Abstract
Osteoarthritis (OA) is the most common degenerative joint disease, and there is no disease-modifying therapy for OA currently available. Targeting of articular cartilage alone may not be sufficient to halt this disease progression. Articular cartilage and subchondral bone act as a functional unit. Increasing evidence indicates that transforming growth factor β (TGFβ) plays a crucial role in maintaining homeostasis of both articular cartilage and subchondral bone. Activation of extracellular matrix latent TGFβ at the appropriate time and location is the prerequisite for its function. Aberrant activation of TGFβ in the subchondral bone in response to abnormal mechanical loading environment induces formation of osteroid islets at onset of osteoarthritis. As a result, alteration of subchondral bone structure changes the stress distribution on the articular cartilage and leads to its degeneration. Thus, inhibition of TGFβ activity in the subchondral bone may provide a new avenue of treatment for OA. In this review, we will respectively discuss the role of TGFβ in homeostasis of articular cartilage and subchondral bone as a novel target for OA therapy.
Keywords: Osteoarthritis, TGFβ, Subchondral bone, Articular cartilage
Current understanding of osteoarthritis and treatment
Osteoarthritis (OA) is a noninflammatory degenerative joint disease and the leading cause of physical disability1. There are approximately 27 million people that are suffering with this disease in the USA alone2. OA represents an enormous societal burden that increases greatly as the population ages. Clinically, OA is described by joint pain and functional impairment including tenderness and limitation of movement3; pathologically, OA is characterized by degeneration of cartilage, sclerosis of subchondral bone and marginal osteophytes (Glossary) 4. Preclinical and clinical studies have primarily focused on articular cartilage for decades. Various signaling mechanisms have been suggested to be responsible for the degeneration of articular cartilage, including complement C5, hypoxia-inducible factor-2α, syndecan-4, in addition to the well established ADAMTS5 and matrix metalloproteinase 13 (MMP13) 4–13. Accordingly, a wide array of agents have been designed and tested in different clinical trials, including glucosamine sulfate, chondroitin sulfate, sodium hyaluronan, doxycycline, and MMP inhibitors14. Although various levels of efficacy of these interventions have been reported, none of them successfully ceased OA progression or reverse the pathological changes. To date, OA is still treated by medications and life-style modifications to alleviate pain and reduce functional impairment in clinics14. OA management guidelines advocate the use of acetaminophen, NSAIDs, serotonin/norepinephrine reuptake inhibitors and opioids. When pain becomes disabling, surgery may be performed, such as arthroscopy, osteotomy, joint resurfacing, or whole joint replacement15–16.
The dilemma in OA treatment is that targeting of articular cartilage alone may not be sufficient to halt disease progression. Indeed, increasing evidence indicates that articular cartilage and subchondral bone act in concert as a functional unit17. Articular cartilage prevents biomechanical damage caused by severe loading, whereas its homeostasis and integrity relies on the biochemical and biomechanical interplay with subchondral bone17. Because of the relatively greater stiffness and strength in comparison with the overlying articular cartilage, the subchondral bone absorbs most of the mechanical force transmitted by diarthrodial joints and provides the mechanical support for overlying articular cartilage18–19. Relative to the slower turnover rate of articular cartilage, subchondral bone undergoes more rapid modeling and remodeling in response to the changes of the mechanical environment20. The reduced ability of subchondral bone to dissipate the load would be expected to alter the stress distribution on articular cartilage and signaling pathways in chondrocytes in maintaining cartilage homeostasis21. It is therefore reasonable to consider osteoarthritis not as simply a disease of cartilage. Transforming growth factor β (TGFβ) is a homeostasis regulator for both subchondral bone and articular cartilage, and increasing evidence indicates altered TGFβ signaling is involved in the pathogenesis of OA development. In this review, we describe the role of TGFβ in maintaining homeostasis of subchondral bone, articular cartilage. Alterations of TGFβ signaling in these tissues impair their integrity as a function unit and initiate osteoarthritic pathology. The potential and associated challenges in the development and application of therapy targeting TGFβ signaling are also discussed.
Temporal-spatial activation of extracellular matrix latent TGFβ
There are more than 40 members in the TGFβ superfamily, which is further classified into four major subfamilies22–23. The TGFβ subfamily contains three closely related mammalian isoforms, TGF-β1, -β2 and -β3, that all function through the same receptor signaling systems24–25. TGFβs are different from other cytokines and factors in that, upon secretion, they are deposited into the extracelluar matrix (ECM) of different tissues in an inactive, latent form. TGFβ is synthesized as a large precursor molecule which forms a homodimer that interacts with two other polypeptides, latent TGFβ binding protein (LTBP) and latency-associated peptide (LAP), forming a complex named large latent complex (LLC). The LAP is noncovalently linked to active TGFβ, masking the receptor-binding domains of the TGFβ and rendering it inactive26–29. Storage of inactive TGFβs in the matrix enables temporal-spatial regulation of TGFβ activation during tissue homeostasis. Precise activation of latent TGFβ is the prerequisite for it to function in the right locations at a specific time. The TGFβ activation process involves the release of the LLC from the ECM, followed by further proteolysis of LAP to release active TGFβ to its receptors28. There are distinct mechanisms employed in activation of TGFβ in different tissues such as proteolytic cleavage and interaction with integrins30–31. Proteolytic cleavage of LLC and liberation of active TGFβs can be performed by a variety of MMPs, plasmin, plasminogen activators, thrombin, elastase32–37. Independently from proteolytic cleavage, interaction between LAP-β1 and thrombospondin (TSP) 1 and the mannose-6-phosphate receptor also promote latent TGF-β1 activation31, 38. In platelets, a furin-like proprotein convertase appears to extracellularly activate latent TGFβ1 independently from any of the above mentioned mechanisms39. Integrin αvβ5, αvβ6, αvβ8 and an unidentified β1 integrin and possibly αvβ3 integrin have been reported to participate in activating latent TGFβ140–42. Interestingly, all of these identified integrins that be able to active TGFβ share the αv subunit and recognize the same RGD peptide motif of LAP. These data suggest that αv subunit is the key component for TGFβ activation. Targeting the αv-containing integrin mediated molecular pathway could have clinical utility in the treatment of high TGFβ induced disorders.
During tissue injury or remodeling, TGFβs in the matrix are activated and then signal to recruit stem cells for tissue repair. Adult tissues often harbor resident stem cells or progenitor cells for tissue homeostasis43. TGFβs, in consultation with the other signals, appear to regulate stem cells’ decision in differentiation or self-renew.44–47. Mutations in the extracellular proteins that result in premature activation of TGFβs often lead to skeletal disorders such as Camurati-Engelmann disease (CED), Marfan syndrome (MFS), Loeys-Dietz syndrome (LDS) and Shprintzen- Goldberg syndrome (SGS)48–52. Constitutive activation of TGFβ is also associated with tissue fibrosis. Thus, appropriate spatio-temporal TGFβ function is clearly crucial for maintaining healthy skeletal tissue.
Activation latent TGFβ in the matrix maintains bone homeostasis during remodeling
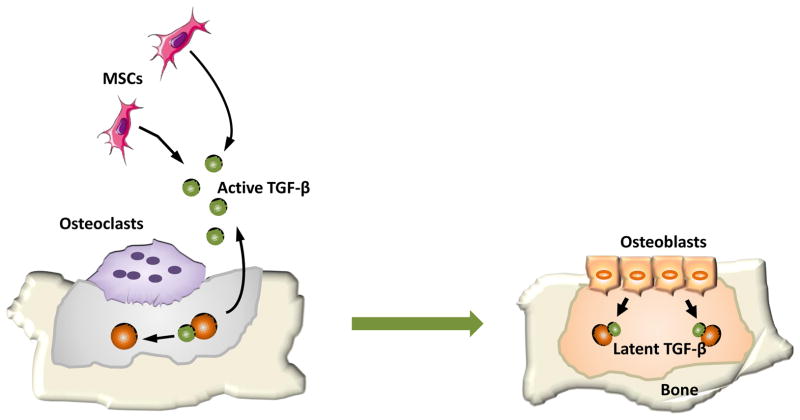
Adult bone is a dynamic tissue in constant remodeling that continuously being formed and resorbed. The remodeling process is necessary to maintain the structural integrity of the skeleton and allows the repair of tissue damage and homeostasis of calcium and phosphorous metabolism53. This bone remodeling is accomplished by precise coordination of osteoblasts and osteoclasts54. Bone resorption and formation do not occur randomly along the bone surface. Rather, they occur at specific anatomical sites and follow a well-defined sequence of events, the bone remodeling cycle, to maintain bone homeostasis55. It has been demonstrated that active TGFβ1 that released during osteoclast bone resorption directs the migration of mesenchymal stem cells (MSCs) to form the new bone at the resportion site56 (Figure 1). The newly recruited MSCs undergo a cell-lineage specific differentiation that is defined by signals in the microenvironment at the resorptive sites. Both physical properties of the fresh resorption site and soluble factors released from matrix contribute to the differentiation of MSCs. Once the bone mineral matrix is exposed by osteoclast bone resorption, the stiff microenvironment on the rough bare matrix, which lacks a lining of cell coverage, can facilitate the commitment of MSCs into osteoblasts57. Also, osteotropic factors including insulin-like growth factor-1 (IGF-1)s and platelet-derived growth factor (PDGF)s released from exposed bone further stimulates the differentiation of MSCs into osteoblast lineage cells.
Figure 1. Active TGF-β released during bone resorption coordinates bone formation by inducing migration of bone marrow MSCs (mesenchymal stem cells).
Under normal circumstances, TGFβ is stored in the bone matrix in a latent form. During osteoclast bone resorption, active TGFβ is freed from latent protein and diffuses to the marrow cavity. Following the gradient of active TGFβ, bone marrow MSCs are recruited to the bone resportion site. The MSCs then differentiate to osteoblasts and form new bone to fill the resorbed bone cavities.
Accumulating evidence indicates that high levels of active TGFβ in subchondral bone disrupt joint homeostasis and integrity. TGFβ was found to be aberrantly elevated in OA subchondral bone in both human specimen and various animal models58. Abnormal subchondral bone structure and degeneration of articular cartilage were observed in transgenic mice in which active TGFβ1 is constitutively expressed by osteoblastic cells56, 58. Genetically, gain-of-function of Smad3 mutations has been linked with the incidence of hip and knee OA and early onset of this disease59–60. Aberrant elevation of active TGFβ1in subchondral bone is associated with early signs of OA including bone marrow lesions (BMLs)58. High levels of active TGFβ1 induce clustering of MSCs/osteoprogenitor in the subchondral bone marrow and formation of marrow osteoid islets. Indeed, OA progression was attenuated in the mouse anterior cruciate ligament transection (ACLT) model when the TGFβ type II receptor was deleted in MSCs58.
Dynamic changes in bone microenvironment during bone remodeling
It is known that bone marrow has an organized and structured architecture, and the behavior of MSCs is precisely regulated in this highly dynamic microenvironment. MSCs serve to replenish the differentiated compartment of various cell types for bone formation, angiogenesis, adipogenesis and chondrogenesis61–62, 43, 63–65. Extrinsic signals in the surrounding microenvironment that are transmitted to the stem cell niche substantially influence MSC self-renewal or differentiation. TGFβ regulates stem cell quiescence through its direct or indirect effects in modulating the bone marrow microenvironment66. In the context of different mophogenetic events, epithelial cells undergo epithelial to mesenchymal transition (EMT) which recapitulated under pathological conditions such as fibrosis and metastasis of carcinomas67,68. Recently, endothelial to mesenchymal transition (EndoMT) has emerged as another possible source of tissue myofibroblasts. TGFβ signaling has been shown to play an important role in both EMT and EndoMT67, 69. In a microenvironment with aberrant elevated active TGFβs, MSCs are recruited in the marrow to form osteoid islets and agiogensis. TGFβ was found to be associated with almost all histological characteristics of BML such as less-well mineralized bone, increased marrow perfusion, and marrow fibrosis70–72. Increased angiogenesis in OA subchondral bone provide resources of epithelia and endothelia. Whether EMT is involved in TGFβ-induced MSC clustering and BML formation is worthy of further investigation.
Activation of matrix TGFβ in articular cartilage homeostasis
The indispensable role of TGFβ in maintenance of articular cartilage metabolic homeostasis and structural integrity has been well established73. TGFβ stimulates early events in chondrogenesis, including chondrogenic condensation, chondroprogenitor cell proliferation and differentiation74–77. It also inhibits terminal differentiation of chondrocytes, thereby blocking cartilage matrix calcification and vascular to maintain extracellular matrix (ECM) integrity78. Interruption of TGFβ signaling in the articular cartilage results in loss of proteoglycans and cartilage degeneration79. The effects of TGFβ on articular cartilage can be regulated at different levels: activation of matrix latent TGFβ, expression of different receptors as well as downstream intracellular signaling components. Dysregulation of any factor involved in TGFβ signaling transduction may affect cartilage integrity.
The extracellular matrix of cartilage stores abundant latent TGFβ (~300 ng/ml) that fulfill the needs for sufficient supply of active TGFβ80. Exogenous active TGFβ has limited effects on articular cartilage81–83. Factors that participated in the activation process of latent TGFβ are often found to be disregulated in OA. The expression of LTBPs is upregulated in both mouse OA model and human OA cartilage84–86. OA phenotypes are also observed in LTBP-3 knockout mice similar to impaired TGFβ signaling mouse models87–88. Most of the factors and mechanisms have been implicated in the activation process of TGFβ in other tissue and organ also applies to articular cartilage, such as MMPs-, Furin, Transglutaminase, Plasmin, TSP-1 and lysophospholipid32–33, 89–92. However, the precise context and mechanistic details in activation of cartilage matrix TGFβ are still unclear.
Articular cartilage is a tissue that resistant to mechanical stress and undergoes atrophy with loading deprivation93. Physiological mechanical stimulation promotes chondrocyte ECM protein synthesis critical for maintenance of articular cartilage function and integrity94. The effect of shear stress induces an increase of protein synthesis in the superficial zone of articular cartilage, which can can be abolished by treating with TβRI specific inhibitor95–96. TGFβ also mediate shear force-stimulated chondrocyte proliferation. These findings indicate the important role of TGFβ signaling in the mechanism of chondrocyte mechanotransduction. Combined with the finding that the latent TGFβ can be activated by shearing forces in synovial fluid97, it is likely that the TGFβ activation process in articular cartilage is directly or indirectly regulated by mechanical stress. The fact that cells can activate TGFβ in their surrounding ECM through integrin-mediated contractile forces98–99 implicates a potential mechanism of TGFβ activation by chondrocytes in response to mechanical stress. Thus, integrins may mediate chondrocyte-activation of TGFβ, which is known to stimulate expression of integrins100–102. In addition, the integrins appears to play a role in mediating chondrocyte response to TGFβ via regulating its adhering capacity to the type II collagen100–102. It would be interesting to investigate whether integrins mediate TGFβ activation via cell-matrix interactions and the potential positive feedback loop between them in articular cartilage.
TGFβ expression is upregulated in the early phase of OA which stimulates chondrocyte proliferation and proteoglycan synthesis in attempting to repair injured cartilage103–104. Yet the response of chondrocytes to TGFβ also relies on their differentiation status and TGFβ receptors expression conditions105. In general, TGFβ signals via heteromeric complexes of two related transmembrane type I and type II serine/threonine kinase receptors that activate smad-dependent gene transcription. Rather than a TβRII unique to its ligand, the TGFβ type I receptors, also termed activin receptor-like kinases (ALKs), act downstream of type II receptors and determine receptor specificity106. SMAD2 and SMAD3 are substrates of ALK5, whereas ALK1 utilize SMAD1, SMAD5 and SMAD8. During degeneration of articular cartilage, TGFβ signaling pathways are dysregulated with differential expression of TGFβ receptors in the chondrocytes. The mRNA level for TGFβ receptor II was dramatically reduced at early stage OA in a rabbit model107. Expression of mutant TGFβ type II receptor (TβRII) promotes terminal chondrocyte differentiation108. Increased TβRII degradation and down-regulated TβRI expression lead to decreased sensitivity of articular chondrocytes to TGFβ and accelerate OA development109–111. Conditional deletion of Smad3 in chondrocytes induced Runx2 expression and ended up with cartilage degeneration73, 112.
Moreover, TGFβ was found to signal not only via activin receptor-like kinase 5 (ALK5)-induced Smad2/3 phosphorylation, but also via ALK1-induced Smad1/5/8 phosphorylation113. These two main intracellular signaling pathways are often found to act in an opposing or even antagonizing fashion114. Both OA and the aging process itself change the pattern of TβRI expression115. In a shift to dominant usage of the receptor from ALK5 to ALK1, TGFβ stimulates the catabolic pathway in chondrocytes111. Therefore, TGFβ may actas a double edged saw; it is anabolic when signaling through ALK5 in maintenance of articular cartilage homeostasis and catabolic when ALK1 expression is upregulated during progression of OA105. A specific ALK1 antagonist could be promising to reduce progression of OA.
In addition, TGFβ co-receptors betaglycan (also termed type III TGFβ receptor), endoglin (CD105) and CD109116 are emerging as important regulators of TGFβ signaling. Endoglin is a transmenbrane glycoprotein that facilitates TGFβ binding to TβRII with preferential recruitment of ALK1117. Betaglycan, a homologue of endoglin, has been shown to direct clathrin-mediated endocytosis of TβRI and TβRII, and enhance TGFβ signaling via Smad and MAP kinase pathways118–120. Betaglycan also increases the sensitivity of TβRII to its ligands and equalizes the affinities across TGFβ isoforms, thus maximizing TGFβ signaling121. CD109 has been identified as a TGFβ co-receptor and inhibiting Smad2/3 signaling by promoting TGFβ receptor internalization and degradation in a Smad7/Smurf2-dependent manner122. Thus, Endoglin, Betaglycan and CD109 may also be considered as potential pharmaceutical targets for OA treatment.
High level of active TGFβ in the subchondral bone at onset of OA
The structure of bone dynamically changes in response to mechanical loading, particularly, when joint stability is decreased in patients during aging or with ligament injury or obesity. Recent studies show that osteocytes regulate the dynamic nature of bone through diverse functions123. Osteocytes are now recognized as the principal sensors for mechanical loading and are able to transduce mechanical signals into biological responses124. The activities of both osteoblasts and osteoclasts are regulated by the signaling molecules that released by osteoblasts and osteocytes such as osteoprotegerin (OPG), receptor activator of nuclear factor-κB ligand (RANKL) and sclerostin123. Alterations in the RANKL/OPG ratio are central in the pathogenesis of bone loss. Denosumab, a monoclonal antibody to RANKL used in the treatment of OA, mimics the function of OPG to induce a sustained inhibition of bone resorption125 and improve the bone structure 126. The finding that osteocytes express a much higher amount of RANKL and have a greater capacity to support osteoclastogenesis than osteoblasts and bone marrow stromal cells provides functional evidence that osteocytes control osteoclastogenesis127–128. Therefore, elevated osteoclast activity and turnover rate in OA subchondral bone could be one of the responses of osteocytes to aberrant mechanical loading. Indeed, osteoclastic bone resorption in the subchondral bone was significantly increased as early as 7 days post surgery of ACLT OA mice58. In parallel, large quantity of active TGFβ1 released to the marrow during subchondral bone resorption recruits nestin+ MSCs to form marrow osteoid islets and angiogenesis58. Notably, osteoclastic bone resorption was uncoupled with TGFβ1-induced recruitment of MSCs in the marrow where they undergo aberrant bone formation. Such responses of subchondral bone alter its microarchitecture and functional integrity with articular cartilage129. This notion was substantiated by the development of osteoarthritic-like changes in a transgenic mouse model with osteoblastic expression of active TGFβ158.
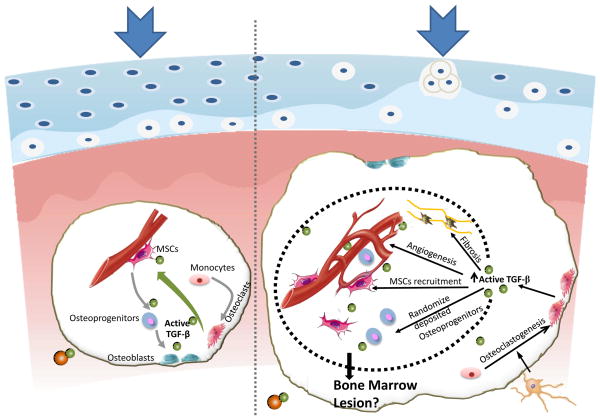
The subchondral bone volume and subchondral bone plate (SBP) thickness fluctuated substantially in ACLT rodent models130. In human osteoarthritis joints, SBP is markedly thicker relative to those of healthy subjects. It is likely that the formation of osteoid islets and abnormal bone formation induced by TGFβ1 changes micro-architecture of subchondral bone58. The changes in subchondral bone structure and stiffness may diminish structural support for the overlying cartilage (Figure. 2). For example, expansion of 1–2% subchondral bone significantly changes the distribution of articular cartilage stress in a computerized simulation model for human knee joints. The normal function of articular cartilage relies on the structural integrity and biochemical composition of the extracellular matrix, mainly collagen and proteoglycan. In an mechanical active environment, the balance and organization of these extracellualr matrix macromolecules may be disrupted when biomechanical factors in articular cartilage are altered. Indeed, abnormal mechanical stress induced cartilage structural damage and morphological changes such as clefts, proteoglycan loss and collagen breakdown, have been well documented in previous literatures. Cell death, water content and fibronectin content in the cartilage explants were increased in a load duration and magnitude dependent manner131. Vigorous cyclic loading leads to cartilage matrix damage such as collagen fiber broken and proteoglycan depletion possibly due to increased MMP-3132. Although intermittent articular loading seems to be necessary for normal cartilage metabolism, abnormal loading patterns likely activate TGFβ irregularly which further induce progressive cartilage degeneration133. Therefore, the fluctuation of subchondral bone mechanical property inevitably influences its capacity to dissipate the mechanical stimuli from the joint surface and consequently leads to cartilage degeneration in OA.
Figure 2. Association of articular cartilage degeneration and pathological changes in subchondral bone at onset of OA.
Left panel: Maintenance of homeostasis in articular cartilage and subchondral bone at normal conditions. Right panel: Increased bone turn-over at onset of OA results in elevated active TGFβ levels in subchondral bone. Increased active TGFβ stimulates angiogenesis, marrow fibrosis, clustering of MSC and osteoprogenitors. These cellular pathologies further lead to uncoupled bone remodeling and potentially bone marrow lesion formation. Disrupted architecture of subchondral bone changes its mechanical property and the reduced ability of subchondral bone to dissipate the load contribute to the articular cartilage degeneration.
Modulation of TGFβ activity in subchondral bone as a potential therapy for OA
Several studies of human OA have pointed to subchondral bone as a site for pharmaceutical intervention. Increased osteoclast activity and bone turnover rate are known pathological characteristics of subchondral bone in OA, particularly at early stage. For this reason, the common anti-resorptive medicine, bisphosphonate, has been tested its efficacy for treating OA in many clinical trials134. Though the outcome in human subjects was not as encouraging as in animal OA models135–139, specific drugs within the bisphosphonate class did show benefit effects in a few human studies. In the most recent prospective 2-year trial, alendronate treatment successfully improved WOMAC pain score, decrease in biochemical markers in hip osteoarthritis patients140. Elderly women being treated with alendronate had a significantly decreased prevalence of knee OA-related subchondral bone lesion and associated with a reduction in knee pain141. However, in the recent studies, risedronate failed to improve signs or symptoms of osteoarthritis or alter progression of OA, although a reduction in the level of a marker of cartilage degradation was observed142–143. Based on these findings, alendronate seems to be more effective than risedronate for treating OA patients. Yet differences in the study design, such as duration of bisphosphonate use, the dose and route of administration may also affect the results. Moreover, X-ray progression of joint space narrowing may not be sensitive enough to be used in end point judgment. More sensitive and reliable parameters such as BMLs should be considered as end point definition. Future more targeted studies are required to appreciate the value of bisphosphonates in treating osteoarthritis.
Although the approved bisphosphonates differ in structure and activity, they all inhibit osteoclast bone resorption144–145, a process that allows active TGFβ to be released from bone matrix. Aberrantly activated TGFβ signaling in subchondral bone was found to contribute to OA progression. High levels of active TGFβ were detected in subchondral bone through osteoclast bone resorption at the onset of OA in animal models. Inhibiting bone resorption prevented subsequent activation of TGFβ from matrix. This at least partially suggests a rationale for treating OA with bisphosphonates. Indeed, inhibition of TGFβ signaling in subchondral bone attenuated degeneration of articular cartilage in the ACLT OA rodent models58. However, as a critical growth factor, TGFβ plays important role in a wide range of biological processes such as growth inhibition, cell migration, invasion, epithelial–mesenchymal transition (EMT) and immune-regulation. Inhibiting TGFβ activity systemically may therefore affect tissue homeostasis in other organs including articular cartilage. Thus, tissue-oriented therapy that specifically inhibits TGFβ activity in subchondral bone would be a novel approach for treating OA.
High levels of active TGFβ alter the microenvironment of subchondral bone, leading to formation of cluster of osteoprogenitors, osteroid islets and increased angiogenesis. Improving the osteogenic microenvironment may help restore coupling by enhancing the osteogenic potential of MSCs during the reversal phase of bone remodeling, as another potential therapeutic approach. As a hormone that developed during evolution for vertebrates to adapt their terrestrial life, parathyroid hormone (PTH) regulates bone remodeling and improves marrow environment by orchestrating signaling of local factors, including TGF-β, Wnts, bone morphogenetic protein (BMP), and IGF-1146–148. During the interactions with TGFβ signaling pathway, PTH induces the recruitment of TβRII as an endocytic activator. TβRII directly phosphorylates the cytoplasmic domain of PTH 1 receptor (PTH1R) and facilitates PTH-induced endocytosis of the PTH1R-TβRII complex in downregulation of TGF-β signaling146. PTH also stimulates the commitment of MSCs to the osteoblast lineage by enhancing BMP and Wnt signaling148. Moreover, PTH has been shown to spatially relocating small blood vessels closer to sites of new bone formation, likely secondary to PTH-mediated upregulation of VEGFA and neuropilin 1 and 2149. In addition, PTH has been shown to induce cartilage matrix synthesis, suppress chondrocytes hypertrophy and reduce progression of OA in different animal models150–151. Thus, PTH’s beneficial effects on both articular cartilage and subchondral bone implicate its potential to be developed as a pharmaceutical intervention for OA.
Concluding remarks
Articular cartilage and subchondral bone constantly interact as a functional unit during joint movements. TGFβ plays a critical role in maintenance of both bone and articular cartilage homeostasis. Aberrant activation TGFβ1 in the subchondral bone leads to abnormal bone remodeling and formation of marrow osteroid islets. Importantly, the abnormal subchondral bone structure alters the stress distribution on the articular cartilage and results degeneration of articular cartilage. The concept of the wholism is essential for exploring the therapeutic strategies for OA. Improving mechanical properties of subchondral bone and its physiological function is at least equally important to directly targeting articular cartilage. Therapies that attenuate TGF-β signaling, either directly by neutralizing TGF-β activity or indirectly by PTH-mediated modulation of the bone marrow microenvironment, may serve as potential therapies for these joint disorders. OA is a disease of the whole joint. Therefore, pharmacological interventions that improve interaction between subchondral bone and articular cartilage and their homeostasis could be effective disease modifying treatment for OA.
Highlights.
Articular cartilage and subchondral bone act as a function unit.
TGFβ maintains homeostasis of articular cartilage and subchondral bone.
Spatial and temporal activation of TGFβ is the prerequisite for its function.
Aberrant activation of TGFβ contributes to onset of osteoarthritis.
Improvement of microenvironment in subchondral bone has potential for OA treatment.
GLOSSARY
- ADAMTS
Abbreviation of “a disintegrin and metalloproteinase with thrombospondin motifs”. A family of peptidase function to process of procollagens and von Willebrand factors as well as cleavage of aggrecan, versican, brevican and neurocan
- Arthroscopy
A minimally invasive surgical procedure in which an examination and sometimes treatment of damage of the interior of a joint is performed using an arthroscope, a type of endoscope that is inserted into the joint through a small incision
- Articular cartilage
Cartilage that covers the articular surfaces of bones
- Bone remodeling
A lifelong process where maturebone tissue is removed from the skeleton (a process called bone resorption) and new bone tissue is formed (a process called new bone formation). These processes control the reshaping or replacement of bone following injuries like fractures but also micro-damage, which occurs during normal activity. Remodeling responds also to functional demands of the mechanical loading
- Bone marrow lesions
Ill-defined hyperintensities seen on short T1 inversion-recovery images and on fat-suppressed proton density and T2-weighted fast spin echo magnetic resonance images
- Bone mineral density (BMD)
A medical term normally referring to the amount of mineral matter per square centimeter of bones. BMD is used in clinical medicine as an indirect indicator of osteoporosis and fracture risk
- Chondrocyte
The only cells found in healthy cartilage. They produce and maintain the cartilaginous matrix, which consists mainly of collagen and proteoglycans
- Collagen
An insoluble fibrous protein of vertebrates that is the chief constituent of the fibrils of connective tissue and of the organic substance of bones and yields gelatin and glue on prolonged heating with water
- Complement
The thermolabile group of proteins in normal blood serum and plasma that in combination with antibodies causes the destruction especially of particulate antigens
- Diarthrodial joints
The most common and movable type of joint which is characterized by the presence of a layer of fibrocartilage or hyaline cartilage that lines the opposing bony surfaces, as well as a lubricating synovial fluid within the synovial cavity
- Elastase
An enzyme especially of pancreatic juice that digests elastin
- Extracelluar matrix
Extracellular part of multicellular structure that typically provides structural and biochemical support to the surrounding cells
- Fibronectin
A group of glycoproteins of cell surfaces, blood plasma, and connective tissue that promote cellular adhesion and migration
- Glycosidases
An enzyme that catalyzes the hydrolysis of a bond joining a sugar of a glycoside to an alcohol or another sugar unit
- Matrix metalloproteinase
A group of zinc-dependent endopeptidases that capable of degrading extracellular matrix proteins and process a number of bioactive molecules
- Mesenchymal stem cell
Mutipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts, chondrocytes and adipocytes
- Mitogen
A substance that induces mitosis
- OPG
osteoprotegerin. a secreted member of the TNF receptor superfamily that negatively regulates osteoclastogenesis. It is a soluble decoy receptor of RANKL that inhibits both cell differentiation and function of osteoclasts by inhibiting the interaction between RANKL and RANK
- Osteoblast
Cell with single nuclei that synthesize bone
- Osteoclast
The large multinucleate cells closely associated with areas of bone resorption
- Osteocyte
Cell that is characteristic of adult bone and is isolated in a lacuna of the bone substance
- Osteoid
Unmineralized, organic portion of the bone matrix that forms prior to the maturation of bone tissue
- Osteoid islet
Pathological changes in the subchondral bone marrow cavity with under-mineralized osteoid-like structure containing osterix+ osteoprogenitors, fibrous tissue and vasculature
- Osteophyte
A pathological bony outgrowth; bony projections that form along joint margins
- Osteoporosis
A condition that affects especially older women and is characterized by decrease in bone mass with decreased density and enlargement of bone spaces producing porosity and brittleness
- Osteotomy
A surgical operation in which a bone is divided or a piece of bone is excised (as to correct a deformity)
- Plasmin
A proteolytic enzyme that originally found to be able to dissolves the fibrin of blood clots
- Plasminogen
The precursor of plasmin that is found in blood plasma and serum; also name as profibrinolysin
- Proteoglycan
A class of glycoproteins of high molecular weight that are found in the extracellular matrix of connective tissue. Proteoglycan are made up mostly of carbohydrate consisting of various polysaccharide side chains linked to a protein, and resemble polysaccharides rather than proteins in their properties
- RANKL
Receptor activator of nuclear factor kappa-B ligand. A transmembrane protein belonging to the tumor necrosis factor superfamily that specifically binds receptor activator of nuclear factor -kappa Band osteoprotegerin. It plays an important role in regulating osteoclast differentiation and activation
- Stem cell
An unspecialized cell population that gives rise to differentiated cells
- Subchondral bone
The layer of bone just below the cartilage which provide support for the cartilage of the articular surface
- Subchondral bone Plate
The bone structure that immediately beneath the calcified cartilage. which is a 1–3 mm thick plate of corticalized bone that is physiologically and mechanically similar to cortical bone in other skeletal locations, but somewhat less stiff than diaphyseal cortical bone
- Synovial fluid
A transparent viscid lubricating fluid secreted by a membrane of an articulation, bursa, or tendon sheath
- Thrombin
A proteolytic enzyme formed from prothrombin that facilitates the clotting of blood by catalyzing conversion of fibrinogen to fibrin and that is used in the form of a powder as a topical hemostatic
- Thrombosondin
A secreted protein with antiangiogenic abilities
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kean WF, et al. Osteoarthritis: symptoms, signs and source of pain. Inflammopharmacology. 2004;12:3–31. doi: 10.1163/156856004773121347. [DOI] [PubMed] [Google Scholar]
- 4.Pitsillides AA, Beier F. Cartilage biology in osteoarthritis--lessons from developmental biology. Nat Rev Rheumatol. 2011;7:654–663. doi: 10.1038/nrrheum.2011.129. [DOI] [PubMed] [Google Scholar]
- 5.Aigner T, et al. Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis--structure, chaos and senescence. Nat Clin Pract Rheumatol. 2007;3:391–399. doi: 10.1038/ncprheum0534. [DOI] [PubMed] [Google Scholar]
- 6.Kizawa H, et al. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet. 2005;37:138–144. doi: 10.1038/ng1496. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 9.Echtermeyer F, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 10.Glasson SS, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 11.Neuhold LA, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, et al. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci. 2011;1240:61–69. doi: 10.1111/j.1749-6632.2011.06258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee AS, et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527:440–447. doi: 10.1016/j.gene.2013.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter DJ. Pharmacologic therapy for osteoarthritis--the era of disease modification. Nat Rev Rheumatol. 2011;7:13–22. doi: 10.1038/nrrheum.2010.178. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg VM, et al. Recommendations of the OARSI FDA Osteoarthritis Devices Working Group. Osteoarthritis Cartilage. 2011;19:509–514. doi: 10.1016/j.joca.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–49. doi: 10.1038/nrrheum.2010.197. [DOI] [PubMed] [Google Scholar]
- 18.Layton MW, et al. Examination of subchondral bone architecture in experimental osteoarthritis by microscopic computed axial tomography. Arthritis Rheum. 1988;31:1400–1405. doi: 10.1002/art.1780311109. [DOI] [PubMed] [Google Scholar]
- 19.Madry H. The subchondral bone: a new frontier in articular cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2010;18:417–418. doi: 10.1007/s00167-010-1071-y. [DOI] [PubMed] [Google Scholar]
- 20.Goldring SR. Alterations in periarticular bone and cross talk between subchondral bone and articular cartilage in osteoarthritis. Ther Adv Musculoskelet Dis. 2012;4:249–258. doi: 10.1177/1759720X12437353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burr DB. The importance of subchondral bone in osteoarthrosis. Curr Opin Rheumatol. 1998;10:256–262. doi: 10.1097/00002281-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Assoian RK, et al. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155–7160. [PubMed] [Google Scholar]
- 23.Burt DW, Law AS. Evolution of the transforming growth factor-beta superfamily. Prog Growth Factor Res. 1994;5:99–118. doi: 10.1016/0955-2235(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 24.Roberts AB, Sporn MB. Transforming growth factor beta. Adv Cancer Res. 1988;51:107–145. [PubMed] [Google Scholar]
- 25.Cupp AS, et al. Expression and action of transforming growth factor beta (TGFbeta1, TGFbeta2, and TGFbeta3) during embryonic rat testis development. Biol Reprod. 1999;60:1304–1313. doi: 10.1095/biolreprod60.6.1304. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009;21:616–622. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins G. The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol. 2008;40:1068–1078. doi: 10.1016/j.biocel.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Annes JP, et al. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 30.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 32.D’Angelo M, et al. Authentic matrix vesicles contain active metalloproteases (MMP). a role for matrix vesicle-associated MMP-13 in activation of transforming growth factor-beta. J Biol Chem. 2001;276:11347–11353. doi: 10.1074/jbc.M009725200. [DOI] [PubMed] [Google Scholar]
- 33.Maeda S, et al. The first stage of transforming growth factor beta1 activation is release of the large latent complex from the extracellular matrix of growth plate chondrocytes by matrix vesicle stromelysin-1 (MMP-3) Calcif Tissue Int. 2002;70:54–65. doi: 10.1007/s002230010032. [DOI] [PubMed] [Google Scholar]
- 34.Lyons RM, et al. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol. 1990;110:1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu TM, Kawinski E. Plasmin, substilisin-like endoproteases, tissue plasminogen activator, and urokinase plasminogen activator are involved in activation of latent TGF-beta 1 in human seminal plasma. Biochem Biophys Res Commun. 1998;253:128–134. doi: 10.1006/bbrc.1998.9760. [DOI] [PubMed] [Google Scholar]
- 36.Taipale J, et al. Release of transforming growth factor-beta 1 from the pericellular matrix of cultured fibroblasts and fibrosarcoma cells by plasmin and thrombin. J Biol Chem. 1992;267:25378–25384. [PubMed] [Google Scholar]
- 37.Karonen T, et al. Transforming growth factor beta 1 and its latent form binding protein-1 associate with elastic fibres in human dermis: accumulation in actinic damage and absence in anetoderma. Br J Dermatol. 1997;137:51–58. [PubMed] [Google Scholar]
- 38.Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A. 1991;88:580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blakytny R, et al. Latent TGF-beta1 activation by platelets. J Cell Physiol. 2004;199:67–76. doi: 10.1002/jcp.10454. [DOI] [PubMed] [Google Scholar]
- 40.Munger JS, et al. Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphavbeta1. Mol Biol Cell. 1998;9:2627–2638. doi: 10.1091/mbc.9.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munger JS, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 42.Mu D, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalinina NI, et al. Mesenchymal stem cells in tissue growth and repair. Acta Naturae. 2011;3:30–37. [PMC free article] [PubMed] [Google Scholar]
- 44.Watabe T, Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 45.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21:1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 46.Zhao L, Hantash BM. TGF-beta1 regulates differentiation of bone marrow mesenchymal stem cells. Vitam Horm. 2011;87:127–141. doi: 10.1016/B978-0-12-386015-6.00042-1. [DOI] [PubMed] [Google Scholar]
- 47.Studer D, et al. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. Eur Cell Mater. 2012;24:118–135. doi: 10.22203/ecm.v024a09. discussion 135. [DOI] [PubMed] [Google Scholar]
- 48.Kinoshita A, et al. Domain-specific mutations in TGFB1 result in Camurati-Engelmann disease. Nat Genet. 2000;26:19–20. doi: 10.1038/79128. [DOI] [PubMed] [Google Scholar]
- 49.Neptune ER, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 50.Mizuguchi T, et al. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet. 2004;36:855–860. doi: 10.1038/ng1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loeys BL, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 52.Doyle AJ, et al. Mutations in the TGF-beta repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat Genet. 2012;44:1249–1254. doi: 10.1038/ng.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 54.Mundy GR, Elefteriou F. Boning up on ephrin signaling. Cell. 2006;126:441–443. doi: 10.1016/j.cell.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 55.Cao X. Targeting osteoclast-osteoblast communication. Nat Med. 2011;17:1344–1346. doi: 10.1038/nm.2499. [DOI] [PubMed] [Google Scholar]
- 56.Tang Y, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Engler AJ, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 58.Zhen G, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loughlin J. Genetics of osteoarthritis. Curr Opin Rheumatol. 2011;23:479–483. doi: 10.1097/BOR.0b013e3283493ff0. [DOI] [PubMed] [Google Scholar]
- 60.van de Laar IM, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–126. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 61.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 62.Friedenstein AJ, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 63.Zaidi N, Nixon AJ. Stem cell therapy in bone repair and regeneration. Ann N Y Acad Sci. 2007;1117:62–72. doi: 10.1196/annals.1402.074. [DOI] [PubMed] [Google Scholar]
- 64.Gregory CA, et al. How Wnt signaling affects bone repair by mesenchymal stem cells from the bone marrow. Ann N Y Acad Sci. 2005;1049:97–106. doi: 10.1196/annals.1334.010. [DOI] [PubMed] [Google Scholar]
- 65.Fibbe WE. Mesenchymal stem cells. A potential source for skeletal repair. Ann Rheum Dis. 2002;61(Suppl 2):ii29–31. doi: 10.1136/ard.61.suppl_2.ii29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruscetti FW, et al. Autocrine transforming growth factor-beta regulation of hematopoiesis: many outcomes that depend on the context. Oncogene. 2005;24:5751–5763. doi: 10.1038/sj.onc.1208921. [DOI] [PubMed] [Google Scholar]
- 67.Xu J, et al. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bi WR, et al. Transforming growth factor-beta1 induced epithelial-mesenchymal transition in hepatic fibrosis. Hepatogastroenterology. 2012;59:1960–1963. doi: 10.5754/hge11750. [DOI] [PubMed] [Google Scholar]
- 69.Piera-Velazquez S, et al. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daheshia M, Yao JQ. The bone marrow lesion in osteoarthritis. Rheumatol Int. 2011;31:143–148. doi: 10.1007/s00296-010-1454-x. [DOI] [PubMed] [Google Scholar]
- 71.Hunter DJ, et al. Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res Ther. 2009;11:R11. doi: 10.1186/ar2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aaron RK, et al. Perfusion abnormalities in subchondral bone associated with marrow edema, osteoarthritis, and avascular necrosis. Ann N Y Acad Sci. 2007;1117:124–137. doi: 10.1196/annals.1402.069. [DOI] [PubMed] [Google Scholar]
- 73.Yang X, et al. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldring MB, et al. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 75.Onyekwelu I, et al. Chondrogenesis, joint formation, and articular cartilage regeneration. J Cell Biochem. 2009;107:383–392. doi: 10.1002/jcb.22149. [DOI] [PubMed] [Google Scholar]
- 76.Kawakami Y, et al. The role of TGFbetas and Sox9 during limb chondrogenesis. Curr Opin Cell Biol. 2006;18:723–729. doi: 10.1016/j.ceb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Quintana L, et al. Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering. Tissue Eng Part B Rev. 2009;15:29–41. doi: 10.1089/ten.teb.2008.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Kraan PM, et al. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: modulation and integration of signaling pathways through receptor-Smads. Osteoarthritis Cartilage. 2009;17:1539–1545. doi: 10.1016/j.joca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 79.Shen J, et al. Deletion of the Transforming Growth Factor beta Receptor Type II Gene in Articular Chondrocytes Leads to a Progressive Osteoarthritis-like Phenotype in Mice. Arthritis Rheum. 2013;65:3107–3119. doi: 10.1002/art.38122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morales TI, et al. Transforming growth factor-beta in calf articular cartilage organ cultures: synthesis and distribution. Arch Biochem Biophys. 1991;288:397–405. doi: 10.1016/0003-9861(91)90212-2. [DOI] [PubMed] [Google Scholar]
- 81.Albro MB, et al. Accumulation of exogenous activated TGF-beta in the superficial zone of articular cartilage. Biophys J. 2013;104:1794–1804. doi: 10.1016/j.bpj.2013.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hildebrand A, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302 (Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paralkar VM, et al. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev Biol. 1991;143:303–308. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- 84.Appleton CT, et al. Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum. 2007;56:1854–1868. doi: 10.1002/art.22711. [DOI] [PubMed] [Google Scholar]
- 85.Wei T, et al. Analysis of early changes in the articular cartilage transcriptisome in the rat meniscal tear model of osteoarthritis: pathway comparisons with the rat anterior cruciate transection model and with human osteoarthritic cartilage. Osteoarthritis Cartilage. 2010;18:992–1000. doi: 10.1016/j.joca.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 86.Aigner T, et al. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54:3533–3544. doi: 10.1002/art.22174. [DOI] [PubMed] [Google Scholar]
- 87.Dabovic B, et al. Bone abnormalities in latent TGF-[beta] binding protein (Ltbp)-3-null mice indicate a role for Ltbp-3 in modulating TGF-[beta] bioavailability. J Cell Biol. 2002;156:227–232. doi: 10.1083/jcb.200111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dabovic B, et al. Bone defects in latent TGF-beta binding protein (Ltbp)-3 null mice; a role for Ltbp in TGF-beta presentation. J Endocrinol. 2002;175:129–141. doi: 10.1677/joe.0.1750129. [DOI] [PubMed] [Google Scholar]
- 89.Moldovan F, et al. Modulation of collagenase 3 in human osteoarthritic cartilage by activation of extracellular transforming growth factor beta: role of furin convertase. Arthritis Rheum. 2000;43:2100–2109. doi: 10.1002/1529-0131(200009)43:9<2100::AID-ANR22>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 90.Rosenthal AK, et al. Participation of transglutaminase in the activation of latent transforming growth factor beta1 in aging articular cartilage. Arthritis Rheum. 2000;43:1729–1733. doi: 10.1002/1529-0131(200008)43:8<1729::AID-ANR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 91.Pedrozo HA, et al. Potential mechanisms for the plasmin-mediated release and activation of latent transforming growth factor-beta1 from the extracellular matrix of growth plate chondrocytes. Endocrinology. 1999;140:5806–5816. doi: 10.1210/endo.140.12.7224. [DOI] [PubMed] [Google Scholar]
- 92.Gay I, et al. Lysophospholipid regulates release and activation of latent TGF-beta1 from chondrocyte extracellular matrix. Biochim Biophys Acta. 2004;1684:18–28. doi: 10.1016/j.bbalip.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 93.Vanwanseele B, et al. Knee cartilage of spinal cord-injured patients displays progressive thinning in the absence of normal joint loading and movement. Arthritis Rheum. 2002;46:2073–2078. doi: 10.1002/art.10462. [DOI] [PubMed] [Google Scholar]
- 94.Zuscik MJ, et al. Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest. 2008;118:429–438. doi: 10.1172/JCI34174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Malaviya P, Nerem RM. Fluid-induced shear stress stimulates chondrocyte proliferation partially mediated via TGF-beta1. Tissue Eng. 2002;8:581–590. doi: 10.1089/107632702760240508. [DOI] [PubMed] [Google Scholar]
- 96.Neu CP, et al. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis Rheum. 2007;56:3706–3714. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- 97.Albro MB, et al. Shearing of synovial fluid activates latent TGF-beta. Osteoarthritis Cartilage. 2012;20:1374–1382. doi: 10.1016/j.joca.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishimura SL. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol. 2009;175:1362–1370. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aluwihare P, Munger JS. What the lung has taught us about latent TGF-beta activation. Am J Respir Cell Mol Biol. 2008;39:499–502. doi: 10.1165/rcmb.2008-0003ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yeh YC, et al. Transforming growth factor-{beta}1 induces Smad3-dependent {beta}1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am J Pathol. 2010;177:1743–1754. doi: 10.2353/ajpath.2010.091183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wahl SM, et al. Transforming growth factor beta enhances integrin expression and type IV collagenase secretion in human monocytes. Proc Natl Acad Sci U S A. 1993;90:4577–4581. doi: 10.1073/pnas.90.10.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee JW, et al. The involvement of beta1 integrin in the modulation by collagen of chondrocyte-response to transforming growth factor-beta1. J Orthop Res. 2002;20:66–75. doi: 10.1016/S0736-0266(01)00073-0. [DOI] [PubMed] [Google Scholar]
- 103.van der Kraan PM, et al. Early elevation of transforming growth factor-beta, decorin, and biglycan mRNA levels during cartilage matrix restoration after mild proteoglycan depletion. J Rheumatol. 1997;24:543–549. [PubMed] [Google Scholar]
- 104.Pombo-Suarez M, et al. Differential upregulation of the three transforming growth factor beta isoforms in human osteoarthritic cartilage. Ann Rheum Dis. 2009;68:568–571. doi: 10.1136/ard.2008.090217. [DOI] [PubMed] [Google Scholar]
- 105.van der Kraan PM, et al. Age-dependent alteration of TGF-beta signalling in osteoarthritis. Cell Tissue Res. 2012;347:257–265. doi: 10.1007/s00441-011-1194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heldin CH, et al. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 107.Boumediene K, et al. Decrease of cartilage transforming growth factor-beta receptor II expression in the rabbit experimental osteoarthritis--potential role in cartilage breakdown. Osteoarthritis Cartilage. 1998;6:146–149. doi: 10.1053/joca.1997.0104. [DOI] [PubMed] [Google Scholar]
- 108.Serra R, et al. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andriamanalijaona R, et al. Mediation of interleukin-1beta-induced transforming growth factor beta1 expression by activator protein 4 transcription factor in primary cultures of bovine articular chondrocytes: possible cooperation with activator protein 1. Arthritis Rheum. 2003;48:1569–1581. doi: 10.1002/art.11020. [DOI] [PubMed] [Google Scholar]
- 110.Bauge C, et al. Regulatory mechanism of transforming growth factor beta receptor type II degradation by interleukin-1 in primary chondrocytes. Biochim Biophys Acta. 2012;1823:983–986. doi: 10.1016/j.bbamcr.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 111.Blaney Davidson EN, et al. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Immunol. 2009;182:7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- 112.Chen CG, et al. Chondrocyte-intrinsic Smad3 represses Runx2-inducible matrix metalloproteinase 13 expression to maintain articular cartilage and prevent osteoarthritis. Arthritis Rheum. 2012;64:3278–3289. doi: 10.1002/art.34566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Finnson KW, et al. ALK1 opposes ALK5/Smad3 signaling and expression of extracellular matrix components in human chondrocytes. J Bone Miner Res. 2008;23:896–906. doi: 10.1359/jbmr.080209. [DOI] [PubMed] [Google Scholar]
- 114.Goumans MJ, et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 115.van der Kraan PM, et al. A role for age-related changes in TGFbeta signaling in aberrant chondrocyte differentiation and osteoarthritis. Arthritis Res Ther. 2010;12:201. doi: 10.1186/ar2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kirkbride KC, et al. Cell-surface co-receptors: emerging roles in signaling and human disease. Trends Biochem Sci. 2005;30:611–621. doi: 10.1016/j.tibs.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 117.Finnson KW, et al. Endoglin differentially regulates TGF-beta-induced Smad2/3 and Smad1/5 signalling and its expression correlates with extracellular matrix production and cellular differentiation state in human chondrocytes. Osteoarthritis Cartilage. 2010;18:1518–1527. doi: 10.1016/j.joca.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 118.McLean S, Di Guglielmo GM. TGF beta (transforming growth factor beta) receptor type III directs clathrin-mediated endocytosis of TGF beta receptor types I and II. Biochem J. 2010;429:137–145. doi: 10.1042/BJ20091598. [DOI] [PubMed] [Google Scholar]
- 119.Finger EC, et al. Endocytosis of the type III transforming growth factor-beta (TGF-beta) receptor through the clathrin-independent/lipid raft pathway regulates TGF-beta signaling and receptor down-regulation. J Biol Chem. 2008;283:34808–34818. doi: 10.1074/jbc.M804741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Santander C, Brandan E. Betaglycan induces TGF-beta signaling in a ligand-independent manner, through activation of the p38 pathway. Cell Signal. 2006;18:1482–1491. doi: 10.1016/j.cellsig.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 121.Lopez-Casillas F, et al. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991;67:785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- 122.Bizet AA, et al. The TGF-beta co-receptor, CD109, promotes internalization and degradation of TGF-beta receptors. Biochim Biophys Acta. 2011;1813:742–753. doi: 10.1016/j.bbamcr.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 123.Schaffler MB, Kennedy OD. Osteocyte signaling in bone. Curr Osteoporos Rep. 2012;10:118–125. doi: 10.1007/s11914-012-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klein-Nulend J, et al. Mechanosensation and transduction in osteocytes. Bone. 2013;54:182–190. doi: 10.1016/j.bone.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 125.Hamdy NA. Denosumab: RANKL inhibition in the management of bone loss. Drugs Today (Barc) 2008;44:7–21. doi: 10.1358/dot.2008.44.1.1178467. [DOI] [PubMed] [Google Scholar]
- 126.Bogado CE, et al. Denosumab: an update. Drugs Today (Barc) 2011;47:605–613. doi: 10.1358/dot.2011.47.8.1603507. [DOI] [PubMed] [Google Scholar]
- 127.Nakashima T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 128.O’Brien CA, et al. Osteocyte control of osteoclastogenesis. Bone. 2013;54:258–263. doi: 10.1016/j.bone.2012.08.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Burr DB, Radin EL. Microfractures and microcracks in subchondral bone: are they relevant to osteoarthrosis? Rheum Dis Clin North Am. 2003;29:675–685. doi: 10.1016/s0889-857x(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 130.Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8:665–673. doi: 10.1038/nrrheum.2012.130. [DOI] [PubMed] [Google Scholar]
- 131.Farquhar T, et al. Swelling and fibronectin accumulation in articular cartilage explants after cyclical impact. J Orthop Res. 1996;14:417–423. doi: 10.1002/jor.1100140312. [DOI] [PubMed] [Google Scholar]
- 132.Lin PM, et al. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthritis Cartilage. 2004;12:485–496. doi: 10.1016/j.joca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 133.Ni GX, et al. Matrix metalloproteinase-3 inhibitor retards treadmill running-induced cartilage degradation in rats. Arthritis Res Ther. 2011;13:R192. doi: 10.1186/ar3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cohen SB. An update on bisphosphonates. Curr Rheumatol Rep. 2004;6:59–65. doi: 10.1007/s11926-004-0084-2. [DOI] [PubMed] [Google Scholar]
- 135.Zhu S, et al. Alendronate protects against articular cartilage erosion by inhibiting subchondral bone loss in ovariectomized rats. Bone. 2013;53:340–349. doi: 10.1016/j.bone.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 136.Panahifar A, et al. Potential mechanism of alendronate inhibition of osteophyte formation in the rat model of post-traumatic osteoarthritis: evaluation of elemental strontium as a molecular tracer of bone formation. Osteoarthritis Cartilage. 2012;20:694–702. doi: 10.1016/j.joca.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 137.Shirai T, et al. Chondroprotective effect of alendronate in a rabbit model of osteoarthritis. J Orthop Res. 2011;29:1572–1577. doi: 10.1002/jor.21394. [DOI] [PubMed] [Google Scholar]
- 138.Zhang L, et al. Enhancement of subchondral bone quality by alendronate administration for the reduction of cartilage degeneration in the early phase of experimental osteoarthritis. Clin Exp Med. 2011;11:235–243. doi: 10.1007/s10238-011-0131-z. [DOI] [PubMed] [Google Scholar]
- 139.Hayami T, et al. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50:1193–1206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 140.Nishii T, et al. Alendronate treatment for hip osteoarthritis: prospective randomized 2-year trial. Clin Rheumatol. 2013;32:1759–1766. doi: 10.1007/s10067-013-2338-8. [DOI] [PubMed] [Google Scholar]
- 141.Carbone LD, et al. The relationship of antiresorptive drug use to structural findings and symptoms of knee osteoarthritis. Arthritis Rheum. 2004;50:3516–3525. doi: 10.1002/art.20627. [DOI] [PubMed] [Google Scholar]
- 142.Bingham CO, 3rd, et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 2006;54:3494–3507. doi: 10.1002/art.22160. [DOI] [PubMed] [Google Scholar]
- 143.Buckland-Wright JC, et al. A 2 yr longitudinal radiographic study examining the effect of a bisphosphonate (risedronate) upon subchondral bone loss in osteoarthritic knee patients. Rheumatology (Oxford) 2007;46:257–264. doi: 10.1093/rheumatology/kel213. [DOI] [PubMed] [Google Scholar]
- 144.Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Colucci S, et al. Alendronate reduces adhesion of human osteoclast-like cells to bone and bone protein-coated surfaces. Calcif Tissue Int. 1998;63:230–235. doi: 10.1007/s002239900519. [DOI] [PubMed] [Google Scholar]
- 146.Qiu T, et al. TGF-beta type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat Cell Biol. 2010;12:224–234. doi: 10.1038/ncb2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wan M, et al. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22:2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu B, et al. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. J Bone Miner Res. 2012;27:2001–2014. doi: 10.1002/jbmr.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Prisby R, et al. Intermittent PTH(1–84) is osteoanabolic but not osteoangiogenic and relocates bone marrow blood vessels closer to bone-forming sites. J Bone Miner Res. 2011;26:2583–2596. doi: 10.1002/jbmr.459. [DOI] [PubMed] [Google Scholar]
- 150.Orth P, et al. Parathyroid hormone [1–34] improves articular cartilage surface architecture and integration and subchondral bone reconstitution in osteochondral defects in vivo. Osteoarthritis Cartilage. 2013;21:614–624. doi: 10.1016/j.joca.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 151.Sampson ER, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011;3:101ra193. doi: 10.1126/scitranslmed.3002214. [DOI] [PMC free article] [PubMed] [Google Scholar]