Highlights
-
•
Emergence of MERS-CoV demonstrates the need for novel vaccine strategies against coronaviruses.
-
•
Production of novel nanoparticle vaccine containing full spike protein of MERS-CoV and SARS-CoV.
-
•
Higher titer neutralizing antibody produced in vaccinated mice.
-
•
Vaccination in combination with a new adjuvant, Matrix M1, boosts neutralizing antibody titer.
Keywords: Middle East Respiratory Syndrome Coronavirus, Severe Acute Respiratory Syndrome Coronavirus, Neutralizing antibody, Vaccine
Abstract
Development of vaccination strategies for emerging pathogens are particularly challenging because of the sudden nature of their emergence and the long process needed for traditional vaccine development. Therefore, there is a need for development of a rapid method of vaccine development that can respond to emerging pathogens in a short time frame.
The emergence of severe acute respiratory syndrome coronavirus (SARS-CoV) in 2003 and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in late 2012 demonstrate the importance of coronaviruses as emerging pathogens. The spike glycoproteins of coronaviruses reside on the surface of the virion and are responsible for virus entry. The spike glycoprotein is the major immunodominant antigen of coronaviruses and has proven to be an excellent target for vaccine designs that seek to block coronavirus entry and promote antibody targeting of infected cells.
Vaccination strategies for coronaviruses have involved live attenuated virus, recombinant viruses, non-replicative virus-like particles expressing coronavirus proteins or DNA plasmids expressing coronavirus genes. None of these strategies has progressed to an approved human coronavirus vaccine in the ten years since SARS-CoV emerged. Here we describe a novel method for generating MERS-CoV and SARS-CoV full-length spike nanoparticles, which in combination with adjuvants are able to produce high titer antibodies in mice.
1. Introduction
Coronaviruses infect a range of mammals and birds, causing respiratory tract and gastrointestinal tract infections. Coronaviruses were known to cause severe and, therefore, economically important diseases in chickens [1] and pigs [2], but, while a number of coronaviruses were known to infect humans, the symptoms are usually mild in healthy adults, akin to a common cold, and only rarely cause more severe pneumonia. In 2003, however, severe acute respiratory syndrome coronavirus (SARS-CoV) emerged, causing 8273 confirmed infections, of which 775 resulted in death [3], [4], [5]. Most of the cases were linked to China, Hong Kong and Singapore, with the only major outbreak outside of this area occurring in Toronto, Canada. SARS-CoV had a zoonotic origin, having emerged from bats, via civet cats, to infect humans [6], [7]. Although there have been no reported cases of SARS-CoV infection in humans after this, a recent study has shown that the parental virus still exists in bats in China [8].
In late 2012, a novel betacoronavirus named Middle East Respiratory Syndrome Coronavirus (MERS-CoV) was identified in a sample from a severe respiratory infection patient in The Kingdom of Saudi Arabia (KSA) [9], [10]. Since then, 238 cases have been positively identified, of which 92 have resulted in death (www.who.org). All of the cases have been linked to countries on or near the Arabian peninsula (KSA, Jordan, Qatar, Egypt, Oman and United Arab Emirates). Cases in other parts of the world, notably Europe, involved recent travelers to the Middle East region or were closely linked with people who did [11]. Patients infected with MERS-CoV present at the hospital with symptoms consistent with a severe lower respiratory tract infection and, in some cases, develop kidney failure. MERS-CoV is closely related to bat coronaviruses found in China, Europe and Africa, suggesting a zoonotic origin, similar to SARS-CoV, however the reservoir of MERS-CoV has not yet been identified.
Coronaviruses are enveloped viruses with large single-stranded positive sense RNA genomes which encode 4 major structural proteins: spike (S), membrane (M), envelope (E) and nucleocapsid (N) [12]. The S protein is a type I trans-membrane glycoprotein expressed on the surface of coronaviruses that is responsible for receptor binding and virion entry to cells [13]. The location of S on the virion surface, the role of S in binding to coronavirus receptors and the finding that S can induce neutralizing antibodies in vivo [14] have made it an attractive target for vaccine development strategies [15], [16].
Previous efforts to create a vaccine for SARS-CoV have utilized a number of approaches, but none is currently licensed for use and a recent study of four putative SARS-CoV vaccines yielded negative results [17]. Initial studies suggested that whole inactivated SARS-CoV could be used as an effective vaccination [18], [19], [20], however further work has suggested that the level of protection induced by inactivated SARS-CoV is incomplete and fails to prevent SARS-CoV symptoms, while also inducing increased eosinophilia in vaccinated animals [17], [21]. Therefore, the most likely candidates for coronavirus vaccine platforms are based on spike subunits [22], [23], recombinant viruses expressing SARS-CoV proteins [24], [25], [26], DNA plasmids expressing SARS-CoV proteins [27], [28], [29] or virus-like particle (VLP) based vaccines [30], [31], [32], [33], [34], however all of these approaches come with their own safety concerns and approval processes. There are currently no approved vaccines for MERS-CoV, but early studies using a modified vaccinia virus and replication deficient MERS-CoV have been shown to induce antibodies capable of neutralizing MERS-CoV in vitro [35], [36]. Ideally, vaccines for highly pathogenic viruses, including coronaviruses, should be able to be made rapidly, on demand and in conjunction with approved adjuvants using approved techniques [37].
The emergence of both SARS-CoV and MERS-CoV highlight the importance of coronaviruses as potential human pathogens that can emerge at any time. Therefore, rapid methods for treatment and vaccination are required for this important group of viruses. In this study we describe a novel method for creating SARS-CoV S and MERS-CoV S nanoparticles that in conjunction with adjuvant are able to induce a neutralizing antibody response in mice.
2. Materials and methods
2.1. Cell and viruses
Vero E6 cells were originally obtained from the American Type Culture Collection (ATCC) and maintained in Minimal Eagles Medium (MEM; Corning) supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS; SAFC), 1% l-Glutamine (Gibco) and 1% Penicillin/Streptomycin (Gemini Bio-Products). Spodoptera frugiperda Sf9 insect cells (ATCC CRL-1711) were maintained as suspension cultures in HyQ-SFX insect serum free medium (HyClone, Logan, UT) at 27 ± 2 °C.
Mouse adapted SARS-CoV (MA15) has been previously described [38] and was grown in Vero E6 cells and stored at −80 °C. MERS-CoV (Jordan) was obtained from the NIH in conjunction with AFHSC-GEIS and NAMRU-3, with special assistance from Dr. Mohareb. All experiments with live virus were performed under biosafety level 3 conditions at the University of Maryland, Baltimore.
MERS-CoV (Jordan) Spike protein is 99.8% identical to the MERS-CoV (Al Hassa1) Spike protein sequence used in the nanoparticle cloning. We consider MERS-CoV (Al Hassa1) and MERS-CoV (Jordan) to be homologous in their Spike proteins. SARS-CoV (Urbani) Spike protein is 99.9% identical to the SARS-CoV (MA15) Spike protein sequence used in the nanoparticle cloning. We consider SARS-CoV (Urbani) and SARS-CoV (MA15) Spike proteins to be homologous.
2.2. Recombinant baculovirus
The MERS-CoV S protein sequence was from isolate Al-Hasa_1_2013 with NCBI accession #AGN70962. The SARS-CoV S protein sequence was from Urbani strain with NCBI accession #AAP13441. The genes were codon optimized for optimal expression in insect cells and biochemically synthesized for MERS-CoV S (Genscript, Piscataway, NJ) and SARS-CoV S (Geneart AG, Regensburg, Germany). Full length S genes was cloned between BamHI – HindIII sites in pFastBac1 baculovirus transfer vector plasmid (Invitrogen, Carlsbad, CA) under the transcriptional control of the Autographa californica Multiple Nuclear Polyhedrosis Virus (AcMNPV) polyhedrin promoter. Recombinant baculovirus construct was plaque purified and master seed stocks prepared, characterized for identity, and used to prepare working virus stocks. The titers of baculovirus master and working stocks were determined by using rapid titration kit (BacPak Baculovirus Rapid Titer Kit; Clontech, Mountain View, CA). Recombinant baculovirus stocks were prepared by infecting Sf9 cells at a low multiplicity of infection (MOI) of ≤0.01 plaque forming units (pfu) per cell and harvested at 68–72 h post infection (hpi).
2.3. Recombinant MERS and SARS S protein
MERS-CoV and SARS-CoV S protein antigens were produced in Sf9 cells at 2–3 × 106 cells/ml infected with specific recombinant baculovirus. Infected Sf9 cells were incubated with continuous agitation at 27 ± 2 °C and harvested at 68–72 hpi by centrifugation at 4000 × g for 15 min. S proteins were extracted from cellular membranes with a non-ionic detergent and insoluble material removed by centrifugation at 10,000 × g for 30 min. S proteins oligomers were purified using a combination of anion exchange, affinity and size exclusion chromatography. During purification the majority of the detergent is removed allowing S trimers to form higher ordered protein–protein micellular nanoparticles. Purified S nanoparticles were 0.2 micron filtered and stored −80 °C.
2.4. Analytical methods
MERS-CoV and SARS-CoV S were analyzed by SDS-PAGE using 4–12% gradient polyacrylamide gels (Invitrogen), stained with GelCode Blue stain reagent (Pierce, Rockford, IL) and quantified by scanning densitometry using OneDscan system (BD Biosciences, Rockville, MD). Purified S proteins were tested for total protein concentration (BCA bicinchoninic acid protein assay, Pierce Biochemicals) and particle size by dynamic light scattering using ZETASizer Nano (Malvern Instruments, PA) using standard, manufacturer recommended methods.
2.5. Electron microscopy
Purified MERS-CoV and SARS-CoV S proteins were adsorbed by flotation for 2 min onto a freshly discharged 400-mesh carbon parlodion-coated copper grid (Poly-Sciences, Warrington, PA). The grids were rinsed with buffer containing 20 mM Tris, pH 7.4, and 120 mM KCl and negatively stained with 1% phosphotungstic acid, then dried by aspiration. Virus-like particles were visualized on a Hitachi H-7600 transmission electron microscope (Hitachi High Technologies America, Schaumburg, IL) operating at 80 kV and digitally captured with a CCD camera at 1 K × 1 K resolution (Advanced Microscopy Techniques Corp., Danvers, MA).
2.6. Mouse immunization
The immunogenicity of purified MERS-CoV and SARS-CoV S protein nanoparticles were evaluated in BALB/c mice (6–8 weeks old), purchased from Harlan Laboratories, Inc. (Fredrick, MD). Mice (10 per group) received 2 vaccinations by intramuscular injection in the quadriceps muscles on days 0 and 21. Mice immunized with MERS-CoV S protein received either 1, 3 or 10 μg of S protein alone or 1 or 3 μg MERS-CoV S protein with either 120 μg aluminum hydroxide (AlHydrogel, Brenntag AG) or 5 μg Matrix M1 (Isconova, Sweden). Mice immunized with SARS-CoV S protein received 3 μg S protein alone or S protein with either 120 μg aluminum hydroxide (Alum) or 5 μg Matrix M1. Sera were collected on day 0, 21 and 45 for serological analysis. Blood was collected via the retro-orbital route and serum was obtained by centrifugation of whole blood.
All animal procedures were in accordance with the NRC Guide for the Care and Use of Laboratory Animals, the Animal Welfare Act, and the CDC/NIH Biosafety in Microbiological and Biomedical Laboratories.
2.7. Coronavirus neutralization assays
Mouse sera were diluted 1:20 in Vero E6 cell growth media and further diluted down to 1:40960 in a two-fold series. MERS-COV-Jordan or SARS-CoV (MA15) was added at a final concentration of 3950 TCID50/ml and incubated for 30 min at room temperature. Mock infected mouse sera and MERS-CoV-Jordan or SARS-CoV in Vero E6 media served as negative and positive controls respectively. The inhibitory capacity of each serum dilution was assessed by TCID50 assay and the serum dilution at which MERS-CoV-Jordan or SARS-CoV was inhibited by 50% was recorded. The geometric mean titer (GMT) for each group of mice was calculated and presented ± the 95% confidence interval (CI).
2.8. Coronavirus TCID50
Vero E6 cells were seeded into 96-well plates (USA scientific) at 1 × 104 cells per well and were cultured overnight at 37 °C. Cells were infected with a 5-fold dilution series of virus containing media in triplicate and cultured for a further 48 h. Plates were fixed in 4% paraformaldehyde (Sigma–Aldrich) for 5 min at room temperature and then stained with 0.05% crystal violet (Sigma–Aldrich) in 20% methanol (Sigma–Aldrich) for 30 min at room temperature. Plates were then washed twice in water and cell death was assessed by the presence (live cells) or absence (dead cells) of crystal violet stain. Viral load was calculated based on the dilution at which the media killed 50% of the cells using the TCID50 formula [39].
2.9. Statistical analysis
Neutralization titers were analyzed using a non-parametric one-way ANOVA. Statistical significance was assumed when p < 0.05.
3. Results
3.1. MERS and SARS S protein characterization
Full-length recombinant MERS and SARS S protein were produced in the baculovirus insect cell expression system. The S proteins were extracted from infected cells pellet with non-ionic detergent and purified by column chromatography as previously described [30]. Purified full-length MERS and SARS S proteins had an apparent molecular weight of 180 kDa and 160 kDa by SDS-PAGE, respectively (Fig. 1A and B). Purified MERS and SARS S proteins formed ∼25 nm diameter particles consisting of multiple S protein molecules when observed by electron microscopy (Fig. 1C and D) and dynamic light scattering (data not shown).
Fig. 1.
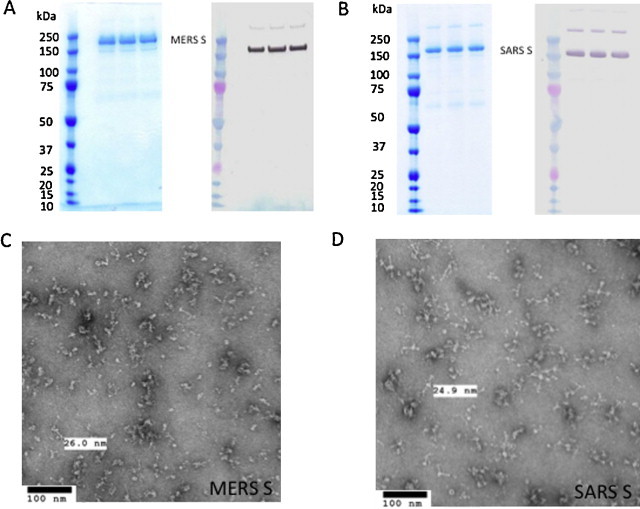
Purification of coronavirus spike proteins and generation of spike nanoparticles. (A and B) Purified MERS-CoV (A) and SARS-CoV (B) S protein micelle, each protein is loaded on the gel in triplicate lanes. Left panel: coomassie blue stain, right panel: western blot using rabbit anti-MERS S (A) or rabbit anti-SARS S (B) as primary antibody. Protein marker: precision plus protein molecular weight marker (Bio-Rad, Hercules, CA). (C and D) Phosphotungstic acid negative stain electron microscopy images of MERS (C) and SARS (D) S protein micelle. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
3.2. Immunogenicity of S protein nanoparticles
In vaccine development, an effective vaccine should have a strong neutralizing response against the target, i.e. the dilution of antibody at which it effectively neutralizes the virus should be high. In these experiments, 10 mice per group were inoculated with MERS-CoV or SARS-CoV S proteins and serum was tested against the homologous virus.
3.3. Generation of neutralizing antibodies to SARS-CoV in BALB/c mice using S nanoparticles
Previous work has shown production of a protective neutralizing antibody response in mice inoculated with chimeric SARS-CoV S nanoparticles in combination with Alum adjuvant [30]. In this study we also used Matrix M1 adjuvant to assess induction of neutralizing antibody responses in mice.
When 3 μg of SARS-CoV S protein is used without adjuvant the neutralizing antibody concentration in the sera at day 21 post-inoculation is low (GMT = 86) and this does not change at day 45 post-inoculation (GMT = 80), indicating that SARS-CoV S protein without adjuvant does not effectively induce anti-SARS-CoV neutralizing antibody (Table 1 and Fig. 1). When Alum is used as an adjuvant in conjunction with 3 μg of SARS-CoV S protein, there is a statistically significant 7–11 fold increase in neutralizing antibody (for example, at 21 days post-inoculation GMT = 970; p < 0.01) and when Matrix M1 adjuvant is used in conjunction with 3 μg of SARS-CoV S, there is a statistically significant 36–39 fold increase in neutralizing antibody (for example, at 21 days post-inoculation, GMT = 3152; p < 0.001), indicating that Matrix M1 is a more effective adjuvant than Alum. None of the vaccinations with SARS-CoV S, showed any significant difference in neutralizing antibody titer at 45 days post-inoculation compared to 21 days post-inoculation, suggesting that the neutralizing antibody response does not change over the vaccination course (Fig. 2 ).
Table 1.
Coronavirus neutralizing antibody titers.
| Vaccine (S protein) | Dose (μg) | Adjuvant | Day | GMT | 95% LCL | 95% UCL |
|---|---|---|---|---|---|---|
| MERS-CoV | 1 | None | 21 | 49 | 17 | 140 |
| 45 | 320 | 110 | 934 | |||
| 3 | 21 | 43 | 22 | 85 | ||
| 45 | 299 | 193 | 461 | |||
| 10 | 21 | 65 | 33 | 126 | ||
| 45 | 243 | 104 | 567 | |||
| 1 | Alum | 21 | 735 | 419 | 1,291 | |
| 45 | 640 | 251 | 1,630 | |||
| 3 | 21 | 368 | 171 | 792 | ||
| 45 | 905 | 505 | 1,624 | |||
| 1 | Matrix M1 | 21 | 3,378 | 1,804 | 6,325 | |
| 45 | 1,940 | 1,139 | 3,306 | |||
| 3 | 21 | 1,194 | 542 | 2,634 | ||
| 45 | 1,689 | 831 | 3,432 | |||
| SARS-CoV | 3 | None | 21 | 86 | 32 | 228 |
| 45 | 80 | 38 | 168 | |||
| Alum | 21 | 970 | 497 | 1,894 | ||
| 45 | 640 | 330 | 1,240 | |||
| Matrix M1 | 21 | 3,152 | 1,624 | 6,117 | ||
| 45 | 3,152 | 1,864 | 5,329 |
The vaccine groups are shown that were tested for neutralizing antibody titer. Dose, adjuvant type, day of bleeding and geometric mean titer with confidence intervals are shown for each group. N = 10 for each dosing/adjuvant group shown.
Fig. 2.
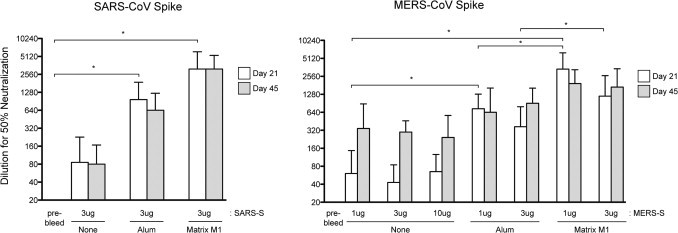
Neutralization titers of coronavirus spike vaccinated mice. Serum from mice vaccinated with the noted mix of spike protein and adjuvant was analyzed by TCID50 assay for neutralization capability and geometric mean titer (GMT) graphed for all groups (10 mice per group). Error bars indicate 95% confidence interval. Stars denote statistically significant differences (p < 0.05) noted in the text as described in Section 2.
These data confirm previous observations that Alum significantly enhances the production of anti-SARS-CoV neutralizing antibodies in mice [30]. This also demonstrates that the Matrix M1 adjuvant is more effective at inducing an anti-SARS-CoV neutralizing antibody response in mice, suggesting that Matrix M1 can be used as an effective vaccine adjuvant with SARS-CoV S.
3.4. Generation of neutralizing antibodies to MERS-CoV in BALB/c mice using S nanoparticles
When MERS-CoV S was inoculated into Balb/C mice without adjuvant, levels of neutralizing antibody to MERS-CoV (Jordan) at day 21 post-inoculation were low (for example 1 μg S GMT = 49). These data demonstrate that inoculation with MERS-CoV S protein alone is not an effective method for development of a robust neutralizing antibody response. At day 45 post-inoculation without adjuvant, levels of neutralization of MERS-CoV-Jordan are 3–7 fold higher than at day 21 and are not statistically significant (for example 1 μg MERS-S GMT = 320; p > 0.05). The amount of MERS-S protein did not have any significantly effect the GMT of the neutralizing responses, but there is a trend to more consistent responses with higher doses of MERS-S.
When the adjuvant Alum is used in conjunction with MERS-CoV S for inoculation, the levels of neutralizing antibody at day 21 post-inoculation are significantly higher than found in sera from mice inoculated with MERS-S alone (for example 1 μg MERS-S alone GMT = 49 compared to 1 μg MERS-S with Alum GMT = 735; p < 0.05), indicating that Alum promotes production of anti-MERS-CoV-S neutralizing antibodies. There is no significant difference between dosing regimens consisting of 1 μg of MERS-CoV-S or 3 μg of MERS-CoV-S in the presence of Alum (for example, at day 21, 1 μg MERS-S plus Alum GMT = 735 compared to 3 μg MERS-S plus Alum GMT = 368; p > 0.05), suggesting that lower doses of MERS-S can be used for effective vaccination in the presence of Alum. Furthermore, there is no significant difference between neutralizing antibodies in the sera from day 21 compared to day 45 (for example 1 μg MERS-S plus Alum at day 21 GMT = 735 compared to day 45 GMT = 640; p > 0.05), indicating that, in the presence of Alum, the vaccination course causes early production of neutralizing antibodies that are maintained.
When Matrix M1 adjuvant is used in conjunction with MERS-CoV S for inoculation, the increase in levels of neutralizing antibody at day 21 post-inoculation are significantly higher compared to the sera from mice inoculated with MERS-S alone (27–68 fold) and 3–4 fold higher than sera from mice inoculated with MERS-S plus Alum (for example 1 μg MERS-S alone GMT = 49 compared to 1 μg MERS-S with Alum GMT = 735 compared to 1 μg MERS-S with Matrix M1 GMT = 3378; p < 0.001), indicating that Matrix M1 promotes production of anti-MERS-CoV-S neutralizing antibodies higher than that seen when Alum is used as the adjuvant. There is no significant difference between dosing mice with 1 μg of MERS-CoV-S or 3 μg of MERS-CoV-S in the presence of Matrix M1 (for example, at day 21, 1 μg MERS-S plus Matrix M1 GMT = 3378 compared to 3 μg MERS-S plus Matrix M1 GMT = 1194; p > 0.05), again suggesting that lower doses of MERS-S can be used for effective vaccination in the presence of Matrix M1. Furthermore, there is no significant difference between neutralizing antibodies in the sera from day 21 compared to day 45 (for example 1 μg MERS-S plus Matrix M1 at day 21 GMT = 3378 compared to day 45 GMT = 1940; p > 0.05), suggesting that, in the presence of Matrix M1, the vaccination course causes early production of neutralizing antibodies that are maintained. These data demonstrate that the MERS-S vaccine platform can be used to induce a neutralizing antibody response in mice. We also demonstrate that the Matrix M1 adjuvant significantly enhances the level of neutralizing antibody produced compared to Alum.
3.5. Cross-protection activity of anti-MERS-CoV and anti-SARS-CoV sera
To determine if there is cross-neutralization of SARS-CoV S antisera against MERS-CoV infection, and vice versa, we tested sera that offered strong protection against the homologous virus against the heterologous virus. Therefore, sera from mice inoculated with 3 μg of MERS-S or SARS-S in combination with Matrix M1 adjuvant were tested against SARS-CoV and MERS-CoV respectively.
The sera from mice inoculated with 3 μg MERS-S in combination with Matrix M1 was unable to neutralize SARS-CoV, as none of the dilutions tested had any effect on the titer of SARS-CoV. The sera from mice inoculated with 3 μg SARS-S in combination with Matrix M1 was only minimally able to neutralize MERS-CoV above background, with 9 out of 10 mice showing low level neutralizing effects against MERS-CoV, but none showing complete neutralization and all neutralizing to a much lower extent than anti-SARS-CoV sera against SARS-CoV or anti-MERS-CoV sera against MERS-CoV (data not shown).
These data demonstrate that there is only limited, if any, cross-protection in mouse sera containing neutralizing antibodies induced against MERS-S or SARS-S.
4. Discussion
There is a need in vaccine production for a rapid process of manufacturing vaccines to emerging pathogens [37]. The recent emergences of SARS-CoV, in 2003, and MERS-CoV, in 2012, have demonstrated the importance of the Coronaviridae virus family as an important group of emerging pathogens. Previous efforts to develop vaccines to SARS-CoV have encompassed many methods, but have yielded variable results. In this study we describe a novel method for development of protein nanoparticles carrying the spike proteins from SARS-CoV and MERS-CoV that were able to induce significant neutralizing antibody responses in mice. The nanoparticles contain only MERS-S or SARS-S and no other potentially immunogenic viral or insect proteins. The formation of amphiphilic membrane proteins aggregates (protein nanoparticles), used in this study, which are relatively homogeneous in size, soluble in aqueous media, and used as a platform for vaccines was first described by Simons et al. [40]. Recently a respiratory syncytial virus (RSV) fusion (F) glycoprotein nanoparticle vaccine was shown to be highly immunogenic, inducing protective immune responses against conformational epitopes [41]. In a phase 1 human study the RSV F nanoparticle vaccine was well tolerated and induced neutralizing antibodies at levels associated with decreased hospitalization from RSV in children [42].
We have shown that mice vaccinated with coronavirus S nanoparticles produced high levels of neutralizing antibodies against the homologous virus, but did not offer cross-protection against the heterologous virus. These data suggest that the coronavirus S nanoparticles are able to elicit specific anti-SARS-CoV or MERS-CoV antibody responses in mice, but that there is not a general anti-coronavirus response that can be used to protect against multiple coronaviruses. Given that, so far, only one coronavirus has emerged at a time, this should be sufficient, but further work will determine if nanoparticle vaccines can be used in combination.
We also investigated the use of adjuvants to boost production of neutralizing antibody to coronavirus S. When we used Alum or Matrix M1, neutralizing antibody levels were significantly boosted (15 fold with Alum and 68 fold with Matrix M1) over levels of neutralizing antibody produced from S nanoparticles alone. Alum is a common adjuvant that has been used previously to boost neutralizing antibodies to SARS-CoV in a VLP vaccine strategy [30], but here we show that Alum also significantly boosts responses to SARS-CoV S nanoparticles and also to MERS-CoV S nanoparticles in mice.
Matrix M1 is a saponin based adjuvant manufactured by Novavax, AB (formally Isconova AB, Uppsala Sweden). Quillaja saponins are extracted from the bark of the tree Quillaja saponaria Molina and two fractions, A and C, are independently formulated with phospholipids and cholesterol to create matrix particles (∼40 nm cage like structures). Matrix M1 is comprised of two components, Matrix-A and Matrix-C at an 85/15 ratio [43] and has been shown to be a potent adjuvant in clinical trials [44]. The data in this study demonstrate that Matrix M1 is more effective than Alum at boosting the neutralizing antibody response to coronavirus S nanoparticles in mice, especially with MERS-CoV spike.
Further studies will be needed to determine if coronavirus S nanoparticle vaccinations block coronavirus replication and pathogenesis in vivo, as animal models are developed. No small animal model currently exists, as MERS-CoV does not replicate in mice, ferrets, or hamsters. [45], [46]. We have demonstrated that coronavirus S nanoparticles are able to stimulate neutralizing antibody responses in mice, an important first step towards vaccine development.
Acknowledgement
This project has been funded in part by a supplement to NIH RO1 AI095569 (MBF).
References
- 1.Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56(4):634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- 2.Song D., Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44(2):167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding Y. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drosten C. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 5.Kuiken T. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau S.K. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A. 2005;102(39):14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poon L.L. Identification of a novel coronavirus in bats. J Virol. 2005;79:2001–2009. doi: 10.1128/JVI.79.4.2001-2009.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge X.Y. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Boheemen S. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio. 2012;3(6) doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaki A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 11.Mailles A. First cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections in France, investigations and implications for the prevention of human-to-human transmission, France, May 2013. Euro Surveill. 2013;18(24) [PubMed] [Google Scholar]
- 12.Masters P.S. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belouzard S. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu M. Antibody responses to individual proteins of SARS coronavirus and their neutralization activities. Microbes Infect. 2005;7(5–6):882–889. doi: 10.1016/j.micinf.2005.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du L. The spike protein of SARS-CoV – a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X. Receptor-binding domain as a target for developing SARS vaccines. J Thorac Dis. 2013;5(Suppl 2):S142–S148. doi: 10.3978/j.issn.2072-1439.2013.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng C.T.et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PloS One. 2012;7(4):e35421. doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spruth M. A double-inactivated whole virus candidate SARS coronavirus vaccine stimulates neutralising and protective antibody responses. Vaccine. 2006;24(5):652–661. doi: 10.1016/j.vaccine.2005.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin E. Immunogenicity and protective efficacy in monkeys of purified inactivated Vero-cell SARS vaccine. Vaccine. 2006;24(7):1028–1034. doi: 10.1016/j.vaccine.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J.T. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther. 2007;12(7):1107–1113. [PubMed] [Google Scholar]
- 21.Bolles M. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85(23):12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J. Immunogenicity and protection efficacy of monomeric and trimeric recombinant SARS coronavirus spike protein subunit vaccine candidates. Viral Immunol. 2013;26(2):126–132. doi: 10.1089/vim.2012.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun. 2004;324(2):773–781. doi: 10.1016/j.bbrc.2004.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shim B.S. Sublingual immunization with recombinant adenovirus encoding SARS-CoV spike protein induces systemic and mucosal immunity without redirection of the virus to the brain. Virol J. 2012;9:215. doi: 10.1186/1743-422X-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisht H. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci U S A. 2004;101(17):6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Q. Baculovirus surface display of SARS coronavirus (SARS-CoV) spike protein and immunogenicity of the displayed protein in mice models. DNA Cell Biol. 2006;25(12):668–673. doi: 10.1089/dna.2006.25.668. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z.Y. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin J.E. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26(50):6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu B. Humoral and cellular immune responses induced by 3a DNA vaccines against severe acute respiratory syndrome (SARS) or SARS-like coronavirus in mice. Clin Vaccine Immunol. 2009;16(1):73–77. doi: 10.1128/CVI.00261-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y.V. Chimeric severe acute respiratory syndrome coronavirus (SARS-CoV) S glycoprotein and influenza matrix 1 efficiently form virus-like particles (VLPs) that protect mice against challenge with SARS-CoV. Vaccine. 2011;29(38):6606–6613. doi: 10.1016/j.vaccine.2011.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu X. Immune responses against severe acute respiratory syndrome coronavirus induced by virus-like particles in mice. Immunology. 2007;122(4):496–502. doi: 10.1111/j.1365-2567.2007.02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai B.et al. Virus-like particles of SARS-like coronavirus formed by membrane proteins from different origins demonstrate stimulating activity in human dendritic cells. PloS One. 2008;3(7):e2685. doi: 10.1371/journal.pone.0002685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lokugamage K.G. Chimeric coronavirus-like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protect mice against challenge with SCoV. Vaccine. 2008;26(6):797–808. doi: 10.1016/j.vaccine.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu B. Effect of mucosal and systemic immunization with virus-like particles of severe acute respiratory syndrome coronavirus in mice. Immunology. 2010;130(2):254–261. doi: 10.1111/j.1365-2567.2010.03231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almazan F. Engineering a replication-competent: propagation-defective Middle East respiratory syndrome coronavirus as a vaccine candidate. mBio. 2013;4(5):e00650–e713. doi: 10.1128/mBio.00650-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song F. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus ankara efficiently induces virus-neutralizing antibodies. J Virol. 2013;87(21):11950–11954. doi: 10.1128/JVI.01672-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Groot A.S. Making vaccines on demand: a potential solution for emerging pathogens and biodefense? Human Vaccines Immunother. 2013;9(9) doi: 10.4161/hv.25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts A. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathogens. 2007;3(1):e5. doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27(3):493–497. [Google Scholar]
- 40.Simons K. Formation of protein micelles from amphiphilic membrane proteins. Proc Natl Acad Sci U S A. 1978;75(11):5306–5310. doi: 10.1073/pnas.75.11.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith G. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS One. 2012;7(11):e50852. doi: 10.1371/journal.pone.0050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glenn G.M. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine. 2013;31(3):524–532. doi: 10.1016/j.vaccine.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Lovgren Bengtsson K., Morein B., Osterhaus A.D. ISCOM technology-based Matrix M adjuvant: success in future vaccines relies on formulation. Expert Rev Vaccines. 2011;10(4):401–403. doi: 10.1586/erv.11.25. [DOI] [PubMed] [Google Scholar]
- 44.Cox R.J. Evaluation of a virosomal H5N1 vaccine formulated with Matrix M adjuvant in a phase I clinical trial. Vaccine. 2011;29(45):8049–8059. doi: 10.1016/j.vaccine.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 45.Devitt E. Lack of small animal model hinders MERS coronavirus research. Nat Med. 2013;19(8):952. doi: 10.1038/nm0813-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coleman C.M. Wild type and innate immune deficient mice are not susceptible to the Middle East Respiratory Syndrome Coronavirus. J Gen Virol. 2013;95:408–412. doi: 10.1099/vir.0.060640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


