Abstract
The success of peripheral nerve regeneration is governed by the rate and quality of axon bridging and myelination that occurs across the damaged region. Neurite growth and the migration of Schwann cells is regulated by neurotrophic factors produced as the nerve regenerates, and these processes can be enhanced by mesenchymal stem cells (MSCs), which also produce neurotrophic factors and other factors that improve functional tissue regeneration. Our laboratory has recently identified a population of mesenchymal progenitor cells (MPCs) that can be harvested from traumatized muscle tissue debrided and collected during orthopaedic reconstructive surgery. The objective of this study was to determine whether the traumatized muscle-derived MPCs exhibit neurotrophic function equivalent to that of bone marrow-derived MSCs. Similar gene- and protein-level expression of specific neurotrophic factors was observed for both cell types, and we localized neurogenic intracellular cell markers (brain-derived neurotrophic factor and nestin) to a subpopulation of both MPCs and MSCs. Furthermore, we demonstrated that the MPC-secreted factors were sufficient to enhance in vitro axon growth and cell migration in a chick embryonic dorsal root ganglia (DRG) model. Finally, DRGs in co-culture with the MPCs appeared to increase their neurotrophic function via soluble factor communication. Our findings suggest that the neurotrophic function of traumatized muscle-derived MPCs is substantially equivalent to that of the well-characterized population of bone marrow-derived MPCs, and suggest that the MPCs may be further developed as a cellular therapy to promote peripheral nerve regeneration.
Keywords: mesenchymal stem cells, nerve regeneration, neurotrophic factors, dorsal root ganglia, brain-derived neurotrophic factor
1. Introduction
Peripheral nerve injury is currently a challenging complication of musculoskeletal trauma, and the functional outcome of surgical reconstruction is often limited by the ability to repair the injured nerves. Success of nerve regeneration depends primarily on the speed and efficiency of axonal growth and myelination to bridge the damaged region (Gordon et al., 2009; Lee et al., 2000), and these processes are mediated by the native neuroglial Schwann cells (Madduri and Gander, 2010). Several recent studies have investigated the use of adult mesenchymal stem cells (MSCs) as Schwann cell surrogates that can enhance the rate of peripheral nerve regeneration (Bossolasco et al., 2005; Brohlin et al., 2009; Keilhoff et al., 2006b; Mahay et al., 2008a; Pan et al., 2007; Ribeiro-Resende et al., 2009). Although the precise role of undifferentiated MSCs in these processes is not completely understood, it has previously been shown that these cells exhibit immunomodulatory effects that enable them to regulate injury-initiated inflammation (Le Blanc, 2006; Le Blanc and Ringden, 2007). Similarly, MSCs appear to have trophic characteristics to enhance regeneration by expressing factors that are pro-angiogenic, limit scar formation and promote functional tissue formation by local wound-healing cells (Caplan and Dennis, 2006; Ozaki et al., 2007; Passweg and Tyndall, 2007; Tomchuck et al., 2008). MSCs have also been shown to secrete specific neurotrophic factors that are characteristic of Schwann cells (Caplan, 2007; Crigler et al., 2006; Hu et al., 2007; Munoz et al., 2005; Scuteri et al., 2006; Woodbury et al., 2000). Therefore, MSCs are a desirable candidate cell type for use in cell-based tissue-engineered constructs for peripheral nerve regeneration. However, the clinical use of autologous bone marrow-derived MSCs usually requires additional surgical procedures, and the availability of these cells at the point of care is relatively low. Thus, alternative autologous sources of cells would be clinically useful.
Our laboratory has recently identified a population of multipotent mesenchymal progenitor cells (MPCs) in traumatized muscle tissue, with characteristics similar to those of MSCs (Nesti et al., 2008). The MPCs are available at high concentrations in tissue that has been debrided from the zone of injury following musculo-skeletal trauma (Jackson et al., 2009a). In addition to having multilineage differentiation potential, the MPCs appear to exhibit trophic and pro-regeneration properties that are associated with MSC populations (Jackson et al., 2010). For example, the MPCs are capable of modulating inflammatory responses and they also participate in specific mechanisms of angiogenesis and vascular maintenance. We have also recently demonstrated that MPCs express neurotrophic factors, including nerve growth factor (NGF), brain-derived growth factor (BNDF), ciliary neurotrophic factor (CNTF) and neurotrophin 3 (NT-3) (Bulken-Hoover et al., 2011), all of which have specific functions in peripheral nerve regeneration to promote the growth of axons and migration of Schwann cells into the site of injury (Gordon, 2009; Xiao et al., 2009). Culturing the MPCs in a defined medium for neurotrophic induction can also enhance the expression of these factors (Bulken-Hoover et al., 2011).
Given the general pro-regeneration functions of traumatized muscle-derived MPCs and their expression of specific neurotrophic factors, these cells represent a possible alternative cell type for use in strategies to promote nerve repair. Although MPCs and MSCs exhibit many similar regenerative functions, differences between these cell types have been observed, including their response to particular extracellular stimuli and some mechanisms by which they interact with other cell types (Jackson et al., 2010). The expression of neurotrophic factors by MPCs has not been compared directly to a well-characterized population of MSCs to establish a quantitative measurement of their neurotrophic potential. Furthermore, the effect of the factors secreted by MPCs on neurons has not yet been evaluated to verify their ability to promote nerve regeneration. Therefore, the objective of this study was to determine whether the neurotrophic properties of traumatized muscle-derived MPCs are equivalent to those of bone marrow-derived MSCs. Our specific aims were: (a) to compare the gene- and protein-level expression of neurotrophic factors by MPCs and MSCs; (b) to evaluate the functional effect of the secreted neurotrophic factors on axon growth; and (c) to determine whether the neurotrophic function of MPCs and MSCs are enhanced by cellular communication with neuronal cells. The findings of this study will be useful to determine whether traumatized muscle-derived MPCs will be a sufficient substitute cell type for tissue-engineering applications of peripheral nerve repair.
2. Materials and methods
2.1. Cell-harvesting procedure
Institutional Review Board (IRB) approval from the Walter Reed Army Medical Center (WRAMC) was obtained to collect debrided muscle tissue following orthopaedic injury to the extremities. MPCs were harvested from the viable edge of muscle samples along the wound margin, as previously described (Jackson et al., 2009b; Nesti et al., 2008). In brief, approximately 200 mg muscle tissue was minced and gently agitated for 2 h at 37°C in digestion medium (DMEM with 0.5 mg/ml collagenase 2; Worthington Biosciences, Lakewood, NJ, USA). The cells, pelleted by centrifugation, were plated in a T150 tissue culture flask in DMEM containing 10% v/v fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA) and 5 U/ml penicillin, streptomycin and fungizone (PSF; Invitrogen). After 2 h, non-adherent cells were removed by washing with phosphate-buffered saline (PBS; Invitrogen) and the adherent cells were cultured in growth medium (GM; DMEM supplemented with 10% v/v FBS and 1 U/ml PSF). Bone marrow-derived MSCs were harvested from femoral heads obtained from total hip arthroplasties, following an IRB approved protocol at the University of Washington and using an established technique (Caterson et al., 2002).
2.2. Neurotrophic induction
The cells were plated at a density of 1000 cells/cm2 and maintained in GM for 3 days. On day 4, the cells were cultured in a pretreatment medium consisting of α-MEM (Sigma, St. Louis, MO, USA), 10% v/v FBS and 1 U/ml PSF, which was supplemented with 1 mm β-mercaptoethanol (Sigma) for 24 h and then 35 ng/ml retinoic acid (Sigma) for 2 additional days. Seven days after the initial plating, the cells were incubated for an additional 7 days in a previously optimized neurotrophic induction medium (NM) consisting of DMEM/HAM’s-F12 supplemented with 2% v/v B27 Supplement (Invitrogen), 2% v/v FBS, 20 µµ retinoic acid and 10 ng/ml basic fibroblast growth factor (bFGF) (Bulken-Hoover et al., 2011), and the medium was replaced with fresh NM every 2–3 days.
2.3. Immunocytochemistry
The cells were seeded in eight-well chamber slides and cultured according to the 14 day neurotrophic induction protocol described above. Control cells were maintained in GM for 14 days. The cells were washed once with HBSS, fixed in phosphate-buffered 3% w/v paraformaldehyde for 20 min, permeabilized with 0.5% v/v Triton X-100 (Invitrogen) for 10 min and blocked in 2% w/v bovine serum albumin (BSA; Sigma) for 30 min. The fixed cells were incubated with 10 µg/ml mouse anti-nestin IgG (Abcam, Cambridge, MA, USA) and 5 µg/ml chicken anti-BDNF IgY (Abcam) in phosphate-buffered saline (PBS) with 1% v/v goat non-immune IgG for 2 h at room temperature or overnight at 4°C, and then with 5 µg/ml Alexa 488-conjugated goat anti-mouse IgG and 5 µg/ml Alexa 548-conjugated goat anti-chicken IgG (diluted 1:100; Jackson ImmunoResearch, West Grove, PA, USA) in PBS for 30 min. Primary antibodies were reactive against the human antigens, and the secondary antibodies exhibited minimal cross-reactivity between mouse IgG and chicken IgY. Negative controls were performed to rule out non-specific or cross-reactive staining. Nuclear staining was carried out using Hoechst 33342 (Sigma). The coverslips were then mounted to slides with VectaShield (Vector Labs, Burlingame, CA, USA) and viewed with a Zeiss Observer Z1 microscope (Carl Zeiss Microimaging, Thornwood, NY, USA).
2.4. Histomorphometry
Cell density was extrapolated by counting the number of nuclei in each of five images taken at × 10 magnification. Each image captured approximately 0.13 mm2 of the surface area and the five images were taken from the same region of each culture, distributed over a total area of approximately 3.1 mm2. The number of cells in each image that were positive for BDNF and nestin was determined by uniformly thresholding the red and green channels and counting the number of cells with fluorescence intensity above the set threshold.
2.5. Gene expression assays
RNA was extracted by phase separation, using TRIzol (Invitrogen) according to the manufacturer’s protocol and purified using RNeasy Mini-columns (Qiagen). RNA concentrations were estimated with UV spectrophotometry on the basis of A260/280 and A260/230. Genomic DNA elimination, first-strand cDNA synthesis and real-time RT-PCR analysis was performed using the RT2 PCR assay system (SA Biosciences, Frederick, MD, USA) and a BioRad iCycler iQ real-time PCR detection system. Gene expression was normalized using β-actin (ACTB) as an internal housekeeping control (Bulken-Hoover et al., 2011; Jackson et al., 2010). All primers were obtained from SA Biosciences.
2.6. ELISA assays
Cells were plated in 12-well dishes, and neurotrophic induction was performed as described above. On day 14 of the neurotrophic induction protocol, cell supernatants were collected and the concentration of NGF, BDNF, CNTF and NT-3 in each sample was measured using ELISA kits (DuoSet ELISA systems, R&D Systems, Minneapolis, MN, USA), using a Synergy HT Plate reader (Bio-Tek, Winooski, VT, USA).
2.7. Dorsal root ganglion (DRG) assays
DRGs (L1-L6) were harvested from embryonic day 9 chicken embryos, using a previously described protocol (Nishi, 1996), and one DRG was positioned into the centre of each well of a 12-well plate with laminin-treated culture surfaces (~1 µg/ml, Sigma). The DRGs were cultured overnight in DRG medium, consisting of Eagle’s MEM (Invitrogen), 10% v/v horse serum (Sigma), 2 mm glutamine (Invitrogen), 1 U/ml PSF and 1 ng/ml ciliary neurotrophic factor (CNTF; Sigma). After 24 h, DRGs were prepared for either the conditioned media assay or for co-culture. GM or NM used for culture of MPCs and MSCs during days 14–17 of the neurotrophic induction protocol were collected as cell supernatants, mixed at a 1:1 ratio with fresh DRG medium and used as conditioned media. GM and NM, handled identically but placed in wells without cells for 3 days, were used as ‘no cell’ conditioned medium controls. Co-culture was performed using MPCs and MSCs, plated into Transwell inserts (Corning, NY, USA), cultured with GM or NM and then transferred to the wells containing the DRGs on day 14 of the neurotrophic induction protocol. The co-culture was maintained in fresh GM or NM, mixed at a 1:1 ratio with fresh DRG medium. After an additional 2 days of culture with conditioned media or co-culture, the DRGs were fixed with 4% w/v paraformaldehyde (FD NeuroTechnologies, Catonville, MD, USA) and imaged with phase-contrast and interference light microscopy. Neurite extension was compared to the average neurite length of neurites that extended from DRGs cultured in DRG medium for 3 days (1.75 mm).
2.8. Statistics
Statistical significance for each of the indicated tests was assigned to p < 0.05. All assays were performed with a minimum of three biological replicates consisting of cells derived from different donors.
3. Results
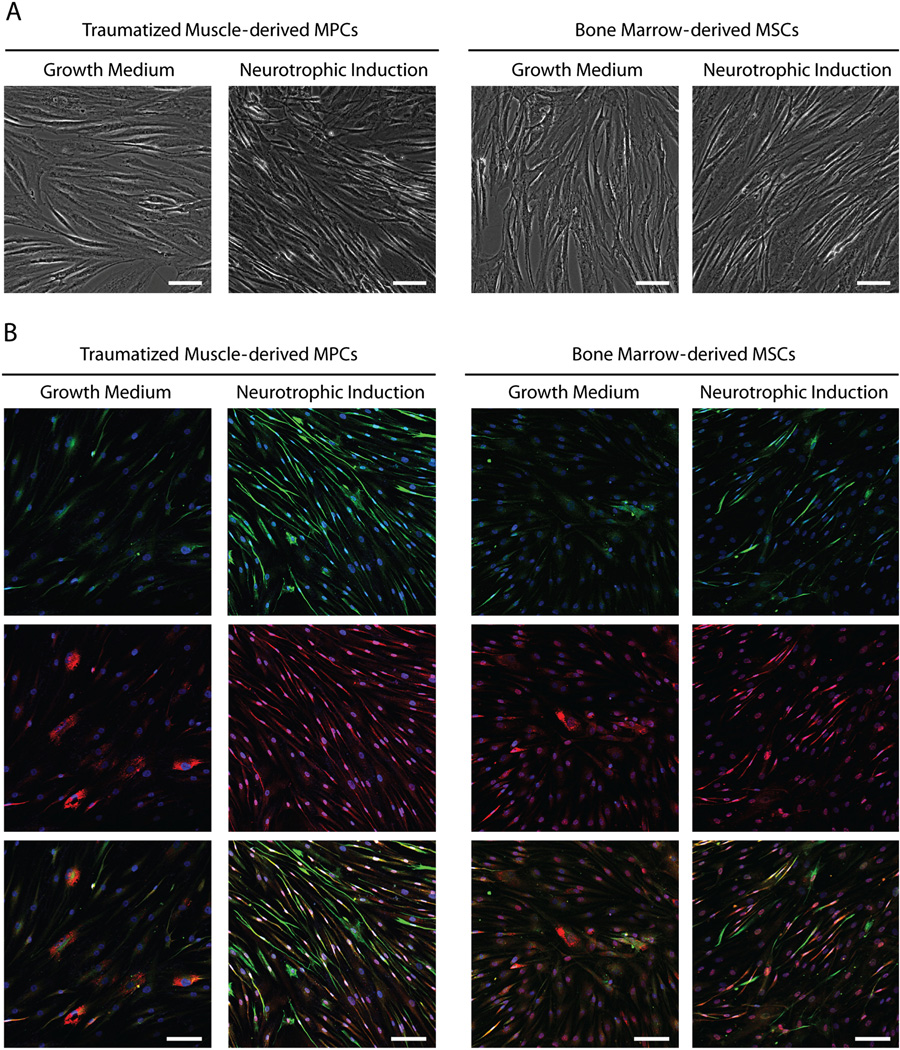
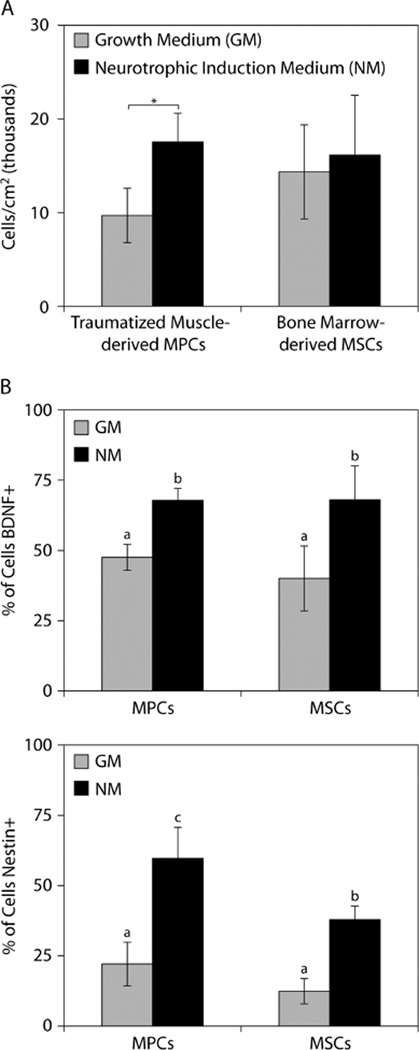
As a result of neurotrophic induction, the traumatized muscle-derived MPCs and bone marrow-derived MSCs both assumed a smaller and more spindle-shaped morphology (Figure 1A). Under growth medium conditions, both cell types displayed nestin-positive cells and a subpopulation of cells with BNDF staining associated with cytoplasmic vesicles located adjacent to the nuclei (Figure 1B). Following neurotrophic induction, more intense nestin and BNDF staining was evident in a greater number of both the MPCs and MSCs. Most of the nestin- and BDNF-positive cells had an elongated, spindle-shaped morphology. Immunostaining of nestin and BDNF appeared to be highly co-localized in the traumatized muscle-derived MPCs, whereas many of the bone marrow-derived MSCs were positive for either BDNF or nestin. Using histomorphometry, we determined that there was a significant increase in the number of MPCs following neurotrophic induction, but not for MSCs (Figure 2A). The percentage of BDNF-positive cells increased to approximately the same extent for MPCs and MSCs, while the percentage of nestin-positive cells increased significantly more for MPCs than for MSCs (Figure 2B).
Figure 1.
Morphology of MSCs and MPCs cultured in either growth medium or neurotrophic induction medium: (A) phase-contrast microscopy; (B) immunocytochemistry; both × 10 magnification. Cells were stained for nestin (green, top row), BNDF (red, middle row) and with Hoechst 33342 (blue). Red and green merged panels (bottom row) indicate cells with co-localized nestin and BDNF. All scale bars=100 µm
Figure 2.
Histomorphometric analysis of MPCs and MSCs cultured in growth medium (GM) and neurotrophic induction medium (NM). (A) Cell densities after 14 days of culture;*p < 0.05, Student’s t-test with n = 3. (B) Percentage of cells that stained positively for BNDF or nestin; a,bp < 0.05, b,cp < 0.05, a,cp < 0.01, one-way ANOVA with SNK post hoc comparisons and n = 3
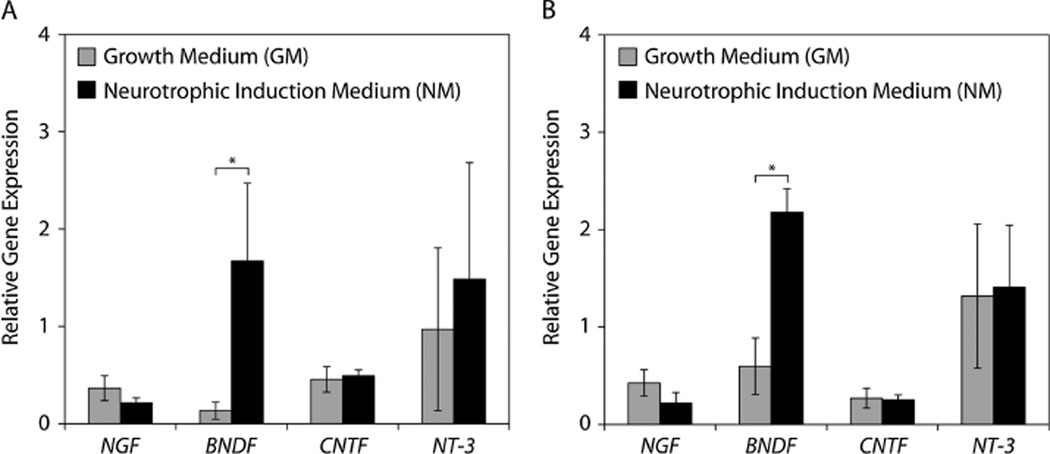
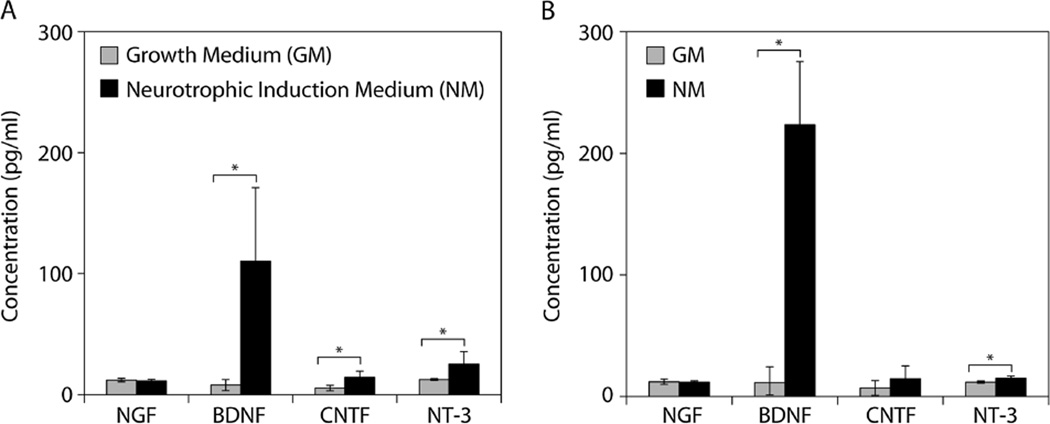
We evaluated the gene- and protein-level expression of four neurotrophic factors: NGF, BDNF, CNTF and NT-3. Only BDNF exhibited significant gene-level upregulation in response to neurotrophic induction for both traumatized muscle-derived MPCs and bone marrow-derived MSCs (Figure 3). However, significantly increased protein levels of CNTF and NT-3, in addition to BDNF, were detected in the culture supernatants of the traumatized muscle-derived MPCs maintained under neurotrophic induction conditions (Figure 4A). Similarly, bone marrow-derived MSCs exhibited significantly elevated protein levels of BDNF and NT-3 following neurotrophic induction (Figure 4B).
Figure 3.
Neurotrophic factor gene expression: (A) traumatized muscle-derived MPCs and (B) bone marrow-derived MSCs were cultured in either growth medium (GM) or neurotrophic induction medium (NM) for 14 days, and gene expression was assayed using real-time RT-PCR; *p < 0.01, Student’s t-tests with n = 4. NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; NT-3, neurotrophin-3
Figure 4.
Neurotrophic factor production: (A) traumatized muscle-derived MPCs and (B) bone marrow-derived MSCs were cultured in either growth medium (GM) or neurotrophic induction medium (NM) for 14 days, and the concentration of neurotrophic factors secreted in the cell supernatants during the final 3 days was measured using ELISA; *p < 0.05, Student’s t-tests with n = 4
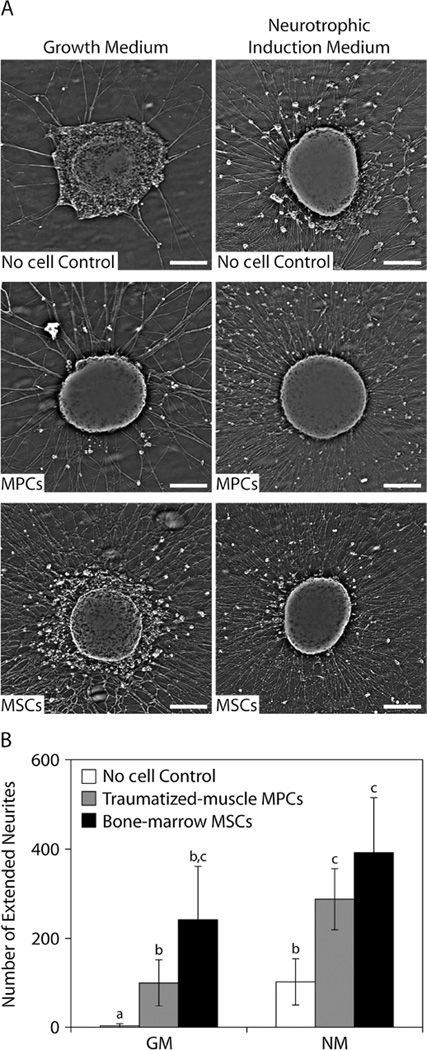
The effects of these secreted factors on neuron function were evaluated using a conditioned medium-DRG assay. The density of neurites appeared to increase for DRGs that were cultured in medium conditioned by either traumatized muscle-derived MPCs or bone marrow-derived MSCs, with the latter having a greater effect on neurite densities (Figure 5A). Neurite density also appeared to be higher for DRGs that were cultured in conditioned medium derived using NM. The number of neurites that extended beyond the average neurite length of DRGs cultured in DRG medium alone (1.75 mm) was significantly increased in cultures exposed to factors secreted by the MPCs and MSCs in both GM and NM, compared to the ‘no cell’ control condition (Figure 5B).
Figure 5.
Neurotrophic activity assay of MPC and MSC conditioned media using cultured DRGs. (A) Neurite density was imaged using ×4 interference microscopy after culture for 3 days with growth medium or neurotrophic induction media that had been conditioned with factors secreted by MPCs or MSCs; scale bar = 250 µm. (B) The number of neurites that extended beyond the average neurite length in control DRG medium (1.75 mm) as a result of culture with growth medium or neurotrophic induction medium conditioned by MPCs or MSCs; a,bp < 0.05, b,cp < 0.05, a,cp < 0.01, one-way ANOVA with SNK post hoc comparisons and n = 4
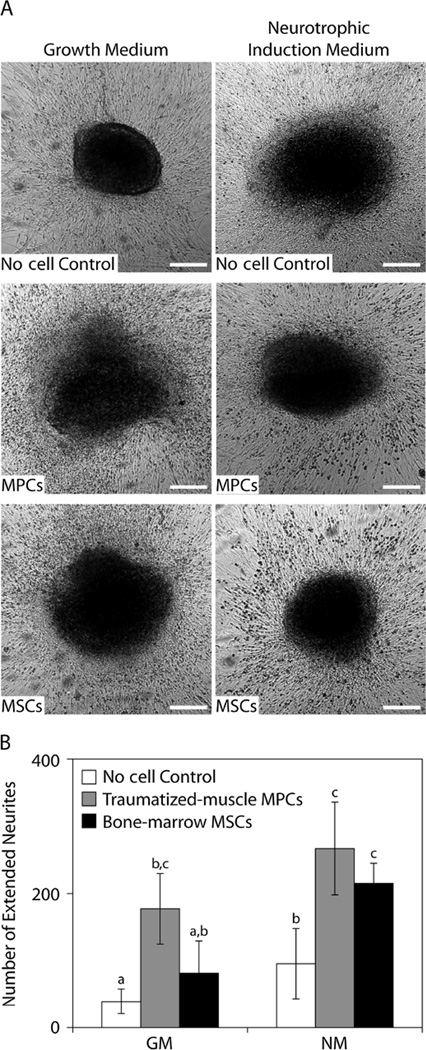
The co-culture medium conditions affected the length of neurites and the proliferation of fibroblastic cells in the DRG (Figure 6A). The DRGs cultured in the ‘no cell’ growth medium condition exhibited the least fibroblastic cell proliferation and were able to maintain their shape throughout the culture period, while fibroblastic cell density appeared to be greater for the DRGs that were co-cultured with either traumatized muscle-derived MPCs or bone marrow-derived MSCs. Under neurotrophic induction conditions, the fibroblastic cell density did not appear to depend on the co-culture cell type for DRGs that were cultured. The number of extended neurites was also significantly greater for the DRGs that were co-cultured with either MPCs or MSCs under either medium condition compared to the ‘no cell’ controls (Figure 6B), although the effect of MSC co-culture did not appear to be as significant as co-culture with the traumatized muscle-derived MPCs.
Figure 6.
Neurotrophic activity of MPCs and MSCs in DRG co-culture assays. (A) The DRGs were imaged using ×4 phase-contrast microscopy after co-culture with MPCs or MSCs for 3 days in growth medium or neurotrophic induction medium; scale bar = 250 µm. (B) The number of neurites that extended beyond the average neurite length in DRG medium (1.75 mm) as a result of co-culture with MPCs or MSCs in growth medium or neurotrophic induction media; a,b,cp < 0.05, one-way ANOVA with SNK post hoc comparisons and n = 4
4. Discussion
Traumatized muscle-derived MPCs are readily available following traumatic musculoskeletal injuries, and these cells could provide a substitute for bone marrow-derived MSCs for use in cellular therapies to promote functional tissue regeneration (Jackson et al., 2010). The objective of this study was to determine whether the neurotrophic properties of traumatized muscle-derived MPCs are equivalent to those of bone marrow-derived MSCs. Our results showed that both the MPCs and the MSCs contain a subpopulation of cells with neurotrophic properties, and that treatment with an optimized neurotrophic induction medium resulted in increasing the percentage of both cell types that express nestin and BNDF. This treatment resulted in an overall increase in the number of MPCs but did not alter the proliferation of MSCs. Neurotrophic induction also led to an increase in the gene- and protein-level expression of BDNF in both cell types. Increased protein levels of CNTF and NT-3 were detected in conditioned medium of MPCs after neurotrophic induction, which was likely due to a higher number of cells expressing these factors. A similar result was observed for NT-3 expression by MSCs. These secreted factors led to an increase in the density and length of neurites that had extended from chick embryonic DRGs. Similar results were observed in a co-culture assay, although the effect of the MPC-secreted factors appeared to be more significant, suggesting crosstalk involving soluble factors between these cells and the DRG cells. In summary, while there appeared to be some differences between traumatized muscle-derived MPCs and bone marrow-derived MSCs in terms of specific neurotrophic interactions, the two cell types appear to be functionally similar in their ability to produce neurotrophic factors and to enhance the growth and proliferation of neurogenic cell types.
Several aspects of this study contribute to the strength of our findings. First, the expression of neurotrophic factors by the entire population of traumatized muscle-derived MPCs was assessed at both gene and protein levels. We also used immunocytochemistry to substantiate the expression of BNDF by localizing the protein to intracellular regions of the MPCs, and further identified a subpopulation of cells that express higher levels of this neurotrophic factor. Second, the trophic effect of secreted neurotrophic factors on neuronal cell types was evaluated using a DRG model. We demonstrated not only that the MPCs express specific neurotrophic factors, but also that these cytokines work in concert with other secreted factors to promote the growth of neurites and the migration of fibroblastic cells away from the DRG. The results of the co-culture experiment also suggest that there is crosstalk and communication via secreted soluble factors between the neuronal cell types and the MPCs to further enhance trophic factor production. Finally, all our experiments were carried out using MPCs derived from traumatized human muscle and the results are compared against human bone marrow-derived MSCs, a well characterized population of adult stem cells. Our findings are thus directly applicable to developing clinical therapies using traumatized muscle-derived MPCs, with their potential therapeutic effects evaluated in the context of MSCs, a cell type that is currently in clinical use.
A few caveats of our study should be noted. First, the neurotrophic functional assays were made using an in vitro model based on chick embryonic DRGs. Although this is a well-characterized system for the study of neurotrophic effects, it will be necessary to verify the neurotrophic activities of the MPCs in vivo. Second, the experimental outputs of our functional neurotrophic assays were based on measurement of neurite length and cell migration of fibroblastic cells away from the DRG, which are morphological observations made without the identification of associated cell-type specific markers. While this observation allows us to describe general neurotrophic effects of soluble, secreted factors derived from MPCs, immunocytochemical detection of specific cellular markers, such as S100 for Schwann cells, is needed for more specific characterization of the effects and cellular targets within the DRG. Finally, this is primarily a descriptive study and not an investigation into the mechanism of the neurotrophic enhancement activity of the traumatized muscle-derived MPCs. We believe that these findings serve as a foundation for further development of clinical therapies based on the trophic function of the MPCs and, in parallel, we have initiated amechanistic investigation into the trophic interactions between the MPCs and neuronal cell types.
It is noteworthy that the neurotrophic potential of MSCs has previously been demonstrated in vitro and in vivo using several animal models. Even without treatment to enhance their glial cell-like properties, MSCs express glial cell markers, such as glial fibulary acidic protein (GFAP) and nestin (Deng et al., 2006; Tondreau et al., 2004), as well as neurotrophic factors that can promote the growth and survival of neurons in vitro (Pan et al., 2007; Ribeiro-Resende et al., 2009; Wright et al., 2010; Yang et al., 2009). Untreated MSCs have also been shown to promote nerve regeneration in vivo (Pan et al., 2007). Similar to our findings described here, the previously reported neurotrophic behaviour appears to arise from subpopulations of MSCs that exhibit baseline neurogenic characteristics (Crigler et al., 2006). Treatment with defined induction factors can promote glial cell functions and generate an in vitro phenotype suggesting their trans-differentiation into Schwann cells (Brohlin et al., 2009; Keilhoff et al., 2006a; Mahay et al., 2008b). These Schwann cell-like populations of MSCs function similarly to Schwann cells in vitro and can also be used to promote nerve regeneration in vivo (Keilhoff et al., 2006b). The results of the previous in vitro functional assays corroborate our findings, suggesting that traumatized muscle-derived MPCs exhibit neurotrophic potential similar to that of MSCs. While few studies have directly compared untreated MSCs to differentiated MSCs to assess whether the transplanted cells are effective in the in vivo environment of the injured nerve, a recent study in a non-human primate model demonstrated the long-term safety and efficacy of using MSCs to promote peripheral nerve regeneration (Wakao et al., 2010).
There is currently conflicting evidence as to whether MSCs can undergo non-mesenchymal trans-differentiation into a Schwann or glial cell type. There are several reports indicating that Schwann cells can be generated from MSCs using an induction protocol involving defined factors that yields morphological and functional properties that are lineage-characteristic for glial cells (Brohlin et al., 2009; Caddick et al., 2006). However, some studies indicate that the induction condition is merely triggering transient expression of these characteristics, and that the MSCs will eventually revert to their previous phenotype and function (Rooney et al., 2009). An alternative description of the neurotrophic induction is that treatment with defined factors promotes the proliferation and survival of the neurogenic MSC subpopulations, thus resulting in a population of MSCs with Schwann cell-like characteristics by means of selection rather than trans-differentiation (Phinney and Prockop, 2007). In our study, the defined induction medium used appears to emulate the latter method of neurotrophic enhancement of MPC and MSC populations, although it does not substantially affect the survival of the non-neurogenic cells. The resulting cell population is thus able to continue providing the general trophic functions that are characteristic of MSCs, but with a higher percentage of cells that produce BDNF and, presumably, the other neurotrophic factors as well. Furthermore, our data suggest that MPCs and MSCs respond to cells in the DRG by exhibiting greater neurotrophic function, and this effect has been observed previously in MSCs co-cultured with DRG neurons (Yang et al., 2008). By extension, we speculate that untreated MSCs or traumatized muscle-derived MPCs may be used as a cellular therapy to promote peripheral nerve regeneration, with subpopulations of these cells assuming Schwann cell-type functions while they are exposed to the wound-response factors in the nerve (Bulken-Hoover et al., 2011).
Several differences were noted that distinguish the neurotrophic function of traumatized muscle-derived MPCs from that of bone marrow-derived MSCs. First, treatment of the MPCs with the defined neurotrophic induction medium resulted in an increase in their proliferation and, in particular, there was an increase in the percentage of nestin-positive cells. There also appeared to be a higher number of MPCs expressing both BNDF and nestin. These findings suggest that there may be differences in the neurogenic subpopulations between MPCs and MSCs, likely reflecting their different tissue origin. Second, the overall protein-level expression of BDNF was lower for the traumatized muscle-derived MPCs, and the functional neurotrophic enhancement from MPC-secreted factors, as measured by the density of extended DRG neurites, appeared to be less effective than that of the MSC-secreted factors. However, the MPCs also appeared to communicate via soluble factors with the DRG cells in co-culture and, in response, the neurotrophic function of the MPCs was enhanced. These observations corroborate our previous studies suggesting that the MPCs should become more neurotrophic when they are in the environment of an injured peripheral nerve (Bulken-Hoover et al., 2011). We have previously described the similarities between the MPCs and MSCs in terms of differentiation potential and pro-regeneration activities, and this study extends our previous findings to neurotrophic functions as well.
In summary, the findings of our study show that the neurotrophic characteristics of MPCs isolated from traumatized muscle tissue appear to be substantially equivalent to the well-characterized population of bone marrow-derived MSCs. Both cell types exhibit gene- and protein-expression of neurotrophic factors, which act on neuronal cell types to enhance DRG cell migration and neurite growth. The MPCs and MSCs are also both responsive to neurotrophic induction via defined factors, and our immunocytochemistry results suggest that different subpopulations of these cell types may be responsible for the enhanced neurotrophic function. Our current studies are aimed at investigating the mechanism by which the MPCs respond to the neurotrophic induction factors, as well as the factors secreted by the DRG neurons, and to differentiate the responses of specific MPC subpopulations. We are also evaluating the ability of the traumatized muscle-derived MPCs to improve peripheral nerve regeneration in vivo, using a segmental peroneal nerve defect model. We believe that the findings of the present study strongly suggest that MPCs derived from traumatized muscle tissue may be developed as an alternative cell type to bone marrow-derived MSCs as a cellular therapy to promote neurite growth and cell migration during peripheral nerve regeneration.
Acknowledgements
This study was supported in part by the National Institutes of Health (NIH) Intramural Research Program (Grant No. Z01 AR41131), the Department of Defense Military Amputee Research Program at WRAMC (Department of the Army, U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office, Grant No. PO5-A011), the Comprehensive Neurosciences Program (The Uniformed Services University of the Health Sciences (USU), 4301 Jones Bridge Rd. Bethesda, MD 20814-4799 is the awarding and administering office, Grant No. CNP-2008-CR01) and the Peer-reviewed Orthopedic Research Program (Department of the Army, U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office, Grant No. W81XWH-10-2-0084), and from the Commonwealth of Pennsylvania Department of Health. The authors thank Dr Paul Manner, University of Washington, for providing human skeletal tissues, and Ibardo Zambrano and Richard Booth for technical assistance.
Footnotes
The views expressed in this manuscript are those of the authors alone and do not represent the views, policies or official positions of, nor should any official endorsement be inferred on the part of, the United States government, the United States Army, the Uniformed Services University of the Health Sciences (USU), or the Department of Defense. Nor do they represent those of the National Institutes of Health or the Department of Health and Human Services.
References
- Bossolasco P, Cova L, Calzarossa C, et al. Neuroglial differentiation of human bone marrow stem cells in vitro. Exp Neurol. 2005;193:312–325. doi: 10.1016/j.expneurol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Brohlin M, Mahay D, Novikov LN, et al. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci Res. 2009;64:41–49. doi: 10.1016/j.neures.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Bulken-Hoover JD, Jackson WM, Ji Y, et al. Inducible expression of neurotrophic factors by mesenchymal progenitor cells derived from human muscle after traumatic injury. Mol Biotechnol. 2011 doi: 10.1007/s12033-011-9445-z. [DOI] [PubMed] [Google Scholar]
- Caddick J, Kingham PJ, Gardiner NJ, et al. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia. 2006;54:840–849. doi: 10.1002/glia.20421. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Caterson EJ, Nesti LJ, Danielson KG, et al. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002;20:245–256. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- Crigler L, Robey RC, Asawachaicharn A, et al. Human mesenchymal stem cell sub-populations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Deng J, Petersen BE, Steindler DA, et al. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006;24:1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26:E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- Gordon T, Chan KM, Sulaiman OA, et al. Accelerating axon growth to overcome limitations in functional recovery after peripheral nerve injury. Neurosurgery. 2009;65:A132–A144. doi: 10.1227/01.NEU.0000335650.09473.D3. [DOI] [PubMed] [Google Scholar]
- Hu J, Zhu QT, Liu XL, et al. Repair of extended peripheral nerve lesions in rhesus monkeys using acellular allogenic nerve grafts implanted with autologous mesenchymal stem cells. Exp Neurol. 2007;204:658–666. doi: 10.1016/j.expneurol.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Jackson WM, Aragon AB, Bulken-Hoover JD, et al. Putative heterotopic ossification progenitor cells derived from traumatized muscle. J Orthop Res. 2009a;27:1645–1651. doi: 10.1002/jor.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WM, Aragon AB, Djouad F, et al. Mesenchymal progenitor cells derived from traumatized human muscle. J Tissue Eng Regen Med. 2009b;3:129–138. doi: 10.1002/term.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WM, Lozito T, Djouad F, et al. Differentiation and regeneration potential of mesenchymal progenitor cells derived from traumatized muscle tissue. J Cell Mol Med. 2010;1511:2377–2388. doi: 10.1111/j.1582-4934.2010.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhoff G, Goihl A, Stang F, et al. Peripheral nerve tissue engineering: autologous Schwann cells vs. transdifferentiated mesenchymal stem cells. Tissue Eng. 2006a;12:1451–1465. doi: 10.1089/ten.2006.12.1451. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Stang F, Goihl A, et al. Transdifferentiated mesenchymal stem cells as alternative therapy in supporting nerve regeneration and myelination. Cell Mol Neurobiol. 2006b;26:1235–1252. doi: 10.1007/s10571-006-9029-9. [DOI] [PubMed] [Google Scholar]
- Le Blanc K. Mesenchymal stromal cells: tissue repair and immune modulation. Cytotherapy. 2006;8:559–561. doi: 10.1080/14653240601045399. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- Lee JY, Qu-Petersen Z, Cao B, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madduri S, Gander B. Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J Peripher Nerv Syst. 2010;15:93–103. doi: 10.1111/j.1529-8027.2010.00257.x. [DOI] [PubMed] [Google Scholar]
- Mahay D, Terenghi G, Shawcross SG. Growth factors in mesenchymal stem cells following glial-cell differentiation. Biotechnol Appl Biochem. 2008a;51:167–176. doi: 10.1042/BA20070212. [DOI] [PubMed] [Google Scholar]
- Mahay D, Terenghi G, Shawcross SG. Schwann cell mediated trophic effects by differentiated mesenchymal stem cells. Exp Cell Res. 2008b;314:2692–2701. doi: 10.1016/j.yexcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Munoz JR, Stoutenger BR, Robinson AP, et al. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci USA. 2005;102:18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesti LJ, Jackson WM, Shanti RM, et al. Differentiation potential of multipotent progenitor cells derived from war-traumatized muscle tissue. J Bone Joint Surg Am. 2008;90:2390–2398. doi: 10.2106/JBJS.H.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi R. Autonomic and sensory neuron cultures. Methods Cell Biol. 1996;51:249–263. doi: 10.1016/s0091-679x(08)60632-9. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Sato K, Oh I, et al. Mechanisms of immunomodulation by mesenchymal stem cells. Int J Hematol. 2007;86:5–7. doi: 10.1532/IJH97.07003. [DOI] [PubMed] [Google Scholar]
- Pan HC, Cheng FC, Chen CJ, et al. Post-injury regeneration in rat sciatic nerve facilitated by neurotrophic factors secreted by amniotic fluid mesenchymal stem cells. J Clin Neurosci. 2007;14:1089–1098. doi: 10.1016/j.jocn.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Passweg J, Tyndall A. Autologous stem cell transplantation in autoimmune diseases. Semin Hematol. 2007;44:278–285. doi: 10.1053/j.seminhematol.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Resende VT, Pimentel-Coelho PM, Mesentier-Louro LA, et al. Trophic activity derived from bone marrow mononuclear cells increases peripheral nerve regeneration by acting on both neuronal and glial cell populations. Neuroscience. 2009;159:540–549. doi: 10.1016/j.neuroscience.2008.12.059. [DOI] [PubMed] [Google Scholar]
- Rooney GE, Howard L, O’Brien T, et al. Elevation of cAMP in mesenchymal stem cells transiently upregulates neural markers rather than inducing neural differentiation. Stem Cells Dev. 2009;18:387–398. doi: 10.1089/scd.2008.0080. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Cassetti A, Tredici G. Adult mesenchymal stem cells rescue dorsal root ganglia neurons from dying. Brain Res. 2006;1116:75–81. doi: 10.1016/j.brainres.2006.07.127. [DOI] [PubMed] [Google Scholar]
- Tomchuck SL, Zwezdaryk KJ, Coffelt SB, et al. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondreau T, Lagneaux L, Dejeneffe M, et al. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72:319–326. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- Wakao S, Hayashi T, Kitada M, et al. Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp Neurol. 2010;223:537–547. doi: 10.1016/j.expneurol.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, et al. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wright KT, Griffiths GJ, Johnson WE. A comparison of high-content screening versus manual analysis to assay the effects of mesenchymal stem cell-conditioned medium on neurite outgrowth in vitro. J Biomol Screen. 2010;15:576–582. doi: 10.1177/1087057110367959. [DOI] [PubMed] [Google Scholar]
- Xiao J, Kilpatrick TJ, Murray SS. The role of neurotrophins in the regulation of myelin development. Neurosignals. 2009;17:265–276. doi: 10.1159/000231893. [DOI] [PubMed] [Google Scholar]
- Yang J, Lou Q, Huang R, et al. Dorsal root ganglion neurons induce transdifferentiation of mesenchymal stem cells along a Schwann cell lineage. Neurosci Lett. 2008;445:246–251. doi: 10.1016/j.neulet.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Yang J, Wu H, Hu N, et al. Effects of bone marrow stromal cell-conditioned medium on primary cultures of peripheral nerve tissues and cells. Neurochem Res. 2009;34:1685–1694. doi: 10.1007/s11064-009-9963-2. [DOI] [PubMed] [Google Scholar]