Abstract
Purpose
To describe the demographic and clinical features and outcomes for children and adolescents with primary CNS lymphoma (PCNSL).
Experimental Design
A retrospective series of children and adolescents with PCNSL was assembled from ten cancer centers in three countries.
Results
Twenty-nine patients with a median age of 14 years were identified. Sixteen (55%) had Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≥ 1. Front line therapy consisted of chemotherapy (CT) only in twenty patients (69%), while 9 (31%) had CT plus cranial radiotherapy. Most patients received methotrexate (MTX)-based regimens. Overall response rate was 86% (CR 69%, PR 17%). The 2 year PFS and OS rates were 61% and 86%, respectively; the 3 year OS was 82%. Univariate analyses were conducted for age (≤ 14 vs > 14 years), PS (0 or 1 vs >1), deep brain lesions, MTX dose, primary treatment with CT alone, intrathecal chemotherapy and high-dose therapy. Primary treatment with CT alone was associated with better overall response rates with an OR of 0.125 (p=0.02). There was a marginally significant relationship between higher doses of MTX and response (OR =1.5, p = 0.06). ECOG-PS of 0–1 was the only factor associated with better outcome with hazard ratios of 0.136 (p = 0.017) and 0.073(p = 0.033) for PFS and OS, respectively.
Conclusion
This is the largest series collected of pediatric PCNSL. The outcome of children and adolescents appears to be better than in adults. PS of 0–1 is associated with better survival.
INTRODUCTION
Primary CNS lymphoma (PCNSL) is a rare brain tumor in childhood. The incidence is unknown, however, among 596 cases of PCNSL reported to the Brain Tumor Registry of Japan (1969–1990) only nine pediatric cases (1.5%) were documented 1. In the Surveillance, Epidemiology and End Results (SEER, USA, 1973–1998) program 1% of all reported PCNSL cases were in patients younger than 19 years of age, giving an estimated incidence of 15–20 cases per year in North America 2. Patients with congenital or acquired immune deficiencies are at increased risk for developing PCNSL3,4, although most of the 50 pediatric cases reported over the last decade were immunocompetent 5. Because of the rarity of the disease and the lack of large prospective studies the most appropriate therapy for pediatric PCNSL has not yet been determined. Sporadic case reports have shown long-term survival with chemotherapy (CT) alone 6,7. The largest pediatric PCNSL case-series suggested that most children can achieve long-term remission with chemotherapy-based regimens, without whole brain radiotherapy (WBRT). However, this study was limited by the small number of patients (12), its retrospective nature, the heterogeneous patient population (one third were immunodeficient) and individualized treatment regimens 8. The International PCNSL Collaborative Group (IPCG) is a multidisciplinary group of neuro-oncologists, neurologists, neurosurgeons, radiation oncologists, hematologists, pathologists and pediatric oncologists from North America, Europe, Australia and New Zealand. We report a large retrospective analysis of 29 children and adolescents with PCNSL assembled from 10 institutions in three countries.
Patients And Methods
Study Population
A data collection form regarding PCNSL was sent to investigators affiliated with the IPCG and to selected pediatric oncology centers. Requested information from each participating institution included patient characteristics (age, gender), Eastern Cooperative Oncology Group (ECOG) performance status (PS), immune status, presenting symptoms, initial lactate dehydrogenase (LDH) levels, pathology, cerebrospinal fluid (CSF) cytology and protein, ocular involvement, number of brain lesions and location, treatment, site and date of progression, second-line therapy, long-term neurotoxicity and survival. Ten cancer centers with at least one case of pediatric or adolescent (≤ 21 years of age) PCNSL responded. Each center received ethics committee approval for the release of anonymized patient information. The inclusion criteria were histologic or cytologic diagnosis of lymphoma localized exclusively to the brain, meninges or spinal cord. Central pathology review was not feasible due to logistics and time elapsed from initial diagnosis. In 23 cases, the original pathology reports were available for central review. Cytologic features, immunophenotyping and final diagnosis were abstracted from these reports.
Statistical Analyses
Logistic regression models were used to assess predictors of radiographic response. Univariate Cox regression models were used to assess predictors of overall survival (OS) and progression free survival (PFS). Kaplan Meier curves were calculated to display the distributions of OS and PFS. Stratification variables included gender, age (≤ 14 years vs > 14), ECOG PS (0–1 vs > 1), number of brain lesions (multiple vs single), lesion location (deep brain vs cerebral hemisphere), CSF cytology for lymphoma cells (positive vs negative), initial LDH levels (high vs normal), primary treatment (chemotherapy only vs chemotherapy plus WBRT), receipt of intrathecal therapy (no vs yes), high-dose therapy (no vs yes) and methotrexate (MTX) dose.
Results
Case Identification
A total of 29 cases were identified from 6 IPCG centers and 4 pediatric oncology centers. All cases were diagnosed between 1978 and 2008. All patients had their disease confined to the brain (n = 26) or meninges (n = 3) with no evidence of systemic lymphoma at presentation. Three patients described in a previous pediatric PCNSL report 8 were included in this series.
Demographics and Clinical Features
There were 21 males (73%) and 8 females (27%). Median age at diagnosis was 14 years (range 2 to 21). Three patients were immunodeficient; one had congenital combined immunodeficiency, one had acquired immunodeficiency with PCNSL developing 4 months post-renal transplant and one patient with lupus erythematous had immunosuppressive therapy with Mycophenolate for one year prior to developing PCNSL. ECOG PS was available in 19 cases (65%) and was abnormal in 16 (84%), 3 of whom had an ECOG of 2 or worse. Slit-lamp examination of the eyes was recorded in 7 patients (24%), 2 had positive findings. Initial LDH was available in 17 cases, 9 of whom (53%) had elevated levels defined as above institutional normal values. CSF cytology was documented from 26 patients (90%) and was positive for lymphoma in 8 cases (31%); 3 of these had primary leptomeningeal lymphoma (PLML). The CSF protein was available in 11 cases (38%) and was high in 8. Baseline computed tomography (CT) and/or magnetic resonance imaging (MRI) of the brain with gadolinium contrast enhancement were obtained in all patients before starting therapy. Eleven patients (38%) had multiple lesions at diagnosis. Twelve patients (41%) had involvement of deep brain structures (basal ganglia, cerebellum or brain stem), 14 had cerebral hemispheres involved while three had isolated meningeal involvement. The most common presenting symptoms were those of increased intracranial pressure (headaches, nausea/vomiting), followed by cerebellar symptoms such as ataxia, dysarthria and dysmetria. Seizures and hemiparesis were also common. Some patients had associated blurring of vision, photophobia, nystagmus, diplopia and proptosis. One patient presented with cranial polyneuropathy, and another patient, diagnosed with pineal PCNSL, presented with Parinaud syndrome. One patient presented at age 14 years with short stature, diabetes insipidus and a thickened pituitary stalk on brain MRI. She was treated with growth hormone therapy for one year and repeat MRI showed progression of the lesions which on biopsy were confirmed to be diffuse large B-cell lymphoma (DLBCL)-PCNSL. Another patient had a 4-year prodrome with headaches and dysarthria; meningeal biopsy showed a DLBCL-PLML after being treated for hydrocephalus and shunt infections for years. None of the 29 patients had initial “B” symptoms.
Pathologic Features
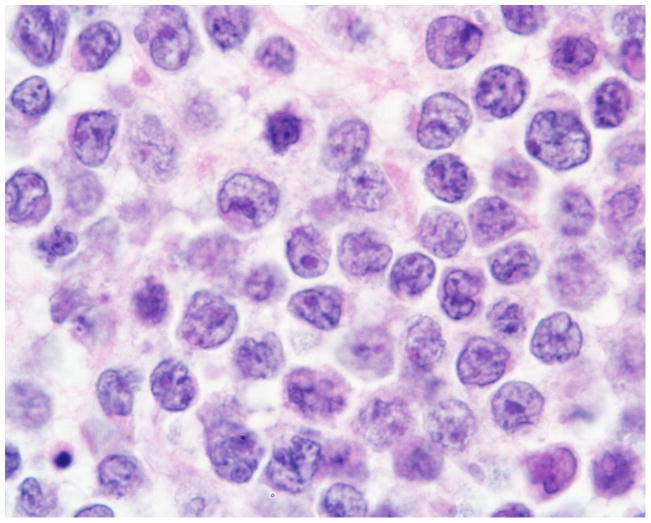
Diagnosis was confirmed by stereotactic brain biopsy in 59% (17 patients), surgical resection in 31% (9 patients, 4 total and 5 subtotal) and by cytologic and immunophenotypic analyses of the CSF in 10% (3 patients). In 6 cases, the diagnosis of PCNSL was retrieved from lymphoma databases at individual institutions. The original anonymized pathology reports were available in the other 23 cases. All patients were diagnosed as primary CNS non-Hodgkin’s lymphoma (NHL). A total of 20 patients (69%) had DLBCL (Figures 1–2), 5 patients (17%) had anaplastic large T-cell lymphoma (ALCL), 2 patients (7%) had lymphoblastic lymphoma (one precursor-B and one not specified) and in 2 patients (7%) the diagnosis was Burkitt-like lymphoma. The diagnosis of PCNSL was confirmed by immunophenotyping in all patients, combined with immunoglobulin (IgH) gene rearrangement RT- PCR in two cases.
Figure 1.
Primary diffuse large B-cell lymphoma of the CNS in a 12 year old patient. High magnification (100x): homogeneous tumor cell population, cells medium sized to large, prominent nucleoli, CD20+, kappa-light chain restricted, CD10−, CD30−, K167+>90%.
Figure 2.
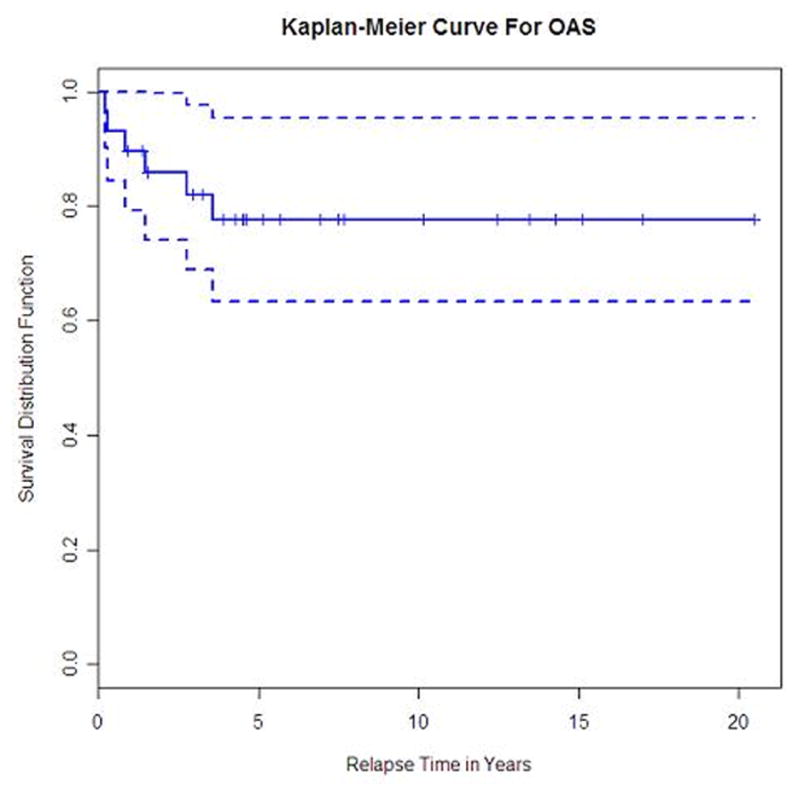
Progression-free survival for all patients with primary CNS lymphoma
Therapeutic Strategies
Treatment data was available for all 29 patients. Steroid use before primary therapy was documented in 12 patients (41%). Dexamethasone dose ranged from 4 to 16 mg/day. Primary treatment consisted of chemotherapy (CT) alone in 18 patients (62%), 2 of whom had intra-arterial CT with blood-brain barrier disruption (BBBD). Two patients (7%) had chemo-immunotherapy; 9 patients (31%) had CT followed by WBRT. Nine patients had initial surgical resection followed by chemotherapy or chemo-radiotherapy combinations (Table 1). Details of primary treatment strategies are summarized in Table 2.
Table 1.
Front-line treatment of PCNSL patients
| Treatment | Patients no. (%) |
|---|---|
| Chemotherapy only | 18 patients (62%) |
| Chemo-immunotherapy only | 2 patients (7%) |
| Chemotherapy and WBRT | 9 patients (31%) |
| Surgical resection* | 9 patients (31%) |
| ASCT | 2 patients (7%) |
Immunotherapy = rituximab.
WBRT = whole brain radiotherapy.
These patients had surgical resection prior to definitve treatment.
ASCT = autologous stem cell transplantation
Table 2.
Chemotherapy regimens at PCNSL presentation
| Treatment | Patients, no. |
|---|---|
| MTX-based | 27 |
| FAB/LMB-96 | 9 |
| Bonn protocol | 2 |
| CALGB-50202 | 1 |
| POG-9906 | 1 |
| COG-ANHL0131/A | 1 |
| IA-MTX/IV Cy + BBBD | 2 |
| Methotrexate (225 mg) + vincristine (2.5 mg) | 1 |
| Methotrexate/vincristine + other regimens# | 3 |
| HD-MTX (5 g/m2) + HD-Ara-C (3 g/m2) | 3 |
| HD-MTX (3.5–5 g/m2) + other chemotherapy drugs* | 4 |
| Non-MTX based | 2 |
| CCNU | 1 |
| Vincristine/prednisone/doxorubicin | 1 |
-FAB (French-American-British) LMB 96 = COPADM x 2: cyclophosphamide, vincristine, prednisone, cytarabine, doxorubicin, intravenous methotrexate (5–8 g/m2); CYVE x 2: cytarabine (3 g/m2), etoposide.
-Bonn protocol = methotrexate (3 g/m2), vincristine, ifosfamide, dexamethasone, intra-omaya chemotherapy, cytarabine (3g/m2) and vindesine.
-CALGB-50202: methotrexate (3.5 g/m2), rituximab, temozolomide, cytarabine, etoposide
-POG (pediatric oncology group) 9906-Acute lymphoblastic leukemia protocol: vincristine, prednisone, doxorubicin, L-asparaginase; cyclophosphamide, cytarabine, 6-mercaptopurine plus intrathecal methotrexate; intravenous methotrexate (5 g/m2), vincristine; oral 6-mercaptopurine plus oral methotrexate in maintenance. Patient received also 1200 cGy WBRT.
-COG (children’s oncology group) ANHL0131-regimen A-anaplastic T-large cell lymphoma protocol: vincristine, prednisone, L-asparaginase, doxorubicin plus intrathecal methotrexate; oral methotrexate plus 6-mercaptopurine/prednisone in maintenance.
-IA-MTX: intra-arterial methotrexate (1.5–2.5 g/m2) × 2 days, IV Cy: intravenous cyclophosphamide (15 mg/kg/day) × 2 days, BBBD: blood-brain-barrier disruption for a total 12 months
Other regimens included cyclophosphamide, doxorubicin, ifosfamide, cytarabine, and etoposide
Other chemotherapy drugs included thiotepa (35 mg/m2)/cytarabine (1), vincristine/procarbazine (1), vincristine/cyclophosphamide/dexamethasone (1), rituximab/ifosfamide/carboplatin/etoposide (1).
Methotrexate (MTX) was the most commonly used drug in combination with other agents (n = 27). The intravenous (or intra-arterial) MTX dose ranged from 60 mg/m2 to 8 g/m2 per course, and the most common intravenous doses ranged from 5 to 8 g/m2. In 4 patients, the MTX dose was unknown. MTX therapy was interrupted in 2 patients due to anaphylaxis and severe hepatitis, respectively. Two patients had non-MTX-based regimens. Intrathecal (IT) chemotherapy was given to 17 patients (59%), one had IT cytarabine (Ara-C) alone and 16 had IT MTX either alone (n = 4) or in combination with Ara-C (n = 3), hydrocortisone (n = 1) or both (n = 8). Two patients had intraventricular MTX and Ara-C/ rituximab, respectively. Two patients had front-line high-dose chemotherapy and autologous stem-cell transplantation (ASCT) following MTX-based regimens. Primary treatment with cranial irradiation combined with chemotherapy was administered to 9 patients, and consisted of whole brain irradiation in all; in one of them, the irradiation field also included the eyes. In 8 of these 9 patients, WBRT was delivered after chemotherapy; in one patient WBRT was given initially alone without any response. This patient was subsequently switched to a MTX- based chemotherapy regimen. The irradiation dose in all patients ranged from 12 to 50 Gy (median, 24 Gy).
Response rate was determined based on consensus criteria for brain tumours at the time of assessment (Macdonald criteria9 prior to 2006 and IPCG criteria10 after 2006).
Outcome
The median time to end of follow-up is 5.7 years (95% CI, 4.3 – 10.1 years). Overall response rate was 86% with 20 patients (69%) achieving complete remission (CR)and 5 patients (17%) partial remission (PR). One patient had stable disease (SD) after initial therapy and three had progressive disease (PD). Six patients have died, five due to lymphoma (one progressive disease and four relapses) and one as a result of infectious toxicity. The 5- and 10-year OS were not estimable since the longest time to death from diagnosis was slightly over 3.5 years. Similarly, the 5- and 10-year PFS were not estimable since all patients who relapsed did so within slightly over 2 years. The 2-year PFS (Figure 2) and OS (Figure 3) were 61% (95% CI, 40% - 76%) and 86% (95% CI, 66% – 94%), respectively. The 3-year OS was 82% (95% CI, 61% – 92%). Ten patients (35%) have relapsed at a median 12 months from diagnosis (range, 1 to 25 months); eight relapsed in the brain alone, one in the leptomeninges alone and one in the brain and leptomeninges. One patient was salvaged with WBRT alone, and one had no further treatment. Eight patients received systemic chemotherapy-based regimens as salvage therapy; three of them had systemic chemotherapy alone, while WBRT followed chemotherapy in 4 patients and partial brain irradiation in one. High dose chemotherapy with ASCT was used in 4 patients as part of their salvage strategy; all are alive at a median 45 months from diagnosis (range 18–56 months) and 26 months from relapse (range 14–30 months). Among the six patients who relapsed after primary treatment with chemotherapy alone, five were salvaged with either WBRT alone or chemotherapy followed by ASCT plus irradiation. Overall, 6 of the 10 relapsed patients were still alive at the time of data submission. Details on salvage treatment for the relapsed PCNSL patients are summarized in Table 3.
Figure 3.
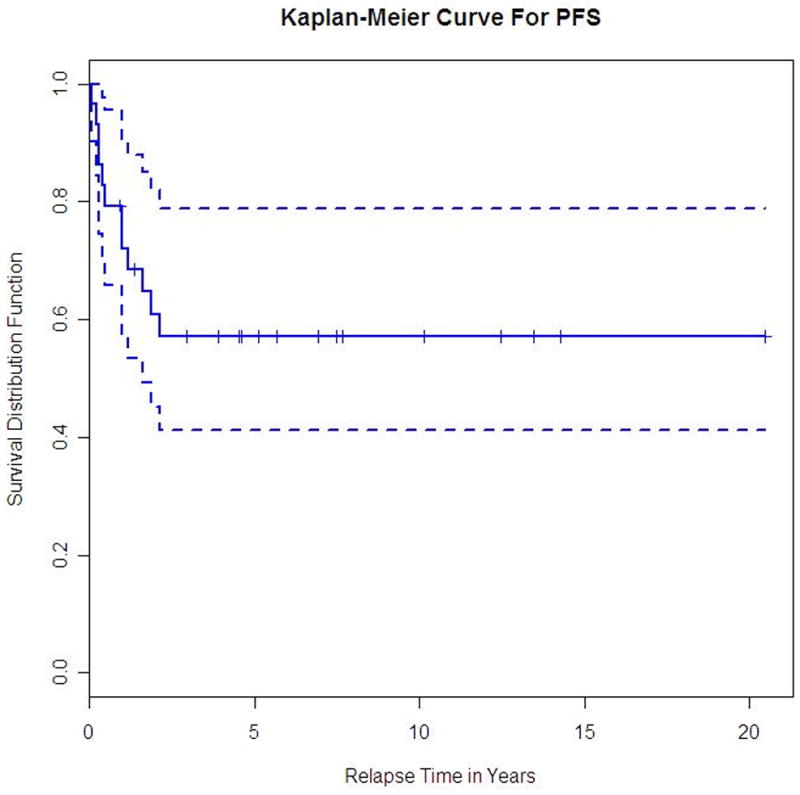
Overall-survival for all PCNSL patients.
Table 3.
Treatment of PCNSL Relapses
| Modality | Outcome |
|---|---|
| WBRT only: 1 patient (54 Gy) | (alive) |
| WBRT+Chemotherapy: 5 patients | |
| -MTX/Ara-c/Ritux/Ifos/VP16 → 24 Gy + boost 12 Gy | (died) |
| -ICE (x 4) → 45 Gy | (died) |
| -ICE (x 3) + BEAM-ASCT → 50 Gy | (alive) |
| -BEAM + Ritux → ASCT + partial brain 45 Gy | (alive) |
| -MTX/Gem/Ritux/Ifos + IO → BEAM-ASCT + WBRT | (alive) |
| Chemotherapy only: 3 patients | |
| -HD-MTX Bu/Cy/Thio → ASCT | (alive) |
| -MTX (IA) + Cy + BBBD + IO (2) | (1 died & 1 alive) |
| No further therapy: 1 patient | (died) |
WBRT: whole brain radiotherapy; MTX: methotrexate, Ara-C: cytarabine, Ritux: rituximab, Ifos: ifosfamide, VP16: etoposide; ICE: ifosfamide, carboplatin, etoposide; BEAM: BCNU, etoposide, cytarabine, melphalan; ASCT: autologous stem cell transplantation; Gem: gemcitabine; IO: intra-omaya chemotherapy; HD-MTX: 8 g/m2 methotrexate; Bu: busulfan, Cy: cyclophosphamide, Thio: thiotepa; IA: intra-arterial, BBBD: blood-brain-barrier disruption.
Univariate analyses of twelve predictor variables showed that the only variable that had significant association with response was primary treatment with chemotherapy alone, which had an odds ratio (OR) of 0.125 – Ct plus WBRT vs CT alone (p = 0.02). Higher doses of MTX, analyzed as a continuous variable, had a marginally significant relationship with response rate with an OR of 1.5 (p = 0.06). Univariate Cox models showed that only ECOG PS was significantly associated with PFS, where a lower ECOG score (0–1) was associated with 0.136 hazard ratio of progression or death than is higher ECOG (>1) (p = 0.017). Age > 14 years did not have any impact on response rates, PFS or OS (RR: OR = 1.63, p = 0.55; PFS: HR = 0.85, p = 0.79; OS: HR = 1.21, p = 0.81). Lesions involving deep brain structures have been previously associated with a worse outcome 9. In our cohort, this was not shown (RR: OR = 2.5, p = 0.33; PFS: HR = 2.3, p = 0.20; OS: HR = 0.46, p = 0.50). Other well known adverse prognostic factors for PCNSL are high LDH and elevated CSF protein 9; data regarding these 2 factors were available in only few patients. The IELSG score (ECOG PS, tumor location, CSF protein, age and serum LDH) could not be assessed, since data on all 5 parameters was missing in few patients. Furthermore, the collection of data on treatment-related acute toxicities was not feasible due to the retrospective nature of the study and time from diagnosis in most patients.
Neurotoxicity
Long-term neurotoxicity data was available in 7 patients (24%). Three have developed learning disability, one of them with chronic headaches, depression and aggressiveness. Only one of these had received cranial irradiation (1200 cGy) as part of his primary treatment. Other neurological symptoms included seizures and visual field loss (1), hearing loss (1), esotropia and tremors (1) and periodic stroke-like migraine after radiation therapy-SMART (1). The two patients who presented with diabetes insipidus and Parinaud syndrome, respectively, have persistent symptoms in the presence of radiographic complete remission.
Discussion
The exact incidence of pediatric PCNSL is unknown, and it is likely that many cases are not being reported. Although mostly retrospective, our international collaboration within the IPCG allowed us to collect the largest series of pediatric and adolescent PCNSL to date. The dataset was not comprehensive, contained missing values and heterogeneous treatments across different centers and throughout 3 decades. Nevertheless, some patient characteristics and treatment-related findings can be helpful for clinicians managing very young patients with PCNSL The median age of our cohort was 14 years and, similar to previous reports 5,8, there was male predominance. The majority of patients (49%) presented with lesions in the cerebral hemispheres, and, unlike the situation in adult PCNSL 11, involvement of deep brain structures was not associated with a difference in outcome. The most frequent pathological subtype was DLBCL (69%) which is consistent with previous pediatric PCNSL reports 5,8. In adults, 90% of PCNSL is represented by DLBCL 12. The pathology subtype, however, did not affect response rates or survival in our series.
Age and PS have been reported as the 2 most important prognostic factors in PCNSL11,13. Furthermore, age ≥ 15 years was associated with worse outcome in adolescents with systemic NHL treated on the French-LMB 89 study 14 and the international French-American-British (FAB)-LMB 96 study 15. In our series, only ECOG PS was a strong predictor of survival, while age >14 years was not. High-dose methotrexate (HD-MTX) has been the single most active agent in PCNSL to date16. Use of MTX did not have an influence on PFS or OS in this series, however, there was a marginally significant relationship between higher doses of this drug and response (p = 0.06).
The prognosis of childhood PCNSL depends on the intensity and type of CNS-directed therapy. A previous review of cases treated between 1975 and 1991 demonstrated a mean survival time of 17 months with WBRT alone or combined with chemotherapy 1. A more recent pediatric PCNSL retrospective series demonstrated improved survival with 70% 5-year EFS in patients treated with chemotherapy alone, mostly consisting of HD-MTX and HD-Ara-C combinations 8. In the present study, the 3-year OS was 82% and at least 15 patients received HD-MTX plus HD-Ara-C. Two of these received the Bonn protocol (consisting of HD-MTX, Ara-C, vinca alkaloids, ifosfamide and cyclophosphamide with intraventricular MTX, prednisolone and Ara-C), and 9 had FAB-LMB 96 based regimens (Table 2); both protocols contain MTX and Ara-c in high doses. The Bonn protocol in adulthood PCNSL has a 5 year survival of 75% 17. Children and adolescents with CNS positive B-NHL who were treated on the FAB-LMB 96 study, received therapy that included HD-ARA-C as well as 8 gm/m2 of MTX plus IT therapy without WBRT. The 4 year EFS for CNS+ patients in this study was 75% 18.
In our study, primary treatment with chemotherapy did not have a statistically significant effect on PFS (OR = 0.51, p = 0.31) and OS (OR = 1.75, p = 0.49). The, response rate for PCNSL in patients who received chemotherapy alone, however, was better than that seen in patients who received combined chemoradiotherapy. The likely explanation for this finding is the lower doses of chemotherapy, particularly of methotrexate, given to patients receiving combined therapy. In addition, five of the 6 patients who relapsed after initial treatment with chemotherapy were salvaged with either chemotherapy alone or with chemoradiotherapy combinations and ASCT. These results, together with the well-known devastating late effects of cranial irradiation in children (secondary brain tumors, neurocognitive deficits, hypothyroidism, early puberty and short stature)19, 20, suggest that young patients with PCNSL could be treated initially with chemotherapy as a single modality, without WBRT.
The prognosis of childhood and adolescent PCNSL appears to be better than most adult series (25–40% 5 year EFS) 21. This could be due to the fact that very young patients can tolerate higher doses of MTX more than adults, as well as to different biology. Pediatric DLBCL has a moderate to high proliferation index, decreased Bcl2 protein expression and an increased frequency (75%) of the germinal center (GC) phenotype (Bcl6+) which may contribute to the excellent prognosis 22. Since most cases of childhood PCNSL are pathologically DLBCL (69% in our study) and if most of them are of the GC phenotype then this could explain, at least partially, the favorable outcome in children. Where data was available, all DLBCL cases had moderate to high proliferative indices (60–90%); Bcl2 showed weak focal expression in 2 patients, and Bcl6 was strongly positive in these.
Our descriptive study has some limitations. Not all IPCG members had treated pediatric cases of PCNSL, and some did not reply. Most cases were retrospectively collected (only 2 were prospective) and detailed data was not available. Pathology slides were not reviewed centrally and treatments were not standardized among the individual patients. Nevertheless, this study confirms our previous observation that children with PCNSL can be treated initially with chemotherapy only, and that cranial irradiation can be reserved for refractory or relapsed disease. Of interest, was also the correlation (although not statistically significant) between higher doses of MTX and response rates. Finally, our study is the first to demonstrate a correlation between ECOG-PS and outcome in pediatric and adolescent PCNSL. This may suggest that patients with an ECOG > 1 at diagnosis could benefit from more intensive therapy including front-line ASCT.
Due to the rarity of pediatric PCNSL and the need for many years of follow-up to detect late relapses, it is clear that no meaningful prospective phase III trials can be conducted through the North American Children’s Oncology Group (COG) alone. Thus, a prospective collaboration between IPCG members and the international pediatric lymphoma groups (French- LMB, German NHL-BFM and COG-NHL committee) might lead to better therapeutic strategies for pediatric and adolescent PCNSL. Furthermore, biology and molecular studies are warranted for this rare entity.
Statement of Translational Relevance.
The outcome of children and adolescents with PCNSL appears to be better than in adults. Front line therapy with chemotherapy alone, mainly HD-MTX containing regimens, is associated with better overall response rates. This is the first study that correlates ECOG performance status with outcome in pediatric PCNSL. An ECOG of 0–1 at diagnosis was associated with better progression-free survival and overall survival. Children and adolescents with PCNSL could be treated initially with chemotherapy only, and cranial irradiation may be reserved to refractory or relapsed patients. Furthermore, patients with an ECOG > 1 at diagnosis may benefit from more intensive therapy including Autologous Stem Cell Transplant (ASCT). Multinational prospective studies are needed for this rare entity.
References
- 1.Kai Y, Kuratsu J, Ushio Y. Primary Malignant Lymphoma of the Brain in Childhood-Case report- Neurol Med Chir (TOKYO) 1998;38:232–7. doi: 10.2176/nmc.38.232. [DOI] [PubMed] [Google Scholar]
- 2.Kadan-Lottic NS, Skluzacek MC, Gurney JG. Decreasing Incidence Rates of Primary central Nervous System Lymphoma. Cancer. 2002;95:193–202. doi: 10.1002/cncr.10643. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez M, Delgado P, Petito CK. Epstein-Barr Virus-associated Primary Central Nervous System Lymphoma in a child With the Acquired Immunodeficiency Syndrome. Arch Pathol Lab Med. 1997;121:1287–91. [PubMed] [Google Scholar]
- 4.Schabet M. Epidemiology of Primary CNS Lymphoma. J Neurooncol. 1999;43:199–201. doi: 10.1023/a:1006290032052. [DOI] [PubMed] [Google Scholar]
- 5.Abla O, Weitzman S. Primary CNS lymphoma in children. Neurosurg Focus. 2006;21:E8. doi: 10.3171/foc.2006.21.5.9. [DOI] [PubMed] [Google Scholar]
- 6.Cohen IJ, Vogel R, Matz S, et al. Successful non-neurotoxic therapy (without radiation) of a multifocal primary brain lymphoma with a methotrexate, vincristine and BCNU protocol (DEMOB) Cancer. 1986;57:6–11. doi: 10.1002/1097-0142(19860101)57:1<6::aid-cncr2820570104>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Kirov I, Shen V, Lones MA. Primary central nervous system B-cell lymphoma in childhood. J Pediatr Hematol Oncol. 2003;25:S11. Abstract # 46. [Google Scholar]
- 8.Abla O, Sandlund JT, Sung L, et al. A case series of Pediatric Primary Central nervous System Lymphoma- Favourable Outcome Without Cranial Irradiation. Pediatr Blood Cancer. 2006;47:880–5. doi: 10.1002/pbc.20736. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald D, Cascino T, Schold SJ, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–80. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 10.Abrey LE, Batchelor TT, Ferreri AJM, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–43. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 11.Ferreri AJM, Blay JY, Reni M, et al. Prognostic Scoring System for Primary CNS Lymphomas: The International Extranodal Lymphoma Study Group Experience. J Clin Oncol. 2003;21:266–72. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 12.Kleihues P, Cavanee WK. Pathology and Genetics of Tumours of the Nervous System. Lyon, France: IARC Press; 2000. World Health Organization Classification of Tumors. [Google Scholar]
- 13.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711–5. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 14.Patte C, Auperin A, Michon J, et al. The Societe Francaise d’Oncologie Pediatrique LMB 89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–9. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- 15.Cairo M, Sposto R, Gerrard M, et al. Tumor histology, advanced stage, and primary site, explain the increased risk of failure in adolescents (Age greater than or equal to 15 years) with newly diagnosed B-NHL: Results of the FAB/LMB 96. Pediatric Blood & Cancer. O.120:52. (abstract); SIOP-2008. [Google Scholar]
- 16.Watanabe T, Katayama Y, Yoshino A, et al. Long-term remission of primary central nervous system lymphoma by intensified methotrexate chemotherapy. J Neurooncol. 2003;63:87–95. doi: 10.1023/a:1023760824739. [DOI] [PubMed] [Google Scholar]
- 17.Pels H, Schmidt-Wolf I, Galsmacher A, et al. Primary central nervous lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21:4489–95. doi: 10.1200/JCO.2003.04.056. [DOI] [PubMed] [Google Scholar]
- 18.Patte C, Auperin A, Gerrard M, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–80. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loning L, Zimmermann M, Reiter A, et al. Secondary neoplasms subsequent to Berlin-Frankfurt-Munster therapy of acute lymphoblastic leukemia in childhood: Significantly lower risk without cranial radiotherapy. Blood. 2000;95:2770–5. [PubMed] [Google Scholar]
- 20.Said JA, Waters BG, Cousens P, et al. Neuropsychological sequelae of central nervous system prophylaxis in survivors of childhood acute lymphoblastic leukemia. J Consult Clin Psychol. 1989;57:251–6. doi: 10.1037//0022-006x.57.2.251. [DOI] [PubMed] [Google Scholar]
- 21.McAllister LD, Doolittle ND, Guastadisegni PE, et al. Cognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphoma. Neurosurgery. 2000;46:51–60. [PubMed] [Google Scholar]
- 22.Miles R, Raphael M, McCarthy K, et al. Pediatric Diffuse Large B-Cell Lymphoma Demonstrates a High Proliferation Index, Frequent c-Myc Protein Expression, and a High Incidence of Germinal Center Subtype: Report of the French-American-British (FAB) International Study Group. Pediatr Blood Cancer. 2008;51:369–74. doi: 10.1002/pbc.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]