Abstract
Half of the recovered expanded criteria donor (ECD) kidneys are discarded in the United States. A new kidney allocation system offers kidneys at higher risk of discard, Kidney Donor Profile Index (KDPI) >85%, to a wider geographic area to promote broader sharing and expedite utilization. Dual kidney transplantation (DKT) based on the KDPI is a potential option to streamline allocation of kidneys which otherwise would have been discarded. To assess the clinical utility of the KDPI in kidneys at higher risk of discard, we analyzed the OPTN/UNOS Registry that included the deceased donor kidneys recovered between 2002 and 2012. The primary outcomes were allograft survival, patient survival and discard rate based on different KDPI categories (<80%, 80–90% and >90%). Kidneys with KDPI >90% were associated with increased odds of discard (OR = 1.99, 95% CI 1.74–2.29) compared to ones with KDPI <80%. DKTs of KDPI >90% were associated with lower overall allograft failure (HR = 0.74, 95% CI 0.62–0.89) and better patient survival (HR = 0.79, 95% CI 0.64–0.98) compared to single ECD kidneys with KDPI >90%. Kidneys at higher risk of discard may be offered in the up-front allocation system as a DKT. Further modeling and simulation studies are required to determine a reasonable KDPI cutoff percentile.
Keywords: Deceased donor, discard, dual kidney transplant, KDPI, KDRI, outcomes, UNOS, utilization
Introduction
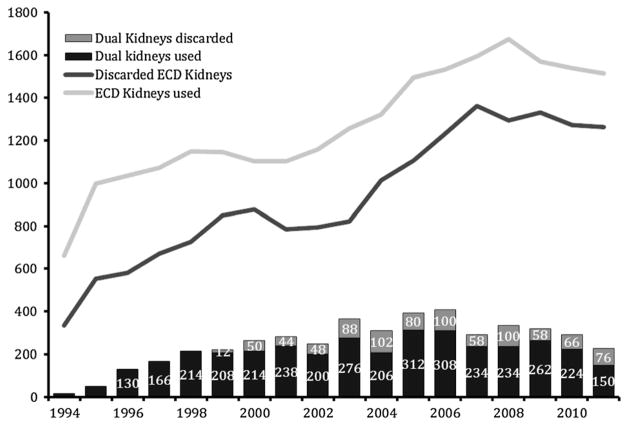
The percentage of expanded criteria donor (ECD) and dual kidneys recovered but not transplanted in the United States remains significantly high (42% and 20%, respectively; Figure 1). This is particularly striking when compared to the 8% overall kidney discard rate reported by the Eurotransplant Consortium (1–3). There exists high discard rates of kidneys, significant survival overlap among standard criteria donor (SCD) and ECD kidneys, variability in access to transplantation by blood group and geographic location, and survival longevity mismatch between donor and recipient pairs. As a result, the United Network of Organ Sharing (UNOS) Kidney Transplantation Committee has approved a new allocation system on June 25, 2013, with the implementation date expected in 2014 (4). The primary goal of the new system is better matching of graft and recipient longevity and more efficient organ utilization through introduction of the Kidney Donor Profile Index (KDRI; a percentage score derived from the Kidney Donor Risk Index [KDRI]) and estimated posttransplant survival formulae. The new system may prove to be a disadvantage to older end-stage renal disease patients because the highest quality kidneys will be allocated to candidates with the highest estimated posttransplant survival. Therefore, there is a real need to expand the marginal donor pool by minimizing discard of high-risk kidneys (KDPI >85%) to accommodate older end-stage renal disease patients in the growing waiting list, of which 20% were 65 years or older in 2012 (5).
Figure 1.
Deceased donor kidneys transplanted and discarded for single ECD and dual kidney transplantation based on the OPTN/UNOS Registry, between 1994 and 2012. ECD, expanded criteria donor.
A potential option to decrease discard of marginal kidneys is to allocate them as a dual kidney transplantation (DKT) (6). The concept of DKT was first introduced by Dr. Ratner and his colleagues in 1993 (7). Currently, DKT is not a part of the allocation system in the United States and comprises less than 2% of the deceased donor transplants performed (5). According to the UNOS policy 3.5.6.5 (Double Kidney Allocation-UNOS-DKT criteria), an Organ Procurement Organization (OPO) must offer kidneys individually through the allocation sequence before offering both kidneys to a single candidate, unless the donor meets at least two of the following criteria: (1) donor age >60 years, (2) estimated creatinine clearance <65 mL/min on admission, (3) rising serum creatinine, >2.5 mg/dL, at the time of organ recovery, (4) donor medical comorbidities (hypertension and/or diabetes mellitus) or (5) adverse renal histopathology (15% < glomerulosclerosis [GS] <50%) at procurement) (8).
Potential factors associated with low DKT utilization include differences in practice among OPOs, longer operation time, increased surgical complications and the fact that these kidneys are considered suboptimal (more so than those in the ECD pool) (9–15). However, DKTs have similar short-term survival outcomes to those of ECD kidneys despite having high-risk characteristics (6). In the United States, new strategies are urgently needed for more effective and efficient use of ECD kidneys and selecting appropriate recipients to eventually alleviate the organ shortage for seniors (13,15–20).
Determining whether to use kidneys with high KDPI as a single ECD kidney versus DKT is complex due to the following reasons: (1) DKT is not a part of the up-front allocation system; (2) transplant centers factor additional information (anatomy, full histology features and machine perfusion characteristics) into their decision to accept or decline kidneys; (3) there is a significant disparity among practices of transplant centers and OPOs (21); and (4) the UNOS-DKT criteria do not effectively identify ECD kidneys that would provide allograft and patient survival benefit if transplanted as DKT. Currently, ECD kidneys are offered first locally and candidates who elect to receive ECD kidneys are rank-ordered only according to waiting time. Generally, OPOs with longer waiting times tend to procure and transplant more ECD kidneys than OPOs with shorter waiting times, suggesting that utilization of these kidneys is driven by more demand and lengthier waiting times than quality.
DKT based on the KDPI is a potential option to streamline allocation of kidneys which otherwise would have been discarded. We conducted this study using the Organ Procurement and Transplantation Network (OPTN)/UNOS data to address three clinically relevant issues: (1) describe utilization and variation of single ECD and dual kidneys across the United States; (2) compare contemporary (since 2002) deceased donor, recipient and transplant characteristics and recipient outcomes between DKT and ECD transplants based on the KDPI categories, <80%, 80–90% and >90% (corresponding KDRI-UNOS categories <1.7, 1.7–2.0 and >2.0); and (3) assess the clinical utility of KDPI in identifying kidneys with better outcomes when transplanted as dual compared to the current UNOS-DKT criteria.
Materials and Methods
Kidney Donor Risk Index
In this article, we use three interrelated KDRI scores (KDRI-UNOS, KDRI-median and KDPI) to express the quality of the deceased donor kidneys relative to other donors. Original KDRI is defined as a single metric that combines 15 donor and transplant factors to estimate relative risk of allograft failure posttransplant in an average adult recipient (Online Supplement, Methods section) (22). KDRI-UNOS, displayed in DonorNet (UNOS Centralized Computer Network between OPO and transplant centers), is the deceased donor–only version (based on 10 donor factors) of original KDRI (23). A version of the KDRI-UNOS, KDRI-median, is scaled to a value of 1.0 corresponding to the median donor among all deceased donors recovered in the prior calendar year (KDRI-median = KDRI − UNOS/[scaling factor]). The KDRI-median has been reported on a cumulative percentage scale, the KDPI, in the DonorNet since March 2012. When performing long-term trend analyses with KDPI, it is important that the scaling factor remains constant (23). Therefore, all the KDPI calculations in this article are scaled to a factor of 1.22219212347775, a median KDRI-UNOS value among all deceased kidney donors recovered during 2012.
Study cohort
This is a retrospective cohort study using the OPTN/UNOS data of kidneys recovered from deceased donors between 2002 and 2012 (discarded [Dual n = 368 and ECD n = 13 543] and transplanted [Dual n = 1160 and ECD = 15 448 kidneys]). Our analysis was restricted to first ECD and DKT kidney transplants and excluded all deceased donors younger than 50 years old, recipient age <18 years, living renal transplants, multiorgan transplants and patients with missing data to calculate the KDRI score. To evaluate variation in regional utilization, we analyzed 11 OPTN regions in the United States. We used donor-only KDRI scores for discard, kidney and patient survival analysis stratified by the KDPI categories <80%, 80–90%, >90%, corresponding to the KDRI-UNOS groups of <1.7, 1.7–2.0, >2.0, respectively. The DKT and the ECD pairs under each KDPI category were compared to each other.
Outcomes
The primary outcomes were: (1) allograft failure (defined as the need for renal replacement therapy, preemptive re-transplantation or death with functioning graft); (2) patient survival; and (3) donor kidney discard, both single and dual (the latter refers to those instances when both kidneys were procured and neither was transplanted). Secondary outcomes included delayed allograft function (DGF; need for at least one dialysis session within first week of kidney transplantation), early allograft failure (a graft never achieving sufficient function, estimated glomerular filtration rate [eGFR] <20 mL/min, or not allowing cessation of dialysis within 3 months after transplantation), and low eGFR (<30 mL/min/1.73 m2) at last follow-up visit. To address clinical utility of current UNOS-DKT criteria and the KDPI categories (<80% [<1.7], 80–90% [1.7–2.0], >90% [>2.0]), we fitted multivariable Cox and logistic regression models for outcomes of DKT, relative to single ECD kidney transplants based on the UNOS data between 2002 and 2012.
Statistical analysis
Donor and recipient characteristics were described using mean and standard deviation or frequencies as needed. Comparisons between groups were made using t-test, Kruskal–Wallis or chi-square test, as appropriate. Allograft survival was estimated by the Kaplan–Meier method and the test for equality of survival curves was performed using the log-rank test. Univariate and multivariable Cox proportional-hazard regression models were used to estimate relative risk of allograft failure. Logistic regression models were fitted to examine the risk of DGF and early allograft failure in deceased donor transplants that fulfill UNOS-DKT criteria and determinants of discard in ECD kidneys. A p-value <0.05 is considered statistically significant. Statistical analyses were performed with Stata 12/MP2 (Stata Corp., College Station, TX).
Results
Utilization of kidneys with high discard rates
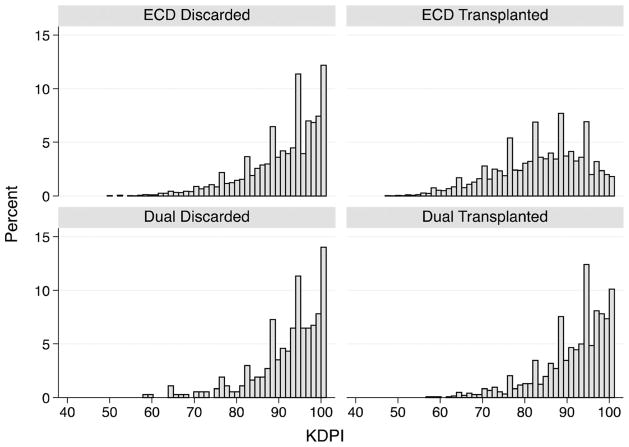
The distribution of the KDPI of ECD and dual kidneys by their disposition is graphically illustrated in Figure 2. There was considerable overlap in the KDPI scores for both groups between transplanted and discarded kidneys. This is particularly striking for dual kidney donors.
Figure 2.
Histograms showing KDPI distribution of the deceased donor kidneys stratified by their donor and disposition type between 2002 and 2012. KDPI, Kidney Donor Profile Index.
Utilization by the OPTN regions
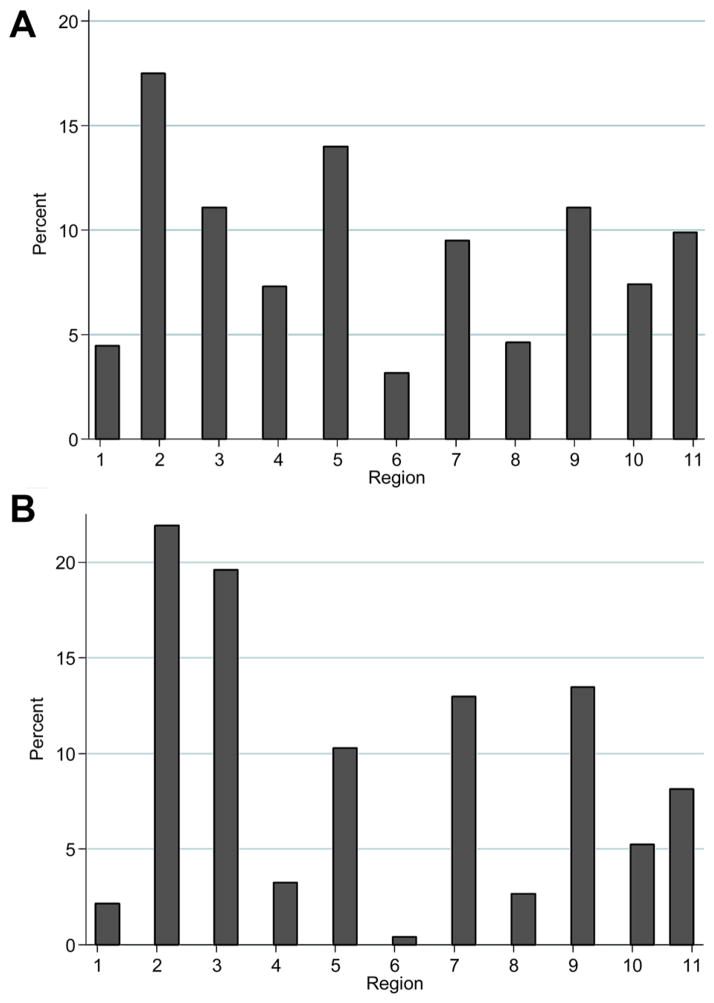
There was a great interregional disparity in utilization of these high KDPI kidneys among regions between 2002 and 2012 (see Figure 3A, B). Although all of the regions performed at least one DKT, Regions 1, 4, 6 and 8 performed fewer DKTs. These four regions also performed the fewest ECD transplants.
Figure 3.
(A, B) Utilization of dual kidney transplantation and expanded criteria donor kidneys by the OPTN regions between 2002 and 2012.
Determinants and organ quality of discards
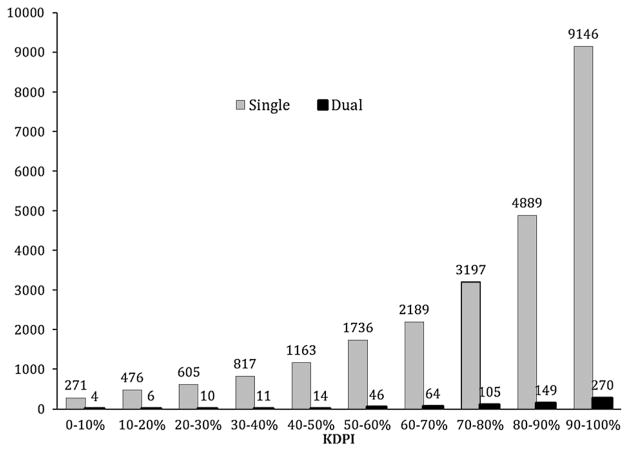
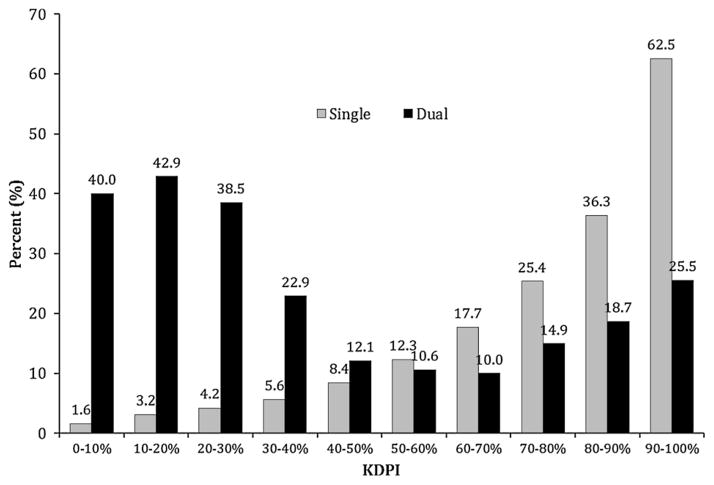
The absolute numbers of discards for single ECD and dual kidneys are shown in Figure 4. For single ECD kidneys, discard rates were 12.1%, 17.7%, 25.4%, 36.3% and 62.5%, across KDPI deciles 50–60%, 60–70%, 70–80%, 80–90% and 90–100%, respectively, while dual kidney discards ranged between 12.1% and 25.5% (Figure 5). The process of capturing reasons for organ discard is highly subjective and the recorded reason does not always reflect the actual reason for discard. Table 1 lists the common causes of discard and kidney quality. The most common reason for organ discard reported to UNOS was biopsy findings, which accounted for 48% of discards of the kidneys from donors ≥50 years old. The mean KDPI of discarded ECD and dual kidneys was approximately 90% regardless of cause of discard. Table 2 displays the results of the univariate and multivariate logistic regression models examining predictors of discard in ECD kidneys. Factors associated with an increased likelihood of discard were fulfilling UNOS-DKT criteria (OR 1.73, 95% CI 1.54–1.93), KDPI 80–90% (OR 1.17, 95% CI 1.03–1.33), KDPI > 90% (OR 1.99, 95% CI 1.74–2.29) and Region 10 (OR 1.36, 95% CI 1.01–1.83). Variance inflation factor (VIF) for the model was not significant for multicollinearity (mean VIF = 2.30).
Figure 4.
Absolute number of discarded single and dual kidneys by the KDPI deciles between 2002 and 2012. KDPI, Kidney Donor Profile Index.
Figure 5.
Discard rate of expanded criteria donor and dual kidneys by the KDPI deciles between 2002 and 2012. KDPI, Kidney Donor Profile Index.
Table 1.
Common causes of discard and their donor quality between 2002 and 2012
| Biopsy findings | Poor kidney function | No recipient located | Long cold ischemia time | No reason specified | p-Value | |
|---|---|---|---|---|---|---|
| Dual1 | ||||||
| % of discarded kidneys | 48.4 | 13.0 | 13.2 | 0.5 | 7.4 | <0.001 |
| KDRI-UNOS | 2.23 ± 0.53 | 2.08 ± 0.42 | 2.23 ± 0.54 | 2.01 | 2.11 ± 0.39 | 0.268 |
| KDPI (%)2 | 91.0 ± 10.2 | 89.1 ± 10.0 | 91.1 ± 9.4 | 90.5 | 90.1 ± 11.7 | 0.510 |
| ECD3 | ||||||
| % of discarded kidneys | 48.2 | 9.6 | 9.7 | 1.5 | 12.3 | <0.001 |
| KDRI-UNOS | 2.19 ± 0.47 | 2.13 ± 0.45 | 2.28 ± 0.46 | 2.1 ± 0.41 | 2.13 ± 0.46 | <0.001 |
| KDPI (%)2 | 91.3 ± 8.4 | 90.2 ± 8.8 | 92.9 ± 7.5 | 88.5 ± 9.9 | 90.0 ± 9.2 | <0.001 |
ECD, expanded criteria donor; KDPI, Kidney Donor Profile Index; KDRI, Kidney Donor Risk Index; UNOS, United Network of Organ Sharing.
Dual kidney donor age ≥50 years.
KDPI is calculated based on a scaling factor of 1.22219212347775, a median KDRI-UNOS value among all deceased kidney donors recovered during 2012.
ECD kidneys include donation after cardiac death kidneys.
Table 2.
Logistic regression for predictors of discard in ECD kidneys recovered between 2002 and 2012
| Variables (reference) | Univariate
|
Multivariable1
|
||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Pretransplant biopsy (No) | 1.25 (1.09–1.43) | 0.001 | 1.07 (0.93–1.24) | 0.330 |
| Fulfilling UNOS-DKT criteria (No) | 2.28 (2.07–2.52) | <0.001 | 1.73 (1.54–1.93) | <0.001 |
| KDPI %2 (corresponding KDRI-UNOS categories) | ||||
| <80% (<1.7) | Referent | – | Referent | – |
| ≥80% and ≤90% (≥1.7 and ≤2.0) | 1.41 (1.25–1.60) | <0.001 | 1.17 (1.03–1.33) | 0.018 |
| >90% (>2.0) | 2.81 (2.50–3.17) | <0.001 | 1.99 (1.74–2.29) | <0.001 |
| Transplant center volume 100 kidney transplants/year (<100/year) | 1.06 (0.97–1.18) | 0.192 | 1.03 (0.93–1.15) | 0.553 |
| OPTN regions | ||||
| Region 1 | Referent | – | Referent | – |
| Region 2 | 1.24 (0.96–1.61) | 0.103 | 1.20 (0.92–1.56) | 0.182 |
| Region 3 | 0.94 (0.71–1.24) | 0.652 | 1.00 (0.75–1.33) | 0.984 |
| Region 4 | 1.06 (0.79–1.42) | 0.645 | 1.16 (0.86–1.57) | 0.329 |
| Region 5 | 0.99 (0.75–1.29) | 0.940 | 1.05 (0.79–1.38) | 0.746 |
| Region 6 | 0.76 (0.51–1.11) | 0.158 | 0.88 (0.59–1.30) | 0.522 |
| Region 7 | 1.28 (0.97–1.69) | 0.076 | 1.29 (0.97–1.71) | 0.078 |
| Region 8 | 0.95 (0.68–1.32) | 0.751 | 1.12 (0.80–1.57) | 0.522 |
| Region 9 | 1.39 (1.06–1.81) | 0.017 | 1.31 (0.99–1.72) | 0.059 |
| Region 10 | 1.22 (0.91–1.63) | 0.168 | 1.36 (1.01–1.83) | 0.041 |
| Region 11 | 1.20 (0.91–1.58) | 0.204 | 1.26 (0.95–1.67) | 0.104 |
| Mean VIF (variance inflation factor)3 | – | – | 2.30 | – |
DKT, dual kidney transplantation; KDPI, Kidney Donor Profile Index; KDRI, Kidney Donor Risk Index; OPTN, Organ Procurement and Transplantation Network; UNOS, United Network of Organ Sharing.
Total 16 931 observations were included for multivariable analysis.
KDPI is calculated based on a scaling factor of 1.22219212347775, a median KDRI-UNOS value among all deceased kidney donors recovered during 2012.
VIF <10 shows no significant multicolinearity among independent predictors.
Organ quality of all recovered kidneys
When comparing the organ quality of dual discards versus DKTs, the mean KDPI percentile was 89% for both groups (Table 3). However, discarded ECD kidneys had a higher mean KDPI percentile compared to those that were transplanted (91.0% vs. 84.2%, p <0.001).
Table 3.
Donor quality of recovered ECD and dual kidneys between 2002 and 2012
| Recovered but not transplanted | Recovered and transplanted | p-Value | |
|---|---|---|---|
| Dual1 | |||
| n | 368 | 1 160 | – |
| KDRI-UNOS2 | 2.16 ± 0.49 | 2.11 ± 0.46 | 0.299 |
| KDPI3 (%) | 89.8 ± 10.8 | 89.4 ± 10.4 | 0.883 |
| ECD4 | |||
| n | 13 543 | 15 448 | – |
| KDRI-UNOS2 | 2.18 ± 0.46 | 1.87 ± 0.35 | <0.001 |
| KDPI3 (%) | 91.0 ± 8.6 | 84.2 ± 10.2 | <0.001 |
ECD, expanded criteria donor; KDPI, Kidney Donor Profile Index; KDRI, Kidney Donor Risk Index; UNOS, United Network of Organ Sharing.
Dual kidney donor age ≥50 years.
KDRI-UNOS based on 10 donor variables reported in the UNOS DonorNet.
KDPI scaled for a factor of 1.22219212347775, a median KDRI-UNOS value among all deceased kidney donors recovered during 2012.
ECD kidneys include donation after cardiac death kidneys.
DKT and ECD transplant characteristics and outcomes
Donor, recipient and transplant characteristics across KDPI categories are shown in Table 4. The higher KDPI categories were more likely to fulfill the UNOS-DKT criteria and had lower terminal creatinine clearance. The KDPI >90% category had more African-American (AA) and diabetic donors and tended to be older for both groups. The DKT tended to have a higher degree of GS and longer cold ischemia time (CIT).
Table 4.
Characteristics of deceased donors and recipients by donor KDPI categories based on the OPTN/UNOS Registry between 2002 and 2012
| KDPI1 <80% (KDRI-UNOS <1.7)
|
80% ≤ KDPI1 ≤ 90% (1.7 ≤ KDRI-UNOS ≤ 2.0)
|
KDPI1 >90% (KDRI-UNOS >2.0)
|
||||
|---|---|---|---|---|---|---|
| ECD | DKT | ECD | DKT | ECD | DKT | |
| n | 5721 | 212 | 5463 | 300 | 4264 | 648 |
| Donor factors | ||||||
| Mean age2 (years) | 56.2 ± 4.7* | 55.2 ± 4.7* | 59.3 ± 5.0* | 60.7 ± 5.7* | 64.0 ± 6.1* | 68.0 ± 6.4* |
| BMI | 28.8 ± 5.5 | 28.4 ± 5.9 | 28.4 ± 5.8 | 28.6 ± 6.0 | 28.3 ± 5.9* | 27.1 ± 5.7* |
| Female (%) | 36.0 | 32.1 | 36.4 | 35.3 | 36.1 | 38.9 |
| African-American (%) | 2.4 | 2.4 | 11.0 | 10.0 | 30.9* | 19.6* |
| History of diabetes2 (%) | 4.4* | 11.0* | 14.6 | 16.6 | 28.2* | 24.6* |
| History of hypertension2 (%) | 66.5* | 45.4* | 71.7* | 64.2* | 84.4* | 74.0* |
| Death due to CVA (%) | 77.7* | 53.7* | 80.1* | 63.3* | 87.6* | 83.5* |
| Terminal creatinine (mg/dL) | 1.0 ± 0.8* | 1.3 ± 0.8* | 1.1 ± 0.9 | 1.2 ± 0.6 | 1.3 ± 1.1 | 1.3 ± 1.3 |
| Terminal creatinine >2.5 mg/dL2 (%) | 1.9* | 9.9* | 1.6 | 2.7 | 1.7 | 1.7 |
| Terminal creatinine clearance2 (mL/min) | 109.4 ± 49.4* | 96.3 ± 51.1* | 88.8 ± 38.5 | 85.0 ± 39.9 | 74.1 ± 29.9* | 66.5 ± 27.5* |
| Terminal creatinine clearance <65 mL/min (%) | 14.6* | 25.0* | 26.2* | 36.0* | 43.7* | 56.6* |
| Pretransplant biopsy (%) | 79.2* | 90.6* | 84.4* | 90.0* | 89.5 | 90.3 |
| Glomerulosclerosis2 (15–50%) | 10.5* | 38.3* | 13.1* | 30.6* | 14.0* | 27.5* |
| KDRI-UNOS | 1.55 ± 0.11* | 1.52 ± 0.13* | 1.84 ± 0.09* | 1.85 ± 0.08* | 2.27 ± 0.25* | 2.43 ± 0.35* |
| KDPI1 | 72.8 ± 6.5* | 71.4 ± 8.6* | 86.3 ± 2.9* | 86.8 ± 2.7* | 95.1 ± 2.6* | 96.5 ± 2.7* |
| Fulfilling UNOS-DKT criteria (%) | 20.4* | 41.0* | 52.3* | 66.3* | 81.2* | 90.1* |
| Recipient factors | ||||||
| Mean age1 (years) | 58.9 ± 10.7 | 58.5 ± 9.1 | 60.1 ± 10.2 | 60.1 ± 10.4 | 61.9 ± 10.1* | 62.7 ± 9.0* |
| BMI | 28.5 ± 5.0* | 27.8 ± 4.7* | 28.3 ± 5.1* | 27.5 ± 4.7* | 28.0 ± 5.0* | 27.3 ± 4.6* |
| Female (%) | 36.0 | 32.1 | 36.4 | 35.3 | 36.1 | 38.9 |
| African-American (%) | 28.7* | 36.3* | 32.0 | 33.0 | 35.1 | 36.0 |
| Diabetes (%) | 45.1 | 39.2 | 46.4 | 41.7 | 47.2 | 46.5 |
| Pretransplant dialysis (year) | 4.0 ± 2.5 | 3.7 ± 2.6 | 4.0 ± 2.4* | 3.4 ± 2.1* | 3.8 ± 2.3* | 3.2 ± 2.1* |
| Peak PRA (%) | 9.4 ± 20.5 | 7.7 ± 19.6 | 9.3 ± 20.4 | 12.5 ± 24.7 | 8.7 ± 19.9 | 9.7 ± 19.9 |
| Primary insurance (%) | ||||||
| Private | 26.0 | 31.9 | 26.1 | 32.4 | 24.5* | 26.3* |
| Medicaid | 4.0 | 3.8 | 4.2 | 3.7 | 3.8* | 5.9* |
| Medicare | 70.0 | 64.3 | 69.7 | 63.9 | 71.7* | 67.8* |
| Transplant factors | ||||||
| DCD donor (%) | 4.1* | 13.2* | 6.4* | 11.3* | 5.4 | 6.9 |
| Kidney pumped by OPO (%) | 54.8 | 60.7 | 60.0* | 66.4* | 64.0 | 64.4 |
| Cold ischemia time (CIT), hour | 18.1 ± 9.6* | 25.4 ± 15.1* | 18.8 ± 9.6* | 24.1 ± 13.3* | 19.4 ± 9.3* | 24.1 ± 13.3* |
| HLA mismatch (%) | 4.4 ± 1.5* | 4.6 ± 1.1* | 4.5 ± 1.4 | 4.3 ± 1.3 | 4.6 ± 1.3 | 4.5 ± 1.2 |
CVA, cerebrovascular accident; DCD, donation after cardiac death; DKT, dual kidney transplantation; ECD, expanded criteria donor; KDPI, Kidney Donor Profile Index; KDRI, Kidney Donor Risk Index; OPO, Organ Procurement Organization; OPTN, Organ Procurement and Transplantation Network; PRA, panel reactive antibody; UNOS, United Network of Organ Sharing.
p-Value <0.05.
KDPI is calculated based on a scaling factor of 1.22219212347775, a median KDRI-UNOS value among all deceased kidney donors recovered during 2012.
DKT criteria per UNOS guidelines.
Outcomes of the recipients transplanted with DKT and ECD kidneys are described in Table 5. While the incidence of primary nonfunction and graft thrombosis was similar between DKT and ECD groups, rates of acute rejection and DGF at 1 year were lower in DKT recipients compared to the ECD recipients. Three-year death-censored graft survival among DKT recipients was persistently superior compared to ECD recipients across all KDPI categories; however, only the >90% category reached statistical significance (72.9% vs. 67.6%, p <0.05). In our study cohort, DGF, primarily resulted from CIT longer than 20 h, significantly increased early allograft failure while pulsatile perfusion decreased the risk of DGF by 34% compared to cold storage (Table S1).
Table 5.
Outcomes ECD transplants and DKTs by donor KDPI categories between 2002 and 2012
| KDPI1 <80% (KDRI-UNOS <1.7)
|
80% ≤ KDPI1 ≤90% (1.7 ≤KDRI-UNOS ≤2.0)
|
KDPI1 >90% (KDRI-UNOS >2.0)
|
||||
|---|---|---|---|---|---|---|
| ECD | DKT | ECD | DKT | ECD | DKT | |
| n | 5721 | 212 | 5463 | 300 | 4264 | 648 |
| DGF (%) | 30.9 | 28.8 | 32.5 | 28.7 | 33.5* | 25.0* |
| Acute rejection (%) | ||||||
| At discharge | 3.1 | 0.6 | 3.1 | 2.8 | 3.5* | 1.0* |
| At 1 year | 11.9 | 9.0 | 12.8 | 10.1 | 13.2 | 10.2 |
| Causes of graft failure (% of failed allografts) | ||||||
| Primary nonfunction | 13.5 | 8.8 | 14.8 | 9.4 | 14.6 | 12.6 |
| Graft thrombosis | 5.0 | 8.8 | 6.2 | 7.8 | 5.1 | 6.3 |
| Length of stay at the time of transplant (day) | 8.5 ± 9.8 | 9.2 ± 6.6 | 8.8 ± 11* | 10.1 ± 10.4* | 9.1 ± 12.8 | 9.6 ± 98.7 |
| Follow-up time (year) | 3.7 ± 2.8 | 3.8 ± 2.9 | 3.6 ± 2.6 | 3.8 ± 2.7 | 3.3 ± 2.5* | 4.1 ± 2.7* |
| One-year death-censored allograft survival | 89.0 | 89.2 | 86.0 | 88.1 | 83.3* | 85.3* |
| One-year death-censored allograft survival if fulfilled the UNOS-DKT criteria | 88.8 | 90.1 | 84.0* | 88.8* | 83.3* | 85.3* |
| Three-year death-censored allograft survival | 77.5 | 77.8 | 73.8 | 75.7 | 67.6* | 72.9* |
| Three-year death-censored allograft survival if fulfilled the UNOS-DKT criteria | 76.5* | 80.0* | 72.0* | 76.1* | 67.2* | 72.5* |
| Ninety-day mortality (%) | 3.2 | 4.4 | 4.7 | 4.1 | 4.8 | 5.4 |
| One-year patient survival | 92.5 | 92.5 | 89.5* | 91.6* | 88.4 | 88.7 |
| Three-year patient survival | 83.9 | 83.5 | 78.8* | 80.9* | 76.0* | 78.6* |
DGF, delayed allograft function; DKT, dual kidney transplantation; ECD, expanded criteria donor; KDPI, Kidney Donor Profile Index; KDRI, Kidney Donor Risk Index; UNOS, United Network of Organ Sharing.
p-Value <0.05.
KDPI is calculated based on a scaling factor of 1.22219212347775, a median KDRI-UNOS value among all deceased kidney donors recovered during 2012.
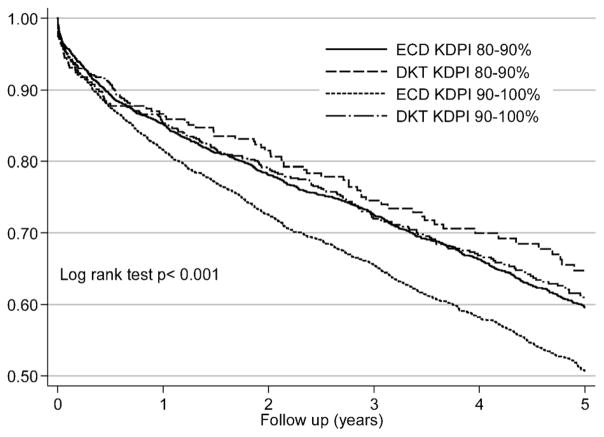
Results from the Kaplan–Meier survival models are shown in Figure 6. Five-year graft survival rates for the DKT and ECD kidneys were 64.7% versus 59.6% and 60.9% versus 50.7% in the KDPI 80–90% and >90% categories, respectively. The result of the Cox model is displayed in Table 6. DKT was associated with 24% survival benefit with kidneys originated form the KDPI >90% (HR 0.76, 95% CI 0.65–0.90) compared to ECD kidneys.
Figure 6.
Death-censored Kaplan–Meier survival estimates by the KDRI categories for DKT and ECD transplants, the OPTN/UNOS Registry, between 2002 and 2012. DKT, dual kidney transplantation; ECD, expanded criteria donor; KDPI, Kidney Donor Profile Index; KDRI, Kidney Donor Risk Index.
Table 6.
Cox proportional-hazard model for death-censored allograft failure based on OPTN/UNOS Registry between 2002 and 2012
| Variables (reference) | Univariate
|
Multivariable
|
||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Donor type: DKT (ECD) | 0.90 (0.82–0.99) | 0.042 | 0.76 (0.65–0.90) | 0.001 |
| Recipient age | 1.011 (1.009–1.013) | <0.001 | 1.011 (1.007–1.016) | <0.001 |
| Recipient diabetes (no diabetes) | 1.31 (1.25–1.38) | <0.001 | 1.22 (1.13–1.33) | <0.001 |
| Recipient blood type (blood type O) | 1.01 (0.98–1.04) | 0.353 | – | – |
| Recipient race (non-African-American) | 1.15 (1.09–1.21) | <0.001 | 1.07 (0.98–1.17) | 0.144 |
| Recipient gender (female) | 1.15 (1.09–1.22) | <0.001 | 1.17 (1.07–1.27) | 0.001 |
| Recipient BMI (<30 kg/m2) | 1.012 (1.007–1.017) | <0.001 | 1.011 (1.004–1.020) | 0.004 |
| Primary insurance (private) | ||||
| Medicaid | 1.18 (1.03–1.35) | 0.016 | 0.97 (0.78–1.29) | 0.811 |
| Medicare | 1.25 (1.17–1.32) | <0.001 | 1.04 (0.94–1.14) | 0.465 |
| Peak PRA (%) | 1.001 (1.000–1.003) | 0.141 | 1.001 (0.999–1.002) | 0.980 |
| HLA mismatch | 1.02 (1.05–1.07) | <0.001 | 1.01 (0.98–1.05) | 0.641 |
| Dialysis duration | 1.023 (1.007–1.040) | 0.006 | 1.03 (1.01–1.05) | 0.003 |
| Cold ischemia time (h) | 1.006 (1.004–1.009) | <0.001 | 1.002 (0.999–1.006) | 0.231 |
| Machine perfusion (ice storage) | 1.10 (1.03–1.16) | 0.002 | 1.07 (0.99–1.17) | 0.096 |
| Donor diabetes (no diabetes) | 1.26 (1.18–1.35) | <0.001 | 1.32 (1.18–1.46) | <0.001 |
| Donor age (year) | 1.014 (1.010–1.018) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| Glomerulosclerosis 15–50% (<15%) | 1.07 (0.98–1.15) | 0.095 | 1.07 (0.95–1.20) | 0.260 |
| KDRI-UNOS (<1.7) | ||||
| 1.7–2.0 | 1.19 (1.12–1.27) | <0.001 | – | – |
| >2.0 | 1.46 (1.36–1.56) | <0.001 | – | – |
DKT, dual kidney transplantation; ECD, expanded criteria donor; KDRI, Kidney Donor Risk Index; PRA, panel reactive antibody; UNOS, United Network of Organ Sharing.
Clinical utility of the UNOS-DKT criteria and its alternative, the KDPI
Patient and graft survival
In multivariable Cox analysis, DKT with KDPI >90% was associated with significantly better patient survival (HR = 0.79, 0.64–0.98) and graft survival (HR = 0.74, 95% CI 0.62–0.89) compared to single ECD transplants. There were no differences in patient and graft survival with the use of DKT with kidneys from lower KDPI categories. Similar to the KDPI >90%, fulfilling UNOS-DKT criteria was also associated with significant protective effects on allograft (HR = 0.79, 95% CI 0.68–0.92) and patient survival (HR = 0.80, 95% CI 0.66–0.97; Table 7).
Table 7.
Multivariable1 models for outcomes of DKT, relative to ECD kidney transplants, across the KDPI and UNOS-DKT categories, based on the UNOS data between 2002 and 2012
| KDPI2 categories (corresponding KDRI-UNOS groups)
|
|||
|---|---|---|---|
| KDPI2 <80% (KDRI-UNOS <1.7) | 80% ≤ KDPI2 ≤ 90% (1.7 ≤ KDRI-UNOS ≤ 2.0) | KDPI2 >90% (KDRI-UNOS >2.0) | |
| Adjusted hazard ratio (95% CI) | |||
| Overall graft failure | 0.92 (0.67–1.27) | 0.82 (0.63–1.08) | 0.74 (0.62–0.89) |
| Death-censored graft failure | 0.90 (0.65–1.26) | 0.87 (0.66–1.14) | 0.75 (0.63–0.90) |
| Patient death | 1.16 (0.79–1.70) | 0.83 (0.54–1.09) | 0.79 (0.64–0.98) |
| Adjusted odds ratio (95% CI) | |||
| DGF | 0.89 (0.59–1.32) | 0.92 (0.66–1.27) | 0.76 (0.60–0.97) |
| Early allograft failure posttransplant 3 months (including patient death) | 0.45 (0.11–1.90) | 0.88 (0.42–1.85) | 1.53 (0.97–2.40) |
| eGFR2 ≤ 30 mL/min/1.73 m2 at last follow-up visit | 0.49 (0.32–0.75) | 0.51 (0.36–0.71) | 0.46 (0.36–0.59) |
| UNOS-DKT criteria
|
||
|---|---|---|
| Not fulfilling | Fulfilling | |
| Adjusted hazard ratio (95% CI) | ||
| Overall graft failure | 0.85 (0.64–1.12) | 0.79 (0.68–0.92) |
| Death-censored graft failure | 0.84 (0.63–1.12) | 0.82 (0.70–0.96) |
| Patient death | 1.00 (0.72–1.39) | 0.80 (0.66–0.97) |
| Adjusted odds ratio (95% CI) | ||
| DGF | 0.86 (0.62–1.20) | 0.80 (0.65–0.98) |
| Early allograft failure posttransplant 3 months (including patient death) | 0.90 (0.39–2.10) | 1.15 (0.77–1.73) |
| eGFR3 <30 mL/min/1.73 m2 at last follow-up visit | 0.58 (0.41–0.82) | 0.45 (0.37–0.56) |
DKT, dual kidney transplantation; eGFR, estimated glomerular filtration rate; ECD, expanded criteria donor; KDPI, Kidney Donor Profile Index; KDRI, Kidney Donor Risk Index; PRA, panel reactive antibody; UNOS, United Network of Organ Sharing.
Adjusted for recipient’s age, gender, race, diabetes status, BMI at listing, peak PRA, HLA mismatch, dialysis duration, cold ischemia time, pulsatile perfusion and insurance coverage.
KDPI is calculated based on a scaling factor of 1.22219212347775, a median KDRI-UNOS value among all deceased kidney donors recovered during 2012.
eGFR is calculated by using the modification of diet in renal disease 4 variable equation.
Graft function
In the multivariable logistic regression model, DKT had a protective effect against DGF with kidneys designated as the KDPI >90% (OR = 0.76, 95% CI 0.60–0.97) and fulfilling UNOS criteria (OR = 0.80, 0.65–0.98). Even though DKT was associated with increased odds of early allograft failure with the highest KDRI-UNOS category and with kidneys fulfilling the UNOS-DKT criteria, this did not reach statistical significance. DKT was associated with decreased odds, advanced chronic kidney disease (eGFR <30 mL/min/1.73 m2) across all the KDRI-UNOS categories, and any UNOS-DKT criteria groups compared to single ECD transplants.
Discussion
The practice of transplanting both kidneys from an older donor with reduced renal function into a single recipient was introduced as a means of utilizing kidneys that were not considered acceptable for transplantation as single kidneys and would otherwise have been discarded, thus increasing the number of organs available for transplantation. While the rate of discarded kidneys recovered from older donors remains high, DKTs from these donors still represent a very small proportion of overall deceased donor transplants and is not increasing despite the ever more pressing need to find new sources of kidneys for transplantation. Several studies have reported comparable allograft survival, better eGFR and lower rejection rates in DKT compared to single ECD transplants (24–27). Better renal function and lower DGF rates in DKTs may be related to transplanting more nephrons (28,29). However, it is difficult to tease out a reason for lower rejection rates since it may be influenced by a combination of immunologic protection, higher physiological reserve and/or center-specific practices (30).
Gill et al (6) reviewed the UNOS database and compared outcomes of kidneys transplanted between 2000 and 2005 from donors >50 years old, used either as single SCD, single ECD or dual transplants. In that population, DKTs accounted for approximately 4% of transplants. Despite the fact that the donors whose kidneys were used as DKTs were older, more likely to be AA, diabetic and have longer CITs, DGF rates were lower, and 3-year outcomes were equal compared with ECD recipients. They found inconsistent application of UNOS-DKT criteria with many kidneys meeting DKT criteria being used as single ECD kidneys and those not meeting DKT criteria being used as DKT. They urged a more aggressive and consistent approach to the use of DKT. More recently, Klair et al (31) analyzed the UNOS database from 1995 to 2010 and found better graft survival for DKT for organs with KDRI >2.2, suggesting that the utilization of these marginal organs as dual organs should be considered for certain recipient categories to improve outcomes and resource utilization.
We analyzed the UNOS data between 2002 and 2012, compared DKT and ECD and found: (1) significant overlap in KDPI between utilized DKTs and discarded duals; (2) high rates of discard of ECD at highest KDPI—these could be used as DKT rather than discarding one kidney using the other as single with inferior outcome; (3) inconsistent use of DKT criteria; and (4) the fact that histology is the most common reason for discard despite the lack of any rigorous evidence-based data to validate this practice.
According to the new allocation system, kidneys with KDPI >85% would be offered on a wider geographic area (local and regional lists) in order to promote broader sharing of kidneys that are currently at high risk for discard. While this approach is likely to improve organ sharing and utilization, it does not address the issue of optimal use of organs with the highest KDPI.
Which kidneys should be used as DKT?
Approximately one-third of the DKT kidneys did not originate from donors who met the UNOS criteria. In contrast, 46.7% of ECD kidneys fulfilled at least two of the UNOS-DKT criteria and could therefore have been offered as DKT with superior outcomes. This suggests that there are significant inconsistencies in implementation of the UNOS-DKT criteria among OPOs. In DKT utilization, there are two main goals: (1) allocating ECD kidneys to DKT that otherwise would have been discarded; and (2) achieving allograft survival and function at least as good as single ECD transplants. The relative risk of allograft failure exponentially increases when the KDPI reaches 90% (break point) based on the data on deceased donors recovered in 2009 (32). Our analysis showed that the mean KDPI percentile of discarded kidneys, regardless of cause of discard and intended use (single vs. dual), was in the range of 90–100% and odds of discard were twice as much in organs with KDPI >90% compared to the organs with KDPI <80%. We also found that the discard rate of kidneys with KDPI >90% would be almost three times lower if organs were offered dual compared to single kidney. Death-censored estimated allograft survivals at 1 and 3 years between single ECD kidneys with KDPI of 80–90% and DKTs with KDPI >90% were same. These results suggest that a KDPI >90% (KDRI-UNOS >2.0) may distinguish ECD kidneys that should preferentially be offered as a DKT early on during the allocation system. Further advanced modeling and simulation studies are required to better define the KDPI cutoff for DKT utilization.
Role of procurement biopsies in predicting discard and subsequent function
Kidneys that undergo pretransplant renal biopsy are at increased risk of discard. Despite the limitations and variability in interpretation of wedge kidney biopsies (over estimating GS due to more subcapsular sampling) in defining the quality of the donor organs, this finding alone is one of the common reasons for refusal of the organ in the United States (odds of kidney discard is 12 times more likely when it has GS >20% than if GS is <5%) (15). In our analysis, the death-censored adjusted Cox model, GS 15–50% was not associated with higher risk of allograft failure than the reference group with GS <15%. Overall, GS does not appear to be an accurate histologic indicator of subsequent renal function. Many studies successfully linked other biopsy findings to clinical outcomes (19,33–35). Arterial hyalinosis, fibrous intimal thickening, tubular atrophy and interstitial fibrosis showed better correlation with long-term renal function and longevity of allograft. The Dual Kidney Transplant Group in Italy conducted a prospective study to evaluate long-term allograft survival among 62 patients who received single or dual kidneys based on renal histology (preimplantation biopsy 12-point scoring system) (36), from donor age ≥60 years (37). They reported that histologically evaluated kidneys had excellent 3-year allograft survival compared to ideal kidney transplants, at 90% vs. 95%, respectively. In the future, more uniform and inclusive biopsy report system (vessels, glomeruli, tubules and interstitium) will be necessary to utilize renal histopathology more efficiently in the allocation system.
DGF and early allograft failures
DGF has remained an important predictor of early allograft failure and may be more detrimental on older recipients who received older donor kidneys (19,38). It was previously shown that utilization of pulsatile organ perfusion-preservation, if routinely used, could decrease the incidence of DGF, improve allograft survival and potentially reduce the ECD discard rate by up to 30% in the United States (39–41). Carter et al (19) reported on a center-specific ECD allocation program that demonstrated significantly decreased CIT (from 16.4 to 7.4 h) and DGF (from 43% to 15%) compared to historical control group. Their improvement and efficiency primarily resulted from short CIT, less DGF, not relying on biopsy and careful recipient selection (including high-risk mortality candidates and excluding re-transplants, recipients with panel reactive antibody [PRA] >10% and obese patients). In this regard, widespread efforts to reduce CIT (subsequently DGF) by efficient allocation and routine use of pulsatile perfusion and appropriate recipient selection could significantly improve allograft survival.
Potential DKTs could be performed
To estimate impact of using dual kidneys on expansion of the marginal donor pool, we calculated potential DKTs that could have been performed based on the OPTN/UNOS data between 2002 and 2012 including transplanted and discarded kidneys with KDPI >90%. Potential DKTs = (9146 [total number of discarded kidneys] +4264 [transplanted kidneys as single] +[648×2 (transplanted kidneys as dual)]/2 [two kidneys used as DKT]) × 74.5% (rate of dual kidney use for KDPI >90%) = (14 706/2) × 0.745 = 5478 for 10 years and 548 annually. On average, the number of potential DKTs (548) that could be performed annually is 58 transplants more than those (426 single +64 duals) performed during the same period.
Potential to improve the DKT policy and minimize discard rate
Standardizing objective and practical criteria for DKT allocation is an important issue. The KDPI >90% may be a reasonable predictor of discard compared to the UNOS-DKT criteria. Using a KDPI threshold may give us an opportunity to simplify the allocation of kidneys at high risk of discard as a single organ or DKT. Since the KDPI will be fully implemented in 2014 into the allocation system and strongly correlates with patient and allograft survival as well as kidney discard rate, it would be possible at that time to replace the UNOS criteria with KDPI to effectively classify ECD kidneys that would provide allograft and patient survival benefit if transplanted as a DKT.
We observed a decline in DKT transplants performed since 2008. The fact that this was not matched by an increase in the number of ECD kidneys indicates that these high KDPI kidneys are not being offered and/or used as single ECD kidneys. The problem appears to be a systemic issue and could be attributed to the increasingly risk-averse climate based on center performance metrics, as measured in the Scientific Registry Transplant Recipients (SRTR) reports. Potential consequences of negative reports resulting from inferior outcomes related to marginal kidneys can affect the transplant center’s approach. Exclusion of outcomes of high-risk kidneys from a center’s SRTR performance reports might facilitate utilization of these organs.
Strengths and limitations of analysis of the UNOS Registry
Strengths of our study include the fact that it evaluates a contemporary cohort of DKT and ECD in which short and long-term outcomes are explored in detail. In addition, we assessed the donor quality and related risk of allograft failure by using a more comprehensive tool, KDRI/KDPI, over SCD/ECD dichotomy. Finally, in our multivariable Cox model, we examined an extensive set of covariates. Despite these strengths, our study has some limitations that are inherent in observational studies using the registry data. First, the process of capturing reasons for organ discard and disposition of kidneys is highly subjective, and the recorded reason does not always reflect the actual reason for discard. Second, practice of UNOS-DKT criteria is not standard and mostly under the latitude of OPO (selection bias). Third, detailed kidney biopsy findings, as a major determinant of discard, are not captured in the data set (except GS) and not incorporated into KDRI scoring. Fourth, center-specific transplant techniques and details about immunosuppressive regimens are not reported to the UNOS. Fifth, transplant centers may not accurately and timely report their complications, causes of allograft and patient loss and outcomes to the UNOS (reporting errors). Finally, it is unclear if discarded organs were even offered as DKT during the allocation process.
Conclusion
DKT is not part of the deceased kidney allocation system and is an underutilized option for transplantation of kidneys obtained from expanded donors and given to older recipients. The implementation of KDRI/KDPI in the allocation system may result in improvements of donor risk assessment as a continuum that may overcome limitations of the SCD/ECD dichotomy. A prospective, comprehensive and uniform registry for the procurement biopsy reporting is urgently needed to delineate when and how to utilize histopathology in the allocation system. Routine use of machine perfusion/preservation for expanded donor kidneys should lead to a significant decrease in DGF. A more efficient allocation system based on the KDPI and offering higher percentile kidneys as a DKT, up front, may potentially decrease discard of organs. Exclusion of outcomes of high-risk kidneys from the transplant center’s SRTR performance reports might promote greater utilization of these organs. Appropriate recipient selection (avoidance of sensitized patients, re-transplants, donation after cardiac death [DCD] donors and large patients) for DKT may significantly improve outcomes.
Supplementary Material
Multivariable risk analysis for delayed graft function (DGF) and early graft loss within 3 months posttransplant in ECD transplants and DKTs which met UNOS-DKT criteria (including death as an allograft failure) and their characteristics.
Acknowledgments
This work was supported by NIH grant KM1CA156709-01 (BT), the Columbia University Irving Institute for Clinical and Translational Research (grant no.: UL1 TR000040) and in part by NIH T32HL007854-17 (MAH) and Health Resources and Services Administration (HRSA) contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services (HHS), nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Abbreviations
- AA
African-American
- BMI
body mass index
- CIT
cold ischemia time
- DCD
donation after cardiac death
- DGF
delayed allograft function
- DKT
dual kidney transplantation
- DonorNet
UNOS Centralized Computer Network between OPO and transplant centers
- ECD
expanded criteria donor
- eGFR
estimated glomerular filtration rate
- GS
glomerulosclerosis
- HLA mm
human leukocyte antigen mismatch
- KDPI
Kidney Donor Profile Index
- KDRI
Kidney Donor Risk Index (based on 15 donor and transplant variables)
- KDRI-median
Kidney Donor Risk Index (based on KDRI-UNOS adjusted for a scaling factor)
- KDRI-UNOS
Kidney Donor Risk Index (based on 10 donor variables)
- MDRD
modification of diet in renal disease
- OPO
Organ Procurement Organization
- OPTN
Organ Procurement and Transplantation Network
- PRA
panel reactive antibody
- SCD
standard criteria donor
- SRTR
Scientific Registry Transplant Recipients
- UNOS
United Network of Organ Sharing
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Vinkers MT, Smits JM, Tieken IC, de Boer J, Ysebaert D, Rahmel AO. Kidney donation and transplantation in Eurotransplant 2006–2007: Minimizing discard rates by using a rescue allocation policy. Prog Transplant. 2009;19:365–370. doi: 10.1177/152692480901900414. [DOI] [PubMed] [Google Scholar]
- 2.Frei U, Noeldeke J, Machold-Fabrizii V, et al. Prospective age-matching in elderly kidney transplant recipients—A 5-year analysis of the Eurotransplant Senior Program. Am J Transplant. 2008;8:50–57. doi: 10.1111/j.1600-6143.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- 3.Smits JM, Persijn GG, van Houwelingen HC, Claas FH, Frei U. Evaluation of the Eurotransplant Senior Program. The results of the first year. Am J Transplant. 2002;2:664–670. doi: 10.1034/j.1600-6143.2002.20713.x. [DOI] [PubMed] [Google Scholar]
- 4.OPTN/UNOS. [Accessed July 21, 2013];Revison to the deceased donor kidney allocation policy by UNOS/OPTN Board. 2013 Available at: http://optn.transplant.hrsa.gov/news/newsDetail.asp?id=1600.
- 5.Atlas of chronic kidney disease and end-stage renal disease in the US National Institute of Health. Vol. 2. National Institute of Diabetes, Digestive and Kidney Diseases; Bethesda, MD: 2012. Data from US Renal Data System, USRDS 2012 Annual Data Report; pp. 283–294. ( http://www.usrds.org/2012/pdf/v2_ch7_12.pdf) Chapter 7. [Google Scholar]
- 6.Gill J, Cho YW, Danovitch GM, et al. Outcomes of dual adult kidney transplants in the United States: An analysis of the OPTN/UNOS database. Transplantation. 2008;85:62–68. doi: 10.1097/01.tp.0000296855.44445.af. [DOI] [PubMed] [Google Scholar]
- 7.Johnson LB, Kuo PC, Schweitzer EJ, et al. Double renal allografts successfully increase utilization of kidneys from older donors within a single organ procurement organization. Transplantation. 1996;62:1581–1583. doi: 10.1097/00007890-199612150-00009. [DOI] [PubMed] [Google Scholar]
- 8.OPTN/UNOS. [Accessed August 3, 2013];Double kidney allocation. 2013 Jul 25; Available at: http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_7.pdf.
- 9.Ruggenenti P, Perico N, Remuzzi G. Ways to boost kidney transplant viability: A real need for the best use of older donors. Am J Transplant. 2006;6:2543–2547. doi: 10.1111/j.1600-6143.2006.01519.x. [DOI] [PubMed] [Google Scholar]
- 10.Schaeffner ES, Rose C, Gill JS. Access to kidney transplantation among the elderly in the United States: A glass half full, not half empty. Clin J Am Soc Nephrol. 2010;5:2109–2114. doi: 10.2215/CJN.03490410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schold JD, Meier-Kriesche HU. Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol. 2006;1:532–538. doi: 10.2215/CJN.01130905. [DOI] [PubMed] [Google Scholar]
- 12.Schold JD, Howard RJ, Scicchitano MJ, Meier-Kriesche HU. The expanded criteria donor policy: An evaluation of program objectives and indirect ramifications. Am J Transplant. 2006;6:1689–1695. doi: 10.1111/j.1600-6143.2006.01390.x. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro R, Halloran PF, Delmonico FL, Bromberg JS. The ‘two, one, zero’ decision: What to do with suboptimal deceased donor kidneys. Am J Transplant. 2010;10:1959–1960. doi: 10.1111/j.1600-6143.2010.03204.x. [DOI] [PubMed] [Google Scholar]
- 14.Ekser B, Furian L, Broggiato A, et al. Technical aspects of unilateral dual kidney transplantation from expanded criteria donors: Experience of 100 patients. Am J Transplant. 2010;10:2000–2007. doi: 10.1111/j.1600-6143.2010.03188.x. [DOI] [PubMed] [Google Scholar]
- 15.Sung RS, Christensen LL, Leichtman AB, et al. Determinants of discard of expanded criteria donor kidneys: Impact of biopsy and machine perfusion. Am J Transplant. 2008;8:783–792. doi: 10.1111/j.1600-6143.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 16.Cecka JM, Cohen B, Rosendale J, Smith M. Could more effective use of kidneys recovered from older deceased donors result in more kidney transplants for older patients? Transplantation. 2006;81:966–970. doi: 10.1097/01.tp.0000216284.81604.d4. [DOI] [PubMed] [Google Scholar]
- 17.Gaston RS, Danovitch GM, Adams PL, et al. The report of a national conference on the wait list for kidney transplantation. Am J Transplant. 2003;3:775–785. doi: 10.1034/j.1600-6143.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 18.Cecka JM, Gritsch HA. Why are nearly half of expanded criteria donor (ECD) kidneys not transplanted? Am J Transplant. 2008;8:735–736. doi: 10.1111/j.1600-6143.2007.02071.x. [DOI] [PubMed] [Google Scholar]
- 19.Carter JT, Chan S, Roberts JP, Feng S. Expanded criteria donor kidney allocation: Marked decrease in cold ischemia and delayed graft function at a single center. Am J Transplant. 2005;5:2745–2753. doi: 10.1111/j.1600-6143.2005.01095.x. [DOI] [PubMed] [Google Scholar]
- 20.Tso PL, Dar WA, Henry ML. With respect to elderly patients: Finding kidneys in the context of new allocation concepts. Am J Transplant. 2012;12:1091–1098. doi: 10.1111/j.1600-6143.2011.03956.x. [DOI] [PubMed] [Google Scholar]
- 21.Garonzik-Wang JM, James NT, Weatherspoon KC, et al. The aggressive phenotype: Center-level patterns in the utilization of suboptimal kidneys. Am J Transplant. 2012;12:400–408. doi: 10.1111/j.1600-6143.2011.03789.x. [DOI] [PubMed] [Google Scholar]
- 22.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation. 2009;88:231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 23.OPTN/UNOS. [Accessed July 31, 2013];Guide to calculating and interpreting KDPI. 2012 Available at: http://optn.transplant.hrsa.gov/ContentDocuments/Guide_to_Calculating_Interpreting_KDPI.pdf.
- 24.Tan JC, Alfrey EJ, Dafoe DC, Millan MT, Scandling JD. Dual-kidney transplantation with organs from expanded criteria donors: A long-term follow-up. Transplantation. 2004;78:692–696. doi: 10.1097/01.tp.0000130452.01521.b1. [DOI] [PubMed] [Google Scholar]
- 25.Moore PS, Farney AC, Sundberg AK, et al. Experience with dual kidney transplants from donors at the extremes of age. Surgery. 2006;140:597–605. doi: 10.1016/j.surg.2006.07.004. discussion 605–606. [DOI] [PubMed] [Google Scholar]
- 26.Lu AD, Carter JT, Weinstein RJ, et al. Outcome in recipients of dual kidney transplants: An analysis of the dual registry patients. Transplantation. 2000;69:281–285. doi: 10.1097/00007890-200001270-00014. [DOI] [PubMed] [Google Scholar]
- 27.Dietl KH, Wolters H, Marschall B, Senninger N, Heidenreich S. Cadaveric “two-in-one” kidney transplantation from marginal donors: Experience of 26 cases after 3 years. Transplantation. 2000;70:790–794. doi: 10.1097/00007890-200009150-00014. [DOI] [PubMed] [Google Scholar]
- 28.Brenner BM, Cohen RA, Milford EL. In renal transplantation, one size may not fit all. J Am Soc Nephrol. 1992;3:162–169. doi: 10.1681/ASN.V32162. [DOI] [PubMed] [Google Scholar]
- 29.Brenner BM, Milford EL. Nephron underdosing: A programmed cause of chronic renal allograft failure. Am J Kidney Dis. 1993;21 (5 Suppl 2):66–72. doi: 10.1016/0272-6386(93)70097-i. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Lorente L, Riera L, Bestard O, et al. Long-term results of biopsy-guided selection and allocation of kidneys from older donors in older recipients. Am J Transplant. 2012;12:2781–2788. doi: 10.1111/j.1600-6143.2012.04153.x. [DOI] [PubMed] [Google Scholar]
- 31.Klair T, Gregg A, Phair J, Kayler LK. Outcomes of adult dual kidney transplants by KDRI in the United States. Am J Transplant. 2013;13:2433–2440. doi: 10.1111/ajt.12383. [DOI] [PubMed] [Google Scholar]
- 32.Friedewald JJ. Utilization and outcomes of marginal kidneys—Using Kidney Donor Risk Index to move beyond the current labels. Am J Transplant. 2012;12:1971–1972. doi: 10.1111/j.1600-6143.2012.04149.x. [DOI] [PubMed] [Google Scholar]
- 33.Munivenkatappa RB, Schweitzer EJ, Papadimitriou JC, et al. The Maryland aggregate pathology index: A deceased donor kidney biopsy scoring system for predicting graft failure. Am J Transplant. 2008;8:2316–2324. doi: 10.1111/j.1600-6143.2008.02370.x. [DOI] [PubMed] [Google Scholar]
- 34.Woestenburg AT, Verpooten GA, Ysebaert DK, Van Marck EA, Verbeelen D, Bosmans JL. Fibrous intimal thickening at implantation adversely affects long-term kidney allograft function. Transplantation. 2009;87:72–78. doi: 10.1097/TP.0b013e31818bbe06. [DOI] [PubMed] [Google Scholar]
- 35.Anglicheau D, Loupy A, Lefaucheur C, et al. A simple clinicohistopathological composite scoring system is highly predictive of graft outcomes in marginal donors. Am J Transplant. 2008;8:2325–2334. doi: 10.1111/j.1600-6143.2008.02394.x. [DOI] [PubMed] [Google Scholar]
- 36.Remuzzi G, Grinyo J, Ruggenenti P, et al. Early experience with dual kidney transplantation in adults using expanded donor criteria. Double Kidney Transplant Group (DKG) J Am Soc Nephrol. 1999;10:2591–2598. doi: 10.1681/ASN.V10122591. [DOI] [PubMed] [Google Scholar]
- 37.Remuzzi G, Cravedi P, Perna A, et al. Long-term outcome of renal transplantation from older donors. N Engl J Med. 2006;354:343–352. doi: 10.1056/NEJMoa052891. [DOI] [PubMed] [Google Scholar]
- 38.Lim WH, Clayton P, Wong G, et al. Outcomes of kidney transplantation from older living donors. Transplantation. 2013;95:106–113. doi: 10.1097/TP.0b013e318277b2be. [DOI] [PubMed] [Google Scholar]
- 39.Moers C, Smits JM, Maathuis MH, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7–19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- 40.Gallinat A, Moers C, Treckmann J, et al. Machine perfusion versus cold storage for the preservation of kidneys from donors ≥65 years allocated in the Eurotransplant Senior Programme. Nephrol Dial Transplant. 2012;27:4458–4463. doi: 10.1093/ndt/gfs321. [DOI] [PubMed] [Google Scholar]
- 41.Schold JD, Kaplan B, Howard RJ, Reed AI, Foley DP, Meier-Kriesche HU. Are we frozen in time? Analysis of the utilization and efficacy of pulsatile perfusion in renal transplantation. Am J Transplant. 2005;5:1681–1688. doi: 10.1111/j.1600-6143.2005.00910.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multivariable risk analysis for delayed graft function (DGF) and early graft loss within 3 months posttransplant in ECD transplants and DKTs which met UNOS-DKT criteria (including death as an allograft failure) and their characteristics.