Abstract
The traditional perception of adipose tissue as a storage organ of fatty acids has been replaced by the notion that adipose tissue is an active endocrine organ, releasing various adipokines that are involved in the pathogenesis of obesity-related metabolic disturbances. Obesity is a well-known risk factor for atherosclerosis, and accelerates atherosclerosis by many mechanisms such as increase in blood pressure and glucose level, abnormal lipid profiles, and systemic inflammation. Furthermore, growing evidence suggests that some adipokines directly mediate the process of atherosclerosis by influencing the function of endothelial cells, arterial smooth muscle cells, and macrophages in vessel walls. In obese patients, the secretion and coordination of such adipokines is abnormal, and the secretion of specific adipokines increases or decreases. Accordingly, the discovery of new adipokines and elucidation of their functions might lead to a new treatment strategy for metabolic disorders related to obesity, including cardiovascular diseases.
Keywords: Adipose tissue, Adipokine, Obesity, Atherosclerosis, Inflammation
Core tip: This review summarizes recent laboratory and clinical studies on the influence of various adipokines, including adiponectin, resistin, adipocyte fatty acid binding protein, omentin-1, and chemerin, on the development of atherosclerosis.
INTRODUCTION
Obesity is an important risk factor for atherosclerosis, but the underlying mechanism for this association is poorly understood. Adipose tissue was considered to be a store of surplus energy, but is now recognized as an independent and active endocrine organ. Various adipokines, such as leptin (a protein secreted by fat cells), tumor necrosis factor-α (TNF-α), resistin, and adiponectin significantly affect obesity-related metabolic diseases by controlling fat metabolism, energy homeostasis, and insulin sensitivity[1]. Independent of their effects on glucose and fat metabolism, some adipokines have been regarded recently as direct links between obesity and atherosclerosis because of their influence on the function of endothelial cells, arterial smooth muscle cells, and macrophages in vessel walls[2] (Figure 1). The identification of a novel adipokine that regulates the atherosclerotic process might provide new opportunities for developing more effective approaches for preventing cardiovascular disease. This review will focus on adipokines that mediate obesity and atherosclerosis, including adiponectin, resistin, adipocyte fatty acid binding protein (A-FABP), omentin-1, and chemerin.
Figure 1.
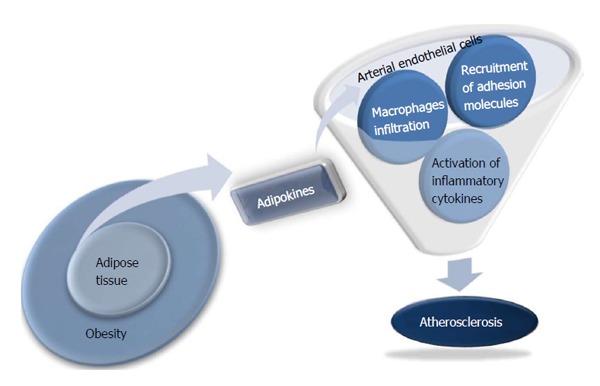
Novel function of adipokines as a direct link between obesity and atherosclerosis.
ADIPONECTIN
Adiponectin was the first 30-kDa protein cloned from fat tissues[3]. Adiponectin is a metabolically active adipokine that is inversely associated with obesity, insulin resistance, and atherosclerosis[4,5]. Adiponectin promotes fatty acid oxidation through the phosphorylation of 5-AMP-activated protein kinase (AMPK), thereby stimulating acetyl-CoA carboxylase. The adiponectin receptors AdipoR1 and AdipoR2 are responsible for adiponectin signaling and biological function. Yamauchi et al[6] reported that insulin resistance occurred in AdipoR1/R2 knockout mice, but when AdipoR1 or AdipoR2 were overexpressed in the liver by using adenovirus, glucose metabolism improved in terms of increase in AMPK vitality and peroxisome proliferator-activated receptors α expression. Adiponectin is a metabolically active adipokine which has anti-inflammatory, antiatherogenic, and antidiabetic properties[7] and is therefore inversely associated with obesity, insulin resistance, and atherosclerosis. Hypoadiponectinemia has been established as an independent risk factor for type 2 diabetes and cardiovascular disease (CVD)[8]. We previously showed that, after adjusting for age, sex, obesity, history of impaired fasting glucose or impaired glucose tolerance, hypertension, and dyslipidemia, lower baseline serum adiponectin concentrations are associated significantly with the development of type 2 diabetes and metabolic syndrome[9]. On the other side, the Health Professionals Follow-Up Study showed that high plasma adiponectin levels were associated with a lower risk of myocardial infarction in men during 6 years of follow-up studies[10].
Experimental studies have shown that adiponectin plays a protective role against the development of inflammation and atherosclerosis. Ouchi et al[11] demonstrated that adiponectin specifically suppressed TNF-α-induced nuclear factor κ light chain enhancer of activated B cells (NF-κB) activation in human aortic endothelial cells (HAECs) through a cAMP-dependent pathway. Furthermore, adiponectin suppressed TNF-α-mediated induction of adhesion molecule expression in HAECs. Recently, we reported that serum adiponectin levels had a significant negative correlation with vascular inflammation as indicated by the mean target to background ratio (TBR), suggesting a cardio-protective effect of adiponectin[12].
RESISTIN
Resistin was originally discovered as an adipokine with a possible link between obesity and insulin resistance in rodents[13]. In contrast to rodents, human resistin is expressed primarily in inflammatory cells and has been shown to be involved in obesity-related subclinical inflammation, atherosclerosis, and CVD[14]. Reilly et al[15] showed that circulating resistin levels are correlated with inflammation markers and are predictive of coronary atherosclerosis, as measured by coronary artery calcification scores, independent of C-reactive protein. Kawanami et al[16] found that resistin induces the expression of adhesion molecules, such as vascular cellular adhesion molecule-1 and intercellular adhesion molecule-1 and that adiponectin inhibit the effect of resistin in vascular endothelial cells. Lee et al[17] observed that resistin promotes foam cell formation via the dysregulation of scavenger receptors macrophages. In men with acute myocardial infarction, a multivariate model revealed that obesity and C-reactive protein were independent variables associated with higher resistin levels[18]. In a cross-sectional study of 3193 Chinese subjects, resistin was more significantly associated with fibrinolytic and inflammatory markers than with obesity or insulin resistance[19]. Moreover, Weikert et al[20] reported that individuals in the highest quartile of resistin levels had a significantly increased risk of myocardial infarction compared with those in the lowest quartile of resistin levels after adjustment for cardiovascular risk factors, including C-reactive protein (RR = 2.09; 95%CI: 1.01-4.31) in 26490 middle-aged subjects. Among 397 South Korean patients with acute myocardial infarction, high resistin level was an significant predictor for all-cause mortality, independent of other confounding risk factors[21]. We also showed that serum resistin levels were positively correlated with vascular inflammation measured using 18F-fluoro-deoxyglucose positron emission tomography[12]. These studies suggest that resistin may represent a novel linkage of metabolic signals, inflammation, and atherosclerosis.
ADIPOCYTE FATTY ACID BINDING PROTEIN
A-FABP is a cytoplasmic protein that combines with saturated and unsaturated fatty acids to control the distribution of fatty acids in various inflammatory response and metabolic pathways[22]. Since Xu et al[23] established that the serum concentration of A-FABP, which is synthesized in cytoplasm and secreted into serum, is significantly correlated with components of metabolic syndrome, the role of A-FABP in metabolic syndrome has been studied with renewed interest. Uysal et al[24] proved through an oral glucose tolerance test that insulin sensitivity was increased in A-FABP knock out ob/ob mice compared with control mice. In prospective studies, circulating A-FABP has been shown to predict the development of metabolic syndrome and type 2 diabetes independent of adiposity and insulin resistance[25,26].
A-FABP has been shown to be a major mediator of vulnerable plaque formation in various animal and in vitro studies. The survival rates of apoE-/- mice null for both A-FABP and mal1 were significantly higher than apoE-/- control mice, primarily because of increased stability of atherosclerotic plaques[27]. In macrophage cell lines, adenovirus-mediated over-expression of A-FABP directly induced foam cell formation by increasing intracellular lipid accumulation, which is an essential step in the formation of atherosclerotic plaques[28]. In contrast, A-FABP-/- macrophages displayed significantly decreased intracellular cholesterol ester accumulation in vitro[29] and suppressed production of inflammatory cytokines, such as TNF-α, monocyte chemoattractant protein-1, and interleukin (IL)-6, compared with wild-type controls[30]. Furthermore, Furuhashi et al[31] reported that an orally active small molecule inhibitor of A-FABP was an effective therapeutic agent against severe atherosclerosis in mouse models. Recently, a few clinical studies have shown that circulating A-FABP levels are closely related to the development of atherosclerosis in humans. In Korean subjects in whom coronary angiograms were performed for evaluation of chest pain, serum A-FABP levels increased as the number of stenotic coronary arteries increased[32]. Serum A-FABP was shown to be independently associated with carotid intima-media thickness (IMT) in Chinese women after adjusting for other risk factors, including age, obesity, and blood pressure[33]. In patients with coronary artery disease recruited to undergo elective percutaneous coronary intervention, Miyoshi et al[34] showed that increased serum A-FABP levels were significantly associated with a greater coronary plaque burden as quantified by intravascular ultrasound. After adjusting for other cardiovascular risk factor in South Korean men without cardiovascular disease or diabetes, we reported that circulating A-FABP levels were independently associated with vascular inflammation as measured by maximum TBR values[35], suggesting A-FABP as a promising key link between different metabolic pathways of adiposity and inflammation.
OMENTIN-1
Omentin is a visceral fat-specific adipokine discovered through expressed sequence tag analysis[36] that has paracrine and autocrine roles in improving insulin sensitivity. Yang et al[37] demonstrated that the addition of recombinant omentin stimulated glucose uptake in human adipocytes via the activation of Akt phosphorylation. Recent studies showed that omentin increased insulin signal transduction and that it was significantly negatively correlated with metabolic risk factors, including obesity and hyperglycemia, thereby suggesting a beneficial role in energy homeostasis[38-40]. In human clinical studies, it has been suggested that serum omentin-1 levels were significantly decreased in metabolically unhealthy states, such as metabolic syndrome, types 2 diabetes mellitus, and polycystic ovarian syndrome[38-40].
Expression of the omentin gene in interstitial and endothelial cells suggests multi-functionality[41,42]. Fain et al[43] were the first to demonstrate the predominant expression of omentin mRNA in human epicardial fat, suggesting that omentin might influence coronary atherogenesis like other periadventitial epicardial adipokines. Some researchers reported that omentin might modulate vascular function through direct action on endothelial cells[44,45]. The vasodilating effect of omentin on isolated rat aorta, mediated by endothelium-derived nitric oxide, was first examined by Yamawaki et al[45]. Treatment of human endothelial cells with omentin prevented TNF-α-induced cyclooxygenase-2 expression by inhibiting c-Jun N-terminal kinase signaling, suggesting an anti-inflammatory function of omentin on endothelial cells[44]. Recently, several in vivo studies that might explain the mechanism underlying the connection between circulating omentin-1 and the atherosclerotic process have been published. In human endothelial cells, omentin significantly decreased C-reactive protein and TNF-α-induced NF-κB[46]. Xie et al[47] reported that adenovirus-mediated overexpression of omentin-1 attenuated arterial calcification in OPG-/- mice, suggesting that increasing concentrations of omentin-1 might be beneficial by protecting arteries. In an in vitro study, treatment of calcifying vascular smooth muscle cells (CVSMs) with omentin inhibited osteoblastic differentiation of CVSMCs via the phosphatidylinositol 3-kinase/Akt signaling pathway[48]. Very recently, Maruyama et al[49] reported that systemic delivery of an adenoviral vector expressing omentin enhanced blood flow recovery and capillary density in ischemic limbs of wild type mice. Taken together, these in vitro data suggest the possibility that lower omentin levels contribute to the development of cardiovascular disease from initiating early endothelial dysfunction to arterial calcification.
There have been many clinical studies examining the significance of correlations of circulating omentin-1 levels with brachial artery vascular reactivity, carotid intima media thickness, and coronary heart disease[50-53]. Moreno-Navarrete et al[50] demonstrated that the concentration of circulating omentin-1 contributes independently to endothelial dysfunction, even after controlling for adiposity, age, and inflammation in subjects with impaired glucose tolerance. In that study, vascular reactivity was measured using high-resolution ultrasound imaging of the brachial artery. Subsequently, two reports on the negative correlation of serum omentin-1 with carotid IMT have suggested cardioprotective and anti-atherosclerotic roles for omentin-1[52,53]. Liu et al[53] demonstrated that the serum omentin-1 level was independently correlated with carotid IMT in metabolic syndrome patients, and Shibata et al[52] showed similar results in apparently healthy men. Recently, El-Mesallamy et al[54] examined the level of circulating omentin-1 in an Egyptian population with type 2 diabetes, with or without ischemic heart disease. Although they did not detect clear differences in serum omentin-1 levels between patients with type 2 diabetes with or without ischemic heart disease, multiple regression analysis showed that the IL-6 level was an independent risk factor influencing serum omentin-1 level. This suggests that omentin-1 is regulated by inflammation. Therefore, omentin is regarded as a novel link between insulin resistance, inflammation, and cardiovascular disease, suggesting its possibility as a novel therapeutic target for the cardiovascular diseases.
CHEMERIN
Chemerin was identified as a chemoattractant that promotes the recruitment of immature dendritic cells and macrophages to lymphoid organs and sites of tissue injury[55]. Goralski et al[56] first reported a high level of chemerin expression in mouse and human adipocytes. They also reported that loss of chemerin expression almost completely abrogated adipogenesis in 3T3-L1 cells, and modified the expression of genes important in glucose and lipid metabolism, such as GLUT4, leptin, and adiponectin[56]. After that, Ernst et al[57] reported that exogenous administration of chemerin exacerbated glucose intolerance, lowered serum insulin levels, and decreased tissue glucose uptake in a mouse model of obesity and diabetes. Growing evidence from human data supports a linkage between chemerin, obesity, and metabolic syndrome. A study of a Mexican-American population showed that circulating chemerin levels were significantly higher in obese subjects compared with non-obese controls. Plasma levels of chemerin were correlated positively with body mass index (BMI), fasting glucose, fasting insulin, and triglycerides levels, and negatively correlated with high-density lipoprotein (HDL)-cholesterol level[58]. Bozaoglu et al[58,59] demonstrated that serum chemerin levels were closely correlated with BMI, fasting serum insulin, triglycerides, and HDL-cholesterol in non-diabetic subjects. Sell et al[60] reported that in patients who had undergone bariatric surgery for weight reduction, the serum chemerin levels were significantly reduced after surgery, indicating that chemerin might mediate the metabolic alterations in obesity.
Although chemerin is a well-known secreted protein with an established role in immune function, recent experimental data indicate that chemerin might provide a link between obesity and chronic inflammation[61]. Recently, Sell et al[62] reported that chemerin activated the NF-κB pathway and impaired glucose uptake in primary human skeletal muscle cells. Moreover, TNF-α treatment of 3T3-L1 adipocytes increased bioactive chemerin levels, suggesting that inflammatory cytokines contribute to the up-regulation of chemerin in obesity[63]. Thus, adipocyte-derived chemerin might be involved in the pathogenesis of obesity-related inflammatory disorders, including atherosclerosis. Although Becker et al[64] showed that the expression of chemerin did not significantly alter the extent of atherosclerosis in low-density lipoprotein cholesterol receptor knockout mice, they hypothesized that chemerin might affect early atherosclerotic plaque development and morphology rather than the extent of the atherosclerotic lesion area. Hart et al[65] showed that chemerin rapidly stimulated the adhesion of macrophages to the extracellular matrix protein, fibronectin, and to the adhesion molecule, vascular cell adhesion molecule-1, suggesting that chemerin might promote the progression of atherosclerosis. Furthermore, Kaur et al[66] demonstrated the novel presence of a G-protein coupled chemerin receptor 1 in human endothelial cells and its significant up-regulation by pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6). Thus, the altered expression of chemerin and its receptors during an inflammatory process might cause dysregulated angiogenesis, leading to the development of cardiovascular disease.
However, there have been very few clinical studies that examined the influence of circulating chemerin on the atherosclerotic process. Lehrke et al[67] showed that circulating chemerin was positively correlated with the atherosclerotic plaque burden, as assessed by multi-slice computed tomography angiography, but that the association was lost after adjusting for established cardiovascular risk factors. Very recently, we showed that the circulating chemerin level was an independent risk factor for arterial stiffness even after adjusting other cardiovascular risk factors[68].
CONCLUSION
Various adipokines have been reported to directly modulate the atherogenic environment of the vessel wall by regulating the function of endothelial, arterial smooth muscle, and macrophage cells. Therefore, the identification of a novel adipokine that regulates the atherosclerotic process might provide new opportunities for developing more effective approaches for preventing cardiovascular disease.
Footnotes
P- Reviewers: Cardoso CRL, Papazoglou D, Tomkin GH S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
References
- 1.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 2.Ntaios G, Gatselis NK, Makaritsis K, Dalekos GN. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis. 2013;227:216–221. doi: 10.1016/j.atherosclerosis.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 4.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 5.Stefan N, Stumvoll M. Adiponectin--its role in metabolism and beyond. Horm Metab Res. 2002;34:469–474. doi: 10.1055/s-2002-34785. [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzawa Y. Adiponectin: Identification, physiology and clinical relevance in metabolic and vascular disease. Atheroscler Suppl. 2005;6:7–14. doi: 10.1016/j.atherosclerosissup.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–146. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi KM, Lee J, Lee KW, Seo JA, Oh JH, Kim SG, Kim NH, Choi DS, Baik SH. Serum adiponectin concentrations predict the developments of type 2 diabetes and the metabolic syndrome in elderly Koreans. Clin Endocrinol (Oxf) 2004;61:75–80. doi: 10.1111/j.1365-2265.2004.02063.x. [DOI] [PubMed] [Google Scholar]
- 10.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 11.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 12.Choi HY, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Association of adiponectin, resistin, and vascular inflammation: analysis with 18F-fluorodeoxyglucose positron emission tomography. Arterioscler Thromb Vasc Biol. 2011;31:944–949. doi: 10.1161/ATVBAHA.110.220673. [DOI] [PubMed] [Google Scholar]
- 13.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 14.Filková M, Haluzík M, Gay S, Senolt L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin Immunol. 2009;133:157–170. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 16.Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314:415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 17.Lee TS, Lin CY, Tsai JY, Wu YL, Su KH, Lu KY, Hsiao SH, Pan CC, Kou YR, Hsu YP, et al. Resistin increases lipid accumulation by affecting class A scavenger receptor, CD36 and ATP-binding cassette transporter-A1 in macrophages. Life Sci. 2009;84:97–104. doi: 10.1016/j.lfs.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Piestrzeniewicz K, Łuczak K, Komorowski J, Maciejewski M, Jankiewicz Wika J, Goch JH. Resistin increases with obesity and atherosclerotic risk factors in patients with myocardial infarction. Metabolism. 2008;57:488–493. doi: 10.1016/j.metabol.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Qi Q, Wang J, Li H, Yu Z, Ye X, Hu FB, Franco OH, Pan A, Liu Y, Lin X. Associations of resistin with inflammatory and fibrinolytic markers, insulin resistance, and metabolic syndrome in middle-aged and older Chinese. Eur J Endocrinol. 2008;159:585–593. doi: 10.1530/EJE-08-0427. [DOI] [PubMed] [Google Scholar]
- 20.Weikert C, Westphal S, Berger K, Dierkes J, Möhlig M, Spranger J, Rimm EB, Willich SN, Boeing H, Pischon T. Plasma resistin levels and risk of myocardial infarction and ischemic stroke. J Clin Endocrinol Metab. 2008;93:2647–2653. doi: 10.1210/jc.2007-2735. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Ha JW, Kim JS, Choi EY, Park S, Kang SM, Choi D, Jang Y, Chung N. Plasma adiponectin and resistin levels as predictors of mortality in patients with acute myocardial infarction: data from infarction prognosis study registry. Coron Artery Dis. 2009;20:33–39. doi: 10.1097/MCA.0b013e328318ecb0. [DOI] [PubMed] [Google Scholar]
- 22.Makowski L, Hotamisligil GS. Fatty acid binding proteins--the evolutionary crossroads of inflammatory and metabolic responses. J Nutr. 2004;134:2464S–2468S. doi: 10.1093/jn/134.9.2464S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, Wat NM, Wong WK, Lam KS. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem. 2006;52:405–413. doi: 10.1373/clinchem.2005.062463. [DOI] [PubMed] [Google Scholar]
- 24.Uysal KT, Scheja L, Wiesbrock SM, Bonner-Weir S, Hotamisligil GS. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology. 2000;141:3388–3396. doi: 10.1210/endo.141.9.7637. [DOI] [PubMed] [Google Scholar]
- 25.Tso AW, Xu A, Sham PC, Wat NM, Wang Y, Fong CH, Cheung BM, Janus ED, Lam KS. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care. 2007;30:2667–2672. doi: 10.2337/dc07-0413. [DOI] [PubMed] [Google Scholar]
- 26.Xu A, Tso AW, Cheung BM, Wang Y, Wat NM, Fong CH, Yeung DC, Janus ED, Sham PC, Lam KS. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation. 2007;115:1537–1543. doi: 10.1161/CIRCULATIONAHA.106.647503. [DOI] [PubMed] [Google Scholar]
- 27.Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, Hotamisligil GS. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation. 2004;110:1492–1498. doi: 10.1161/01.CIR.0000141735.13202.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y, Luo N, Lopes-Virella MF, Garvey WT. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis. 2002;165:259–269. doi: 10.1016/s0021-9150(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 29.Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem. 2005;280:12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuhashi M, Tuncman G, Görgün CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee EJ, Lee WY, Park CY, Oh KW, Kim BJ, Sung KC, Kim BS. The association of serum adipocyte fatty acid-binding protein with coronary artery disease in Korean adults. Eur J Endocrinol. 2009;160:165–172. doi: 10.1530/EJE-08-0665. [DOI] [PubMed] [Google Scholar]
- 33.Yeung DC, Xu A, Cheung CW, Wat NM, Yau MH, Fong CH, Chau MT, Lam KS. Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1796–1802. doi: 10.1161/ATVBAHA.107.146274. [DOI] [PubMed] [Google Scholar]
- 34.Miyoshi T, Onoue G, Hirohata A, Hirohata S, Usui S, Hina K, Kawamura H, Doi M, Kusano KF, Kusachi S, et al. Serum adipocyte fatty acid-binding protein is independently associated with coronary atherosclerotic burden measured by intravascular ultrasound. Atherosclerosis. 2010;211:164–169. doi: 10.1016/j.atherosclerosis.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 35.Yoo HJ, Kim S, Park MS, Choi HY, Yang SJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, et al. Serum adipocyte fatty acid-binding protein is associated independently with vascular inflammation: analysis with (18)F-fluorodeoxyglucose positron emission tomography. J Clin Endocrinol Metab. 2011;96:E488–E492. doi: 10.1210/jc.2010-1473. [DOI] [PubMed] [Google Scholar]
- 36.Yang R, Xu A, Pray J, Hu H, Jadhao S, Hansen B, Shuldiner A, Mc-Lenithan J, Gong D. Cloning of omentin, a new adipocytokine from omental fat tissue in humans. Diabetes. 2003;Suppl1:A1. [Google Scholar]
- 37.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC, Gong DW. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 38.Pan HY, Guo L, Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract. 2010;88:29–33. doi: 10.1016/j.diabres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Tan BK, Adya R, Farhatullah S, Lewandowski KC, O’Hare P, Lehnert H, Randeva HS. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57:801–808. doi: 10.2337/db07-0990. [DOI] [PubMed] [Google Scholar]
- 40.de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, Ndubuizu K, Patil S, Schwartz A, Kligman M, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 41.Tsuji S, Uehori J, Matsumoto M, Suzuki Y, Matsuhisa A, Toyoshima K, Seya T. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J Biol Chem. 2001;276:23456–23463. doi: 10.1074/jbc.M103162200. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki YA, Shin K, Lönnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry. 2001;40:15771–15779. doi: 10.1021/bi0155899. [DOI] [PubMed] [Google Scholar]
- 43.Fain JN, Sacks HS, Buehrer B, Bahouth SW, Garrett E, Wolf RY, Carter RA, Tichansky DS, Madan AK. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes (Lond) 2008;32:810–815. doi: 10.1038/sj.ijo.0803790. [DOI] [PubMed] [Google Scholar]
- 44.Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408:339–343. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 45.Yamawaki H, Tsubaki N, Mukohda M, Okada M, Hara Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun. 2010;393:668–672. doi: 10.1016/j.bbrc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 46.Tan BK, Adya R, Farhatullah S, Chen J, Lehnert H, Randeva HS. Metformin treatment may increase omentin-1 levels in women with polycystic ovary syndrome. Diabetes. 2010;59:3023–3031. doi: 10.2337/db10-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie H, Xie PL, Wu XP, Chen SM, Zhou HD, Yuan LQ, Sheng ZF, Tang SY, Luo XH, Liao EY. Omentin-1 attenuates arterial calcification and bone loss in osteoprotegerin-deficient mice by inhibition of RANKL expression. Cardiovasc Res. 2011;92:296–306. doi: 10.1093/cvr/cvr200. [DOI] [PubMed] [Google Scholar]
- 48.Duan XY, Xie PL, Ma YL, Tang SY. Omentin inhibits osteoblastic differentiation of calcifying vascular smooth muscle cells through the PI3K/Akt pathway. Amino Acids. 2011;41:1223–1231. doi: 10.1007/s00726-010-0800-3. [DOI] [PubMed] [Google Scholar]
- 49.Maruyama S, Shibata R, Kikuchi R, Izumiya Y, Rokutanda T, Araki S, Kataoka Y, Ohashi K, Daida H, Kihara S, et al. Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism. J Biol Chem. 2012;287:408–417. doi: 10.1074/jbc.M111.261818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreno-Navarrete JM, Ortega F, Castro A, Sabater M, Ricart W, Fernández-Real JM. Circulating omentin as a novel biomarker of endothelial dysfunction. Obesity (Silver Spring) 2011;19:1552–1559. doi: 10.1038/oby.2010.351. [DOI] [PubMed] [Google Scholar]
- 51.Shibata R, Ouchi N, Kikuchi R, Takahashi R, Takeshita K, Kataoka Y, Ohashi K, Ikeda N, Kihara S, Murohara T. Circulating omentin is associated with coronary artery disease in men. Atherosclerosis. 2011;219:811–814. doi: 10.1016/j.atherosclerosis.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Shibata R, Takahashi R, Kataoka Y, Ohashi K, Ikeda N, Kihara S, Murohara T, Ouchi N. Association of a fat-derived plasma protein omentin with carotid artery intima-media thickness in apparently healthy men. Hypertens Res. 2011;34:1309–1312. doi: 10.1038/hr.2011.130. [DOI] [PubMed] [Google Scholar]
- 53.Liu R, Wang X, Bu P. Omentin-1 is associated with carotid atherosclerosis in patients with metabolic syndrome. Diabetes Res Clin Pract. 2011;93:21–25. doi: 10.1016/j.diabres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 54.El-Mesallamy HO, El-Derany MO, Hamdy NM. Serum omentin-1 and chemerin levels are interrelated in patients with Type 2 diabetes mellitus with or without ischaemic heart disease. Diabet Med. 2011;28:1194–1200. doi: 10.1111/j.1464-5491.2011.03353.x. [DOI] [PubMed] [Google Scholar]
- 55.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brézillon S, Tyldesley R, Blanpain C, Detheux M, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 57.Ernst MC, Issa M, Goralski KB, Sinal CJ. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology. 2010;151:1998–2007. doi: 10.1210/en.2009-1098. [DOI] [PubMed] [Google Scholar]
- 58.Bozaoglu K, Segal D, Shields KA, Cummings N, Curran JE, Comuzzie AG, Mahaney MC, Rainwater DL, VandeBerg JL, MacCluer JW, et al. Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J Clin Endocrinol Metab. 2009;94:3085–3088. doi: 10.1210/jc.2008-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 60.Sell H, Divoux A, Poitou C, Basdevant A, Bouillot JL, Bedossa P, Tordjman J, Eckel J, Clément K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2010;95:2892–2896. doi: 10.1210/jc.2009-2374. [DOI] [PubMed] [Google Scholar]
- 61.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010;21:660–667. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Sell H, Laurencikiene J, Taube A, Eckardt K, Cramer A, Horrighs A, Arner P, Eckel J. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes. 2009;58:2731–2740. doi: 10.2337/db09-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parlee SD, Ernst MC, Muruganandan S, Sinal CJ, Goralski KB. Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-{alpha} Endocrinology. 2010;151:2590–2602. doi: 10.1210/en.2009-0794. [DOI] [PubMed] [Google Scholar]
- 64.Becker M, Rabe K, Lebherz C, Zugwurst J, Göke B, Parhofer KG, Lehrke M, Broedl UC. Expression of human chemerin induces insulin resistance in the skeletal muscle but does not affect weight, lipid levels, and atherosclerosis in LDL receptor knockout mice on high-fat diet. Diabetes. 2010;59:2898–2903. doi: 10.2337/db10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hart R, Greaves DR. Chemerin contributes to inflammation by promoting macrophage adhesion to VCAM-1 and fibronectin through clustering of VLA-4 and VLA-5. J Immunol. 2010;185:3728–3739. doi: 10.4049/jimmunol.0902154. [DOI] [PubMed] [Google Scholar]
- 66.Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010;391:1762–1768. doi: 10.1016/j.bbrc.2009.12.150. [DOI] [PubMed] [Google Scholar]
- 67.Lehrke M, Becker A, Greif M, Stark R, Laubender RP, von Ziegler F, Lebherz C, Tittus J, Reiser M, Becker C, et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol. 2009;161:339–344. doi: 10.1530/EJE-09-0380. [DOI] [PubMed] [Google Scholar]
- 68.Yoo HJ, Choi HY, Yang SJ, Kim HY, Seo JA, Kim SG, Kim NH, Choi KM, Choi DS, Baik SH. Circulating chemerin level is independently correlated with arterial stiffness. J Atheroscler Thromb. 2012;19:59–66; discussion 67-68. doi: 10.5551/jat.9647. [DOI] [PubMed] [Google Scholar]


