Abstract
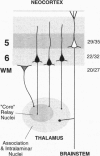
How are long-range axonal projections from the cerebral cortex orchestrated during development? By using both passively and actively transported axonal tracers in fetal and postnatal ferrets, we have analyzed the development of projections from the cortex to a number of thalamic nuclei. We report that the projections of a cortical area to its corresponding thalamic nuclei follow highly cell-specific programs of development. Axons from cells in the deepest layers of the cerebral cortex (layer 6 and superficial subplate neurons) appear to grow very slowly and be delayed for several weeks in the cerebral white matter, reaching the thalamus over a protracted period. Neurons of layer 5, on the other hand, develop their projections much faster; despite being born after the neurons of deeper layers, layer 5 neurons are the first to extend their axons out of the cortical hemisphere and innervate the thalamus. Layer 5 projections are massive in the first postnatal weeks but may become partly eliminated later in development, being overtaken in number by layer 6 cells that constitute the major corticothalamic projection by adulthood. Layer 5 projections are area-specific from the outset and arise as collateral branches of axons directed to the brainstem and spinal cord. Our findings show that the early development of corticofugal connections is determined not by the sequence of cortical neurogenesis but by developmental programs specific for each type of projection neuron. In addition, they demonstrate that in most thalamic nuclei, layer 5 neurons (and not subplate or layer 6 neurons) establish the first descending projections from the cerebral cortex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore C., Molnár Z. Factors involved in the establishment of specific interconnections between thalamus and cerebral cortex. Cold Spring Harb Symp Quant Biol. 1990;55:491–504. doi: 10.1101/sqb.1990.055.01.048. [DOI] [PubMed] [Google Scholar]
- Bourassa J., Pinault D., Deschênes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci. 1995 Jan 1;7(1):19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Dalva M. B., Ghosh A., Shatz C. J. Independent control of dendritic and axonal form in the developing lateral geniculate nucleus. J Neurosci. 1994 Jun;14(6):3588–3602. doi: 10.1523/JNEUROSCI.14-06-03588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. M. Intrinsic differences in the growth rate of early nerve fibres related to target distance. Nature. 1989 Feb 9;337(6207):553–555. doi: 10.1038/337553a0. [DOI] [PubMed] [Google Scholar]
- Davies A. M. Intrinsic programmes of growth and survival in developing vertebrate neurons. Trends Neurosci. 1994 May;17(5):195–199. doi: 10.1016/0166-2236(94)90104-x. [DOI] [PubMed] [Google Scholar]
- De Carlos J. A., O'Leary D. D. Growth and targeting of subplate axons and establishment of major cortical pathways. J Neurosci. 1992 Apr;12(4):1194–1211. doi: 10.1523/JNEUROSCI.12-04-01194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel H., Holländer H. Autoradiographic tracing of developing subcortical projections of the occipital region in fetal rabbits. J Comp Neurol. 1980 Aug 1;192(3):505–518. doi: 10.1002/cne.901920309. [DOI] [PubMed] [Google Scholar]
- Erzurumlu R. S., Jhaveri S. Emergence of connectivity in the embryonic rat parietal cortex. Cereb Cortex. 1992 Jul-Aug;2(4):336–352. doi: 10.1093/cercor/2.4.336. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Shatz C. J. Pathfinding and target selection by developing geniculocortical axons. J Neurosci. 1992 Jan;12(1):39–55. doi: 10.1523/JNEUROSCI.12-01-00039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Kelly J. P. The projections of cells in different layers of the cat's visual cortex. J Comp Neurol. 1975 Sep;163(1):81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- Godement P., Vanselow J., Thanos S., Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987 Dec;101(4):697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- Gottlieb D. I., Cowan W. M. Evidence for a temporal factor in the occupation of available synaptic sites during the development of the dentate gyrus. Brain Res. 1972 Jun 22;41(2):452–456. doi: 10.1016/0006-8993(72)90514-8. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Wilson J. R., Ogren M. P. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. J Comp Neurol. 1978 Nov 1;182(1):123–136. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- Hoogland P. V., Wouterlood F. G., Welker E., Van der Loos H. Ultrastructure of giant and small thalamic terminals of cortical origin: a study of the projections from the barrel cortex in mice using Phaseolus vulgaris leuco-agglutinin (PHA-L). Exp Brain Res. 1991;87(1):159–172. doi: 10.1007/BF00228517. [DOI] [PubMed] [Google Scholar]
- Jackson C. A., Peduzzi J. D., Hickey T. L. Visual cortex development in the ferret. I. Genesis and migration of visual cortical neurons. J Neurosci. 1989 Apr;9(4):1242–1253. doi: 10.1523/JNEUROSCI.09-04-01242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. K., Casagrande V. A. Prenatal development of axon outgrowth and connectivity in the ferret visual system. Vis Neurosci. 1993 Jan-Feb;10(1):117–130. doi: 10.1017/s0952523800003266. [DOI] [PubMed] [Google Scholar]
- Kuang R. Z., Kalil K. Development of specificity in corticospinal connections by axon collaterals branching selectively into appropriate spinal targets. J Comp Neurol. 1994 Jun 8;344(2):270–282. doi: 10.1002/cne.903440208. [DOI] [PubMed] [Google Scholar]
- Luskin M. B., Shatz C. J. Neurogenesis of the cat's primary visual cortex. J Comp Neurol. 1985 Dec 22;242(4):611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- Luskin M. B., Shatz C. J. Studies of the earliest generated cells of the cat's visual cortex: cogeneration of subplate and marginal zones. J Neurosci. 1985 Apr;5(4):1062–1075. doi: 10.1523/JNEUROSCI.05-04-01062.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell S. K. Fates of visual cortical neurons in the ferret after isochronic and heterochronic transplantation. J Neurosci. 1988 Mar;8(3):945–974. doi: 10.1523/JNEUROSCI.08-03-00945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell S. K., Ghosh A., Shatz C. J. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989 Sep 1;245(4921):978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- McConnell S. K., Ghosh A., Shatz C. J. Subplate pioneers and the formation of descending connections from cerebral cortex. J Neurosci. 1994 Apr;14(4):1892–1907. doi: 10.1523/JNEUROSCI.14-04-01892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissirel C., Dehay C., Kennedy H. Transient cortical pathways in the pyramidal tract of the neonatal ferret. J Comp Neurol. 1993 Dec 8;338(2):193–213. doi: 10.1002/cne.903380205. [DOI] [PubMed] [Google Scholar]
- Miller B., Chou L., Finlay B. L. The early development of thalamocortical and corticothalamic projections. J Comp Neurol. 1993 Sep 1;335(1):16–41. doi: 10.1002/cne.903350103. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J., Baker G. E. Development of the thalamic reticular and perireticular nuclei in rats and their relationship to the course of growing corticofugal and corticopetal axons. J Comp Neurol. 1993 Dec 22;338(4):575–587. doi: 10.1002/cne.903380407. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J., Guillery R. W. New views of the thalamic reticular nucleus in the adult and the developing brain. Trends Neurosci. 1993 Jun;16(6):240–245. doi: 10.1016/0166-2236(93)90163-g. [DOI] [PubMed] [Google Scholar]
- Ojima H. Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. Cereb Cortex. 1994 Nov-Dec;4(6):646–663. doi: 10.1093/cercor/4.6.646. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974 Feb 1;183(4123):425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988 Jul 8;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Schwartz M. L., Dekker J. J., Goldman-Rakic P. S. Dual mode of corticothalamic synaptic termination in the mediodorsal nucleus of the rhesus monkey. J Comp Neurol. 1991 Jul 15;309(3):289–304. doi: 10.1002/cne.903090302. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Rakic P. The genesis of efferent connections from the visual cortex of the fetal rhesus monkey. J Comp Neurol. 1981 Feb 20;196(2):287–307. doi: 10.1002/cne.901960208. [DOI] [PubMed] [Google Scholar]
- Sheng X. M., Marotte L. R., Mark R. F. Development of the laminar distribution of thalamocortical axons and corticothalamic cell bodies in the visual cortex of the wallaby. J Comp Neurol. 1991 May 1;307(1):17–38. doi: 10.1002/cne.903070103. [DOI] [PubMed] [Google Scholar]
- Stanfield B. B., O'Leary D. D. The transient corticospinal projection from the occipital cortex during the postnatal development of the rat. J Comp Neurol. 1985 Aug 8;238(2):236–248. doi: 10.1002/cne.902380210. [DOI] [PubMed] [Google Scholar]