Abstract
Introduction
B7 homolog 1 [B7-H1; aka programmed cell death 1 ligand 1 (PD-L1)] is a negative costimulatory molecule that is associated with poor prognosis in many tumor types. Given the poor prognosis and the limited treatments available for mesothelioma, we decided to examine B7-H1 expression and its association with survival in patients with mesothelioma.
Methods
Expression of B7-H1 was determined in 106 patients using a mouse monoclonal anti-human B7-H1 (clone 5H1-A3) antibody with immunohistochemistry. Positive expression was defined as ≥5% positively-stained cells. Clinicopathologic features and survival were compared between B7-H1 positive and negative groups.
Results
Malignant mesotheliomas of 42 patients (40%) expressed B7-H1. Patients with B7-H1-postive tumors were less likely to be offered or undergo therapeutic surgery (p=0.03). All sarcomatoid mesotheliomas except one desmoplastic subtype expressed B7-H1. Survival was significantly decreased for patients whose tumors expressed B7-H1 (5 months median, 2-9.5 months interquartile range) compared to those whose tumors did not (14.5 months, 9.25-19 months; p<0.0001). In a multivariate model, B7-H1 expression and sarcomatoid mesothelioma remained significantly associated with worse survival [risk ratio 1.71, 95% CI 1.03-2.78 (p=0.04) and risk ratio 2.18, 1.08-4.23 (p=0.03) respectively].
Conclusions
B7-H1 is expressed in a substantial proportion of malignant pleural mesotheliomas and is associated with poor survival. Almost all malignant pleural mesotheliomas with sarcomatoid differentiation expressed B7-H1. The expression of B7-H1 may have important therapeutic implications for the management of malignant pleural mesothelioma.
Keywords: mesothelioma, B7-H1, PD-L1, immunology, sarcomatoid
Introduction
Malignant pleural mesothelioma (MPM) is an inexorably progressive malignancy that is almost universally fatal. There are approximately 1.05 cases per 100,000 persons in the United States(1), and the incidence continues to rise in many countries around the world(2). The immune system is capable of mounting a tumor-specific response to mesothelioma(3), and lymphocytic infiltration of mesotheliomas has been associated with improved survival(4). B7 homolog 1 [B7-H1; also known as programmed cell death 1 ligand 1 (PD-L1)] is a negative costimulatory molecule that is constitutively expressed on macrophage-lineage cells and inhibits T-lymphocyte activation by binding to programmed cell death 1 (PD-1) receptor(5). Many human tumors aberrantly express B7-H1, and such expression has been associated with poor prognosis. Given the poor prognosis of mesothelioma and the limited treatment options available, we examined B7-H1 expression in MPM in a cohort with long term follow-up to determine the effects of B7-H1 expression on survival.
Materials and Methods
Patient selection
Patients treated at Mayo Clinic in Rochester, Minnesota and diagnosed with MPM between 01/01/1987 and 12/31/2003 with adequate tissue samples were included in this study. The adequacy of samples for inclusion was determined by a pathologist who was blinded to clinical outcomes (YMS). Tissue used for analysis was obtained through surgical biopsy of suspicious pleural lesions, pleurectomy or extrapleural pneumonectomy. A thoracic pathologist (ACR) who was blinded to B7-H1 status and clinical information independently confirmed the diagnosis of MPM. All cases were classified according to the current World Health Organization classification as epithelioid, biphasic or sarcomatoid type(6). As desmoplastic mesotheliomas are accepted as a subtype of aggressive sarcomatoid mesothelioma, the two cases in our series were included in the sarcomatoid group for analysis(6). Cases without a supportive immunohistochemical staining pattern were excluded from the study. Clinical demographics and outcome information were obtained by a retrospective chart review. This study was approved by the Mayo Clinic Institutional Review Board.
Immunohistochemistry
Paraffin-embedded formalin-fixed tissue blocks were cut at 5 μm and deparaffinized in xylene and rehydrated in a graded series of ethanol. Antigen retrieval was performed by heating tissue sections in Target Retrieval Solution pH 6.0 (Dako #S1699, Carpinteria, CA) to 121°C using a Digital Decloaking Chamber (Biocare Medical, Walnut Creek, CA) cooled to 90°C and incubated for an additional 5 minutes before opening the Decloaking Chamber. Sections were washed in running distilled H20 for 5 minutes and then incubated for 5 minutes in Wash Buffer (Dako #S3006) before being placed on the Autostainer Plus (Dako) for the following protocol utilizing a CSA II kit (Dako #K1497). Sections were blocked for endogenous peroxidase for 5 minutes using Endogenous Blocking Solution washed in wash buffer followed by incubated for 60 minutes in mouse monoclonal anti-human B7-H1 (clone 5H1-A3) (as described and validated previously(7)) diluted 1:300 with antibody diluent with background reducing components. Sections were washed in wash buffer and incubated 15 minutes in horse radish peroxidase (HRP) labeled anti-mouse immunoglobulins washed with wash buffer and incubated for 15 minutes in amplification reagent. Sections were then washed with wash buffer and incubated for 15 minutes in anti-FITC HRP, washed and visualized in 3, 3′-diaminobenzidine (DAB) for 8 minutes. Sections were washed with distilled H20, counterstained with hematoxylin, dehydrated in ethanol, and cleared in xylene. A coverslip was placed with permanent mounting media. The percent of B7-H1 expression was scored for each sample by a pathologist (YMS). Cases with less than 5% expression were considered negative, as per previous studies(8-10).
Statistical analysis
Summary statistics were used to describe the clinicopathologic data. Clinicopathologic differences between B7-H1 positive and negative groups were compared with a Chi-square test. Overall survival was determined from the date of diagnosis to the date of death or last follow-up with the Kaplan-Meier method. Patients alive at the time of last follow-up were censored. Survival comparisons were made with the log-rank test. The associations of B7-H1 expression in the tumor cells with outcome were assessed with Cox proportional hazards regression models in a univariate fashion and after adjusting for any factor with a p value ≤0.2 in a multivariate model. JMP 9.0.1 (SAS Institute Inc. Cary, NC USA) was used for statistical analysis. All tests were two sided and a p value <0.05 was considered statistically significant.
Results
Patient follow-up
One hundred six patients were included in this study, and one hundred four patients had died at the time of last follow-up. Of the two patients whose deaths were not confirmed, one pursued care closer to home due to no evidence of recurrent disease after ten years, and the other returned to his native country and was lost to follow-up after surgical care.
B7-H1 expression
Malignant pleural mesothelioma of 42 patients (40%) expressed B7-H1 (Figure 1). Of the patients who expressed B7-H1 the median percent of B7-H1 positive neoplastic cells was 35% (10-70% interquartile range). While in most cases B7-H1 expression was cytoplasmic (n=18, 43%), in many cases there was cytoplasmic and membranous staining (n=14, 33%). Exclusive membranous staining for B7-H1 was less common (n=10, 24%). There were no significant differences in gender, age, decade of diagnosis, or lymphocytic infiltration between B7-H1 positive and negative tumors; however, patients with B7-H1 positive tumors were less likely to undergo therapeutic surgery primarily due to extent of disease (p=0.03) (Table 1). Additionally, all sarcomatoid mesotheliomas besides a single desmoplastic subtype were in the B7-H1 positive group. Conversely, there were fewer epithelioid mesotheliomas in the B7-H1 positive group (n=14, 33% of the B7-H1 positive group) than the B7-H1 negative group (n=54, 84%; p<0.0001 of the B7-H1 negative group).
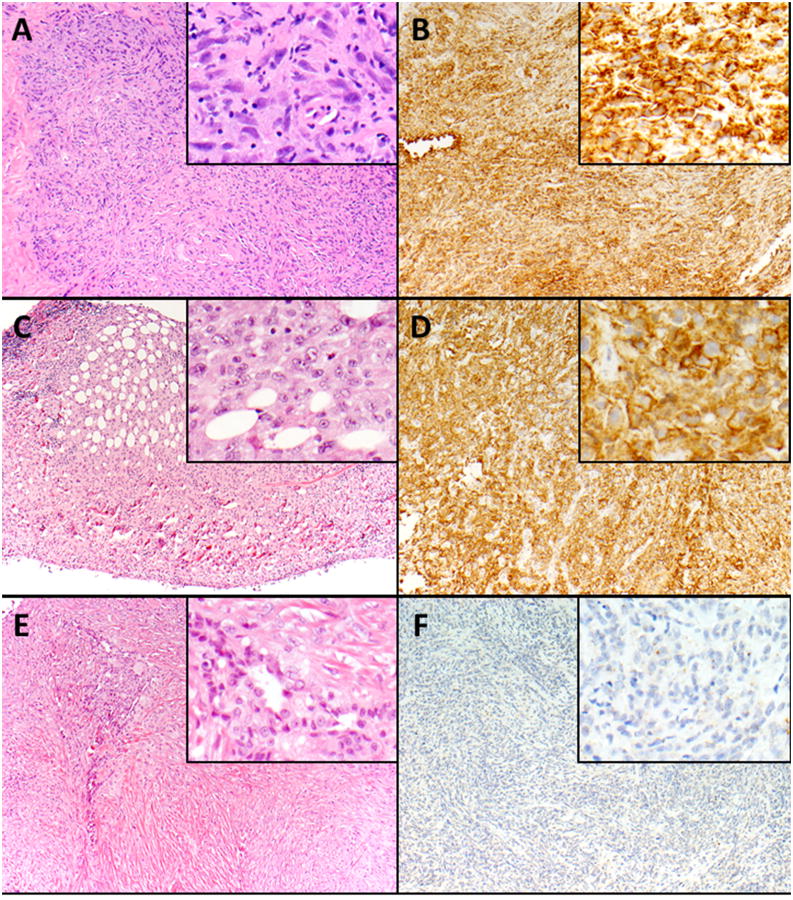
Figure 1.
B7-H1-positive malignant mesothelioma, sarcomatoid type: Neoplastic spindle cells are growing in a haphazard and tumefactive pattern (A, A insert). The neoplastic cells show diffuse and strong membranous and cytoplasmic expression of B7-H1 (B, B insert). B7-H1-positive malignant mesothelioma, epithelioid type: Neoplastic epithelioid cells are invading into adipose tissue (C, C insert). The neoplastic cells are strongly and diffusely positive for B7-H1 with a predominant cytoplasmic and focal membranous staining pattern (D, D insert). B7-H1-negative malignant mesothelioma, biphasic type: Neoplastic spindle cells are growing in a tumefactive pattern. Islands of neoplastic epithelioid cells are also present (E, E insert). The neoplastic cells are negative for B7-H1 (F, F insert). Magnification × 100 (A-F), × 400 (A-F-inserts).
Table 1. Comparison of clinicopathologic factors by tumor B7-H1 expression.
| B7-H1+ | B7-H1- | P-value | |
|---|---|---|---|
| 42 (40%) | 64 (60%) | ||
| Gender | 0.72 | ||
| Male | 35 (83%) | 55 (86%) | |
| Female | 7 (17%) | 9 (14%) | |
| Age (years; median, IQR) | 68 (59-75) | 65 (58-73) | 0.19 |
| Decade of Diagnosis | 0.57 | ||
| 1980's | 0 (0%) | 1 (2%) | |
| 1990's | 22 (52%) | 35 (55%) | |
| 2000's | 20 (48%) | 28 (43%) | |
| Subtype | <0.0001 | ||
| Epithelial | 14 (33%) | 54 (84%) | |
| Sarcomatoid | 16 (38%) | 1 (2%) | |
| Biphasic | 12 (29%) | 9 (14%) | |
| Lymphocytic infiltration | 0.36 | ||
| Yes | 38 (90%) | 54 (84%) | |
| No | 4 (10%) | 10 (16%) | |
| Surgical Treatment | 0.03 | ||
| Yes | 7 (17%) | 23 (36%) | |
| No | 35 (83%) | 41 (64%) |
The results of the univariate analysis are presented in the “P-value” column.
Three patients had multiple samples taken at the same time for review. All three cases demonstrated concordant B7-H1 expression in regards to its presence or absence. Two samples from one patient did not have any B7-H1 expression, and the degree of B7-H1 expression in the two samples varied from 5-90% in one patient, and 10-30% in another. In our cohort there was one patient from the 1980's, 57 patients from the 1990's and 48 patients from the 2000's. There was no difference in the number of patients with B7-H1 expression by decade (p=0.57).
Association of B7-H1 expression with survival
Survival was significantly shorter for patients with B7-H1 expression (5 months median, 2-9.5 months interquartile range) than those without B7-H1 expression (14.5 months, 9.25-19 months; p<0.0001; Figure 2). Amongst patients with epithelioid histology, there was longer survival in patients whose tumors did not express B7-H1 (14 months, 9-18 months) than in patients whose tumors expressed B7-H1 (6 months, 2-17 months), but this difference was not statistically significant (p=0.10). There was no difference in survival between patients with cytoplasmic B7-H1 expression and patients with any membranous expression (p=0.44). In a multivariate model, B7-H1 expression remained significantly associated with worse survival after adjusting for age, histology, and therapeutic surgical intervention (risk ratio 1.71, 95% confidence interval 1.03-2.78; p=0.04). Sarcomatoid histology also remained significantly associated with worse survival (risk ratio 2.18, 1.08-4.23; p=0.03).
Figure 2. Survival in mesothelioma by B7-H1 expression.
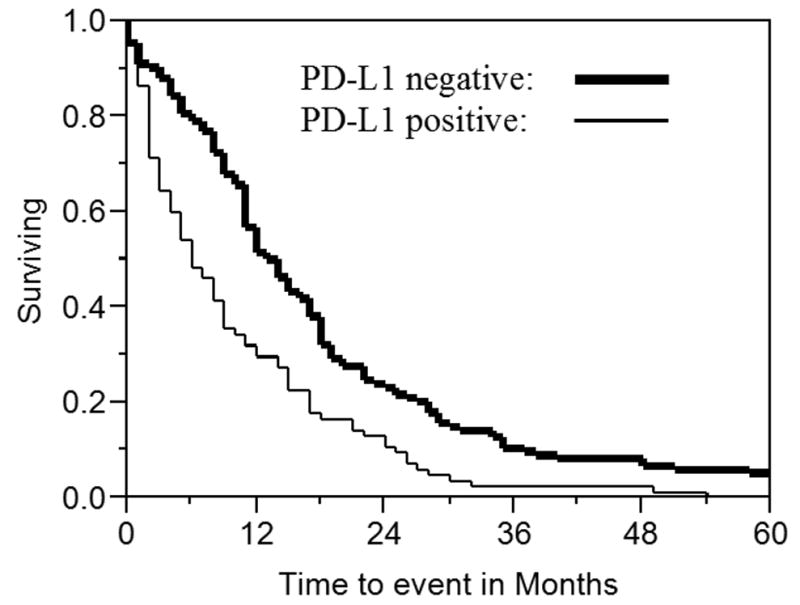
The survival of 106 patients with malignant pleural mesothelioma is shown based on B7-H1 expression. Two patients were censored. Median survival was 5 months (2-9.5 months interquartile range) for patients with B7-H1 expression and 14.5 months (9.25-19 months interquartile range; p<0.0001) for those without B7-H1 expression.
Discussion
We found that B7-H1 is expressed in 40% of MPM in a relatively large cohort with almost complete follow-up. B7-H1 expression was correlated with decreased survival, even after adjusting for multiple factors. Importantly, every case of MPM with sarcomatoid differentiation expressed B7-H1 with the exception of a single case with a desmoplastic subtype. These findings suggest that agents that target B7-H1 or its receptor (PD-1) warrant exploration for the treatment of mesothelioma, especially for patients with sarcomatoid mesothelioma.
Immune surveillance is a critical component of tumor growth control(11) and tumor-infiltrating CD3+ and CD8+ lymphocytes have been associated with improved survival in many tumor types including MPM(12). Anti-tumor immunity is modulated by a combination of positive and negative co-stimulatory signals to ensure appropriate physiologic immune responses and self-tolerance without tissue damage. Tumors co-opt many of the co-inhibitory signals used to dampen immune responses. Recently, a strategy of inhibiting cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), a co-inhibitory signal, has proven effective for the treatment metastatic malignant melanoma(13). Although there are durable responses to CTLA-4 inhibitors, treatment is relatively toxic and CTLA-4 expression is not specific to the tumor microenvironment. Notably, the strategy of CTLA-4 inhibition in mesothelioma is currently under investigation and has shown some encouraging results(14).
B7-H1 is another co-inhibitory molecule and its expression is normally limited to macrophage-lineage cells; however, it has been detected on many tumors. B7-H1 is the ligand for programmed cell death 1 (PD-1) which is expressed on T cells. B7-H1 activation of PD-1 inhibits effector molecules involved with CD3+ T cell signaling(15-17), blunts lymphocyte activation(18) and promotes immune tolerance(19). Furthermore, tumor-specific immune responses are impaired by PD-1-B7-H1 interactions(20, 21). Tumor expression of B7-H1 is a poor prognostic feature in esophageal carcinoma(22), gastric carcinoma(23), hepatocellular carcinoma(24), pancreatic carcinoma(25), renal cell carcinoma(26-29) and ovarian carcinoma(30). The degree of B7-H1 expression we detected in mesothelioma is similar to that reported in a clinical trial for malignant melanoma(31), but higher than that reported for squamous cell carcinoma of the lung(32). Recently, targeting the PD-1/B7-H1 axis in many early phase clinical trials has demonstrated durable responses in patients with various solid tumors(8, 31, 33-36). Given the degree of expression of B7-H1 in mesothelioma, especially for patients with sarcomatoid differentiation, and its association with poor survival, we believe that there is a potential role for targeting PD-1/B7-H1 interactions in this malignancy.
In this study we evaluated patient samples that were fixed in formalin and embedded in paraffin. In renal cell carcinoma the proportion of patients with B7-H1 expression has been higher in studies that use fresh-frozen tissue instead of formalin-fixed paraffin-embedded(10, 27, 28). However, given the rarity of MPM and the often rather small amount of tissue that was needed for diagnostic purposes in its entirety, it was not feasible for us to conduct as large of a study with fresh-frozen tissue. Regardless, we may have underestimated the incidence of B7-H1 expression in MPM and our results should be prospectively validated using fresh-frozen tissue. Additionally, we used strict criteria for the inclusion of cases for this analysis as the diagnosis of MPM has been improved by the use immunohistochemistry in more recent times than in previous decades. Although this limited our sample size, we confidently excluded from our analysis other malignancies that spread to the pleura.
Conclusion
B7-H1 is expressed in a substantial proportion of MPM and is associated with poor survival. Almost all malignant pleural mesotheliomas with sarcomatoid differentiation expressed B7-H1. The expression of B7-H1 may have important therapeutic implications for the management of malignant pleural mesothelioma.
Acknowledgments
Research Support: This study was supported in part by NIH R01 CA134345, K23 CA159391 and the Richard M. Schulze Family Foundation.
Footnotes
Previous presentation: Some of these data were accepted for an oral presentation at the European Lung Cancer Conference 2014, (Geneva, Switzerland March 26-29, 2014)
Disclaimers: Some authors of this manuscript have patents related to B7-H1 as disclosed.
References
- 1.Henley SJ, Larson TC, Wu M, et al. Mesothelioma incidence in 50 states and the District of Columbia, United States, 2003-2008. Int J Occup Environ Health. 2013;19:1–10. doi: 10.1179/2049396712Y.0000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stayner L, Welch LS, Lemen R. The worldwide pandemic of asbestos-related diseases. Annu Rev Public Health. 2013;34:205–16. doi: 10.1146/annurev-publhealth-031811-124704. [DOI] [PubMed] [Google Scholar]
- 3.Sterman DH, Recio A, Carroll RG, et al. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin Cancer Res. 2007;13:4456–66. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- 4.Leigh RA, Webster I. Lymphocytic infiltration of pleural mesothelioma and its significance for survival. S Afr Med J. 1982;61:1007–9. [PubMed] [Google Scholar]
- 5.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, costimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 6.Travis WD World Health Organization., International Agency for Research on Cancer., International Association for the Study of Lung Cancer., International Academy of Pathology. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: Oxford: IARC Press, Oxford University Press (distributor); 2004. [Google Scholar]
- 7.Frigola X, Inman BA, Lohse CM, et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–23. doi: 10.1158/1078-0432.CCR-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 11.Disis ML. Immune regulation of cancer. J Clin Oncol. 2010;28:4531–8. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calabro L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14:1104–11. doi: 10.1016/S1470-2045(13)70381-4. [DOI] [PubMed] [Google Scholar]
- 15.Chemnitz JM, Parry RV, Nichols KE, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki T, Maeda A, Nishimura H, et al. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–71. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheppard KA, Fitz LJ, Lee JM, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 18.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–92. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 21.Fourcade J, Kudela P, Sun Z, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240–9. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–53. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–9. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 25.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–7. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 26.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–15s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 27.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104:2084–91. doi: 10.1002/cncr.21470. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson RH, Kwon ED. Significance of B7-H1 overexpression in kidney cancer. Clin Genitourin Cancer. 2006;5:206–11. doi: 10.3816/CGC.2006.n.038. [DOI] [PubMed] [Google Scholar]
- 30.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boland JM, Kwon ED, Harrington SM, et al. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer. 2013;14:157–63. doi: 10.1016/j.cllc.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–8. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]