Abstract
Type 2 diabetes is a complicated metabolic disorder with both short- and long-term undesirable complications. In recent years, there has been growing evidence that functional foods and their bioactive compounds, due to their biological properties, may be used as complementary treatment for type 2 diabetes mellitus. In this review, we have highlighted various functional foods as missing part of medical nutrition therapy in diabetic patients. Several in vitro, animal models and some human studies, have demonstrated that functional foods and nutraceuticals may improve postprandial hyperglycemia and adipose tissue metabolism modulate carbohydrate and lipid metabolism. Functional foods may also improve dyslipidemia and insulin resistance, and attenuate oxidative stress and inflammatory processes and subsequently could prevent the development of long-term diabetes complications including cardiovascular disease, neuropathy, nephropathy and retinopathy. In conclusion available data indicate that a functional foods-based diet may be a novel and comprehensive dietary approach for management of type 2 diabetes.
Keywords: Type 2 diabetes, Insulin resistance, Functional foods, Whole grain, Legumes, Nuts, Fruits, Herbs or spices, Vegetables, Prebiotics, Probiotics
Core tip: Medical nutrition therapy (MNT) is a main part of type 2 diabetes management. Apparently the therapeutic and medicinal properties of foods maybe a missing step during MNT process, and could enhance the effectiveness of dietary management of type 2 diabetes.
INTRODUCTION
Type 2 diabetes is a metabolic disorder characterized by hyperglycemia, developing insulin resistance, β-cell dysfunction and impaired insulin secretion[1,2]. Multiple metabolic disorders including impaired lipid and lipoprotein metabolism, oxidative stress (over production of free radicals and defect in endogenous antioxidant defense system), sub-clinical inflammation, vascular endothelial dysfunction and hypertension are commonly accompanied by type 2 diabetes[3-5]; these metabolic disorders lead to long-term pathogenic conditions such as micro- and macro-vascular complications including neuropathy, retinopathy, nephropathy, and a decreased quality of life and an increased mortality rate[6,7].
Despite availability of many pharmacological interventions including oral hypoglycemic agents and insulin therapy for diabetes management, current evidence shows an alarming rising trend in the occurrence of undesirable complications among these patients[1].
Medical nutrition therapy (MNT) is also a main part of type 2 diabetes management; estimation of energy and nutrients requirements, carbohydrate counting as well as glycemic index and glycemic load, recommendation for dietary fats and cholesterol and protein intakes, explanation the foods exchange list for patients and common important recommendations for a healthy diet are the main components of diet planning in type 2 diabetic patients[8,9]; however it is not clear whether this approach per se is sufficiently adequate for prevention of long-term complications of diabetes. Administration of various supplements, including antioxidant vitamins, fibers, ω3 fatty acids, numerous nutraceuticals, and herbs has also been proposed for glycemic control but data available supporting these recommendations for diabetic patients are insufficient[10-14]. Apparently the therapeutic and medicinal properties of foods maybe a missing step during MNT process, and could enhance the effectiveness of dietary management of type 2 diabetes.
During the past two decades, the concept of functional food is fast expanding; functional foods beyond the basic nutritional functions have potential benefits to promote health and reduce the risk of chronic diseases and have hence been given much attention[15,16]. In recent years, researchers have focused on properties of the bioactive compounds of functional foods in the control of various aspects of diabetes mellitus; some protective effects of these compounds and food sources have been investigated in vitro and in vivo, and several clinical trials have even confirmed these advantages in diabetic patients[17-19].
Here, based on the multiple biological properties of functional foods and their bioactive compounds, a functional foods-based diet has been hypothesized as a novel and comprehensive dietary approach for management of type 2 diabetes and prevention of long-term complications.
RESEARCH
The evidence cited in this review was obtained through searches in PubMed, Scopus, and Google scholar using the following key words: “Type 2 diabetes or hyperglycemia”, “insulin resistance”, “cardiovascular disease”, “obesity”, “metabolic syndrome”, “oxidative stress”, “inflammation”, long-term diabetic complications” in combination with “functional foods”, “nutraceuticals”, “bioactive food compounds”, “fiber”, “polyphenols”, “whole grain”, “legumes”, “nuts”, “fruits”, “herbs or spices” “vegetables”, “prebiotics”, “probiotics”, and “bioactive peptides”. Relevant articles of acceptable quality were used. Briefly, in this article we tried to highlight some of the following important functional foods including whole grains, phytochemical-rich fruits and vegetables, legumes, nuts, dairy products, green tea and some spices, as required components of a health-promoting diet for diabetic patients.
Whole grains
Grains and cereal-based products are the basic sources providing energy and carbohydrate in human diets. Since the dietary carbohydrate sources in type 2 diabetic patients play a determining role in glycemic and insulin secretary response, the use of functional grains including whole grain cereals, and bakery products prepared using whole wheat, rye, oat, and barley is the first step in planning of a functional foods-based diet.
Some previous studies report that dietary carbohydrate modification in patients with metabolic syndrome resulted in favorable metabolic consequences especially increased insulin sensitivity, decreased adipocyte cell size, and modulated expression of adipose tissue genes involved in insulin signaling pathways (insulin-like-growth-factor binding protein-5, insulin receptors, hormone-sensitive lipase[20,21].
Compared to refined grains, whole grains (WGs) have more non-digestible complex polysaccharides including soluble and insoluble fibers, inulin, β-glucan, and resistant starches, as well as non-carbohydrate functional components including carotenoids, phytates and phytoesterogens, phenolic acids (ferulic acid, vanilic acid, caffeic acid, syringic acid, P-cumaric acid), and tocopherols. The most well-known protective effects of whole grain-based products against obesity, type 2 diabetes, cardiovascular diseases, hypertension, metabolic syndrome and various types of cancer, have been attributed to these bioactive compounds[22-25]. Among the several mechanisms available in current data regarding the beneficial effects of WGs and cereal-based products in diabetic patients, some of the more important are that bioactive compounds of WGs could effectively regulate glycemic response, increase insulin sensitivity, improve pancreatic β-cell functions and increase insulin secretion[26,27]. High contents of inulin and β-glucan, main soluble and fermentable fibers in WGs, in addition to their hypolipidemic and hypoglycemic effects, act as prebiotics in the gut and modulate gut microbiota via stimulation of growth and activity of bifidobacteria and lactic acid bacteria[28,29], effects leading to more metabolic responses (Figure 1).
Figure 1.
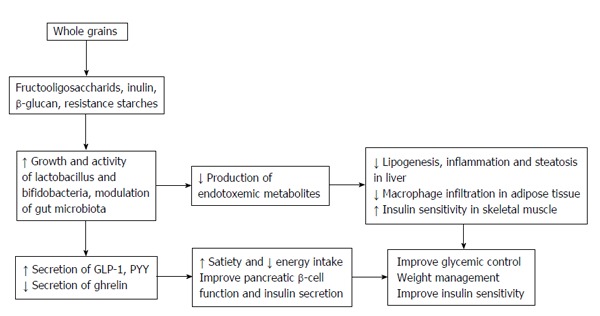
Role of prebiotic compounds of whole grains and cereal-based products in modulation of gut microbiota and con sequent metabolic effects could lead to better glycemic control.
Long-term follow-ups of diabetic patients indicate that higher consumption of whole grain, cereal fiber, bran, and germ were associated with decreased all-cause and cardiovascular disease-cause mortality[30]. Epidemiological studies also confirmed that regular consumption of WGs products could modify the main risk factors of atherosclerotic diseases including triglyceride and LDL-C levels, blood pressure and serum homocysteine levels, as well as vascular functions, and oxidative and inflammatory status[31].
Rye, a widely used grain especially in Northern and Eastern Europe, is considered a functional grain. The high fiber content of rye products decreases digestion and absorption of dietary carbohydrates, and increase metabolites derived from colonic fermentation of the soluble fiber of rye products, including propionic and butyric acids which effectively stimulate secretion of insulin from β-cells; studies have indicated that the bioactive compounds of rye (phenolic acids, tannins, benzoic acid, phenylalanine) derivates have a similar efficacy with anti-diabetic drugs in insulin secretion[26,32]. In one study, the consumption of rye products in the breakfast meal increased colonic fermentation, decreased ghrelin levels and satiety rating in the late postprandial phase after breakfast as well as energy intake at a subsequent lunch meal, and improved acute glucose and insulin responses[32].
Oat meal products have also been investigated as healthy carbohydrate sources for diabetic patients; they are rich sources of soluble fiber especially β-glucan, antioxidants and bioactive compounds including carotenoids, phytic acid, phenolic acids (hydroxycinammic acids, caffeic acid, ferulic acid), flavonoids and phytosterols[33]. Studies show that consumption of oat products improves glycemic, insulinemic, and lipidemic responses in diabetic patients, and act as active ingredient reducing postprandial glycemia[34,35]. In diabetic animal models, oat products attenuated hyperglycemia-induced retinal oxidative stress, increased glycogen content of liver, decreased plasma free fatty acids and succinate dehydrogenase activity and inhibited pancreatic β-cell apoptosis as well[36].
The beneficial effects of barley and its by products for diabetic patients are mainly attributed to its high content of β-glucan; Administration of barley β-glucan extract in pre-diabetic subjects improved glucose tolerance and insulin resistance index[27]. In addition, barley may use as base of a meal; the use of barley combined with refined grains such as white rice maybe a practical way to attenuate their undesirable effects on glycemic control; in a randomized crossover study, combination of cooked barley with white rice dose-dependently reduced the area under the curves of plasma glucose and insulin concentrations, suppressed postprandial decrease of plasma desacyl ghrelin levels and consequently increased satiety[37]. The hypolipidemic properties, antioxidant and anti inflammatory activities of barley products have also been investigated[38,39]. In animal diabetic models, barley improved some features of fatty liver, decreased lipid content of the liver, increased fatty acid oxidation and adiponectin levels[40].
Several positive effects of whole wheat and its byproducts on carbohydrate and insulin metabolism have also been reported; wheat bran and whole wheat products are rich sources of dietary fiber, magnesium (main cofactor of enzymes involved in glucose metabolism and insulin secretion), potassium, phenolic acids, α-tocopherols, carotenoids and antioxidants[41]. It is believed that the majority of beneficial effects of whole wheat grain are related to bran and germ fractions; wheat bran is a main source of fiber, lignans, phenolic acid and alkylresorcinol, and beyond the health promotion of gastrointestinal tract and weight management, could improve postprandial glycemic response, glycosylated hemoglobin, lipid disorders and other cardiovascular risk factors in diabetic patients[42]. Studies showed that alkylresorcinol of wheat bran inhibited platelet activity and aggregation, decreased triglyceride de novo synthesis, and decreased cardiovascular disease risk factors[43]. Wheat germ is rich in non-digestible oligosaccharides, phytosterols, benzoquinone and flavonoids that play a potent role in induction of antioxidant and anti-inflammatory properties and modulation of immunity responses[44]. Avemar, fermented wheat germ extract, had interesting properties in the treatment of cardiovascular disease, and improved metabolic abnormalities including hyperglycemia, lipid peroxidation and abdominal fat gain[45].
Brown rice and its byproducts is another grain investigated as a functional food. Compared to white rice, brown rice has lower glycemic load and glycemic index, and higher content of fiber, vitamins and minerals, phytic acids, polyphenols, tocopherols, tocotrienols, and other bioactive compounds[46]; consumption of brown rice has benefits on glycemic control, dyslipidemia, endothelial function, abdominal obesity and liver functions in type 2 diabetic patients[47]. Studies show that γ-orizanol found in brown rice modulates high-fat diet induces oxidative stress, improves β-cell function, enhances glucose-stimulated insulin secretion and prevents the development of type 2 diabetes[48]. Germinated and pre-germinated brown rice, as more interesting functional foods, have unique components including γ-amino butyric acid, and bioactive acylated steryl glucosides with potent anti-diabetic properties; these bioactive components attenuate oxidative-induced peripheral nervous system, prevent diabetic neuropathy, inhibit oxidative-induced pancreatic β-cell apoptosis and enhance insulin secretion[49-51]. Bran rice, a byproduct of brown rice, contains within 31% fiber (mainly insoluble fiber), β-glucan, pectin, tocopherols, orizanol, ferulic acid, lutein, xanthine, vitamin K, thiamin, niacin, pantothenic acid, α-lipoic acid, coenzyme Q10 and other nutraceuticals; administration of bran rice in diabetic patients reduced glycosylated hemoglobin, LDL-C and total cholesterol as well as increased HDL-C[52].
In conclusion, replacement of whole grain and cereal-based products with refined grains in diet planning may be an effective and practical strategy for MNT in type 2 diabetic patients; this approach beyond the improvement of glycemic control, leads to more benefits for management of other aspects of diabetes, attenuation of diabetes-induced metabolic disorders, and prevents long-term complications especially atherosclerosis and cardiovascular disease.
PHYTOCHEMICAL-RICH FRUITS AND VEGETABLES
Fruits and vegetables are rich sources of dietary fiber (soluble and insoluble fiber), vitamins, and various phytochemicals and play a vital role in health promotion and prevention of chronic disease[53]. Dietary modification based on fruits and vegetables certainly is a definitely important strategy for management of type 2 diabetes and prevention of its complications; several studies indicate that regular consumption of various fruits and vegetables in diabetic patients can lead to an improved glycemic control, reduced HbA1c and triglyceride levels, enhanced antioxidant defense system, attenuated oxidative stress and inflammatory markers, decreased risk of diabetic retinopathy, and a lower burden of carotid atherosclerosis[54-57]. Since various fruits and vegetables provide many different micronutrients and bioactive compounds, consumption of varied fruits and vegetables is mainly recommended; it should be noted that the color of fruits and vegetables reflects predominant pigmented phytochemicals, and considering the colors in selection of these food groups provide a wide range of nutraceuticals. In Table 1, some phytochemical-rich fruits and vegetables, their bioactive compounds and favorable effects on diabetic related conditions are reviewed. Studies showed that tomato and its by products, as main sources of lycopene, β-carotene, flavonoids and other bioactive components, could attenuate blood pressure and dyslipidemia, decrease cardiovascular risk factors and enhance antioxidant defense system; other sources of lycopene and carotenoids such as grapefruit and watermelon have also beneficial properties to regulate lipid and lipoprotein metabolism, blood pressure and vascular function. Anthocyanins-rich fruits including red apple, berries family, grapes, cherries, red cabbage, and pomegranate have mainly hypoglycemic effects (↓ digestion and absorption of dietary carbohydrates, ↓ postprandial glycemic response and ↓ glycosylated hemoglobin) as well as protective properties against oxidative damages (Table 1).
Table 1.
Bioactive compounds and functional properties of some of favorable fruits and vegetables
| Ref. | Possible functional properties in diabetes | Main bioactive components and phytochemicals | Fruits and vegetables |
| [58-62] | ↓ Systolic and diastolic blood pressure ↑ apolipoprotein a1 and HDL-C ↓ LDL oxidation, improve diabetes-induced lipid disorders ↓ cardiovascular risk factors ↓ aldose reductase activity and cataract ↑ antioxidative enzymes activity | Lycopene, β-carotene, flavonoids, anthocyanins, phytoan, phyto flava, quercetin, kampferol | Tomato and its by products |
| [63-65] | ↓ Triglyceride levels, enhance endogenous antioxidant defense system, regulation of appetite | Lycopene, pectin, naringin, hesperidin | Grapefruit |
| [66-69] | ↑ Nitric oxide biosynthesis, improve endothelial function ↓ blood pressure ↑ plasma arginine levels and consequently ↓ insulin resistance and adipocyte size | Lycopene, carotenoids, cytrolin | Watermelon |
| [70-73] | ↓ Absorption of dietary carbohydrate ↓ postprandial glycemia, improve pancreatic β-cell function ↓ free radical generation ↓ lipid peroxidation ↑ plasma total antioxidant capacity, prevent vascular damage, improve dyslipidemia | Soluble fiber, quercetin, catechins, epicatechin, P-cumaric acid, chlorogenic acid, gallic acid, phlordizin, procyanidins | Red apple, apple peel, apple and its by products |
| [74-81] | Glycemic control, inhibit α-glucosidase and α-amylase activity ↓ digestion and absorption of dietary carbohydrates ↓ insulin resistance, improve dyslipidemia ↓ postprandial oxidative stress ↓ lipid peroxidation ↑ plasma total antioxidant capacity ↓ systolic blood pressure ↑ antioxidative enzymes activity ↑ adipocytes lipolysis ↓ inflammatory processes, modulation of peroxisome proliferator-activated receptors | Anthocyanins, tannins, ellagitanins, α-carotene, β-carotene, lutein, delphinidins, pelargonidins, ciyanidins, catechins, hydroxy-cinnamic acid | Berries; cranberry, blackberry, black raspberry, blueberry, red raspberry, strawberries |
| [82-86] | Protective effects on vascular system ↓ platelet hyperactivity and aggregation ↓ cardiovascular diseases ↓ oxidative damage ↓ rennin-angiotensin activity ↑ production of nitric oxide ↓ blood pressure ↑ bone-marrow-derived endothelial progenitor cells | Anthocyanins, resveratrol | Grapes, grape by products |
| [87-91] | ↓ Hyperglycemia ↓ HbA1c, improve lipid disorders, anti-inflammatory properties (inhibit cyclooxygenase) ↓ abdominal fat ↓ microalbuminuria, improve metabolic syndrome and fatty liver features ↓ oxidative stress ↓ production of cytokines, induction of PPARγ ↓ diabetic neuropathy | Anthocyanins, quercetin, hydroxy-cinnamic acid, carotenoids, melatonin, phenolic acids, gallic acid, lutein, xanthine, β-carotene | Cherries |
| [92-95] | ↓ Hyperglycemia, attenuate hyperglycemia-induced metabolic disorders ↓ lipid peroxidation, induction of gluthathione reductase, glutathione peroxidase, superoxide dismutase, delay progression of nephropathy ↓ inflammatory processes, improve dyslipidemia | Isothiocyanates, anthocyanins (red cabbage), carotenoids, lutein, β-carotene | Cabbage, Cauliflower |
| [96-100] | ↓ Hyperglycemia ↑ endothelial nitric oxide synthase activity, inhibit angiotensin converting enzyme ↓ blood pressure, improve vascular function ↓ cholesterol and atherogenic lipids ↓ lipid peroxidation ↓ progression of atherosclerosis ↑ plasma total antioxidant capacity, modulate activation of PPARγ and nuclear factor κB ↑ activity of paraxonase 1 and HDL-C levels ↓ serum resistin levels and ameliorate obesity-induced insulin resistance | Anthocyanins, tannins, catechins, gallocatechins, punicalagin acid, ellagic acid, gallic acid, oleanolic acid, ursolic acid, uallic acid | Pomegranate and its by products, pomegranate peel and seeds |
| [101-105] | ↓ Hyperglycemia, induce insulin secretion from β-cell ↓ blood pressure, inhibit enzyme involved in cholesterol biosynthesis, improve dyslipidemia, prevent atherosclerosis ↓ lipid peroxidation ↓ platelet hyperactivity and aggregation, regulate glycolysis, gluconeogenesis and carbohydrate metabolism pathways ↑ insulin sensitivity | Allyl sulfors, flavonoids, quercetin, dihydroflavonols, anthocyanins (red onion) | Garlic, onions |
| [106-111] | ↓ Endothelial macrophage activation ↓ hyperactivity and aggregation of platelet, improve vascular function ↓ oxidative stress and inhibit stress-sensitive signaling pathways ↓ digestion of dietary lipids, improve dyslipidemia ↓ pro-inflammatory cytokines ↓ lipid peroxidation | Lutein, xanthine, α-cryptoxanthin, β-cryptoxanthin, naringenin, hesperidin, β-carotene, phytosterols | Citrus fruits |
| [112-113] | ↓ Free radical generation and lipid peroxidation, binding to bile acids ↑ cholesterol excretion, improve lipid profile ↑ plasma total antioxidant capacity | Lutein, betaine, violaxanthine, opioid peptides (rubisculins), P-cumaric acid, ferulic acid | Spinach |
| [114-115] | Improve glycemic and insulinemic response ↓ systemic inflammation ↓ cardiovascular disease risk factors | Carotenoids, pectin, oleic and linolenic acids | Pumpkin |
| [116] | Improve hyperglycemia and dyslipidemia ↑ adiponectin, antioxidant and anti-inflammatory effect | Fiber, polyphenols, chlorogenic acid, flavonoids, anthocyanins | Plums |
| [117-119] | Improve dyslipidemia, anti-inflammatory properties ↓ lipid peroxidation ↑ plasma total antioxidant capacity | Soluble fiber (pectin), α-carotene, β-carotene lutein, phenolic acids, stilbenes | Carrots |
| [120-122] | Inhibit α-amylase ↓ postprandial glycemia ↑ glycogen synthesis, improve dyslipidemia ↓ lipid peroxidation, protective effect against diabetic nephropathy | Carotenoids, quercetin, kampferol, gallic acid, caffeic acid, catechins, tannins, mangiferin | Mango |
| [123-127] | Regulate carbohydrate metabolism (↑ glucokinase and glucose-6-phosphate dehydrogenase activity ↓ glucose-6-phosphatase activity) ↓ lipid peroxidation ↓ protein carbonylation ↑ antioxidant enzyme activity, improve metabolic syndrome features ↑ insulin sensitivity ↓carbohydrate absorption ↓ plasma free fatty acid | Anthocyanins, alkaloid compounds (berberine, oxycontin) | Barberry |
| [128-131] | Protective effects against diabetic neuropathy ↓ lipid peroxidation, induce antioxidant enzymes, protect liver and kidney against oxidative damage | Dietary fiber, polyphenols, acid cinnamic, melatonin | Date fruit |
| [132-133] | Improve lipid and lipoprotein metabolism ↑ insulin sensitivity ↓ blood pressure | Dietary fiber, pectin, flavonoids, gallic acid, chlorogenic acid, catechins, anthocyanins | Figs |
PPARγ: Peroxisome proliferator-activated receptor γ.
LEGUMES
Legumes (peas, beans, lentils, peanuts) are valuable sources of dietary protein, non-digestible carbohydrates including dietary fiber, resistance starches, oligosaccharides, and bioactive compounds such as functional fatty acids (linoleic acid, α-linolenic acid), isoflavones (daidzein, genistein, glycitein), phenolic acids, saponins, and phytic acid; some polyphenols including pelargonidin, cyanidin, delphinidin, and malvidin are also found in legumes[134,135]. Legumes are considered a component of a healthy diet and there is much evidence showing that regular consumption of legumes has protective effects against obesity, type 2 diabetes, and cardiovascular disease[136]. Legumes may be considered as an important component of a functional-foods based diet for management of type 2 diabetes. α-amylase inhibitory peptides are one of the bioactive compounds in legumes and beans that reduce digestion and absorption of dietary carbohydrates, and modulate postprandial glycemic response; other bioactive peptides of grain legumes including the 7S globulin α chain and conglutin γ have unique properties to regulate lipid metabolism and normalize lipid and lipoprotein levels[137]. Low glycemic index, high fiber and phytochemical content of legumes have made them functional food for diabetic patients.
Lentils (Lens culinaris), the most consumed legume grains, are rich sources of dietary fiber, slowly digestible starch and resistant starch, tannins, β-glucan, functional antioxidant ingredients, a wide range of phenolic acids including gallic acid, proanthcyanidins, prodelphinidin, procyanidins, catechins, epicatechin, kampferol, quercetin, cinapic acid and apigenin[138]. Studies show that bioactive proteins of lentil reduce plasma levels of LDL-C, triglyceride content of the liver, and adipose tissue lipoprotein lipase activity; moreover, polyphenols of lentil could prevent angiotensin II-induced hypertension, and pathological changes including vascular remodeling and vascular fibrosis[139,140].
Beans are also other important legume grains in the human diet with high content of fiber, phytate, ω3 fatty acids, antioxidants, phenolic compounds. The hypoglycemic effect of beans (via inhibition of α-amylase and β-glucosidase activity) has been reported as being similar to those of anti-diabetic drugs[141-143]. Including beans (pinto, dark red kidney, black beans) in diet planning for type 2 diabetic patients effectively helps weight management, attenuates postprandial glycemic response, and improves dyslipidemia[144-146].
Soybean, a rich source of unique phytoesterogens (genistein, daidzein, glycitein), is another important functional food which has been considered in diabetes; the isoflavones and bioactive peptides of soybean have favorable effects on glycemic control and insulin sensitivity, dyslipidemia, and kidney function[147-149]. It seems that the anti-diabetic effects of soybean mainly occur through interaction with estrogen receptors (ERs); studies show that soy isoflavones selectively bind to both α and β estrogen receptors; ERα is considered as key modulator of glucose and lipid metabolism, and regulate insulin biosynthesis and secretion as well as pancreatic β-cell survival[150]. Soy protein could induce insulin sensitivity and improve lipid homeostasis via activation of peroxisome proliferator-activated receptor and liver X receptors, and inhibition of the sterol regulatory element binding protein-1c[151]. Regular consumption of soy products could help diabetic patients in the management of dyslipidemia[152]. Soy protein and isoflavones decrease production of atherogenic apolipoproteins such as apo B, increase biosynthesis of HDL-C, induce LDL-C receptors, increase biosynthesis and excretion of bile acids, decrease gastrointestinal absorption of steroids, induce favorable changes in hormonal status, including the insulin to glucagon ratio, and thyroid hormones which lead to improvement of dyslipidemia[153,154]. Recently two bioactive peptides, identified in glycinin (a main soy protein), have unique hypolipidemic properties. These peptides inhibit 3-hydroxy-3methyl glutaryl CoA reductase, key enzyme involved in cholesterol biosynthesis. β-conglycinin, another main soy bioactive protein with anti-atherogenic properties via regulation of lipogenesis, decease liver lipogenic enzyme activity, inhibits fatty acid biosynthesis in liver, and facilitates fatty acid β-oxidation; other biological activities of soy peptides include antioxidant, anti-inflammatory, and hypotensive effect[155].
Another feature of soybean and soy products as well as other legumes which may highlight them as main part of a functional foods-based diet, is their established effectiveness in weigh management; since the overweight and obesity are the common problems in diabetic patients and main contributors in development of insulin resistance, benefit from anti-obesity properties of legumes is considered another key approach in these patients. Thermogenic effects, induction of satiety through some important appetite regulatory gut peptides, mediation in gene expression and secretion of key adipocytokines such as leptin and adiponectin, as well as inhibitory effects on proliferation and differentiation of adipocytes are some of the mechanisms that could explain the role of legumes on weight management[140,156-159]. In conclusion, considering the potential benefits of legumes and its by products, regular consumption of these functional foods may be an effective strategy for management of various aspects of type 2 diabetes.
NUTS
Based on current evidence, nuts may play a protective effect against cardiovascular disease risk factors. Almonds, pistachios, walnuts and hazelnuts are commonly used nuts; these functional foods are considered as rich sources of high-biological value proteins, bioactive peptides, functional fatty acids (mono and poly unsaturated fatty acids), fiber, phytosterols, polyphenols, tocopherols and other antioxidant vitamins; the antioxidative effect of nuts mainly is related to a high content of α and γ tocopherol, phenolic acids, melatonin, oleic acid and selenium, while the anti-inflammatory effect is related to ellagic acid, α-linolenic acid and magnesium[160,161].
Most current evidence reveals that consumption of nuts in type 2 diabetic patients other than improving the overall diet quality also has beneficial effects on postprandial glycemic response following high-carbohydrate meals, attenuates postprandial oxidative stress and inflammatory processes, normalizes lipid and lipoprotein levels and decreases lipid atherogenicity, and improves insulin resistance[162,163]. Moreover, habitual intake of nuts could help to effectively manage weight especially in diabetic patients; the anti-obesity effects of nuts investigated in some studies may be attributed to thermogenic effects, induction of satiety, decreased dietary fat absorption, and increased fat excretion; bioactive components of nuts also modulate regulatory appetite neurotransmitters and adipose tissue metabolism, as well as decrease proliferation and differentiation of adipocytes, inhibit lipogenesis and induce fatty acid β-oxidation[164,165]. Studies show that consumption of nuts effectively decreases serum levels of high-sensitivity C-reactive protein; a well measure of systemic low-grade inflammation, interleukin 6 (a potent pro-inflammatory cytokine) and fibrinogen while increase plasma concentration of adiponectin, a potent anti-inflammatory cytokine released from adipose tissue; dietary patterns, high in nuts, were also related to lower levels of soluble inflammatory and cardiovascular risk markers including intercellular adhesion molecule 1 and vascular cell adhesion molecule 1[166,167]. Another beneficial effect of nuts which is important especially in diabetic patients is favorably influence on endothelial function; high content of L-arginine, a main precursor of nitric oxide, as well as antioxidants and polyphenols could contribute to this effect[161].
In conclusion, it seems that a diet enriched with nuts may be an effective strategy to improve glycemic control and prevent cardiovascular disease in type 2 diabetic patients.
OTHER BENEFICIAL FUNCTIONAL FOODS AND BIOACTIVE COMPONENTS FOR DIABETIC PATIENTS
Although there are a large number of natural foods, nutraceuticals or bioactive components that could be considered as functional ingredients and have beneficial effects for diabetes management, addressing all these issues is beyond the scope of this article. Table 2 shows some of these potential functional foods including dairy products and probiotics, fish meat, green tea, spices are presented.
Table 2.
Bioactive compounds and functional properties of some of favorable functional foods
| Ref. | Possible functional properties in diabetes | Main bioactive components and nutraceuticals | Functional foods |
| [168-179] | Improve the features of metabolic syndrome, modulate gut microbiota, regulate satiety and food intake ↑ adiponectin, modulate adipocytokines, induce thermogenesis, lipolysis and β-oxidation ↑ dietary fat excretion ↓ adiposity and body weight ↓ oxidative stress and inflammatory markers, hypo-lipidemic and anti-thrombotic effects ↑ insulin sensitivity, modulate immune responses in diabetic patients ↑ total antioxidant capacity ↓ lipid peroxidation ↓ HbA1c | Calcium, vitamin B, bioactive proteins such as casein and whey, immunoglobulines, bioactive peptides (α- and β-lactorphines, lactoferrin, lactoferricin, α-lactalbumin, β-lactoglobulin, growth factors), conjugated linoleic acids, lactic acid bacteria and bifidobacteria | Dairy products and probiotics |
| [180-185] | Improve hypertriglyceridemia and hypertension ↓ cardiovascular disease ↓ insulin resistance and inflammation, improve glycemic management ↓ proteinuria ↓ oxidative stress, inhibit lipogenesis and induce lipolysis, induce PPARα and PPARβ ↓ adiposity and weight management ↑ thermogenesis and energy expenditure, inhibit angiotensin converting enzyme and modulate blood pressure | Bioactive peptides, antioxidant compounds, ω3 fatty acids (docosahexaenoic acid, eicosapentaenoic acid), selenium, taurine | Fish and seafood |
| [186-189] | Regulate cholesterol metabolism ↓ LDL oxidation, protect vascular endothelium against atherogenesis, inhibit platelet aggregation ↓ atherosclerosis development ↓ pro-inflammatory cytokines, activate PPARγ, improve sub-clinical inflammation | Oleic acid, ω3 fatty acids, Flavonoids, cinnamic acid, benzoic acid, lignans, cumaric acid, ferulic acid, tocopherols, carotenoids, oleuropein, oleocanthal | Olive oil |
| [190-193] | Promote endogenous antioxidant defense system, induce superoxide dismutase and catalase ↓ lipid peroxidation, improve glycemic control ↑ insulin sensitivity ↓ gluconeogenesis ↑ glycogen content ↓ glycation of collagen and fibrosis, protect cardiac muscle, regulate lipid metabolism as well as adipose tissue metabolism, inhibit lipogenic enzymes ↓ satiety ↑ thermogenesis ↓ proliferation and differentiation of adipocytes ↓ pro-inflammatory cytokines ↓ monocyte chemotactic protein-1 | Polyphenols, phenolic acids, catechins, epigallocatechin-3-gallat, chlorophyll, carotenoids, pectin, plant sterols | Green tea |
| [194-196] | ↑ Iinsulin sensitivity, improve peripheral uptake of glucose, increase glycolysis and gluconeogenesis, hypoglycemic and hypolipidemic effects, antioxidant and anti-inflammatory properties | Cinnamaldehyde, cinnamic acid, coumarin, catechins, epicatechin, procyanidins B-2 | Cinnamon |
| [197-199] | Inhibit enzymes involved in inflammation including cyclooxygenase-2, lipoxygenase, and nuclear factor κB, inhibit α-glucosidase and α-amylase activity ↓ postprandial glycemic response ↓ proteinuria, activate PPARγ and regulate carbohydrate and lipid metabolism, prevent diabetic cataract | Curcuminoids, stigmasterol, β-sitosterol, 2-hydroxy methyl anthraquinone, bioactive peptide turmerin | Turmeric |
| [200-203] | Attenuate oxidative stress, protective effects against oxidative damage ↓ serum creatinine and urea, improve dyslipidemia ↓ atherogenic lipoprotein levels ↓ lipid peroxidation in renal tissue, inhibit α-glucosidase activity ↓ carbohydrate digestion and absorption, protect liver against diabetes-induced oxidative damage | Tannins, flavonoids, anthocyanins, phenolic acid, gallic acid | Sumac |
PPAR: Peroxisome proliferator-activated receptor.
CONCLUSION
Type 2 diabetes is a complicated metabolic disorder with both short- and long-term undesirable complications as well as various pathogenic conditions including dyslipidemia, vascular dysfunction, oxidative stress, sub-clinical inflammation, and altered signaling pathways. Ineffectiveness of the current medical treatments in management of long-term diabetes complications confirms that other complementary approaches are required; the use of functional foods and bioactive compounds is one of these new approaches. Functional foods and their bioactive compounds could attenuate carbohydrate metabolism and hyperglycemia, improve pancreatic β-cell function and insulin secretion as well as insulin resistance, regulate lipid and lipoprotein metabolism and adipose tissue metabolism, modulate oxidative/antioxidative balance and inflammatory processes, improve weight management and prevent micro and macro vascular complications.
Considering the beneficial properties of functional foods, it seems that diet planning based on these healthy foods may be considered an effective strategy for management of various aspects of diabetes and promotion of health in diabetic patients.
ACKNOWLEDGMENTS
The authors express to acknowledge the assistance given by Ms Niloofar Shiva for critical editing of English grammar and syntax. None of the authors had any personal or financial conflicts of interest.
Footnotes
Supported by Research Institute of Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
P- Reviewers: Hiroyuki T, Karoly R, Nahid P, Semir O S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
References
- 1.Santaguida PL, Balion C, Hunt D, Morrison K, Gerstein H, Raina P, Booker L, Yazdi H. Diagnosis, prognosis, and treatment of impaired glucose tolerance and impaired fasting glucose. Evid Rep Technol Assess (Summ) 2005;(128):1–11. [PMC free article] [PubMed] [Google Scholar]
- 2.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 3.Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 4.Bekyarova GY, Ivanova DG, Madjova VH. Molecular mechanisms associating oxidative stress with endothelial dysfunction in the development of various vascular complications in diabetes mellitus. Folia Med (Plovdiv) 2007;49:13–19. [PubMed] [Google Scholar]
- 5.Gadi R, Samaha FF. Dyslipidemia in type 2 diabetes mellitus. Curr Diab Rep. 2007;7:228–234. doi: 10.1007/s11892-007-0036-0. [DOI] [PubMed] [Google Scholar]
- 6.Constantino MI, Molyneaux L, Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, Twigg SM, Yue DK, Wong J. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36:3863–3869. doi: 10.2337/dc12-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd A, Sawyer W, Hopkinson P. Impact of long-term complications on quality of life in patients with type 2 diabetes not using insulin. Value Health. 2001;4:392–400. doi: 10.1046/j.1524-4733.2001.45029.x. [DOI] [PubMed] [Google Scholar]
- 8.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31 Suppl 1:S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 9.Dämon S, Schätzer M, Höfler J, Tomasec G, Hoppichler F. Nutrition and diabetes mellitus: an overview of the current evidence. Wien Med Wochenschr. 2011;161:282–288. doi: 10.1007/s10354-011-0888-4. [DOI] [PubMed] [Google Scholar]
- 10.Perera PK, Li Y. Functional herbal food ingredients used in type 2 diabetes mellitus. Pharmacogn Rev. 2012;6:37–45. doi: 10.4103/0973-7847.95863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahadoran Z, Mirmiran P, Hosseinpanah F, Hedayati M, Hosseinpour-Niazi S, Azizi F. Broccoli sprouts reduce oxidative stress in type 2 diabetes: a randomized double-blind clinical trial. Eur J Clin Nutr. 2011;65:972–977. doi: 10.1038/ejcn.2011.59. [DOI] [PubMed] [Google Scholar]
- 12.Bahadoran Z, Mirmiran P, Hosseinpanah F, Rajab A, Asghari G, Azizi F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Diabetes Res Clin Pract. 2012;96:348–354. doi: 10.1016/j.diabres.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Bahadoran Z, Tohidi M, Nazeri P, Mehran M, Azizi F, Mirmiran P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: a randomized double-blind clinical trial. Int J Food Sci Nutr. 2012;63:767–771. doi: 10.3109/09637486.2012.665043. [DOI] [PubMed] [Google Scholar]
- 14.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 15.Ballali S, Lanciai F. Functional food and diabetes: a natural way in diabetes prevention? Int J Food Sci Nutr. 2012;63 Suppl 1:51–61. doi: 10.3109/09637486.2011.637487. [DOI] [PubMed] [Google Scholar]
- 16.Gregori D, Gafare CE. Multifunctional food: medical evidence and methodological notes on substantiating health claims. Int J Food Sci Nutr. 2012;63 Suppl 1:29–36. doi: 10.3109/09637486.2011.653553. [DOI] [PubMed] [Google Scholar]
- 17.Rudkowska I. Functional foods for health: focus on diabetes. Maturitas. 2009;62:263–269. doi: 10.1016/j.maturitas.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Diabetes Metab Disord. 2013;12:43. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahadoran Z, Mirmiran P, Azizi F. Potential efficacy of broccoli sprouts as a unique supplement for management of type 2 diabetes and its complications. J Med Food. 2013;16:375–382. doi: 10.1089/jmf.2012.2559. [DOI] [PubMed] [Google Scholar]
- 20.Laaksonen DE, Toppinen LK, Juntunen KS, Autio K, Liukkonen KH, Poutanen KS, Niskanen L, Mykkänen HM. Dietary carbohydrate modification enhances insulin secretion in persons with the metabolic syndrome. Am J Clin Nutr. 2005;82:1218–1227. doi: 10.1093/ajcn/82.6.1218. [DOI] [PubMed] [Google Scholar]
- 21.Kallio P, Kolehmainen M, Laaksonen DE, Kekäläinen J, Salopuro T, Sivenius K, Pulkkinen L, Mykkänen HM, Niskanen L, Uusitupa M, et al. Dietary carbohydrate modification induces alterations in gene expression in abdominal subcutaneous adipose tissue in persons with the metabolic syndrome: the FUNGENUT Study. Am J Clin Nutr. 2007;85:1417–1427. doi: 10.1093/ajcn/85.5.1417. [DOI] [PubMed] [Google Scholar]
- 22.Borneo R, León AE. Whole grain cereals: functional components and health benefits. Food Funct. 2012;3:110–119. doi: 10.1039/c1fo10165j. [DOI] [PubMed] [Google Scholar]
- 23.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr. 2012;142:1304–1313. doi: 10.3945/jn.111.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venn BJ, Mann JI. Cereal grains, legumes and diabetes. Eur J Clin Nutr. 2004;58:1443–1461. doi: 10.1038/sj.ejcn.1601995. [DOI] [PubMed] [Google Scholar]
- 25.Okarter N, Liu RH. Health benefits of whole grain phytochemicals. Crit Rev Food Sci Nutr. 2010;50:193–208. doi: 10.1080/10408390802248734. [DOI] [PubMed] [Google Scholar]
- 26.Rosén LA, Silva LO, Andersson UK, Holm C, Ostman EM, Björck IM. Endosperm and whole grain rye breads are characterized by low post-prandial insulin response and a beneficial blood glucose profile. Nutr J. 2009;8:42. doi: 10.1186/1475-2891-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen RL, Cai FL, Dong JL, Hu XZ. Hypoglycemic effects and biochemical mechanisms of oat products on streptozotocin-induced diabetic mice. J Agric Food Chem. 2011;59:8895–8900. doi: 10.1021/jf200678q. [DOI] [PubMed] [Google Scholar]
- 28.Martínez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, Louk JA, Rose DJ, Kyureghian G, Peterson DA, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013;7:269–280. doi: 10.1038/ismej.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson A, Tengblad S, Karlström B, Kamal-Eldin A, Landberg R, Basu S, Aman P, Vessby B. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J Nutr. 2007;137:1401–1407. doi: 10.1093/jn/137.6.1401. [DOI] [PubMed] [Google Scholar]
- 30.He M, van Dam RM, Rimm E, Hu FB, Qi L. Whole-grain, cereal fiber, bran, and germ intake and the risks of all-cause and cardiovascular disease-specific mortality among women with type 2 diabetes mellitus. Circulation. 2010;121:2162–2168. doi: 10.1161/CIRCULATIONAHA.109.907360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson JW. Whole grains protect against atherosclerotic cardiovascular disease. Proc Nutr Soc. 2003;62:135–142. doi: 10.1079/PNS2002222. [DOI] [PubMed] [Google Scholar]
- 32.Rosén LA, Ostman EM, Björck IM. Effects of cereal breakfasts on postprandial glucose, appetite regulation and voluntary energy intake at a subsequent standardized lunch; focusing on rye products. Nutr J. 2011;10:7. doi: 10.1186/1475-2891-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadiq Butt M, Tahir-Nadeem M, Khan MK, Shabir R, Butt MS. Oat: unique among the cereals. Eur J Nutr. 2008;47:68–79. doi: 10.1007/s00394-008-0698-7. [DOI] [PubMed] [Google Scholar]
- 34.VandenLangenberg GM, Brady WE, Nebeling LC, Block G, Forman M, Bowen PE, Stacewicz-Sapuntzakis M, Mares-Perlman JA. Influence of using different sources of carotenoid data in epidemiologic studies. J Am Diet Assoc. 1996;96:1271–1275. doi: 10.1016/S0002-8223(96)00332-X. [DOI] [PubMed] [Google Scholar]
- 35.Tapola N, Karvonen H, Niskanen L, Mikola M, Sarkkinen E. Glycemic responses of oat bran products in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2005;15:255–261. doi: 10.1016/j.numecd.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Bays H, Frestedt JL, Bell M, Williams C, Kolberg L, Schmelzer W, Anderson JW. Reduced viscosity Barley β-Glucan versus placebo: a randomized controlled trial of the effects on insulin sensitivity for individuals at risk for diabetes mellitus. Nutr Metab (Lond) 2011;8:58. doi: 10.1186/1743-7075-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakuma M, Yamanaka-Okumura H, Naniwa Y, Matsumoto D, Tsunematsu M, Yamamoto H, Taketani Y, Takeda E. Dose-dependent effects of barley cooked with white rice on postprandial glucose and desacyl ghrelin levels. J Clin Biochem Nutr. 2009;44:151–159. doi: 10.3164/jcbn.08-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gremmel H, Konrad RM. [Postoperative results in alloplastic closure of diaphragmatic defects] Fortschr Geb Rontgenstr Nuklearmed. 1964;100:703–710. [PubMed] [Google Scholar]
- 39.Kofuji K, Aoki A, Tsubaki K, Konishi M, Isobe T, Murata Y. Antioxidant Activity of β-Glucan. ISRN Pharm. 2012;2012:125864. doi: 10.5402/2012/125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brockman DA, Chen X, Gallaher DD. Consumption of a high β-glucan barley flour improves glucose control and fatty liver and increases muscle acylcarnitines in the Zucker diabetic fatty rat. Eur J Nutr. 2013;52:1743–1753. doi: 10.1007/s00394-012-0478-2. [DOI] [PubMed] [Google Scholar]
- 41.Whent M, Huang H, Xie Z, Lutterodt H, Yu L, Fuerst EP, Morris CF, Yu LL, Luthria D. Phytochemical composition, anti-inflammatory, and antiproliferative activity of whole wheat flour. J Agric Food Chem. 2012;60:2129–2135. doi: 10.1021/jf203807w. [DOI] [PubMed] [Google Scholar]
- 42.Stevenson L, Phillips F, O’Sullivan K, Walton J. Wheat bran: its composition and benefits to health, a European perspective. Int J Food Sci Nutr. 2012;63:1001–1013. doi: 10.3109/09637486.2012.687366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross AB, Kamal-Eldin A, Aman P. Dietary alkylresorcinols: absorption, bioactivities, and possible use as biomarkers of whole-grain wheat- and rye-rich foods. Nutr Rev. 2004;62:81–95. doi: 10.1111/j.1753-4887.2004.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 44.Brandolini A, Hidalgo A. Wheat germ: not only a by-product. Int J Food Sci Nutr. 2012;63 Suppl 1:71–74. doi: 10.3109/09637486.2011.633898. [DOI] [PubMed] [Google Scholar]
- 45.Iyer A, Brown L. Fermented wheat germ extract (avemar) in the treatment of cardiac remodeling and metabolic symptoms in rats. Evid Based Complement Alternat Med. 2011;2011:508957. doi: 10.1093/ecam/nep090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Callegaro Mda D, Tirapegui J. [Comparison of the nutritional value between brown rice and white rice] Arq Gastroenterol. 1996;33:225–231. [PubMed] [Google Scholar]
- 47.Shimabukuro M, Higa M, Kinjo R, Yamakawa K, Tanaka H, Kozuka C, Yabiku K, Taira S, Sata M, Masuzaki H. Effects of the brown rice diet on visceral obesity and endothelial function: the BRAVO study. Br J Nutr. 2014;111:310–320. doi: 10.1017/S0007114513002432. [DOI] [PubMed] [Google Scholar]
- 48.Zhang P, Tang H, Chen K, Chen Y, Xu D. Biological variations of hematologic parameters determined by UniCel DxH 800 hematology analyzer. Arch Pathol Lab Med. 2013;137:1106–1110. doi: 10.5858/arpa.2012-0377-OA. [DOI] [PubMed] [Google Scholar]
- 49.Kozuka C, Yabiku K, Takayama C, Matsushita M, Shimabukuro M. Natural food science based novel approach toward prevention and treatment of obesity and type 2 diabetes: recent studies on brown rice and γ-oryzanol. Obes Res Clin Pract. 2013;7:e165–e172. doi: 10.1016/j.orcp.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Torimitsu M, Nagase R, Yanagi M, Homma M, Sasai Y, Ito Y, Hayamizu K, Nonaka S, Hosono T, Kise M, et al. Replacing white rice with pre-germinated brown rice mildly ameliorates hyperglycemia and imbalance of adipocytokine levels in type 2 diabetes model rats. J Nutr Sci Vitaminol (Tokyo) 2010;56:287–292. doi: 10.3177/jnsv.56.287. [DOI] [PubMed] [Google Scholar]
- 51.Usuki S, Tsai YY, Morikawa K, Nonaka S, Okuhara Y, Kise M, Yu RK. IGF-1 induction by acylated steryl β-glucosides found in a pre-germinated brown rice diet reduces oxidative stress in streptozotocin-induced diabetes. PLoS One. 2011;6:e28693. doi: 10.1371/journal.pone.0028693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng HH, Huang HY, Chen YY, Huang CL, Chang CJ, Chen HL, Lai MH. Ameliorative effects of stabilized rice bran on type 2 diabetes patients. Ann Nutr Metab. 2010;56:45–51. doi: 10.1159/000265850. [DOI] [PubMed] [Google Scholar]
- 53.Heber D. Vegetables, fruits and phytoestrogens in the prevention of diseases. J Postgrad Med. 2004;50:145–149. [PubMed] [Google Scholar]
- 54.Hegde SV, Adhikari P, M N, D’Souza V. Effect of daily supplementation of fruits on oxidative stress indices and glycaemic status in type 2 diabetes mellitus. Complement Ther Clin Pract. 2013;19:97–100. doi: 10.1016/j.ctcp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka S, Yoshimura Y, Kawasaki R, Kamada C, Tanaka S, Horikawa C, Ohashi Y, Araki A, Ito H, Akanuma Y, et al. Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology. 2013;24:204–211. doi: 10.1097/EDE.0b013e318281725e. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi K, Kamada C, Yoshimura H, Okumura R, Iimuro S, Ohashi Y, Araki A, Umegaki H, Sakurai T, Yoshimura Y, et al. Effects of total and green vegetable intakes on glycated hemoglobin A1c and triglycerides in elderly patients with type 2 diabetes mellitus: the Japanese Elderly Intervention Trial. Geriatr Gerontol Int. 2012;12 Suppl 1:50–58. doi: 10.1111/j.1447-0594.2011.00812.x. [DOI] [PubMed] [Google Scholar]
- 57.Chan HT, Yiu KH, Wong CY, Li SW, Tam S, Tse HF. Increased dietary fruit intake was associated with lower burden of carotid atherosclerosis in Chinese patients with Type 2 diabetes mellitus. Diabet Med. 2013;30:100–108. doi: 10.1111/j.1464-5491.2012.03764.x. [DOI] [PubMed] [Google Scholar]
- 58.Ali MM, Agha FG. Amelioration of streptozotocin-induced diabetes mellitus, oxidative stress and dyslipidemia in rats by tomato extract lycopene. Scand J Clin Lab Invest. 2009;69:371–379. doi: 10.1080/00365510802658473. [DOI] [PubMed] [Google Scholar]
- 59.Canene-Adams K, Campbell JK, Zaripheh S, Jeffery EH, Erdman JW. The tomato as a functional food. J Nutr. 2005;135:1226–1230. doi: 10.1093/jn/135.5.1226. [DOI] [PubMed] [Google Scholar]
- 60.Pollack A, Oren P, Stark AH, Eisner Z, Nyska A, Madar Z. Cataract development in sand and galactosemic rats fed a natural tomato extract. J Agric Food Chem. 1999;47:5122–5126. doi: 10.1021/jf9900231. [DOI] [PubMed] [Google Scholar]
- 61.Shidfar F, Froghifar N, Vafa M, Rajab A, Hosseini S, Shidfar S, Gohari M. The effects of tomato consumption on serum glucose, apolipoprotein B, apolipoprotein A-I, homocysteine and blood pressure in type 2 diabetic patients. Int J Food Sci Nutr. 2011;62:289–294. doi: 10.3109/09637486.2010.529072. [DOI] [PubMed] [Google Scholar]
- 62.Upritchard JE, Sutherland WH, Mann JI. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care. 2000;23:733–738. doi: 10.2337/diacare.23.6.733. [DOI] [PubMed] [Google Scholar]
- 63.Goldwasser J, Cohen PY, Yang E, Balaguer P, Yarmush ML, Nahmias Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: role of PPARalpha, PPARgamma and LXRalpha. PLoS One. 2010;5:e12399. doi: 10.1371/journal.pone.0012399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gorinstein S, Caspi A, Libman I, Lerner HT, Huang D, Leontowicz H, Leontowicz M, Tashma Z, Katrich E, Feng S, et al. Red grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: studies in vitro and in humans. J Agric Food Chem. 2006;54:1887–1892. doi: 10.1021/jf058171g. [DOI] [PubMed] [Google Scholar]
- 65.Owira PM, Ojewole JA. The grapefruit: an old wine in a new glass? Metabolic and cardiovascular perspectives. Cardiovasc J Afr. 2010;21:280–285. doi: 10.5830/CVJA-2010-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu G, Collins JK, Perkins-Veazie P, Siddiq M, Dolan KD, Kelly KA, Heaps CL, Meininger CJ. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr. 2007;137:2680–2685. doi: 10.1093/jn/137.12.2680. [DOI] [PubMed] [Google Scholar]
- 67.Figueroa A, Sanchez-Gonzalez MA, Perkins-Veazie PM, Arjmandi BH. Effects of watermelon supplementation on aortic blood pressure and wave reflection in individuals with prehypertension: a pilot study. Am J Hypertens. 2011;24:40–44. doi: 10.1038/ajh.2010.142. [DOI] [PubMed] [Google Scholar]
- 68.Fatema K, Habib B, Afza N, Ali L. Glycemic, non-esterified fatty acid (NEFA) and insulinemic responses to watermelon and apple in type 2 diabetic subjects. Asia Pac J Clin Nutr. 2003;12 Suppl:S53. [Google Scholar]
- 69.Martins MJ, Negrão MR, Azevedo I. Watermelon: the value of higher plasma arginine concentrations. Nutrition. 2007;23:517. doi: 10.1016/j.nut.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 70.Setorki M, Asgary S, Eidi A, Rohani AH, Esmaeil N. Effects of apple juice on risk factors of lipid profile, inflammation and coagulation, endothelial markers and atherosclerotic lesions in high cholesterolemic rabbits. Lipids Health Dis. 2009;8:39. doi: 10.1186/1476-511X-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutr J. 2004;3:5. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chai SC, Hooshmand S, Saadat RL, Payton ME, Brummel-Smith K, Arjmandi BH. Daily apple versus dried plum: impact on cardiovascular disease risk factors in postmenopausal women. J Acad Nutr Diet. 2012;112:1158–1168. doi: 10.1016/j.jand.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 73.Hyson DA. A comprehensive review of apples and apple components and their relationship to human health. Adv Nutr. 2011;2:408–420. doi: 10.3945/an.111.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaume L, Howard LR, Devareddy L. The blackberry fruit: a review on its composition and chemistry, metabolism and bioavailability, and health benefits. J Agric Food Chem. 2012;60:5716–5727. doi: 10.1021/jf203318p. [DOI] [PubMed] [Google Scholar]
- 75.Seymour EM, Tanone II, Urcuyo-Llanes DE, Lewis SK, Kirakosyan A, Kondoleon MG, Kaufman PB, Bolling SF. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J Med Food. 2011;14:1511–1518. doi: 10.1089/jmf.2010.0292. [DOI] [PubMed] [Google Scholar]
- 76.Suh JH, Romain C, González-Barrio R, Cristol JP, Teissèdre PL, Crozier A, Rouanet JM. Raspberry juice consumption, oxidative stress and reduction of atherosclerosis risk factors in hypercholesterolemic golden Syrian hamsters. Food Funct. 2011;2:400–405. doi: 10.1039/c1fo10047e. [DOI] [PubMed] [Google Scholar]
- 77.Apostolidis E, Kwon YI, Shetty K. Potential of cranberry-based herbal synergies for diabetes and hypertension management. Asia Pac J Clin Nutr. 2006;15:433–441. [PubMed] [Google Scholar]
- 78.Basu A, Fu DX, Wilkinson M, Simmons B, Wu M, Betts NM, Du M, Lyons TJ. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr Res. 2010;30:462–469. doi: 10.1016/j.nutres.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen CF, Li YD, Xu Z. [Chemical principles and bioactivities of blueberry] Yaoxue Xuebao. 2010;45:422–429. [PubMed] [Google Scholar]
- 80.Morimoto C, Satoh Y, Hara M, Inoue S, Tsujita T, Okuda H. Anti-obese action of raspberry ketone. Life Sci. 2005;77:194–204. doi: 10.1016/j.lfs.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 81.Basu A, Rhone M, Lyons TJ. Berries: emerging impact on cardiovascular health. Nutr Rev. 2010;68:168–177. doi: 10.1111/j.1753-4887.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castilla P, Echarri R, Dávalos A, Cerrato F, Ortega H, Teruel JL, Lucas MF, Gómez-Coronado D, Ortuño J, Lasunción MA. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am J Clin Nutr. 2006;84:252–262. doi: 10.1093/ajcn/84.1.252. [DOI] [PubMed] [Google Scholar]
- 83.Felice F, Zambito Y, Di Colo G, D’Onofrio C, Fausto C, Balbarini A, Di Stefano R. Red grape skin and seeds polyphenols: Evidence of their protective effects on endothelial progenitor cells and improvement of their intestinal absorption. Eur J Pharm Biopharm. 2012;80:176–184. doi: 10.1016/j.ejpb.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Gollücke AP, Ribeiro DA. Use of grape polyphenols for promoting human health: a review of patents. Recent Pat Food Nutr Agric. 2012;4:26–30. [PubMed] [Google Scholar]
- 85.Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutr Res. 2008;28:729–737. doi: 10.1016/j.nutres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 86.Vislocky LM, Fernandez ML. Biomedical effects of grape products. Nutr Rev. 2010;68:656–670. doi: 10.1111/j.1753-4887.2010.00335.x. [DOI] [PubMed] [Google Scholar]
- 87.Kim DO, Heo HJ, Kim YJ, Yang HS, Lee CY. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J Agric Food Chem. 2005;53:9921–9927. doi: 10.1021/jf0518599. [DOI] [PubMed] [Google Scholar]
- 88.Seymour EM, Lewis SK, Urcuyo-Llanes DE, Tanone II, Kirakosyan A, Kaufman PB, Bolling SF. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J Med Food. 2009;12:935–942. doi: 10.1089/jmf.2008.0270. [DOI] [PubMed] [Google Scholar]
- 89.Traustadóttir T, Davies SS, Stock AA, Su Y, Heward CB, Roberts LJ, Harman SM. Tart cherry juice decreases oxidative stress in healthy older men and women. J Nutr. 2009;139:1896–1900. doi: 10.3945/jn.109.111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ataie-Jafari A, Hosseini S, Karimi F. Effects of sour cherry juice on blood glucose and some cardiovascular risk factors improvements in diabetic women: A pilot study. Nutr Food Sci. 2008;38:355–360. [Google Scholar]
- 91.Zhou Z, Nair MG, Claycombe KJ. Synergistic inhibition of interleukin-6 production in adipose stem cells by tart cherry anthocyanins and atorvastatin. Phytomedicine. 2012;19:878–881. doi: 10.1016/j.phymed.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 92.Islam MS, Choi H. Antidiabetic effect of Korean traditional Baechu (Chinese cabbage) kimchi in a type 2 diabetes model of rats. J Med Food. 2009;12:292–297. doi: 10.1089/jmf.2008.0181. [DOI] [PubMed] [Google Scholar]
- 93.Kataya HA, Hamza AA. Red Cabbage (Brassica oleracea) Ameliorates Diabetic Nephropathy in Rats. Evid Based Complement Alternat Med. 2008;5:281–287. doi: 10.1093/ecam/nem029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sankhari JM, Thounaojam MC, Jadeja RN, Devkar RV, Ramachandran AV. Anthocyanin-rich red cabbage (Brassica oleracea L.) extract attenuates cardiac and hepatic oxidative stress in rats fed an atherogenic diet. J Sci Food Agric. 2012;92:1688–1693. doi: 10.1002/jsfa.5532. [DOI] [PubMed] [Google Scholar]
- 95.Dinkova-Kostova AT, Kostov RV. Glucosinolates and isothiocyanates in health and disease. Trends Mol Med. 2012;18:337–347. doi: 10.1016/j.molmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 96.Aviram M, Dornfeld L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis. 2001;158:195–198. doi: 10.1016/s0021-9150(01)00412-9. [DOI] [PubMed] [Google Scholar]
- 97.Basu A, Penugonda K. Pomegranate juice: a heart-healthy fruit juice. Nutr Rev. 2009;67:49–56. doi: 10.1111/j.1753-4887.2008.00133.x. [DOI] [PubMed] [Google Scholar]
- 98.Banihani S, Swedan S, Alguraan Z. Pomegranate and type 2 diabetes. Nutr Res. 2013;33:341–348. doi: 10.1016/j.nutres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 99.Mathew AS, Capel-Williams GM, Berry SE, Hall WL. Acute effects of pomegranate extract on postprandial lipaemia, vascular function and blood pressure. Plant Foods Hum Nutr. 2012;67:351–357. doi: 10.1007/s11130-012-0318-9. [DOI] [PubMed] [Google Scholar]
- 100.Medjakovic S, Jungbauer A. Pomegranate: a fruit that ameliorates metabolic syndrome. Food Funct. 2013;4:19–39. doi: 10.1039/c2fo30034f. [DOI] [PubMed] [Google Scholar]
- 101.Slimestad R, Fossen T, Vågen IM. Onions: a source of unique dietary flavonoids. J Agric Food Chem. 2007;55:10067–10080. doi: 10.1021/jf0712503. [DOI] [PubMed] [Google Scholar]
- 102.Taj Eldin IM, Ahmed EM, Elwahab H M A. Preliminary Study of the Clinical Hypoglycemic Effects of Allium cepa (Red Onion) in Type 1 and Type 2 Diabetic Patients. Environ Health Insights. 2010;4:71–77. doi: 10.4137/EHI.S5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gabler NK, Osrowska E, Imsic M, Eagling DR, Jois M, Tatham BG, Dunshea FR. Dietary onion intake as part of a typical high fat diet improves indices of cardiovascular health using the mixed sex pig model. Plant Foods Hum Nutr. 2006;61:179–185. doi: 10.1007/s11130-006-0030-8. [DOI] [PubMed] [Google Scholar]
- 104.Ashraf R, Aamir K, Shaikh AR, Ahmed T. Effects of garlic on dyslipidemia in patients with type 2 diabetes mellitus. J Ayub Med Coll Abbottabad. 2005;17:60–64. [PubMed] [Google Scholar]
- 105.Ashraf R, Khan RA, Ashraf I. Garlic (Allium sativum) supplementation with standard antidiabetic agent provides better diabetic control in type 2 diabetes patients. Pak J Pharm Sci. 2011;24:565–570. [PubMed] [Google Scholar]
- 106.Yamada T, Hayasaka S, Shibata Y, Ojima T, Saegusa T, Gotoh T, Ishikawa S, Nakamura Y, Kayaba K. Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the Jichi Medical School cohort study. J Epidemiol. 2011;21:169–175. doi: 10.2188/jea.JE20100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramful D, Tarnus E, Rondeau P, Robert Da Silva C, Bahorun T, Bourdon E. Citrus Fruit Extracts Reduce Advanced Glycation End Products (AGEs)- and H2O2-Induced Oxidative Stress in Human Adipocytes. J Agric Food Chem. 2010;58:11119–11129. doi: 10.1021/jf102762s. [DOI] [PubMed] [Google Scholar]
- 108.Mulvihill EE, Huff MW. Citrus flavonoids and the prevention of atherosclerosis. Cardiovasc Hematol Disord Drug Targets. 2012;12:84–91. doi: 10.2174/1871529x11202020084. [DOI] [PubMed] [Google Scholar]
- 109.Buscemi S, Rosafio G, Arcoleo G, Mattina A, Canino B, Montana M, Verga S, Rini G. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am J Clin Nutr. 2012;95:1089–1095. doi: 10.3945/ajcn.111.031088. [DOI] [PubMed] [Google Scholar]
- 110.Chanet A, Milenkovic D, Manach C, Mazur A, Morand C. Citrus flavanones: what is their role in cardiovascular protection? J Agric Food Chem. 2012;60:8809–8822. doi: 10.1021/jf300669s. [DOI] [PubMed] [Google Scholar]
- 111.Assini JM, Mulvihill EE, Huff MW. Citrus flavonoids and lipid metabolism. Curr Opin Lipidol. 2013;24:34–40. doi: 10.1097/MOL.0b013e32835c07fd. [DOI] [PubMed] [Google Scholar]
- 112.Bergman M, Varshavsky L, Gottlieb HE, Grossman S. The antioxidant activity of aqueous spinach extract: chemical identification of active fractions. Phytochemistry. 2001;58:143–152. doi: 10.1016/s0031-9422(01)00137-6. [DOI] [PubMed] [Google Scholar]
- 113.Moser B, Szekeres T, Bieglmayer C, Wagner KH, Mišík M, Kundi M, Zakerska O, Nersesyan A, Kager N, Zahrl J, et al. Impact of spinach consumption on DNA stability in peripheral lymphocytes and on biochemical blood parameters: results of a human intervention trial. Eur J Nutr. 2011;50:587–594. doi: 10.1007/s00394-011-0167-6. [DOI] [PubMed] [Google Scholar]
- 114.Yadav M, Jain S, Tomar R, Prasad GB, Yadav H. Medicinal and biological potential of pumpkin: an updated review. Nutr Res Rev. 2010;23:184–190. doi: 10.1017/S0954422410000107. [DOI] [PubMed] [Google Scholar]
- 115.Yoshinari O, Sato H, Igarashi K. Anti-diabetic effects of pumpkin and its components, trigonelline and nicotinic acid, on Goto-Kakizaki rats. Biosci Biotechnol Biochem. 2009;73:1033–1041. doi: 10.1271/bbb.80805. [DOI] [PubMed] [Google Scholar]
- 116.Utsunomiya H, Yamakawa T, Kamei J, Kadonosono K, Tanaka S. Anti-hyperglycemic effects of plum in a rat model of obesity and type 2 diabetes, Wistar fatty rat. Biomed Res. 2005;26:193–200. doi: 10.2220/biomedres.26.193. [DOI] [PubMed] [Google Scholar]
- 117.Potter AS, Foroudi S, Stamatikos A, Patil BS, Deyhim F. Drinking carrot juice increases total antioxidant status and decreases lipid peroxidation in adults. Nutr J. 2011;10:96. doi: 10.1186/1475-2891-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramezani A, Tahbaz F, Rasooli Sh, Rashidkhani B, Gharavi Noori A, Moslemi N, Hedayati M. Effects of ß -carotene-fortified carrot juice on the lipid profile in type-2 diabetes patients. Iranian Journal of Nutrition Sciences and Food Technology. 2010;5:57–66. [Google Scholar]
- 119.Metzger BT, Barnes DM, Reed JD. Purple carrot (Daucus carota L.) polyacetylenes decrease lipopolysaccharide-induced expression of inflammatory proteins in macrophage and endothelial cells. J Agric Food Chem. 2008;56:3554–3560. doi: 10.1021/jf073494t. [DOI] [PubMed] [Google Scholar]
- 120.Lucas EA, Li W, Peterson SK, Brown A, Kuvibidila S, Perkins-Veazie P, Clarke SL, Smith BJ. Mango modulates body fat and plasma glucose and lipids in mice fed a high-fat diet. Br J Nutr. 2011;106:1495–1505. doi: 10.1017/S0007114511002066. [DOI] [PubMed] [Google Scholar]
- 121.Masibo M, He Q. Mango Bioactive Compounds and Related Nutraceutical Properties: A Review. Food Rev. 2009;25:346–370. [Google Scholar]
- 122.Li X, Cui X, Sun X, Li X, Zhu Q, Li W. Mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Phytother Res. 2010;24:893–899. doi: 10.1002/ptr.3045. [DOI] [PubMed] [Google Scholar]
- 123.Zhou J, Zhou S, Tang J, Zhang K, Guang L, Huang Y, Xu Y, Ying Y, Zhang L, Li D. Protective effect of berberine on beta cells in streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Eur J Pharmacol. 2009;606:262–268. doi: 10.1016/j.ejphar.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 124.Fatehi M, Saleh TM, Fatehi-Hassanabad Z, Farrokhfal K, Jafarzadeh M, Davodi S. A pharmacological study on Berberis vulgaris fruit extract. J Ethnopharmacol. 2005;102:46–52. doi: 10.1016/j.jep.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 125.Fatehi-Hassanabad Z, Jafarzadeh M, Tarhini A, Fatehi M. The antihypertensive and vasodilator effects of aqueous extract from Berberis vulgaris fruit on hypertensive rats. Phytother Res. 2005;19:222–225. doi: 10.1002/ptr.1661. [DOI] [PubMed] [Google Scholar]
- 126.Gao CR, Zhang JQ, Huang QL. [Experimental study on berberin raised insulin sensitivity in insulin resistance rat models] Zhongguo Zhongxiyi Jiehe Zazhi. 1997;17:162–164. [PubMed] [Google Scholar]
- 127.Yin J, Hu R, Chen M, Tang J, Li F, Yang Y, Chen J. Effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51:1439–1443. doi: 10.1053/meta.2002.34715. [DOI] [PubMed] [Google Scholar]
- 128.Al-Farsi MA, Lee CY. Nutritional and functional properties of dates: a review. Crit Rev Food Sci Nutr. 2008;48:877–887. doi: 10.1080/10408390701724264. [DOI] [PubMed] [Google Scholar]
- 129.Saafi EB, Louedi M, Elfeki A, Zakhama A, Najjar MF, Hammami M, Achour L. Protective effect of date palm fruit extract (Phoenix dactylifera L.) on dimethoate induced-oxidative stress in rat liver. Exp Toxicol Pathol. 2011;63:433–441. doi: 10.1016/j.etp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 130.Vayalil PK. Date fruits (Phoenix dactylifera Linn): an emerging medicinal food. Crit Rev Food Sci Nutr. 2012;52:249–271. doi: 10.1080/10408398.2010.499824. [DOI] [PubMed] [Google Scholar]
- 131.Zangiabadi N, Asadi-Shekaari M, Sheibani V, Jafari M, Shabani M, Asadi AR, Tajadini H, Jarahi M. Date fruit extract is a neuroprotective agent in diabetic peripheral neuropathy in streptozotocin-induced diabetic rats: a multimodal analysis. Oxid Med Cell Longev. 2011;2011:976948. doi: 10.1155/2011/976948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Slatnar A, Klancar U, Stampar F, Veberic R. Effect of drying of figs (Ficus carica L.) on the contents of sugars, organic acids, and phenolic compounds. J Agric Food Chem. 2011;59:11696–11702. doi: 10.1021/jf202707y. [DOI] [PubMed] [Google Scholar]
- 133.Josef B, Raj S. Pharmacognostic and phytochemical properties of Ficus carica Linn: An overview. Int J Pharma Tech Res. 2011;3:8–12. [Google Scholar]
- 134.Duranti M. Grain legume proteins and nutraceutical properties. Fitoterapia. 2006;77:67–82. doi: 10.1016/j.fitote.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 135.Madar Z, Stark AH. New legume sources as therapeutic agents. Br J Nutr. 2002;88 Suppl 3:S287–S292. doi: 10.1079/BJN2002719. [DOI] [PubMed] [Google Scholar]
- 136.Flight I, Clifton P. Cereal grains and legumes in the prevention of coronary heart disease and stroke: a review of the literature. Eur J Clin Nutr. 2006;60:1145–1159. doi: 10.1038/sj.ejcn.1602435. [DOI] [PubMed] [Google Scholar]
- 137.López-Amorós ML, Hernández T, Estrella I. Estrella I. Effect of germination on legume phenolic compounds and their antioxidant activity. J Food Comp Anal. 2006;19:277–283. [Google Scholar]
- 138.Zou Y, Chang SK, Gu Y, Qian SY. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions. J Agric Food Chem. 2011;59:2268–2276. doi: 10.1021/jf104640k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Boualga A, Prost J, Taleb-Senouci D, Krouf D, Kharoubi O, Lamri-Senhadji M, Belleville J, Bouchenak M. Purified chickpea or lentil proteins impair VLDL metabolism and lipoprotein lipase activity in epididymal fat, but not in muscle, compared to casein, in growing rats. Eur J Nutr. 2009;48:162–169. doi: 10.1007/s00394-009-0777-4. [DOI] [PubMed] [Google Scholar]
- 140.Yao F, Sun C, Chang SK. Lentil polyphenol extract prevents angiotensin II-induced hypertension, vascular remodelling and perivascular fibrosis. Food Funct. 2012;3:127–133. doi: 10.1039/c1fo10142k. [DOI] [PubMed] [Google Scholar]
- 141.Barrett ML, Udani JK. A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): a review of clinical studies on weight loss and glycemic control. Nutr J. 2011;10:24. doi: 10.1186/1475-2891-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yao Y, Cheng XZ, Wang LX, Wang SH, Ren G. Major Phenolic Compounds, Antioxidant Capacity and Antidiabetic Potential of Rice Bean (Vigna umbellata L.) in China. Int J Mol Sci. 2012;13:2707–2716. doi: 10.3390/ijms13032707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Helmstädter A. Beans and diabetes: Phaseolus vulgaris preparations as antihyperglycemic agents. J Med Food. 2010;13:251–254. doi: 10.1089/jmf.2009.0002. [DOI] [PubMed] [Google Scholar]
- 144.Geil PB, Anderson JW. Nutrition and health implications of dry beans: a review. J Am Coll Nutr. 1994;13:549–558. doi: 10.1080/07315724.1994.10718446. [DOI] [PubMed] [Google Scholar]
- 145.Preuss HG. Bean amylase inhibitor and other carbohydrate absorption blockers: effects on diabesity and general health. J Am Coll Nutr. 2009;28:266–276. doi: 10.1080/07315724.2009.10719781. [DOI] [PubMed] [Google Scholar]
- 146.Thompson SV, Winham DM, Hutchins AM. Bean and rice meals reduce postprandial glycemic response in adults with type 2 diabetes: a cross-over study. Nutr J. 2012;11:23. doi: 10.1186/1475-2891-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Anderson JW, Bush HM. Soy protein effects on serum lipoproteins: a quality assessment and meta-analysis of randomized, controlled studies. J Am Coll Nutr. 2011;30:79–91. doi: 10.1080/07315724.2011.10719947. [DOI] [PubMed] [Google Scholar]
- 148.Azadbakht L, Shakerhosseini R, Atabak S, Jamshidian M, Mehrabi Y, Esmaill-Zadeh A. Beneficiary effect of dietary soy protein on lowering plasma levels of lipid and improving kidney function in type II diabetes with nephropathy. Eur J Clin Nutr. 2003;57:1292–1294. doi: 10.1038/sj.ejcn.1601688. [DOI] [PubMed] [Google Scholar]
- 149.Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 150.Gilbert ER, Liu D. Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic β-cell function. Food Funct. 2013;4:200–212. doi: 10.1039/c2fo30199g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ronis MJ, Chen Y, Badeaux J, Badger TM. Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. J Nutr. 2009;139:1431–1438. doi: 10.3945/jn.109.107029. [DOI] [PubMed] [Google Scholar]
- 152.Yang B, Chen Y, Xu T, Yu Y, Huang T, Hu X, Li D. Systematic review and meta-analysis of soy products consumption in patients with type 2 diabetes mellitus. Asia Pac J Clin Nutr. 2011;20:593–602. [PubMed] [Google Scholar]
- 153.Borradaile NM, de Dreu LE, Wilcox LJ, Edwards JY, Huff MW. Soya phytoestrogens, genistein and daidzein, decrease apolipoprotein B secretion from HepG2 cells through multiple mechanisms. Biochem J. 2002;366:531–539. doi: 10.1042/BJ20020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Potter SM. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J Nutr. 1995;125:606S–611S. doi: 10.1093/jn/125.3_Suppl.606S. [DOI] [PubMed] [Google Scholar]
- 155.Liu CF, Pan TM. Beneficial Effects of Bioactive Peptides Derived from Soybean on Human Health and Their Production by Genetic Engineering. Soybean and health. 2011:311–329. [Google Scholar]
- 156.Frigolet ME, Torres N, Uribe-Figueroa L, Rangel C, Jimenez-Sanchez G, Tovar AR. White adipose tissue genome wide-expression profiling and adipocyte metabolic functions after soy protein consumption in rats. J Nutr Biochem. 2011;22:118–129. doi: 10.1016/j.jnutbio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 157.Cheik NC, Rossi EA, Guerra RL, Tenório NM, Oller do Nascimento CM, Viana FP, Manzoni MS, Carlos IZ, Leão da Silva P, Vendramini RC, et al. Effects of a ferment soy product on the adipocyte area reduction and dyslipidemia control in hypercholesterolemic adult male rats. Lipids Health Dis. 2008;7:50. doi: 10.1186/1476-511X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang Z, Lanza E, Ross AC, Albert PS, Colburn NH, Rovine MJ, Bagshaw D, Ulbrecht JS, Hartman TJ. A high-legume low-glycemic index diet reduces fasting plasma leptin in middle-aged insulin-resistant and -sensitive men. Eur J Clin Nutr. 2011;65:415–418. doi: 10.1038/ejcn.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Segura R, Javierre C, Lizarraga MA, Ros E. Other relevant components of nuts: phytosterols, folate and minerals. Br J Nutr. 2006;96 Suppl 2:S36–S44. doi: 10.1017/bjn20061862. [DOI] [PubMed] [Google Scholar]
- 160.Kendall CW, Esfahani A, Truan J, Srichaikul K, Jenkins DJ. Health benefits of nuts in prevention and management of diabetes. Asia Pac J Clin Nutr. 2010;19:110–116. [PubMed] [Google Scholar]
- 161.Ros E. Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr. 2009;89:1649S–1656S. doi: 10.3945/ajcn.2009.26736R. [DOI] [PubMed] [Google Scholar]
- 162.Jenkins DJ, Hu FB, Tapsell LC, Josse AR, Kendall CW. Possible benefit of nuts in type 2 diabetes. J Nutr. 2008;138:1752S–1756S. doi: 10.1093/jn/138.9.1752S. [DOI] [PubMed] [Google Scholar]
- 163.Li TY, Brennan AM, Wedick NM, Mantzoros C, Rifai N, Hu FB. Regular consumption of nuts is associated with a lower risk of cardiovascular disease in women with type 2 diabetes. J Nutr. 2009;139:1333–1338. doi: 10.3945/jn.108.103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tey SL, Brown R, Gray A, Chisholm A, Delahunty C. Nuts improve diet quality compared to other energy-dense snacks while maintaining body weight. J Nutr Metab. 2011;2011:357350. doi: 10.1155/2011/357350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mattes RD, Dreher ML. Nuts and healthy body weight maintenance mechanisms. Asia Pac J Clin Nutr. 2010;19:137–141. [PubMed] [Google Scholar]
- 166.Mantzoros CS, Williams CJ, Manson JE, Meigs JB, Hu FB. Adherence to the Mediterranean dietary pattern is positively associated with plasma adiponectin concentrations in diabetic women. Am J Clin Nutr. 2006;84:328–335. doi: 10.1093/ajcn/84.1.328. [DOI] [PubMed] [Google Scholar]
- 167.Jiang R, Jacobs DR, Mayer-Davis E, Szklo M, Herrington D, Jenny NS, Kronmal R, Barr RG. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163:222–231. doi: 10.1093/aje/kwj033. [DOI] [PubMed] [Google Scholar]
- 168.Al-Salami H, Butt G, Fawcett JP, Tucker IG, Golocorbin-Kon S, Mikov M. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur J Drug Metab Pharmacokinet. 2008;33:101–106. doi: 10.1007/BF03191026. [DOI] [PubMed] [Google Scholar]
- 169.Ataie-Jafari A, Larijani B, Alavi Majd H, Tahbaz F. Cholesterol-lowering effect of probiotic yogurt in comparison with ordinary yogurt in mildly to moderately hypercholesterolemic subjects. Ann Nutr Metab. 2009;54:22–27. doi: 10.1159/000203284. [DOI] [PubMed] [Google Scholar]
- 170.Chang BJ, Park SU, Jang YS, Ko SH, Joo NM, Kim SI, Kim CH, Chang DK. Effect of functional yogurt NY-YP901 in improving the trait of metabolic syndrome. Eur J Clin Nutr. 2011;65:1250–1255. doi: 10.1038/ejcn.2011.115. [DOI] [PubMed] [Google Scholar]
- 171.Guo C, Zhang L. [Cholesterol-lowering effects of probiotics--a review] Weishengwu Xuebao. 2010;50:1590–1599. [PubMed] [Google Scholar]
- 172.Guzel-Seydim ZB, Kok-Tas T, Greene AK, Seydim AC. Review: functional properties of kefir. Crit Rev Food Sci Nutr. 2011;51:261–268. doi: 10.1080/10408390903579029. [DOI] [PubMed] [Google Scholar]
- 173.Lin MY, Yen CL. Reactive oxygen species and lipid peroxidation product-scavenging ability of yogurt organisms. J Dairy Sci. 1999;82:1629–1634. doi: 10.3168/jds.S0022-0302(99)75391-9. [DOI] [PubMed] [Google Scholar]
- 174.Liu S, Choi HK, Ford E, Song Y, Klevak A, Buring JE, Manson JE. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care. 2006;29:1579–1584. doi: 10.2337/dc06-0256. [DOI] [PubMed] [Google Scholar]
- 175.Lopitz-Otsoa F, Rementeria A, Elguezabal N, Garaizar J. Kefir: a symbiotic yeasts-bacteria community with alleged healthy capabilities. Rev Iberoam Micol. 2006;23:67–74. doi: 10.1016/s1130-1406(06)70016-x. [DOI] [PubMed] [Google Scholar]
- 176.Marteau PR. Probiotics in clinical conditions. Clin Rev Allergy Immunol. 2002;22:255–273. doi: 10.1007/s12016-002-0011-0. [DOI] [PubMed] [Google Scholar]
- 177.Pfeuffer M, Schrezenmeir J. Milk and the metabolic syndrome. Obes Rev. 2007;8:109–118. doi: 10.1111/j.1467-789X.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 178.Shab-Bidar S, Neyestani TR, Djazayery A. Efficacy of vitamin D3-fortified-yogurt drink on anthropometric, metabolic, inflammatory and oxidative stress biomarkers according to vitamin D receptor gene polymorphisms in type 2 diabetic patients: a study protocol for a randomized controlled clinical trial. BMC Endocr Disord. 2011;11:12. doi: 10.1186/1472-6823-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Teruya K, Yamashita M, Tominaga R, Nagira T, Shim SY, Katakura Y, Tokumaru S, Tokumaru K, Barnes D, Shirahata S. Fermented milk, Kefram-Kefir enhances glucose uptake into insulin-responsive muscle cells. Cytotechnology. 2002;40:107–116. doi: 10.1023/A:1023926407877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Boukortt FO, Girard A, Prost JL, Ait-Yahia D, Bouchenak M, Belleville J. Fish protein improves the total antioxidant status of streptozotocin-induced diabetes in spontaneously hypertensive rat. Med Sci Monit. 2004;10:BR397–BR404. [PubMed] [Google Scholar]
- 181.Farmer A, Montori V, Dinneen S, Clar C. Fish oil in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2001;(3):CD003205. doi: 10.1002/14651858.CD003205. [DOI] [PubMed] [Google Scholar]
- 182.Jacques H, Gascon A, Bergeron N, Lavigne C, Hurley C, Deshaies Y, Moorjani S, Julien P. Role of dietary fish protein in the regulation of plasma lipids. Can J Cardiol. 1995;11 Suppl G:63G–71G. [PubMed] [Google Scholar]
- 183.Lankinen M, Schwab U, Kolehmainen M, Paananen J, Poutanen K, Mykkänen H, Seppänen-Laakso T, Gylling H, Uusitupa M, Orešič M. Whole grain products, fish and bilberries alter glucose and lipid metabolism in a randomized, controlled trial: the Sysdimet study. PLoS One. 2011;6:e22646. doi: 10.1371/journal.pone.0022646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.McEwen B, Morel-Kopp MC, Tofler G, Ward C. Effect of omega-3 fish oil on cardiovascular risk in diabetes. Diabetes Educ. 2010;36:565–584. doi: 10.1177/0145721710372675. [DOI] [PubMed] [Google Scholar]
- 185.Pecis M, de Azevedo MJ, Gross JL. Chicken and fish diet reduces glomerular hyperfiltration in IDDM patients. Diabetes Care. 1994;17:665–672. doi: 10.2337/diacare.17.7.665. [DOI] [PubMed] [Google Scholar]
- 186.Cicerale S, Conlan XA, Sinclair AJ, Keast RS. Chemistry and health of olive oil phenolics. Crit Rev Food Sci Nutr. 2009;49:218–236. doi: 10.1080/10408390701856223. [DOI] [PubMed] [Google Scholar]
- 187.Lucas L, Russell A, Keast R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr Pharm Des. 2011;17:754–768. doi: 10.2174/138161211795428911. [DOI] [PubMed] [Google Scholar]
- 188.Madigan C, Ryan M, Owens D, Collins P, Tomkin GH. Dietary unsaturated fatty acids in type 2 diabetes: higher levels of postprandial lipoprotein on a linoleic acid-rich sunflower oil diet compared with an oleic acid-rich olive oil diet. Diabetes Care. 2000;23:1472–1477. doi: 10.2337/diacare.23.10.1472. [DOI] [PubMed] [Google Scholar]
- 189.Wardhana ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138–143. [PubMed] [Google Scholar]
- 190.Babu PV, Sabitha KE, Srinivasan P, Shyamaladevi CS. Green tea attenuates diabetes induced Maillard-type fluorescence and collagen cross-linking in the heart of streptozotocin diabetic rats. Pharmacol Res. 2007;55:433–440. doi: 10.1016/j.phrs.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 191.Fiorino P, Evangelista FS, Santos F, Motter Magri FM, Delorenzi JC, Ginoza M, Farah V. The effects of green tea consumption on cardiometabolic alterations induced by experimental diabetes. Exp Diabetes Res. 2012;2012:309231. doi: 10.1155/2012/309231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kumar B, Gupta SK, Nag TC, Srivastava S, Saxena R. Green tea prevents hyperglycemia-induced retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Ophthalmic Res. 2012;47:103–108. doi: 10.1159/000330051. [DOI] [PubMed] [Google Scholar]
- 193.Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diabetes Metab J. 2013;37:173–175. doi: 10.4093/dmj.2013.37.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Anderson RA. Chromium and polyphenols from cinnamon improve insulin sensitivity. Proc Nutr Soc. 2008;67:48–53. doi: 10.1017/S0029665108006010. [DOI] [PubMed] [Google Scholar]
- 195.Gruenwald J, Freder J, Armbruester N. Cinnamon and health. Crit Rev Food Sci Nutr. 2010;50:822–834. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- 196.Kirkham S, Akilen R, Sharma S, Tsiami A. The potential of cinnamon to reduce blood glucose levels in patients with type 2 diabetes and insulin resistance. Diabetes Obes Metab. 2009;11:1100–1113. doi: 10.1111/j.1463-1326.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- 197.Honda S, Aoki F, Tanaka H, Kishida H, Nishiyama T, Okada S, Matsumoto I, Abe K, Mae T. Effects of ingested turmeric oleoresin on glucose and lipid metabolisms in obese diabetic mice: a DNA microarray study. J Agric Food Chem. 2006;54:9055–9062. doi: 10.1021/jf061788t. [DOI] [PubMed] [Google Scholar]
- 198.Khajehdehi P, Pakfetrat M, Javidnia K, Azad F, Malekmakan L, Nasab MH, Dehghanzadeh G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol. 2011;45:365–370. doi: 10.3109/00365599.2011.585622. [DOI] [PubMed] [Google Scholar]
- 199.Lekshmi PC, Arimboor R, Raghu KG, Menon AN. Turmerin, the antioxidant protein from turmeric (Curcuma longa) exhibits antihyperglycaemic effects. Nat Prod Res. 2012;26:1654–1658. doi: 10.1080/14786419.2011.589386. [DOI] [PubMed] [Google Scholar]
- 200.Rayne S, Mazza G. Biological activities of extracts from sumac (Rhus spp.): a review. Plant Foods Hum Nutr. 2007;62:165–175. doi: 10.1007/s11130-007-0058-4. [DOI] [PubMed] [Google Scholar]
- 201.Shafiei M, Nobakht M, Moazzam AA. Lipid-lowering effect of Rhus coriaria L. (sumac) fruit extract in hypercholesterolemic rats. Pharmazie. 2011;66:988–992. [PubMed] [Google Scholar]
- 202.Giancarlo S, Rosa LM, Nadjafi F, Francesco M. Hypoglycaemic activity of two spices extracts: Rhus coriaria L. and Bunium persicum Boiss. Nat Prod Res. 2006;20:882–886. doi: 10.1080/14786410500520186. [DOI] [PubMed] [Google Scholar]
- 203.Pourahmad J, Eskandari MR, Shakibaei R, Kamalinejad M. A search for hepatoprotective activity of aqueous extract of Rhus coriaria L. against oxidative stress cytotoxicity. Food Chem Toxicol. 2010;48:854–858. doi: 10.1016/j.fct.2009.12.021. [DOI] [PubMed] [Google Scholar]


