Abstract
Aberrant activation of the Raf/MEK/MAPK pathway plays a key role in breast cancer development and progression. Dysregulation of Raf/MEK/MAPK oncogenic signaling often results from overexpression of the HER-2/Neu tyrosine kinase receptor leading to chemoendocrine resistance, development of distant metastases and ultimately poor prognosis in breast cancer patients. HER-2/Neu overexpression is also linked to activation of the epithelial to mesenchymal transition (EMT) pathway, loss of adhesion molecules and metastasis. Recently, it has been demonstrated that cancer cells that undergo EMT acquire a CD44+/CD24−/low basal cancer stem cell-like phenotype and are characterized by activation of HER-2/Neu and TGFβ oncogenic signaling pathways with increased capacity of self-renewal, drug resistance, invasion and distant metastases. Following meta-static dissemination, cancer cells re-activate certain epithelial properties through mesenchymal to epithelial transition (MET) to establish neoplastic lesions at secondary sites, although the molecular mechanisms regulating MET remain elusive. In this study we demonstrate that constitutive activation of Raf-1 oncogenic signaling induces HER-2/Neu overexpression leading to the development of distant metastases in ERα+ MCF-7 breast cancer xenografts. Importantly, development of distant metastases in xenograft models was linked to activation of the MET pathway characterized by reduced expression of EMT inducer genes (TGFB2, TWIST1 and FOXC1) and overexpression of BMB7, CXCR7 and EGR family of transcription factors. In summary, our results demonstrate for the first time that amplification of Raf/MEK/MAPK oncogenic signaling during tumor growth promotes the genesis of metastatic lesions from primary tumors by activating the mesenchymal epithelial transition.
Keywords: Raf-1, breast cancer, Raf/MEK/MAPK pathway, HER-2/Neu, tyrosine kinase receptor
Introduction
Each year more than 210,000 new cases of breast cancer are diagnosed in the USA (1). More than two-thirds of these patients display initially positivity for the estrogen receptor (ERα) making them eligible for endocrine therapy (2). Usually ERα+ breast tumors show a better outcome compared to ERα− tumors because they display a more differentiated epithelial phenotype, are sensitive to endocrine therapy and show a moderately invasive behavior (3). Tamoxifen has been the most widely used adjuvant anti-estrogen agent for the past two decades, achieving a 39% reduction in disease recurrence and a 31% reduction in mortality in ERα early stage breast carcinomas (4). Despite the clinical benefit of hormonal treatment in patients with ERα+ breast cancer, primary and secondary resistance to endocrine therapy remains a significant clinical problem (5). One of the molecular mechanisms by which ERα+ breast tumors become resistant to endocrine manipulations is through overexpression of the receptor tyrosine kinase HER-2/Neu (6-8). Overexpression of HER-2/Neu signaling pathway induces phosphorylation and constitutive activation of ERα leading to aberrant cell proliferation (9,10). HER-2/Neu is overexpressed in 20-30% of invasive breast tumors and is associated with poor clinical outcome with reduced disease-free and overall survival rates (11,12). Cellular plasticity occurs in cancer progression in a similar fashion of embryonic development. The importance of cellular transitions in development is first apparent during gastrulation when the process of epithelial to mesenchymal transition (EMT) transforms polarized epithelial cells into migratory mesenchymal cells that constitute the embryonic and extra-embryonic mesoderm (13). It is now widely accepted that this developmental pathway is exploited in various disease states, including cancer. The loss of epithelial characteristics and the acquisition of a mesenchymal-like migratory phenotype that characterize EMT are crucial to the development of invasive carcinomas and metastasis (14).
Metastatic dissemination is an extremely complex and highly organized process that is organ-specific and involves numerous reciprocal interactions between the cancer cells and the host (15). Breast cancer progression is dependent on the capacity to metastasize to distant organs, and loss of cancer cell adhesion through EMT plays a key role in this process (16,17). Importantly, HER-2/Neu overexpression is linked to loss of the adhesion molecule E-cadherin during breast cancer progression, dissemination and metastasis (18). However, given the morphological similarities between primary tumor and metastatic lesions, it is likely that tumor cells re-activate certain epithelial properties through a mesenchymal to epithelial transition (MET) at the secondary site, although the molecular mechanisms regulating MET pathway remain elusive (19).
One of the major problems in eradicating metastatic cancer cells consists in their ability to self-renewal and to display higher resistance to conventional chemo-endocrine therapy (20,21). Recently, the discovery that breast tumors contain a CD44+/CD24−/low sub-population of cells harboring stem cell properties has generated excitement because these cancer stem cells may represent the source of therapeutic failures, distant metastases and ultimately poor outcome (22-24). It has also been demonstrated that cancer cells that undergo EMT acquire a basal cancer stem cell-like phenotype and display increased expression of the enzyme aldehyde dehydrogenase (ALDH) increasing tumorigenicity, chemoresistance and invasiveness (25,26). Moreover, tumors enriched with cancer stem cells are characterized by activation of HER-2/Neu and TGFβ oncogenic signaling pathways with increased capacity of self-renewal, drug resistance, invasion and distant metastases (27,28). Transcription factors such as TWIST1, SNAIL, SLUG, FOXC1, ZEB1 and SMADs play a key role in orchestrate the nuclear reprogramming leading to activation of EMT and stemness of cancer cells (29-31).
In this study we have employed an ERα+ MCF-7 breast cancer cell line engineered to express constitutive active Raf-1 oncoprotein to characterize the role of aberrant Raf/MEK/MAPK signaling pathway in breast cancer progression. Our results demonstrate that constitutive activation of Raf-1 confers a more aggressive phenotype characterized by increased cell proliferation and migration, chemo-endocrine resistance and ultimately development of distant metastases. Importantly, development of distant metastases in xenograft models was linked to overexpression of HER-2/Neu and activation of MET pathway leading to the establishment of secondary neoplastic lesions. Taken together, our results demonstrate for the first time that amplification of Raf/MEK/MAPK oncogenic signaling during tumor growth promotes the genesis of metastatic lesions from primary breast cancer cells by activating MET signaling.
Materials and methods
Human breast cancer cell lines
The human breast cancer cell line MCF-7 was obtained from ATCC (Manassas, VA, USA). The MCF-7 cells overexpressing the Raf-1 oncoprotein were generated as described previously (32). All cell lines were maintained in EMEM medium containing 5 mM glutamine, 1% penicillin/streptomycin, 20 microgram insulin/ml and 10% FBS at 37°C in 5% CO2 atmosphere.
Cell proliferation assay
For cell proliferation assay, breast cancer cells were cultured in a 96-well plate at a density of 2,000 cells in each plate and treated with 1 μm daunorubicin (Sigma). Following a 7-day incubation, cell viability was determined using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA), performed according to the manufacturer's protocol.
Cell cycle profile, indirect immunofluorescence and immuno-blotting
For fluorescence-activated cell sorting (FACS), Indirect immunofluorescence and protein expression analyses, breast cancer cells were treated as previously described (33-35).
Antibodies
Antibodies employed in this study were the following: Cell Signaling Technology (tMAPK, p-MAPK, HER-2/Neu, E-cadherin); Santa Cruz Biotechnology (ERα) and Sigma (β-actin).
In vitro cell migration assay
In vitro migration assay of MCF-7 and vMCF-7Raf-1 cells was performed by employing the xCELLigence System (Roche) according to the manufacturer's procedure.
Human breast cancer xenografts
Procedures established by the Institutional Animal Care and Use Committee based on US NIH guidelines for the care and use of laboratory animals were followed for all experiments. Four-week-old non-ovariectomized female NCR/Nu/Nu nude mice were anesthetized by exposure to 3% isoflurane and injected subcutaneously with 2×106 cells suspended in 50 μl of 50% Matrigel (BD Bioscience, Bedford, MA, USA). Tumor localization and growth was monitored using the IVS imaging system from the ventral view 10 min after luciferin injection. After 12 weeks, mice were sacrificed and xenograft tumors were processed for histology, immunohistochemistry and immunofluorescence analyses as previously described (34,35). To re-establish cultures from 1GX explants, tumors were excised from the animals, minced using sterile scissors, transferred to complete culture medium and fibroblast-free tumor cell lines were established by serial passages in culture.
Gene microarray analysis
Total RNA was extracted from breast cancer cells using TRizol according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). One microgram of total RNA (A260/A280 ratio of 1.8-2.2) was used to probe for global genome expression employing Affimetrix U133 Plus 2.0 chips (Affimetrix, Santa Clara, CA). The raw data (CEL files) were pre-processed using GCRMA algorithm using Partek GS 6.5 software (St. Louis, MO). Partek built in tools were used to perform analysis of variance (ANOVA) on groups of samples. Experiments were performed in duplicates.
Results and Discussion
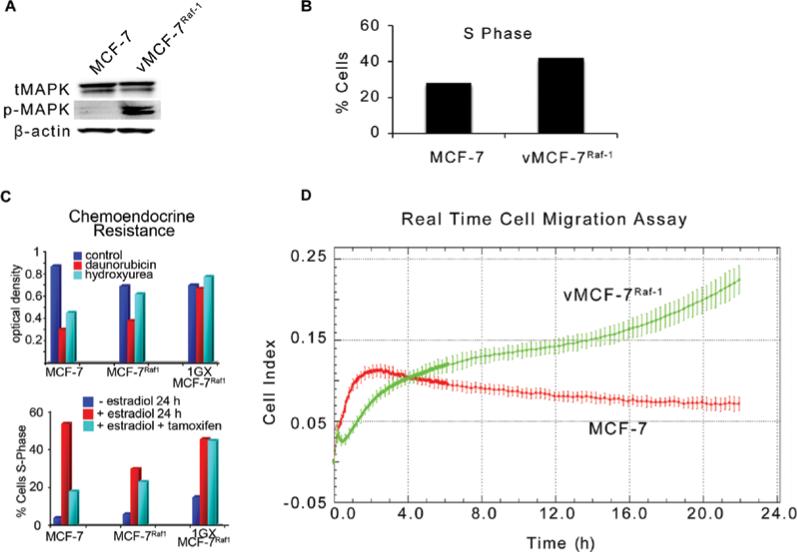
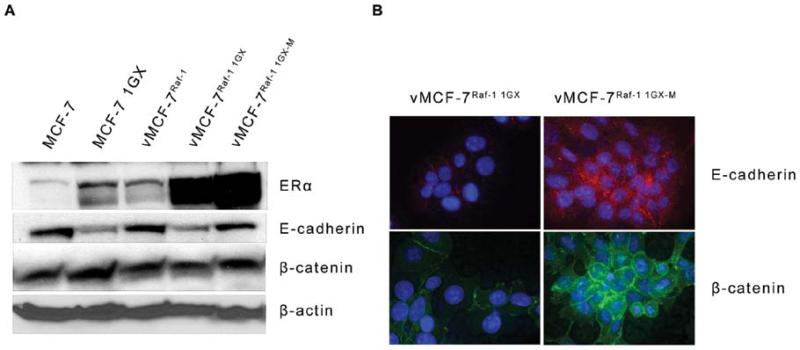
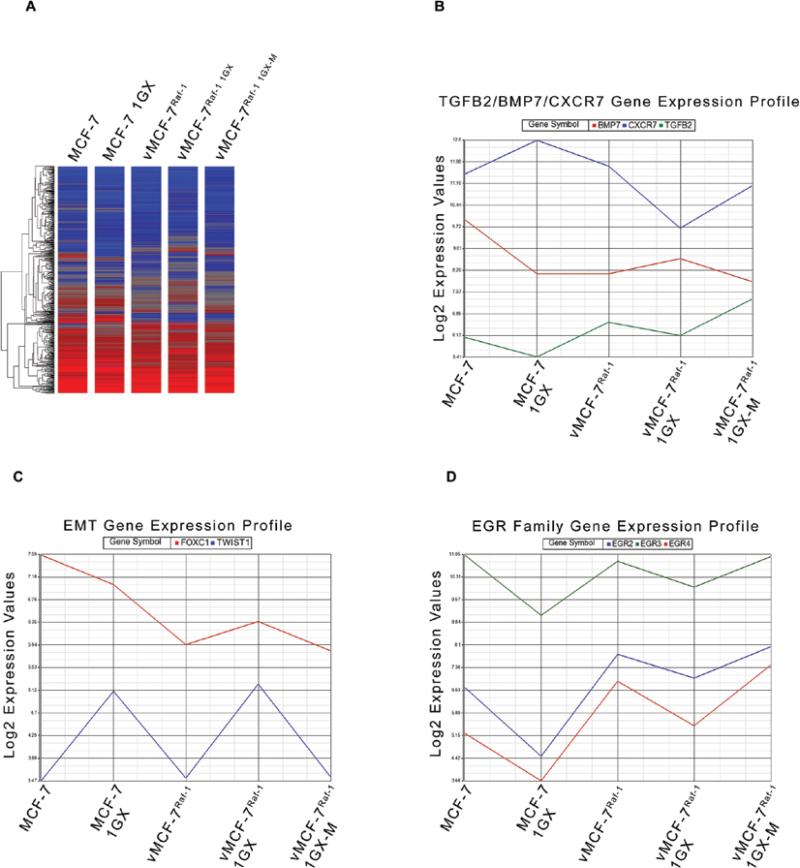
To characterize the mechanistic role of aberrant activation of MAPK signaling pathway in breast cancer progression, we employed a variant MCF-7 cell line overexpressing a constitutive active Raf-1 oncoprotein (vMCF-7ΔRaf-1) as previously described (32). Hyper-phosphorylation of MAPK demonstrated constitutive activation of Raf/MEK/MAPK pathway in vMCF-7ΔRaf-1 compared to parental MCF-7 cells (Fig. 1A). To determine the extent to which aberrant Raf/MEK/MAPK signaling pathway confers an advantage on cell cycle progression, we performed a FACS cell cycle analysis on MCF-7 and vMCF-7ΔRaf-1 cells. vMCF-7ΔRaf-1 displayed a higher percentage of cells in the S phase fraction of the cell cycle compared to parental MCF-7 cells, indicating that constitutive MAPK activation accelerates G1/S cell cycle progression (Fig. 1B). Since aberrant activation of Raf/MEK/MAPK signaling pathway is linked to increased chemo-endocrine resistance in breast cancer, we treated MCF-7 and vMCF-7ΔRaf-1 cells with anti-cancer drugs targeting the DNA replication process (hydroxyurea and daunorubicin) and ERα mitogenic pathway (tamoxifen). Resistance to chemo-endocrine treatment was observed in vMCF-7ΔRaf-1 compared to parental MCF-7 cells, indicating that aberrant Raf/MEK/MAPK signaling activation induced chemo-endocrine resistance in estrogen-sensitive breast cancer cells (Fig. 1C). Since tumor progression and development of distant metastases is linked to increased cell motility and consequent invasion, we employed an innovative real-time migratory assay to establish whether constitutive Raf/MEK/MAPK signaling activation conferred higher motility properties of MCF-7 cells. Importantly, vMCF-7ΔRaf-1 cells showed a higher migration index than MCF-7 cells during a 24-h migration assay (Fig. 1D) suggesting that activation of Raf-1 signaling pathway promotes an invasive phenotype. To determine in vivo the extent to which vMCF-7ΔRaf-1 cells gave rise to invasive breast tumors, we established murine MCF-7 and vMCF-7ΔRaf-1 xenografts (Fig. 2). vMCF-7ΔRaf-1 xenografts developed larger tumors than parental MCF-7 xenografts following a 4-week cancer cells injection into the mammary fat pad (Fig. 2A). Breast tumors were surgically removed 12 weeks after implantation without sacrificing the animals to monitor the development of distant metastases (Fig. 2B). Significantly, 8 weeks following surgical removal, only vMCF-7ΔRaf-1 xenografts developed frank visceral metastases demonstrating that aberrant Raf/MEK/MAPK signaling pathway induces metastatic dissemination (Fig. 2B). Because development of chemo-endocrine resistance and distant metastases is often associated to HER-2/Neu overexpression in breast cancer, we performed immunohistochemistry analysis of HER-2/Neu staining on primary tumors and metastatic lesions (Fig. 2C). While MCF-7 primary tumors did not show positivity for HER-2/Neu, vMCF-7ΔRaf-1 primary tumors displayed HER-2/Neu heterogeneity and vMCF-7ΔRaf-1 metastatic tumors showed strong positivity for HER-2/Neu expression. These results demonstrate that aberrant Raf/MEK/MAPK pathway induces HER-2/Neu expression during tumor growth, suggesting a key role of HER-2 oncogenic signaling pathway in the development of breast cancer metastases and tumor progression. Furthermore, to characterize the molecular mechanisms responsible for the development of distant metastases in vMCF-7ΔRaf-1 xenografts, we re-cultured cancer cells from MCF-7 and vMCF-7ΔRaf-1 xenografts (primary and metastatic) and termed them first generation xenografts (1GX). Immunoblot analysis revealed that primary and metastatic vMCF-7ΔRaf-1 1GX cells overexpressed ERα compared to parental cells (Fig. 3A). However primary vMCF-7ΔRaf-1 1GX cells displayed higher resistance to chemo-endocrine therapy (Fig. 1C) indicating that aberrant activation of Raf-1/MEK/MAPK pathway and HER-2/Neu overexpression activates ERα pathway and confers higher resistance to conventional anti-cancer therapy during in vivo growth. To establish the extent to which development of breast cancer metastases was linked to EMT, we analyzed the expression of key EMT markers (E-cadherin and β-catenin) in breast cancer cells. Loss of E-cadherin expression was observed in MCF-7 1GX and vMCF-7ΔRaf-11GX cells while cultured MCF-7, vMCF-7ΔRaf-1 and metastatic vMCF-7ΔRaf-11GX cells expressed high levels of E-cadherin. Similarly, expression of the epithelial marker β-catenin was increased in MCF-7, MCF-7 1GX and metastatic vMCF-7ΔRaf-11GX cells compared to its expression level in vMCF-7ΔRaf-1 and vMCF-7ΔRaf-11GX cells (Fig. 3A). Immunofluorescence analysis also demonstrated that metastatic vMCF-7ΔRaf-11GX cells re-express the epithelial markers E-cadherin and β-catenin compared to primary vMCF-7ΔRaf-11GX cells (Fig. 3B). These results demonstrate that HER-2/Neu overexpression and amplification of MAPK signaling pathway in vMCF-7ΔRaf-1 xenografts induces development of EMT and metastatic dissemination, while re-expression of epithelial markers and activation of MET pathway is essential for the establishment of secondary neoplastic lesions. To determine whether activation of EMT and MET pathways during in vivo growth of vMCF-7ΔRaf-1 cells were linked to specific transcriptome changes that are critical for tumor progression, we employed human Affimetrix micro-arrays to assess global gene expression of breast cancer cells (Fig. 4A). From this comparison, we identified a group of 8 differentially expressed genes involved in tumor growth (TGFB2, BMB7, EGR2, EGR3, EGR4), EMT (TWIST1, FOXC1) and metastasis (CXCR7) correlated with tumor progression (Fig. 4B-D). Specifically, TGFB2 and CXCR7 genes were overexpressed in the metastatic vMCF-7ΔRaf-11GX cells while BMP7 gene was down-regulated compared to the primary vMCF-7ΔRaf-11GX cells (Fig. 4B). These results indicate that TGFB2 and BMP7 signaling pathways are positive and negative regulators of distant metastases in breast cancer, respectively. Furthermore, we identified genes encoding for nuclear transcription factors involved in the induction of EMT such as TWIST1 and FOXC1 that were down-regulated in metastatic vMCF-7ΔRaf-11GX compared to primary vMCF-7ΔRaf-11GX cells (Fig. 4C). These findings substantiate suppression of EMT, activation of MET pathway and consequent re-expression of epithelial markers observed in metastatic vMCF-7ΔRaf-11GX cells compared to primary tumors (Fig. 3). Importantly, vMCF-7ΔRaf-11GX cells that lack epithelial markers displayed a decreased expression of EGR2, EGR3 and EGR4 nuclear transcription factors compared to MCF-7, vMCF-7ΔRaf-1 and metastatic vMCF-7ΔRaf-11GX cells that expressed high levels of E-cadherin and β-catenin (Fig. 4D). These results highlight a novel role for the EGR family of transcription factors in the activation of MET pathway and establishment of breast cancer metastases.
Figure 1.
Molecular characterization of MCF-7 cells harboring constitutive activation of Raf/MEK/MAPK pathway. (A) Immunoblot assay showing MAPK phosphorylation in vMCF-7ΔRaf-1 compared to parental MCF-7 cells. (B) Cell cycle analysis (FACS) showing increased S phase fraction in vMCF-7ΔRaf-1 compared to parental MCF-7 cells. (C) MTT assay and FACS analysis of variants and parental MCF-7 cells treated with chemo-endocrine therapy. (D) Real-time cell migration assay showing higher motility of vMCF-7ΔRaf-1 compared to parental MCF-7 cells.
Figure 2.
Establishment of MCF-7 and vMCF-7ΔRaf-1 breast cancer xenografts. (A) Tumor imaging in live animals of MCF-7 and vMCF-7ΔRaf-1 xenografts expressing the firefly luciferase reporter lenti-vector at 4 weeks after mammary fat pad injection. (B) Tumor imaging in live animals of vMCF-7ΔRaf-1 xenografts expressing the firefly luciferase reporter lenti-vector at 12 weeks after mammary fat pad injection and 8 weeks following surgical removal. (C) Paraffin sections of xenograft tumors showing expression of HER-2 receptor in primary and metastatic vMCF-7ΔRaf-1 xenografts.
Figure 3.
Characterization of EMT in human breast cancer cell lines. (A) Immunoblot assay showing expression of ERα, E-cadherin and β-catenin proteins in variants and parental MCF-7 cells. (B) Immunofluorescence analysis showing expression of E-cadherin and β-catenin in metastatic vMCF-7ΔRaf-1 1GX cells.
Figure 4.
Global gene expression profile in human breast cancer cell lines. (A) Heat map representing the unsupervised cluster analysis of global gene expression of MCF-7, MCF-7 1GX, vMCF-7ΔRaf-1, vMCF-7ΔRaf-1 1GX and metastatic vMCF-7ΔRaf-1 1GX cells. (B-D) Expression profile of TGFB2, BMP7, CXCR7, FOXC1, TWIST1, EGR2, EGR3 and EGR4 genes in MCF-7, MCF-7 1GX, vMCF-7ΔRaf-1, vMCF-7ΔRaf-1 1GX and metastatic vMCF-7ΔRaf-1 1GX cells.
Taken together these findings demonstrate that constitutive activation of Raf-1 oncoprotein leads to HER-2/Neu overexpression and consequent amplification of MAPK signaling pathway during breast cancer progression. Importantly, metastatic spreading to distant organs is characterized by activation of EMT pathway and loss of E-cadherin and β-catenin epithelial markers during tumor growth. Activation of MET pathway and re-expression of epithelial markers is essential for the establishment of secondary neoplastic lesions. Activation of MET signaling is characterized by reduced expression of EMT inducer genes (TGFB2, TWIST1 and FOXC1) and overexpression of BMB7, CXCR7 and EGR family of transcription factors. In conclusion, this study highlighted for the first time the mechanistic linkage between Raf/MEK/MAPK oncogenic signaling and development of distant metastases through activation of MET pathway.
Acknowledgements
This study was supported by USAMRMC BC02276, the Intramural RECDA Award and the Mayo Clinic Breast Cancer Specialized Program of Research Excellence NIH CA116201 to ABD, and the Mayo Clinic School of Medicine. We also wish to acknowledge the TACMA and CTSA facilities for performing the immunohistochemistry and gene microarray assays and assisting us with the interpretation of the results.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21:28–34. doi: 10.1200/JCO.2003.03.088. [DOI] [PubMed] [Google Scholar]
- 3.Colleoni M, Sun Z, Martinelli G, Basser RL, Coates AS, Gelber RD, Green MD, Peccatori F, Cinieri S, Aebi S, Viale G, Price KN, Goldhirsch A. The effect of endocrine responsiveness on high-risk breast cancer treated with dose-intensive chemotherapy: results of International Breast Cancer Study Group Trial 15-95 after prolonged follow-up. Ann Oncol. 2009;20:1344–1351. doi: 10.1093/annonc/mdp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11:643–658. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 6.Leung E, Kannan N, Krissansen GW, Findlay MP, Baguley BC. MCF-7 breast cancer cells selected for tamoxifen resistance acquire new phenotypes differing in DNA content, phospho-HER2 and PAX2 expression, and rapamycin sensitivity. Cancer Biol Ther. 2010;9:717–724. doi: 10.4161/cbt.9.9.11432. [DOI] [PubMed] [Google Scholar]
- 7.Johnston S, Pippen J, Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ, Press MF, Maltzman J, Florance A, O'Rourke L, Oliva C, Stein S, Pegram M. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 8.Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008;456:663–666. doi: 10.1038/nature07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghayad SE, Vendrell JA, Ben Larbi S, Dumontet C, Bieche I, Cohen PA. Endocrine resistance associated with activated ErbB system in breast cancer cells is reversed by inhibiting MAPK or PI3K/Akt signaling pathways. Int J Cancer. 2010;126:545–562. doi: 10.1002/ijc.24750. [DOI] [PubMed] [Google Scholar]
- 10.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, Weiss H, Rimawi M, Schiff R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett AI, Starcyznski J, Robson T, Maclellan A, Campbell FM, van de Velde CJ, Hasenburg A, Markopoulos C, Seynaeve C, Rea D, Bartlett JM. Heterogeneous HER2 gene amplification: impact on patient outcome and a clinically relevant definition. Am J Clin Pathol. 2011;136:266–274. doi: 10.1309/AJCP0EN6AQMWETZZ. [DOI] [PubMed] [Google Scholar]
- 12.Al-Azawi D, Leong S, Wong L, Kay E, Hill AD, Young L. HER-2 positive and p53 negative breast cancers are associated with poor prognosis. Cancer Invest. 2011;29:365–369. doi: 10.3109/07357907.2011.584586. [DOI] [PubMed] [Google Scholar]
- 13.Acloque H, Ocaña OH, Matheu A, Rizzoti K, Wise C, Lovell-Badge R, Nieto MA. Reciprocal repression between Sox3 and snail transcription factors defines embryonic territories at gastrulation. Dev Cell. 2011;21:546–558. doi: 10.1016/j.devcel.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Foroni C, Broggini M, Generali D, Damia G. Epithelial-mesenchymal transition and breast cancer: role, molecular mechanisms and clinical impact. Cancer Treat Rev. 2011 Nov 25; doi: 10.1016/j.ctrv.2011.11.001. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 17.Takebe N, Warren RQ, Ivy SP. Breast cancer growth and metastasis: interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition. Breast Cancer Res. 2011;13:211. doi: 10.1186/bcr2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freudenberg JA, Wang Q, Katsumata M, Drebin J, Nagatomo I, Greene MI. The role of HER2 in early breast cancer metastasis and the origins of resistance to HER2-targeted therapies. Exp Mol Pathol. 2009;87:1–11. doi: 10.1016/j.yexmp.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ, Garcia-Blanco MA. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9:997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scopelliti A, Cammareri P, Catalano V, Saladino V, Todaro M, Stassi G. Therapeutic implications of cancer initiating cells. Expert Opin Biol Ther. 2009;9:1005–1016. doi: 10.1517/14712590903066687. [DOI] [PubMed] [Google Scholar]
- 21.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 22.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS, Polyak K: Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed MA, Aleskandarany MA, Rakha EA, Moustafa RZ, Benhasouna A, Nolan C, Green AR, Ilyas M, Ellis IO. A CD44(−)/CD24 (+) phenotype is a poor prognostic marker in early invasive breast cancer. Breast Cancer Res Treat. 2011 Nov 27; doi: 10.1007/s10549-011-1865-8. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 24.Olsson E, Honeth G, Bendahl PO, Saal LH, Gruvberger-Saal S, Ringnér M, Vallon-Christersson J, Jönsson G, Holm K, Lövgren K, Fernö M, Grabau D, Borg A, Hegardt C. CD44 isoforms are heterogeneously expressed in breast cancer and correlate with tumor subtypes and cancer stem cell markers. BMC Cancer. 2011;11:418. doi: 10.1186/1471-2407-11-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Croker AK, Allan AL. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDH(hi)CD44 (+) human breast cancer cells. Breast Cancer Res Treat. 2011 Aug 5; doi: 10.1007/s10549-011-1692-y. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 26.Reuben JM, Lee BN, Gao H, Cohen EN, Mego M, Giordano A, Wang X, Lodhi A, Krishnamurthy S, Hortobagyi GN, Cristofanilli M, Lucci A, Woodward WA. Primary breast cancer patients with high risk clinicopathologic features have high percentages of bone marrow epithelial cells with ALDH activity and CD44+/CD24low/− cancer stem cell phenotype. Eur J Cancer. 2011;47:1527–1536. doi: 10.1016/j.ejca.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soini Y, Tuhkanen H, Sironen R, Virtanen I, Kataja V, Auvinen P, Mannermaa A, Kosma VM. Transcription factors zeb1, twist and snai1 in breast carcinoma. BMC Cancer. 2011;11:73. doi: 10.1186/1471-2407-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Saleh S, Sharaf LH, Luqmani YA. Signalling pathways involved in endocrine resistance in breast cancer and associations with epithelial to mesenchymal transition. Int J Oncol. 2011;38:1197–1217. doi: 10.3892/ijo.2011.942. [DOI] [PubMed] [Google Scholar]
- 31.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis JM, Navolanic PM, Weinstein-Oppenheimer CR, Steelman LS, Hu W, Konopleva M, Blagosklonny MV, McCubrey JA. Raf-1 and Bcl-2 induce distinct and common pathways that contribute to breast cancer drug resistance. Clin Cancer Res. 2003;9:1161–1170. [PubMed] [Google Scholar]
- 33.D'Assoro AB, Barrett SL, Folk C, Negron VC, Boeneman K, Busby R, Whitehead C, Stivala F, Lingle WL, Salisbury JL. Amplified centrosomes in breast cancer: a potential indicator of tumor aggressiveness. Breast Cancer Res Treat. 2002;75:25–34. doi: 10.1023/a:1016550619925. [DOI] [PubMed] [Google Scholar]
- 34.D'Assoro AB, Busby R, Suino K, Delva E, Almodovar-Mercado GJ, Johnson H, Folk C, Farrugia DJ, Vasile V, Stivala F, Salisbury JL. Genotoxic stress leads to centrosome amplification in breast cancer cell lines that have an inactive G1/S cell cycle checkpoint. Oncogene. 2004;23:4068–4075. doi: 10.1038/sj.onc.1207568. [DOI] [PubMed] [Google Scholar]
- 35.D'Assoro AB, Busby R, Acu ID, Quatraro C, Reinholz MM, Farrugia DJ, Schroeder MA, Allen C, Stivala F, Galanis E, Salisbury JL. Impaired p53 function leads to centrosome amplification, acquired ERalpha phenotypic heterogeneity and distant metastases in breast cancer MCF-7 xenografts. Oncogene. 2008;27:3901–3911. doi: 10.1038/onc.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]