Abstract
Tricalcium silicate cements have been successfully employed in the biomedical field as bioactive bone and dentin substitutes, with widely acclaimed osteoactive properties. This research analyzed the effects of different tricalcium silicate cement formulations on the temporal osteoactivity profile of human bone marrow-derived mesenchymal stem cells (hMW-MSCs). These cells were exposed to 4 commercially-available tricalcium silicate cement formulations in osteogenic differentiation medium. After 1, 3, 7 and 10 days, quantitative real time-polymerase chain reaction and Western blotting were performed to detect the expression of target osteogenic markers ALP, RUNX2, OSX, OPN, MSX2, and OCN. After 3, 7, 14 and 21 days, alkaline phosphatase assay was performed to detect changes in intracellular enzyme level. Alizarin Red S assay was performed after 28 days to detect extracellular matrix mineralization. In the presence of tricalcium silicate cements, target osteogenic markers were downregulated at the mRNA and protein levels at all time-points. Intracellular alkaline phosphatase enzyme levels and extracellular mineralization of the experimental groups were not significantly different from the untreated control. Quantitative polymerase chain reaction results showed increases in downregulation of RUNX2, OSX, MSX2 and OCN with increase in time of exposure to the tricalcium silicate cements, while ALP showed peak downregulation at day 7. For Western blotting, OSX, OPN, MSX2 and OCN showed increased downregulation with increased exposure time to the tested cements. Alkaline phosphatase enzyme levels generally declined after day 7. Based on these results, it is concluded that tricalcium silicate cements do not induce osteogenic differentiation of hBM-MSCs in vitro.
Keywords: bone marrow, human origin, mesenchymal stem cells, osteogenic differentiation, tricalcium silicate cements
1. Introduction
Bioactivity is one of the most coveted properties of tricalcium silicate cements (TSCs) that is responsible for the continuously-expanding clinical applications of these cements in biomedical tissue engineering. By definition, bioactivity is the ability of a biomaterial to induce a specific biologic response [1]. Tricalcium silicate cements are hydraulic cements wherein their major components, calcium di- and tri-silicates, react with water to form calcium silicate hydrate and calcium hydroxide [2]. The water that is required for these reactions may be derived in vivo from interstitial tissue fluid in the vicinity of the area of application of the TSCs.
Of particular interest to clinicians and researchers is the ability of TSCs to induce osteogenic responses when they are applied to bone defects (i.e. osteoactivity). The literature is replete with studies addressing the osteoactivity of TSCs with the overall results being supportive of their osteogenic potential [3-11]. However, a closer inspection of this body of literature raises important concerns. For instance, the aforementioned studies employed cell lines from different species to study the osteogenic properties of TSCs. While consensus on the cytotoxicity of a biomaterial may be reached because cytotoxicity ranking is preserved among different cell lines [12-14], the same cannot be extended to cell differentiation studies, since different cell lines react differently to the same stimulus [4, 12]. Intraspecies variation is another salient issue; for example, murine MSCs not only from differ from human MSCs, but also differ among various strains in their genetic marker expressions and culture behavior [15-17]. Another source of confusion arises from the frequent use of fully- or partially-differentiated cell lines with established secretory functions and hard tissue forming potential for analysis of the osteogenic differentiation potential of a biomaterial. Results from these studies only show how well-differentiated cells perform their normal physiologic secretory functions in presence of the biomaterial of interest; they do not provide information on how that biomaterial affects the differentiation of those cells from their immature counterparts.
Bone marrow is a promising source of mesenchymal stem cells (MSCs) for a broad range of cellular therapies in regenerative medicine. Human bone marrow-derived MSCs (hBM-MSCs) provide an excellent model for studying the in vitro osteoactivity of TSCs. They are among the most thoroughly characterized cell lines in the field of regenerative medicine [18-21]. These cells are pluripotent, undifferentiated adult stem cells with multi-lineage differentiation potential [22-24]. They may be harvested from the bone marrow relatively easily [25], and have the ability to differentiate into mature, specialized cells with chondrogenic, osteogenic and adipogenic lineages [22-24]. To the best of our knowledge, the osteoactive effects of TSCs on hBM-MSCs have not been studied previously. To address this gap in knowledge and to better understand the bioactivity of TSCs, the objective of the present study was to evaluate the effects of several commercial TSC formulations on osteogenic differentiation of hBM-MSCs. Two null hypotheses were tested: i) TSC exposure has no osteogenic effects on hBM-MSCs, and ii) there are no differences among the different commercial TSC formulations in their prospective influences on osteogenic differentiation of hBM-MSCs.
2. Materials and methods
2.1 Materials
The main constituents and primary phases of the tested TSCs are summarized in Table I. White (WMTA) and gray (GMTA) ProRoot MTA (Dentsply Tulsa Dental Specialties, Tulsa, OK) were mixed with distilled water. White (WMTAP) and gray (GMTAP) MTA Plus (Avalon Biomed Inc, Bradenton, FL) were mixed with the proprietary hydrogel supplied by the manufacturer. These TSCs were mixed in a liquid to powder ratio of 0.3 according to their respective manufacturer's instructions. The mixed materials were placed in pre-sterilized Teflon molds (5-mm diameter and 3-mm thick), covered with pre-sterilized Mylar sheets, and allowed to set in a 100% humidity chamber for 24 hours.
Table I. Comparison of gray and white varieties of ProRoot MTA and MTA Plus.
| Characteristics | White ProRoot MTA | Gray ProRoot MTA | White MTA Plus | Gray MTA Plus |
|---|---|---|---|---|
| Liquid | Water | Water-based gel with water-soluble thickening agents and polymers a | ||
|
| ||||
| Powder:liquid ratio (by weight) | 3:1 | Variable from 1:1 to 4:1 depending on indication | ||
|
| ||||
| Primary Phases | 3CaO·SiO2 | 3CaO·SiO2 | 3CaO·SiO2 | 3CaO·SiO2 |
| 2CaO·SiO2 | 2CaO·SiO2 | 2CaO·SiO2 | 2CaO·SiO2 | |
| Bi2O3 b | Bi2O3 b | Bi2O3 b | Bi2O3 b | |
| 3CaO·Al2O3 | 3Ca·OAl2O3 | 3CaO·Al2O3 | 3CaO·Al2O3 | |
| CaSO4 | CaSO4 | CaSO4 | CaSO4 | |
| Ca2(Al,Fe)2O5 c | Ca2(Al,Fe)2O5 c | |||
contents are GRAS (generally regarded as safe);
bismuth oxide added for radiopacity;
calcium aluminoferrite is only present in the gray variety of both cements. It is involved in the hydraulic phase and is more radiopaque than calcium silicate hydrate.
To avoid the initial cytotoxic effects of TSCs as demonstrated in a previous study [26], a previously-published aging/elution protocol based on the cements' specific cytotoxic profiles was adopted to render the cement discs non-cytotoxic prior to initiation of the experiment [26, 27]. According to this protocol, cement discs were sterilized by UV light for 4 hours, aseptically placed in their respective sterile Transwell® inserts (3.0 micron porous filter, Corning Inc., MA), and immersed in complete culture medium (CCM) for 2 weeks at 37°C in 100% humidity. The CCM consisted of alpha minimum essential medium (αMEM) and 20% fetal bovine serum (Atlanta Biologicals, GA), supplemented with 2 mmol/L L-glutamine (Lonza, Wakersville, MD) and 100 U/mL penicillin G/streptomycin sulfate (Invitrogen Corp, Carlsbad, CA). The culture medium was changed every 3 days to eliminate cytotoxic eluents that may interfere with their bioactive properties.
2.2 Cell culture
Previously characterized hBM-MSCs with CD73+/CD105+/CD45− immunophenotype [28] were used in the present study. The cells were received from the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White, through a grant from NCRR of the National Institute of Health, Grant #P40RR017447. This cell line has a subpopulation of small, rapidly-proliferating cells with high multi-differentiation potential that have been identified as rapidly self-renewing cells, among larger mature MSCs with limited differentiation potential and slow proliferation rate [29]. The multi-lineage potential of this cell line has been confirmed in previous studies [18]. For the current experiment, cells from passages 4 to 6 were plated in CCM at 1x104 cells/cm2. After 24 hours, or after the cells reached ≈70%-80% confluence, Transwells® containing the pre-treated cement discs were placed in their respective wells, and the medium was changed to osteogenic differentiation medium (ODM). The latter consisted of the original CCM, supplemented with 50 mg/mL ascorbic acid, 10 mmol/L β-glycerophosphate, and 100 nmol/L dexamethasone (Sigma-Aldrich, St Louis, MO). The ODM was changed every 3 days.
2.3 RNA isolation and quantitative real time-polymerase chain reaction
Quantitative real time-polymerase chain reaction (qRT-PCR) was used to detect the relative expression of target markers of osteogenic differentiation among the test groups. The procedures were performed by isolating total RNA from hBM-MSCs after 1, 3, 7 and 10 days of exposure to the test materials. Total RNA was isolated and purified using QIAshredder and RNeasy kit (Qiagen, Valencia, CA) following the manufacturer's recommended protocol for RNA purification from animal cells. The purity and quantity of the resultant RNA were assessed from 2-μL samples by using a NanoDrop1000 spectrophotometer (ThermoScientific, Wilmington, DE). Equal amounts of total RNA (0.1 mg RNA/mL) were then reverse-transcribed into single-stranded complementary DNA (cDNA) by using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) in a thermal cycler by using the recommended settings (25°C for 10 min, 37°C for 120 min, 85°C for 5 min). The resultant cDNA was stored at -20°C until commencement of the qRT-PCR procedures.
For qRT-PCR, alkaline phosphatase (ALP), osteocalcin (OCN), osteopontin (OPN), Runt-related transcription factor 2 (RUNX2), osterix (OSX) and muscle segment homeobox 2 (MSX2) were selected as target markers for osteogenic differentiation. Untreated hBM-MSCs in ODM (designated as MM) were assigned as the experimental control. The cDNA was mixed with Taqman® PCR universal master mix II (Invitrogen Corp.), added in 20 μL portions to 96-well custom TaqMan array standard plates preloaded with the pre-designated primers and housekeeping gene, loaded into a 7300 Real Time PCR System (Applied Biosystems), and run in a regular cycle. Each cycle consisted of 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of denaturing/annealing at 95°C for 15 sec followed by 60°C for 1 min. One hundred nanograms of cDNA were loaded in each 20 μL reaction. Each specimen was run in triplicates, with 18S rRNA used as the housekeeping gene. Relative quantification of gene expression was performed by using the comparative threshold cycle method (ΔΔCT), in which the expression level of each target marker was normalized to the 18S rRNA endogenous control used as an active reference. Data output was expressed as fold changes of mRNA expression levels, given by 2-ΔΔCT [30]. Expression profiles of target gene markers were compared among test groups.
2.4 Western blotting
Western blotting was used to analyze the expression levels of OCN, OPN, OSX, MSX2 and RUNX2 proteins following exposure of the hBM-MSCs to the test TSCs. After 1, 3, 7 and 10 days, cells exposed to different treatments were lysed using Radio Immuno Precipitation Assay buffer with a protease inhibitor cocktail (Sigma-Aldrich). Bicinchoninic acid assay was used to determine the protein concentration per specimen using bovine serum albumin as a standard. Protein denaturing and reduction was accomplished by adding 4X Laemmli buffer (Bio-Rad Laboratories, Hercules, CA) to the specimens, after standardization of the total amount of protein to be loaded, followed by boiling the samples at 100°C for 5 min. Forty microliter specimens were loaded onto 18-well 4–20% gradient Criterion XT Bis-Tris Gel (Bio-Rad) and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was run for 1 hour at 150 volts on a Criterion™ electrophoresis cell (Bio-Rad) with β-actin (43 kDa) as the loading control. The proteins were transferred from the gel to a PVDF membrane (Immobilon-FL PVDF, 0.45µm/Immobilon-PSQ Membrane, PVDF, 0.2 µm, Millipore, MA) using a Criterion™ Blotter and PowerPac™ HC power supply (Bio-Rad) running at 100 volts for 30 min. The membranes were blocked with Odyssey® blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 hour, and the blocked membranes were incubated with the primary antibodies diluted in Odyssey® blocking buffer overnight. Table II represents the target primary antibodies, molecular weights in kDa and the dilutions used. The membranes were then washed at room temperature in phosphate-buffered saline (PBS) and 0.1% Tween 20 (PBST) with gentle shaking. Fluorescent-labeled secondary antibodies, IRDye® 800CW Goat anti-Mouse IgG2b-Specific and 680LT Goat anti-Rabbit IgG (H + L) infrared dyes (LI-COR), were diluted with Odyssey® blocking buffer in the dilutions listed in Table II. The membranes were incubated with the diluted secondary antibodies for 60 min in the dark, at room temperature with gentle shaking. The membranes were then washed with PBST for 4 times and after a final wash with PBS, they were scanned with an Odyssey® CLx infrared imager using the standard scanning parameters. Experiment was conducted in triplicates. Relative protein expression analyses were performed using the Image Studio Lite software (LI-COR).
Table II. List of antibodies used for Western blot analysis including molecular weights, isotypes, clonality, localization, secondary antibodies and concentrations used.
| Name | Code | Description | Clonality | Isotype | Concentration | Molecular weight | Cellular localization | Secondary antibody | Additional bands |
|---|---|---|---|---|---|---|---|---|---|
| Anti MSX2 | ab69058 | Rabbit polyclonal to Msx2/Hox8 | polyclonal | IgG | 1 μg/mL | 29 kDa | nuclear | Goat polyclonal to rabbit IgG at 1/3000 dilution | 48, 55 |
| Anti-RUNX2 | ab23981 | Rabbit polyclonal to RUNX2 | polyclonal | IgG | 1 μg/mL | 57 kDa | nuclear | Goat polyclonal to rabbit IgG at 1/10000 dilution | |
| Anti-Sp7 / osterix antibody | ab22552 | Rabbit polyclonal to Sp7/osterix – ChIP grade | polyclonal | IgG | 2.5 μg/mL | 46 kDa | nuclear | Goat anti-rabbit IgG | |
| Anti-osteopontin antibody | ab91655 | Rabbit monoclonal [EPR3688] to osteopontin | monoclonal | IgG | 1/1000 | 35 kDa | secreted | HRP-conjugated goat anti-rabbit at 1/2000 dilution | |
| Anti-osteocalcin antibody | ab13420 | Mouse monoclonal [OCG3] to osteocalcin | monoclonal | IgG3 | 3 μg/mL | 12 kDa | secreted | HRP-conjugated goat anti-mouse IgG polyclonal at 1/5000 dilution | |
| Anti-actin antibody | ab3280 | Mouse monoclonal [ACTN05 (C4)] to actin | monoclonal | IgG1 | 0.5 μg/mL | 42 kDa | cytoplasm > cytoskeleton | HRP-conjugated goat anti-mouse IgG polyclonal at 1/5000 dilution |
2.5 Alkaline phosphatase enzyme activity
Intracellular release of ALP enzyme was assessed using the Quantichrom ALP assay kit (Bioassay Systems, Hayward, CA). Colorimetric determination was based on hydrolysis of p-nitrophenyl phosphate by ALP into inorganic phosphate and p-nitrophenol, a yellow-colored product [31]. After 3, 7, 14 and 21 days of exposure, the cement discs and their respective Transwell® inserts were retrieved. The cells were washed with PBS, lysed with 0.2% Triton X-100 for 20 min. Working solution was added to a clear-bottomed 96 well plate (150 μL/well), and cell lysate was quickly added to the working solution using a multi-channel pipettor. Absorbance of p-nitrophenol at 405 nm was recorded every min for a maximum of 16 min by using a microplate reader. The level of ALP activity of the cell lysate in IU/L was calculated from the equation:
Where OD is the optical density at 405 nm, and t is the time in min. ODSample 0 is the optical density at 0 min. The resultant data were plotted on a graph, and the peak value for each material was selected and compared with the other materials.
2.6 Alizarin red S assay
Extracellular matrix mineralization was assessed both qualitatively and quantitatively by Alizarin Red S assay [32]. Alizarin red S (AR-S) stains calcium-rich deposits (nodules) produced by cultured cells; the dye may be extracted from the stained nodules and assayed by spectrophotometry. After the hBM-MSCs were exposed to cement discs in ODM for 28 days, the cement discs were retrieved and cells were washed with PBS, fixed in 10% formaldehyde and stained with AR-S (40 mmol/L solution, pH 4.2) for 20 min at room temperature under shaking. Images of the specimens were taken for qualitative assessment of the stained nodules. The stained nodules were then incubated in 10% acetic acid for 30 min and neutralized with 10% ammonium hydroxide. The supernatant was pipetted into a clear-bottom 96-well plate, and the absorbance of the supernatants was determined at OD = 405 nm. The level of AR-S staining in the specimens (mg/L) was determined according to a linear regression equation derived from a pre-equilibration standard curve of known AR-S concentrations.
2.7 Statistical analyses
For qRT-PCR, the effect of materials on expression of each osteogenic marker was analyzed separately for each time-point by using one-factor analysis of variance (ANOVA) and post-hoc Tukey test or their nonparametric equivalents. Similar tests were used for analyzing the protein levels obtained from Western blotting, as well as the results obtained from the ALP enzyme assay and the Alizarin red assay. Parametric versions of these tests were used after evaluation of the normality (Shapiro–Wilk test) and equal variance assumptions (modified Levene test) of the individual data tests. If those assumptions were violated, the data were nonlinearly-transformed to satisfy those assumptions before using parametric testing methods. If those assumptions remained violated after nonlinear transformation, the original data set was analyzed using Kruskal–Wallis ANOVA and Dunn's multiple comparison tests. Statistical significances for all analyses were set at α = 0.05.
3. Results
3.1 qRT-PCR
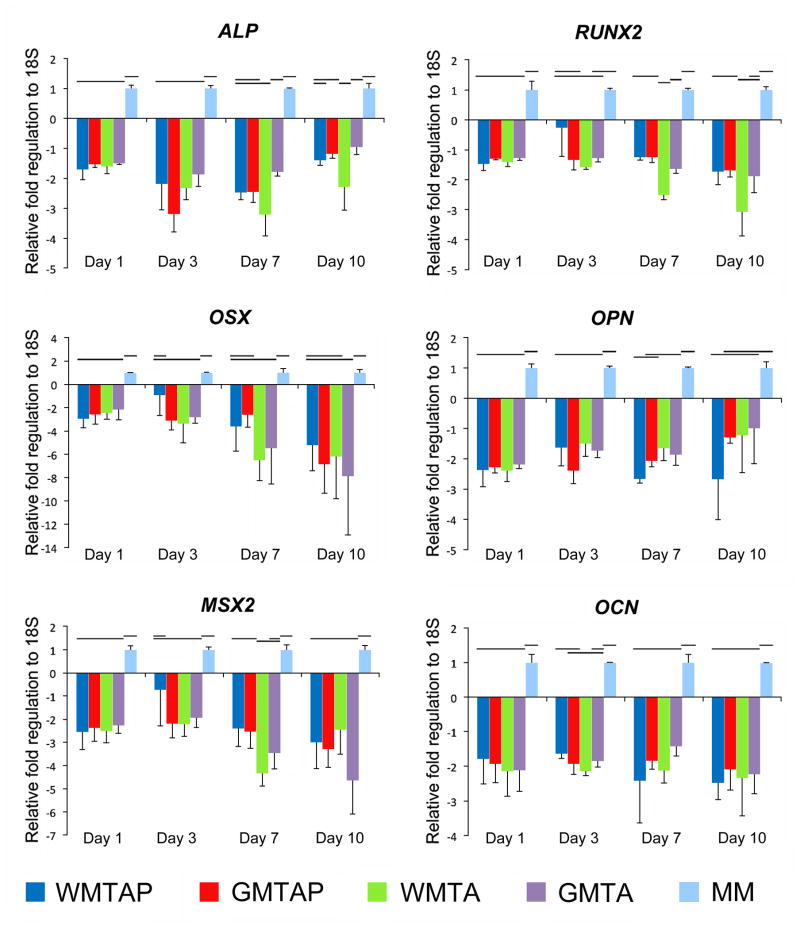
A detailed presentation of the fold regulation of the expression of target mineralization markers can be found in Figure 1. Expression of ALP was significantly downregulated at the 4 time-points in all treatment groups compared to the MM control, with maximum downregulation noted at day 7 for all treatment groups. No significant differences were found between ALP expression in treatment groups except between GMTA and WMTA at days 7 and 10, where ALP expression was significantly more downregulated in WMTA compared to GMTA and GMTAP. Expression of RUNX2 was significantly downregulated for all treatment groups at all time-points compared to the MM control, except for day 3, where the downregulation was not statistically significant for all groups except for WMTA. White ProRoot MTA (WMTA) also showed significantly more downregulation of RUNX2 expression at day 7 compared to other treatment groups. At day 10, RUNX2 expression was significantly more downregulated in WMTA when compared to GMTAP and WMTAP, but not to GMTA. Expression of OSX was significantly downregulated in WMTAP only at day 1, in GMTAP at days 1 and 3, in WMTA at days 1, 3 and 7, and in GMTA at all time-points. Expression of OPN in all treatment groups was significantly downregulated compared to the MM control at days 1, 3 and 7. At day 10, no differences were found between treatment groups and the MM control except for WMTAP, where OPN expression was significantly downregulated compared to the control. The other 3 groups were not significantly different from the MM control or WMTAP. Expression of MSX2 was significantly downregulated in all treatment groups at day 1. At day 3, MSX2 was downregulated in all treatment groups except for WMTAP, which was not different from both the MM control and the other treatment groups. At days 7 and 10, all groups showed significant downregulation of MSX2 compared to the MM control. Expression of OCN was significantly downregulated in all treatment groups at all time-points compared to the MM control. No difference in OCN expression was identified among treatment groups at each time-point except between WMTAP and WMTA at day 3.
Figure 1.
Temporal profiles of mRNA expression of target osteogenic markers in hBM-MSCs after exposure to different TSCs in osteogenic differentiation medium, using qRT-PCR with 18S as the endogenous control. Groups connected with a horizontal bar are not significantly different (p>0.05). WMTAP: White MTA Plus; GMTAP: Gray MTA Plus; WMTA: White MTA; GMTA: Gray MTA; MM: untreated cells in osteogenic differentiation medium.
3.2 Western blotting
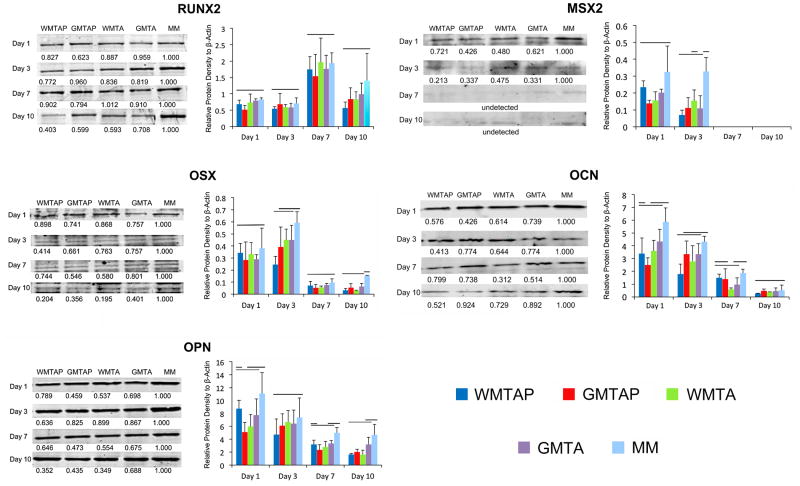
Figure 2 shows the results of the Western blotting. Expression of RUNX2 among groups was not significantly different at all time-points. Nevertheless, peak values were noted at day 7, after which the protein expression level declined at day 10. For OSX, no differences were found at day 1 and 7 among groups, while WMTAP was significantly downregulated at day 3. All groups were significantly downregulated at day 10. For OPN, only GMTA was significantly downregulated at day 1. No differences were noted among all groups at day 3. WMTA and GMTAP were significantly downregulated at day 7. All groups except GMTA were significantly downregulated at day 10. For MSX2, no differences were found among groups at day 1, while significant downregulation was found in groups GMTA, GMTAP and WMTAP at day 3. MSX2 expression was not detected at days 7 and 10. For OCN, GMTAP was significantly downregulated at day 1, WMTAP at day 3, WMTA at day 7. No difference was found among all groups at day 10.
Figure 2.
Temporal profiling of the expression of target osteogenic marker proteins in hBM-MSCs after exposure to different TSCs in osteogenic differentiation medium. Figures on the left side of each figure-pair for the respective protein marker are representative Western blot scans of each protein, with peak protein densities normalized to the experimental control at each time-point. Different letters in each row indicate statistical significance. Figures on the right side of each figure-pair for the respective protein marker are graphical representations of mean protein densities in arbitrary units at each time-point. Groups connected with a horizontal bar are not significantly different (p>0.05). WMTAP: White MTA Plus; GMTAP: Gray MTA Plus; WMTA: White MTA; GMTA: Gray MTA; MM: untreated cells in osteogenic differentiation medium.
3.3 ALP assay
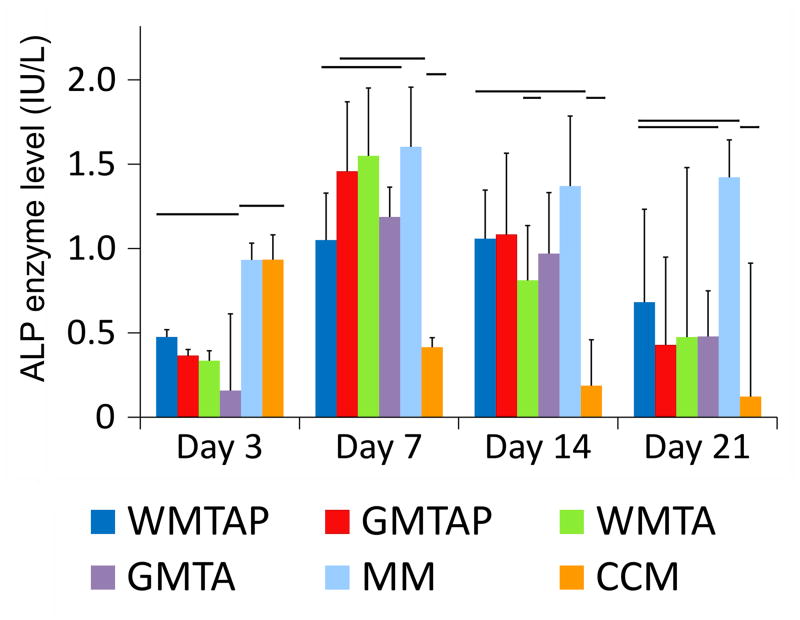
Figure 3 represent the expression of ALP by the MM control and TSC groups over the 4 time-periods. At day 3, ALP levels in all treatment groups were significantly less than both the CCM and MM controls. At day 7, ALP levels in all treatment groups were not significantly different from MM except for WMTAP. At day 14, ALP levels dropped compared with the activity observed at day 7. The ALP levels in all treatment groups were not significantly different from MM but significantly higher than CCM. Further reduction in ALP activity was observed for all experimental groups at day 21, with the results not significantly different from either control group.
Figure 3.
Intracellular alkaline phosphatase (ALP) enzyme level in hBM-MSCs after exposure to different TSCs in osteogenic differentiation medium for 3, 7, 14 and 21 days. Groups connected with a horizontal bar are not significantly different (p>0.05). WMTAP: White MTA Plus; GMTAP: Gray MTA Plus; WMTA: White MTA; GMTA: Gray MTA; MM: untreated cells in osteogenic differentiation medium; CCM: untreated cells in complete culture medium.
3.4 Alizarin red S assay
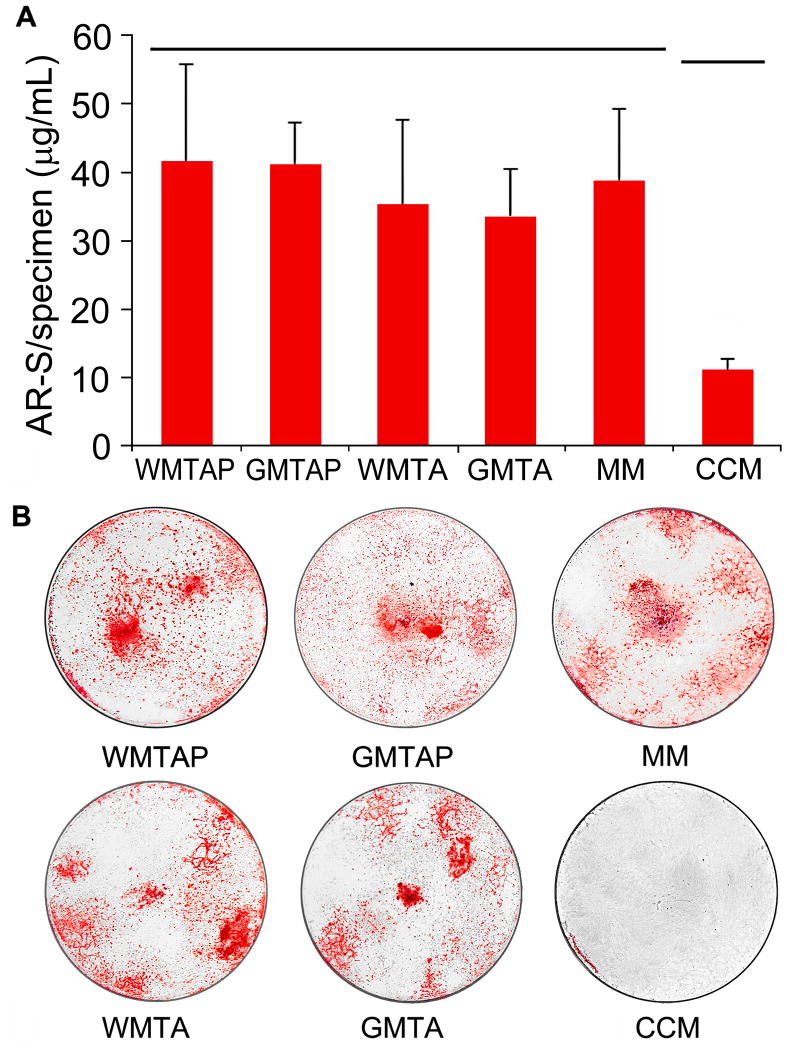
After 28 days of incubation with the TSCs, WMTAP and GMTAP showed higher AR-S levels than WMTA, GMTA and the MM control, but the difference was not statistically significant (Figure 4A). All treatment groups showed significantly higher AR-S levels compared to the CCM control (Figures 4A and 4B).
Figure 4.
A. Alizarin red-S stain level in the extracellular matrix secreted by hBM-MSCs after 28 days of exposure to different TSCs in osteogenic differentiation medium. Groups connected with a horizontal bar are not significantly different (p>0.05). B. Representative images showing macroscopic evaluation of Alizarin red-S staining in the specimens before stain extraction and quantification. WMTAP: White MTA Plus; GMTAP: Gray MTA Plus; WMTA: White MTA; GMTA: Gray MTA; MM: untreated cells in osteogenic differentiation medium; CCM: untreated cells in complete culture medium.
4. Discussion
Human BM-MSCs were employed for the present study due to their robust stemness, pleuripotency, relative ease of harvesting and their clinical significance [18, 20, 25]. Previous studies have reported that TSCs increase the osteogenic potential of osteoblast precursors [33], pre-odontoblasts [34], human osteosarcoma cells [3], periodontal ligament and gingival fibroblast cells [4], osteoblasts [5], osteoblast precursor cells [9], dental pulp stem cells [10], human tooth germ stem cells [35]. To our knowledge, this is the first study that examined the effects of TSCs on the osteoactivity of a pleuripotent, human adult MSC cell line derived from the bone marrow.
The present study employed a multi-parameter approach to study the temporal expression profiles of osteogenic differentiation determinants by tracking the four major molecular events in osteogenesis: mRNA expression of osteogenic markers using qRT-PCR, protein expression using Western blotting, and intracellular ALP enzyme level, all at strategic time points following exposure to the test cements. This was followed by quantification of extracellular calcium deposition and matrix mineralization using AR-S assay after 28 days of exposure to the test cements. This enabled us to monitor the intra- and extra-cellular events that altered the translational, transcriptional, enzymatic and hard tissue forming levels over the length of the experiment.
The following 6 markers, ALP, RUNX2, OCN, OPN, MSX2 and OSX were chosen for examination of osteogenic differentiation in the present study. ALP appears to be intimately related to pre-osseous cellular metabolism and to the elaboration of a calcifiable bone matrix [36]. RUNX2 is a transcription factor involved in osteoblastic differentiation and skeletal morphogenesis. This transcription factor has been shown to effectuate the expression of OSX, collagen type 1 alpha-1 (Col1a1), OCN and OPN by binding to the promoters of these genes [37]. Generally, RUNX2, ALP, Col1a1, transforming growth factor-beta 1 (TGF-β1), osteonectin and bone morphogenetic protein-2 are known to be early markers of osteoblastic differentiation, whereas OCN and OPN are expressed later in the differentiation process [38]. MSX2 promotes osteoblast differentiation and bone formation by upregulating osterix in a RUNX-dependent manner [39]. OSX is a zinc finger-containing transcription factor, specifically expressed in all developing bones. The protein produced is a bone specific transcription factor and is required for osteoblast differentiation and bone formation [40].
Although significant differences in the mRNA and protein levels of some target osteogenic markers among experimental groups existed at several time points, they constitute little if any evidence that one material is different from the other. In fact, the inhibition of target markers in all experimental groups compared to the control group was found to be the only constant finding throughout the allocated time points. Thus, the results of the qRT-PCR and Western blotting are suggestive of the lack of evidence to support that: 1) TSCs may possess osteoactive properties on hBM-MSCs in vitro; and 2) notable differences are different in the osteogenic effects of different formulations of TSCs exist on hBM-MSCs in vitro. The results of ALP and AR-S assays also confirmed the aforementioned results. Accordingly, the first null hypothesis that TSC exposure has no osteogenic effects on hBM-MSCs cannot be rejected. Also, the second null hypothesis that there are no differences among the different commercial TSC formulations in their prospective influences on osteogenic differentiation of hBM-MSCs cannot be rejected.
Interpretation of the results of the present study requires in-depth understanding of the major molecular regulators of BM-MSC differentiation. Because of the significance of BM-MSCs in the field of regenerative medicine, these cells have been studied extensively. Two of those regulators are of particular interest to the present work: the Wnt/β-catenin signaling pathway and the TGF-β superfamily pathways and their roles in MSC differentiation.
The Wnt/β-catenin pathway has been associated in skeletogenesis, bone development and homeostasis [41]. Wnt signaling pathway has differential effects on osteogenesis, which are dependent on the stage of cellular differentiation of the recipient cells [42]. Activation of Wnt/β-catenin signaling pathway has been shown to prevent transdifferentiation of osteoblast to chondrocytes [43], while at the same time inhibiting osteogenic differentiation of BM-MSCs [44]. In a recent article investigating the relationship between the intracellular pH and the Wnt/β-catenin signaling in cancer cells, cross-talk was found between intracellular pH and Wnt signaling pathway activation [45].
A more recent article studied the effects of silicon (Si) on the sonic hedgehog and Wnt signaling pathways, as well as expression of osteogenic markers in BMSCs. AXIN2 and β-catenin, two WNT/SHH signaling related genes, were upregulated in response to Si [46]. Furthermore, Si counteracted the effect of cardamonin, a major Wnt signaling pathway inhibitor. A noteworthy observation in that study is that the Si concentration at which β-catenin was upregulated - the highest concentration (5 mM Si) corresponded to the least ALP activity and cell viability. These observations suggest that TSCs may activate the Wnt/β-catenin pathway partly due to the change of cellular microenvironmental pH and partly due to Si release, leading to inhibition of osteogenic differentiation of BM-MSCs. It should be noted that all the TSCs examined produced highly alkaline extracellular environments (pH ≥ 10) after setting by the release of Ca(OH)2 from the set cements.
Another major regulator of MSC differentiation is the TGF-β superfamily signaling pathway. TGF-β signaling pathway promotes cartilage-specific gene expression through intracellular signaling cascades involving SMAD proteins, the mitogen activated protein (MAP) kinases, p38, extracellular-signal regulated kinase (ERK)-1, and c-Jun N-terminal kinase (JNK) [47]. Tuli and colleagues [48] demonstrated that TGF-β1 treatment initiates and maintains chondrogenesis of MSCs, through the differential chondrostimulatory activities of p38, ERK-1 and JNK in the early steps of MSC chondrogenesis. Transcriptional profiling of MSCs revealed that TGF-β was significant in chondrogenesis, but axonal guidance pathway was significant in adipogenesis and osteogenesis. Furthermore, inhibition of ALK-5 (TGF-β type I receptor)– mediated TGF-β signaling resulted in enhanced adipogenic and osteogenic differentiation, but a complete lack of chondrogenic differentiation [49]. These findings suggest that TGF-β signaling pathway is strongly involved in chondrogenesis of MSCs, and that their role in osteogenic differentiation of MSCs is somewhat less critical. Since TSCs have been found to release TGF- β from gingival fibroblasts [50, 51], it is possible that TSCs may not favor osteogenesis in MSCs in vitro due to activation of TGF-β signaling pathway.
5. Conclusion
In conclusion, the present study did not find any in vitro evidence that TSCs can induce osteogenic differentiation of hBM-MSCs. The results suggest that the previously-reported osteoactivity of TSCs may be dependent upon the cellular differentiation stage, with possible connection to the activation of Wnt canonical and TGF-β signaling pathways in MSCs. This also suggests that fully- or partially-differentiated cell types with established secretory functions such as osteoblasts and pre-osteoblasts may play a more substantial role in the reported in vivo osteogenicity of TSCs, rather than the osteogenic differentiation of pluripotent stem cells. Future research should be directed at examining the direct effects of TSCs on Wnt canonical and TGF-β signaling pathways. Identification of the roles of various subpopulations of bone/hard tissue progenitor cells in bone formation/healing is also of prime importance. Research in this direction is in order.
Acknowledgments
This work was supported by grant R01 DE015306-06 from NIDCR (PI. David H Pashley). The authors thank Dr. Carolyn Primus for supplying the gray and white MTA Plus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams DF. Definitions in Biomaterials: Progress in Biomedical Engineering. Amsterdam: Elsevier; 1987. [Google Scholar]
- 2.Nielsen EP, Herfort D, Geiker MR. Phase equilibria of hydrated Portland cement. Cement Concrete Res. 2005;35:109–115. [Google Scholar]
- 3.Chen CL, Huang TH, Ding SJ, Shie MY, Kao CT. Comparison of calcium and silicate cement and mineral trioxide aggregate biologic effects and bone markers expression in MG63 cells. J Endod. 2009;35:682–685. doi: 10.1016/j.joen.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Bonson S, Jeansonne BG, Lallier TE. Root-end filling materials alter fibroblast differentiation. J Dent Res. 2004;83:408–413. doi: 10.1177/154405910408300511. [DOI] [PubMed] [Google Scholar]
- 5.Koh ET, Torabinejad M, Pitt Ford TR, Brady K, McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res. 1997;37:432–439. doi: 10.1002/(sici)1097-4636(19971205)37:3<432::aid-jbm14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Perinpanayagam H, Al-Rabeah E. Osteoblasts interact with MTA surfaces and express Runx2. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:590–596. doi: 10.1016/j.tripleo.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Masuda-Murakami Y, Kobayashi M, Wang X, Yamada Y, Kimura Y, Hossain M, Matsumoto K. Effects of mineral trioxide aggregate on the differentiation of rat dental pulp cells. Acta Histochem. 2010;112:452–458. doi: 10.1016/j.acthis.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Kuratate M, Yoshiba K, Shigetani Y, Yoshiba N, Ohshima H, Okiji T. Immunohistochemical analysis of nestin, osteopontin, and proliferating cells in the reparative process of exposed dental pulp capped with mineral trioxide aggregate. J Endod. 2008;34:970–974. doi: 10.1016/j.joen.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Li K, Zheng X, He D, Ye X, Wang M. In vitro and in vivo evaluation of zinc-modified Ca-Si-based ceramic coating for bone implants. PLoS One. 2013;8:e57564. doi: 10.1371/journal.pone.0057564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding SJ, Shie MY, Wei CK. In vitro physicochemical properties, osteogenic activity, and immunocompatibility of calcium silicate-gelatin bone grafts for load-bearing applications. ACS Appl Mater Interfaces. 2011;3:4142–4153. doi: 10.1021/am201017v. [DOI] [PubMed] [Google Scholar]
- 11.Lin K, Xia L, Li H, Jiang X, Pan H, Xu Y, Lu WW, et al. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials. 2013;34:10028–10042. doi: 10.1016/j.biomaterials.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 12.Lee DH, Lim BS, Lee YK, Yang HC. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol. 2006;22:39–46. doi: 10.1007/s10565-006-0018-z. [DOI] [PubMed] [Google Scholar]
- 13.Thonemann B, Schmalz G, Hiller KA, Schweikl H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent Mater. 2002;18:318–323. doi: 10.1016/s0109-5641(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 14.Mantellini MG, Botero T, Yaman P, Dennison JB, Hanks CT, Nör JE. Adhesive resin and the hydrophilic monomer HEMA induce VEGF expression on dental pulp cells and macrophages. Dent Mater. 2006;22:434–440. doi: 10.1016/j.dental.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 15.Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, Selig M, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 2009;183:993–1004. doi: 10.4049/jimmunol.0900803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 17.Sung JH, Yang HM, Park JB, Choi GS, Joh JW, Kwon CH, Chun JM, et al. Isolation and characterization of mouse mesenchymal stem cells. Transplant Proc. 2008;40:2649–2654. doi: 10.1016/j.transproceed.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 19.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 20.Gregory CA, Ylostalo J, Prockop DJ. Adult bone marrow stem/progenitor cells (MSCs) are preconditioned by microenvironmental “niches” in culture: a two-stage hypothesis for regulation of MSC fate. Sci STKE. 2005;2005:pe37. doi: 10.1126/stke.2942005pe37. [DOI] [PubMed] [Google Scholar]
- 21.Ylostalo J, Pochampally R, Prockop DJ. Assays of MSCs with microarrays. Methods Mol Biol. 2008;449:133–151. doi: 10.1007/978-1-60327-169-1_10. [DOI] [PubMed] [Google Scholar]
- 22.Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells. 2004;22:823–831. doi: 10.1634/stemcells.22-5-823. [DOI] [PubMed] [Google Scholar]
- 23.Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, O'Connor KC. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 25.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 26.Eid AA, Gosier JL, Primus CM, Hammond BD, Susin LF, Pashley DH, Tay FR. In vitro biocompatibility and oxidative stress profiles of different hydraulic calcium silicate cements. J Endod. 2013 doi: 10.1016/j.joen.2013.07.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eid AA, Nikonov SY, Looney SW, Didato A, Niu LN, Levin MD, Rueggeberg FA, et al. In vitro biocompatibility evaluation of a root canal filling material that expands on water sorption. J Endod. 2013;39:883–888. doi: 10.1016/j.joen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Bessey OA, Lowry OH, Brock MJ. A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J Biol Chem. 1946;164:321–329. [PubMed] [Google Scholar]
- 32.Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Bryan TE, Khechen K, Brackett MG, Messer RL, El-Awady A, Primus CM, Gutmann JL, et al. In vitro osteogenic potential of an experimental calcium silicate-based root canal sealer. J Endod. 2010;36:1163–1169. doi: 10.1016/j.joen.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 34.Eid AA, Niu LN, Primus CM, Opperman LA, Pashley DH, Watanabe I, et al. In vitro osteogenic/dentinogenic potential of an experimental calcium aluminosilicate cement. J Endod. 2013;39:1161–1166. doi: 10.1016/j.joen.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guven EP, Tasli PN, Yalvac ME, Sofiev N, Kayahan MB, Sahin F. In vitro comparison of induction capacity and biomineralization ability of mineral trioxide aggregate and a bioceramic root canal sealer. Int Endod J. 2013 doi: 10.1111/iej.12115. in press. [DOI] [PubMed] [Google Scholar]
- 36.Siffert RS. The role of alkaline phosphatase in osteogenesis. J Exp Med. 1951;93:415–426. doi: 10.1084/jem.93.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero-Prado M, Blázquez C, Rodríguez-Navas C, Muñoz J, Guerrero I, Delgado-Baeza E, García-Ruiz JP. Functional characterization of human mesenchymal stem cells that maintain osteochondral fates. J Cell Biochem. 2006;98:1457–1470. doi: 10.1002/jcb.20778. [DOI] [PubMed] [Google Scholar]
- 38.Long MW. Osteogenesis and bone-marrow-derived cells. Blood Cells Mol Dis. 2001;27:677–690. doi: 10.1006/bcmd.2001.0431. [DOI] [PubMed] [Google Scholar]
- 39.Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- 40.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 41.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21:1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 42.Quarto N, Behr B, Longaker MT. Opposite spectrum of activity of canonical Wnt signaling in the osteogenic context of undifferentiated and differentiated mesenchymal cells: implications for tissue engineering. Tissue Eng Part A. 2010;16:3185–3197. doi: 10.1089/ten.tea.2010.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 44.de Boer J, Siddappa R, Gaspar C, et al. Wnt signaling inhibits osteogenic differentiation of human mesenchymal stem cells. Bone. 2004;34:818–826. doi: 10.1016/j.bone.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Serafino A, Moroni N, Psaila R, Zonfrillo M, Andreola F, Wannenes F, Mercuri L, et al. Anti-proliferative effect of atrial natriuretic peptide on colorectal cancer cells: evidence for an Akt-mediated cross-talk between NHE-1 activity and Wnt/beta-catenin signaling. Biochim Biophys Acta. 2012;1822:1004–1018. doi: 10.1016/j.bbadis.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 46.Han P, Wu C, Xiao Y. The effect of silicate ions on proliferation, osteogenic differentiation and cell signalling pathways (WNT and SHH) of bone marrow stromal cells. Biomater Sci. 2013;1:379–392. doi: 10.1039/c2bm00108j. [DOI] [PubMed] [Google Scholar]
- 47.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 48.Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278:41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 49.Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 50.Guven G, Cehreli ZC, Ural A, Serdar MA, Basak F. Effect of mineral trioxide aggregate cements on transforming growth factor beta1 and bone morphogenetic protein production by human fibroblasts in vitro. J Endod. 2007;33:447–450. doi: 10.1016/j.joen.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 51.Tomson PL, Grover LM, Lumley PJ, Sloan AJ, Smith AJ, Cooper PR. Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J Dent. 2007;35:636–642. doi: 10.1016/j.jdent.2007.04.008. [DOI] [PubMed] [Google Scholar]