Abstract
Satellite-based remote sensing of marine microorganisms has become a useful tool in predicting human health risks associated with these microscopic targets. Early applications were focused on harmful algal blooms, but more recently methods have been developed to interrogate the ocean for bacteria. As satellite-based sensors have become more sophisticated and our ability to interpret information derived from these sensors has advanced, we have progressed from merely making fascinating pictures from space to developing process models with predictive capability. Our understanding of the role of marine microorganisms in primary production and global elemental cycles has been vastly improved as has our ability to use the combination of remote sensing data and models to provide early warning systems for disease outbreaks. This manuscript will discuss current approaches to monitoring cyanobacteria and vibrios, their activity and response to environmental drivers, and will also suggest future directions.
Introduction
The first satellite-based remote sensing of earth can be traced to the Explorer-6 satellite that sent back images in August 1959. This was quickly followed by the world’s first weather satellite TIROS 1 that beamed back images of cloud formations and ocean storms in 1960. Astronauts on board various orbital missions starting in the 1960s and continuing through the era of the Space Shuttle to the International Space Station today have also provided spectacular photographs of blooms of cyanobacteria in the oceans. Remote sensing of biota, including bacteria, in the oceans exploit changes to the electromagnetic spectrum caused by the presence of biota that can be detected by space-based sensors. The first regular, near daily, global, high-resolution (1 km) satellite maps of ocean properties first became available in 1978 by the Advanced Very High Resolution Radiometer (AVHRR) that had four channels in the infrared and the Coastal Zone Color Scanner that had four channels in the visible. These instruments provided a new view of the oceans and revolutionized our understanding of ocean biota.
Background
The modes of observation can be classified into direct, indirect, and inference or model based. Direct observation is possible when bacteria can be seen by the space-based sensor and identified uniquely by their optical properties. There are few instances of direct observation of bacteria, the two most spectacular examples being mapping of massive surface accumulations of colonial cyanobacteria that can cover 100 s of thousands of square kilometers [12, 38] (Fig. 1a, b) and detection of bioluminescence attributed to a bloom of bacteria that covered more than 15,000 km2 [50]. Indirect observations are those involving identifying and quantifying bacteria based on physical or physiological properties, including optical properties of pigments such as chlorophyll or phycoerythrin found in phototrophic cyanobacteria [47]. An example of inferred or model-based detection of bacteria from space is the detection of Enterococcus. Here, a correlation is derived between Enterococcus counts and particle concentration and the particle concentration is estimated from satellite measurements [73]. Similarly, Vibrio concentrations can be estimated using a model that links Vibrio abundance to various remotely sensed environmental parameters such as sea surface temperature and phytoplankton abundance [13].
Fig. 1.
a True color SeaWiFS image showing a bloom of Trichodesmium in the Capricorn Channel, Great Barrier Reef. b A space shuttle photograph of a Trichodesmium bloom in the same region
Most satellite-based remote sensing of ocean biota is accomplished by recording changes to the electromagnetic spectrum by active or passive sensors. Passive sensors detect radiation—light or heat—emanating from the ocean. For example, passive ocean color sensors measure sunlight scattered back from below the sea surface and detected by a satellite-based sensor. Infrared passive sensors are used to measure sea surface temperature (SST). Active sensors, on the other hand, have their own source of “illumination” that is beamed down to the ocean and the reflected signal is used to infer properties about the surface of the ocean. Currently there are active sensors that work in the microwave part of the electromagnetic spectrum and these are used to study sea surface roughness and sea surface salinity (SSS), both properties of interest when inferring information about bacteria. Synthetic Aperture Radar (SAR), an active sensor, has the advantage of being able to “see” through clouds and has been used to map massive surface blooms (Fig. 2c). There are plans to deploy an ocean color satellite that will carry a laser to induce a fluorescence signal to quantify phytoplankton biomass as well as physiology.
Fig. 2.
a Swedish Coastal aerial photograph of a cyanobacterial bloom in the Baltic Sea. The wake of a ship sailing through the bloom is seen as a line. b SeaWiFS true color image shows the bloom extending over most of the Baltic proper. c A SAR image of the bloom shows the fine structure of the bloom. The bright dot on the right side of the image is a ship and its wake can be seen as a line behind as well as another wake to its left
The most frequently employed method to study ocean biota uses visible band sensors to detect changes in the color of water caused by constituents in the water, the most common being chlorophyll. When sunlight enters the ocean, it is either absorbed or scattered. The light that is scattered in the backward (upward) direction reemerges from the ocean surface and travels through the atmosphere before being detected by the satellite sensor in space. The processes of absorption and scattering of light in the water by water molecules, particles (including bacteria), and dissolved matter change the wavelength of light that emanates from the sea surface—the color of ocean. Thus, the characteristic color of open ocean waters that have relatively low concentrations of material other than seawater itself is blue while coastal waters that are rich in phytoplankton are green or brown if there is a lot of dissolved material. Satellite sensors have detectors—a few at select 10–20 nm wide bands for multispectral sensors or several narrower bands that span the spectrum from 400 to 700 nm for hyperspectral sensors—to measure light emanating from the sea surface at these specific wavelengths. Algorithms have been developed to deconvolute relationships between the amount of light at specific wavelengths and estimated concentrations of the various constituents, such as chlorophyll, particles, and colored dissolved organic matter. A major challenge to ocean color remote sensing is the interference of atmosphere on the signal received by the sensor in space. About 90-95 % of the signal detected by the sensor is light scattered by the atmosphere which has no oceanic origin. The atmospheric signal has to be removed in order to derive quantitative information about the ocean. This challenge is especially difficult in coastal waters that are more complex because of the heterogeneity in both constituents of the water and the atmosphere. Although tremendous progress has been made in solving this problem over the last 25 years, it remains one of the greatest sources of uncertainty in ocean color remote sensing.
To identify and quantify a given species of bacteria from space, the organism must have characteristic properties that can be remotely sensed. Phototrophic bacteria such as cyanobacteria carry auxiliary pigments, including phycoeryrthin (PEB) and phycocyanin that have unique optical properties. Researchers [9, 64] have proposed techniques that exploit changes in absorption of light caused by these pigments to identify and quantify cyanobacteria, such as the filamentous, non-heterocyctous Trichodesmium spp. However, it should be noted that Garver and Siegel [27] analyzed about 400 in situ absorption spectra and concluded that more than 99 % of the variance found in absorption spectra of particulate matter found in the sea was related to the total amount of material and only a very small signal (less than 0.5 %) was caused by auxiliary pigments—i.e., variance in the amount of phytoplankton biomass was overwhelmingly more important than the relatively subtle changes in the makeup of the pigments. In other words, they concluded that for typical conditions with mixed populations homogeneously mixed through the upper water column, it was unlikely that ocean color remote sensing would be able to uniquely identify particular phytoplankton groups. Morel [51] analyzed optical data collected during a dense bloom (3×108 cells per liter) of Synechococcus, a cyanobacterium that contains PEB, and contributed more than half the phytoplankton found at that location. He concluded that it would be “illusory to expect more than the possibility of producing an index or a flag indicating the presence of PEB bearing organisms” from ocean color sensors.
Another use of remote sensing is to infer the presence of bacteria by employing a combination of satellite-based observations, knowledge of environmental parameters that control bacterial presence and activity, and ecosystem models. For example, although there are no algorithms at present for direct detection and quantification of Richelia, a nitrogen-fixing symbiotic cyanobacterium found in association with diatoms, Wilson [71] used a combination of remotely sensed chlorophyll, sea surface height (SSH), and SST to conclude that late summer blooms in the North Pacific Subtropical Gyre were associated with this organism. There are efforts underway to use the relationship between bacteria attached to particles and remotely sensed particle concentration to model and predict water quality in coastal waters. Similar efforts, described in detail below, have been successfully used to predict cholera caused by Vibrio cholerae, health risks associated with Vibrio parahaemolyticus in raw oysters, and health risks associated with Vibrio vulnificus in raw oysters and seawater. And most recently, the European Centre for Disease Prevention and Control has has posted a Vibrio page in their site at https://e3geoportal.ecdc.europa.eu/SitePages/VibrioRiskMap.aspx
Mapping V. cholerae
The ability to predict outbreaks and epidemics of cholera months in advance has implications far beyond the immediate morbidity and mortality associated with the disease. This includes the potential to mitigate the wide range of socioeconomic factors that are inevitable consequences of an epidemic. In addition, longer term health burdens from malnutrition, such as stunted growth, can be decreased with rapid intervention to mitigate effects of diarrheal diseases such as cholera [24].
Most research on use of satellite imaging for cholera prediction has focused on Bangladesh and the Bay of Bengal, but more recent studies include the Mediterranean and African coastal countries. Since 1960, researchers have noted a relationship between coastal algal blooms and seasonality of cholera ([59]; reviewed by Colwell and Spira [14]). This in turn has led to extensive research on the relationship between the causative organism of the disease, V. cholerae, and a suite of environmental parameters that promote its growth and distribution, as well as timing of epidemics. In particular, the relationship between V. cholerae and zooplankton was established by Colwell and colleagues in their seminal work in the 1980s [13, 31]. This relationship suggests that the ability to remotely monitor algal blooms represents a powerful predictive tool for cholera epidemics.
One of the earliest publications directly addressing the use of remote sensing for detection of V. choleraewas published in 2000 by Lobitz and colleagues [43]. These authors argued that linking algal blooms to cholera epidemics requires field measurements of seawater temperature, nutrient concentration, and plankton biomass, and cholera case data. These oceanographic data are expensive and time-consuming to measure and as a consequence are often sparse in time and space. The scarcity of environmental data makes it difficult to extrapolate to a broader geographic region. Remote sensing of large areas of the ocean offered a very attractive alternative to field surveys. Of particular interest in the study was SST because of its relationship to phytoplankton growth [22] and SSH that has an association with coastal flooding and the potential for increased human exposure. At the time of the Lobitz study, SST was measured by the AVHRR sensor and SSH by TOPEX/Poseidon radar altimeter. Data from both sensors were made available through the NASA Physical Oceanography Distributed Active Archive Center at the Jet Propulsion Lab. The authors observed patterns of cholera outbreaks that were consistent with SST data, with SSH explaining, in part, inconsistencies between cholera and SST. In 1997, the launching of the Sea-viewing Wide Field-of-view Sensor allowed chlorophyll concentration data to be accessed and allowed for more sophisticated analysis of seasonal trends.
However, it has become apparent that cholera outbreak prediction cannot be based on a single remote sensing parameter and recent publications have focused on composite models to improve the predictive power of remote sensing data for cholera outbreaks (Fig. 3). Studies have modeled cholera prediction using a combination of chlorophyll, SST, and rainfall, and demonstrated a relationship between environmental variables and cholera epidemics with a 1-month lag [16]. However, they also found regional differences that required different models for prediction that were in part due to local differences in river flow and tidal intrusion. For the Bay of Bengal, studies have demonstrated major roles for hydroclimatic influences on cholera transmission cycles, with the two characteristic seasonal peaks in cholera cases each year attributed to different environmental drivers. Jutla, Akanda and colleagues [1, 34, 35] have argued that minimal dry season discharge resulting in saltwater and plankton intrusion is a driver of spring epidemics, whereas fall epidemics are more closely related to rain-induced flooding and subsequent contamination. Evidence of two independent physical drivers for phytoplankton abundance has been documented, the first is phytoplankton blooming produced by upwelling of cold, nutrient-rich deep ocean water and the second, explaining the Bay of Bengal findings, includes coastal phytoplankton blooming during high river discharges containing terrestrial nutrients. Thus, freshwater discharge can play a significant role [34].
Fig. 3.
The use of SST, SSH, and chlorophyll a to predict cholera incidence in the Bay of Bengal. (Figure adapted from Ford et al. [25] and from Colwell and Calkins, unpublished data)
Although much of the research on remote sensing to predict cholera outbreaks has focused on the Bay of Bengal, limited work has also been conducted in other regions of the world susceptible to cholera. Reyburn et al. [57] present an interesting analysis of climate variability and cholera outbreaks in Zanzibar, East Africa. These authors used a seasonal autoregressive integrated moving average or SARIMA model to examine associations between climatic variables and the outbreaks. Their model demonstrated an association between cholera clusters and minimum temperature with a 4-month lag, and an association between an increase in rainfall with a 2-month lag. The interaction between rainfall and temperature resulted in a positive association with cholera with a 1-month lag. SSH and SST were not associated with cholera clusters in this study. Instead, the authors concluded increased human exposure through rain-induced flooding and the effect of temperature stimulating V. cholerae survival and growth were important. However, care should be taken in interpreting these time lag data. Another study in South Africa [48] found a strong association between a cholera epidemic and SST with a 0-month lag, and a “moderately strong” association with chlorophyll a with a 6-month lag. A weak relationship was also shown with SSH with a 5-month lag. Although there may be a rational hypothesis to explain these associations, spurious associations would no doubt be found between environmental drivers and epidemics if data were examined using multi-year time lags. An example is a study of cholera in the Republic of Senegal showing strong relationship between rainfall patterns and the pattern of cholera outbreaks in several regions of thecountry, after comparing rainfall patterns between 2002 and 2005 and the relationship between the seasonal temperature gradient in the tropical Atlantic Ocean and population over Senegal for 2005 [17].
Although coastal phytoplankton and subsequent zooplankton blooms have become predictable events (although not yet predictive) preceding cholera epidemics in coastal areas of the Bay of Bengal, it has become increasingly obvious that these blooms alone are insufficient for assessment of future risk. Hydroclimatic influences on plankton blooms are important, with nutrients from river discharge playing a greater role in certain regions, while in other areas upwelling of nutrient-rich colder water may stimulate these blooms (e.g., [23]). Human exposure is obviously a critical factor, in addition to rainfall and consequent flooding, as well as saltwater and plankton intrusion playing key roles. Each study provides new information to consider in predictive modeling of cholera outbreaks, and we are clearly getting closer to building specific models for each region that enables remote sensing data to be coupled with local conditions that reflect region-specific drivers of human exposure. The real strength in this approach to predictive modeling is not so much in the geographical locations where cholera is endemic, and vigilance is necessary to mitigate the burden of diarrheal disease, but rather in providing an early warning system with the potential to prepare for future outbreaks/epidemics in coastal regions with little or no prior history of the disease, as has occurred recently in Haiti, the Dominican Republic, and Cuba. Given the rate of climate change, with the potential for rapid ocean warming and sea level rise, the ability to use remote sensing data to provide early warning of emergence of a plankton bloom in coastal areas is invaluable. Coupled with an understanding of hydrodynamic and hydroclimatic influences, this information would be important not only for cholera prevention but also for risk assessments concerning algae toxins, eutrophication, and other deleterious effects on the marine ecosystem and consequent implication for human health.
Viewing V. parahaemolyticus
Following the pioneering work of Colwell and her colleagues, Grimes and DePaola decided to investigate the feasibility of predicting another Vibrio sp. using satellite remote sensing of SST (Grimes, personal communication). They used an algorithm developed by the US Food and Drug Administration (FDA) that incorporated SST to predict health risks associated with consuming raw oyster meats. Specifically, the algorithm used real-time SST to “now cast” V. parahaemolyticus levels in the meats. Andrea Phillips-Zimmerman worked with DePaola and Bowers (both with FDA) and carried out a project to further develop the algorithm and drive it with SST obtained by satellite RS.
Phillips-Zimmerman employed real-time temperature measurements (YSI salinometer, Oxford, Ohio) and colony DNA hybridization employing a probe for the thermolabile hemolysin gene (tlh) to measure levels of V. parahaemolyticus in oyster meats [74]. The relationship between real-time SSTand satellite-derived SST was significant (R=0.86, p≤0.001, Fig. 4) which allowed use of satellite-derived SST to drive the algorithm [56]. Salinity was added to the algorithm [mean log V. parahaemolyticus per gram=-1.904+0.084×(SST)+ 0.242×(salinity)-0.006×(salinity2)], improving its accuracy. In general, SST explained 50 % of the annual variation of V. parahaemolyticus in oysters harvested from the northern Gulf of Mexico [74]. The effect of salinity was nonlinear, i.e., salinity was significantly related to V. parahaemolyticus density. There was a direct linear relationship to a point (optimal water salinity for tlh was 22 %thou and for oysters the optimum was 24 %thou), after which the relationship decreased as salinity values increased [32].
Fig. 4.
Significant correlation (R=0.86, p≤0.001) between real-time and satellite-derived SST (Phillips, A.M.B., 2005. M.S. Thesis, USM)
NOAA Oceans and Human Health Initiative provided funds for work (DJG) with the National Center for Atmospheric Research to create a predictive website. The improved algorithm created by Zimmerman et al. [74] was the basis for the website; satellite-derived data were downloaded, first by USM scientists and subsequently by NOAA/NOS scientists [63], and these data were used to drive the algorithm. Until recently, NOAA/NOS scientists downloaded satellite-derived data, ran the algorithm, and provided a map for NCAR to refresh the website and this was done approximately twice a month (see Fig. 5 and website at http://www.eol.ucar.edu/projects/ohhi/vibrio/). In the near future, it is hoped that this entire process will be automated so that the downloaded remotely sensed data populates the website. Finally, the website is not intended to be used by regulatory agencies for enforcement. It is simply a predictive website to alert users that there may (or may not) be risk associated with consumption of raw oysters harvested from one or more indicated sites and that those oysters should be tested for the presence and abundance of V. parahaemolyticus.
Fig. 5.
Mean log10 V. parahaemolyticus per gram of oyster meat at harvest based on SST and salinity (log10(V. parahaemolyticus/g)=−2.05+0.097*SST+0.2*SAL−0.0055*SAL2). See complete web site and additional maps at http://www.eol.ucar.edu/projects/ohhi/vibrio/
Development of algorithms to predict distribution and abundance of V. parahaemolyticus and V. vulnificus at bathing beaches in the Mississippi Sound and V. vulnificus in oyster meats from Mississippi oyster reefs has begun. The data that will be used to create the algorithms will be obtained from NSF Ecology of Infectious Diseases grant that include Colwell and Grimes as co-PIs. These data include distribution and abundance of both vibrio species using tlh for V. parahaemolyticus and V. vulnificus hemolysin gene (vvh) for V. vulnificus, as well as a variety of environmental parameters [including SST, bottom temperature, surface salinity, bottom salinity, 18 pigments (including chlorophyll a, fucoxanthin, and alloxanthin), suspended particulate matter, and dissolved organic carbon]. Also included are wind direction and speed because it is hypothesized that strong southerly winds resuspend shallow sediments containing vibrios (Grimes, unpublished data). Wind data have been collected by the GCRL Beach Monitoring program and posted on their website for several years.
Several other studies have utilized remote sensing to analyze emergence of V. parahaemolyticus disease, particularly in areas where the infections are rare or at least considered to be “non endemic.” Significantly, the studies provide striking evidence of warm water incursions that temporally and spatially correspond closely to reported disease outbreaks. These studies include V. parahaemolyticus outbreaks in Alaska in 2005 [46], Northwest Spain in 1999 [3], and South America, the latter during El Niño events [44]. Martinez-Urtaza et al. [45] also carried out an analysis of zooplankton and seawater, showing occurrence of V. parahaemolyticus in offshore areas to be almost exclusively associated with zooplankton and present in 80 % of the sample abundance, with occurrence of downwelling periods promoting zooplankton patchiness. Their findings, coupled with those of deMagney et al. [16] showing specificity of particular zooplankton groups to be consistently associated with V. cholerae, namely copepods, cladocerans, and rotifers, suggest that remote sensing is becoming increasingly more effective in prediction of blooms of those bacteria associated with human disease. Because SST alone provides, relatively speaking, such a useful measure of potential risk in temperate regions, remote sensing will, in all likelihood, provide a highly effective early warning system to manage public health risk.
With respect to climate change and ocean warming, the long-term effect of increasing temperature of the ocean on microbial populations was investigated using historical data, i.e., applying a retrospective molecular analysis of the bacterial community using formalin-fixed samples from the historical Continuous Plankton Recorder archive, one of the longest (1961-2005) and most geographically extensive collections of marine biological samples in the world. Vezzulli et al. [68] were able to demonstrate the effect of ocean warming during the last half century on vibrios, including V. cholerae. Increased dominance within the plankton-associated bacterial community of the North Sea where an unprecedented increase in bathing infections has been reported. SST accounted for 45 % of the variance in vibrio data. This finding indicates that more precise data and improvement in hindcast and forecast analyses will allow even more precise prediction for public health application.
Viewing V. vulnificus—Examples from Europe and the USA
A number of recent reports have documented increasing Vibrio wound infections in Europe, notably linked to heat wave events in the Baltic and North Sea, such as those experienced in 1994, 2003, and 2006 [2]. Significantly, several of the fastest warming marine systems anywhere in the world are found in Northern Europe, including the North, White, and Baltic Sea, suggesting these areas may be undergoing a transition into “at risk” regions for Vibriodisease. Of the clinical cases reported in the Baltic and North Sea, the most serious infections appear to have been caused by V. vulnificus. Because this species has well-characterized ecological preferences (e.g., temperature and salinity optima), it is possible to develop remote sensing-enabled risk mapping of areas to identify regions where these pathogens may be proliferating during anomalously warm climatic events.
To this end, Baker-Austin et al. [2] developed a risk-based matrix using temperature and salinity to map risk in this region. Using past “at risk” episodes, such as the summers of 1994 and 2006, they were able to provide a mechanism to scrutinize retrospectively when and where clinical cases were reported utilizing a simple method based on SST and SSS (Fig. 6).
Fig. 6.
a Basic schematic of remote sensing-enabled Vibrio risk mapping, Baltic Sea, summer (May-September) 2006. Because pathogens of interest (e.g., V. vulnificus) have well-defined temperature and salinity optima, a combination of these two variables can be used to build a basic risk map. During 2006, a record number of Vibrio wound infections were reported in and around the Baltic Sea, which corresponded with a significant heat wave event [2]. Remote sensing-enabled risk mapping can be used to predict similar anomalous climatic events in the future, and potentially as a risk management tool. Figure courtesy Joaquin Trinanes (NOAA). b Predictive hindcast depicting mean probability of V. vulnificus during 1991. The scale on the right represents the probability of occurrence, with 25 % as high and 0 % as the low probability [4]
A separate and novel extension of this work is to forecast future risk using statistical predictions of SST changes (e.g., driven by climate change) as well as population density (an indirect measure of exposure), thus providing a measure of possible changes in risk in coming decades. The regional-specific risk maps are being expanded to include other geographical areas and, by incorporating past outbreak data, to validate models for other Vibrio species of interest. More sophisticated remote sensing-based risk mapping is also being developed that integrates a range of the most pertinent satellite-derived fields (SSS, SST, chlorophyll a, turbidity) with relevant epidemiology variables—such as population density, susceptible populations, recreational water usage, etc.—into a useable “nowcasting” approach similar in scope and vision to efforts in the USA. This technology is of particular application in coastal regions undergoing rapid warming, such as Europe, the Far East, and Canada [42], and where vibriosis represents an emerging problem.
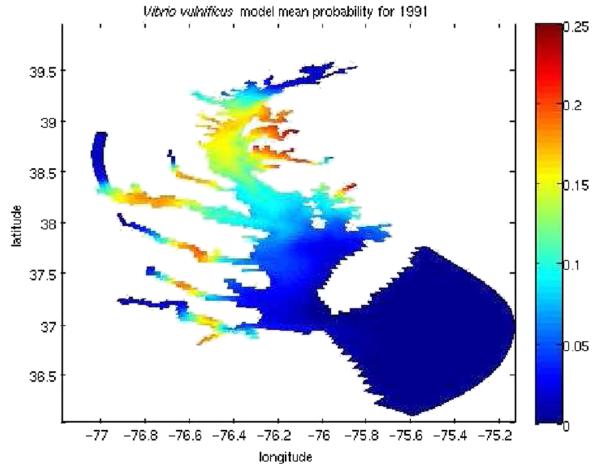
In the USA, V. vulnificus is officially recognized to be a serious health threat, accounting for a 50 % fatality rate in patients at risk (http://www.fda.gov/Food/ResourcesForYou/HealthEducators/ucm085365.htm). The highest number of cases has been reported in summer months [60]. The Chesapeake Bay of the USA in particular is a region sensitive to climate change events, such as increased water temperature, precipitation, streamflow, and sea level rise [53]. These factors influence the geographical and seasonal variation of several vibrios including V. vulnificus [33]. Hindcast prediction was undertaken by Banakar et al. [4] to determine spatial and temporal variability in the likelihood of occurrence of V. vulnificus in surface waters of the Chesapeake Bay (Fig. 7). The hindcast predictor yielded an improved understanding of environmental conditions associated with occurrence of V. vulnificus, notably temperature and salinity.
Fig. 7.
Predicted hindcast depicting the mean probability of V. vulnificus during 1991. The scale on the right represents the probability of occurrence, with 25 % as high and 0 % as low probability (from Banakar et al. [4])
Ocean Color and Marine Microbial Ecology
In biological oceanography, the most pervasive use of satellites has been to characterize and detail the spatial and seasonal patterns of chlorophyll in the upper ocean [19]. At high latitudes, the chlorophyll signal largely derives from eukaryotic algae, such as diatoms, coccolithophorids, and dinoflagellates. However, in the low latitude oligotrophic oceans, much of the basic chlorophyll signal derives from the coccoid cyanobacteria of the genera Synechococcusand Prochlorococcus, numerically dominant phytoplankton in those systems [6, 55]. Efforts are underway to develop advanced algorithms that may be able to exploit reflectance spectra to detect the presence of diagnostic accessory pigments in order to identify specific algal and cyanobacterial taxa and functional groups [9, 18, 52]. Similarly, efforts are also ongoing to derive upper ocean primary production from these data [5]. Thus, satellite remote sensing is contributing directly to understanding of the role of the oceans and their picophytoplankton populations in the global carbon cycle.
Cyanobacterial Case Studies
Trichodesmium
Trichodesmium, as noted above, has several unique attributes that have allowed for its unique identification under specific conditions, namely in addition to its absorption characteristics derived from the presence of PEB, it also contains gas vesicles that scatter light, making surface blooms of this organism “brighter.” Indeed, senescing blooms have been described as silvery gray in color [20]. Water molecules absorb strongly in the red/near-infrared region of the spectrum and the optical signature of subsurface blooms in this region of the spectrum are lost due to absorption by water. However, gas vesicles in Trichodesmium cause it to accumulate at the surface as a microlayer above the water when wind stress is low, thereby creating high reflectance in the near-infrared—so high that surface blooms of Trichodesmium often are classified as clouds in standard satellite data processing analyses. This high reflectance of surface blooms of Trichodesmium was exploited by Subramaniam et al. [65] to detect the organism using visible and infrared bands of the AVHRR sensor. Capone et al. [12] used the same method to map a bloom of Trichodesmiumin the Arabian Sea that covered an area greater than 2 million square kilometers. The combination of absorption, fluorescence, and scattering properties of Trichodesmium, in addition to high-infrared reflectance, has been used by researchers to develop satellite algorithms for its detection and quantification [7, 21, 30, 47, 65, 66, 69].
Nodularia Blooms in the Baltic Sea
Each summer during upper water column warming, a sequence of cyanobacterial blooms occurs in the Baltic Ocean, usually culminating in a major bloom of Nodularia spumigena (Fig. 2a, b, c). Nodularia, a nitrogen-fixing cyanobacteria, is also associated with Nodularin, a hepatotoxin [62], and beta methyl amino alanine (BMAA), a neurotoxic amino acid [15]. Kahru [36] showed that surface blooms of these organisms can be detected using the AVHRR sensor and he compiled a time series of images to explore factors that contribute to formation and transport of these blooms [37]. Metsamaa et al. [49] developed algorithms for specifically identifying these organisms.
Microcystis Blooms in Lakes
Many of the cyanobacteria that bloom in freshwater lakes can be toxic and understanding bloom dynamics is a critical issue for water quality management, especially for those bodies that serve as drinking water reservoirs [72]. Remote sensing techniques have been developed for remote sensing of freshwater cyanobacteria based on their absorption and scattering characteristics. Sims et al. [61] developed a technique for quantifying concentrations of phycocyanin, the marker pigment for fresh-water cyanobacteria, and found that their algorithm could allow for assessment of cyanobacterial risk to water quality and public health following the World Health Organization [70] guidelines in about 70 % of the cases they considered. Budd et al. [10] exploited the enhanced scattering of light by surface blooms of the toxic cyanobacterium Microcystis to map blooms of this organism using visible band channels on the AVHRR and Landsat Thematic Mapper (TM) sensors. Both these instruments have very broad band sensors and are not optimal for ocean color sensing. But the AVHRR series of sensors have operated without any breaks for almost 30 years, providing daily global coverage and so are ideal for developing long-term time series. The Landsat TM sensor has high spatial resolution (30 m vs. 1 km for AVHRR) and is ideal for small inland water bodies but its frequency is once every 16 days at best, less frequent in cloudy areas and seasons. Nevertheless, using this combination, Budd et al. [11] constructed a time series of satellite-based turbidity maps for Lake Erie from 1987 to 1993, representing a period from before to after zebra mussel (Dreissena polymorpha) establishment in these waters. They showed changes in water quality in Lake Erie and attributed a decrease in turbidity arising from suspended sediment and subsequent increase in incidence of Microcystis blooms to the presence of zebra mussels.
Ocean Bioluminescence
A distinct phenomenon which has been reported by mariners for many centuries is the occurrence of glowing “milky seas” in the surface ocean at night. Research by Lapota et al. [40] suggested that this phenomenon might be due to luminescent bacteria, such as Vibrio harveyi, rather than the well-known bioluminescent dinoflagellates. A ship report by a British merchantman in 1995 was followed up by Miller et al. [50], who were able to exploit assets of the US Defense Meteorological Satellite Program and their polar-orbiting highly sensitive Operational Linescan System to capture the extent of the surface manifestation of this event, which occurred off the coast of Somalia in the northwest Arabian Sea and covered about 15,000 km2 (Fig. 8). Nealson and Hastings [54] recount how this study came to be and discuss it as an example of scaling a molecular phenomenon happening at the microscale to the global scale at which it was detected. Haddock et al. [28] provide a detailed review of the bioluminescence phenomenon. Another application of bioluminescence is reviewed by Frischer et al. [26] who suggest that this phenomenon can be used as a tool for mapping chemical contamination in coastal waters. They use the ratio of bioluminescent to total bacteria (bioluminescent ratio) as a simple and reliable initial indicator of chemical contamination of estuarine systems. While there are no reports currently available in the literature on the use of satellites for this application, it is entirely possible that the Day/Night Band channel on the Suomi National Polar-Orbiting Partnership satellite has the radiometric sensitivity and the spatial resolution for this application.
Fig. 8.
Bioluminescence due to V. harveyi detected by the Operational Line Scanner sensor on the Defense Meteorological Satellite Program in the Indian Ocean [50]
Harmful Algal Blooms
Satellite color remote sensing has been long been employed as a tool to detect harmful algal blooms (HABs) [39, 63]. While a major focus of HAB work has been on certain dinoflagellates and diatoms in coastal waters, certain bloom-forming marine cyanobacteria contain toxins and are also considered HABs (see Baltic Sea and lakes, above) (Fig. 8). Trichodesmium contains toxins [29] and it has been implicated in fish kills [58]. In the Gulf of Mexico, Trichdesmium has also been implicated in conditioning water, allowing subsequent growth of dinoflagellates such as Karenia brevis. This finding derives from correlations constructed from remote sensing data [41]. Similarly, remote sensing has been used to detail HABs in coastal waters of Vietnam and China [67].
Conclusions/Future Directions
The use of remote sensing to observe microbes in the open ocean has evolved extensively since the inception of remote sensing as a tool, which began in the 1960s. Improvements in sensors that capture important physicochemical parameters (temperature, salinity, turbidity, chlorophyll a, etc.), coupled with basic and fundamental knowledge regarding the biology, ecology, and growth dynamics of marine microorganisms as well as bio-optical theory, have undoubtedly underpinned this progress. However, several key gaps exist that hinder progress in using remote sensing to observe bacteria. Our ability to monitor coastal waters with satellites is limited by both the lack of sensors with the high spatial, temporal, and spectral resolution in the case of ocean color remote sensing, as well as the lack theoretical approaches to interpret these observations. For example, a space-based salinity sensor cannot map closer than about 200 km from the coast and there are no satellites that can measure coastal sea surface salinity. Similarly, satellite-based wind products are not reliable close to the coast. Equally important, there are few satellite sensors that can provide high spatial and temporal resolution data essential to study dynamic coastal regions that are influenced by tides and can change at hourly increments. The Korean Geostationary sensor, GOCI, is an example of what future sensors such as GEO-CAPE being planned for US coastal waters might be able to provide— several looks a day at a high spatial resolution of 500 m. Future hyperspectral imagers might also provide the tools to unravel the complexity of coastal waters. Fundamentally, a more globally integrated and cohesive approach to coastal environments should be encouraged that mirrors coverage of the open ocean by satellites. Daily output from high-resolution coastal physical/biogeochemical models that include parameters relevant to management of public health should be made available on a regular basis. Pathogenic bacteria and algae species in the marine environment are not limited by geographical boundaries and many disease outbreaks require transborder and multiregional cooperation in terms of clinical, epidemiological, and risk management response. Indeed, microbial oceanography offers an extraordinary opportunity to appreciate the sensitivity of the ocean’s response to climate change and human health, especially as the predictive power of climate models is enhanced [8]. We conclude remote sensing tools can be used to predict waterborne diseases like cholera and other vibrio-caused diseases and the development of remote sensing as a public health weapon against those diseases should transcend borders. To this end, we advocate greater national, regional, and international collaborations in this increasingly important research area. Such collaborations will require availability and sharing of large databases of oceanic parameters coupled with reliable epidemiological information to achieve significant progress in our understanding of the role of oceans in human disease dynamics.
Acknowledgments
The authors are grateful to the National Science Foundation (EF-0813285/EF-0813066/EF-1003943 to DJG and RRC), National Oceanic and Atmospheric Administration (NA04-OAR-4600214 and NA-06-OAR4310119, UCAR Sub Award No. S09-75034 to DJG), National Aeronautics and Space Administration (NNX09AR57G to DJG and NNX10AT99G to AS), and National Institutes of Health (2R01A1039129-11A2 to A. Huq and RRC) for their support.
Contributor Information
D. Jay Grimes, Gulf Coast Research Laboratory, The University of Southern Mississippi, 703 East Beach Drive, Ocean Springs, MS 39564, USA.
Tim E. Ford, University of New England, 716 Stevens Avenue, Portland, ME 04103, USA, tford@une.edu
Rita R. Colwell, Center for Bioinformatics and Computational Biology, UMIACS, University of Maryland, 3103 Biomolecular Sciences Building #296, College Park, MD 20742, USA, rcolwell@umiacs.umd.edu
Craig Baker-Austin, Centre for Environment, Fisheries and Aquaculture Science (Cefas), Barrack Road, Weymouth, Dorset, UK, craig.baker-austin@cefas.co.uk.
Jaime Martinez-Urtaza, Department of Biology and Biochemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK, martinez-urtaza@gmail.com.
Ajit Subramaniam, Lamont-Doherty Earth Observatory, Columbia University, Palisades, NY 10964, USA, ajit@ldeo.columbia.edu.
Douglas G. Capone, Wrigley Institute for Environmental Studies, University of Southern California, Los Angeles, CA 90089-0371, USA, capone@usc.edu
References
- 1.Akanda AS, Jutla AS, Alam M, deMagny GC, Siddique A, Sack RB, Huq A, Colwell RR, Islam S. Hydroclimatic influences on seasonal and spatial cholera transmission cycles: implications for public health intervention in the Bengal Delta. Water Resour Res. 2011:47. doi: 10.1029/2010WR009914. [Google Scholar]
- 2.Baker-Austin C, Trinanes JA, Taylor NG, Hartnell R, Siitonen A, Martinez-Urtaza J. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat Clim Chang. 2012;3:73–77. [Google Scholar]
- 3.Baker‐Austin C, Stockley L, Rangdale R, Martinez‐Urtaza J. Environmental occurrence and clinical impact of Vibrio vulnificusand Vibrio parahaemolyticus: a European perspective. Environ Microbiol Rep. 2010;2:7–18. doi: 10.1111/j.1758-2229.2009.00096.x. [DOI] [PubMed] [Google Scholar]
- 4.Banakar V, deMagny GC, Jacobs J, Murtugudde R, Huq A, Wood RJ, Colwell RR. Temporal and spatial variability in thedistribution of Vibrio vulnificus in the Chesapeake Bay: a hindcast study. Ecohealth. 2011;8:456–467. doi: 10.1007/s10393-011-0736-4. [DOI] [PubMed] [Google Scholar]
- 5.Behrenfeld MJ, Falkowski PG. A consumer’s guide to phytoplankton primary productivity models. Limnol Oceanogr. 1997;42:1479–1491. [Google Scholar]
- 6.Binder BJ, Chisholm SW, Olson RJ, Frankel SL, Worden AZ. Dynamics of picophytoplankton, ultraphytoplankton and bacteria in the central equatorial Pacific. Deep Sea Res II: Top Stud Oceanogr. 1996;43:907–931. [Google Scholar]
- 7.Borstad GA, Gower JFR, Carpenter EJ. Development of algorithms for remote sensing of Trichodesmium blooms. In: Carpenter EJ, Capone DG, Rueter JG, editors. Marine pelagic cyanobacteria: Trichodesmium and other diazotrophs. Vol. 362. Dordrecht; Kluwer: 1992. pp. 193–210. [Google Scholar]
- 8.Bowler C, Karl DM, Colwell RR. Microbial oceanography in a sea of opportunity. Nature. 2009;459:180–184. doi: 10.1038/nature08056. [DOI] [PubMed] [Google Scholar]
- 9.Bracher A, Vountas M, Dinter T, Burrows JP, Rottgers R, Peeken I. Quantitative observation of cyanobacteria and diatoms from space using PhytoDOAS on SCIAMACHY data. Biogeosciences. 2009;6:751–764. [Google Scholar]
- 10.Budd JW, Beeton AM, Stumpf RP, Culver DA. Kerfoot WC Presented at the International Association of Theoretical and Applied Limnology. Stuttgart: [Google Scholar]
- 11.Budd JW, Drummer TD, Nalepa TF, Fahnenstiel GL. Remote sensing of biotic effects: Zebra mussels (Dreissena polymorpha) influence on water clarity in Saginaw Bay, Lake Huron. Limnol Oceanogr. 2001;46:213–223. [Google Scholar]
- 12.Capone DG, Subramaniam A, Montoya JP, Humborg C, Voss M, Pollehne F, Carpenter EJ. An extensive bloom of the diazotrophic cyanobacterium, Trichodesmium, in the Central Arabian Sea during the spring intermonsoon. Mar Ecol Prog Ser. 1998;172:281–292. [Google Scholar]
- 13.Colwell RR. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 14.Colwell RR, Spira WM, William BG., III . The ecology of Vibrio cholerae. In: Barua D, editor. Cholera. Plenum; New York: 1992. pp. 107–127. [Google Scholar]
- 15.Cox PA, Banack SA, Murch SJ, Rasmussen U, Tien G, Bidigare RR, Metcalf JS, Morrison LF, Codd GA, Bergman B. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. Proc Natl Acad Sci U S A. 2005;102:5074–5078. doi: 10.1073/pnas.0501526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.deMagny GC, Mozumder PK, Grim CJ, Hasan NA, Naser MN, Alam M, Sack RB, Huq A, Colwell RR. Role of zooplankton diversity in Vibrio choleraepopulation dynamics and in the incidence of cholera in the Bangladesh Sundarbans. Appl Environ Microbiol. 2011;77:6125–6132. doi: 10.1128/AEM.01472-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.deMagny GC, Thiaw W, Kumar V, Manga NM, Diop BM, Gueye L, Kamara M, Roche B, Murtugudde R, Colwell RR. Cholera outbreak in Senegal in 2005: was climate a factor? PloS ONE. 2012;7:e44577. doi: 10.1371/journal.pone.0044577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Moraes Rudorff N, Kampel M. Orbital remote sensing of phytoplankton functional types: a new review. Int J Remote Sens. 2012;33:1967–1990. [Google Scholar]
- 19.Demarcq H, Reygondeau G, Alvain S, Vantrepotte V. Monitoring marine phytoplankton seasonality from space. Remote Sens Environ. 2011;117:211–222. [Google Scholar]
- 20.Devassy VP, Bhattathiri PMA, Qasim SZ. Trichodesmium phenomenon. Indian J Mar Sci. 1978;7:168–186. [Google Scholar]
- 21.Dupouy C, Neveux J, Dirberg G, Röttgers R, Barboza Tenório MM, Ouillon S. Bio-optical properties of the marine cyanobacteria Trichodesmium spp. J Appl Remote Sens. 2008;2:023503. [Google Scholar]
- 22.Epstein PR, Ford TE, Colwell RR. Marine ecosystems. Lancet. 1993;342:1216–1219. doi: 10.1016/0140-6736(93)92191-u. [DOI] [PubMed] [Google Scholar]
- 23.Fawcett S, Ward B. Phytoplankton succession and nitrogen utilization during the development of an upwelling bloom. Mar Ecol Prog Ser. 2011;428:13–31. [Google Scholar]
- 24.Ford T, Colwell R. A global decline in microbiological quality of water: a call for action. American Academy of Microbiology; Washington, DC: 1996. [Google Scholar]
- 25.Ford TE, Colwell RR, Rose JB, Morse SS, Rogers DJ, Yates TL. Using satellite images of environmental changes to predict infectious disease outbreaks. Emerg Infect Dis. 2009;15:1341. doi: 10.3201/eid/1509.081334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frischer M, Danforth J, Foy T, Juraske R. Bioluminescent bacteria as indicators of chemical contamination of coastal waters. J Environ Qual. 2005;34:1328–1336. doi: 10.2134/jeq2004.0245. [DOI] [PubMed] [Google Scholar]
- 27.Garver SA, Siegel DA. Variability in near-surface particulate absorption spectra: what can a satellite ocean color imager see? Limnol Oceanogr. 1994;39:1349–1367. [Google Scholar]
- 28.Haddock SH, Moline MA, Case JF. Bioluminescence in the sea. Ann Rev Mar Sci. 2010;2:443–493. doi: 10.1146/annurev-marine-120308-081028. [DOI] [PubMed] [Google Scholar]
- 29.Hawser SP, Codd GA, Carpenter EJ, Capone DG. A neurotoxic factor associated with the bloom-forming cyanobacterium Trichodesmium. Toxicon. 1991;29:277–278. doi: 10.1016/0041-0101(91)90231-f. [DOI] [PubMed] [Google Scholar]
- 30.Hu CM, Cannizzaro J, Carder KL, Muller-Karger FE, Hardy R. Remote detection of Trichodesmium blooms in optically complex coastal waters: examples with MODIS full-spectral data. Remote Sens Environ. 2010;114:2048–2058. [Google Scholar]
- 31.Huq A, West P, Small E, Huq M, Colwell R. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar 01 associated with live copepods in laboratory microcosms. Appl Environ Microbiol. 1984;48:420–424. doi: 10.1128/aem.48.2.420-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson CN, Flowers AR, Noriea N, Zimmerman A, Bowers J, DePaola A, Grimes DJ. Relationships between environmental factors and pathogenic Vibrios in the Northern Gulf of Mexico. Appl Environ Microbiol. 2010;76:7076–7084. doi: 10.1128/AEM.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson CN, Bowers JC, Griffitt KJ, Molina V, Clostio RW, Pei S, Laws E, Paranjpye RN, Strom MS, Chen A, Hasan NA, Huq A, Noriea NF, III, Grimes DJ, Colwel RR. Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States) Appl Environ Microbiol. 2012;78:7249–7257. doi: 10.1128/AEM.01296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jutla AS, Akanda AS, Griffiths JK, Colwell R, Islam S. Warming oceans, phytoplankton, and river discharge: implications for cholera outbreaks. Am J Trop Med Hyg. 2011;85:303. doi: 10.4269/ajtmh.2011.11-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jutla AS, Akanda AS, Islam S. Tracking cholera in coastal regions using satellite observations1. JAWRA J Am Water Resour Assoc. 2010;46:651–662. doi: 10.1111/j.1752-1688.2010.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahru M, Leppanen J-M, Rud O. Cyanobacterial blooms cause heating of the sea surface. Mar Ecol Prog Ser. 1993;191:1–7. [Google Scholar]
- 37.Kahru M, Leppanen JM, Rud O, Savchuk OP. Cyanobacteria blooms in the Gulf of Finland triggered by saltwater inflow into the Baltic Sea. Mar Ecol Prog Ser. 2000;207:13–18. [Google Scholar]
- 38.Kuchler DA, Jupp DLB. Shuttle photograph captures massive phytoplankton bloom in the Great Barrier Reef. Int J Remote Sens. 1988;9:1299–1301. [Google Scholar]
- 39.Kutser T. Passive optical remote sensing of cyanobacteria and other intense phytoplankton blooms in coastal and inland waters. Int J Remote Sens. 2009;30:4401–4425. [Google Scholar]
- 40.Lapota D, Galt C, Losee JR, Huddell HD, Orzech JK, Nealson KH. Observations and measurements of planktonic bioluminescence in and around a milky sea. J Exp Mar Biol Ecol. 1988;119:55–81. [Google Scholar]
- 41.Lenes JM, Heil CA. A historical analysis of the potential nutrient supply from the N2 fixing marine cyanobacterium Trichodesmium spp. to Karenia brevis blooms in the eastern Gulf of Mexico. J Plankton Res. 2010;32:1421–1431. [Google Scholar]
- 42.Lima FP, Wethey DS. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat commun. 2012 doi: 10.1038/ncomms1713. doi: 10.1038/ncomms1713. [DOI] [PubMed] [Google Scholar]
- 43.Lobitz B, Beck L, Huq A, Wood B, Fuchs G, Faruque ASG, Colwell R. Climate and infectious disease: use of remote sensing for detection of Vibrio choleraeby indirect measurement. Proc Natl Acad Sci U S A. 2000;97:1438–1443. doi: 10.1073/pnas.97.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez-Urtaza J, Huapava B, Gavilan RG, Blanco-Abad V, Ansede-Bermejo J, Cadarso-Suarez C, Figueiras A, Trinanes J. Emergence of Asiatic vibrio diseases in South America in phase with El Niño. Epidemiology. 2008;19:829–837. doi: 10.1097/EDE.0b013e3181883d43. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Urtaza J, Blanco-Abad V, Rodriguez-Castro A, Ansede-Bermejo J, Miranda A, Rodriguez-Alvarez MX. Ecological determinants of the occurrence and dynamics of Vibrio parahaemolyticus in offshore areas. ISME J. 2011;6:994–1006. doi: 10.1038/ismej.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez-Urtaza J, Bowers JC, Trinanes J, DePaola A. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int. 2010;43:1780–1790. [Google Scholar]
- 47.McKinna LIW, Furnas MJ, Ridd PV. A simple, binary classification algorithm for the detection of Trichodesmium spp. within the Great Barrier Reef using MODIS imagery. Limnol Oceanogr: Methods. 2011;9:50–66. [Google Scholar]
- 48.Mendelsohn J, Dawson T. Climate and cholera in KwaZulu-Natal, South Africa: the role of environmental factors and implications for epidemic preparedness. Int J Hyg Environ Health. 2008;211:156–162. doi: 10.1016/j.ijheh.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Metsamaa L, Kutser T, Strombeck N. Recognising cyanobacterial blooms based on their optical signature: a modelling study. Boreal Environ Res. 2006;11:493–506. [Google Scholar]
- 50.Miller SD, Haddock SHD, Elvidge CD, Lee TF. Detection of a bioluminescent milky sea from space. Proc Natl Acad Sci U S A. 2005;102:14181–14184. doi: 10.1073/pnas.0507253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morel A. Consequences of a Synechococcus bloom upon the optical properties of oceanic (case 1) waters. Limnol Oceanogr. 1997;42:1746–1754. [Google Scholar]
- 52.Nair A, Sathyendranath S, Platt T, Morales J, Stuart V, Forget MH, Devred E, Bouman H. Remote sensing of phytoplankton functional types. Remote Sens Environ. 2008;112:3366–3375. [Google Scholar]
- 53.Najjar RG, Pyke CR, Adams MB, Breitburg D, Hershner C, Kemp M, Howarth R, Mulholland MR, Paolisso M, Secor D. Potential climate-change impacts on the Chesapeake Bay. Estuar Coast Shelf Sci. 2010;86:1–20. [Google Scholar]
- 54.Nealson KH, Hastings J. Quorum sensing on a global scale: massive numbers of bioluminescent bacteria make milky seas. Appl Environ Microbiol. 2006;72:2295–2297. doi: 10.1128/AEM.72.4.2295-2297.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olson RJ, Chisholm SW, Zettler ER, Altabet MA, Dusenberry JA. Spatial and temporal distributions of prochlorophyte picoplankton in the North Atlantic Ocean. Deep Sea Research Part A. Oceanogr Res Pap. 1990;37:1033–1051. [Google Scholar]
- 56.Phillips A, DePaola A, Bowers J, Ladner S, Grimes DJ. An evaluation of the use of remotely sensed parameters for prediction of incidence and risk associated with Vibrio parahaemolyticus in Gulf Coast oysters (Crassostrea virginica) J Food Prot. 2007;70:879–888. doi: 10.4315/0362-028x-70.4.879. [DOI] [PubMed] [Google Scholar]
- 57.Reyburn R, Kim DR, Emch M, Khatib A, von Seidlein L, Ali M. Climate variability and the outbreaks of cholera in Zanzibar, East Africa: a time series analysis. Am J Trop Med Hyg. 2011;84:862. doi: 10.4269/ajtmh.2011.10-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rörig LR, Yunes JS, Kuroshima KN, Schetinni CAF, Pezzuto PR, Proença L. Studies on the ecology and toxicity of Trichodesmium spp. blooms in southern Brazilian coastal waters. Harmful Algae. 1998;1:22–25. [Google Scholar]
- 59.Samadi A, Chowdhury N, Huq M, Khan M. Seasonality of classical and El Tor cholera in Dhaka, Bangladesh: 17 year trends. Trans R Soc Trop Med Hyg. 1983;77:853. doi: 10.1016/0035-9203(83)90306-1. [DOI] [PubMed] [Google Scholar]
- 60.Shapiro R, Altekruse S, Hutwagner L, Bishop R, Hammond R, Wilson S, Ray B, Thompson S, Tauxe R, Griffin P. The role of Gulf Coast oysters harvested in warmer months in Vibrio vulnificus infections in the United States, 1988-1996. J Infect Dis. 1998;178:752–759. doi: 10.1086/515367. [DOI] [PubMed] [Google Scholar]
- 61.Simis SGH, Ruiz-Verdú A, Domínguez-Gómez JA, Peña-Martinez R, Peters SWM, Gons HJ. Influence of phytoplankton pigment composition on remote sensing of cyanobacterial biomass. Remote Sens Environ. 2007;106:414–427. [Google Scholar]
- 62.Sivonen K, Kononen K, Carmichael W, Dahlem A, Rinehart K, Kiviranta J, Niemela S. Occurrence of the hepatotoxic cyanobacterium Nodularia spumigena in the Baltic Sea and structure of the toxin. Appl Environ Microbiol. 1989;55:1990–1995. doi: 10.1128/aem.55.8.1990-1995.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stumpf RP, Tomlinson MC. Remote sensing of harmful algal blooms. In: Miller RL, Del Castillo CE, McKee BA, editors. Remote sensing of coastal aquatic environments. Springer; Dordrecht: 2005. pp. 277–296. [Google Scholar]
- 64.Subramaniam A, Carpenter EJ. An empirically derived protocol for the detection of blooms of the marine cyanobacrerium Trichodesmium using CZCS imagery. Int J Remote Sens. 1994;15(8):1559–1569. [Google Scholar]
- 65.Subramaniam A, Carpenter EJ, Falkowski PG. Bio-optical properties of the marine diazotrophic cyanobacteria Trichodesmium spp. II. A reflectance model for remote sensing. Limnol Oceanogr. 1999;44:618–627. [Google Scholar]
- 66.Subramaniam A, Hood RR, Brown CW, Carpenter EJ, Capone DG. Detecting Trichodesmium blooms in SeaWiFS imagery. Deep_Sea Research, Part II, 1st Special Issue on the U.S. JGOFS Synth Model Proj. 2002;49:107–121. [Google Scholar]
- 67.Tang DL, Di BP, Wei G, Ni IH, Oh IS, Wang SF. Spatial, seasonal and species variations of harmful algal blooms in the South Yellow Sea and East China Sea. Hydrobiologia. 2006;568:245–253. [Google Scholar]
- 68.Vezzulli L, Brettar I, Pezzati E, Reid PC, Colwell RR, Höfle MG, Pruzzo C. Long-term effects of ocean warming on the pro-karyotic community: evidence from the vibrios. ISME J. 2011;6:21–30. doi: 10.1038/ismej.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westberry TK, Siegel DA, Subramaniam A. An improved biooptical model for the remote sensing of Trichodesmium spp. blooms. J. Geophys. Res. 2005;110:C06012. doi:10.1029/2004JC002517. [Google Scholar]
- 70.WHO . Guidelines for safe recreational water environments. Vol. 1. World Health Organization; Geneva: 2003. pp. 136–158. [Google Scholar]
- 71.Wilson C. Late summer chlorophyll blooms in the oligotrophic North Pacific Subtropical Gyre. Geophys Res Lett. 2003;30:OCE 4-1–4-4. doi:1029/2003GL017770. [Google Scholar]
- 72.Xu H, Zhu G, Qin B, Paerl HW. Growth response of Microcystis spp. to iron enrichment in different regions of Lake Taihu, China. Hydrobiologia. 2013;700:187–202. [Google Scholar]
- 73.Zhang Z, Deng Z-Q, Rusch K, Gutierrez WM, Chenier K. Remote sensing algorithms for estimating enterococcus concentration in coastal Louisiana Beaches; The 5th International Conference on Environmental Science and Technology; Houston, Texas. 2010. [Google Scholar]
- 74.Zimmerman A, DePaola A, Bowers J, Krantz J, Nordstrom J, Johnson CN, Grimes DJ. Variability of total and pathogenic Vibrio parahaemolyticus densities in northern Gulf of Mexico water and oysters. Appl Environ Microbiol. 2007;73:7589–7596. doi: 10.1128/AEM.01700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]