Abstract
AIM: To give a comprehensive review of current literature on robotic rectal cancer surgery.
METHODS: A systematic review of current literature via PubMed and Embase search engines was performed to identify relevant articles from january 2007 to november 2013. The keywords used were: “robotic surgery”, “surgical robotics”, “laparoscopic computer-assisted surgery”, “colectomy” and “rectal resection”.
RESULTS: After the initial screen of 380 articles, 20 papers were selected for review. A total of 1062 patients (male 64.0%) with a mean age of 61.1 years and body mass index of 24.9 kg/m2 were included in the review. Out of 1062 robotic-assisted operations, 831 (78.2%) anterior and low anterior resections, 132 (12.4%) intersphincteric resection with coloanal anastomosis, 98 (9.3%) abdominoperineal resections and 1 (0.1%) Hartmann’s operation were included in the review. Robotic rectal surgery was associated with longer operative time but with comparable oncological results and anastomotic leak rate when compared with laparoscopic rectal surgery.
CONCLUSION: Robotic colorectal surgery has continued to evolve to its current state with promising results; feasible surgical option with low conversion rate and comparable short-term oncological results. The challenges faced with robotic surgery are for more high quality studies to justify its cost.
Keywords: Rectal cancer, Robotics, Minimal invasive surgery, Systematic review, Rectal surgery
Core tip: This systematic review summarizes current evidence on the role of robotic surgery for the treatment of rectal cancer. It is a timely article as minimal invasive surgery has proven to benefit patients with colonic cancers but conventional laparoscopic surgery for the treatment for rectal cancer remains controversial due to its steep learning curve. Robotic-assisted surgery has technological advances, which may have the potential to overcome some of the limitations of conventional laparoscopic surgery.
INTRODUCTION
Minimally invasive surgery over the past two decades has revolutionised surgical management of colorectal cancers. Despite its initial scepticism, various randomised controlled trials have now demonstrated its short-term and long-term benefits over conventional open surgery in the treatment of colonic cancer such as faster recovery, decreased morbidity and reduced hospital length of stay with comparable oncological result and survival outcome[1-4]. However, laparoscopic colorectal surgery has limitations. These concerns were high-lighted not only by the high conversion rate but also the initially high proportion of circumferential resection margin (CRM) positive rates in the medical research council colorectal cancer (MRC-CLASICC) trial for laparoscopic rectal surgery[5]. The ability to perform total mesorectal excision (TME) laparoscopically requires intensive training. Limitations of conventional laparoscopic surgery include: 2-dimension view, unstable assistant controlled camera, poor ergonomics, straight tip instruments, fulcrum effect and enhanced tremor effect.
Various attempts have been made to seek alternative techniques to overcome some of these limitations. For example, single incision laparoscopic surgery has reduced the number of incisions and ports required for minimal invasive colonic surgery producing a better cosmetic result and reduction in wound pain[6]. Natural orifice translumenal endoscopic surgery (NOTES) aims to eliminate external incision by gaining access using the transvaginal, transgastric, transvesical and transrectal approach, which has been shown to be feasible on animal models[7-9]. However, there are still many hurdles in NOTES (e.g., determining a safe access into the peritoneal cavity, developing a reliable method on the closure of viscotomy, minimising the infection and tumour seedling risk, developing a stable and versatile platform for suturing, managing complications from NOTES and training issues), which need to be addressed before its routine application on Human subjects.
The da Vinci® robot is the first robotic surgical system approved by the United States Food and Drug Administration in 2000. It has evolved from its first generation robot in 1999, the da Vinci standard®, to the current third generation da Vinci-Si HD®, which was launched in 2009. The da Vinci Si-HD® has features such as: (1) dual operating console capability for combined operating and training; (2) enhanced operator-controlled 3D high-definition vision; (3) endowrist™ technology allowing 7 degrees of freedom intra-abdominally; and (4) tremor elimination with improved dexterity. Weber et al[10] and Hashizume et al[11] first performed colorectal robotics surgery in 2002[10,11]. Prior to this, robotic surgery was already successfully performed on cardiothoracic, urological and general surgical[12-14] patients.
Robotic rectal surgery has potential advantages over conventional laparoscopic rectal surgery: Surgeon motion filter for tremor-free surgery, high definition three-dimensional images, surgeon control camera on a stable platform and increased degree of freedom of the operating instruments. The master and slave system allows improved ergonomics for the surgeon. As the surgical field mainly confines to the pelvic cavity, it allows a stable platform for precision surgery to be performed in a confined space. For the above reasons, robotic technology may be more suitable and may translate more benefits when used for rectal cancers than colonic cancers.
Several review articles have attempted to summarize up-to-date practice and results of robotic colorectal surgery. However some studies included data from both robotic colonic and rectal resections, which may not give a focused overview of the benefits and risks of robotic rectal surgery[15-17]. Other studies included more than one study from the same institute with overlapping period of assessment, which may cause duplication of results[15,18]. Although meta-analysis of robotic rectal resection have been published, studies included were from non-randomised studies[19,20]. Hence we feel that an up-to-date systematic review on robotic rectal surgery is most appropriate and warranted.
This article aims to compare robotic-assisted rectal surgery with conventional laparoscopic rectal surgery for patients with rectal cancers. The current status of robotic rectal surgery focusing on its efficacy, feasibility and oncological safety will also be discussed.
MATERIALS AND METHODS
Two reviewers independently (T.M. and K.F.) performed a literature search via PubMed, Google Scholar, Cochrane Library and Embase database during the period between January 2007 to November 2013. Search terms such as “robotic surgery”, “surgical robotics”, “laparoscopic computer-assisted surgery” and “rectal resection” were used. Only english language published studies were considered. In addition, the reference lists of selected articles were searched manually. Abstract publications from conferences were excluded from this review. Published data from robotic rectal surgery using the Da Vinci® Surgical System (Intuitive Surgical, Mountain View, Sunnyvale, CA, United States) were only included in order to reduce clinical heterogeneity and the authors recognise that currently it is the only operating system available.
Inclusion criteria for search include randomised and non-randomised controlled trials, comparison studies, case series and case report. The target population consists of patients aged > 18 years with histologically proven rectal cancers.
This systematic review was conducted according to a guidance from the Centre for Reviews and Dissemination[21] and the Cochrane Handbook[22]. The review is reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement[23]. Selected articles were screened independently by two reviewers for bias using The Cochrane Collaboration’s tool for assessing risk of bias[22].
Two reviewers (T.M and K.F.) extracted data from the manuscripts of selected articles including the study design, patient demographics, clinical characteristics, site of malignancy, types of intervention, peri-operative details, pathological results, and post-operative outcomes.
RESULTS
After the initial screen of 380 articles, 60 articles met the predefined inclusion criteria. 15 articles with inseparable data from colonic cancers, 15 articles with benign colorectal disease and 10 articles from the same institutes with overlapping study period were excluded to avoid duplication. 20 studies were selected for review, which comprised of: 13 comparison studies and 7 case series (Figure 1). A large proportion of these studies came from South Korea[24-31] (40.0%) followed by United States[32-36] (25.0%), Italy[37-39] (15.0%), Singapore[40,41] (10.0%) and Turkey[42] (5.0%) and Romania[43] (5.0%) (Table 1).
Figure 1.
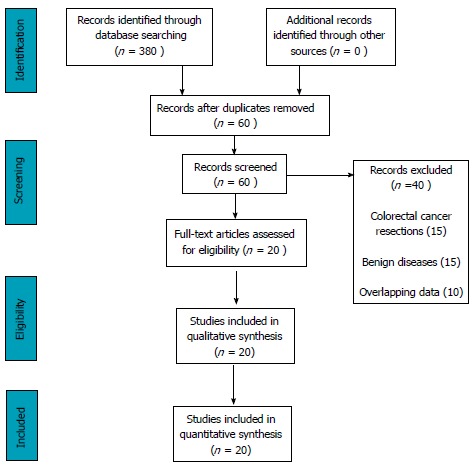
Systematic review Prisma flow diagram.
Table 1.
Characteristics of studies on robotic rectal surgery
| Ref. | Country | Year | Study type | No. of robotic patients | Gender M:F | Mean age (yr) | BMI (kg/m2) | Robotic Technique |
Type of operation |
|||
| AR/LAR | ISR | APR | Hartmann's operation | |||||||||
| Baik et al[24] | South Korea | 2009 | Comparison | 56 | 37:19 | 60.0 | 23.4 | Hybrid | 56 | - | - | - |
| Ng et al[40] | Singapore | 2009 | Case Series | 8 | 5:3 | 55.01 | - | Hybrid | 8 | - | - | - |
| Patriti et al[37] | Italy | 2009 | Comparison | 29 | 11:18 | 68.0 | 24.0 | Hybrid | 19 | 5 | 5 | - |
| Bianchi et al[38] | Italy | 2010 | Comparison | 25 | 18:7 | 69.0 | 24.6 | Total/hybrid | 18 | - | 7 | - |
| Pigazzi et al[32] | United States, Italy | 2010 | Case Series | 143 | 87:56 | 62.0 | 26.5 | Total/hybrid | 80 | 32 | 31 | - |
| Zimmern et al[33] | United States | 2010 | Case Series | 58 | 34:24 | 60.9 | 27.5 | Hybrid | 47 | - | 11 | - |
| Baek et al[34] | United States | 2011 | Comparison | 41 | 25:16 | 63.6 | 25.7 | Hybrid | 33 | 2 | 6 | - |
| Koh et al[41] | Singapore | 2011 | Case Series | 20 | 13:8 | 61.0 | 23.8 | Total | 19 | - | 1 | - |
| Kwak et al[25] | South Korea | 2011 | Comparison | 59 | 39:20 | 60.01 | 23.3 | Total | 54 | 5 | - | - |
| Leong et al[26] | South Korea | 2011 | Case Series | 29 | 23:6 | 61.51 | 23.3 | Total | - | 29 | - | - |
| Park et al[27] | South Korea | 2011 | Comparison | 52 | 28:24 | 57.3 | 23.7 | Hybrid | 52 | - | - | - |
| Kim et al[28] | South Korea | 2012 | Comparison | 100 | 71:29 | 57.0 | 24.0 | Total | 100 | - | - | - |
| Park et al[35] | United States | 2012 | Case Series | 30 | 16:14 | 58.01 | 27.6 | Reverse-hybrid | 5 | 19 | 6 | - |
| Shin et al[29] | South Korea | 2012 | Comparison | 17 | - | - | - | Total/hybrid | 17 | - | - | - |
| Erguner et al[42] | Turkey | 2013 | Comparison | 27 | 14:13 | 54.0 | 28.3 | Total | 27 | - | - | - |
| Kang et al[30] | South Korea | 2013 | Comparison | 165 | 104:61 | 61.2 | 23.1 | Total | 164 | - | - | 1 |
| Park et al[31] | South Korea | 2013 | Comparison | 40 | 28:12 | 57.3 | 23.9 | Hybrid | - | 40 | - | - |
| Stanciulea et al[43] | Romania | 2013 | Case Series | 100 | 66:34 | 62.0 | 26.0 | Total/Hybrid | 77 | - | 23 | - |
| D’Annibale et al[39] | Italy | 2013 | Comparison | 50 | 30:20 | 66.0 | - | Total | 502 | - | - | - |
| Fernandez et al[36] | United States | 2013 | Comparison | 13 | 13:0 | 67.9 | - | Hybrid | 5 | - | 8 | - |
| Total | 1062 | 680:382 | 61.1 | 24.9 | 831 | 132 | 98 | 1 | ||||
median value;
TME: Paper did not specify operation. AR: Anterior resection; LAR: Low anterior resection; ISR: Intersphincteric resection; APR: Abdominoperineal resection.
Surgical technique
There are generally two recognised techniques for Robotic Rectal surgery; the hybrid technique or the total robotic technique. The hybrid technique involves a combination of laparoscopic and robotic techniques to be used in different stages of the operation. The advantage of this method allows a shorter operative time, in particular for rectal cancer operation where the left colon and splenic flexure are mobilised by conventional laparoscopic technique followed by the robotic pelvic dissection[24,27,31,33,34,36-38,40]. Total robotic technique allows the entire operation to the carried out robotically which can either be via: (1) single docking technique- which only requires one docking of the robotic cart with repositioning of the robotic arms according to the operative field[25,26,28,39,41,42]; or (2) dual docking technique which requires the operating table to be positioned twice to the desired operative field[30]. Amongst the selected articles, there was 8 Hybrid, 7 Total robotic, 4 combinations of hybrid and total robotic and 1 reverse-hybrid techniques. Study from Park et al[35] reported a reverse-hybrid whereby robotic lymphovascular (inferior mesenteric artery) and pelvic dissection is performed before laparoscopic mobilisation of left colon and splenic flexure mobilisation.
Clinical outcomes
Patient demographics: A total of 1062 patients were included in the study. The mean age was 61.1 years and 64.0% were male. The average Body mass index BMI was 24.9 kg/m2. Out of 1062 robotic-assisted operations, there were 831 (78.2%) anterior and low anterior resections, 132 (12.4%) intersphincteric resection with coloanal anastomosis, 98 (9.3%) abdominoperineal resections and 1 (0.1%) Hartmann’s operation.
Operative procedures: The review identified 1062 and 706 robotic and laparoscopic rectal operations respectively (Table 2). Mean operation time in the robotic group was 281.8 min (range, 180.0-528.0) compared with the laparoscopic group 242.6 min (range, 158.1-344.0). 7 out of the 11 comparison studies found robotic rectal surgery to have a significantly longer operative time when compared to the laparoscopic surgery[25,27,29-31,36,42]. The remaining 4 studies found laparoscopic rectal surgery to be longer but none were statistically significant[24,34,37,39]. Most authors identified the longer time taken with robotic surgery to be due to docking and changing of the robotic arms.
Table 2.
Perioperative and postoperative outcomes
| Ref. |
No. of patients |
Conversion (%) |
Mean OR time (min) |
Blood loss (mL) |
Overall post-op morbidity (%) |
Anastomotic leak (%) |
Erectile dysfunction (%) |
Voiding dysfunction (%) |
LOS (d) |
|||||||||
| Rob | Lap | Rob | Lap | Rob | Lap | Rob | Lap | Rob | Lap | Rob | Lap | Rob | Lap | Rob | Lap | Rob | Lap | |
| Baik et al[24] | 56 | 57 | 0 | 10.5 | 190.1 | 191.1 | - | - | 10.7 | 19.3 | 1.8 | 7.0 | - | - | - | - | 5.7 | 7.6 |
| Ng et al[40] | 8 | NA | 0 | NA | 193.8 | NA | min | NA | 12.5 | NA | 0 | NA | - | - | - | - | 5.0 | NA |
| Patriti et al[37] | 29 | 37 | 0 | 18.9 | 202.0 | 208.0 | 137.0 | 127.0 | 26.0 | 32.8 | 6.8 | 2.7 | 5.5 | 16.6 | - | - | 11.9 | 9.6 |
| Bianchi et al[38] | 25 | 25 | 0 | 4.0 | 240.0 | 237.0 | - | - | 16.0 | 24.0 | 4.0 | 8.0 | - | - | - | - | 6.5 | 6.0 |
| Pigazzi et al[32] | 143 | NA | 4.7 | NA | 297.0 | NA | min | NA | 41.3 | NA | 10.5 | NA | - | - | - | - | 8.3 | NA |
| Zimmern et al[33] | 58 | NA | 3.7 | NA | 338.0 | NA | 232.0 | NA | 25.9 | NA | 3.4 | NA | - | - | - | - | 6.0 | NA |
| Baek et al[34] | 41 | 41 | 7.3 | 22.0 | 296.0 | 315.0 | - | - | 22.0 | 26.8 | 7.3 | 2.4 | - | - | - | - | 6.5 | 6.6 |
| Koh et al[41] | 20 | NA | 0 | NA | 306.0 | NA | - | - | 23.8 | NA | 0 | NA | - | - | - | - | 6.4 | NA |
| Kwak et al[25] | 59 | 60 | 0 | 3.4 | 270.0 | 228.0 | - | - | 32.2 | 26.7 | 13.6 | 10.2 | - | - | - | - | - | - |
| Leong et al[26] | 29 | NA | 0 | NA | 325.0 | NA | - | - | 37.9 | NA | 10.3 | NA | - | - | - | - | 9.01 | NA |
| Park et al[27] | 52 | 123 | 0 | 0 | 232.6 | 158.1 | - | - | 19.2 | 12.2 | 9.6 | 5.6 | - | - | 0 | 1.6 | 10.4 | 9.8 |
| Kim et al[28] | 100 | NA | 0 | NA | 188.0 | NA | - | - | 11.0 | NA | 2.0 | NA | 36.6 | NA | 6.0 | NA | 7.1 | NA |
| Park et al[35] | 30 | NA | 0 | NA | 369.0 | NA | 100.0 | NA | 36.7 | NA | 4.2 | NA | 0 | NA | 0 | NA | 4.01 | NA |
| Shin et al[29] | 17 | 12 | 0 | 1.0 | 396.5 | 298.8 | 188.8 | 229.2 | 16.72 | 20.02 | 0 | 0 | - | - | 1.0 | 2.0 | 10.7 | 9.6 |
| Erguner et al[42] | 27 | 37 | 0 | 0 | 280.0 | 190.0 | 50.0 | 125.0 | 11.1 | 21.6 | 0 | 8.1 | 0 | 2.7 | - | - | 4.0 | 5.0 |
| Kang et al[30] | 165 | 165 | 0.6 | 1.8 | 309.7 | 277.8 | 133.0 | 140.1 | 20.6 | 27.9 | 7.3 | 10.8 | - | - | 2.4 | 4.2 | 10.8 | 13.5 |
| Park et al[31] | 40 | 40 | 0 | 0 | 225.0 | 183.7 | 45.7 | 59.2 | 15.0 | 12.5 | 7.5 | 5.0 | A | A | A | A | 10.6 | 11.3 |
| Stanciulea et al[43] | 100 | NA | 4.0 | NA | 180.01 | NA | 150.01 | NA | 30.0 | NA | 9.0 | NA | 3.8 | NA | 7.7 | NA | 10.01 | NA |
| D’Annibale et al[39] | 50 | 50 | 0 | 12.0 | 270.01 | 280.01 | - | - | 10.0 | 22.0 | 10.0 | 22.0 | 5.6 | 56.5 | A | A | 8.01 | 10.01 |
| Fernandez et al[36] | 13 | 59 | 8.0 | 17.0 | 528.01 | 344.0 | 157.01 | 200.0 | - | - | 20.0 | 7.0 | - | - | - | - | 13.01 | 8.01 |
Median;
Overall figures for colorectal resections (not just rectal). OR: Operating room; LOS: Length of stay; A: Erectile and voiding dysfunction was assessed and scored with the International Index of Erectile Function score and/or the International Prostate Symptom score respectively; NA: Not available.
Conversion rates for the robotic group ranges from 0% to 8.0% compared to 1.8% to 22% in the laparoscopic group. Both groups cited reasons for conversion such as obesity, difficulty anatomy, bulky tumour, narrow pelvis, adhesions from previous surgery, equipment malfunction and intra-operative complications (e.g., massive bleeding, rectal perforation). In 10 comparison studies, there were no conversions in the robotic group when compared to the laparoscopic group[24,25,27-29,31,37-39,42].
Intraoperative blood loss was compared in 6 studies in this review[29-31,36,37,42]. Five studies found the laparoscopic group had more blood loss when compared to the robotic group but only two of these studies were found to be statistically significant[29,42].
Post-operative outcome
The overall post-operative morbidity in both groups was found to be similar with median of 20.0% (range 10.7%-41.3%) in the robotic group compared with 22.3% (range 12.2%-32.8%) in the laparoscopic group (Table 2). These include anastomotic leak, chest infection, urinary tract infection, postoperative ileus, urinary retention, DVT, wound dehiscence and intra-abdominal collection. Anastomotic leak was also assessed separately as it carries a significant morbidity and mortality. It has been postulated that with the advanced technology, robotic assisted surgery may reduce its incidence with better operative vision and a more precise dissection technique. In this review, median anastomotic leak rate was found to be similar with mean of 6.4% (range, 0%-20.0%) in robotic group compared to 7.4% (range, 0%-22.0%) in laparoscopic group. Preservation of the pelvic autonomic nerves during pelvic surgery is important in order to prevent erectile and voiding dysfunctions. In this review, 7 studies[28,31,35,37,39,42,43] assessed erectile dysfunction and found the incidence of complication ranged from 0% to 36.6% in the robotic group compared to 2.7% to 56.5% in the laparoscopic group. Four of these papers were comparative studies, where Patriti et al[37] found a higher proportion of erectile dysfunction in the laparoscopic group (16.6% vs 5.5% respectively) but this was not significant. Two papers reported sexual and voiding function using the International Index of Erectile Function score (IIEF-5) and the International Prostate Symptom score respectively[31,39]. In the study by Park et al[31], patients were asked to complete the questionnaires preoperatively, 3 and 6 mo postoperatively. In terms of erectile dysfunction, the laparoscopic group had a significantly higher incidence than the robotic group. The robotic group also shown a faster rate of improvement when assessed at 3 and 6 mo. However there was no difference found in terms of voiding function. D’Annibale et al[39] reported 1-year follow-up assessment of erectile dysfunction and found a significant proportion of sexually active patients in the laparoscopic group (13 out of 23; 56.5%) reported erectile dysfunction when compared with the robotic group (1 out of 17; 5.6%). However this result may need to be interpreted with caution as there were a high non-participation rate in the 30 patients selected in each group (laparoscopic group = 23.3% vs robotic assisted group = 40.0%).
Length of stay found the median stay of 7.1 d (range 4-13.0 d) in the robotic procedures compared with median of 9.6 d (range 5-13.5 d) performed by the laparoscopic procedures. Only 2 out of 11 studies showed significantly shorter hospital stay in the robotic group[24,30].
Oncological outcome
Robotic rectal surgery achieved comparable results with laparoscopic surgery in terms of percentage of CRM positivity, mean distal resection margin (Table 3). All studies documented that rectal cancer patients who were preoperatively diagnosed to have T3 or T4 tumour +/- lymph node invasion were given neoadjuvant chemoradiotherapy. Percentage of patients who received neoadjuvant chemoradiotherapy was documented in 11 comparative studies, varying from 8.9% to 80.5% in the robotic group compared with 5.4% to 56.0% in the laparoscopic group[24,25,30,31,34,36-39,42]. The quality of the TME was also assessed. Two studies comparing TME quality after robotic and laparoscopic dissection found the former to be significantly superior[24,42] whereas the study by Fernandez et al[36] found the laparoscopic group to be superior but this was not statistically significant. The studies showed there was minimal difference between the number of lymph nodes retrieved with robotic assisted (range, 10.3 to 20.0) and laparoscopic rectal resection (range, 11.2 to 21). Recurrence of cancer from 6 studies ranged from no recorded recurrence to 5.5%. In a study by Kwak et al[25], there were no significant differences found between the robotic-assisted group and laparoscopy assisted group in terms of loco-regional recurrence, distant metastasis and total recurrence. Three-year disease free survival ranges from 77.6% to 100% with overall survival between 90% to 97%. The study by Kang et al[30] found no difference in 2-year survival between robotic assisted group (83.5%), laparoscopy group (81.9%) and open surgery (79.7%) (P = 0.855).
Table 3.
Oncological outcomes
| Ref. |
No. of patients |
Mean follow-up (mths) |
NeoCRT (%) |
Lymph nodes harvested (mean) |
TME grade complete (%) |
CRM +ve (%) |
DRM (cm) |
Robotic Recurrence (%) |
3 yr Robotic Survival (%) |
||||||||
| Rob | Lap | Rob | Lap | Rob | Lap | Rob | Lap | Rob | Lap | Rob | Lap | Rob | Lap | DS | OS | ||
| Baik et al[24] | 56 | 57 | 14.3 (both) | 8.9 | 12.2 | 18.4 | 18.7 | 92.9 | 75.4 | 7.1 | 8.8 | 4.0 | 3.6 | - | - | 7.6 | |
| Ng et al[40] | 8 | NA | 1.5 | NA | - | - | 12.9 | NA | - | - | 0 | NA | > 2.0 | NA | - | - | NA |
| Patriti et al[37] | 29 | 37 | 29.2 | 18.7 | 24.1 | 5.4 | 10.3 | 11.2 | - | - | 0 | 0 | 2.1 | 4.5 | None | 100.0 | 9.6 |
| Bianchi et al[38] | 25 | 25 | 10.0 (both) | 52.0 | 40.0 | 19.7 | 18.2 | - | - | 0 | 4.0 | 2.0 | 2.0 | None | - | 6.0 | |
| Pigazzi et al[32] | 143 | NA | 17.4 | NA | 65.1 | - | 14.1 | NA | - | - | 0.7 | NA | 2.9 | NA | 1.5 | 77.6 | NA |
| Zimmern et al[33] | 58 | NA | 13.2 | NA | 39.7 | NA | 14.1 | NA | - | - | 0 | NA | - | - | 5.2 | - | NA |
| Baek et al[34] | 41 | 41 | - | - | 80.5 | 43.9 | 13.1 | 16.2 | - | - | 2.4 | 4.9 | 3.6 | 3.8 | - | - | 6.6 |
| Koh et al[41] | 20 | NA | - | - | 9.5 | NA | 17.8 | NA | - | - | 5.3 | - | 3.7 | - | - | - | NA |
| Kwak et al[25] | 59 | 60 | 17.0 | 13.0 | 13.6 | 8.5 | 20.0 | 21.0 | - | - | 1.7 | 0 | - | - | - | - | - |
| Leong et al[26] | 29 | NA | - | - | 37.9 | NA | 16.0 | NA | - | - | 7.0 | NA | 0.8 | NA | - | - | NA |
| Park et al[27] | 52 | 123 | - | - | 23.1 | 8.1 | 19.4 | 15.9 | - | - | 1.9 | 2.4 | 2.8 | 3.2 | - | - | 9.8 |
| Kim et al[28] | 100 | NA | 24.0 | NA | 32.0 | NA | 20.0 | NA | - | - | 1.0 | NA | 2.7 | NA | - | - | NA |
| Park et al[35] | 30 | NA | - | - | 66.7 | NA | 20.0 | NA | 83.3 | NA | 0 | NA | - | - | - | - | NA |
| Shin et al[29] | 17 | 12 | - | - | - | - | 18.42 | 15.92 | - | - | - | - | - | - | - | - | 9.6 |
| Erguner et al[42] | 27 | 37 | - | - | 14.8 | 21.6 | 16.0 | 16.0 | 100.0 | 70.6 | 0 | 0 | 4.0 | 4.0 | - | - | 5.0 |
| Kang et al[30] | 165 | 165 | 22.41 (both) | 23.6 | 21.8 | 15.0 | 15.6 | - | - | 4.2 | 6.7 | 1.9 | 2.0 | - | - | - | |
| Park et al[31] | 40 | 40 | 6.0 | 6.0 | 80.0 | 50 | 12.9 | 13.3 | - | - | 7.5 | 5.0 | 1.4 | 1.3 | - | - | - |
| Stanciulea et al[43] | 100 | NA | 24.01 | NA | 58.0 | NA | 14.01 | NA | - | - | 1.0 | - | 3.0 | - | 2.0 | NA | 90.0 |
| D’Annibale et al[39] | 50 | 50 | 12.0 | 12.0 | 68.0 | 56.0 | 16.5 | 13.8 | - | - | 0 | 0 | 3.0 | 3.0 | - | - | - |
| Fernandez et al[36] | 13 | 59 | - | - | 77.0 | 54.0 | 16.0 | 20.0 | 69.0 | 73.0 | 0 | 2.0 | - | - | - | - | - |
Median;
Overall figures for colorectal resections (not just rectal). Rob: Robotic-assisted surgery; Lap: Conventional laparoscopic surgery; NeoCRT: Neoadjuvant chemoradiotherapy; TME: Total mesorectal excision; CRM: Circumferential resection margin; DRM: Distal resection margin; DS: Disease free survival; NA: Not available.
Learning curve
Within the selected articles, there were only 3 papers which looked into learning curve for robotic rectal surgery[31,32,39]. Pigazzi et al[32] found operative time decreased significantly after 20 cases. With intersphincteric resections, Park et al[31] found the learning curve plateau after 17 cases by using the moving average method. In one paper the author’s opinion was that the numbers of cases require for learning can be as low as two cases if performed by an already skilled laparoscopic surgeon[38]. D’Annibale et al[39] found mean operative time decreased from 312.5 min in the first 25 procedures to 238.2 min in the last 10 procedures (P = 0.002). Following cusum analysis, this study showed that learning curve in robot group was achieved after 22 cases[39].
Cost
A review of the selected articles found four studies, which looked into the cost of robotic surgery (Table 4). In two of the studies, the cost of robotic rectal surgery was estimated to be three times more expensive than laparoscopic rectal surgery[25,26]. The remaining two studies found also robotic rectal surgery to be more expensive when compared to laparoscopic and open rectal surgery but the figures in these studies did not show statistical significance[28,34]. Authors also highlighted the fact that the provision of health is different between countries such as in South Korea.
Table 4.
Cost of Robotic rectal surgery
| Ref. | Country | Year | Study type |
No. of rectal cancer patients |
Average total hospitalisation cost (United States $) |
P value | ||||
| Robotic | Laparoscopic | Open | Robotic | Laparoscopic | Open | |||||
| Baik et al[24] |
United States |
2011 |
Comparison |
41 |
41 |
- |
83915 |
62601 |
- |
0.092 |
| Kwak et al[25] |
South Korea |
2011 |
Comparison |
59 |
59 |
- |
Robotic x3 Laparoscopic cost |
NA |
NA |
|
| Leong et al[26] |
South Korea |
2011 |
Case Series |
29 |
- |
- |
Robotic x3 Laparoscopic cost |
- |
- |
|
| Kim et al[28] | South Korea | 2012 | Comparison | 100 | - | 100 | 12-15000 | 5000 | - | - |
Rob: Robotic-assisted surgery; Lap: Conventional laparoscopic surgery; NA: Not available.
DISCUSSION
This systematic review suggests robotic-assisted surgery to be feasible and safe. We have selected 20 articles for review out of 380 articles, which met our selection criteria. We deliberately set the inclusion period to be within the past 6 years as it will exclude small case series where authors may not have attained the desired learning curve and also a more recent data-set may give a more accurate reflection of the current practice and capability of the da Vinci robotic systems.
Previous systematic reviews have reported similar outcomes to our study[15,16,18]. They concluded robotic-assisted rectal surgery to be feasible and safe. Similar to our review, conversion rates tend to be lower in the robotic-assisted group when compared to the laparoscopic group. This may have important implications as converted cases are associated with greater morbidity and tumour recurrence[3]. Many authors identified lower conversion rates in the robotic group to be associated with superior visualisation, better exposure and endowrist™ technology.
In our review we found overall complication rates between robotic and laparoscopic group to be similar. These perceived advantages also did not translate to lower anastomotic leaks in the robotic group, which may be due to the fact that the aetiology for anastomotic leak is multifactorial (e.g., patient nutrition, underlying comorbidity, neoadjuvant chemoradiotherapy, surgical technique, blood supply, tension to anastomosis, etc.) and therefore an adequately powered study is required. Intraoperative blood loss only resulted in two studies, which found laparoscopic group to have a statistically greater blood loss than the robotic group[29,42].
The short-term oncological outcome using conventional surgical yardsticks for rectal cancer dissection seems to be comparable between the two groups. CRM and distal resection margins are comparable to laparoscopic group. Quality of the TME dissection is important as breach of the TME envelope may increase local and distant recurrence. In this review, only three studies assessed the quality of the TME specimen macroscopically with two comparative studies found robotic dissection to be superior. With emerging data favouring TME via minimal invasive approach over open surgery[5,44], robotic surgery may offer additional advantage.
Traditionally long operative times are related with increased morbidity, which is likely to be related to the difficulty of the operation[45]. Robotic surgery has been found to have a longer operative time when compared to laparoscopic or open rectal surgery. Attempts have been made to reduce robotic operating time by adopting the hybrid approach. However this will require the surgeon to be skilled at both robotic as well as conventional laparoscopic surgery. Also the perceived advantage of robotic surgery may be lost during inferior mesenteric artery dissection, which may increase the chance of nerve damage as well as additional cost of laparoscopic instruments. Prolonged operative times are most likely to be related to technical aspects of the operation (time taken to dock and redock the robot as well as changing of robotic arms) rather than the operative difficulty. Indeed the overall complication rates between the robotic and the laparoscopic groups have been shown to be similar in this review, which further supports the theory that longer robotic operative time may not necessarily increase operative morbidity.
Cost of robotic surgery remained to be an important issue. Most papers identified the cost of the robot to be around United States $1.65 to 2 million, disposable robotic instruments costing United States $2000 each as well as the yearly maintenance cost United States $150000[24]. In this review article, it was not possible to include cost-effectiveness analysis studies. Baek et al[34] highlighted the fact that caution needs to be taken when interpreting costs as it may differ significantly between hospitals. Different healthcare system between countries will also have an impact on costs. However, maximising the use of the robot by different surgical specialties within the hospital might make savings to the overall running costs.
Identification and preservation of the pelvic autonomic nerves may be better with robotic surgery due to high definition 3-D image, tremor free surgery, surgeon operated camera platform and endowrist™ technology. Common sites of potential pelvic nerve damage leading to sexual dysfunction are: (1) superior hypogastric plexus, leading to ejaculation dysfunction on male patients and impaired lubrication in females; and (2) pelvic splanchnic nerves or the pelvic plexus- leading to erectile dysfunction in men. These perceived advantages may translate to decreased incidence of erectile dysfunction in male patients and urinary dysfunction as the CLASICC trial reported a 41% sexual dysfunction in men after laparoscopic rectal surgery when compared with 23% in the open rectal surgery group[46] (Figure 2). However, in this review although there were some encouraging results to suggest that robotic-assisted surgery is superior to conventional laparoscopic surgery in preventing sexual or urinary dysfunction, the evidence is not entirely clear due to high non-participation rates and possible type II error. Kim et al[47] also reported similar results where although the robotic-assisted group reported earlier recovery of erectile, sexual desire and urinary function when compared with the laparoscopic group, there was no difference in long-term follow-up.
Figure 2.
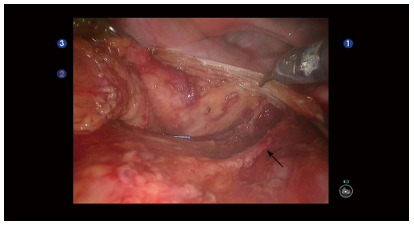
Robotic pelvic dissection. High definition 3-D view of the pelvis with the right hypogastric nerve (arrow) identified and protected.
In this review, we were unable to draw strong conclusion on the learning curve required for robotic surgery. However the range of 17-25 cases of robotic-assisted rectal surgery from experienced surgeons skilled at both open and laparoscopic surgery are quoted as the number required to achieve competency. The cases selected were very heterogeneous; only few studies used recognised method on assessing learning curve and one of studies were from expert’s comment.
Although the da Vinci® robotic platform has produced promising results with at least comparable benefits to laparoscopic colorectal surgery, good quality studies are still required to demonstrate its benefits. The ROLARR (RObotic versus LAparoscopic Resection for Rectal cancer) study is a multicentre international randomised control trial with the primary aim to assess technical ease of robotic rectal operations. The secondary aims are to assess the quality of life, cost-effectiveness analysis and oncological outcome on disease-free and overall survival and local recurrence at 3-year follow-up. The study began recruiting in february 2011 and therefore results will not be available for sometime[48]. Other Robotic rectal surgical clinical trials currently registered on www.clinicaltrials.gov include centres from South Korea[49,50], China[51] and Hong Kong[52].
In summary, from this systematic review, in the authors’ opinion we can draw conclusions on the following: (1) robotic-assisted rectal surgery is feasible and safe; (2) it has a lower conversion rate when compared to laparoscopic group; (3) intra-operative blood loss resulted significantly less in the robotic group in 2 of the comparison studies; (4) postoperative morbidity and long-term voiding and sexual functions remain similar in both groups; (5) quality of the TME dissection is significantly better in some studies but nevertheless there were no significant differences found in short-term of oncological outcomes in both groups; and (6) robotic-assisted is more expensive than laparoscopic surgery. Hence the current challenges will be to justify the benefits of robotic rectal surgery over high costs.
COMMENTS
Background
The incidence of rectal cancers is increasing owing to the elderly population, westernised lifestyle and other environmental factors. Prognosis in rectal cancer can be related to the quality of surgery such as mesorectal integrity, margin status, and adequate lymph node dissection. Laparoscopic has been proven to reduce hospital stay, less pain and less bleeding but its role in rectal cancer surgery remains controversial due to its steep learning-curve. Da Vinci robotic-assisted rectal cancer surgery may be an effective tool but its effectiveness over laparoscopic surgery is unclear.
Research frontiers
Robotic-assisted rectal cancer surgery has technical advantages over conventional laparoscopic method such as tremor free surgery, high definition 3-D vision, stable platform and surgeon-control camera. These technological advances seem to be ideally suited for rectal cancer surgery as it may minimize inadvertent pelvic neurovascular injury and achieve good oncological results.
Innovations and breakthroughs
Conventional laparoscopic rectal surgery has been known to have a steep learning curve owing to 2-Dimensional view, assistant navigated camera and instruments with limited freedom of movement. Robotic-assisted rectal surgery has overcome some of these limitations with 3-Dimensional view, stable platform, surgeon-controlled camera and tremor-free surgery. However further high quality research is required see whether these advances can be translated to benefit patient care.
Applications
Readers will be able to have an unbiased view on the pros and cons of robotic-assisted rectal surgery. This systematic review has identified current evidence is based on case series and comparative reports and that has demonstrated robotic-assisted rectal surgery is feasible and safe. However as these studies demonstrated potential benefits of robotic surgery are not yet proven and that whether the high cost justify these benefits is still under debate.
Terminology
Laparoscopic surgery and robotic-assisted surgery are a form of minimal invasive surgery which has advantages over open operations such as less blood loss, faster recovery, less complications and better cosmetic results.
Peer review
This manuscript is an interesting and well done systematic review on robotic rectal surgery. Authors reported data according to the Prisma guidelines for systematic reviews and meta-analyses. This paper deserves publication.
Footnotes
P- Reviewers: Denadai R, Fiori E, Jani K, Lirici MM S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248:1–7. doi: 10.1097/SLA.0b013e31816a9d65. [DOI] [PubMed] [Google Scholar]
- 2.Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–1645. doi: 10.1002/bjs.7160. [DOI] [PubMed] [Google Scholar]
- 3.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 4.Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 5.Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 6.Gaujoux S, Bretagnol F, Ferron M, Panis Y. Single-incision laparoscopic colonic surgery. Colorectal Dis. 2011;13:1066–1071. doi: 10.1111/j.1463-1318.2010.02404.x. [DOI] [PubMed] [Google Scholar]
- 7.Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114–117. doi: 10.1016/s0016-5107(04)01309-4. [DOI] [PubMed] [Google Scholar]
- 8.Swain P. Nephrectomy and natural orifice translumenal endoscopy (NOTES): transvaginal, transgastric, transrectal, and transvesical approaches. J Endourol. 2008;22:811–818. doi: 10.1089/end.2007.9831. [DOI] [PubMed] [Google Scholar]
- 9.Park PO, Bergström M, Ikeda K, Fritscher-Ravens A, Swain P. Experimental studies of transgastric gallbladder surgery: cholecystectomy and cholecystogastric anastomosis (videos) Gastrointest Endosc. 2005;61:601–606. doi: 10.1016/s0016-5107(04)02774-9. [DOI] [PubMed] [Google Scholar]
- 10.Weber PA, Merola S, Wasielewski A, Ballantyne GH. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum. 2002;45:1689–194; discussion 1689-1694;. doi: 10.1007/s10350-004-7261-2. [DOI] [PubMed] [Google Scholar]
- 11.Hashizume M, Shimada M, Tomikawa M, Ikeda Y, Takahashi I, Abe R, Koga F, Gotoh N, Konishi K, Maehara S, et al. Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc. 2002;16:1187–1191. doi: 10.1007/s004640080154. [DOI] [PubMed] [Google Scholar]
- 12.Loulmet D, Carpentier A, d’Attellis N, Berrebi A, Cardon C, Ponzio O, Aupècle B, Relland JY. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg. 1999;118:4–10. doi: 10.1016/S0022-5223(99)70133-9. [DOI] [PubMed] [Google Scholar]
- 13.Menon M, Tewari A, Baize B, Guillonneau B, Vallancien G. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology. 2002;60:864–868. doi: 10.1016/s0090-4295(02)01881-2. [DOI] [PubMed] [Google Scholar]
- 14.Cadière GB, Himpens J, Vertruyen M, Bruyns J, Germay O, Leman G, Izizaw R. Evaluation of telesurgical (robotic) NISSEN fundoplication. Surg Endosc. 2001;15:918–923. doi: 10.1007/s004640000217. [DOI] [PubMed] [Google Scholar]
- 15.Mirnezami AH, Mirnezami R, Venkatasubramaniam AK, Chandrakumaran K, Cecil TD, Moran BJ. Robotic colorectal surgery: hype or new hope? A systematic review of robotics in colorectal surgery. Colorectal Dis. 2010;12:1084–1093. doi: 10.1111/j.1463-1318.2009.01999.x. [DOI] [PubMed] [Google Scholar]
- 16.Kanji A, Gill RS, Shi X, Birch DW, Karmali S. Robotic-assisted colon and rectal surgery: a systematic review. Int J Med Robot. 2011;7:401–407. doi: 10.1002/rcs.432. [DOI] [PubMed] [Google Scholar]
- 17.Pucci MJ, Beekley AC. Use of Robotics in Colon and Rectal Surgery. Clin Colon Rectal Surg. 2013;26:39–46. doi: 10.1055/s-0033-1333660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scarpinata R, Aly EH. Does robotic rectal cancer surgery offer improved early postoperative outcomes? Dis Colon Rectum. 2013;56:253–262. doi: 10.1097/DCR.0b013e3182694595. [DOI] [PubMed] [Google Scholar]
- 19.Lin S, Jiang HG, Chen ZH, Zhou SY, Liu XS, Yu JR. Meta-analysis of robotic and laparoscopic surgery for treatment of rectal cancer. World J Gastroenterol. 2011;17:5214–5220. doi: 10.3748/wjg.v17.i47.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trastulli S, Farinella E, Cirocchi R, Cavaliere D, Avenia N, Sciannameo F, Gullà N, Noya G, Boselli C. Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis. 2012;14:e134–e156. doi: 10.1111/j.1463-1318.2011.02907.x. [DOI] [PubMed] [Google Scholar]
- 21.Centre for Reviews and Dissemination (CRD) Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2009. p. 292. [Google Scholar]
- 22.JPT H, S G. Cochrane Handbook for Systematic Reviews on Interventions, version 5.1. 0 [updated March 2011]: The Cochrane Collaboration; 2011. [Google Scholar]
- 23.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho CH, Kim H. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol. 2009;16:1480–1487. doi: 10.1245/s10434-009-0435-3. [DOI] [PubMed] [Google Scholar]
- 25.Kwak JM, Kim SH, Kim J, Son DN, Baek SJ, Cho JS. Robotic vs laparoscopic resection of rectal cancer: short-term outcomes of a case-control study. Dis Colon Rectum. 2011;54:151–156. doi: 10.1007/DCR.0b013e3181fec4fd. [DOI] [PubMed] [Google Scholar]
- 26.Leong QM, Son DN, Cho JS, Baek SJ, Kwak JM, Amar AH, Kim SH. Robot-assisted intersphincteric resection for low rectal cancer: technique and short-term outcome for 29 consecutive patients. Surg Endosc. 2011;25:2987–2992. doi: 10.1007/s00464-011-1657-6. [DOI] [PubMed] [Google Scholar]
- 27.Park JS, Choi GS, Lim KH, Jang YS, Jun SH. S052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc. 2011;25:240–248. doi: 10.1007/s00464-010-1166-z. [DOI] [PubMed] [Google Scholar]
- 28.Kim JC, Yang SS, Jang TY, Kwak JY, Yun MJ, Lim SB. Open versus robot-assisted sphincter-saving operations in rectal cancer patients: techniques and comparison of outcomes between groups of 100 matched patients. Int J Med Robot. 2012;8:468–475. doi: 10.1002/rcs.1452. [DOI] [PubMed] [Google Scholar]
- 29.Shin JY. Comparison of Short-term Surgical Outcomes between a Robotic Colectomy and a Laparoscopic Colectomy during Early Experience. J Korean Soc Coloproctol. 2012;28:19–26. doi: 10.3393/jksc.2012.28.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang J, Yoon KJ, Min BS, Hur H, Baik SH, Kim NK, Lee KY. The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison--open, laparoscopic, and robotic surgery. Ann Surg. 2013;257:95–101. doi: 10.1097/SLA.0b013e3182686bbd. [DOI] [PubMed] [Google Scholar]
- 31.Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP. Short-term clinical outcome of robot-assisted intersphincteric resection for low rectal cancer: a retrospective comparison with conventional laparoscopy. Surg Endosc. 2013;27:48–55. doi: 10.1007/s00464-012-2405-2. [DOI] [PubMed] [Google Scholar]
- 32.Pigazzi A, Luca F, Patriti A, Valvo M, Ceccarelli G, Casciola L, Biffi R, Garcia-Aguilar J, Baek JH. Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol. 2010;17:1614–1620. doi: 10.1245/s10434-010-0909-3. [DOI] [PubMed] [Google Scholar]
- 33.Zimmern A, Prasad L, Desouza A, Marecik S, Park J, Abcarian H. Robotic colon and rectal surgery: a series of 131 cases. World J Surg. 2010;34:1954–1958. doi: 10.1007/s00268-010-0591-4. [DOI] [PubMed] [Google Scholar]
- 34.Baek JH, Pastor C, Pigazzi A. Robotic and laparoscopic total mesorectal excision for rectal cancer: a case-matched study. Surg Endosc. 2011;25:521–525. doi: 10.1007/s00464-010-1204-x. [DOI] [PubMed] [Google Scholar]
- 35.Park IJ, You YN, Schlette E, Nguyen S, Skibber JM, Rodriguez-Bigas MA, Chang GJ. Reverse-hybrid robotic mesorectal excision for rectal cancer. Dis Colon Rectum. 2012;55:228–233. doi: 10.1097/DCR.0b013e31823c0bd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez R, Anaya DA, Li LT, Orcutt ST, Balentine CJ, Awad SA, Berger DH, Albo DA, Artinyan A. Laparoscopic versus robotic rectal resection for rectal cancer in a veteran population. Am J Surg. 2013;206:509–517. doi: 10.1016/j.amjsurg.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 37.Patriti A, Ceccarelli G, Bartoli A, Spaziani A, Biancafarina A, Casciola L. Short- and medium-term outcome of robot-assisted and traditional laparoscopic rectal resection. JSLS. 2009;13:176–183. [PMC free article] [PubMed] [Google Scholar]
- 38.Bianchi PP, Ceriani C, Locatelli A, Spinoglio G, Zampino MG, Sonzogni A, Crosta C, Andreoni B. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc. 2010;24:2888–2894. doi: 10.1007/s00464-010-1134-7. [DOI] [PubMed] [Google Scholar]
- 39.D’Annibale A, Morpurgo E, Fiscon V, Trevisan P, Sovernigo G, Orsini C, Guidolin D. Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum. 2004;47:2162–2168. doi: 10.1007/s10350-004-0711-z. [DOI] [PubMed] [Google Scholar]
- 40.Ng KH, Lim YK, Ho KS, Ooi BS, Eu KW. Robotic-assisted surgery for low rectal dissection: from better views to better outcome. Singapore Med J. 2009;50:763–767. [PubMed] [Google Scholar]
- 41.Koh DC, Tsang CB, Kim SH. A new application of the four-arm standard da Vinci® surgical system: totally robotic-assisted left-sided colon or rectal resection. Surg Endosc. 2011;25:1945–1952. doi: 10.1007/s00464-010-1492-1. [DOI] [PubMed] [Google Scholar]
- 42.Erguner I, Aytac E, Boler DE, Atalar B, Baca B, Karahasanoglu T, Hamzaoglu I, Uras C. What have we gained by performing robotic rectal resection? Evaluation of 64 consecutive patients who underwent laparoscopic or robotic low anterior resection for rectal adenocarcinoma. Surg Laparosc Endosc Percutan Tech. 2013;23:316–319. doi: 10.1097/SLE.0b013e31828e3697. [DOI] [PubMed] [Google Scholar]
- 43.Stănciulea O, Eftimie M, David L, Tomulescu V, Vasilescu C, Popescu I. Robotic surgery for rectal cancer: a single center experience of 100 consecutive cases. Chirurgia (Bucur) 2013;108:143–151. [PubMed] [Google Scholar]
- 44.van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 45.Kurmann A, Vorburger SA, Candinas D, Beldi G. Operation time and body mass index are significant risk factors for surgical site infection in laparoscopic sigmoid resection: a multicenter study. Surg Endosc. 2011;25:3531–3534. doi: 10.1007/s00464-011-1753-7. [DOI] [PubMed] [Google Scholar]
- 46.Jayne DG, Brown JM, Thorpe H, Walker J, Quirke P, Guillou PJ. Bladder and sexual function following resection for rectal cancer in a randomized clinical trial of laparoscopic versus open technique. Br J Surg. 2005;92:1124–1132. doi: 10.1002/bjs.4989. [DOI] [PubMed] [Google Scholar]
- 47.Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol. 2012;19:2485–2493. doi: 10.1245/s10434-012-2262-1. [DOI] [PubMed] [Google Scholar]
- 48.Collinson FJ, Jayne DG, Pigazzi A, Tsang C, Barrie JM, Edlin R, Garbett C, Guillou P, Holloway I, Howard H, et al. An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis. 2012;27:233–241. doi: 10.1007/s00384-011-1313-6. [DOI] [PubMed] [Google Scholar]
- 49.Choi GS. A Trial to Assess Robot-assisted Surgery and Laparoscopy-assisted Surgery in Patients with Mid or Low Rectal Cancer (COLRAR). ClinicalTrails.gov identifier: NCT01423214. Secondary A Trial to Assess Robot-assisted Surgery and Laparoscopy-assisted Surgery in Patients with Mid or Low Rectal Cancer (COLRAR). ClinicalTrails.gov identifier: NCT01423214. Available from: http://clinicaltrials.gov/ct2/show/NCT01423214.
- 50.Park JW. Clinical Assessment of Laparoscopic and Robotic Surgery for Rectal Cancer-Randomized Phase II Trial. Available from: http://clinicaltrials.gov/ct2/show/NCT01591798.
- 51.Xu J. A Multicentre, Prospective, Randomised, Controlled, Unblinded, Parallel-group Trail of Robotic-assisted Versus Laparoscopic Versus Open Abdominoperineal Resection for the Curative Treatment of Low Rectal Cancer. ClinicalTrials.gov identifier: NCT01985698. Secondary A Multicentre, Prospective, Randomised, Controlled, Unblinded, Parallel-group Trail of Robotic-assisted Versus Laparoscopic Versus Open Abdominoperineal Resection for the Curative Treatment of Low Rectal Cancer. ClinicalTrials.gov identifier: NCT01985698. Available from: http://clinicaltrials.gov/ct2/show/NCT01985698.
- 52.Law WL. Randomized Trial on Robotic Assisted Resection for Rectal Cancer. ClinicalTrials.gov identifier: NCT01130233. Secondary Randomized Trial on Robotic Assisted Resection for Rectal Cancer. ClinicalTrials.gov identifier: NCT01130233. Available from: http://clinicaltrials.gov/ct2/show/NCT01130233.


