Boldon, UK) suspended on a loose 8-O nylon suture is used to retract the scleral flap. The scleral flap is polyhedral, to facilitate wound apposition during closure. A deep sclerotomy is made, ∼1 mm posterior to the limbus and 1–2 mm within the outer scleral incision, so as to create a stepped wound edge. This is extended with blunt-tipped, spring corneoscleral scissors (ie, A1415, Altomed) (Figure 1c). Toothed colibri forceps (ie, A3822, Altomed) are used to grasp the tissues. Gentle bipolar diathermy is applied to the uveal blood vessels posterior and lateral to the tumour.
Figure 1.
Surgical procedure of cyclo-iridectomy. After a localized conjunctival peritomy, the tumour is localized by trans-illumination and a thick, polyhedral, scleral flap is prepared (a, b). Next, a deep scleral incision is made with blunt scissors posteriorly, laterally, and anteriorly around the tumour base (c–e). Toothed colibri forceps are used to grasp the scleral flap, which serves as a handle during tumour excision (f, g). The scleral flap is closed with interrupted nylon sutures (h) and conjunctiva with vicryl sutures (i). The treated patient was a 76-year-old man. Histopathological examination revealed an epithelioid melanoma with chromosome 3 loss. Six weeks after uneventful surgery the vision was 6/19.
If the tumour involves the iris or angle, an iridotomy is made adjacent to the iris root and extended around the anterior margin of the tumour and back to the angle on the other side of the lesion. If the tumour is located entirely posterior to the iris root, then the uvea is perforated in the region of the pars plicata and the wound is extended with blunt-tipped, spring scissors around the tumour margin, first posteriorly (Figure 1d) and then anteriorly (Figure 1e) so that the iris is spared if it is not involved. The surgical clearance margin is ∼0.5–1 mm wide in the iris and 1–2 mm wide in the ciliary body. The tumour is lifted out of the eye using the deep scleral lamella as a handle (Figure 1f). The specimen is transported to the pathology laboratory in formalin.
Fresh instruments are used for wound closure. If the intact vitreous bulges through the scleral opening (Figure 1g), it can gently be pushed back while suturing the sclera in a zip-like fashion; otherwise, a limited open-sky vitrectomy is performed. The sclera is closed with interrupted, 8-O nylon sutures (Figure 1h) and the conjunctiva is closed with 7-O vicryl sutures (Figure 1i). It is not usually necessary to inject fluid into the anterior chamber, which re-forms spontaneously.
At the end of the operation, 1% atropine and 0.5% chloramphenicol drops are instilled. A pressure bandage is applied. The duration of the procedure is ∼45 min.
Postoperative care
Postoperatively, patients were treated with the following topical agents: 1% atropine, twice daily, for 1 week; 0.5% chloramphenicol, four times daily; and dexamethasone, six to eight times daily, for one month. With medial tumours, chloramphenicol ointment was administered instead of drops, to prevent dellen formation. If the intraocular pressure was less than 5 mm Hg on the first postoperative day, a pressure bandage was applied with a double pad to compress the eye. Adjunctive brachytherapy was administered if histology showed the melanoma to be of high-grade malignancy or if surgical clearance was inadequate or uncertain. If genetic studies showed chromosome 3 loss, which indicates a high risk of metastatic disease, the patient was referred to an oncologist for appropriate management, which usually involved screening for metastatic disease and long-term surveillance.
Statistical methods
The baseline features and outcomes of irido-cyclectomy and cyclo-iridectomy were compared using the χ2-test (STATISTICA, version 10; StatSoft, Inc. (2011), Tulsa, OK, USA).
Results
The patients comprised 8 (33%) men and 16 (67%) women, with a median age of 57 years (range, 14.2–80.7) (Table 1). The tumour affected the right eye in 12 patients and the left eye in 12. The initial visual acuity was 6/6–6/12 in 20 (83%) patients and 6/18–6/60 in 4 (17%). Concurrent ocular disease included high myopia in one patient. The circumferential extent of the tumour had a median of 1.8 clock hours (range, 1–4). The largest basal tumour diameter and the tumour thickness had medians of 4.8 mm (range, 1.8–12.0) and 2.2 mm (range, 0.9–7.3) respectively (Table 1).
Table 1. Baseline characteristics of patients treated by irido-cyclectomy or cyclo-iridectomy.
| Baseline data | Total (N=24) | Irido-cyclectomy (N=9) | Cyclo-iridectomy (N=15) | P value (χ2) |
|---|---|---|---|---|
| Sex | ||||
| Male | 8 (33%) | 3 (33%) | 5 (33%) | |
| Female | 16 (67%) | 6 (67%) | 10 (67%) | 1.000 |
| Age | ||||
| <40 | 5 (21%) | 2 (22%) | 3 (20%) | |
| 40–60 | 11(46%) | 6 (67%) | 5 (33%) | |
| >60 | 8 (33%) | 1 (11%) | 7 (47%) | 0.173 |
| Eye | ||||
| Left | 12 (50%) | 3 (33%) | 9 (60%) | |
| Right | 12 (50%) | 6 (67%) | 6 (40%) | 0.400 |
| Visual acuity | ||||
| 6/6–6/12 | 21 (88%) | 7 (78%) | 14 (93%) | |
| 6/18–6/60 | 3 (12%) | 2 (22%) | 1 (7%) | 0.533 |
| Anterior margin | ||||
| Angle | 9 (37%) | 2 (22%) | 7 (47%) | |
| Iris | 14 (59%) | 7 (78%) | 7 (47%) | |
| Ciliary body | 1 (4%) | 0 | 1 (6%) | 0.297 |
| Posterior margin | ||||
| Iris | 0 | 0 | 0 | |
| Ciliary body | 24 (100%) | 9 (100%) | 15 (100%) | |
| Circumferential spread: iris | ||||
| <1 | 4 (16%) | 0 | 4 (27%) | |
| 1–3 | 18 (75%) | 8 (89%) | 10 (67%) | |
| >3 | 2 (8%) | 1 (11%) | 1 (6%) | 0.234 |
| Circumferential spread: angle | ||||
| <1 | 4 (17%) | 3 (33%) | 1 (7%) | |
| 1–3 | 18 (75%) | 5 (55%) | 13 (87%) | |
| >3 | 2 (8%) | 1 (11%) | 1 (7%) | 0.196 |
| Circumferential spread: ciliary body | ||||
| <1 | 2 (8%) | 1 (11%) | 1 (7%) | |
| 1–3 | 20 (84%) | 7 (78%) | 13 (86%) | |
| >3 | 2 (8%) | 1 (11%) | 1 (7%) | 0.852 |
| Basal tumour diameter (n=21) | ||||
| <5.0 mm | 11 (52%) | 1 (16%) | 10 (67%) | |
| >5.0 mm | 10 (48%) | 5 (84%) | 5 (33%) | 0.064 |
| Tumour thickness (n=21) | ||||
| <3.0 mm | 12 (58%) | 3 (50%) | 9 (60%) | |
| >3.0 mm | 9 (42%) | 3 (50%) | 6 (40%) | 1.000 |
| Melanoma cytomorphology | ||||
| Spindle | 15 (63%) | 4 (44%) | 11 (73%) | |
| Mixed/epithelioid | 9 (37%) | 5 (56%) | 4 (27%) | 0.212 |
The resection was performed antero-posteriorly in nine (37%) patients and postero-anteriorly or circumferentially in 15 (63%). Preoperatively, the pupil was dilated in nine patients, constricted in seven, and left untreated in eight. Adjunctive brachytherapy was administered in six patients, using a 15 mm plaque in five patients and a 20 mm plaque in one patient. This radiotherapy was delivered at the time of the local resection in one patient and after 1 month in three patients, 2 months in one patient and 4 months in one patient. Vitreous loss, which was inconsequential, occurred in two patients.
Histology showed the melanoma to be mixed or epithelioid in nine patients and spindle in 15. One melanoma proved to be of spindle, balloon cell type. Genetic studies were performed in 10 patients, showing chromosome 3 loss in 2.
The median time follow-up was 2.4 years, ranging from 6 months to 14.3 years. It was >5 years in 6 patients, between 2 and 5 years in 7 patients and <2 years in 11 patients.
The last known visual acuity was 6/6–6/12 in 20 (83%) patients and 6/18–6/60 in 2 (8%). Two (8%) patients required secondary enucleation. The reasons for enucleation were tumour recurrence in one patient and bullous keratopathy in another.
Postoperative complications
Hypotony developed in nine (37%) patients, with the lowest intraocular pressure reaching 3 mm Hg in two patients and 4–7 mm Hg in seven patients. The hypotony resolved within 4 weeks in seven patients but persisted >3 months in two, returning to normal by 6 months in both of these. At the close of the study no patient had hypotony. Transient, secondary effects of the hypotony included choroidal effusion in four patients and macular oedema in two. The last known visual acuity in the two patients with macular oedema was 6/12 and 6/6, respectively.
Cataract developed in eight (33%) patients, occurring a median of 6.3 months after the surgical resection (range, 2–12). Cataract surgery was performed in all eight patients, with the use of a tension ring in two patients.
Other postoperative complications were hyphaema in eight (33%) patients, cyclodialysis in one (4%) patient, wound dehiscence in one (4%) patient and bullous keratopathy in one (4%) patient (Table 2). Local tumour recurrence developed in one (4%) patient. This was a 66-year-old woman with an 8.4 mm-diameter tumour involving 4 clock hours of the iris and 4 clock hours of the ciliary body. The surgery was performed in 2002, postero-anteriorly, with the pupil left untreated and adjunctive brachytherapy with 15 mm Ru-106 plaque 1 month after surgery. The recurrence developed 6 months postoperatively and was treated by enucleation.
Table 2. Postoperative complications and final visual acuity according to the surgical approach (ie, irido-cyclectomy or cyclo-iridectomy).
| Outcomes | Total (N=24) | Irido-cyclectomy (N=9) | Cyclo-iridectomy (N=15) | P value (χ2) |
|---|---|---|---|---|
| Visual acuity (n=22) | ||||
| 6/6–6/12 | 20 (92%) | 6 (75%) | 14 (100%) | |
| 6/18–6/60 | 2 (8%) | 2 (25%) | 0 | 0.130 |
| Ocular conservation | ||||
| Yes | 22 (92%) | 8 (89%) | 14 (93%) | |
| No | 2 (8%) | 1(11%) | 1 (7%) | 1.000 |
| Local tumour recurrence | ||||
| Yes | 1 (4%) | 0 | 1 (7%) | |
| No | 23 (96%) | 9 (100%) | 14 (93%) | 1.000 |
| Hypotony | ||||
| Yes | 9 (37%) | 4 (44%) | 5 (33%) | |
| No | 15 (63%) | 5 (56%) | 10 (68%) | 0.679 |
| Hyphaema | ||||
| Yes | 8 (33%) | 4 (44%) | 4 (27%) | |
| No | 16 (67%) | 5 (55%) | 11 (73%) | 0.412 |
| Cataract | ||||
| Yes | 8 (33%) | 5 (55%) | 3 (20%) | |
| No | 16 (67%) | 4 (44%) | 12 (80%) | 0.099 |
Comparison of irido-cyclectomy with cyclo-iridectomy showed no differences between the two groups.
Three patients died, the cause of death being metastatic disease in two (8%) patients. Histology showed the tumour to be of epithelioid cell type in both cases. Genetic studies were not performed in either of these cases because such investigations were not available at the time.
Discussion
Main results
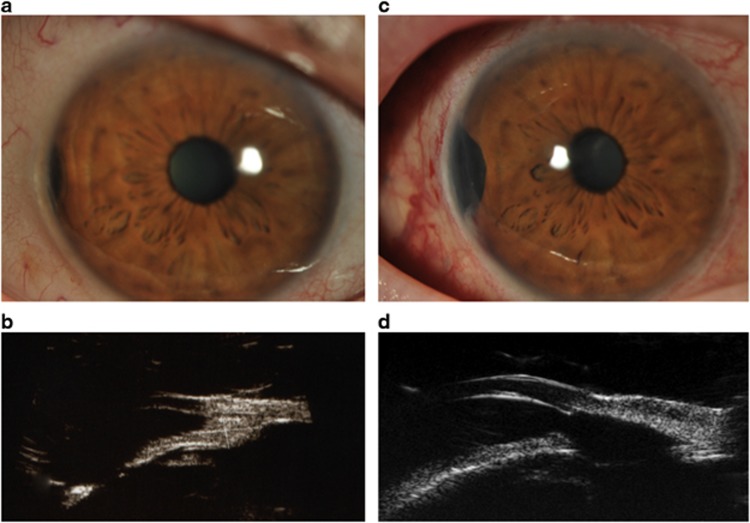
Our study indicates high rates of ocular conservation, local tumour control, and conservation of vision after irido-cyclectomy and cyclo-iridectomy for selected iris and ciliary body melanomas (as shown in Figure 2).
Figure 2.
Iris and ciliary body tumour in the right eye of a 72-year-old woman (a) treated with cyclo-iridectomy. On high-frequency ultrasonography the tumour measured 3.0 by 3.5 mm basally, with a thickness of 1.5 mm (b). One clock hour of ciliary body was involved. Six weeks after surgery, the visual acuity was 6/9 (c). Ultrasound scan showing the resection site (d). Histopathology showed the melanoma to be of spindle-cell type with no chromosome 3 loss.
The patient numbers were not large enough to perform meaningful statistical comparisons between irido-cyclectomy with mydriasis and cyclo-iridectomy with miosis. Because of the low rate of complications with both methods, many patients would have been required for such analyses. It would be difficult to achieve cohort sizes that are large enough, because many patients now undergo proton beam or plaque radiotherapy. In any case, this study provides useful information about the techniques and the outcomes that can be expected using current technology.
Discussion of surgical technique
Preoperative miosis stretches the iris, making the dissection easier, thereby reducing the size of the iris coloboma and increasing the chances of conserving the iris sphincter.
When performing postero-anterior resection of a ciliary body tumour, some tumours peel away from the iris, which can be left intact, with a better cosmetic and functional result. If the tumour proves to be benign, as has occurred in a small number of patients, no further treatment is necessary, despite the absence of safety margins.
In most patients, it was possible to avoid vitreous loss. Although listed as a complication, vitreous loss did not seem to influence the outcome adversely. It may even prevent malignant glaucoma, which was seen in one patient (not included in this series because the operation was performed by the senior author (BD) before 1993 at another hospital).
By commencing the tumour resection at the iris root or in the ciliary body, rather than at the tumour margin, there is no need to place instruments deeply in the anterior chamber and, furthermore, the size of the surgical wound can be smaller. This surgical approach is no more difficult than conventional irido-cyclectomy.
It is often possible to conserve most or all of the zonular fibres in the resected area; however, such zonular fibre preservation does not prevent subluxation of the lens if the resection is extensive. If the subluxated lens comes into contact with the cornea, it can cause keratopathy and opacification.
Some authors have advocated full-thickness eyewall resection because of concerns about intra-scleral recurrence;9 however, this complication is rare, even when adjunctive brachytherapy is not administered.
Viscoelastic materials are not required as the anterior chamber re-forms spontaneously. Although such viscoelastics may prevent postoperative hypotony, the low intraocular pressure is only transient in most cases, without sequelae.
Strengths and weaknesses
The main strengths of the study are the prospective data collection and documentation using a computer database. As mentioned before, the main weakness is the small number of patients. Another weakness is the lack of randomization, but this would have been unethical as equipoise was lost once the good results of the initial cyclo-iridectomies became apparent. Out of seven patients treated between 2001 and 2007 with both methods of excision but with pupil left untreated preoperatively, none had photophobia or any major ocular complaints apart from grittiness at 6-month follow-up.
Comparison with previous studies
Naumann and Rummelt9 developed a technique of full-thickness eye-wall resection with a tectonic corneoscleral graft and reported their results in a group of 68 patients with iris and ciliary body tumours. The main intraoperative problem was vitreous haemorrhage (35%). The rate of postoperative cataract was 32%. The globe was retained in 94% of cases, with the vision being better than 20/60 in 53% of patients.
Daubner et al10 reported local tumour recurrence in 10% of 39 irido-cyclectomies performed between 1980 and 2002. However, in their series only 30% of procedures were done with a lamellar scleral flap. A visual acuity of 0.5 or better was retained in only 57% of patients. Three patients required enucleation.
In 1986, Damato and Foulds reported a series of 60 patients treated by exoresection of a ciliary body tumour during a period of 16 years. That study included cilio-choroidal melanomas, which were excluded from the present investigation. Eye globe retention rate was 78% and 15% patients developed metastases with a mean follow-up of 34 months. The main postoperative complications were vitreous haemorrhage (40%), retinal detachment (26%), and incomplete tumour excision in 20% of cases.11
In a series of 52 patients who underwent irido-cyclectomy between 1960 and 1978 and whose records were submitted to the Registry of Armed Forces Institute of Pathology, 29% globes had to be enucleated and 11% of patients died of metastatic disease at 8-year follow-up.12
Current indications and contraindications
Our current approach is to perform cyclo-iridectomy if (1) the tumour involves the angle or ciliary body; (2) it is possible to conserve the iris sphincter; (3) the circumferential extent does not exceed 2 clock hours; and if (4) the posterior tumour margin is located anterior to the ora serrata.
We prefer proton beam radiotherapy for melanomas confined to the iris. This is because our audit has shown the results to be relatively good, albeit with cataract and glaucoma requiring treatment in patients with more extensive tumours.13, 14
We also prefer proton beam for ciliary body melanomas whose circumferential extent exceeds 2 clock hours. This is because extensive cyclectomy is more likely to be complicated by lens subluxation and persistent hypotony.
If excision is performed for tumours extending to the ora or choroid, the operation is performed differently, with the lamellar scleral flap hinged posteriorly and the uvea perforated posterior to the ora so as to make it easier to conserve the ciliary epithelium over pars plana.8
Plaque radiotherapy is avoided with thick tumours, because a high basal dose of radiation has been reported to cause scleral necrosis.15, 16 We prefer proton beam radiotherapy unless this is expected to cause canalicular damage, that is, if the tumour is medial and extends far posteriorly.
Conclusions
The results of this audit indicate high rates of visual conservation and local tumour control, both with irido-cyclectomy and with cyclo-iridectomy. These results have resulted in a shift away from radiotherapy to tumour resection, which has the additional advantage of providing a better tissue sample for histological and genetic studies.

The authors declare no conflict of interest.
References
- Vail DT. Iridocyclectomy. A review. Gleanings from the literature. Am J Ophthalmol. 1971;71:161–168. [PubMed] [Google Scholar]
- Reese AB, Jones IS, Cooper WC. Surgery for tumors of the iris and ciliary body. Am J Ophthalmol. 1968;66 (2:173–184. doi: 10.1016/0002-9394(68)92062-x. [DOI] [PubMed] [Google Scholar]
- Stallard HB. Surgery of malignant melanoma of the iris. Br J Ophthalmol. 1951;35 (12:774–778. doi: 10.1136/bjo.35.12.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damato BE. Treatment selection for uveal melanoma. Dev Ophthalmol. 2012;49:16–26. doi: 10.1159/000328251. [DOI] [PubMed] [Google Scholar]
- Damato EM, Damato B, Sibbring JS, Coupland SE. Ciliary body melanoma with partial deletion of chromosome 3 detected with multiplex ligation-dependent probe amplification. Graefes Arch Clin Exp Ophthalmol. 2008;246 (11:1637–1640. doi: 10.1007/s00417-008-0855-y. [DOI] [PubMed] [Google Scholar]
- Schalenbourg A, Coupland S, Kacperek A, Damato B. Iridocyclectomy for neovascular glaucoma caused by proton-beam radiotherapy of pigmented ciliary adenocarcinoma. Graefes Arch Clin Exp Ophthalmol. 2008;246 (10:1499–1501. doi: 10.1007/s00417-008-0852-1. [DOI] [PubMed] [Google Scholar]
- Zografos L.IridocyclectomieIn: Zografos L, (ed).Tumeurs intraoculaires Société Française d'Ophthalmologie et Masson: Paris; 2002 [Google Scholar]
- Damato BE. Local resection of uveal melanoma. Dev Ophthalmol. 2012;49:66–80. doi: 10.1159/000328261. [DOI] [PubMed] [Google Scholar]
- Naumann GOH, Rummelt V. Block excision of tumours of anterior uvea. Report on 68 consecutive patients. Ophthalmology. 1996;103:2017–2023. doi: 10.1016/s0161-6420(96)30392-8. [DOI] [PubMed] [Google Scholar]
- Daubner D, Prokosch V, Busse H, Stupp T. Long-term results of iridocyclectomy for iris tumours. Klin Monbl Augenheilkd. 2008;225 (12:1045–1050. doi: 10.1055/s-2008-1027609. [DOI] [PubMed] [Google Scholar]
- Damato BE, Foulds WS. Cilary body tumours and their management. Trans Ophthalmol Soc UK. 1986;105:257–264. [PubMed] [Google Scholar]
- Memmen JE, McLean IW. The long term outcome of patients undergoing iridocyclectomy. Ophthalmology. 1990;97:429–432. doi: 10.1016/s0161-6420(90)32562-9. [DOI] [PubMed] [Google Scholar]
- Damato B, Kacperek A, Chopra M, Sheen MA, Campbell IR, Errington RD, et al. Proton beam radiotherapy of iris melanoma. Int J Radiat Oncol Biol Phys. 2005;63 (1:109–115. doi: 10.1016/j.ijrobp.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Konstantinidis L, Roberts D, Errington RD, Kacperek A, Damato B. Whole anterior segment proton beam radiotherapy for diffuse iris melanoma. Br J Ophthalmol. 2013;97 (4:471–474. doi: 10.1136/bjophthalmol-2012-302659. [DOI] [PubMed] [Google Scholar]
- Gunduz K, Shields CL, Shields JA, Cater J, Brady L. Plaque radiotherapy of uveal melanoma with predominant ciliary body involvement. Arch Ophthalmol. 1999;117 (2:170–177. doi: 10.1001/archopht.117.2.170. [DOI] [PubMed] [Google Scholar]
- Groenewald C, Konstantinidis L, Damato B. Effects of radiotherapy on uveal melanomas and adjacent tissues. Eye (Lond) 2012;27 (2:163–171. doi: 10.1038/eye.2012.249. [DOI] [PMC free article] [PubMed] [Google Scholar]