Abstract
Background
Although patient time costs are recommended for inclusion in cost-effectiveness analyses, these data are not routinely collected. We used nationally representative data and a medical service-based approach to estimate annual patient time costs among cancer survivors.
Methods
We identified 6,699 cancer survivors and 86,412 individuals without a cancer history ≥ 18 years from the 2008–2011 Medical Expenditure Panel Survey (MEPS). Service use was categorized as hospitalizations, emergency room (ER) use, provider visits, ambulatory surgery, chemotherapy, and radiation therapy. Service time estimates were applied to frequencies for each service category and the U.S. median wage rate in 2011 was used to value time. We evaluated the association between cancer survivorship and service use frequencies and patient time costs with multivariable regression models, stratified by age group (18–64 and 65+ years). Sensitivity analyses evaluated different approaches for valuing time.
Results
Cancer survivors were more likely to have hospitalizations, ER visits, ambulatory surgeries, and provider visits in the past year than individuals without a cancer history in adjusted analyses (p<0.05). Annual patient time was higher for cancer survivors than individuals without a cancer history among those ages 18–64 (30.2 vs. 13.6 hours; p<0.001) and ages 65+ (55.1 vs. 36.6 hours; p<0.001), as were annual patient time costs (18–64 years: $500 vs. $226; p<0.001 and 65+ years: $913 vs. $607; p<0.001).
Conclusions
Cancer survivors had greater annual medical service use and patient time costs than individuals without a cancer history. This medical service-based approach for estimating annual time costs can also be applied to other conditions.
Keywords: cost of illness, costs and cost analysis, utilization, neoplasms, Medical Expenditure Panel Survey, time cost
INTRODUCTION
In 2012, more than 13.7 million individuals in the United States were alive with a history of cancer (1). The prevalence of cancer survivors is expected to increase in the future due to population growth, the aging of the US population, and improved survival following diagnosis (2). Cancer survivors receive medical care throughout the trajectory of their cancer experience, including the initial period following diagnosis, long-term survivorship, and for some, recurrence, and end-of-life care. In addition to being at greater risk for additional cancers (3;4), many survivors will experience lasting or late effects of disease or its treatment (5) requiring medical care.
Much work has been devoted to describing the medical costs of cancer care in the United States (2;6–9). However, less attention has been paid to describing the patient time costs associated with medical care . Time spent traveling to and from care, waiting for appointments, consulting with physicians and other providers, and receiving care represents time not spent pursuing other, usual activities, including work and leisure. The opportunity costs associated with patient time have been recommended for inclusion in cost-effectiveness analyses of health interventions since 1996 by the Panel for Cost-Effectiveness in Health (10), yet these data are rarely considered, potentially resulting in a bias towards underestimating the costs of interventions that place a higher burden on cancer patients and families (11).
Prior work estimating patient time costs among cancer survivors has used the linked SEER registry-Medicare claims data and combined the frequency of medical services by category (e.g., hospitalizations, ambulatory surgery) with service-specific time estimates and hourly values of time (12;13). This work was conducted in the elderly population aged 65 years and older living in 11 SEER geographic areas with Medicare fee-for-service coverage. To our knowledge, patient time costs have not been reported for a nationally representative sample of cancer survivors of all ages, or among cancer survivors with all types of health insurance coverage, as well as the uninsured. In this study, we build on prior work (12;13) and use nationally representative data to estimate the per-capita annual time costs associated with cancer survivorship in the United States. These estimates can be used as inputs in health care decision making and cost-effectiveness analyses. Importantly, this service-based approach with publicly available data can also be used to estimate time costs for patients with other conditions, such as heart disease or diabetes.
METHODS
Sample
The sample was selected from the 2008–2011 Medical Expenditure Panel Survey (MEPS) Household Component. The MEPS is an ongoing nationally representative survey of healthcare expenditures, insurance, utilization, and access to care in the U.S. civilian non-institutionalized population. In-person interviews are conducted with a family member who typically responds for all family members in the household. Additional information about health care utilization, charges, and payments by source, including out-of-pocket payments, is collected from medical care providers for members of each household. The 2008–2011 MEPS had a combined average annual response rate ranging from 54–59%. More information about survey design and content is available from http://www.meps.ahrq.gov/mepsweb/.
We identified 6,699 adult cancer survivors from a question asking if a doctor or other health professional had ever told the person they had cancer or a malignancy of any kind. Respondents were asked about the cancer type and age at diagnosis, for each diagnosis. The remaining 86,412 adults with no cancer history were the comparison group. Prior studies have not classified individuals diagnosed solely with non-melanoma skin cancer as cancer survivors, but included them in the comparison group (9;14). To evaluate the impact of classifying individuals with only non-melanoma skin (N=1,179) as without a cancer history rather than excluding them from analyses, we conducted all analyses with and without these individuals. Findings were not perceivably different and we present data with non-melanaoma skin cancers included in the comparison group for consistency with prior studies.
Measures
Sample characteristics included age, gender, race/ethnicity, marital status, educational attainment, employment in the past 12 months, and health insurance status and type. Conditions other than cancer were identified with a series of questions about whether a doctor or other health professional ever told the person they had any MEPS priority conditions, including arthritis, asthma, hypertension, angina, coronary heart disease, stroke, diabetes, high cholesterol, heart attack, and emphysema. Conditions were categorized by the number of priority conditions for each individual.
Time since cancer diagnosis was calculated as the difference between age at first diagnosis and age at the interview (i.e., <2 years, 2–5 years, 6–10 years, and 11+ years). Cancer survivors with a missing age at diagnosis or with an implausible age at diagnosis (N=589) were excluded from the time-since-diagnosis analyses only.
Medical service categories were identified from the MEPS visit files and consolidated files, and included overnight hospitalizations, emergency room visits, ambulatory surgery, provider office-based or hospital outpatient visits (including MEPS zero-night inpatient visits), chemotherapy, and radiation therapy. Service frequency for the year was measured for each category. The annual hospital length of stay was a summary of inpatient days from all hospitalizations for the year.
Time estimates for medical service categories are listed in Table 1. Estimates of patient time associated with round-trip travel to care, waiting for care, and receiving care were calculated separately for each service category, as in prior studies (12;13). We used the most recently available national data sources to estimate time. The average time spent with a physician during an office visit was calculated from the 2010 National Ambulatory Medical Care Survey (NAMCS) (12;15). Patient time for emergency room visits was calculated as the difference between arrival time and discharge time from the 2010 National Hospital Ambulatory Medical Care Survey Emergency Department Patient Record (NHAMCS-ED) (16). Chemotherapy duration was estimated from the linked SEER-Medicare data in 1995–2001. We identified cancer patients receiving chemotherapy during the year following diagnosis, calculated frequencies of CPT-4 chemotherapy infusion codes with time descriptions (e.g., for CPT-4 96410 infusion technique, up to 1 hour was counted at the midpoint of 30 minutes) and then calculated a weighted average of infusion time (13). Time for radiation therapy was estimated from the 2006–2007 NAMCS (17). Patient time in the hospital was measured as the difference between admission and discharge dates, and multiplied by 16 hours, an estimate of waking hours that could alternatively be spent pursuing usual activities (12;13). Patient time spent in ambulatory surgery and recovery was calculated as the difference between admission time and discharge time for outpatient surgeries from the 2001 Medicare Current Beneficiary Survey (18).
Table 1.
Patient Time Estimates for Service Categories (Including Wait Time) in Minutes
| Urban (MSA) | Rural (non-MSA) | Data sources | |
|---|---|---|---|
| Hospitalizations | Number of nights*960 minutes (16 hours) | Estimated directly | |
| Emergency room visits – ages 18–64 | 203.3 | 130.8 | 2010 NHAMCS-ED |
| Emergency room visits – ages 65+ | 232.0 | 166.7 | 2010 NHAMCS-ED |
| Office/outpatient visits | |||
| Outpatient surgery | 274.2 | 292.5 | 1992 NHIS; 2001 MCBS 1992 NHIS; weighted average of CPT-4 infusion codes 1995–2001 |
| Chemotherapy visits | 168.8 | 171.6 | SEER-Medicare 1992 NHIS; 2006–2007 |
| Radiation therapy visits | 58.8 | 61.6 | NAMCS |
| Other office/outpatient visits – ages 18–64 | 51.6 | 53.4 | 1992 NHIS; 2010 NAMCS |
| Other office/outpatient visits ages 65+ | 51.7 | 52.6 | 1992 NHIS; 2010 NAMCS |
| Round trip travel per visit | 35.2 | 38.8 | 2008–2010 MEPS |
NOTE: Wait time estimates from the 1992 NHIS (30.19 minutes urban and 33.02 minutes rural) added to chemotherapy visits, radiation therapy visits, and office and outpatient visits. Chemotherapy duration was estimated from the linked SEER-Medicare data. Among cancer patients receiving chemotherapy during the first year following diagnosis, frequencies of CPT-4 chemotherapy infusion codes with time descriptions were calculated and then a weighted average of chemotherapy duration was calculated. Round-trip travel time estimates added to all services.
MEPS = Medical Expenditure Panel Survey, NHAMCS-ED = National Hospital Ambulatory Medical Care Survey – Emergency Department, NHIS = National Health Interview Survey, NAMCS = National Ambulatory Medical Care Survey, MCBS = Medicare Current Beneficiary Survey
Round-trip travel time to usual source of medical care was estimated from responses to a question from the 2008–2010 MEPS about how long it takes to get to the usual medical provider . Travel time was added to all service time estimates. Waiting time was estimated from the 1992 National Health Interview Survey (NHIS) (19), the most recent year this question was included in the NHIS. Waiting time was added to office-based or hospital outpatient visits, chemotherapy, and radiation therapy estimates. Time estimates for emergency room visits, hospitalizations, and ambulatory surgeries were based on the difference between admission and discharge time, so waiting time was not added to these estimates separately. All patient time estimates were calculated separately by MSA and non-MSA status to reflect any differences in urban and rural travel, wait time, or practice patterns. Round-trip travel time to usual source of care, provider visits, and ER visits was also stratified by age group (18–64, 65+).
As in previous studies (12;13), we used the median U.S. wage rate from the Bureau of Labor Statistics, $16.57 per hour in 2011 (20) to value patient time in our primary base case analyses of all services as well as for service-specific estimates. In sensitivity analyses we evaluated a longer duration for hospitalization time and an approach for valuing time using actual hourly wages for the MEPS participants employed for wages. For those who were not employed, we used the hourly value of home production, $5.32 per hour (adjusted to 2011 dollars) (21), to value their time.
Analyses
Distributions of descriptive characteristics were compared for cancer survivors and individuals without a cancer history stratified by age group (18–64 and 65+ years) with chi-square statistics. We estimated service category frequencies for cancer survivors and individuals without a cancer history. We then applied time estimates for each type of service, summarized patient time, and applied the hourly value of patient time.
All multivariable analyses of service frequencies, patient time, and time costs controlled for age, gender, educational attainment, and number of comorbid conditions. Wald statistics were used to test the statistical significance of differences between cancer survivors and individuals without a cancer history. We present unadjusted bivariate estimates and adjusted predicted marginals from the multivariable regression analyses. The predictive margins method (22) directly standardizes the outcome of each group to the covariate distribution of the overall population. These standardized results can be compared like percentages. All tests of statistical significance were two-sided. All estimates were weighted to account for the MEPS complex survey design and survey non-response using SUDAAN (23)
RESULTS
Cancer survivors were more likely to be older, non-Hispanic white, have at least some college education, and have more MEPS priority conditions than individuals without a cancer history (Table 2). Cancer survivors were less likely to be employed and uninsured than those without a cancer history. The most common cancer diagnoses were breast and prostate cancers (data not shown). Most cancer survivors were diagnosed 6 or more years prior to the survey (52.7% and 59.7% for 18–64 and 65+ age groups, respectively), with fewer cancer survivors diagnosed within 2 years (13.7% and 8.9%) or 2–5 years (28.5% and 19.2%) prior to the survey.
Table 2.
Characteristics of Cancer Survivors and Individuals without a Cancer History, 2008–2011 Medical Expenditure Panel Survey
| 18–64 years | 65+ years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer survivor |
No cancer history |
Cancer survivor |
No cancer history |
|||||||
| n | Wtd % |
n | Wtd % |
Chi-square p-value |
n | Wtd % |
n | Wtd % |
Chi-square p-value |
|
| Age group (years) | ||||||||||
| 18–44 | 884 | 23.7 | 46,046 | 59.7 | <.001 | |||||
| 45–49 | 400 | 11.3 | 8,422 | 11.0 | ||||||
| 50–54 | 555 | 17.2 | 8,121 | 11.6 | ||||||
| 55–59 | 677 | 21.2 | 6,922 | 9.7 | ||||||
| 60–64 | 824 | 26.6 | 5,403 | 8.0 | ||||||
| 65–69 | 826 | 23.6 | 3,931 | 33.0 | <.001 | |||||
| 70–74 | 719 | 22.2 | 2,699 | 23.1 | ||||||
| 75–79 | 703 | 19.6 | 2,059 | 18.1 | ||||||
| 80+ | 1,111 | 34.6 | 2,809 | 25.8 | ||||||
| Sex | ||||||||||
| Male | 1,005 | 34.4 | 35,603 | 50.2 | <.001 | 1,638 | 48.7 | 4,756 | 41.9 | <.001 |
| Female | 2,335 | 65.6 | 39,311 | 49.8 | 1,721 | 51.3 | 6,742 | 58.1 | ||
| Race / ethnicity | ||||||||||
| Non-Hispanic white only | 2,203 | 81.4 | 31,827 | 64.5 | <.001 | 2,575 | 88.0 | 6,628 | 76.6 | <.001 |
| Non-Hispanic black only | 506 | 7.9 | 14,620 | 12.3 | 443 | 6.0 | 2,165 | 9.4 | ||
| Hispanic | 466 | 7.1 | 21,172 | 16.0 | 202 | 3.6 | 1,648 | 8.4 | ||
| Non-Hispanic other / multiple | 165 | 3.5 | 7,295 | 7.3 | 139 | 2.4 | 1,057 | 5.6 | ||
| Marital status | ||||||||||
| Married | 1,864 | 61.4 | 37,853 | 52.5 | <.001 | 1,793 | 54.5 | 5,993 | 54.6 | 0.950 |
| Not married | 1,476 | 38.6 | 37,061 | 47.5 | 1,566 | 45.5 | 5,505 | 45.4 | ||
| Education when first entered MEPS | ||||||||||
| Less than high school grad | 573 | 12.0 | 17,009 | 15.7 | <.001 | 807 | 18.2 | 3,621 | 22.9 | <.001 |
| High school graduate | 1,044 | 28.9 | 23,202 | 29.1 | 1,126 | 35.2 | 3,743 | 34.7 | ||
| Some college or more | 1,723 | 59.1 | 34,703 | 55.2 | 1,426 | 46.7 | 4,134 | 42.5 | ||
| Employed at any time in past 12 months | ||||||||||
| No | 1,152 | 30.4 | 16,663 | 18.6 | <0.001 | 2,712 | 79.3 | 8,835 | 74.8 | <0.001 |
| Yes | 2,138 | 68.1 | 57,567 | 80.6 | 512 | 16.5 | 2,329 | 21.9 | ||
| Health insurance | ||||||||||
| Age <65, any private | 2,179 | 75.0 | 45,465 | 70.8 | <0.001 | |||||
| Age <65, public only | 715 | 14.7 | 10,969 | 10.4 | ||||||
| Age <65, uninsured | 446 | 10.3 | 18,480 | 18.8 | ||||||
| Age 65+, Medicare only | 1,250 | 35.7 | 4,338 | 38.0 | <.001 | |||||
| Age 65+, Medicare and private | 1,684 | 55.2 | 5,011 | 50.0 | ||||||
| Age 65+, Medicare and public | 396 | 8.2 | 1,920 | 10.6 | ||||||
| Number of known MEPS priority conditions | ||||||||||
| 0 | 884 | 26.8 | 40,930 | 53.1 | <.001 | 198 | 6.2 | 997 | 8.4 | <.001 |
| 1 | 789 | 25.0 | 17,021 | 23.7 | 450 | 14.0 | 1,837 | 15.9 | ||
| 2 | 692 | 20.9 | 8,795 | 12.4 | 743 | 21.6 | 2,671 | 24.4 | ||
| 3+ | 975 | 27.3 | 8,168 | 10.8 | 1,968 | 58.2 | 5,993 | 51.2 | ||
| Years since first cancer diagnosis (excluding non-melanoma skin cancer) | ||||||||||
| Missing | 193 | 5.0 | 396 | 12.2 | ||||||
| <2 | 452 | 13.7 | 298 | 8.9 | ||||||
| 2–5 | 942 | 28.5 | 662 | 19.2 | ||||||
| 6–10 | 675 | 19.3 | 676 | 20.3 | ||||||
| >10 | 1,078 | 33.4 | 1,327 | 39.4 | ||||||
Health care utilization
In adjusted analyses, a higher percentage of cancer survivors received medical care in every service category (overnight hospitalizations, ambulatory surgery, ER visits, and provider office-based or outpatient hospital visits) than individuals without a cancer history (Table 3; all p<0.01). For example, in the 18–64 age group 81.4% of cancer survivors and 69.6% of individuals without a cancer history had a provider office-based or hospital outpatient visit, and in the 65+ age group, 95.5% and 90.7% had a provider visit, respectively. Among individuals with a particular service in the past year, the adjusted frequency of the service or length of stay was higher among cancer survivors compared to individuals without a cancer history (Table 3), although the differences were not always statistically significant. For example, cancer survivors aged 18–64 had an average of 10.3 provider office-based or hospital outpatient visits, whereas individuals without a cancer history had an average of 7.2 visits (p<0.001).
Table 3.
Health Care Utilization in Cancer Survivors and Individuals without a Cancer History, 2008–2011 Medical Expenditure Panel Survey
| Percentage with service | Mean service use among individuals with service | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer survivor | No cancer history |
Wald F p-value |
Cancer survivor | No cancer history | Wald F p-value |
||||||||
| Wted % |
Adj %* | Wted % |
Adj %* |
n | Wted Mean |
Pred. Marg.* |
n | Wted Mean |
Pred. Marg.* |
||||
| Ages 18–64 years | Emergency room visits | 18.6 | 16.7 | 12.3 | 12.4 | <.0001 | 677 | 1.5 | 1.5 | 9,340 | 1.4 | 1.4 | 0.0037 |
| Ambulatory surgery | 25.7 | 18.4 | 9.7 | 9.9 | <.0001 | 751 | 1.9 | 1.8 | 5,757 | 1.5 | 1.5 | <.0001 | |
| Hospitalizations/ total length of stay | 13.4 | 10.1 | 6.1 | 6.2 | <.0001 | 467 | 8.7 | 7.5 | 4,591 | 5.3 | 5.4 | 0.0028 | |
| Chemotherapy | 3.9 | 3.2 | 0.0 | 0.0 | <.0001 | 145 | 8.5 | 8.5 | |||||
| Radiation therapy | 3.3 | 2.2 | 0.1 | 0.1 | <.0001 | 98 | 16.1 | 15.6 | |||||
| Other provider office-based and hospital outpatient visits** | 89.0 | 80.4 | 68.4 | 69.0 | <.0001 | 2,912 | 11.1 | 9.2 | 46,561 | 6.9 | 7.0 | <.0001 | |
| Ages 65+ years | Emergency room visits | 20.6 | 19.7 | 16.9 | 17.2 | 0.0038 | 728 | 1.5 | 1.5 | 1,942 | 1.4 | 1.4 | 0.0303 |
| Ambulatory surgeries | 35.2 | 34.1 | 22.4 | 22.7 | <.0001 | 1,071 | 2.3 | 2.2 | 2,160 | 1.9 | 1.9 | 0.0011 | |
| Hospitalizations/ total length of stay | 22.8 | 21.7 | 15.6 | 15.9 | <.0001 | 742 | 9.8 | 9.7 | 1,682 | 8.5 | 8.5 | 0.1369 | |
| Chemotherapy | 4.0 | 4.2 | 0.1 | 0.1 | <.0001 | 141 | 9.0 | 8.9 | |||||
| Radiation therapy | 2.4 | 2.4 | 0.3 | 0.3 | <.0001 | 86 | 16.8 | 16.8 | |||||
| Other provider office-based and hospital outpatient visits** | 95.7 | 95.1 | 90.1 | 90.5 | <.0001 | 3,182 | 13.9 | 13.6 | 10,133 | 10.6 | 10.6 | <.0001 | |
NOTE: Percentage with service calculated among all cancer survivors and individuals without a cancer history ages 18–64 and 65+ years. Estimates for mean service use for chemotherapy and radiation therapy not reported for individuals without a cancer history due to small sample size.
Predicted marginals from a regression model with age, number of comorbid conditions, educational attainment and gender as covariates
Hospital outpatient visits include MEPS zero-night inpatient visits
Patient time costs
In the 18–64 age group, annual patient time for cancer survivors and individuals without a cancer history was 30.2 hours and 13.6 hours, respectively, in adjusted analyses (data not shown). In the older group, annual patient time was 55.1 hours and 36.6 hours, respectively. Of all service types, in-patient hospitalization days contributed the most to the time estimates. In the base case analysis using median US wage rate to value time, annual patient time costs were higher in cancer survivors compared to individuals without a cancer history in the 18–64 ($500 vs. $226) and the 65+ ($913 vs. $607) age groups (Table 4). The sensitivity analyses using actual reported wages in the MEPS and home production for those not employed to value time had a large impact on the magnitude of time cost estimates, reflecting the lower likelihood that cancer survivors were employed than individuals without cancer. This effect was particularly dramatic in the 65+ age group, where time cost estimates declined from the base case of $913 and $607 annually in cancer survivors and individuals without a cancer history, to $359 and $241 annually, respectively.
Table 4.
Patient Time Costs* in Cancer Survivors and Individuals without a Cancer History, 2008–2011 Medical Expenditure Panel Survey
| Cancer survivors |
Individuals without a cancer history |
Wald F p-value |
|||
|---|---|---|---|---|---|
| Ages 18–64 years | All services | Base case: median hourly wage rate from BLS ($16.57) and hospitalization time of 16 hours per day | 500.28 | 225.97 | <.0001 |
| Sensitivity analysis: Median hourly wage rate ($16.57) and hospitalization time of 24 hours per day |
615.18 | 268.08 | <.0001 | ||
| Actual hourly wage rate from MEPS for employed for wages, value of home production for not employed for wages and hospitalization time of 16 hours per day | 384.44 | 194.88 | <.0001 | ||
| Service- specific estimates | Emergency room visits | 16.47 | 10.64 | <.0001 | |
| Ambulatory surgery | 35.09 | 13.06 | <.0001 | ||
| Hospitalizations | 235.77 | 86.91 | <.0001 | ||
| Chemotherapy | 18.14 | 0.07 | <.0001 | ||
| Radiation therapy | 13.15 | 0.22 | <.0001 | ||
| Other provider office-based and hospital outpatient visits** | 186.17 | 117.01 | <.0001 | ||
| Ages 65+ years | All services | Base case: median hourly wage rate from BLS ($16.57) and hospitalization time of 16 hours per day | $912.96 | $606.91 | <.0001 |
| Sensitivity analysis: Median hourly wage rate ($16.57) and hospitalization time of 24 hours per day |
$1,149.02 | $762.89 | <.0001 | ||
| Actual hourly wage rate from MEPS for employed for wages, value of home production for not employed for wages and hospitalization time of 16 hours per day | $358.80 | $240.76 | <.0001 | ||
| Service- specific estimates | Emergency room visits | 19.72 | 15.96 | 0.0016 | |
| Ambulatory surgery | 68.66 | 37.91 | <.0001 | ||
| Hospitalizations | 483.08 | 319.32 | 0.0014 | ||
| Chemotherapy | 20.25 | 0.30 | <.0001 | ||
| Radiation therapy | 10.24 | 1.00 | <.0001 | ||
| Other provider office-based and hospital outpatient visits** | 319.32 | 237.76 | <.0001 |
Predicted marginals from regression models with age, number of comorbid conditions, educational attainment, and gender as covariates
Hospital outpatient visits include MEPS zero-night inpatient visits
NOTE: Service-specific estimates were calculated using the base case median wage rate from BLS ($16.57) to value patient time.
Among cancer survivors, annual time costs varied by time-since-diagnosis (Figure 1), although the pattern by time-since-diagnosis differed by age group. In the 18–64 age group, time costs were highest in those diagnosed within 2 years of the survey ($1,188) and lowest among those diagnosed 6–10 years ($459) and more than 10 years prior to the survey ($489). Patient time costs were intermediate in those diagnosed 2–5 years prior to the survey ($630). In the 65+ age group, annual time costs were highest in those diagnosed within 2 years of the survey ($1,542), but more similar in the other time periods since diagnosis ($955, $831, and $905 for 2–5, 6–10 and >10 years since diagnosis, respectively), likely reflecting older median age and more comorbidities in the later times-since-diagnoses categories. In both age groups, hospitalization was the largest component of time cost estimates, at all times since diagnosis (Supplemental Appendix).
Figure 1.
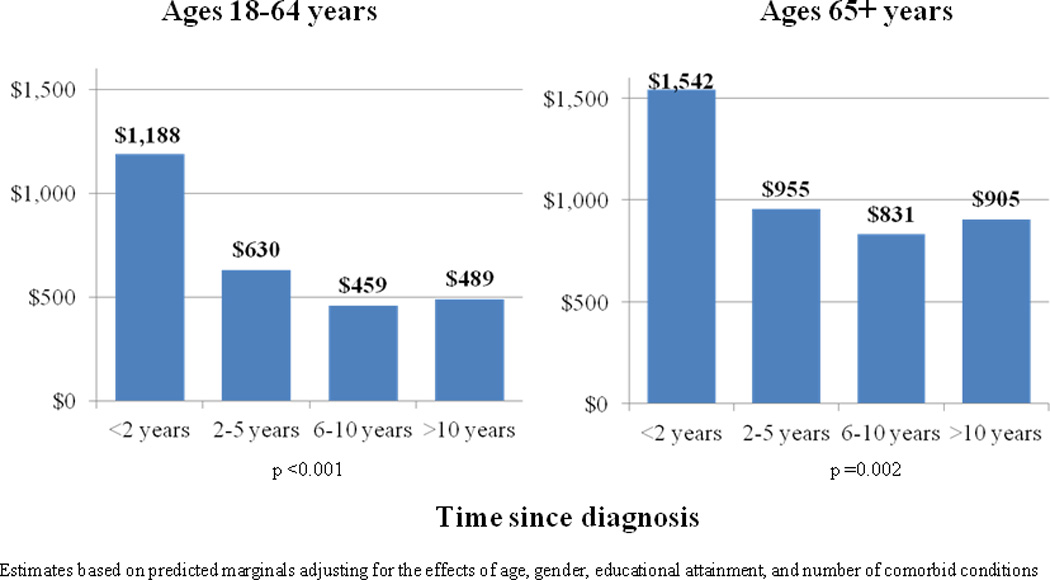
Estimated Annual Patient Time Costs Among Cancer Survivors, by Age and Time Since Diagnosis
DISCUSSION
In this study, we applied a medical service-based approach for estimating annual patient time costs associated with medical care among cancer survivors in a nationally representative sample of adults of all ages in the U.S. Because the MEPS contains comprehensive information about utilization, employment, and expenditures and the forthcoming MEPS Experiences with Cancer Survivorship Supplement (24) contains questions focused on cancer survivorship, our annual patient time cost estimates can be compared with other estimates of direct medical (6;9) and indirect productivity costs (9) associated with cancer for a more complete understanding of differences in burden of illness. Our time cost estimates can also be used as inputs for cost-effectiveness analyses of interventions aimed at improving care for cancer survivors. Importantly, the medical service-based approach we used in this study can also be applied to developing annual time cost estimates for patients with other conditions, such as diabetes or cardiovascular disease. Thus, the medical service-based approach with publically available data may help address the absence of routinely collected patient time cost data for use in cost-effectiveness analyses.
Annual patient time costs were about $300 higher in cancer survivors than in individuals without a cancer history, similar in magnitude to annual out-of-pocket expenditures (9). Others have reported that high out-of-pocket burdens are associated with reduced adherence to cancer care (25). Aspects of patient time, including traveling to and from care, waiting for, and receiving care may also serve as a barrier to adherence to care, particularly for patients and caregivers without paid sick leave from their employers (26). Further work exploring the impact of patient time burdens on adherence to recommended cancer care will be important in prospective studies.
Overall, our findings are consistent with prior service-based studies of cancer patient time costs, which reported higher service use and time costs for elderly cancer survivors compared to individuals without a cancer history in all phases of care - the initial period following diagnosis; the end of life period; and the continuing care phase between diagnosis and end of life. (12;13). In these prior studies, net time costs associated with cancer were highest in the initial and end of life phases, and lowest in the continuing care phase. In the current study, we did not have the complete date of diagnosis (only age at diagnosis) or date of death for classifying the end of life phase, and could only evaluate time costs by year since diagnosis in one-year increments. As a result, our estimates are not directly comparable with phase of care estimates.
An important strength of this study is the ability to compare time costs in adult populations with and without a cancer history in both the 18–64 and 65+ age groups. In prior studies of newly diagnosed cancer patients, younger patients tended to seek more aggressive surgical care (27–29), more adjuvant treatment (30), and greater travel for cancer care (30) than older cancer patients. Additionally, service use tended to be lower in younger compared to older individuals without a cancer history. However, we found that the incremental annual patient time costs were fairly similar in the two age groups, likely reflecting the high proportion of long-term survivors in our prevalence sample.
We did observe other age-related differences in time costs, however. As expected, time costs were highest among those diagnosed within the past 2 years in both age groups. However, annual time costs were more likely to be smaller for individuals with longer time-since-diagnosis intervals in the 18–64 population, whereas estimates were more similar for longer time-since-diagnosis intervals in the population aged 65 and older. Although all estimates were adjusted for age and comorbidity, this difference in patterns of time cost by time-since-diagnosis likely reflects relatively older ages and greater comorbidity in the longer time-since-diagnosis intervals in the population ages 65+. Additional work exploring age differences in service use and time costs by time-since-diagnosis for specific cancer sites will be important for future research.
Another strength of using the MEPS for our study is the availability of systematically collected medical service use and actual wage data for illustrating the impact of different approaches for valuing patient time in sensitivity analyses. In the elderly, annual time cost estimates declined more than 60% when actual wages and home production wages for those who are not paid for their labor were used to value time compared to the base case analysis, which used the median U.S. wage rate to value time. Time costs also declined in the younger population when using actual wages to value time, reflecting the fact that not all individuals work outside the home. Because time spent seeking medical care represents a lost opportunity for usual activities, including both work and leisure, we chose a single median wage rate for our base case approach, valuing each person’s time equally. Methods which value time differently for different populations may lead to inequities when evaluating the costs associated with health interventions, particularly for low-income and other vulnerable populations. It will be important for future work estimating patient time costs to be explicit about assumptions and the approach used for valuing patient time.
Our time cost estimates were based on medical care services, and as a result, did not include preparation time at home prior to medical care (e.g., preparation for colonoscopy (31;32)), post-treatment recovery time spent at home, or time spent addressing health insurance coverage issues. These are important components of patient time that may be best estimated from survey or micro-costing and time-and-motion techniques (31;32). Family or caregiver time spent traveling and waiting with patients for care or addressing other patient care needs, is another important component of burden (33–36), but was not available, and as a result could not be included. For all of these reasons, our net estimates are likely to understate the annual amount of time spent receiving cancer-related care, from the perspective of the patient, their family, and society.
The MEPS did not include information on hourly duration of most services or waiting time prior to receiving care. However hospitalizations were the major component of our time estimates, and their duration was based on the difference between admission and discharge dates measured and reported in the MEPS. In situations where information about service duration was not directly available from the MEPS, we used the most recently available data from national sources for service and travel time estimates, stratified by urban and rural residence. In several cases, namely chemotherapy and ambulatory surgery, data were available only for the elderly population but applied to both age groups. In other cases, only older survey data were available and may not reflect more current practice patterns, possibly resulting in bias of unknown direction. However, in situations where comparisons of time estimates between more recent and older surveys were possible, they were relatively similar. For example, round trip travel time to usual provider in MSAs estimated from the 1992 NHIS was 36.4 minutes (12). Using more recent data from the 2008–2010 MEPS in the current study, round-trip travel to usual provider in MSAs was 35.2 minutes. Additionally, our estimates for waiting and travel time were also similar to those reported for individuals seeking medical care from the 2003–2006 American Time Use Survey (36). Thus, we believe the impact of using older surveys to estimate average patient time is likely small.
There were also several general limitations with our study. Cancer survivors were identified by survey responses and we did not have information about stage at diagnosis, treatment(s), recurrence, or other clinical characteristics. Population-based household surveys generally include only small numbers of newly diagnosed, rare cancers, or cancers associated with short survival, and mainly consist of long-term survivors of common adult cancers; often participating many years after their diagnosis (37;38). Additionally, because the cancer diagnosis question refers to cancer or malignancy of any kind, it may include individuals with pre-invasive disease, likely resulting in an underestimate of differences between cancer survivors and individuals without a cancer history.
In summary, we found that cancer survivors had greater medical service use and patient time costs than did individuals without a cancer history. Our estimates of annual patient time costs can inform understanding of burden of illness and be used as inputs for conducting economic evaluations (e.g., cost-effectiveness) of health interventions in cancer survivors. The medical service-based approach we used in this study with the MEPS can also be applied to developing annual time cost estimates for patients with other chronic conditions.
Supplementary Material
Acknowledgments
This analysis was led by Federal employees with no external source of support for the work. The non-Federal collaborators volunteered their time on this project.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute, the Centers for Disease Control and Prevention, the American Cancer Society, the US Department of Health and Human Services. Emory University, or Massachusetts General Hospital.
Reference List
- 1.Howlander N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Bhatia S. Second primary cancers in survivors of childhood cancer. Lancet. 2009;31(374):1484–1485. doi: 10.1016/S0140-6736(09)61885-7. [DOI] [PubMed] [Google Scholar]
- 4.Ng AK, Travis LB. Second primary cancers: an overview. Hematol Oncol Clin North Am. 2008;22(2):271–289. doi: 10.1016/j.hoc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 6.Short PF, Moran JR, Rajeshwari P. Medical expenditures of adult cancer survivors aged <65 years in the United States. Cancer. 2011;117(12):2791–2800. doi: 10.1002/cncr.25835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard DH, Molinari N-A, Thorpe KE. National estimates of medical costs incurred by nonelderly cancer patients. Cancer. 2004;100:883–891. doi: 10.1002/cncr.20063. [DOI] [PubMed] [Google Scholar]
- 8.Max W, Rice DP, Sung H-Y, Michel M, Breuer W, Zhang X. The economic burden of prostate cancer, California, 1998. Cancer. 2002 Jun 1;94(11):2906–2913. doi: 10.1002/cncr.10532. [DOI] [PubMed] [Google Scholar]
- 9.Guy GP, Jr, Ekwueme DU, Yabroff KR, Dowling EC, Li C, Rodriguez J, et al. The economic burden of cancer survivorship among adults in the United States. J Clin Oncol. 2013;31(30):3749–3757. doi: 10.1200/JCO.2013.49.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 11.Russell L. Completing costs: patients' time. Med Care. 2009;47(7 Suppl 1):S89–S93. doi: 10.1097/MLR.0b013e31819bc077. [DOI] [PubMed] [Google Scholar]
- 12.Yabroff KR, Warren JL, Knopf K, Davis WW, Brown ML. Estimating patient time costs associated with colorectal cancer care. Med Care. 2005 Jul;43(7):640–648. doi: 10.1097/01.mlr.0000167177.45020.4a. [DOI] [PubMed] [Google Scholar]
- 13.Yabroff KR, Davis WW, Lamont EB, Fahey A, Topor M, Brown ML, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14–23. doi: 10.1093/jnci/djk001. [DOI] [PubMed] [Google Scholar]
- 14.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. NAMCS Description. 2013 Available from: URL: http://www.cdc.gov/nchs/about/major/ahcd/namcsdes.htm.
- 16.National Center for Health Statistics. NHAMCS Description. 2013 Available from: URL: www.cdc.gov/nchs/about/major/ahcd/nhamcsds.htm.
- 17.Guy GP, Jr, Richardson LC. Visit duration for outpatient physician office visits among patients with cancer. J Oncol Pract. 2012;8(3S):2S–8S. doi: 10.1200/JOP.2011.000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Medicare & Medicaid Services. MCBS Survey Overview. 2004 Available from: URL: http://www.cms.hhs.gov/MCBSO/overview.asp.
- 19.National Health Interview Survey. Hyattsville, MD: National Center for Health Statistics; 1992. 1995. Report No.: NCHS CD-ROM series 10, no. 6. [Google Scholar]
- 20.U.S.Department of Labor. Bureau of Labor Statistics Data http://www.bls.gov/data. 2013 Available from: URL: http://www.bls.gov/data.
- 21.Grosse SD, Krueger KV, Mvundura M. Economic productivity by age and sex: 2007 estimates for the United States. Med Care. 2009 doi: 10.1097/MLR.0b013e31819c9571. in press. [DOI] [PubMed] [Google Scholar]
- 22.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 23.Research Triangle Institute. SUDAAN Language Manual: Release 10.0. Research Triangle Park, NC: Research Triangle Institute; 2008. [Google Scholar]
- 24.Yabroff KR, Dowling E, Rodriguez J, Ekwueme DU, Meissner H, Soni A, et al. The Medical Expenditure Panel Survey (MEPS) Experiences with Cancer survivorship supplement. J Cancer Surviv. 2012;6(4):407–419. doi: 10.1007/s11764-012-0221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125(1):191–200. doi: 10.1007/s10549-010-0952-6. [DOI] [PubMed] [Google Scholar]
- 26.Peipins LA, Soman S, Berkowitz Z, White MC. The lack of paid sick leave as a barrier to cancer screening and medical care seeking: results from the National Health Interview Survey. BMC Public Health. 2012;12:520. doi: 10.1186/1471-2458-12-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith TJ, Penberthy L, Desch CE, et al. Differences in initial treatment patterns and outcomes of lung cancer in the elderly. Lung Cancer. 1995;13:235–252. doi: 10.1016/0169-5002(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 28.Farrow DC, Hunt WC, Samet JM. Geographic variation in the treatment of localized breast cancer. N Engl J Med. 1992;326:1097–1101. doi: 10.1056/NEJM199204233261701. [DOI] [PubMed] [Google Scholar]
- 29.Satariano ER, Swanson GM, Moll PP. Nonclinical factors associated with surgery received for early stage breast cancer. Am J Public Health. 1992;82:195–198. doi: 10.2105/ajph.82.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazil A, Key CR. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst. 2000 Feb 2;92(3):269–271. doi: 10.1093/jnci/92.3.269. [DOI] [PubMed] [Google Scholar]
- 31.Jonas DE, Russell LB, Sandler RS, Chou J, Pignone M. Value of patient time invested in the colonoscopy screening process: time requirements for colonoscopy study. Med Decis Making. 2008;28:56–65. doi: 10.1177/0272989X07309643. [DOI] [PubMed] [Google Scholar]
- 32.Henry SG, Ness RM, Stiles RA, Shintani AK, Dittus RS. A cost analysis of colonoscopy using microcosting and time-and-motion techniques. J Gen Intern Med. 2007;22(10):1415–1421. doi: 10.1007/s11606-007-0281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayman JA, Langa KM, Kabeto MU, Katz SJ, DeMonner SM, Chernew ME, et al. Estimating the cost of informal caregiving for elderly patients with cancer. J Clin Oncol. 2001 Jul 1;19(13):3219–3225. doi: 10.1200/JCO.2001.19.13.3219. [DOI] [PubMed] [Google Scholar]
- 34.Yabroff KR, Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009 Sep 15;115(18 Suppl):4362–4373. doi: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]
- 35.Hanly P, Ceilleachair AO, Skally M, O'Leary E, Kapur K, FItzpatrick P, et al. How much does it cost to care for survivors of colorectal cancer? Caregiver's time, travel and out-of-pocket costs. Support Care Cancer. 2013;21(19):2583–2592. doi: 10.1007/s00520-013-1834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell LB, Ibuka Y, Carr D. How much time do patients spend on outpatient visits? The American Time Use Survey. Patient. 2008;1(3):211–222. doi: 10.2165/1312067-200801030-00008. [DOI] [PubMed] [Google Scholar]
- 37.Byrne J, Kessler LG, Devesa SS. The prevalence of cancer among adults in the United States: 1987. Cancer. 1992;69(8):2154–2159. doi: 10.1002/1097-0142(19920415)69:8<2154::aid-cncr2820690823>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 38.Hewitt M, Breen N, Devesa S. Cancer prevalence and survivorship issues: analyses of the 1992 National Health Interview Survey. J Natl Cancer Inst. 1999 Sep 1;91(17):1480–1486. doi: 10.1093/jnci/91.17.1480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


