Abstract
Toxoplasma gondii infects all warm-blooded animals, including humans, causing serious public health problems and great economic loss for the food industry. Commonly used serological tests require costly and hazardous preparation of whole Toxoplasma lysate antigens from tachyzoites. Here, we have evaluated an alternative method for antigen production, which involved a prokaryotic expression system. Specifically, we expressed T. gondii dense granular protein-5 (GRA5) in Escherichia coli and isolated it by affinity purification. The serodiagnostic potential of the purified recombinant GRA5 (rGRA5) was tested through Western blot analysis against 212 human patient serum samples. We found that rGRA5 protein was 100% specific for analysis of toxoplasmosis-negative human sera. Also, rGRA5 was able to detect acute and chronic T. gondii infections (sensitivities of 46.8% and 61.2%, resp.).
1. Introduction
Toxoplasmosis is a parasitic disease caused by Toxoplasma gondii (T. gondii) which belongs to phylum Apicomplexa [1]. It is an obligate intracellular protozoan parasite capable of infecting all warm-blooded domestic animals as well as human beings [2]. The infection is globally distributed affecting up to one-third of the world's human population [3]. Infection of T. gondii involves the transmission within and between hosts by zoites [4]. Three infectious stages of the parasite are tachyzoite, bradyzoite, and sporozoite [5]. Humans get infected with such disease through congenital transmission, consumption of raw or undercooked meat contaminated with T. gondii tissue cysts, or uptake of water contaminated with sporulated oocysts from the infected cat feces [6].
Toxoplasmosis in immunocompetent individuals is often asymptomatic [7] but can cause severe clinical outcome to immunocompromised patients leading to multisystem organ failure or even death [2]. Meanwhile, primary infection in pregnant women will likely transmit the parasite to the fetus vertically causing congenital toxoplasmosis and might eventually bring about miscarriage in pregnant women [8, 9]. Besides pregnant women, similar infection does occur in sheep and goats [10], giving rise to similar consequence. Abortions in these animals contribute to great economic loss in livestock industry. Therefore, it is crucial to conduct a rapid, highly accurate, and early diagnostic test for T. gondii infected patients/hosts for prevention or early treatment.
In general, there are few methods available for conducting laboratory diagnosis of toxoplasmosis including serologic assays (antibody detection), polymerase chain reaction (PCR; specific gene detection), histologic examination, and isolation of the parasite followed by inoculation into peritoneal cavities of mice (in vivo) or tissue cultures (in vitro) from biopsy tissue and blood/body fluids, respectively, of the infected patients [11]. However, the most commonly used diagnostic test would be the serological test which relies on the Toxoplasma lysate antigens (TLAs) from tachyzoites propagated in vivo or in vitro. There are several disadvantages pertaining to the usage of antigens originating from tachyzoites: high production cost, time consuming, inconstant quality, contamination with extraparasitic components, and exposure of the staff to the harmful living parasites [12]. To overcome this, recombinant DNA technology plays an important role in producing a larger quantity of recombinant antigenic proteins for serodiagnosis of T. gondii infection in a safer manner with lower production cost. Besides, recombinant tachyzoite proteins production either through prokaryotic or eukaryotic systems can reduce the variation of quality, enabling the development of a more specific and standardized serological assay.
Previous studies have reported the potential of various specific antigens of T. gondii such as the surface proteins [13, 14], microneme proteins [15], rhoptry proteins [16, 17], and dense granule proteins [18, 19] as seromarkers, either as single or multiantigen for detection of T. gondii-specific serum antibodies against acute (recently acquired) or/and chronic (distant past) Toxoplasma infection.
Dense granule (GRA) proteins are proteins with high immunogenicity [20]. They are found abundantly in both tachyzoites and bradyzoites [21] and make up most of the circulating antigens in the blood stream of an infected host which can be detected as early as a few hours postinfection (acute phase) [22]. GRA proteins were also found during the chronic stage of T. gondii infection [20]. As a result, the immunogenicity and prolonged expression of GRA proteins make them one of the promising candidates for recombinant protein production.
A total of 12 GRA proteins with the molecular weight ranging from 21 to 41 kDa have been identified [4, 21, 23–25]. Diagnostic performance of GRA antigens such as GRA2, GRA6, GRA7, and GRA8 has been investigated via ELISA for discriminating acute from chronic Toxoplasma infections [18, 19, 26–28]. Recombinant GRA7 was also shown to detect acute T. gondii infection more strongly compared to chronic infection [29]. Meanwhile, in our previous study, sensitivity and specificity of recombinant GRA2 for serodiagnosis of Toxoplasma-infected patients' sera have also been evaluated through western blot which is capable of discriminating present from past infection [30]. More diagnostic candidates capable of detecting the early acquired phase of toxoplasmosis ought to be determined to improve the efficacy of serodiagnosis especially of pregnant women in order to reduce the risk of transplacental transmission.
Dense granule antigen 5 (GRA5) is a 21 kDa hydrophobic protein consisting of a N-terminal hydrophobic signal peptide and a hydrophobic transmembrane domain [31]. It was reported that GRA5 appears in both soluble and hydrophobic forms [32]. GRA5 is secreted into the parasitophorous vacuole (PV) by T. gondii as a soluble form during the host cell invasion [33] followed by transmembrane insertion into the parasitophorous vacuole membrane (PVM) with its N-terminal projecting into the host cell cytoplasm, while C-terminus remains in the vacuole lumen [32]. A yeast two-hybrid analysis with GRA5 [34] showed that this antigen binds to calcium modulating ligand (CALMG) for regulation of intracellular calcium concentration which helps to inhibit apoptosis [35] and further allows for long-term survival of T. gondii. Besides playing an important role in host cell invasion, maintenance of the PV, and long-term survival of the parasite, GRA5 was found to exist in all life stages of the parasite [36].
However, only limited studies were done on the evaluation of the potential of GRA5 as a diagnostic marker in Toxoplasma infection, thus making it a protein of interest to be studied in this research. Only one study has been conducted showing the suitability of the full-length recombinant GRA5 for use as a component of an antigen cocktail for the detection of anti-T. gondii IgG antibodies [37]. This research study was aimed at the production of recombinant GRA5 (designated rGRA5) antigen in bacteria and at evaluation of its immunogenic properties as a potential single-antigenic diagnostic candidate through western blot. At the same time, we will also find out if GRA5 can detect the early acute stage of human toxoplasmosis through this study.
2. Materials and Methods
2.1. Parasite
T. gondii tachyzoites (RH strain) were maintained by serial intraperitoneal passage in BALB/c mice and were harvested from the peritoneal fluids after 3 to 4 days of infection. The tachyzoites were washed and subsequently resuspended in sterile phosphate buffered saline (PBS) prior to usage.
2.2. Construction of Recombinant Plasmids
The T. gondii GRA5 gene sequence (corresponding to nucleotides 76–360), which encodes the GRA5 antigen, was obtained from Genbank (accession number: EU918733.1). DNA was extracted from tachyzoites of T. gondii (RH strain) and used as the template for PCR amplification of the GRA5 gene with forward (5′-GCGGAATTCGGTTCAACGCGTGAC-3′) and reverse (5′-GACGAATTCCTCTTCCTCGGCAACTTC-3′) primers, which introduced EcoRI restriction sites (underlined) to facilitate cloning. The PCR product was purified and cloned into the pRSET B prokaryotic expression vector (Invitrogen, USA) at the EcoRI site. The resulting recombinant GRA5-pRSET B construct permitted expression of an N-terminally polyhistidine- (His-) tagged rGRA5 (amino acid residues 26–120), lacking its putative N-terminal signal sequence. Both the GRA5-pRSET B construct and the nonrecombinant pRSET B plasmid were transformed into the prokaryotic expression host, Escherichia coli (E. coli) BL21(DE3)pLysS. The recombinant clones were screened and sequenced for verification purposes.
2.3. Optimization of Heterologous Protein Expression in E. coli
Optimal conditions for rGRA5 protein expression in E. coli were determined prior to scaling up the protein production protocol for further study. A single GRA5-pRSET B-containing colony was picked and inoculated into 5 mL of Luria-Bertani (LB) broth supplemented with ampicillin (100 μg/mL) and chloramphenicol (34 μg/mL). The culture was grown overnight at 37°C (200 rpm) and then diluted to a final volume of 10 mL with LB broth to yield an optical density of 0.1 at 600 nm (OD600). The culture was then grown at 37°C (~250 rpm) until reaching an OD600 of 0.5, at which point protein expression was induced by addition of different concentrations (0.1, 0.5, and 1.0 mM) of isopropyl β-D-thiogalactopyranoside (IPTG; Invitrogen, USA) for various incubation periods (0, 2, and 4 h). The cells were harvested every hour by centrifugation at 5,000 ×g for 10 min before assessing protein expression using dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).
2.4. Expression and Purification of rGRA5
Large-scale protein production was achieved by inducing the culture with 1 mM IPTG and incubating it for 2 h before harvesting by centrifugation. The Probond Purification System (Invitrogen, USA) and nitrilotriacetic acid-nickel (Ni-NTA; Qiagen, Germany) resin were then used to purify rGRA5, according to the manufacturers' instructions. Briefly, cell lysate was prepared under denaturing conditions prior to the purification steps. The cell pellet was resuspended in guanidine lysis buffer (6 M guanidine hydrochloride, 500 mM sodium chloride, and 20 mM sodium phosphate, pH 7.8) and rocked slowly for 5 to 10 min at room temperature to ensure thorough cell lysis, followed by sonication on ice with three 5-second pulses (high intensity). After sonication, the lysate was separated from cellular debris by centrifugation at 3,000 ×g for 15 min, added to a column with resin, and allowed to bind for 30 min. Once the resin settled, the supernatant was aspirated, and the column was washed two times with each of the following: denaturing binding buffer (8 M urea, 500 mM sodium chloride, and 20 mM sodium phosphate, pH 7.8), denaturing wash buffer (8 M urea, 500 mM sodium chloride, and 20 mM sodium phosphate, pH 6.0), and denaturing wash buffer (8 M urea, 500 mM sodium chloride, and 20 mM sodium phosphate, pH 5.3). The supernatant was aspirated after each washing step. After the last wash, the rGRA5 protein was eluted from the Ni-NTA resin with denaturing elution buffer (8 M urea, 500 mM sodium chloride, and 20 mM sodium phosphate, pH 4.0). E. coli carrying the empty pRSET B vector was used as a negative control for both expression and purification. The concentration of purified rGRA5 protein was measured with the Bradford Assay Kit (Bio-Rad, USA). The identity of the expressed and purified rGRA5 protein was confirmed by matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry (MS).
2.5. In-Gel Tryptic Digestion of rGRA5
Affinity purified rGRA5 was resolved by SDS-PAGE using 12% polyacrylamide gels, which were stained with Coomassie Brilliant Blue R-250 (Bio-Rad, USA) for 2 h and then incubated with destaining solution (7% acetic acid, 5% methanol) overnight at room temperature. The rGRA5 protein band was then excised from the Coomassie-stained gel (based on size) and further destained with 50 μL of 50% acetonitrile (ACN) in 50 mM ammonium bicarbonate (NH4HCO3). This step was repeated several times (15–20 min washes, discarding the destaining solution after each wash) until the excised gel was completely destained. The rGRA5-containing gel plug was then incubated with 150 μL of 10 mM dithiothreitol (DTT) in 100 mM NH4HCO3 for 30 min at 60°C. The gel was subsequently cooled to room temperature, the DTT solution was discarded, and the band was incubated with 150 μL of 55 mM iodoacetamide (IAA) in 100 mM NH4HCO3 in the dark for 20 min. The gel plug was then washed four times with 50% ACN in 50 mM NH4HCO3 (500 μL washes, 20 min each), dehydrated via incubation with 50 μL of 100% ACN for 15 min, and subjected to speed vacuum for 15 min at ambient temperature to remove the ACN. The gel plug was then incubated with 25 μL of trypsin (6 ng/μL) in 50 mM NH4HCO3 at 37°C. Following overnight digestion, 50 μL of 50% ACN was added to the gel plug, and it was incubated for 15 min in order to disintegrate the trypsin enzyme and extract protein from the gel plug. The resulting liquid (containing the digested protein) was transferred into a new tube (Tube A), and the gel plug, which remained in the old tube, was further incubated with 50 μL of 100% ACN for 15 min. Subsequently, this liquid was also transferred to Tube A. The protein-containing solution in Tube A was then dried completely via speed vacuum. Prior to MALDI-TOF MS analysis, the protein sample was reconstituted in 10 μL of 0.1% formic acid and desalted using a Zip-Tip (Millipore, USA). For this, the Zip-Tip membrane was wetted and equilibrated with 50% ACN and 0.1% formic acid, respectively. The protein sample was bound onto the Zip-Tip membrane, which was then washed with 0.1% formic acid. Finally, the protein was eluted with 0.1% formic acid in 50% ACN and analyzed by MALDI-TOF MS.
2.6. MALDI-TOF MS Analysis
The Zip-Tip-eluted protein sample was mixed at a 1 : 1 ratio. The matrix was provided by UMCPR staff before spotting onto the MALDI plate. The analysis was carried out by University Malaya Center for Proteomics Research (UMCPR).
2.7. SDS-PAGE and Western Blot Analysis
Purified rGRA5 protein was resolved by SDS-PAGE on 12% polyacrylamide gels and transferred onto methanol-activated polyvinylidene difluoride (PVDF; Bio-Rad, USA) membranes, which were then cut into vertical strips. The membranes were incubated with blocking solution (5% nonfat skim milk in Tris Buffered Saline (TBS)) for 2 h at room temperature with constant shaking and were subsequently probed with diluted human serum samples (1 : 200) for 2 h. The membrane strips were washed and then incubated for 1 h with biotinylated goat anti-human IgM/IgG (KPL, USA; 1 : 2500) secondary antibody. Lastly, the membrane strips were washed and incubated with streptavidin-alkaline phosphatase (KPL, USA; 1 : 2,500) at room temperature for 1 h followed by detection using 5-bromo-4-chloro-3-indolyphosphate/nitro blue tetrazolium (BCIP/NBT; Sigma, USA).
2.8. Evaluation of Sensitivity and Specificity of rGRA5
Diagnostic sensitivity and specificity of rGRA5 protein were evaluated by western blot analysis using sera from both toxoplasmosis-diagnosed patients and toxoplasmosis-negative individuals. Toxoplasmosis cases were divided into three groups: (1) patients with early acute toxoplasmosis (n = 44; IgM positive, IgG negative); (2) patients with acute toxoplasmosis (n = 47; IgM positive, IgG positive); and (3) patients with chronic toxoplasmosis (n = 85; IgM negative, IgG positive). A fourth group was comprised of toxoplasmosis-negative control patients (n = 24; IgM negative, IgG negative). These human serum samples were grouped based on results obtained from Novalisa Toxoplasma gondii IgG and Toxoplasma gondii IgM enzyme-linked immunosorbent assay (ELISA) kits (NovaTec, Germany). In addition, specificity of rGRA5 was determined using serum samples from patients diagnosed with amoebiasis (3 samples), cysticercosis (3 samples), filariasis (3 samples), and toxocariasis (3 samples). These sera had given positive results in serological tests for their respective infections. All serum samples were obtained from the Diagnostic Laboratory at the Department of Parasitology, University of Malaya. Sensitivity (number of true positives/[number of true positives + number of false negatives]) and specificity (number of true negatives/[number of true negatives + number of false positives]) were calculated and tabulated in Table 1.
Table 1.
Immunoreactivities (sensitivity and specificity) of the rGRA5 antigen to serum samples from toxoplasmosis-positive and toxoplasmosis-negative patients.
| Serum samples group | Number of human serum samples | Immunoreactivities | |||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Number | % | Number | % | ||
| 1 (Early acute: IgG−ve, IgM+ve) | 44 | 0 | 0 | 44 | 100 |
| 2 (Acute: IgG+ve, IgM+ve) | 47 | 22 | 46.8 | 25 | 53.2 |
| 3 (Chronic: IgG+ve, IgM−ve) | 85 | 52 | 61.2 | 33 | 38.8 |
| 4 (Toxoplasmosis-negative: IgG−ve, IgM−ve) | 24 | 0 | 0 | 24 | 100 |
| Other infections | 12 | 1 | 8.3 | 11 | 91.7 |
| Amoebiasis | 3 | 0 | 0 | 3 | 100 |
| Cysticercosis | 3 | 0 | 0 | 3 | 100 |
| Filariasis | 3 | 0 | 0 | 3 | 100 |
| Toxocariasis | 3 | 1* | 33.3 | 2 | 66.7 |
*One out of three toxocariasis-positive sera samples reacted with the rGRA5 antigen. This particular toxocariasis-positive serum sample was shown to be IgG positive for toxoplasmosis based on the commercial kits.
3. Results
3.1. Cloning of the GRA5 Gene Fragment
We PCR-amplified a fragment of T. gondii GRA5 gene, which encoded amino acids 26–120 of the GRA5 protein (excluding the putative hydrophobic signal peptide). The resulting ~285 bp product was cloned into the pRSET B vector in order to permit prokaryotic expression of N-terminally His-tagged rGRA5, which could be purified using a nickel resin column. Sequence analysis confirmed that the insert within the GRA5-pRSET B plasmid shared 100% identity with the published GRA5 gene.
3.2. Optimization of rGRA5 Expression in E. coli
Production of rGRA5 protein was optimized by altering various parameters, and expression levels were analyzed by SDS-PAGE as shown in Figure 1. Upon induction of rGRA5 expression from GRA5-pRSET B-containing E. coli, we observed a 20 kDa band of increasing intensity, which was absent in the negative control (empty pRSET B). Expression of this protein increased up to two hours after induction and remained constant after four hours. Three different IPTG concentrations were tested, and 1.0 mM was found to result in maximum rGRA5 expression. Taken together, these data suggested that optimal rGRA5 expression was achieved following induction with 1.0 mM IPTG for 2 hours. The same conditions were applied to larger scale production of rGRA5.
Figure 1.
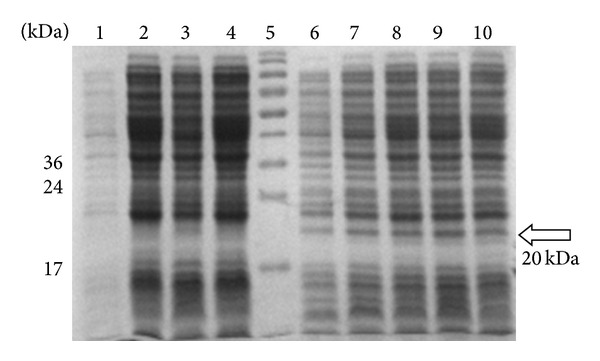
SDS-PAGE analysis on the optimized expression of rGRA5 protein in E. coli BL21 pLysS (DE3), Coomassie blue stained. Lane 5: prestained broad range protein marker. Lane 1, cell pellet fractions of pRSET B clone as negative control before induction (0 hr). Lane 2: cell pellet fractions of pRSET B clone after induction with 0.5 mM IPTG (4 hr). Lanes 3 to 4: cell pellet fractions of pRSET B clone after induction with 1.0 mM IPTG (2, 4 hr). Lane 6: cell pellet fractions of GRA5 clone before induction (0 hr). Lanes 7 to 8: cell pellet fractions of GRA5 clone after induction with 0.5 mM IPTG (2, 4 hr). Lanes 9 to 10: cell pellet fractions of GRA5 clone after induction with 1.0 mM IPTG (2, 4 hr). The GRA5 protein band of interest was observed at molecular weight of 20 kDa (arrow) compared to the negative control. The band intensity increased from 0 to 2 hr after induction and remained constant at the 4th hr with 1.0 mM IPTG, the optimum condition for maximum expression of the protein.
3.3. Expression and Purification of rGRA5 Protein
Following optimization of rGRA5 expression in E. coli, a nickel resin column was used to purify the recombinant protein (Figure 2(a)), which could be detected by western blot analysis using serum from a Toxoplasma-infected patient (Figure 2(b)). This further suggested that the induced 20 kDa band observed prior to purification corresponded to rGRA5 (Figure 1).
Figure 2.
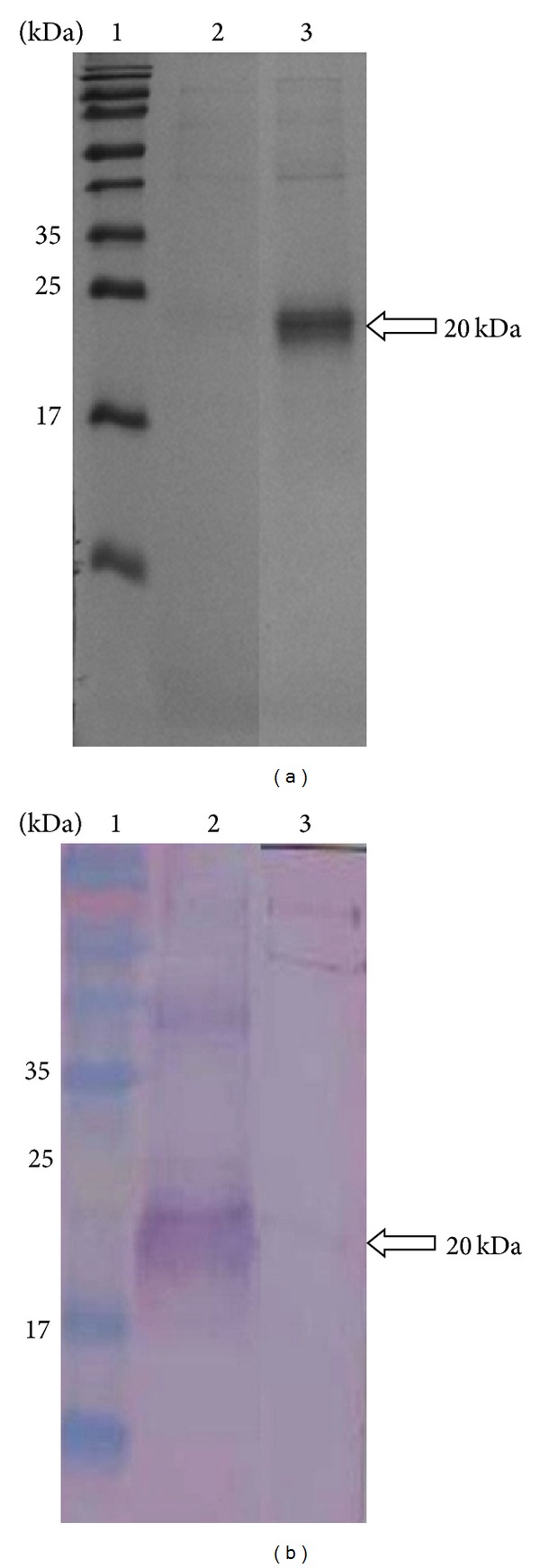
SDS-PAGE analysis on purified rGRA5 protein. (a) Coomassie blue stained. Lane 2: purified pRSET B. Lane 3: purified rGRA5 and (b) western blot probed with toxoplasmosis-infected patient's serum. Lane 2: purified rGRA5. Lane 3: purified pRSET B. Lane 1 (panel a and panel b) is the prestained broad range protein marker. The 20 kDa purified rGRA5 was detected (arrow).
3.4. Confirmation of rGRA5 Protein
Next, we confirmed the identity of our expressed and purified recombinant protein by MALDI-TOF MS analysis. Indeed, the results indicated that the isolated protein was T. gondii GRA5.
3.5. Western Blot Analysis of rGRA5 Protein with Human Serum Samples
The purified rGRA5 protein was tested for its diagnostic sensitivity and specificity through western blot analysis with serum samples from toxoplasmosis-positive (Groups 1, 2, and 3) and toxoplasmosis-negative (Group 4) patients. In addition, specificity was tested using sera from patients infected with other parasites, including amoebiasis, cysticercosis, filariasis, and toxocariasis. We observed that the rGRA5 protein had sensitivities of 0% (0 out of 44 sera), 46.8% (22 out of 47 sera), and 61.2% (52 out of 85 sera) for early acute, acute, and chronic infections, respectively (Table 1). In contrast, 0 out of 24 control sera from the toxoplasmosis-negative patients reacted with rGRA5 (100% specificity). In Figure 3, five example results are shown for each group (positive results for Groups 2 and 3; negative results for Group 4). Also, only 1 (toxocariasis) out of the 12 sera from patients infected with other parasites (data not shown) reacted with the rGRA5 protein (91.7% specificity).
Figure 3.
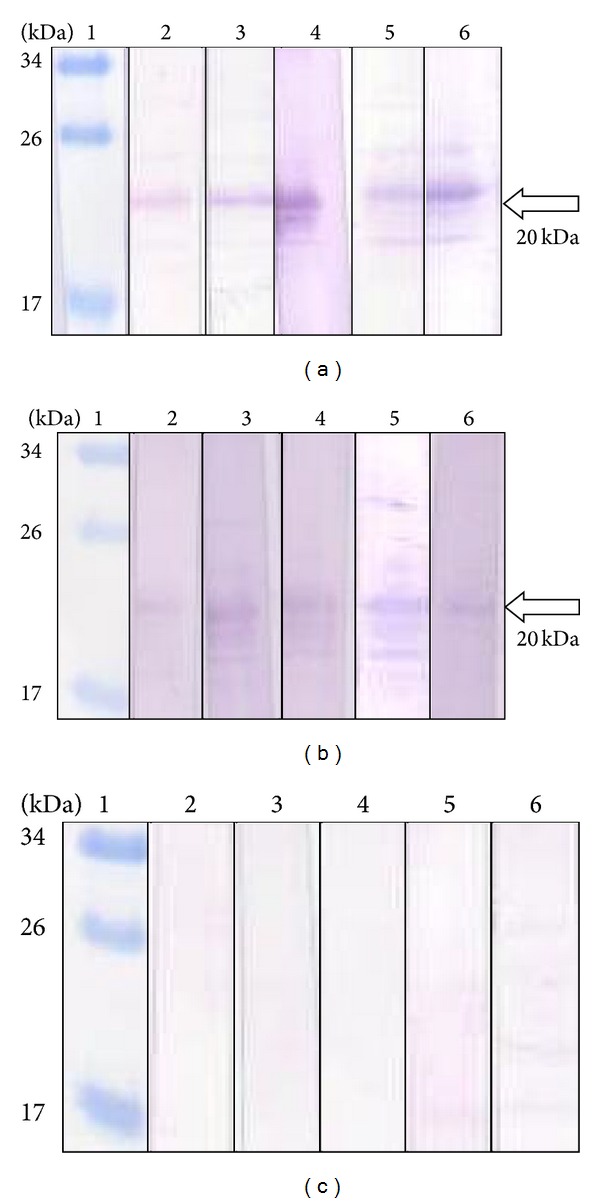
Western blots of purified rGRA5 protein with sera of toxoplasmosis and toxoplasmosis-negative patients. Lane 1 (panels a–c): the prestained broad range protein marker. Lanes 2 (a) to 6 (a): results of 5 sera from chronic-profile patients (Group 3: IgG +ve, IgM −ve). Lanes 2 (b) to 6 (b): results of 5 sera from acute-profile patients (Group 2: IgG +ve, IgM +ve). Lanes 2 (c) to 6 (c): results of 5 sera from toxoplasmosis-negative patients (Group 4: IgG −ve, IgM −ve). The 20 kDa purified rGRA5 was detected by toxoplasmosis-positive sera (arrow).
4. Discussion
A fragment of the T. gondii GRA5 gene was successfully cloned into a prokaryotic expression vector and transformed into E. coli. Full-length recombinant GRA5 protein (rGRA5) was subsequently expressed and purified, yielding a 20 kDa protein. However, the predicted molecular weight of GRA5 is 16 kDa. While this discrepancy between the calculated and observed molecular weights can be partially explained by the presence of the His-tag in rGRA5, it is also possible that this difference stems from common features of GRA proteins, such as proline residue composition [4]. Even though we observed this size discrepancy, the identity of our purified protein was verified by immunoblotting with Toxoplasma-infected sera and MALDI-TOF MS analysis.
Identification of rGRA5 via MALDI-TOF MS involved careful processing, which allowed for reliable confirmation of the purified protein. Briefly, the rGRA5-containing band was excised from a stained SDS-PAGE gel, followed by an in-gel digestion protocol that included seven major steps: (1) destaining of the gel plug, (2) reduction of the protein, (3) alkylation of the protein, (4) dehydration, (5) tryptic digestion of the protein, (6) extraction of the digested protein, and (7) desalting of the digested protein using a Zip-Tip. Reduction and alkylation (aminocarboxymethylation) of the protein at cysteine residues with dithiothreitol (DTT) and iodoacetamide (IAA), respectively, were important for permanent disruption of disulfide linkages, enabling overnight trypsin digestion.
It was demonstrated that the expression of predicted immunodominant epitopes of GRA5 failed to show any immunoreactivity with a pool of T. gondii-positive human sera [13]. Therefore, full-length rGRA5 was constructed and produced in this study. Our evaluation of rGRA5 immunoreactivity revealed high specificities when testing sera from toxoplasmosis-negative patients or from those infected with other parasites (100.0% and 91.7%, resp.). In addition, our findings indicate the sensitivities of 46.8% and 61.2% when analyzing serum samples from patients with acute and chronic Toxoplasma infections, respectively. However, none of the serum samples from the early acute phase patients reacted with the rGRA5 protein. In fact, data from the present study are in agreement with previous results obtained from analysis of rGRA5 antigen-mediated detection of IgG antibodies using ELISA [37]. Specificity of the aforementioned study was shown to be 100.0%, whereas sensitivities of 63.0% and 75.0% were reported for sera from acute and chronic infections, respectively. Thus, it strongly suggested that rGRA5 yields a much higher reactivity towards IgG antibodies in sera from chronically infected patients compared to those with acute infection. Notably, this protein shows no sensitivity towards IgM antibodies found in sera from early acute stage patients. Our study involved the same expression host, BL21(DE3)pLysS, for the expression of full-length rGRA5 as the above mentioned study. In contrast, different expression vectors and evaluation techniques were used. Due to its higher specificity, western blot was chosen to evaluate rGRA5 protein in this study instead of the commonly used ELISA. Also, the chances of obtaining false-positive results via western blot are much lower compared to ELISA [38]. In fact, it has been reported that western blot analysis is superior to ELISA for screening sera samples because this technique gives more information, is less affected by sample degradation, produces results of high confidence with direct visualization of antibodies bound to specific diagnostic antigens, and offers improved determination of diagnostic antigen purity [39].
With regard to the future development of diagnostic tests for T. gondii, the western blot results obtained in this study should be reliable for predicting the efficacy of using rGRA5 antigen in immunochromatographic tests (ICT) due to similarities between the two assays (i.e., western blot and ICT are both immunoassays utilizing nitrocellulose membranes and direct visualization of results). Indeed, ICT is a better serological test for diagnosis of infections (including toxoplasmosis) compared to ELISA, which is commonly used due to its simplicity. However, ICT is a rapid test with high accuracy but lower cost compared to ELISA, which is time consuming and laborious [40]. In addition, ICT can be used in field conditions [40] especially for the diagnosis of farm animals.
Based on our results (Table 1), cross-reactivity was not observed in sera samples from patients infected with amoebiasis, cysticercosis, and filariasis. However, one out of three toxocariasis-positive sera samples reacted with the rGRA5 antigen. This particular toxocariasis-positive serum sample was shown to be IgG positive but IgM negative for toxoplasmosis based on findings from Novalisa Toxoplasma gondii IgG and IgM antibodies ELISA kits. This indicates that there was probably a coinfection of T. gondii and Toxocara spp. in this infected patient [41]. Although T. gondii (a protozoan) and Toxocara spp. (helminths) are two different parasites, they both can be acquired through soil ingestion. Therefore, the chances of coinfection between these two parasites are highly possible [41].
5. Conclusions
Our findings show that rGRA5 lacks sensitivity for detecting IgM antibodies and displays a much lower reactivity towards IgG antibodies in sera from patients with acute infection compared to those with chronic toxoplasmosis (46.8% versus 61.2%). These data indicate that rGRA5 protein is unable to distinguish between current and past infections. Nevertheless, this protein can be combined with other T. gondii antigens (cocktails) in order to improve its sensitivity against toxoplasmosis-positive serum samples [37]. Last but not least, these findings should contribute to the future development of an ICT incorporating this antigen (either alone or in combination with other potential ESA) for diagnosis of T. gondii infection.
Acknowledgments
The authors thank the Diagnostic Laboratory (Para: SEAD), Department of Parasitology, University of Malaya, and University Malaya Medical Centre (UMMC) for providing the serum samples. They also thank the University Malaya Centre for Proteomics Research (UMCPR) for carrying out the MALDI-TOF MS analysis. This research project is supported by University of Malaya under the Postgraduate Research Grant (PS197-2009C) and High Impact Research Fund UM-MOHE UM.C/HIR/MOHE/MED/16 from the Ministry of Higher Education in Malaysia.
Ethical Approval
The authors declared that the experiments comply with the current laws of the country in which they were performed (Malaysia).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Igarashi M, Kano F, Tamekuni K, et al. Toxoplasma gondii: evaluation of an intranasal vaccine using recombinant proteins against brain cyst formation in BALB/c mice. Experimental Parasitology. 2008;118(3):386–392. doi: 10.1016/j.exppara.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Ismael AB, Sekkai D, Collin C, Bout D, Mévélec M-N. MIC3 gene of Toxoplasma gondii is a novel potent vaccine candidate against toxoplasmosis. Infection and Immunity. 2003;71(11):6222–6228. doi: 10.1128/IAI.71.11.6222-6228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. International Journal for Parasitology. 2000;30(12-13):1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercier C, Adjogble KDZ, Däubener W, Delauw M-F. Dense granules: are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites? International Journal for Parasitology. 2005;35(8):829–849. doi: 10.1016/j.ijpara.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clinical Microbiology Reviews. 1998;11(2):267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubey JP. Pathogenicity and infectivity of Toxoplasma gondii oocysts for rats. Journal of Parasitology. 1996;82(6):951–956. [PubMed] [Google Scholar]
- 7.Chen R, Lu S-H, Tong Q-B, et al. Protective effect of DNA-mediated immunization with liposome-encapsulated GRA4 against infection of Toxoplasma gondii . Journal of Zhejiang University: Science B. 2009;10(7):512–521. doi: 10.1631/jzus.B0820300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagne SS. Toxoplasmosis. Primary Care Update for OB/GYNS. 2001;8(3):122–126. doi: 10.1016/s1068-607x(00)00083-4. [DOI] [PubMed] [Google Scholar]
- 9.Montoya JG, Liesenfeld O. Toxoplasmosis. The Lancet. 2004;363(9425):1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 10.Buxton D. Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: recent advances. Veterinary Research. 1998;29(3-4):289–310. [PubMed] [Google Scholar]
- 11.Montoya JG. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. Journal of Infectious Diseases. 2002;185(1, supplement):S73–S82. doi: 10.1086/338827. [DOI] [PubMed] [Google Scholar]
- 12.Gatkowska J, Hiszczynska-Sawicka E, Kur J, Holec L, Dlugonska H. Toxoplasma gondii: an evaluation of diagnostic value of recombinant antigens in a murine model. Experimental Parasitology. 2006;114(3):220–227. doi: 10.1016/j.exppara.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Dai J, Jiang M, Wang Y, Qu L, Gong R, Si J. Evaluation of a recombinant multiepitope peptide for serodiagnosis of Toxoplasma gondii infection. Clinical and Vaccine Immunology. 2012;19(3):338–342. doi: 10.1128/CVI.05553-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau YL, Thiruvengadam G, Lee WW, Fong MY. Immunogenic characterization of the chimeric surface antigen 1 and 2 (SAG1/2) of Toxoplasma gondii expressed in the yeast Pichia pastoris . Parasitology Research. 2011;109(3):871–878. doi: 10.1007/s00436-011-2315-6. [DOI] [PubMed] [Google Scholar]
- 15.Holec L, Gasior A, Brillowska-Dabrowska A, Kur J. Toxoplasma gondii: enzyme-linked immunosorbent assay using different fragments of recombinant microneme protein 1 (MIC1) for detection of immunoglobulin G antibodies. Experimental Parasitology. 2008;119(1):1–6. doi: 10.1016/j.exppara.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Holec-Ģsior L, Kur J, Hiszczyńska-Sawicka E. GRA2 and ROP1 recombinant antigens as potential markers for detection of Toxoplasma gondii-specific immunoglobulin G in humans with acute toxoplasmosis. Clinical and Vaccine Immunology. 2009;16(4):510–514. doi: 10.1128/CVI.00341-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Gelder P, Bosman F, De Meuter F, Van Heuverswyn H, Herion P. Serodiagnosis of toxoplasmosis by using a recombinant form of the 54-kilodalton rhoptry antigen expressed in Escherichia coli . Journal of Clinical Microbiology. 1993;31(1):9–15. doi: 10.1128/jcm.31.1.9-15.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golkar M, Azadmanesh K, Khalili G, et al. Serodiagnosis of recently acquired Toxoplasma gondii infection in pregnant women using enzyme-linked immunosorbent assays with a recombinant dense granule GRA6 protein. Diagnostic Microbiology and Infectious Disease. 2008;61(1):31–39. doi: 10.1016/j.diagmicrobio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Golkar M, Rafati S, Abdel-Latif MS, et al. The dense granule protein GRA2, a new marker for the serodiagnosis of acute Toxoplasma infection: comparison of sera collected in both France and Iran from pregnant women. Diagnostic Microbiology and Infectious Disease. 2007;58(4):419–426. doi: 10.1016/j.diagmicrobio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Mercier C, Cesbron-Delauw MF, Ferguson DJP. Dense granules of the infectious stages of Toxoplasma gondii: their central role in the host/parasite relationship. In: Soldati D, Ajioka J, editors. Toxoplasma: Molecular and Cellular Biology. Horizon Scientific Press; 2007. [Google Scholar]
- 21.Cesbron-Delauw M-F. Dense-granule organelles of Toxoplasma gondii: their role in the Host-Parasite relationship. Parasitology Today. 1994;10(8):293–296. doi: 10.1016/0169-4758(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 22.Hughes HPA, van Knapen F. Characterisation of a secretory antigen from Toxoplasma gondii and its role in circulating antigen production. International Journal for Parasitology. 1982;12(5):433–437. doi: 10.1016/0020-7519(82)90073-x. [DOI] [PubMed] [Google Scholar]
- 23.Ahn H-J, Kim S, Nam H-W. Host cell binding of GRA10, a novel, constitutively secreted dense granular protein from Toxoplasma gondii . Biochemical and Biophysical Research Communications. 2005;331(2):614–620. doi: 10.1016/j.bbrc.2005.03.218. [DOI] [PubMed] [Google Scholar]
- 24.Michelin A, Bittame A, Bordat Y, et al. GRA12, a Toxoplasma dense granule protein associated with the intravacuolar membranous nanotubular network. International Journal for Parasitology. 2009;39(3):299–306. doi: 10.1016/j.ijpara.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Rome ME, Beck JR, Turetzky JM, Webster P, Bradley PJ. Intervacuolar transport and unique topology of GRA14, a novel dense granule protein in Toxoplasma gondii . Infection and Immunity. 2008;76(11):4865–4875. doi: 10.1128/IAI.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs D, Vercammen M, Saman E. Evaluation of recombinant dense granule antigen 7 (GRA7) of Toxoplasma gondii for detection of immunoglobulin G antibodies and analysis of a major antigenic domain. Clinical and Diagnostic Laboratory Immunology. 1999;6(1):24–29. doi: 10.1128/cdli.6.1.24-29.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Maine G, Suzuki Y, et al. Serodiagnosis of recently acquired Toxoplasma gondii infection with a recombinant antigen. Journal of Clinical Microbiology. 2000;38(1):179–184. doi: 10.1128/jcm.38.1.179-184.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redlich A, Müller WA. Serodiagnosis of acute toxoplasmosis using a recombinant form; of the dense granule antigen GRA6 in an enzyme-linked immunosorbent assay. Parasitology Research. 1998;84(9):700–706. doi: 10.1007/s004360050473. [DOI] [PubMed] [Google Scholar]
- 29.Sadeghiani G, Zare M, Babaie J, et al. Heterologous production of dense granule gra7 antigen of Toxoplasma gondii in Escherichia coli . Southeast Asian Journal of Tropical Medicine and Public Health. 2009;40(4):692–700. [PubMed] [Google Scholar]
- 30.Ching XT, Lau YL, Fong MY, Nissapatorn V. Evaluation of Toxoplasma gondii-recombinant dense granular protein (GRA2) for serodiagnosis by western blot. Parasitology Research. 2013;112(3):1229–1236. doi: 10.1007/s00436-012-3255-5. [DOI] [PubMed] [Google Scholar]
- 31.Nam H-W. GRA proteins of Toxoplasma gondii: maintenance of host-parasite interactions across the parasitophorous vacuolar membrane. Korean Journal of Parasitology. 2009;47, supplement:S29–S37. doi: 10.3347/kjp.2009.47.S.S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecordier L, Mercier C, David Sibley L, Cesbron-Delauwz M-F. Transmembrane insertion of the Toxoplasma gondii GRA5 protein occurs after soluble secretion into the host cell. Molecular Biology of the Cell. 1999;10(4):1277–1287. doi: 10.1091/mbc.10.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lecordier L, Mercier C, Torpier G, et al. Molecular structure of a Toxoplasma gondii dense granule antigen (GRA 5) associated with the parasitophorous vacuole membrane. Molecular and Biochemical Parasitology. 1993;59(1):143–153. doi: 10.1016/0166-6851(93)90015-p. [DOI] [PubMed] [Google Scholar]
- 34.Ahn H-J, Kim S, Kim H-E, Nam H-W. Interactions between secreted GRA proteins and host cell proteins across the paratitophorous vacuolar membrane in the parasitism of Toxoplasma gondii . The Korean Journal of Parasitology. 2006;44(4):303–312. doi: 10.3347/kjp.2006.44.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng P, Park J, Lee B-S, Lee S-H, Bram RJ, Jung JU. Kaposi’s sarcoma-associated herpesvirus mitochondrial K7 protein targets a cellular calcium-modulating cyclophilin ligand to modulate intracellular calcium concentration and inhibit apoptosis. Journal of Virology. 2002;76(22):11491–11504. doi: 10.1128/JVI.76.22.11491-11504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tilley M, Fichera ME, Jerome MK, Roos DS, White MW. Toxoplasma gondii sporozoites form a transient parasitophorous vacuole that is impermeable and contains only a subset of dense-granule proteins. Infection and Immunity. 1997;65(11):4598–4605. doi: 10.1128/iai.65.11.4598-4605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holec-Gasior L, Kur J. Toxoplasma gondii: recombinant GRA5 antigen for detection of immunoglobulin G antibodies using enzyme-linked immunosorbent assay. Experimental Parasitology. 2010;124(3):272–278. doi: 10.1016/j.exppara.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Nöckler K, Reckinger S, Broglia A, Mayer-Scholl A, Bahn P. Evaluation of a Western Blot and ELISA for the detection of anti-Trichinella-IgG in pig sera. Veterinary Parasitology. 2009;163(4):341–347. doi: 10.1016/j.vetpar.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 39.Anderson T, DeJardin A, Howe DK, Dubey JP, Michalski ML. Neospora caninum antibodies detected in Midwestern white-tailed deer (Odocoileus virginianus) by Western blot and ELISA. Veterinary Parasitology. 2007;145(1-2):152–155. doi: 10.1016/j.vetpar.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Huang X, Xuan X, Hirata H, et al. Rapid immunochromatographic test using recombinant SAG2 for detection of antibodies against Toxoplasma gondii in cats. Journal of Clinical Microbiology. 2004;42(1):351–353. doi: 10.1128/JCM.42.1.351-353.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones JL, Kruszon-Moran D, Won K, Wilson M, Schantz PM. Toxoplasma gondii and Toxocara spp. co-infection. American Journal of Tropical Medicine and Hygiene. 2008;78(1):35–39. [PubMed] [Google Scholar]


