Abstract
The study of biomaterials for gene delivery in tissue engineering and regenerative medicine is a growing area, necessitating the investigation of new biomaterials and gene delivery vectors. Poly(1,8-octanediol citrate) (POC) and poly(glycerol-sebacate) (PGS) are biodegradable, biocompatible elastomers that have tunable mechanical properties, surface characteristics, and degradation rate. The objective of this work was to investigate whether POC and PGS would support the immobilization and release of lentivirus to allow sustained and localized transgene expression. Porous biomaterials were prepared using salt as a porogen and in vitro and in vivo transgene expression from immobilized and released lentiviruses were assessed. Cells seeded onto biomaterials loaded with lentiviruses yielded titer-dependent transgene expression in vitro. Lentivirus activity on both biomaterials was maintained for at least 5 days. When implanted subcutaneously in rats, POC and PGS with immobilized lentivirus exhibited sustained and localized transgene expression for at least 5 weeks. This research demonstrates that lentivirus immobilization on POC and PGS is feasible and potentially useful for a variety of tissue engineering and regenerative medicine applications.
Keywords: biodegradable polyesters, lentivirus, biomaterials, gene delivery
1. Introduction
Using biomaterials to modulate the microenvironment for tissue repair or regeneration is a fundamental aspect of tissue engineering. In addition to possessing the appropriate mechanical properties for cellular support, biomaterials must also provide suitable biological cues. As such, many researchers have used biomaterials for drug and protein delivery. However, these approaches are often challenging to implement in vivo due to rapid clearance or proteolytic degradation, limited loading capacity, and difficulty in maintaining long-term therapeutic levels [1]. As an alternative, biomaterial-mediated gene delivery is a more versatile approach that allows for the host’s cells to serve as bioreactors and express the proteins for extended periods of time.
Biomaterial-mediated gene delivery offers the opportunity for localized delivery at the injury, which can be achieved by minimizing interactions with unwanted cells and maximizing vector delivery via surface area adjustments of the biomaterial [2, 3]. Compared to biomaterial-mediated gene delivery, vectors delivered intravenously and through direct injection into tissues can lose activity, have limited interstitial convection, and may have off-target gene expression [4, 5]. Lentiviral vectors are an attractive viral vector because they can efficiently transduce both dividing and non-dividing cells, allow for stable long-term gene expression [6, 7], have low genotoxicity [8–10], and lack immunogenic viral proteins [11]. Lentiviral vectors can be delivered by immobilization onto biomaterials through specific or non-specific interactions. Previous studies reported the modification of poly(lactide-co-glycolide) (PLG) biomaterials with phosphatidylserine and the infusion of hydrogels for lentivirus immobilization and delivery [12, 13]. Other work on substrate-mediated lentivirus delivery includes hydroxylapatite-incorporated collagen hydrogels, fibrin hydrogels, and Pluronic F127 gels [14–17].
Poly(1,8-octanediol citrate) (POC) and poly(glycerol-sebacate) (PGS) are attractive polymers for tissue engineering and have been used to repair or regenerate vascular [18–21], bone [22], cartilage [23, 24], neural [25], myocardial [26], and bladder tissue [27]. Although POC, but not PGS, has been used for the delivery of non-viral vectors [28], the efficacy of POC and PGS for gene delivery applications has not been investigated using lentivirus. We hypothesized that the negative charges naturally present on the surface of POC and PGS (i.e. the carboxyl groups) would be conducive to the immobilization and release of active lentivirus, imparting new biofunctionality to these elastomers. This report builds on previous reports in the literature that investigate the use of lentiviruses as a vector for substrate-mediated gene delivery.
2. Materials and Methods
2.1 Biomaterial fabrication and characterization
POC pre-polymer was synthesized according to previously published methods [29]. Briefly, equimolar amounts of citric acid and 1,8-octanediol (Sigma Aldrich, St. Louis, MO) were added to a 100 mL round bottom flask. The monomers were melted under vigorous stirring at 160–165°C. Upon melting, the mixture was polymerized at 140°C for approximately 1 h to yield POC prepolymer. PGS pre-polymer was synthesized by combining equimolar amounts of sebacic acid and glycerol (Sigma Aldrich, St. Louis, MO). The monomers were melted under vigorous stirring at 120°C under 2 Pa vacuum for approximately 5 h. The prepolymers were dissolved in ethanol, mixed with NaCl (sieved 150–250 μm), which served as a porogen (85 wt%), and casted into a Teflon plate. Upon solvent evaporation for 24 h, the Teflon plate was transferred into a vacuum oven (2 Pa) for post-polymerization (120°C, 2 days). The salt in the resulting composite was leached by successive incubations in water (produced by Milli-Q water purification system, Billerica, MA) for 7 days. Biomaterials were cut into disks-shaped pieces (4 mm in diameter, 1 mm thickness) using a cork borer. To remove any unreacted acidic residues, the polymer disks were incubated and rinsed in phosphate buffered saline (PBS) until no further pH changes were detected. The Toluidine Blue O dye binding assay was adapted to measure free carboxyl groups and assumed a 1:1 ratio between carboxyl groups and dye [30]. Biomaterial disks were incubated with 0.25 mM Toluidine Blue O (Sigma Aldrich, St. Louis, MO) at pH 10, 37°C for 30 mins and subsequently washed several times with NaOH at pH 9 to remove non-complexed dye. The dye was eluted from the biomaterial using 50% acetic acid and measured at 633 nm using a UV-visible spectrophotometer and compared to a standard curve of known dye concentrations in 50% acetic acid solution.
2.2 Lentivirus Production
Lentiviruses were produced according to previously established techniques. Lentiviral packaging vectors, pMDL-GagPol, pRSV-Rev, and pIVS-VSV-G, were co-transfected with plenti-CMV-luciferase into HEK-293T cells using Lipofectamine 2000 (Life Technologies, Carlsbad, CA). The cell culture was maintained in DMEM plus 10% FBS at 37°C and 5% CO2. After 48 h of transfection, the supernatant was collected and centrifuged to pellet any residual cells. The supernatant was then concentrated using PEG-it (System Biosciences, Mountain View, CA) and precipitated lentiviruses was resuspended in PBS. Lentivirus titer was determined using an HIV-1 p24 Antigen ELISA Kit (ZeptoMetrix Co., Buffalo, NY).
2.3 Imaging of lentivirus-loaded biomaterials and in vitro transduction of cells
Biomaterial disks were sterilized using 70% ethanol followed by UV sterilization. The disks were allowed to dry, rinsed with sterile PBS, and placed in the wells of ultra-low attachment plates (Corning, Lowell, MA). Lentiviruses encoding for luciferase (Lenti-Luc) were loaded onto the disks and allowed to bind for 30 mins at 4°C. Disks were washed two times with PBS to remove any non-attached or loosely bound viruses and transferred to a new ultra-low attachment plate. Due to the challenging nature of directly quantifying the amount of lentiviruses immobilized onto the biomaterials, the amount of lentiviruses was indirectly quantified using HIV-1 p24 Antigen ELISA Kits. The number of lentiviruses that were initially loaded and released after PBS washes was quantified, and the difference was assumed as the amount of lentiviruses that remain immobilized on the biomaterials. To image the lentiviruses on the disks, disks were fixed in 2.5% paraformaldehyde/2.5% glutaraldehyde, dehydrated using increasing ethanol concentrations, and imaged using scanning electron microscopy (SEM, Hitachi S-4500). For in vitro transduction, HEK-293T cells (6 × 104) were seeded onto each disk. Media was changed the next day to remove unattached cells. After 3 days of transduction, biomaterials were transferred to a new tube. Cells on the biomaterials were lysed with reporter lysis buffer (Promega, Madison, WI) and analyzed for enzymatic activity using Luciferase assay reagent (Promega, Madison, WI) and Synergy Microplate Reader (Biotek, Winooski, VT). The relative light units (RLU) were normalized against protein concentration in the cell extracts, measured by a micro BCA assay kit (Pierce, Rockford, IL).
2.4 Quantification of lentivirus released from biomaterials
Biomaterials with immobilized lentiviruses (Lenti-Luc, 2 × 108 particles) were incubated in DMEM plus 10% FBS at 37°C and 5% CO2 to quantify the amount of lentiviruses released from biomaterials. At different time points, the media was removed (supernatant), stored at −80°C, and fresh media was added to the well. Virus concentration in the supernatant was determined by an HIV-1 p24 Antigen ELISA Kit.
2.5 Activity of lentiviruses released from biomaterials
Biomaterials with immobilized lentiviruses (Lenti-Luc, 2 × 108 particles) were washed two times with PBS and placed into transwell inserts (6.5 mm in diameter, 8 μm pore size) (Corning, Lowell, MA) to determine the activity of the lentiviruses released from the biomaterials. At different time points, inserts with the lentivirus-loaded biomaterials were transferred to new wells pre-seeded with HEK-293T cells. After 3 days of transduction, cells from each well were lysed as previously described and assayed for enzymatic activity.
2.6 Assessment of active lentiviruses retained on biomaterials
Biomaterials with immobilized lentiviruses (Lenti-Luc, 2 × 108 particles) were washed two times with PBS and incubated in DMEM plus 10% FBS at 37°C and 5% CO2 to assess the activity of lentiviruses retained on biomaterials. At different time points, the media were removed, biomaterials transferred to a new well, and HEK-293T cells were seeded on the biomaterials. Three days after cell seeding and transduction, cells on the biomaterials were lysed as previously described and assayed for enzymatic activity.
2.7 Assessment of in vivo transduction via lentivirus-loaded biomaterials
In vivo gene expressions following subcutaneous implantation of lentivirus-loaded biomaterials were determined through bioluminescence imaging. Biomaterials containing lentiviruses (Lenti-Luc, 6 × 108 particles) were prepared as previously described and implanted into Sprague-Dawley rats. Three rats were used per group. Four subcutaneous pockets were created by blunt dissection under deep isoflurane-O2 general anesthesia. Prior to imaging, animals were anesthetized and received an intraperitoneal injection of 30 mg/kg of D-luciferin (Caliper Life Sciences, Hopkinton, MA). Animals were monitored over time for transgene expression using IVIS imaging system (Caliper Life Sciences, Hopkinton, MA). Animals were cared for in compliance with the regulations established by the Northwestern University Institutional Animal Care and Use Committee.
2.8 Statistical Analysis
Statistical analysis was conducted using GraphPad Prism software (La Jolla, CA). Statistical significance between groups was determined after log transformation by one-way ANOVA followed by Tukey’s post test for multiple comparisons and two-tailed Student’s t-test, when appropriate. A level of p < 0.05 was accepted as significant.
3. Results
3.1 POC has greater number of carboxyl groups relative to PGS
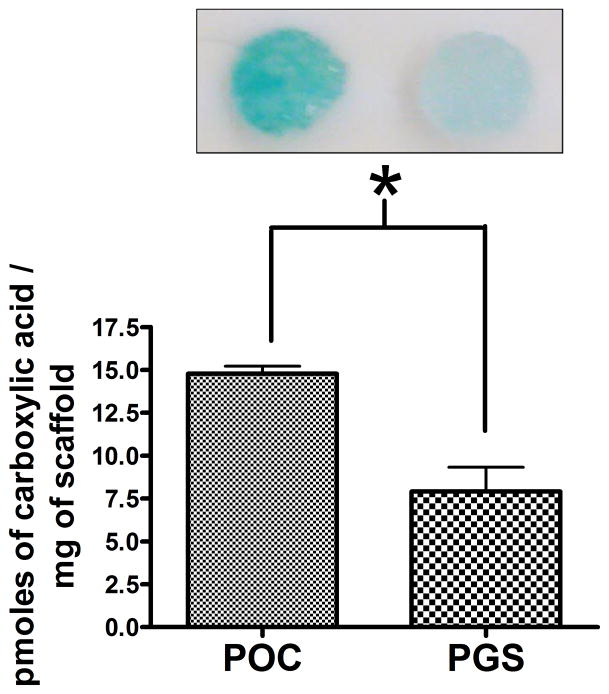
We initially investigated the surface density of carboxyl groups on the biomaterials, as negative charges may influence the adsorption of lentiviruses [12, 14, 17]. Toluidine Blue O is a catonic dye used in histology to bind to negatively charged compounds. The total pmoles of carboxylic acid per mg of biomaterial was approximated to be 14.78 and 7.9 pmoles/mg of biomaterial for POC and PGS, respectively (Fig. 1).
Figure 1.
Toluidine Blue O carboxylic acid determination assay. POC disks had significantly greater dye binding and carboxylic acid compared to PGS (n=4, mean ± SEM). *statistical significance at P<0.005.
3.2 Lentivirus binding to POC and PGS
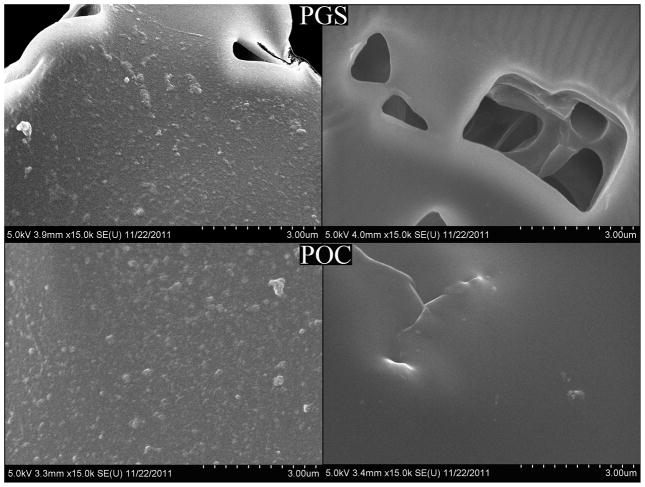
SEM images of POC and PGS samples loaded with lentivirus after washing steps show that particles were absorbed and distributed uniformly on both biomaterials (Fig. 2). The size of the particles are consistent with the reported sizes of lentiviral particles [31]. Particles were not observed on biomaterial disks that were not loaded with lentiviruses.
Figure 2.
SEM images of PGS and POC disks after lentivirus immobilization and washing steps. Disks with immobilized lentiviruses (left) and without lentiviruses (right).
3.3 Titer-dependent in vitro cell transduction on lentivirus-loaded biomaterials
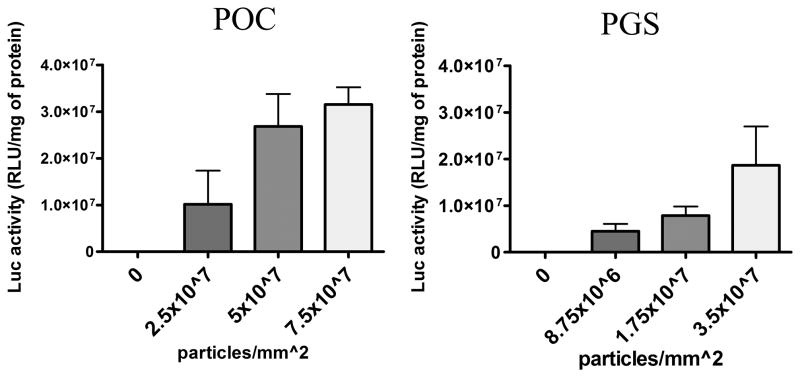
Lenti-Luc were nonspecifically adsorbed onto both biomaterials and were retained following repeated washes with PBS. Lentiviruses retained on the biomaterial were active as they transduced cells that were seeded and cultured on the biomaterial. Transgene expressions on POC and PGS were comparable when similar quantities of Lenti-Luc were deposited on the biomaterials. The luciferase activity increased as the number of lentiviral particles immobilized onto POC and PGS increased (Fig. 3), suggesting that the extent of transgene expression can be modified by varying the density of lentivirus deposited on the biomaterial.
Figure 3.
Titer-dependent transduction on POC and PGS. Multiple titers of Lenti-Luc were immobilized on scaffolds and washed twice to remove unbound viruses. HEK-293T cells were seeded immediately and analyzed for luciferase activity after 3 days of transduction (n=4, mean ± SEM).
3.4 POC exhibited increased lentivirus retention relative to PGS
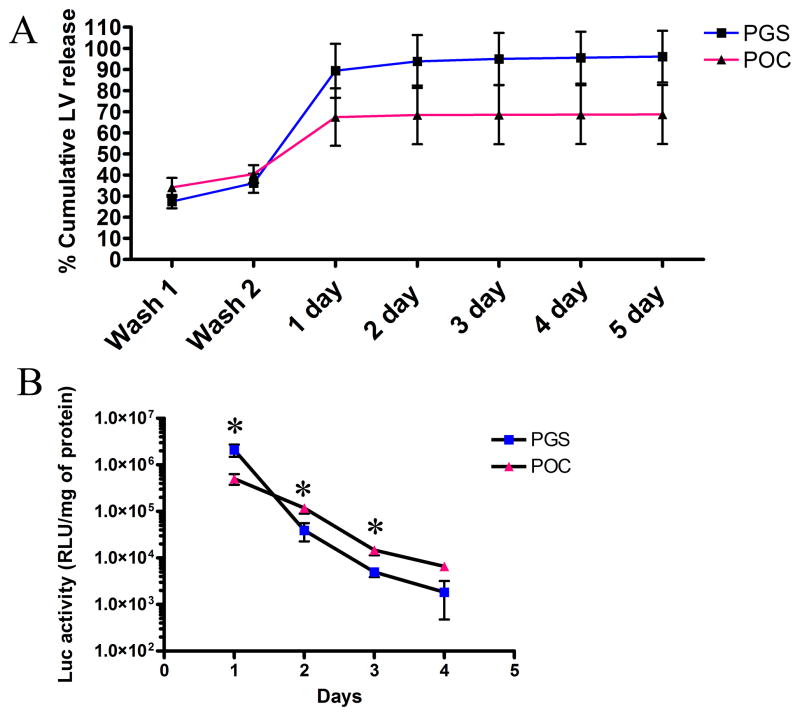
The quantity of lentivirus that was released from the biomaterial was assessed. Biomaterials with immobilized lentiviruses were washed in PBS and incubated in serum-containing media. Released lentiviruses were collected and quantified by ELISA. After PBS washes, 70–80% of lentiviruses loaded onto the biomaterials were retained (Fig. 4a). The greatest loss of lentiviruses occurred on the first day while subsequent days resulted in minimal elution of viruses. Although not statistically significant, POC retained lentiviruses better than PGS.
Figure 4.
Assessment of lentiviruses released from POC and PGS. (A) Cumulative percent release of lentiviruses loaded onto POC and PGS were quantified over several days. (B) Transgene expressions of cells after 3 days of transduction with active lentiviruses released from POC and PGS scaffolds using transwell system (n=4, mean ± SEM). *statistical significance at P<0.05.
3.5 Lentiviruses released from biomaterials are active
Lentiviruses released from the biomaterials were active and capable of transducing surrounding cells (Fig. 4b). At day 1, when there is the greatest release of lentiviruses, the transgene expressions from PGS and POC were 2.11 × 106 and 5.04 × 105 RLU/mg of protein, respectively. The differences between PGS and POC were statistically significant for all days except day 4. The lentiviruses released into the media after day 1 were capable of transducing cells yielding low level of transgene expression that decreased over time. The biggest decrease in transgene expression of approximately 50 fold was between day 1 and 2 for PGS, while POC had a decrease of approximately 4 fold.
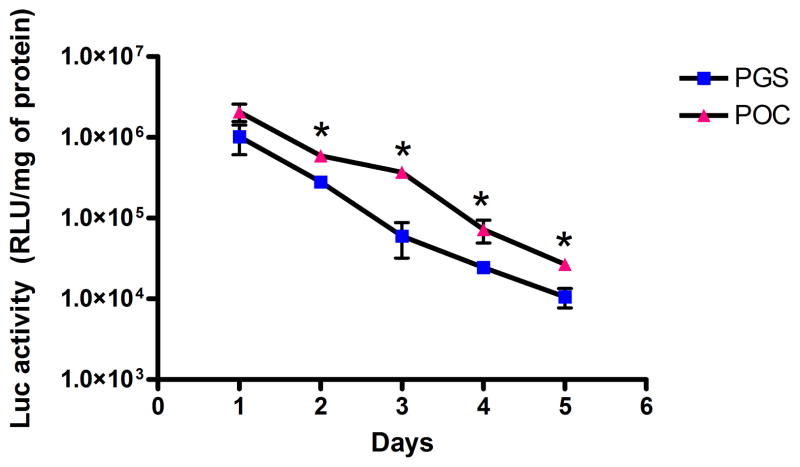
3.6 Lentiviruses on POC and PGS are protected from inactivation
Biomaterials loaded with lentiviruses were incubated in serum-containing media at 37°C for several days before cell seeding. Lentiviruses that are retained on the biomaterial were still active for at least 5 days (Fig. 5). POC and PGS at day 1 resulted in 2.07 × 106 and 1.02 × 106 RLU/mg of protein, respectively. POC yielded statistically higher transgene expression than PGS for all time points except at day 1.
Figure 5.
Activity of retained lentiviruses on POC and PGS. Biomaterials loaded with lentiviruses were incubated in serum-containing media for different durations. Transgene expressions of cells seeded onto the lentivirus-loaded biomaterials were analyzed after 3 days of transduction (n=3, mean ± SEM). *statistical significance at P<0.05.
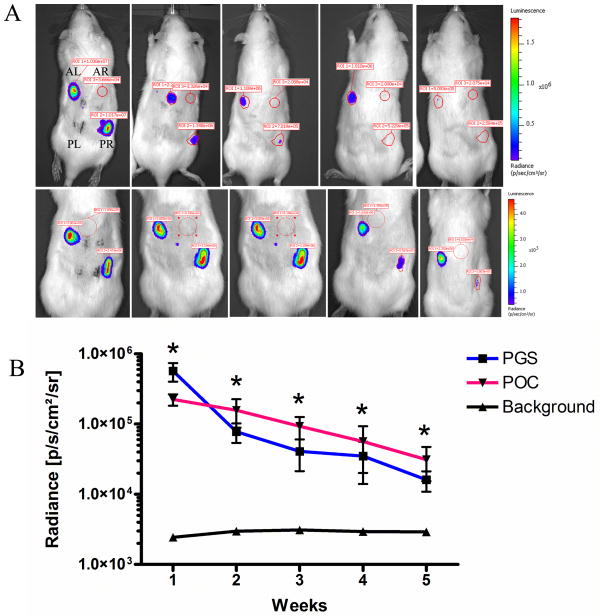
3.7 In vivo transduction via lentivirus-loaded biomaterials
To evaluate sustained and localized transgene expression in vivo, biomaterials containing lentiviruses were implanted into two of the four rat subcutaneous pockets. Based on the bioluminescence imaging, transgene expression persisted for at least 5 weeks (Fig. 6a). Using 3D Diffuse Luminescence Image Tomography (DLIT4) to determine the spatial orientation of the expression, it was confirmed that the transgene expression was localized at the implant site (Fig. 7). At 7 days post-implant, PGS samples exhibited greater luciferase expression than POC samples (5.71 × 105 and 2.24 × 105 radiance, respectively). However, PGS was not significantly different from POC for all time points (Fig. 6b). The greatest decline of 7.3 fold was between week 1 and 2 for PGS, while POC declined by 1.4 fold. Luciferase expression during subsequent weeks dropped by 1–2 fold for both POC and PGS.
Figure 6.
In vivo transgene expressions. (A) Bioluminescence imaging of rats with lentivirus-loaded PGS (top panel) and lentivirus-loaded POC (bottom panel) in the anterior left (AL) and posterior right (PR) subcutaneous pockets. Anterior right (AR) and posterior left (PL) pockets were sham operations and control scaffolds without lentiviruses. Expression was measured using constant-size regions of interest over the implant site. (B) Radiance quantification (photons/sec/cm2/steradian) of luciferase expression for 5 weeks following subcutaneous implantation of PGS and POC loaded with lentiviruses. (n=3, mean ± SEM) *statistical significance at P<0.05 for both POC and PGS versus background
Figure 7.
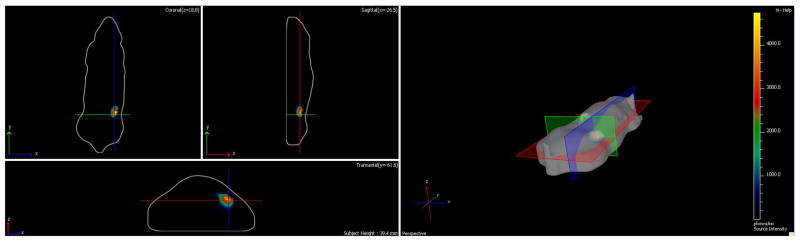
3D Bioluminescence localized at implant site. 3D reconstruction of the rat with lentivirus-loaded POC at week 3, which is also representative of PGS scaffolds. Coronal, sagittal, and transaxial planes intersect the anterior right subcutaneous implant.
4. Discussion
One of the goals of using biomaterials in tissue regeneration applications is to provide microenvironments with appropriate mechanical support and biological cues to modulate tissue formation. In this regard, POC and PGS are promising biodegradable elastomers that have desirable mechanical properties that are comparable to native tissue [32, 33]. Although therapeutic proteins or drugs can be delivered from biomaterials, there are significant challenges in maintaining long-term therapeutic levels due to short half-lives or loss of activity during immobilization. Gene delivery can enhance the bioactivity of biomaterials by allowing for prolonged gene expression. However, a major barrier to clinically successful gene delivery is the lack of a safe and efficient mode of gene delivery. Non-viral vectors such as polyethyleneimine and lipofectamine, the most commonly studied polymer and lipid for nonviral gene delivery, not only elicit low levels of gene expression, but their clinical potential is limited due to their reported toxicity and lack of stable and sustained expression [34, 35]. Lentiviral vectors are a unique type of retroviral vector that was developed to efficiently transduce both dividing and non-dividing cells and allow for long-term gene expression via stable integration of the cargo into host chromosome [6, 7]. The fact that lentiviral vectors have low genotoxicity [8–10] and lack immunogenic viral proteins [11] makes lentiviral vectors one of the most promising gene delivery vectors. Several cardiovascular gene therapy studies have reported that lentiviral vectors are more efficient than adenoviral and adeno-associated vectors at transducing vascular cells [36, 37]. Other studies that utilize lentiviral vectors include the treatment of sickle cell anemia [38], severe combined immunodeficiency [39], melanoma [40], leukemia [41], and Parkinson’s disease [42]. Furthermore, lentiviral vectors are in the planning phase for phase III clinical trials and have shown promise for the treatment of HIV infections [43] and a fatal neurodegenerative disease [10] in humans. However, these studies employ ex vivo transduction or direct injection of lentiviral vectors and reports for biomaterial-mediated lentivirus delivery are scant.
Shin et al demonstrated that lentivirus immobilized on hydroxyapatite particles incorporated into collagen gels effectively transduced cells and proposed that the negatively charged phosphate groups were important for stabilizing the lentivirus [14]. In a different study, they also showed that lentiviruses do not effectively bind to bare poly(lactide-co-glycolide) (PLG) and that anionic head groups provided by phosphatidylserine immobilized onto the polymer were important to allow substrate-mediated transgene delivery [12]. Based on those previous studies, we hypothesize that the negative surface charges of POC and PGS biomaterials are conducive to the immobilization of lentiviruses. Relative to hydrogels and lactide- and glycolide-based polyesters, POC and PGS are tough yet significantly more elastic making them more suitable for soft tissue applications (e.g., bladder, elastic cartilage, blood vessels) [44]. In fact, POC without any reinforcement, showed very promising result as an elastic scaffold in bladder augmentation procedure in small animals [27]. Lentiviruses encoding for growth or transcription factors are expected to increase the bioactivity of POC and PGS and improve tissue regeneration.
In this report, we investigated the use of POC and PGS as substrates for in vitro and in vivo localized lentivirus delivery. According to our data and previous studies [12, 14], it is likely that electrostatic interactions from available, negative carboxyl groups on POC and PGS facilitated the nonspecific lentivirus immobilization. The Toluidine Blue O experiment confirmed that POC had a greater number of carboxyl groups compared to PGS. This is expected as POC utilizes a tricarboxylic acid (citric acid) monomer while PGS utilizes dicarboxylic acid (sebacic acid) monomer. This difference in negative surface charges may have resulted in less lentivirus elution and a lower transgene expression from released lentivirus transduction at day 1 for POC when compared to PGS. Furthermore, POC yielded higher transgene expression from retained lentivirus transduction when compared to PGS. Both POC and PGS showed decreased transgene expressions over time and such finding is expected as lentiviruses’ activity has an inverse relationship with the duration of exposure to serum proteins and heat, factors known to cause lentivirus deactivation [45, 46]. Nevertheless, POC and PGS are suitable substrates for lentivirus delivery and yielded substantial gene expression that can be tailored as suggested by the titer-dependent transduction results.
In vivo, lentiviruses retained on the biomaterial are expected to infect host cells infiltrating the biomaterial while lentiviruses released from the biomaterial are expected to infect tissues and cells surrounding the biomaterial. The in vivo results are consistent with the in vitro experiments, which showed the activity data of retained and released lentiviruses on both POC and PGS. The in vivo bioluminescence imaging experiment demonstrated substantial expression from both biomaterials that was significantly higher than background. Although not statistically significant, PGS yielded greater transgene expression than POC by almost 2 fold at 7 days post-implant. As lentiviruses released more readily from PGS, this could be due to rapid transduction of surrounding tissues and cells as suggested by the in vitro experiments. On the other hand, POC retained more lentiviruses but had not yet been fully infiltrated by host cells for transduction. No significant differences between POC and PGS were detected during the in vivo study. Both biomaterials yielded substantial expression that decreased over the 5-week study. A decrease in the in vivo expression over the course of several weeks has been reported in several studies [12, 14, 17], and is often expected due to natural cell turnover or to an immune response that clears the gene-modified cells [50]. At the end of the 5-week study, POC was better at maintaining transgene expression with 14% of the expression on day 7 compared to 2.8% for PGS.
5. Conclusion
We report the in vitro and in vivo delivery of lentiviral vectors from POC and PGS biomaterials for the purpose of sustained and localized transgene expression. Based on the titer-dependent transduction studies, transgene expressions can be modified on both biomaterials by changing the density of immobilized lentiviruses. However, in vitro, POC was more effective than PGS at retaining lentiviral vectors on the biomaterial. The in vitro data suggest that lentiviruses retained and released from POC and PGS were active for at least 5 and 4 days, respectively. When implanted subcutaneously in rats, both biomaterials resulted in sustained and localized transgene expression for at least 5 weeks. This study demonstrates that POC and PGS, without modification with other compounds, can bind and release bioactive lentiviruses and facilitate transgene expression in cells. Both of these elastomers represent a novel choice for applications that would benefit from substrate-mediated gene delivery.
Supplementary Material
Acknowledgments
The authors thank Jonathan Yuen and Aline Thomas for their assistance in the animal studies. MCJ acknowledges support from the NIH Biotechnology Training Grant.
References
- 1.Tessmar JK, Göpferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Delivery Rev. 2007;59:274–291. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Pannier AK, Shea LD. Controlled Release Systems for DNA Delivery. Mol Ther. 2004;10:19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Jang J-H, Houchin TL, Shea LD. Gene delivery from polymer scaffolds for tissue engineering. Expert Rev Med Devices. 2004;1:127–138. doi: 10.1586/17434440.1.1.127. [DOI] [PubMed] [Google Scholar]
- 4.Heise CC, Williams A, Olesch J, Kirn DH. Efficacy of a replication-competent adenovirus (ONYX-015) following intratumoral injection: Intratumoral spread and distribution effects. Cancer Gene Ther. 1999;6:499. doi: 10.1038/sj.cgt.7700071. [DOI] [PubMed] [Google Scholar]
- 5.Lyons M, Onion D, Green NK, Aslan K, Rajaratnam R, Bazan-Peregrino M, Phipps S, Hale S, Mautner V, Seymour LW, Fisher KD. Adenovirus Type 5 Interactions with Human Blood Cells May Compromise Systemic Delivery. Mol Ther. 2006;14:118–128. doi: 10.1016/j.ymthe.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Trono D. Lentiviral vectors: turning a deadly foe into a therapeutic agent. Gene Ther. 2000;7:20–23. doi: 10.1038/sj.gt.3301105. [DOI] [PubMed] [Google Scholar]
- 7.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In Vivo Gene Delivery and Stable Transduction of Nondividing Cells by a Lentiviral Vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 8.Wang GP, Levine BL, Binder GK, Berry CC, Malani N, McGarrity G, Tebas P, June CH, Bushman FD. Analysis of Lentiviral Vector Integration in HIV+ Study Subjects Receiving Autologous Infusions of Gene Modified CD4+ T Cells. Mol Ther. 2009;17:844–850. doi: 10.1038/mt.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, Sergi LS, Benedicenti F, Ambrosi A, Di Serio C, Doglioni C, von Kalle C, Naldini L. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 10.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, Kiermer V, Mittelstaedt D, Bellesme C, Lahlou N, Lefrère F, Blanche S, Audit M, Payen E, Leboulch P, l’Homme B, Bougnères P, Von Kalle C, Fischer A, Cavazzana-Calvo M, Aubourg P. Hematopoietic Stem Cell Gene Therapy with a Lentiviral Vector in X-Linked Adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 11.Bartosch B, Cosset F. Strategies for retargeted gene delivery using vectors derived from lentiviruses. Curr Gene Ther. 2004;4:427–443. doi: 10.2174/1566523043345995. [DOI] [PubMed] [Google Scholar]
- 12.Shin S, Tuinstra HM, Salvay DM, Shea LD. Phosphatidylserine immobilization of lentivirus for localized gene transfer. Biomaterials. 2010;31:4353–4359. doi: 10.1016/j.biomaterials.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avilés M, Shea L. Hydrogels to modulate lentivirus delivery in vivo from microporous tissue engineering scaffolds. Drug Deliv Transl Res. 2011;1:91–101. doi: 10.1007/s13346-010-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin S, Shea LD. Lentivirus Immobilization to Nanoparticles for Enhanced and Localized Delivery From Hydrogels. Mol Ther. 2010;18:700–706. doi: 10.1038/mt.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raut SD, Lei P, Padmashali RM, Andreadis ST. Fibrin-mediated lentivirus gene transfer: Implications for lentivirus microarrays. J Controlled Release. 2010;144:213–220. doi: 10.1016/j.jconrel.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strappe PM, Hampton DW, Cachon-Gonzalez B, Fawcett JW, Lever A. Delivery of a lentiviral vector in a Pluronic F127 gel to cells of the central nervous system. Eur J Pharm Biopharm. 2005;61:126–133. doi: 10.1016/j.ejpb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Kidd ME, Shin S, Shea LD. Fibrin hydrogels for lentiviral gene delivery in vitro and in vivo. J Controlled Release. 2011;157:80–85. doi: 10.1016/j.jconrel.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K-W, Stolz DB, Wang Y. Substantial expression of mature elastin in arterial constructs. Proc Natl Acad Sci U S A. 2011;108:2705–2710. doi: 10.1073/pnas.1017834108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crapo PM, Wang Y. Hydrostatic pressure independently increases elastin and collagen co-expression in small-diameter engineered arterial constructs. J Biomed Mater Res, Part A. 2011;96A:673–681. doi: 10.1002/jbm.a.33019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kibbe MR, Martinez J, Popowich DA, Kapadia MR, Ahanchi SS, Aalami OO, Jiang Q, Webb AR, Yang J, Carroll T, Ameer GA. Citric acid-based elastomers provide a biocompatible interface for vascular grafts. J Biomed Mater Res, Part A. 2010;93A:314–324. doi: 10.1002/jbm.a.32537. [DOI] [PubMed] [Google Scholar]
- 21.Motlagh D, Yang J, Lui KY, Webb AR, Ameer GA. Hemocompatibility evaluation of poly(glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials. 2006;27:4315–4324. doi: 10.1016/j.biomaterials.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Qiu H, Yang J, Kodali P, Koh J, Ameer GA. A citric acid-based hydroxyapatite composite for orthopedic implants. Biomaterials. 2006;27:5845–5854. doi: 10.1016/j.biomaterials.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 23.Kang Y, Yang J, Khan S, Anissian L, Ameer GA. A new biodegradable polyester elastomer for cartilage tissue engineering. J Biomed Mater Res, Part A. 2006;77A:331–339. doi: 10.1002/jbm.a.30607. [DOI] [PubMed] [Google Scholar]
- 24.Kemppainen JM, Hollister SJ. Tailoring the mechanical properties of 3D-designed poly(glycerol sebacate) scaffolds for cartilage applications. J Biomed Mater Res, Part A. 2010;94A:9–18. doi: 10.1002/jbm.a.32653. [DOI] [PubMed] [Google Scholar]
- 25.Sundback CA, Shyu JY, Wang Y, Faquin WC, Langer RS, Vacanti JP, Hadlock TA. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26:5454–5464. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Park H, Larson BL, Guillemette MD, Jain SR, Hua C, Engelmayr GC, Jr, Freed LE. The significance of pore microarchitecture in a multi-layered elastomeric scaffold for contractile cardiac muscle constructs. Biomaterials. 2011;32:1856–1864. doi: 10.1016/j.biomaterials.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma AK, Hota PV, Matoka DJ, Fuller NJ, Jandali D, Thaker H, Ameer GA, Cheng EY. Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly(1,8-octanediol-co-citrate) based thin films. Biomaterials. 2010;31:6207–6217. doi: 10.1016/j.biomaterials.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X-Q, Tang H, Hoshi R, De Laporte L, Qiu H, Xu X, Shea LD, Ameer GA. Sustained transgene expression via citric acid-based polyester elastomers. Biomaterials. 2009;30:2632–2641. doi: 10.1016/j.biomaterials.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Webb AR, Ameer GA. Novel Citric Acid-Based Biodegradable Elastomers for Tissue Engineering. Adv Mater. 2004;16:511–516. [Google Scholar]
- 30.Gupta B, Plummer C, Bisson I, Frey P, Hilborn J. Plasma-induced graft polymerization of acrylic acid onto poly(ethylene terephthalate) films: characterization and human smooth muscle cell growth on grafted films. Biomaterials. 2002;23:863–871. doi: 10.1016/s0142-9612(01)00195-8. [DOI] [PubMed] [Google Scholar]
- 31.Garnier L, Ratner L, Rovinski B, Cao S-X, Wills JW. Particle Size Determinants in the Human Immunodeficiency Virus Type 1 Gag Protein. J Virol. 1998;72:4667–4677. doi: 10.1128/jvi.72.6.4667-4677.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotech. 2002;20:602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA. Synthesis and evaluation of poly(diol citrate) biodegradable elastomers. Biomaterials. 2006;27:1889–1898. doi: 10.1016/j.biomaterials.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 34.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Controlled Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Gillet JPMB, Fathke RL, Gottesman MM, Kimchi-Sarfaty C. The development of gene therapy: from monogenic recessive disorders to complex diseases such as cancer. Methods Mol Biol. 2009;542:5–54. doi: 10.1007/978-1-59745-561-9_1. [DOI] [PubMed] [Google Scholar]
- 36.Dishart KL, Denby L, George SJ, Nicklin SA, Yendluri S, Tuerk MJ, Kelley MP, Donahue BA, Newby AC, Harding T, Baker AH. Third-generation lentivirus vectors efficiently transduce and phenotypically modify vascular cells: implications for gene therapy. Journal of Molecular and Cellular Cardiology. 2003;35:739–748. doi: 10.1016/s0022-2828(03)00136-6. [DOI] [PubMed] [Google Scholar]
- 37.Céfaï D, Simeoni E, Ludunge KM, Driscoll R, von Segesser LK, Kappenberger L, Vassalli G. Multiply attenuated, self-inactivating lentiviral vectors efficiently transduce human coronary artery cells in vitro and rat arteries in vivo. Journal of Molecular and Cellular Cardiology. 2005;38:333–344. doi: 10.1016/j.yjmcc.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 38.Perumbeti A, Higashimoto T, Urbinati F, Franco R, Meiselman HJ, Witte D, Malik P. A novel human gamma-globin gene vector for genetic correction of sickle cell anemia in a humanized sickle mouse model: critical determinants for successful correction. Blood. 2009;114:1174–1185. doi: 10.1182/blood-2009-01-201863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mortellaro A, Hernandez RJ, Guerrini MM, Carlucci F, Tabucchi A, Ponzoni M, Sanvito F, Doglioni C, Serio CD, Biasco L, Follenzi A, Naldini L, Bordignon C, Roncarolo MG, Aiuti A. Ex vivo gene therapy with lentiviral vectors rescues adenosine deaminase (ADA)-deficient mice and corrects their immune and metabolic defects. Blood. 2006;108:2979–2988. doi: 10.1182/blood-2006-05-023507. [DOI] [PubMed] [Google Scholar]
- 40.Chhabra A, Yang L, Wang P, Comin-Anduix B, Das R, Chakraborty NG, Ray S, Mehrotra S, Yang H, Hardee CL, Hollis R, Dorsky DI, Koya R, Kohn DB, Ribas A, Economou JS, Baltimore D, Mukherji B. CD4+CD25− T Cells Transduced to Express MHC Class I-Restricted Epitope-Specific TCR Synthesize Th1 Cytokines and Exhibit MHC Class I-Restricted Cytolytic Effector Function in a Human Melanoma Model. J Immunol. 2008;181:1063–1070. doi: 10.4049/jimmunol.181.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen E-Y, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Déglon N, Aebischer P. Neurodegeneration Prevented by Lentiviral Vector Delivery of GDNF in Primate Models of Parkinson’s Disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 43.Levine BL, Humeau LM, Boyer J, MacGregor R-R, Rebello T, Lu X, Binder GK, Slepushkin V, Lemiale F, Mascola JR, Bushman FD, Dropulic B, June CH. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci U S A. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb AR, Yang J, Ameer GA. Biodegradable polyester elastomers in tissue engineering. Expert Opin Biol Ther. 2004;4:801–812. doi: 10.1517/14712598.4.6.801. [DOI] [PubMed] [Google Scholar]
- 45.DePolo NJ, Reed JD, Sheridan PL, Townsend K, Sauter SL, Jolly DJ, Dubensky TW. VSV-G Pseudotyped Lentiviral Vector Particles Produced in Human Cells Are Inactivated by Human Serum. Mol Ther. 2000;2:218–222. doi: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- 46.de las Mercedes Segura M, Kamen A, Garnier A. Downstream processing of oncoretroviral and lentiviral gene therapy vectors. Biotechnol Adv. 2006;24:321–337. doi: 10.1016/j.biotechadv.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Jang J-H, Bengali Z, Houchin TL, Shea LD. Surface adsorption of DNA to tissue engineering scaffolds for efficient gene delivery. Journal of Biomedical Materials Research Part A. 2006;77A:50–58. doi: 10.1002/jbm.a.30643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segura T, Shea LD. Surface-Tethered DNA Complexes for Enhanced Gene Delivery. Bioconjugate Chem. 2002;13:621–629. doi: 10.1021/bc015575f. [DOI] [PubMed] [Google Scholar]
- 49.Lee GK, Maheshri N, Kaspar B, Schaffer DV. PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnol Bioeng. 2005;92:24–34. doi: 10.1002/bit.20562. [DOI] [PubMed] [Google Scholar]
- 50.Annoni A, Battaglia M, Follenzi A, Lombardo A, Sergi-Sergi L, Naldini L, Roncarolo MG. The immune response to lentiviral-delivered transgene is modulated in vivo by transgene-expressing antigen-presenting cells but not by CD4+CD25+ regulatory T cells. Blood. 2007;110:1788–1796. doi: 10.1182/blood-2006-11-059873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.