Abstract
The current studies were designed to investigate if the Emotion Context Insensitivity (ECI; Rottenberg & Gottlib, 2004) hypothesis is applicable across the time course of emotion. Recent affective science research has pointed to the importance of considering anticipation and maintenance of emotion. In the current studies, we assessed emotion responses among college students with depression symptoms in anticipation of, during, and after an emotional picture using the emotion modulated startle paradigm. People with and without depression symptoms did not differ in blink magnitude in anticipation of emotional pictures suggesting that some anticipatory processes may not be impaired in with depression symptoms. In contrast, individuals with depression symptoms did not exhibit blink magnitudes that varied by valence, either during viewing or after the pictures were removed from view. These findings suggest that ECI is relevant not only for those diagnosed with Major Depressive Disorder, but also for people with depression symptoms that may not cross the diagnostic threshold. These data point also point to the importance of considering the time course of emotion to better understand emotional deficits in individuals with differing levels of depression symptoms. Identifying where emotion goes awry across the time course of emotion can help inform treatment development.
Keywords: depression, startle modulation, emotion, dysphoria, anticipation, maintenance
Although conventional wisdom suggests that major depressive disorder (MDD) is associated with heightened negative emotion, research supporting this notion is mixed. On the one hand, evidence suggests that people with depression report generally experiencing high negative and low positive affect relative to people with few symptoms of depression (e.g., Clark, Watson, & Mineka, 1994; Watson, Clark, & Carey, 1988), a pattern consistent with the MDD symptoms of sad mood and anhedonia. On the other hand, and perhaps somewhat paradoxically, when faced with emotionally evocative stimuli, people with depression exhibit a different pattern of emotional responding.
More specifically, studies that have investigated expressive, experiential, and physiological responses to emotional stimuli have found that depression is associated with similar or reduced responses to negative stimuli compared to people without depression (Allen, Trinder, & Brennan, 1999; Berenbaum & Oltmanns, 1992; Dunn, Dalgleish, Lawrence, Cusack, & Ogilvie, 2004; Gehricke & Shapiro, 2000; Sloan, Strauss, Quirk, & Sajatovic, 1997; Sloan, Strauss, & Wisner, 2001). In addition, studies have found that depression is associated with reduced responses to positive stimuli (Allen, et al., 1999; Berenbaum & Oltmanns, 1992; Dunn, et al., 2004; Katsikitis & Pilowsky, 1991; Rottenberg, 2005; Rottenberg, Kasch, Gross, & Gotlib, 2002; Shestyuk, Deldin, Brand, & Deveney, 2005; Sloan, et al., 1997; Sloan, et al., 2001). In short, these studies suggest, somewhat counterintuitively, that depression is associated with an overall dampening of both positive and negative emotion responses.
In part to help explain the discrepancy between dampened responses to evocative stimuli amidst reports of heightened negative affect, Rottenberg and Gotlib (2004) proposed the Emotion Context Insensitivity (ECI) hypothesis which states that depressed mood influences a person’s ability to vary emotional response according to context. That is, depressed mood inhibits variation in emotional responses to both positive and negative stimuli. Bylsma and colleagues’ (2008) meta analysis of 19 studies of emotion responsivity among people with MDD generally supported ECI, though there were differences depending upon valence (i.e., positive or negative) and emotion response component (i.e., expression, experience, physiology). More specifically, they reported medium or large effect sizes for dampened positive expression and experience but not physiology. By contrast, they reported medium effect sizes for dampened negative experience and physiology, but not expression. They also suggested that the severity of depression symptoms may influence the observation of ECI. Indeed, people with few depression symptoms do not seem to show the dampened emotional response pattern consistent with ECI (e.g., Vanman, Dawson, & Brennan, 1998). On the other hand, evidence suggests that the clinical significance of depression symptoms does not depend on meeting diagnostic criteria for a depressive episode (Lewinsohn, Solomon, Seeley, & Zeiss, 2000; Solomon, Haaga, & Arnow, 2001). Thus, in order to fully understand when ECI may be observed, it is important to study depression symptoms ranging from moderate to severe. (Beck, Steer, & Carbin, 1988).
The Time Course of Emotion
Another important factor to consider when investigating emotional responding in depression is the time course of emotion. Most prior research on emotional responsivity in depression has focused on responses in the presence of emotional stimuli (i.e., “in-the-moment” responses). Yet, affective science research has identified different aspects of emotional responses as they develop across time (Davidson, 1998). That is, studies using expressive, experiential, and physiological measures have found that emotional responses unfold prior to stimulus presentation, (i.e., in anticipation; e.g., Nitschke, et al., 2002; Poli, Sarlo, Bortoletto, Buodo, & Palomba, 2007; Sabatinelli, Bradley, & Lang, 2001), during stimulus presentation, and through to the post-stimulus period (i.e., maintenance or recovery; e.g., Bradley, Cuthbert, & Lang, 1996; Garrett & Maddock, 2001). While studies with healthy participants and select clinical populations have examined the time course of emotion (e.g., schizophrenia, see Kring & Moran (2008) for a review), studies have only just begun to examine the linkage between depression symptoms, anticipation, and maintenance processes. These processes in particular may be important for understanding the boundaries of ECI that could in turn inform intervention. For example, if depression is also associated with dampened emotion during anticipation, treatment might be improved by focusing on the anticipation of an emotional response in addition to in-the-moment responses. In contrast, if depression is associated with dampened emotion response after emotional stimuli are removed, treatment might be focused on the in-the-moment experience and savoring of emotion.
A useful method for studying the time course of emotion is the startle eyeblink modulation (SEM) paradigm. In this paradigm an abrupt, startling stimulus (e.g., burst of white noise air) is paired with an evocative stimulus and the magnitude of a person’s eyeblink is assessed. During stimulus presentation, blink magnitude is largest when the stimulus is negative and smallest when the stimulus is positive, thus indicating that blink magnitude varies by the valence of the stimulus and the participant’s emotional experience (Lang, Bradley, & Cuthbert, 1990). For the most part, findings from studies using the SEM paradigm in depression are consistent with the ECI hypothesis. That is, prior studies have found diminished emotion modulated blink responses during stimulus presentation (Allen, et al., 1999; Dichter & Tomarken, 2008; Dichter, Tomarken, Shelton, & Sutton, 2004; Kaviani, et al., 2004; Larson, Nitschke, & Davidson, 2007; Mneimne, McDermut, & Powers, 2008). However, severity of depression symptoms appears to be an important moderator. For example, Forbes and colleagues (2005) found that those reporting more MDD episodes failed to show emotion modulated blink response (i.e., an ECI pattern) while those with fewer MDD episodes exhibited emotion modulated blink responses. Other studies have found that depression is associated with an overall reduction in blink magnitude, regardless of whether it varies by valence (Allen, et al., 1999; Sloan & Sandt, 2010).
Anticipation of Emotion and Depression
The SEM paradigm has also been used to assess anticipation. Among people without depression symptoms, blink magnitude is larger in response to anticipatory cues signaling forthcoming positive or negative stimuli than in response to anticipatory cues signaling neutral stimuli (Dichter, Tomarken, & Baucom, 2002; Lipp, Cox, & Siddle, 2001; Sabatinelli, et al., 2001). Potentiated blink magnitude in anticipation of both positive and negative stimuli has been hypothesized to represent a preparatory response for an impending emotional response, regardless of valence (e.g., Dichter, et al., 2002) as well as an attentional response to forthcoming emotionally arousing stimuli (Bradley, Lang, & Cuthbert, 1993; Lipp, et al., 2001).
To our knowledge, only two SEM studies have studied anticipation in depression. Dichter and Tomarken (2008) found that people diagnosed with MDD showed blink attenuation to positive and negative anticipatory cues presented 2s prior to pictures relative to neutral cues. By contrast, people without MDD showed blink potentiation to positive and negative anticipatory cues (relative to neutral). Larson and colleagues (2007), by contrast, found no differences in blink magnitude to cues presented 1s prior to emotional compared to neutral pictures between people with and without anhedonic depression symptoms. Differences in sample characteristics may account for the discrepant findings between the two studies. That is, participants in Larson et al.’s study exhibited moderate anhedonic depression symptoms, and thus other symptoms may be more influential in the anticipation of emotion (e.g., difficulties with attention/concentration). Dichter & Tomarken (2008) studied people with a diagnosis of MDD, suggesting that blink attenuation in anticipation may be associated with depression severity or diagnostic status.
A few studies have examined anticipation of emotion in depression using different methods, including self-report and fMRI yet no consistent findings have emerged, perhaps due to widespread methodological differences. Self-report studies that ask participants to anticipate future positive and negative events found that those with depression symptoms reported anticipating fewer positive (but not negative) events compared to those without depression symptoms (e.g., Abramson, Metalsky, & Alloy, 1989; Bjarehed, Sarkohi, & Andersson, 2009; MacLeod & Salaminiou, 2001). By contrast, fMRI studies assessing anticipation of positive stimuli (monetary reward, positive pictures) found no differences in brain activation or reaction time between people with and without depression symptoms in areas associated with anticipation (e.g., nucleus accumbens, amygdala) (Abler, Erk, Herwig, & Walter, 2007; Knutson, Bhanji, Cooney, Atlas, & Gotlib, 2008; Pizzagalli, et al., 2009). However, two fMRI studies assessing anticipation of negative stimuli (negative pictures, pain) found that people with depression symptoms showed greater amgydala activation compared to people without depression despite no differences in reported experience or reaction times (Abler, et al., 2007; Strigo, Simmons, Matthews, Craig, & Paulus, 2008).
Maintenance of Emotion and Depression
A handful of studies have examined the maintenance of emotional responses after emotional stimuli are removed from view in the context of depression symptoms (Deldin, Deveney, Kim, Casas, & Best, 2001; Rottenberg, et al., 2002; Siegle, Granholm, Ingram, & Matt, 2001; Siegle, Steinhauer, Carter, Ramel, & Thase, 2003). Siegle and colleagues (2001) found that people with depression showed persistent processing of negative words, as indexed by pupil dilation, even after the negative words were no longer present. In a follow-up study, Siegle and colleagues (2002) found continued amygdala activation to negative (but not positive) words in those with depression that lasted up to 30 seconds, while those without depression showed a decay in amygdala activity within 10 seconds following stimulus presentation.
To our knowledge, two studies have used the SEM paradigm to assess maintenance or recovery of emotion response in depression. Forbes et al. (2005) found that people with and without depression exhibited comparable potentiated blink responses during and following the offset (average of 5s post picture) of negative pictures compared to blink responses during and following the offset of positive pictures. However, individuals with more lifetime episodes of MDD exhibited dampened emotion modulated responses during and after pictures, a pattern consistent with ECI. Larson et al. (2007) found that participants with and without anhedonic depression symptoms exhibited blink potentiation to negative pictures compared to positive pictures. However, those without anhedonic depression symptoms exhibited blink attenuation to positive pictures relative to neutral whereas those with anhedonic depression symptoms did not. Thus, findings from Larson and colleagues suggest ECI may be observed for positive, but not negative pictures once emotional stimuli are removed from view. Taken together, findings from SEM studies suggest that those with depression symptoms may exhibit ECI during the maintenance period, particularly for positive stimuli. However, studies using other methods suggest that those with depression symptoms may continue to maintain responses to negative stimuli.
The Present Studies
In summary, although several studies have found that people with depression exhibit dampened responses to both positive and negative stimuli, consistent with the ECI hypothesis, some studies do not find this pattern. One possibility for the discrepant findings is the range of depression symptoms studied. Thus, studies investigating emotion in those with varying levels of depression symptoms are necessary to better understand whether symptom severity plays a role in ECI. In addition, while the affective science literature has pointed to the importance of investigating the time course of emotion, only a handful of studies have taken this approach in the study of depression. Emotion responses unfold over time, and thus there are several points during the time course of emotion where emotion responses may go awry. Given that studies suggest that intact emotional responding is associated with recovery from depression (Geschwind, et al., 2011; Morris, Bylsma, & Rottenberg, 2009; Peeters, Berkhof, Rottenberg, & Nicolson; Rottenberg, et al., 2002), it is important to understand the point at which response dampening may occur or continue.
The present studies take a systematic approach to investigating the time course of emotion in those with and without depression symptoms. In Study 1, we examined emotional responses during the anticipation and presentation of emotionally evocative stimuli. Thus, Study 1 sought to clarify and replicate prior studies reporting a pattern of ECI while viewing emotionally evocative stimuli, along with extending prior research to investigate whether those with depression symptoms exhibit ECI during the anticipation of emotional stimuli. In Study 2 we sought to replicate our findings from Study 1 during picture viewing, while also extending the examination of ECI to the maintenance of emotion. In short, the present studies sought to test the hypothesis that ECI extends to anticipation, in the moment experience and maintenance of emotion responses.
Study 1
In Study 1 we tested several specific hypotheses. First, consistent with ECI, we hypothesized that people with depression symptoms would exhibit dampened emotion responses during the presentation of evocative stimuli, including dampened reports of experienced emotion and dampened blink magnitude relative to people without depression symptoms. Second, we hypothesized that those without depression symptoms would show potentiated blink responses during the anticipation of emotional compared to neutral pictures. In contrast, we hypothesized that people with depression symptoms would not exhibit potentiated blink responses during anticipation of emotional relative to neutral pictures, a pattern consistent with ECI.
Study 1: Methods
Participants
A total of 80 participants were enrolled in the study. Depression symptoms were assessed using the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961). Based on prior research indicating that scores above 17 correspond with clinically significant experiences of depression (Kendall, Hollon, Beck, Hammen, & Ingram, 1987), participants scoring above 17 were assigned to the depression symptom group (DS; i.e., those reporting depression symptoms while not necessarily meeting diagnostic criteria for major depressive disorder). In addition, participants had to endorse symptoms consistent with either sad mood or anhedonia, corresponding to diagnostic criteria for a major depressive episode (APA, 1994). Participants scoring between 1 and 8 on the BDI were assigned to the non-depressed group (ND). Following the Kendall et al. (1987) recommendations, people with a BDI score of 0 were excluded from the ND group as such a low level of depression symptoms may be associated with other forms of psychopathology. Participants’ BDI scores were assessed at two time points: 1) as part of a prescreening survey completed a mean of 4.3 weeks prior to laboratory session 2) at the laboratory session. Participants that changed group status between their BDI 1 and BDI 2 scores were excluded from analyses (n = 13).
Seven participants were excluded (6 of the 7 from the DS group) due to a lack of startle response in more than half of trials (Blumenthal, et al., 2005). Thus, the final number of participants was 33 in the ND (18 female) group and 27 in the DS group (15 female). All participants had normal hearing and normal or corrected vision.
Stimuli
Sixty pictures (20 positive, 20 neutral, and 20 negative and 3 habituation slides) were selected from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2005)1. Pictures were selected based on published rating norms (Lang, et al., 2005) such that mean arousal ratings for positive and negative pictures were comparable (mean = 5.01 and 5.27, respectively). Neutral pictures, such as common household items, were selected based on normative valence ratings placing them midway between positive and negative pictures. Men and women were shown the same set of pictures except for a selection of positive pictures that were selected to ensure comparable normative ratings of arousal and valence between genders.
Procedure
The experimental sessions took place in a dimly lit and quiet laboratory room. After participants provided informed consent, they were positioned in a comfortable chair approximately .5 meters from a 36 cm LCD laptop computer screen. Experimenters blind to participants’ group membership prepared the skin, placed electrodes for recording startle responses, and checked impedance (following recommendations from Berg & Balaban, 1999). After electrode application, participants were asked to complete the Positive and Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988) a measure that includes two 10-item scales that assessed the experience of positive and negative affect over the prior week. Participants were then asked to wear a pair of headphones and were told that they would hear noises over the headphones, but that they could ignore the noises. The acoustic startle probes were digitally generated .WAV files of a 50-ms burst of white noise with instantaneous rise time. The startle probes were amplified to 105-dB by a Radio Shack SA-155 Integrated Stereo Mini-Amplifier and binaurally presented through Sennheiser HD 490 headphones. Probe stimuli were calibrated before each test session. Participants were told that a series of cues and pictures would be presented on the screen and that cues would indicate the type of upcoming picture they would see. The cues indicated the valence (plus sign (+) for positive, minus sign (-) for negative, and a circle (O) for neutral) of the pictures that would be subsequently presented. Participants were told that they would be asked to rate how they felt after viewing each picture. To make these ratings, participants used the Self-Assessment Manikin (SAM; Levenston, Patrick, Bradley, & Lang, 2000), a nonverbal pictorial assessment technique that measures valence and arousal. After viewing each picture, participants were asked to make two ratings (1) valence: how they felt while viewing the picture on a 9-point scale from “unhappy” to “happy” and (2) arousal: how much arousal they felt while viewing the picture on a 9-point scale from “calm” to “aroused.” Participants completed a series of four practice trials to familiarize themselves with the procedure, with three of those trials containing a startle probe. A study assistant walked each participant through the first practice sequence detailing the study procedure, and then participants completed three trials on their own to ensure clarity of study procedures. Presentation of pictures and probes were controlled by VPM software (Cook, Atkinson, & Lang, 1987).
Following the practice and habituation trials, participants began the experimental protocol consisting of 60 trials. Each trial began with a blank screen followed by a 0.5s cue (+, -, or o), followed by a 5s blank screen anticipation period during which participants were asked to anticipate the upcoming picture. Pictures were then displayed for 6s followed by a screen that asked participants to make the two experienced emotion ratings on the computer using the SAM. Intertrial intervals varied between 5 and 7 seconds. Startle probes were pseudo-randomly ordered and presented during either the anticipatory period (2500 ms after the anticipatory cue and prior to picture onset), during the picture presentation period (3500 ms after picture onset), or during the intertrial interval. Unprobed trials were used to minimize predictability. Startle probes were presented in 50 of the 60 trials, 24 in the anticipatory period and 24 during the picture viewing period, and 2 intertrial intervals. No more than one startle probe was presented during a trial and no more than two pictures of the same valence were presented sequentially. Three picture orders were created and used sequentially (there were no differences between order therefore analyses were collapsed across orders).
Physiological Recording and Data Reduction
Electrode placement and skin preparation followed guidelines for human startle research (Blumenthal, et al., 2005). Electromyography (EMG) electrodes were filled with electrolyte gel and placed in the orbicularis oculi region, one directly below the pupil of the left eye and one lateral to this. A third electrode was placed in the middle of the forehead as a grounding electrode. Impedances were kept below 15 kOhm.
EMG signal was filtered using a 13–1000 Hz passband and amplified using a Coulbourn V75-04 Isolated Bioamplifier with Bandpass Filter. EMG was sampled at 1000 HZ for the 50 ms prior to probe onset and ending 300 ms after probe onset. The signal was digitally refiltered offline with a 28–500 Hz passband (Van Boxtel, Boelhouwer, & Bos, 1998) and digitally rectified and integrated using a 30 ms time constant.
Research assistants visually confirmed and scored the EMG data segments using blink scoring software. Blinks were only scored if they fell 20 ms to 150 ms after probe onset. Mean blink magnitude (in microvolts) for each valence (positive, neutral, negative) and time point (anticipatory, in-the-moment) was computed for each individual2. Habituation trials were not included in blink scoring or data analysis.
Data Reduction and Analysis Plan
For SAM valence and arousal ratings, 2 (Group: DS, ND) X 3 (Valence: positive, neutral, negative) repeated measures MANOVAs were conducted with valence as a within subject factor and group as a between subjects factor. For blink magnitude, a 2 (Group: DS, ND) X 2 (Probe Time: anticipatory, in-the-moment) X 3 (Valence: positive, neutral, negative) repeated measures MANOVA was conducted with probe time and valence as within subject factors and group as a between subjects factor. A Greenhouse-Geisser correction was used when appropriate. When applicable, follow up pairwise comparisons were conducted using a Bonferroni correction. All estimates of effect size are reported as partial Eta squared (ηp2). In preliminary analyses, gender and ethnicity were entered as between subject variables but no main effects or interactions with either variable were significant so gender and ethnicity were collapsed in all presented results.
Results
Participant Characteristics
Demographic and symptom information are presented in Table 1. A chi-square test for ethnic status and gender (ps = .62 and .73 respectively) and a t-test for age (p = .72) revealed no significant group differences. As planned, the DS group had significantly higher levels of depression symptoms than the ND group as measured by the BDI, t (58) = 17.83, p < .0001. The mean BDI score for the DS group was comparable to clinically depressed samples (Cox, Enns, & Larsen, 2001) and indicates moderate levels of depressive symptomatology (Kendall, et al., 1987). Consistent with prior work on depression (e.g., Watson, Clark, & Carey, 1988) the DS group reported significantly higher levels of negative affect, t (58) = 6.45, p < .001, and lower levels of positive affect, t (58) = 3.30, p < .001 than the ND group.
Table 1.
Demographic and Symptom Characteristics for Participants in Study 1 and Study 2
| Group | ||||
|---|---|---|---|---|
| Study 1 | Study 2 | |||
| Characteristic | DS | ND | DS | ND |
| Number (n) | 27 | 33 | 31 | 26 |
| Age | 20.1 (2.3) | 20.5 (5.4) | 19.8 (1.4) | 19.6 (1.9) |
| Ethnicity (n) | ||||
| Asian | 14 | 17 | 15 | 15 |
| Caucasian | 9 | 11 | 14 | 4 |
| Latino | 2 | 3 | 3 | 5 |
| African-American | 2 | 2 | 1 | 0 |
| Did not disclose | 0 | 0 | 1 | 1 |
| BDI | 21.0 (5.1) | 3.6 (2.1) | 26.6 (5.9) | 5.74 (2.5) |
| PA | 31.1 (8.1) | 37.2 (6.2) | 27.4 (6.1) | 35.52 (6.8) |
| NA | 31.9 (7.3) | 21.0 (5.8) | 30.8 (7.2) | 19.6 (6.1) |
Note: DS= Depression symptom group; ND: Non-depression symptom group; BDI = Beck Depression Inventory; PA = Positive Affect Scale; NA= Negative Affect Scale;
Self-Reported Emotional Experience
For SAM valence ratings (see Table 2), the valence main effect was significant, F (1, 58) = 267.68, p < .001, ηp2 = .82. All participants reported experiencing more pleasant emotion after seeing positive pictures compared to neutral (p < .001) or negative pictures (p < .001). All participants reported experiencing more unpleasant emotion after seeing negative compared to neutral pictures (p < .001). The group main effect was not significant (p = .55), but there was a significant Group X Valence interaction (F (2, 57) = 35.91, p < .001, ηp2 = .39). Follow-up between group analyses revealed that the DS group rated their experience while viewing positive pictures as less pleasant than the ND group (t (58) = 4.90, p < .001) and experience while viewing negative pictures as less unpleasant (t (58) = 5.10, p < .001). There were no differences between groups in the rating of experience while viewing neutral pictures (p = .32). Thus, contrary to the ECI hypothesis, people with depression symptoms reported emotion experience that varied by valence. However, when compared to those without depression symptoms, people with depression symptoms reported less pleasant experience to positive and less unpleasant experience to negative pictures.
Table 2.
SAM Valence and Arousal Ratings in Study 1
| Valence | Arousal | |||
|---|---|---|---|---|
| DS | ND | DS | ND | |
| Positive | 5.54 (.79) | 6.49 (.71) | 5.14 (1.22) | 5.52 (1.51) |
| Neutral | 4.98 (.49) | 5.11 (.45) | 2.81 (1.31) | 3.73 (1.34) |
| Negative | 3.64 (1.13) | 2.39 (.76) | 5.05 (1.39) | 5.49 (1.46) |
Note: Tabled values are means; standard deviations in parentheses. DS = Depression Symptom group; ND = Non-Depression group. Ratings ranged from 0 to 9 with higher numbers indicating more pleasant or arousing feelings.
For SAM arousal ratings, the valence main effect was significant, F (2, 58) = 89.25, p < .001, ηp2 = .76. All participants reported more arousal while viewing positive and negative pictures than while viewing neutral pictures, a significant quadratic effect, F (1, 58) = 171.75, p < .001. The group main effect was not significant, but there was a significant Group X Valence interaction, F (2,58) = 4.74, p < .05. Follow-up analyses revealed that the DS group reported less arousal to neutral pictures compared to the ND group (t (58) = 2.70, p < .01). Ratings of arousal experience to positive and negative pictures did not differ by group (p > .25).
Blink Magnitude
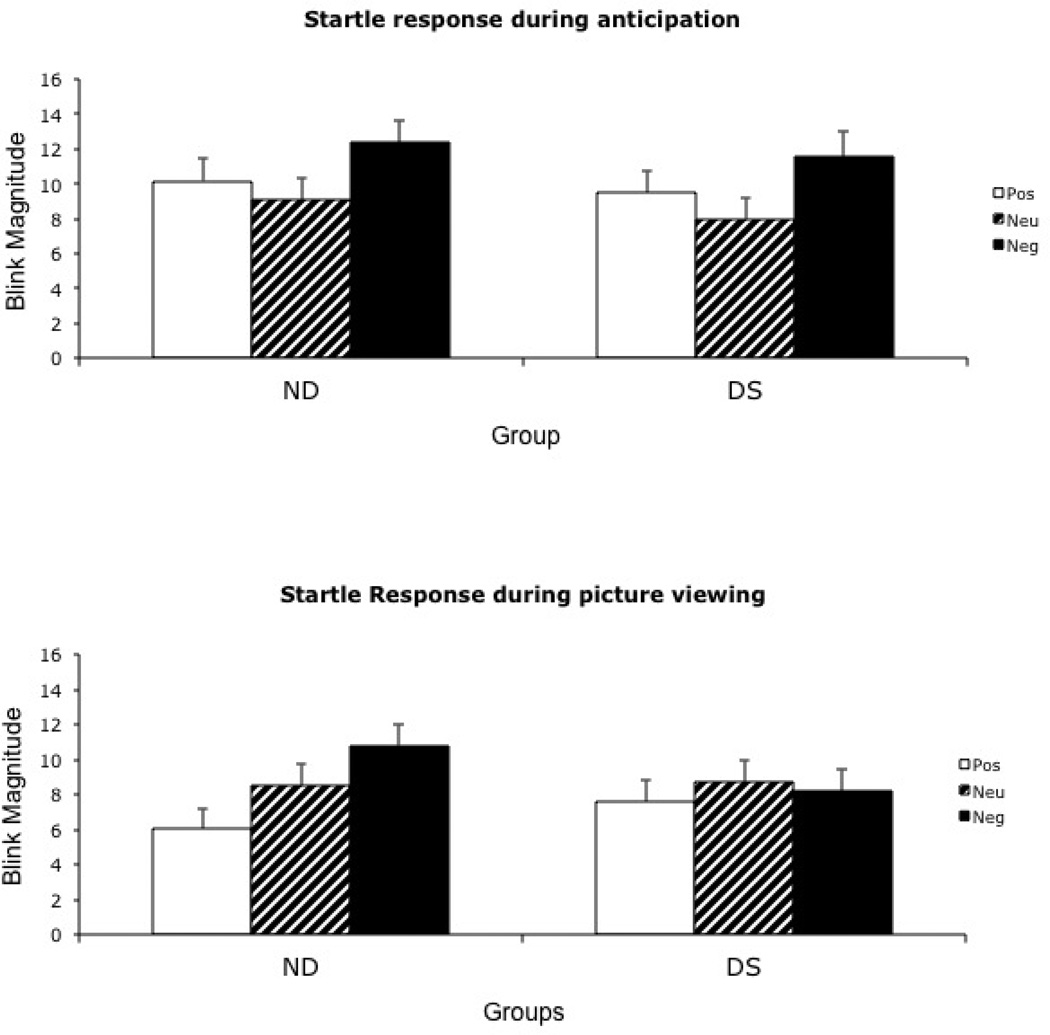
The valence main effect was significant (F (2,114) = 10.22, p < .001, ηp2 = .27) revealing a pattern wherein blink magnitude in response to negative pictures was larger than to either positive (p < .001) or neutral pictures (p < .001). The probe time main effect was also significant (F (1,58) = 8.52, p = .005, ηp2 = .13) indicating that blink magnitude during anticipation of pictures was significantly greater than during picture viewing. There was no main effect for group; however, there was a significant Group X Probe Time X Valence interaction (F (1, 57) = 9.47, p = .003, ηp2 = .15).
To examine blink magnitude during both anticipation and picture viewing, additional 2 (Group: DS, ND) x 3 (Valence: positive, neutral, negative) repeated measures MANOVAs were conducted at each probe time. During anticipation, the valence main effect was significant (F (2,58) = 13.85, p < .001, ηp2 = .33). Posthoc pairwise comparisons indicated that blink magnitude in response to cues signaling forthcoming negative pictures was significantly larger than response to positive and neutral cues (p < .005), and blink magnitude to positive cues was significantly larger than response to neutral cues (p < .05). Neither the group main effect (p = .76) nor the Group X Valence interaction (p = .95) was significant. Thus, both groups showed potentiated blink response during the anticipation of positive and negative pictures compared to the anticipation of neutral pictures.
During picture presentation, the valence main effect (F (2, 58) = 15.94, p < .001, ηp2 = .22) and Group X Valence interaction (F (2, 58) = 7.33, p = .009, ηp2 = .11) were significant. As shown in Figure 1, the ND group exhibited greater blink magnitude in response to negative pictures compared to neutral or positive (p’s < .01). Blink magnitude to positive pictures was smaller than magnitude to neutral pictures (p < .01). The DS group, however, did not show this pattern of emotion modulated startle. This group exhibited no difference in blink magnitude while viewing positive, neutral or negative pictures (p > .32). Between groups, there were no differences in blink magnitude while viewing positive (p = .30), neutral (p = .86) or negative pictures (p = .32).
Figure 1.
Mean blink magnitudes before and during picture presentation in Study 1
Discussion
Study 1 sought to investigate the time course of emotion in those with and without depression symptoms and asked the question of whether anticipation and in-the-moment emotion responses are characterized by emotion context insensitivity. Findings from Study 1 support several of our hypotheses. As predicted, the ND group showed emotion modulated blink responses during picture presentation, while participants in the DS group did not. That is, people without depression symptoms exhibited larger blink magnitudes to negative pictures compared to either neutral or positive pictures, and smaller blink magnitudes to positive compared to neutral pictures. However, people with depression symptoms did not exhibit emotion modulation, a pattern of findings consistent with the ECI hypothesis. Although not all studies of emotion and depression find group differences in reported experience, participants in the DS group differed from the ND group in the present study in that the DS group reported experiencing less pleasantness while viewing the positive pictures, less unpleasantness while viewing the negative pictures, and less arousal while viewing neutral pictures.
Contrary to our hypothesis, we found no difference in blink magnitude between the ND and DS groups during the anticipation of pictures. That is, while individuals with depression symptoms showed diminished blink modulation during picture viewing, their responses were comparable to individuals without depression symptoms during anticipation. Both groups showed a pattern wherein blink magnitude to negative anticipatory cues was largest, followed by response to positive and neutral cues. Our findings are consistent with those of Larson and colleagues (2007) who found no differences in anticipatory blink response between participants with and without anhedonic depression symptoms, but inconsistent with Dichter & Tomarken (2008) who found attenuated blink responses during the anticipation of positive and negative pictures (compared to neutral) in those diagnosed with MDD. It may be the case that the inconsistency in findings is due to severity of symptoms such that those in Dichter & Tomarken’s study were diagnosed with MDD and involved in a depression treatment study while those in the present study and in Larson et al.’s study had moderate levels of depression symptoms. In addition, symptom severity between the samples cannot be easily compared because symptoms were measured differently: BDI in the current study vs. Mood and Anxiety Symptom Questionnaire in the Larson et al. study.
Basic research with the SEM paradigm points to meaningful psychological differences at different probe times. Long lead times in anticipation (e.g., 2000 ms prior to picture presentation) and short lead times during picture viewing (e.g., 300 ms after picture onset) are purported to represent arousal and/or attention in preparation of a response, while short lead times during anticipation (e.g., 750 ms prior to picture presentation) and long lead times after picture presentation are associated with valenced emotional responses (e.g., 2500 during picture viewing). Our findings of intact arousal modulated blink responses in anticipation at a later probe time (2500 ms prior to picture onset) coupled with diminished emotion modulated startle at a later probe time (3500 ms after picture onset) are broadly consistent with those of Dichter et al. (2004) who found a significant difference in startle response between those with and without MDD during a late probe time (i.e., 3500 ms during picture viewing), but no difference during an early (i.e., 300 ms) picture probe time.
In summary, Study 1 findings suggest that depression is associated with a deficit in the in-the-moment emotion responses, consistent with the ECI hypothesis. In contrast, our findings did not support the extension of ECI to the anticipation of emotion.
Study 2
In Study 2, we asked: what happens to an emotional response after the offset of an emotionally meaningful event? Findings from studies of people without depression have shown that emotional modulation of the startle response is maintained following picture presentation, such that blink magnitude following the offset of unpleasant emotional stimuli is larger than blink magnitude following the offset of pleasant emotional stimuli (Bradley, et al., 1996; Germans Gard & Kring, 2007; Jackson, et al., 2003; Larson, Sutton, & Davidson, 1998; Schupp, Cuthbert, Bradley, Birbaumer, & Lang, 1997). As reviewed earlier, prior studies have found evidence for ECI when positive and negative emotional stimuli are removed from view (e.g, Larson et al., 2007; Siegel et al., 2002).
As in Study 1, we measured emotion modulated startle responses and self-reported emotional experience in individuals with high and low levels of depression symptoms. However, in Study 2, we extended the time course to examine startle responses after the evocative pictures were removed from view. Furthermore, we included participants with higher levels of depression symptoms to assess a broader range of symptoms. In Study 2, we tested two hypotheses. First, consistent with ECI (Rottenberg, Gross, & Gotlib, 2005) and findings from Study 1, we predicted that participants with higher depression symptoms would demonstrate less valence modulated startle responses during picture presentation than those without depression symptoms. That is, we expected that the ND group, but not the DS group, would exhibit emotion modulated startle responses, such that blink magnitude to positive pictures would be attenuated and blink magnitude to negative pictures would be potentiated relative to neutral pictures. Second, given that studies of healthy people have found continued emotion modulation of blink response after picture offset, we expected that the ND group would continue to exhibit emotion modulated startle responses after picture offset. However, we predicted that the DS group would not show this modulation pattern, as predicted by ECI, but would instead show potentiated startle response to positive pictures after offset.
Methods
Participants
Similar to Study 1, DS and ND groups were created based on Kendall et al.’s (1987) recommendations. A total of 99 women completed the BDI during an initial prescreening and again during the experimental session (on average 1 month later) in order to address stability of symptoms over time as well as status of symptoms at the time of the experiment. We limited the Study 2 sample to women because depression is more prevalent among women than men and because we did not observe any gender differences in Study 1. In addition, we opted to include participants with BDI scores of 20 or higher for the DS group at both assessments so that we could include participants with moderate depression symptoms based on Kendall et al.’s (1987) guidelines. As in Study 1, participants in the DS group had to endorse a 2 or 3 on either the item assessing sad mood or anhedonia since having one of these two symptoms is required for a diagnosis of depression according to the Diagnostic and Statistical Manual of Mental Disorders (APA, 1994). In addition, participants with scores between 1 and 8 at both assessments comprised the ND group. Participants that changed group status between their BDI 1 and BDI 2 scores (N=39) were excluded from analyses. Following these procedures, 32 participants met criteria for the ND group, and 28 participants met criteria for the DS group. However, distribution analyses of the raw blink data resulted in exclusion of 1 participant from the DS group and 2 participants from the ND group due to outlying scores (>3 SD above mean). Thus, the final sample size was 31 ND and 26 DS participants. As in Study 1, all participants had normal hearing and normal or corrected vision.
Stimuli
Fifty-seven pictures (18 positive, 18 negative, 18 neutral, 3 habituation) from the IAPS set were displayed in one of six orders. Pictures were selected based on normative female self-report valence and arousal ratings (Lang, et al., 2005) such that positive and negative pictures were comparable on arousal (m = 6.36 and 6.45 respectively) but at opposite valence extremes, and neutral pictures had low arousal ratings with valence ratings between those of the positive and negative pictures. Most of the pictures (n=44) in Study 2 were not shown in Study 1, thus giving us the opportunity to examine the generalizability of our findings to different evocative stimuli3. Pictures were presented for 6 seconds each, separated by intertrial intervals (ITI) of 6500–8000 ms.
Procedure
Informed consent, electrode application, and practice trial procedures were the same as in Study 1. Participants received course credit for participating. As in Study 1, participants completed the PANAS, reporting on their experienced positive and negative affect in the past week.
For the experimental trials, participants were told that they would be presented with a series of pictures and were instructed to look at each picture the entire time that the picture was on the screen. They were also told that they would be prompted to make a rating of how they felt while viewing the picture after each picture presentation using the computer. In Study 2, we used the 20-point version of the Self Assessment Manikin (Hodes, Cook Iii, & Lang, 1985), with ratings ranging in value from 0 to 20 with higher ratings reflecting more positive valence or higher arousal. Participants were presented with a sample acoustic probe and were informed that they would hear this noise over the headphones periodically during the task but that they could ignore these noises.
The acoustic startle probes were the same as in Study 1, but the probe times varied. Specifically, probes were presented either during picture presentation (3500 ms after picture onset; n = 20) or after the picture was removed (2500 ms after picture offset; n = 20). No more than one probe was presented per trial. Fourteen trials with no probes or with ITI probes were also included to reduce the predictability of probe presentation. These trials were not included in data analyses. Six stimulus orders were used to counterbalance the presentation of the acoustic probes and picture valence across participants within groups. Pictures of the same valence occurred no more than twice consecutively and trials involving the same probe time occurred no more than three times in a row. No order effects were observed and we thus collapsed across order for the analyses.
Electrode placement, eyeblink measurement, quantification, and scoring procedures and parameters were the same as in Study 1. Mean blink magnitude (microvolts) for each valence (positive, neutral, negative) and time (during picture; after offset) were computed for each individual. Practice and habituation trials were not included in data analysis.
Data Reduction and Analysis Plan
For SAM ratings of pleasantness and arousal separate 2 (Group: DS, ND) X 3 (Valence: positive, negative, neutral) repeated measures MANOVAs were conducted. Significant interactions were further evaluated by conducting a parallel set of follow-up analyses as was done in Study 1. For blink responses, a 2 (Group) X 2 (Probe Time) X 3 (Valence) repeated measures MANOVA was conducted with probe time (during, after) and valence (positive, neutral, negative) as within subject factors and group (DS, ND) as a between subjects factor. A Greenhouse-Geisser correction was used when appropriate. When applicable, follow up pairwise comparisons were conducted using a Bonferroni correction. All estimates of effect size are reported as partial Eta squared. In preliminary analyses, ethnicity was entered as between subject variable but no main effects or interactions with ethnicity were significant so ethnicity was collapsed in all presented results.
Results
Participant Characteristics
Demographic and symptom information for both groups are presented in Table 1. A chi square test for ethnic status and t-test for age did not reveal any significant group differences (ps = .21 and .67 respectively). As planned, the DS group had significantly higher levels of depression symptomatology than the ND group as measured by the BDI, t (55) = 17.83, p < .001. The mean BDI score for the DS group was comparable to clinically depressed samples (Cox, et al., 2001) and indicates moderate to severe levels of depressive symptomatology (Kendall, et al., 1987). Consistent with prior work on depression (e.g., Watson, Clark, & Carey, 1988) and findings from Study 1, the DS group reported significantly higher levels of negative affect, t (55) = 6.41, p < .001, and lower levels of positive affect, t (55) = 4.73, p < .001 than the ND group.
Self-Reported Emotional Experience
For SAM valence ratings, there was a significant main effect for valence, F (2, 54) = 109.42, p < .001, ηp2 = .80. As expected, participants reported experiencing more pleasant emotion in response to positive pictures than neutral pictures, t (55) = 10.92, p < .001 and negative pictures, t (55) = 14.95, p < .001, and they reported experiencing more and negative emotion in response to negative compared to neutral pictures, t (55) = 13.09, p < .001. Neither the group main effect nor the Group X Valence interaction was significant (ps = .88 and .89 respectively). For SAM arousal ratings, there was a significant main effect for valence, F (2, 114) = 102.25, p < .001, ηp2 = .79. Positive and negative pictures elicited more self-reported arousal than neutral pictures, quadratic effect, F (1, 55) = 202.05, p < .001. There was also a significant linear effect indicating that negative pictures elicited significantly more arousal than positive pictures which elicited more arousal than neutral, F (1, 55) = 9.85, p < .01 (see Table 3).
Table 3.
SAM Valence and Arousal Ratings in Study 2
| Valence | Arousal | |||
|---|---|---|---|---|
| DS | ND | DS | ND | |
| Positive | 12.24 (1.76) | 12.22 (1.33) | 11.38 (1.70) | 12.26 (1.33) |
| Neutral | 9.69 (.96) | 9.76 (.70) | 7.95 (1.81) | 8.71 (1.46) |
| Negative | 5.92 (2.85) | 5.76 (1.76) | 13.11 (2.46) | 12.66 (1.98) |
Note: DS = Depression Symptom group; ND = Non-Depression group. Ratings ranged from 0 to 20 with higher numbers indicating more pleasant or arousing feelings.
Blink Magnitude
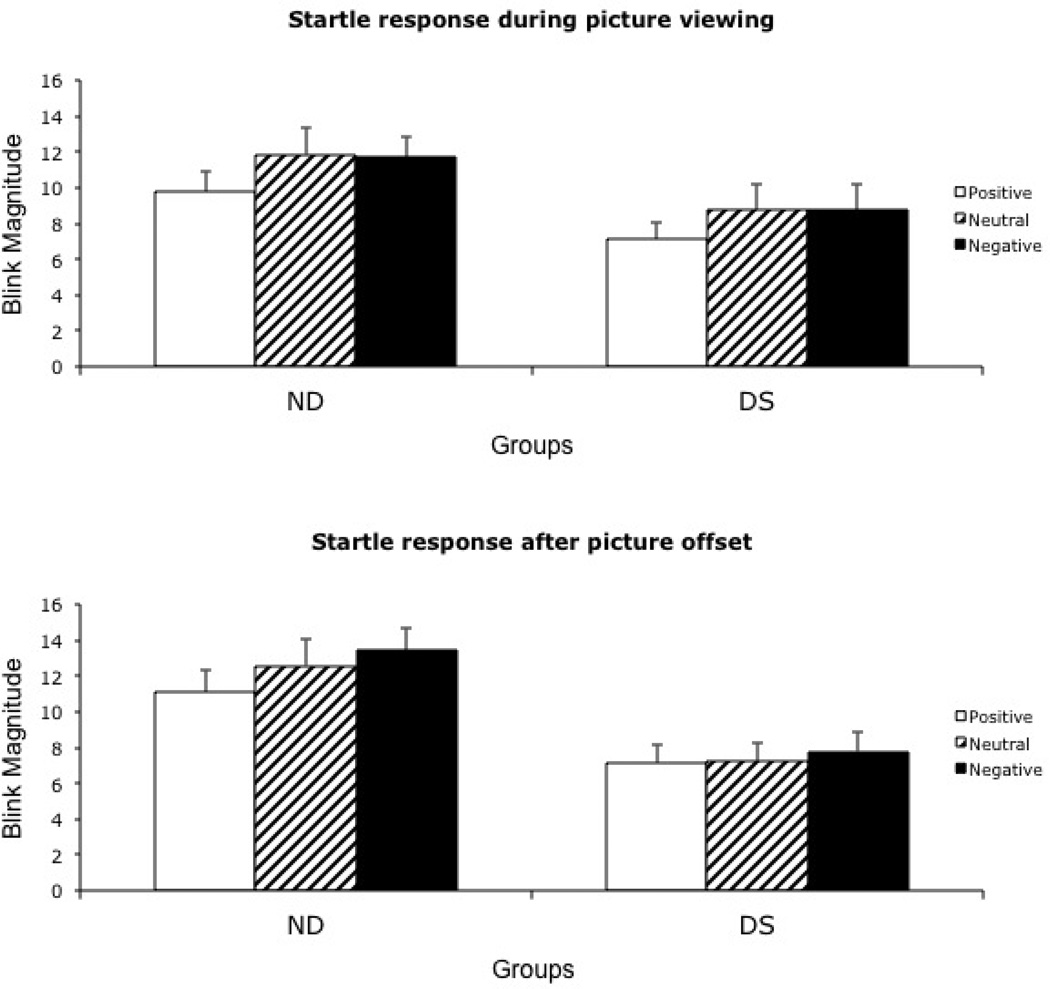
A 2 (Group: DS, ND) X 2 (Probe time: during picture, after picture) X 3 (Valence: positive, neutral, negative) repeated measures MANOVA yielded no significant main effects for group, probe time, or valence and no significant three-way interaction. However, both the Probe Time X Valence interaction (F (2, 54) = 4.98, p = .01, ηp2 = .16) and the Group X Valence interaction (F (2,54) = 3.48, p = .038, ηp2 = .12) were significant (see Figure 2).
Figure 2.
Mean blink magnitudes during and after picture presentation in Study 2
Generally speaking, the Probe Time X Valence interaction indicated that both groups showed greater valence modulation during picture presentation compared to the offset period, consistent with prior studies (Forbes, et al., 2005). That is, during picture presentation, both groups exhibited smaller blink magnitude to positive pictures compared to either neutral or negative pictures (ps < .001), though the difference between negative and neutral was not significant. By contrast, after picture offset, the only significant difference was smaller magnitude after the offset of positive compared to negative pictures (p = .014). However, the Group X Valence interaction indicated that individuals in the ND group showed greater valence modulation across the time points compared to individuals in the DS group. That is, collapsing across probe times, participants in the ND group exhibited smaller blink magnitudes during and after positive pictures compared to neutral and negative pictures (ps = .05 and .005, respectively). Unexpectedly, blink magnitude did not differ between neutral and negative pictures either during of after picture presentation. By contrast, participants in the DS group did not exhibit significantly different blink magnitudes during or after positive, neutral and negative pictures, consistent with the ECI hypothesis. Although blink magnitudes were smaller in the DS group compared to the ND group, these differences were not significant (all p’s > .10).
Discussion
Results from Study 2 replicate and extend the findings from Study 1. As in Study 1, participants in the ND group exhibited smaller blink magnitude while viewing positive pictures compared to while viewing neutral and negative pictures whereas participants in the DS group did not. Extending the time course of responding to after picture offset, the ND group continued to exhibit valence differentiation, with smaller blink magnitudes after positive pictures compared to negative and neutral pictures, but the DS group did not exhibit significant positive attenuation or negative potentiation after picture offset. Therefore, it appears that individuals with moderately high levels of depression symptoms exhibit diminished valence modulation of the startle response both during and after the presentation of emotionally evocative pictures. The overall magnitude of startle responses across probe times was also smaller, albeit nonsignificantly so in the DS group, possibly indicating less overall psychophysiological responsivity to an aversive stimulus (i.e. the acoustic probe) in individuals with depression symptoms.
Although participants with depressive symptoms demonstrated diminished overall emotion modulated startle responses, they exhibited valence-appropriate positive and negative responsivity in their self-reported experience ratings. Therefore, at the experiential level, but not the physiological level, individuals with depression symptoms responded with valence-specific activity. This finding is consistent with previous studies (e.g., Allen, et al., 1999; Berenbaum & Oltmanns, 1992; Dichter & Tomarken, 2008; Dichter, et al., 2004; Forbes, et al., 2005; Gehricke & Shapiro, 2000; Katsikitis & Pilowsky, 1991) and may indicate that depression involves a decoupling of these response systems.
Somewhat surprisingly, while the ND group did exhibit potentiated blink magnitude in response to negative compared to positive pictures, they did not show such potentiation compared to neutral pictures. It is unclear why this pattern was found though prior studies have failed to find significant differentiation between valences (e.g., Germans Gard & Kring, 2007). All participants in Study 2 were women, however, analyses conducted with just the women from Study 1 did not reveal the lack of differentiation between negative and neutral blink magnitude during picture viewing. It seems unlikely that the different pictures used in Study 2 could account for this difference as these pictures have been used in many other SEM studies.
General Discussion
The present studies sought to investigate the time course of emotion in those with and without depression symptoms. While previous studies of emotion in depression have examined emotional responding in the presence of emotional pictures, we sought to understand emotion during the anticipation and maintenance periods. We utilized both self-reported emotional experience and the startle emotion modulation paradigm to measure emotional response. In addition, we sought to extend the ECI hypothesis to different aspects of emotional responding.
Our results confirmed that individuals with depression symptoms exhibit deficits in emotional responsivity compared to asymptomatic individuals. As hypothesized, emotion modulated blink response was robust among people without depression symptoms. However, people with depression symptoms exhibited significantly less differentiated blink response while viewing positive and negative pictures compared to those without symptoms, thus supporting ECI (Rottenberg, et al., 2005). The current studies extend and replicate prior work investigating emotional response in those with depression and suggest that ECI is applicable not just to those diagnosed with MDD, but also to those reporting moderate to high depression symptoms. As Bylsma and colleagues (2008) reported in their meta-analysis, ECI is among the most well-replicated theories of emotion in depression, and the current studies extends this to those with depression symptoms. Diminished emotion modulated responses have also been observed with respect to outward expression in response to emotional stimuli (e.g., Greden, Genero, Price, Feinberg, & Levine, 1986; Rottenberg, et al., 2005).
Our findings differed somewhat with respect to self-reported emotional experience in those with depression symptoms. In Study 1, symptomatic individuals reported feeling less pleasant in response to positive pictures and less unpleasant in response to negative pictures compared to individuals without symptoms. Thus, in Study 1 both startle responsivity and emotional experience during picture viewing showed a pattern consistent with ECI. In contrast, while we found a difference in emotion modulated startle between symptomatic and asymptomatic groups in Study 2, we did not find differences in self-reported emotional experience during picture viewing. Therefore, at the experiential level, but not the physiological level, female participants with moderately severe depression symptoms in Study 2 responded with valence-specific activity.
The two studies differed in a couple of ways that may help to account for these differences. First, participants in Study 2 were all women. While no gender differences were observed in Study 1, it may be that women are more likely to show this decoupling of emotion responses, though there is limited empirical support for this notion. Second, the evocative pictures used in Study 2 had higher normed ratings of valence and arousal. Though we can only speculate on how these factors may have contributed to differences in self-reported valence across studies, it may be the case that the greater valence and arousal of the Study 2 pictures was strong enough to elicit emotion modulation at the experiential level among those with depression symptoms. Future studies might test this hypothesis by utilizing emotion stimuli of varying levels of intensity to observe whether ECI varies by the intensity of the evocative stimuli.
The difference in depression severity between studies, though small, was nonetheless significantly different (p < .001). Thus, dissociation of response modalities (i.e., self-report, startle) may be more apparent in those with slightly more severe symptomatology. While some, but not all, studies of non-depressed participants report response system dissociations (see Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005 for review), emotion response system dissociations are commonly observed in the depression literature, particularly among people with more severe symptoms or a diagnosis of MDD (e.g., Allen, et al., 1999; Berenbaum & Oltmanns, 1992; Brown, Schwartz, & Sweeney, 1978; Dichter, et al., 2004; Forbes, et al., 2005; Gehricke & Shapiro, 2000; Katsikitis & Pilowsky, 1991; Mneimne, et al., 2008; Rottenberg, et al., 2005; Sloan & Sandt, 2010). If intensity of experience is associated with coherence between systems (Mauss, et al., 2005), it may be that dampening of emotion response suggested by ECI is linked with dissociations between emotion response systems.
Anticipation of Emotion in Depression
Participants with depression symptoms showed no deficit during the anticipation of emotional pictures as measured by startle eyeblink response. Because the anticipatory startle response is purported to be a measure of attentional arousal, our findings suggest that those with moderate depression symptoms have intact arousal modulated responses. The pattern of anticipatory startle responses observed in this study is similar to the potentiated startle responses in studies that ask participants to imagine emotional scenes (Miller, Patrick, & Levenston, 2002). In fact, researchers have suggested that this potentiated response during the anticipation of positive and negative stimuli may indicate a period wherein participants are imagining the upcoming stimuli (Dichter & Tomarken, 2008; Lipp, et al., 2001). Though speculative, our findings suggest that people with depression symptoms may be imagining forthcoming emotional stimuli in a manner that is similar to those without depression symptoms.
Nevertheless, our finding of intact anticipatory processes is in contrast to other studies using different methods, such as the anticipation of future events (e.g., Bjarehed, et al., 2009; MacLeod & Salaminiou, 2001). In the current studies, we presented standardized emotional pictures and cues, whereas MacLeod and Salminiou (2001) asked participants to generate future personal positive experiences. Anticipating future positive experiences differs from our task in a number of ways, including having a greater cognitive demand and a greater degree of self-relevance.
While cognitive demand may well be greater in tasks that require generation of future emotional experiences, perhaps the key difference is the use of self-relevant future emotional experiences. Several cognitive theories of depression have long held that depression is characterized by hopelessness related to the self, which in turn influences a person’s hopelessness about the future (e.g., Abramson, et al., 1989; Beck & Sunderland, 1967). Thus, the anticipation of self-relevant emotional scenarios may reveal a deficit during anticipation in those with depression that was not seen in the current study. Indeed, Rottenberg (2005) found that people with depression reported greater negative emotion in response to personally relevant imagery than to normative negative emotion films. Miller and colleagues (2002) found that in healthy controls self-relevant positive imagery was associated with greater startle potentiation compared to general positive imagery. Taken together, these findings suggest the possibility of a distinct physiological pattern of response depending on relation of emotional stimuli to the self. Future studies should investigate the distinction between self-relevant and normative emotion eliciting stimuli in those with depression symptoms to clarify whether the anticipation of self-relevant stimuli would show distinctions in emotion response.
Still, our findings suggest that those reporting moderate symptoms of depression show an intact ability to engage attentional resources to anticipate positive and negative evocative pictures. This replicates findings by Larson and colleagues (2007) who found no differences in anticipatory blink magnitude between people with and without anhedonic depression symptoms. While it is difficult to compare the two samples given that the present study looked at overall depression symptoms, and Larson’s study utilized a measure of anhedonic depression which looks to differentiate depression symptoms not related to anxiety, it is noteworthy that both studies suggest a similar finding suggestive of intact anticipatory responses.
In contrast, Dichter and Tomarken (2008) found that those with MDD had a deficit in anticipatory responding. Given that the anticipation of positive emotion is linked to motivated behavior (Baumeister, Vohs, & Nathan DeWall, 2007; Gard, Gard, Kring, & John, 2006; Klein, 1984), and is associated with well-being (e.g., Quoidbach, Wood, & Hansenne, 2009; Sohl & Moyer, 2009; Vilhauer, et al.), the anticipatory period remains an important area of focus for depression. Should it be the case that the anticipation of emotion remains relatively intact in those with moderate symptoms of depression, including anhedonia, while more severe depression is associated with a deficit, further investigation of anticipatory deficits is in order. Should early interventions be able to target deficits in emotion prior to deficits in anticipation are seen, we may be able to prevent future depression episodes. Additionally, understanding the linkages between the time course of emotion would help explain the ways in which diminished responding in-the-moment impacts maintenance and future anticipation of emotion.
Maintenance of Emotion in Depression
Our findings from Study 2 suggest that participants with depression symptoms exhibited a deficit in the maintenance of emotion, a pattern also consistent with the ECI hypothesis. While the non-depressed group continued to show valence differentiated responses after emotional pictures were removed from view, those with depression symptoms showed an undifferentiated emotion response. Given that people with depression symptoms did not exhibit differentiated responses during picture viewing in either Study 1 or Study 2, it may not be all that surprising that they did not show emotion modulated responses during the maintenance period. Nevertheless, it may be the case that the ability to continue to respond to motivationally salient emotional information in the moments after the information is removed is an adaptive quality that individuals with depression symptoms do not benefit from.
Larson et al. (2007) found that participants within the anhedonic depression symptom group exhibited larger blink magnitude 1.5s after the removal of negative pictures compared to neutral pictures. However, there were no other within or between group differences after the 1.5s probe time. In addition, there were no within or between group differences at 3s or 6s probe times following picture offset. Given that the present study measured blink magnitude 2.5s after picture offset, it may be the case that timing of the startle probe is an important factor to consider when examining emotion maintenance. It could also be the case that there is something distinct about those reporting anhedonic depression symptoms that contribute to the delayed and then rapid diminution of blink responses following the offset of negative pictures. Future studies should investigate these distinctions further by assessing linkages between types of depression symptoms, anhedonia, and more specific timing of responses following the removal of evocative stimuli.
While some of the current studies findings appear inconsistent with some of the aforementioned findings of sustained processing of negative information in depression (Rottenberg, et al., 2002; Siegle, et al., 2001; Siegle, et al., 2002), it is worth noting that prior studies employed measures that also assess attentional resources involved in responding to emotional material. Rottenberg et al. (2002) note that RSA modulation is significantly associated with attentional allocation and Siegle et al. (2001) noted that pupil dilation is influenced by attention. Thus, these previous findings may point to sustained attention to negative information in depression, but the current findings suggest that depression is not associated with sustained emotion modulation to negative information. It would be informative to simultaneously assess attentional and emotional deficits in depression to gain a more integrated understanding of the interactions between cognition and emotion in depression.
Another difference between these previous studies and Study 2 is the diagnostic status of participants. It may be the case that individuals diagnosed with MDD show sustained emotional responding (Rottenberg, et al., 2002; Siegle, et al., 2001; Siegle, et al., 2002) while individuals with moderately severe depression symptoms do not. In other words, it may be the case that severity of depression is related to the potentiation of negative emotion, while those with moderately severe depression symptoms continue to show an overall dampening of emotion response. However, this explanation seems unlikely considering the current sample showed similar levels of depression symptoms comparable to samples diagnosed with MDD (e.g., Dichter, et al., 2004).
Our failure to find the maintenance of negative emotion seconds after removal of negative pictures may be somewhat surprising given the extensive literature showing that depression is associated with rumination (see Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008 for review). Rumination, or the persistent focus on feelings of distress and the reasons and consequences for distressed feelings, has been shown to predict future depression in those with depression symptoms (e.g., Nolen-Hoeksema, 2000) and continued experience of depression (e.g., Kuehner & Weber, 1999; Nolen-Hoeksema, 1991). However, rumination is a self-focused cognitive pattern, and our stimuli may not have evoked a similar internal negative focus. As was the case during anticipation, self-relevant stimuli may evoke a different pattern of emotional response and thus future studies should assess the maintenance of emotion following self-relevant and general, normed emotional stimuli. Should maintenance of negative emotion follow self-relevant emotion stimuli specifically, it provides further support for cognitive models of depression and suggests that emotion following negative stimuli may only be maintained when the stimulus or experience is self-focused.
Although our time course hypotheses were not fully borne out by the data, the SEM findings are significant in that they demonstrate that individuals with moderate to severe levels of depression symptoms exhibit similar emotional deficits as people diagnosed with MDD. In particular, symptomatic individuals demonstrated decreased sensitivity to changes in emotional contexts, a pattern previous studies have shown in clinically depressed individuals (e.g., Dichter, et al., 2004; Kaviani, et al., 2004; Rottenberg, et al., 2002). Given that this pattern of reduced response to emotional stimuli has been linked to poorer concurrent and prospective functioning in clinically depressed samples (e.g., Rottenberg et al., 2002), the findings from this study bring attention to the importance of studying emotional deficits in individuals with moderate to high levels of depression symptoms, regardless of their diagnostic status. When expanding our focus to the time course of emotion, the current findings suggest that those with depression symptoms have intact responses to cues signaling forthcoming emotional stimuli but diminished emotion modulation both during after stimuli are removed from view. Should anticipation be relatively intact, interventions targeting the in-the-moment experience and maintenance of emotion may be key to avoiding the development of deficits in anticipatory emotion. In other words, interventions aimed at the experience of emotion in-the-moment along with the savoring of emotion may be crucial to the treatment of depression given that dampened emotion response consistent with ECI has been linked to poorer outcome and future depression episodes (e.g., O'Brien-Simpson, Di Parsia, Simmons, & Allen, 2009; Rottenberg, et al., 2002).
Limitations and Future Directions
It is important to acknowledge several limitations to the present studies. While our sample sizes were comparable to other studies, they were not particularly large and thus additional replication of these findings is important. Second, measures of emotion experience and responsivity were measured at only one time point during each period of the time course of emotion (i.e., anticipation, in-the-moment, and maintenance). Continuous measurements of response systems could provide additional insight into the temporal patterns of emotional responses in depression. Third, while we utilized well-established emotion eliciting pictures, stimuli other than pictures and measurement of the time course of emotion outside of the laboratory are needed to better understand the real world implications of the current study. Finally, while this was a study of those with depression symptoms, we did not assess for medication status or past depression symptoms, though other studies have found no effect of medications on startle response (Dichter, et al., 2004).
Despite these limitations, the current studies replicate and extend prior work in this area by demonstrating that similar emotional deficits are present in individuals with moderate to high levels of depression symptoms as have been found in clinically depressed populations. In particular, symptomatic individuals demonstrated a lack of sensitivity to changes in emotional contexts as compared to asymptomatic individuals both during and after the presentation of emotional material. These findings suggest a deficit in the ability of individuals with depression symptoms to respond adaptively to motivationally significant emotional material and underscore the importance of studying emotional deficits in a varying range of depression symptoms. In addition, our findings suggest that those reporting depression symptoms show an intact ability to engage arousal systems during the anticipation of positive and negative stimuli. The current studies also highlight the importance of better understanding the relationship between different response systems as well underlying mechanisms linked to the time course of emotional responding when investigating the impact of depression symptoms on emotional responsivity.
Acknowledgments
This research was supported in part by a graduate research fellowship from NSF awarded to Erin K. Moran. We would like to thank Marja Germans Gard, David Gard, Yea-Hung Chen, Akiko Terao, Jen Dobbs, Roxanne Espaldon, Jake Smith, Brian Johnson, and Cara Eberhart for their assistance with various aspects of this project.
Footnotes
IAPS picture numbers used in Study 1 were: POSITIVE 4001m, 4150, 4538f, 4240m, 4542f, 4572f, 4599, 4608f, 4611m, 4640, 4651m, 4656, 4660, 4677, 5621, 5629, 8030, 8034, 8080, 8161, 8178, 8200, 8370, 8490; NEUTRAL: 2393, 2440, 2480, 2840, 5510, 5800, 7000, 7004, 7020, 7034, 7035, 7036, 7041, 7150, 7175, 7179, 7185, 7217, 7491, 7950; NEGATIVE: 1022, 1300, 1930, 2683, 3051, 3168, 3400, 3500, 3530, 6260, 6312, 6530, 6570, 9040, 9252, 9253, 9300, 9400, 9500, 9570; habituation, 7211, 9050, 4650. Note: f denotes pictures shown only to women; m denotes pictures shown only to men.
We also conducted the startle analyses using measures standardized within each individual (i.e., T scores). Specifically, blink magnitude means and standard deviations were computed across the valence conditions (positive, negative, neutral) but within each probe time and converted to T scores (M = 50; SD = 10). The standardization procedure did not change the relative pattern of participants’ responses across the picture types and probe times. Reported results did not differ with the use of T scores in Study 1. Although the direction and pattern of results were similar for Study 2, however, the reported interactions were now outside standard conventions of statistical significance (p = .12).
IAPS picture numbers used in Study 2 were: POSITIVE: 4656*, 4660*, 4670, 4677*, 4680, 4681, 4538, 4572, 4687, 5621*, 5626, 5629*, 8161*, 8180, 8185, 8210, 8300, 8400; NEUTRAL: 2190, 2440*, 2480*, 2570, 5120, 7009, 7025, 7031, 7034*, 7060, 7090, 7110, 7170, 7224, 7234, 7235, 7700, 9360; NEGATIVE: 1050, 1525, 3051*, 3061, 3080, 3530*, 6242, 6243, 6250, 6260*, 6300, 6370, 6510, 6550, 6561, 6570*, 6571, 9250; habituation, 7211*, 9050*, 4650*.
* indicates picture also used in Study 1
References
- Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. Journal of Psychiatric Research. 2007;41(6):511–522. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: A theory-based subtype of depression. Psychological Review. 1989;96(2):358–372. [Google Scholar]
- Allen NB, Trinder J, Brennan C. Affective startle modulation in clinical depression: Preliminary findings. Biological Psychiatry. 1999;46(4):542–550. doi: 10.1016/s0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th ed. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Baumeister RF, Vohs KD, Nathan DeWall C. How emotion shapes behavior: Feedback, anticipation, and reflection, rather than direct causation. Personality and Social Psychology Review. 2007;11(2):167–203. doi: 10.1177/1088868307301033. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- Beck AT, Sunderland H. Depression: Clinical, experimental, and theoretical aspects. Hoeber Medical Division, Harper & Row; 1967. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. Journal of Abnormal Psychology. 1992;101:p37–p44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg WK, Balaban MT. Startle elicitation: Stimulus parameters, recording techniques, and quantification. In: Dawson AM, Schell AM, Boehmelt AH, editors. Startle modification: Implications for neuroscience, cognitive science, and clinical science. 1999. pp. 21–50. [Google Scholar]
- Bjarehed J, Sarkohi A, Andersson G. Less Positive or More Negative? Future-Directed Thinking in Mild to Moderate Depression. Cognitive Behaviour Therapy. 2009;39(1):37–45. doi: 10.1080/16506070902966926. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Lateralized startle probes in the study of emotion. Psychophysiology. 1996;33(2):156–161. doi: 10.1111/j.1469-8986.1996.tb02119.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty, and the startle reflex: Habituation in humans. Behavioral Neuroscience. 1993;107(6):970–980. doi: 10.1037//0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Brown SL, Schwartz GE, Sweeney DR. Dissociation of self-reported and observed pleasure in depression. Psychosomatic Medicine. 1978;40(7):536–548. doi: 10.1097/00006842-197811000-00002. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28(4):676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103:103–116. [PubMed] [Google Scholar]
- Cook EW, Atkinson L, Lang KG. Stimulus control and data acquisition for IBM PCs and compatibles. Psychophysiology. 1987;24(6):726–727. [Google Scholar]
- Cox BJ, Enns MW, Larsen DK. The continuity of depression symptoms: use of cluster analysis for profile identification in patient and student samples. Journal of Affective Disorders. 2001;65(1):67–73. doi: 10.1016/s0165-0327(00)00253-6. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience; Cognition &. Emotion. 1998;12(3):307–330. [Google Scholar]
- Deldin PJ, Deveney CM, Kim AS, Casas BR, Best JL. A slow wave investigation of working memory biases in mood disorders. Journal of Abnormal Psychology. 2001;110(2):267–281. doi: 10.1037//0021-843x.110.2.267. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ. The chronometry of affective startle modulation in unipolar depression. Journal of Abnormal Psychology. 2008;117(1):1–15. doi: 10.1037/0021-843X.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Baucom BR. Startle modulation before, during and after exposure to emotional stimuli. International Journal of Psychophysiology. 2002;43(2):191–196. doi: 10.1016/s0167-8760(01)00170-2. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Shelton RC, Sutton SK. Early-and late-onset startle modulation in unipolar depression. Psychophysiology. 2004;41(3):433–440. doi: 10.1111/j.1469-8986.00162.x. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD, Cusack R, Ogilvie AD. Categorical and dimensional reports of experienced affect to emotion-inducing pictures in depression. Journal of Abnormal Psychology. 2004;113:654–660. doi: 10.1037/0021-843X.113.4.654. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Miller A, Cohn JF, Fox NA, Kovacs M. Affect-modulated startle in adults with childhood-onset depression: Relations to bipolar course and number of lifetime depressive episodes. Psychiatry Research. 2005;134(1):11–25. doi: 10.1016/j.psychres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. Journal of Research in Personality. 2006;40(6):1086–1102. [Google Scholar]
- Garrett AS, Maddock RJ. Time course of the subjective emotional response to aversive pictures: relevance to fMRI studies. Psychiatry Research: Neuroimaging. 2001;108(1):39–48. doi: 10.1016/s0925-4927(01)00110-x. [DOI] [PubMed] [Google Scholar]
- Gehricke JG, Shapiro D. Reduced facial expression and social context in major depression: Discrepancies between facial muscle activity and self-reported emotion. Psychiatry Research. 2000;95(2):157–167. doi: 10.1016/s0165-1781(00)00168-2. [DOI] [PubMed] [Google Scholar]
- Germans Gard M, Kring AM. Sex differences in the time course of emotion. Emotion. 2007;7(2):429–437. doi: 10.1037/1528-3542.7.2.429. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Nicolson NA, Peeters F, van Os J, Barge-Schaapveld D, Wichers M. Early improvement in positive rather than negative emotion predicts remission from depression after pharmacotherapy. European Neuropsychopharmacology. 2011;21(3):241–247. doi: 10.1016/j.euroneuro.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Greden JF, Genero N, Price HL, Feinberg M, Levine S. Facial electromyography in depression: Subgroup differences. Archives of General Psychiatry. 1986;43(3):269–274. doi: 10.1001/archpsyc.1986.01800030087009. [DOI] [PubMed] [Google Scholar]
- Hodes RL, Cook Iii EW, Lang PJ. Individual differences in autonomic response: Conditioned association or conditioned fear? Psychophysiology. 1985;22(5):545–560. doi: 10.1111/j.1469-8986.1985.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Mueller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, et al. Now You Feel It, Now You Don't. Psychological Science. 2003;14(6):612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Katsikitis M, Pilowsky I. A controlled quantitative study of facial expression in Parkinson's disease and depression. The Journal of Nervous and Mental Disease. 1991;179(11):683–688. doi: 10.1097/00005053-199111000-00006. [DOI] [PubMed] [Google Scholar]
- Kaviani H, Gray JA, Checkley SA, Raven PW, Wilson GD, Kumari V. Affective modulation of the startle response in depression: influence of the severity of depression, anhedonia, and anxiety. Journal of Affective Disorders. 2004;83(1):21–31. doi: 10.1016/j.jad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Hollon SD, Beck AT, Hammen CL, Ingram RE. Issues and recommendations regarding use of the Beck Depression Inventory. Cognitive Therapy and Research. 1987;11(3):289–299. [Google Scholar]
- Klein DF. Depression and anhedonia. Anhedonia and affect deficit states. 1984:1–14. [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63(7):686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34(5):819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner C, Weber I. Responses to depression in unipolar depressed patients: An investigation of Nolen-Hoeksema's response styles theory. Psychological Medicine. 1999;29(6):1323–1333. doi: 10.1017/s0033291799001282. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol Rev. 1990;97(3):377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville, FL: University of Florida; 2005. [Google Scholar]
- Larson CL, Nitschke JB, Davidson RJ. Common and distinct patterns of affective response in dimensions of anxiety and depression. Emotion. 2007;7(1):182–191. doi: 10.1037/1528-3542.7.1.182. [DOI] [PubMed] [Google Scholar]
- Larson CL, Sutton SK, Davidson RJ. Affective style, frontal EEG asymmetry and the time course of the emotion-modulated startle. Psychophysiology. 1998;35(Suppl 1):S52. [Google Scholar]
- Levenston GK, Patrick CJ, Bradley MM, Lang PJ. The psychopath as observer: Emotion and attention in picture processing. Journal of Abnormal Psychology. 2000;109(3):373–385. [PubMed] [Google Scholar]
- Lewinsohn PM, Solomon A, Seeley JR, Zeiss A. Clinical Implications of subthreshold depressive symptoms. Journal of Abnormal Psychology. 2000;109(2):345–351. [PubMed] [Google Scholar]
- Lipp OV, Cox D, Siddle DAT. Blink startle modulation during anticipation of pleasant and unpleasant stimuli. Journal of Psychophysiology. 2001;15(3):155–162. [Google Scholar]
- MacLeod AK, Salaminiou E. Reduced positive future-thinking in depression: Cognitive and affective factors; Cognition &. Emotion. 2001;15(1):99–107. [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Miller MW, Patrick CJ, Levenston GK. Affective imagery and the startle response: Probing mechanisms of modulation during pleasant scenes, personal experiences, and discrete negative emotions. Psychophysiology. 2002;39(4):519–529. doi: 10.1017/s0048577202394095. [DOI] [PubMed] [Google Scholar]
- Mneimne M, McDermut W, Powers AS. Affective Ratings and Startle Modulation in People with nonclinical depression. Emotion. 2008;8(4):552–559. doi: 10.1037/a0012827. [DOI] [PubMed] [Google Scholar]
- Morris BH, Bylsma LM, Rottenberg J. Does emotion predict the course of major depressive disorder? A review of prospective studies. British Journal of Clinical Psychology. 2009;48(3):255–273. doi: 10.1348/014466508X396549. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Larson CL, Smoller MJ, Navin SD, Pederson AJC, Ruffalo D, et al. Startle potentiation in aversive anticipation: Evidence for state but not trait effects. Psychophysiology. 2002;39(2):254–258. doi: 10.1017/S0048577202010156. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100(4):569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109(3):504–511. [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- O'Brien-Simpson L, Di Parsia P, Simmons JG, Allen NB. Recurrence of major depressive disorder is predicted by inhibited startle magnitude while recovered. Journal of Affective Disorders. 2009;112(1):243–249. doi: 10.1016/j.jad.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Peeters F, Berkhof J, Rottenberg J, Nicolson NA. Ambulatory emotional reactivity to negative daily life events predicts remission from major depressive disorder. Behaviour Research and Therapy. 2010;48(8):754–760. doi: 10.1016/j.brat.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli S, Sarlo M, Bortoletto M, Buodo G, Palomba D. Stimulus-Preceding Negativity and heart rate changes in anticipation of affective pictures. International Journal of Psychophysiology. 2007;65(1):32–39. doi: 10.1016/j.ijpsycho.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Quoidbach J, Wood AM, Hansenne M. Back to the future: the effect of daily practice of mental time travel into the future on happiness and anxiety. The Journal of Positive Psychology. 2009;4(5):349–355. [Google Scholar]
- Rottenberg J. Mood and emotion in major depression. Current Directions in Psychological Science. 2005;14(3):167–170. [Google Scholar]
- Rottenberg J, Gotlib IH. Socioemotional functioning in depression. Mood disorders: A handbook of science and practice. 2004:61–77. [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114(4):627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2(2):135–146. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ. Affective startle modulation in anticipation and perception. Psychophysiology. 2001;38(4):719–722. [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Birbaumer N, Lang PJ. Probe P3 and blinks: Two measures of affective startle modulation. Psychophysiology. 1997;34(1):1–6. doi: 10.1111/j.1469-8986.1997.tb02409.x. [DOI] [PubMed] [Google Scholar]
- Shestyuk AY, Deldin PJ, Brand JE, Deveney CM. Reduced sustained brain activity during processing of positive emotional stimuli in major depression. Biological Psychiatry. 2005;57(10):1089–1096. doi: 10.1016/j.biopsych.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biological Psychiatry. 2001;49(7):624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003;27(3):365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Sandt AR. Depressed mood and emotional responding. Biological Psychology. 2010;84(2):368–374. doi: 10.1016/j.biopsycho.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Strauss ME, Quirk SW, Sajatovic M. Subjective and expressive emotional responses in depression. Journal of Affective Disorders. 1997;46(2):135–141. doi: 10.1016/s0165-0327(97)00097-9. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Strauss ME, Wisner KL. Diminished response to pleasant stimuli by depressed women. J Abnorm Psychol. 2001;110(3):488–493. doi: 10.1037//0021-843x.110.3.488. [DOI] [PubMed] [Google Scholar]
- Sohl SJ, Moyer A. Refining the conceptualization of a future-oriented self-regulatory behavior: proactive coping. Personality and Individual Differences. 2009;47(2):139–144. doi: 10.1016/j.paid.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Haaga DAF, Arnow BA. Is clinical depression distinct from subthreshold depressive symptoms? A review of the continuity issue in depression research. The Journal of Nervous and Mental Disease. 2001;189(8):498–506. doi: 10.1097/00005053-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Archives of General Psychiatry. 2008;65(11):1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boxtel A, Boelhouwer AJW, Bos AR. Optimal EMG signal bandwidth and interelectrode distance for the recording of acoustic, electrocutaneous, and photic blink reflexes. Psychophysiology. 1998;35(6):690–697. [PubMed] [Google Scholar]
- Vanman EJ, Dawson ME, Brennan PA. Affective reactions in the blink of an eye: Individual differences in subjective experience and physiological responses to emotional stimuli. Personality and Social Psychology Bulletin. 1998;24(9):994–1005. [Google Scholar]
- Vilhauer JS, Young S, Kealoha C, Borrmann J, IsHak WW, Rapaport MH, et al. Treating Major Depression by Creating Positive Expectations for the Future: A Pilot Study for the Effectiveness of Future Directed Therapy (FDT) on Symptom Severity and Quality of Life. CNS Neuroscience & Therapeutics. 2011 doi: 10.1111/j.1755-5949.2011.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. Journal of Abnormal Psychology. 1988;97(3):346–353. doi: 10.1037//0021-843x.97.3.346. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]