Summary
Although thyroid abnormalities are reported with the use of tyrosine kinase inhibitors (TKI), patients rarely require replacement therapy. The initial multicenter studies of sunitinib for metastatic renal cancer did not report hypothyroidism in fatigued patients, and thyroid tests were not routinely monitored. More recent studies however, suggest up to 70% of patients develop thyroid test abnormalities during treatment with sunitinib. Despite these concerns, the clinical relevance of sunitinib-induced hypothyroidism is uncertain since thyroid gland recovery is the norm in most patients .We report a case of a patient with metastatic papillary renal cell cancer on combination anti-angiogenic therapy with sunitinib, who developed unusually high Thyroid Stimulating Hormone levels and severe symptoms despite receiving L-thyroxine. Our case also illustrates the complexity of managing sunitinib associated thyroid dysfunction, which may be accompanied by transient thyroiditis, hyperthyroidism and profound hypothyroidism.
Keywords: sunitinib, extreme hypothyroidism, symptoms
Introduction
Although thyroid abnormalities are reported with the use of tyrosine kinase inhibitors (TKI) such as imatinib, nilotinib, dasatinib1, and pazopanib2, patients rarely require replacement therapy and almost never need to discontinue the TKI. Sorafenib-induced hypothyroidism is reported to occur more frequently (in two thirds of patients with metastatic renal cancer), but only 5.8% develop clinically significant hypothyroidism requiring replacement therapy3.
There is evidence however, that sunitinib may induce thyroid atrophy, transient thyotoxicosis,4 and possibly irreversible thyroid damage5. Despite these concerns, questions have also been raised as to the clinical relevance6of sunitinib-induced hypothyroidism since thyroid gland recovery is the norm in most patients,7 who may experience only transient iodine uptake blockade.8 In addition, few patients develop severe symptoms that can be attributed directly to hypothyroidism. We report a case of a patient on combination anti-angiogenic therapy containing sunitinib, who developed unusually high TSH levels and severe symptoms despite receiving L-thyroxine supplementation.
Case
A 41-year-old female was referred to a comprehensive cancer center for evaluation of progressive metastatic papillary renal cell cancer type II. 12 months after nephrectomy, MRI of the thoracic-spine showed extensive infiltration of the bone marrow and imaging of her chest, abdomen and pelvis showed liver and bone metastases. At presentation she had a low hemoglobin of 10.2 g/dL, placing her in the intermediate-risk category according to Motzer criteria9 ( Karnofsky performance status,lactate dehydrogenase , hemoglobin, corrected serum calcium, and history of nephrectomy).
Over the preceding 6 months, she had reported a 5 pound weight loss, accompanied by insomnia and occasional fatigue. She had no family history of cancer or thyroid disease, but was found to be a carrier of a fumarate hydratase gene mutation (HIF1α), responsible for hereditary leiomyomatosis and renal cell cancer. Renal cell cancers associated with this mutation are very aggressive and often metastatic at presentation, even when the primary lesion is <1 cm.10
Despite trials of bevacizumab, her disease progressed and she was referred for a phase I trial combining sunitinb and AMG-386. Sunitinib was given in courses of 50 mg daily for 4 weeks on, followed by 2 weeks off plus AMG-386 at 10mg/kg once weekly. AMG 386 is an investigational peptide-Fc fusion protein (peptibody) which inhibits angiogenesis by preventing the interaction of angiopoietin-1 and angiopoietin-2 with their receptor11
Thyroid management
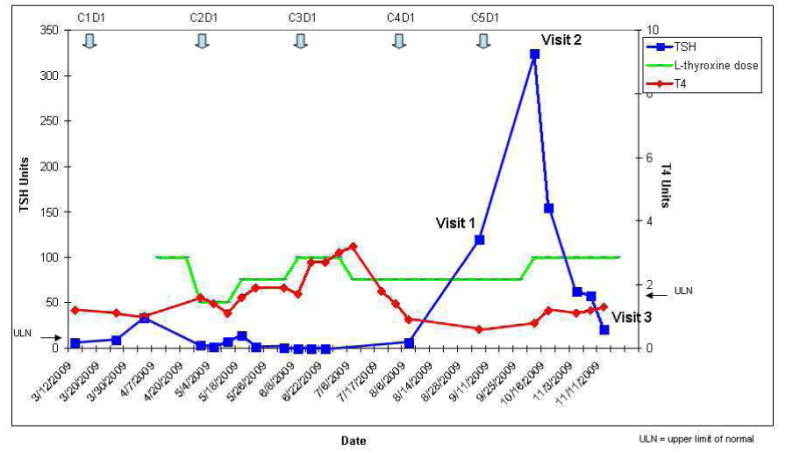
Her initial thyroid tests prior to sunitinib treatment (Figure 1 and 2) showed a marginally elevated thyroid stimulating hormone (TSH) level of 6.5 mU/mL with a normal free T4 and mild symptoms of fatigue. About 4 weeks after starting sunitinib, the TSH had increased to 34.18 mU/mL. Supplementation with 100mcg of L-thyroxine was initiated, but because of a progressively increasing T4 level and low TSH, her L-thyroxine dose was decreased to 75mcg about 4 months after starting sunitinib.
Figure 1.
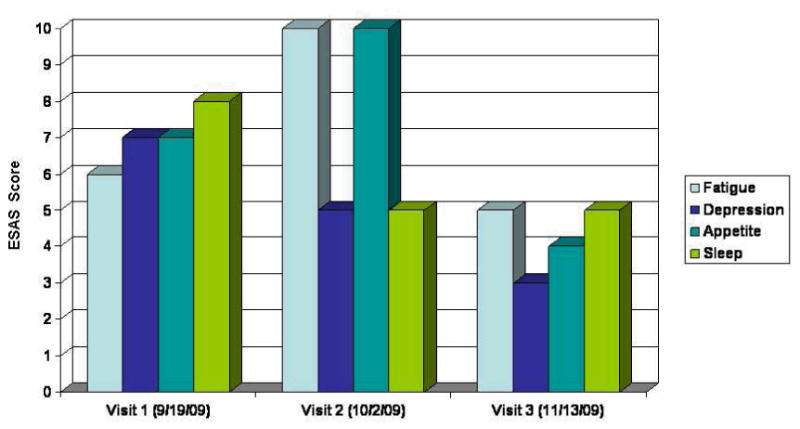
Temporal relationship between TSH, T4, L-thyroxine dose, and sunitinib cycles.
Figure 2.
ESAS scores relative to clinic visit and TSH.
She had no prior history of thyroid disease or radiation therapy to the head and neck. On referral to the supportive care clinic after 6 months of therapy her TSH was 119 mU/mL, T4 was below normal (0.6 ng/dL) and she complained of cold intolerance. She reported compliance with her L-thyroxine replacement therapy at every visit to the clinic. Thyroglobulin levels were normal (41.4ng/mL) and anti-peroxidase antibodies <10 IU/mL. Her symptoms were evaluated using the Edmonton Symptom Assessment Scale (ESAS), a validated assessment tool quantifying a patient's response to symptoms scored for intensity between 0 (best) to 10 (worst).The ESAS scores for poor appetite, fatigue, insomnia and depression during her three visits to the supportive care clinic are shown in figure 2.
By her second supportive care clinic visit, the TSH had risen to 321 mU/mL, and she reported profound fatigue and cold intolerance. Her L-thyroxine dose was increased to 100mcg, and because of disease progression, sunitinib was discontinued.
By her third and final clinic visit, the TSH level had declined to 21mU/mL and her symptom burden had improved considerably. Over the next 4 weeks she remained on L-thyroxine and her TSH declined to normal levels. She died some 6 weeks later, just prior to entering another phase I trial.
Discussion
The clinical management of hypothyroidism is complicated by a lack of clinical data12 since the majority of studies on hypothyroidism as an adverse effect of TKIs are small and observational13. In addition, because severe toxicities have been associated with better survival14, clinicians may be less inclined to investigate the etiology of side-effects associated with hypothyroidism such as fatigue.
Although TKI-related hypothyroidism is thought to be a long-term adverse effect12, studies have reported a variable onset of sunitinb-induced hypothyroidism. A prospective study of Renal Cell Cancer and GIST found a median time to abnormal TSH of 4 weeks15 while another study restricted to those with GI stromal tumors reported a mean time of 50 weeks.16 Since sunitinb may cause fatigue even in the absence of thyroid abnormalities; there is some uncertainty about the frequency of clinically relevant hypothyroidism associated with sunitinib. The initial large multicenter studies17 of sunitinib for metastatic renal cancer did not report hypothyroidism in those patients with grade 3 or 4 fatigue and TSH was not monitored routinely. More recent studies however, suggest up to70% of patients develop thyroid test abnormalities during treatment with sunitinib and between 918 and 30% require thyroid replacement19, 13. Although most patients may experience only transient hypothyroidism, our patient demonstrates that hypothyroidism associated with sunitinib may be severe, accompanied by a high symptom burden, and develop rapidly, even while receiving thyroxine replacement. In our patient, elevated TSH and decreased T4 correlated with symptom intensity, particularly fatigue and poor appetite as shown by the ESAS scores in table 3. Symptom burden was greatest when her TSH increased to an unusually high level of 324 mU/mL despite a dose of 75mcg thyroxine daily. When sunitinib was discontinued, the TSH declined and symptom intensity diminished, suggesting her symptoms were associated with drug-induced hypothyroidism.
Although one study16 reported a resolution of serum TSH abnormalities in all patients after replacement therapy, our case suggests some patients with thyroid dysfunction may be more challenging. Despite adjustments in dosing, figure 1 indicates normal values were difficult to achieve throughout her treatment course. Prior to developing profound hypothyroidism, she had an elevated T4 level suggesting either excessive replacement doses, altered T4 metabolism or even thyroiditis. Studies report thyroiditis accompanied by a low TSH may precede sunitinib14, 19 or sorafenib3 induced hypothyroidism in at least a third of patients. Occasionally patients can develop severe thyrotoxicosis20 but at least one other study involving renal cancer patients9, 16 showed no abnormally low TSH levels. Although a clinical algorithm12, 13 has been proposed to monitor thyroid function in patients on sunitinib, our case suggests the therapeutic management may be complicated by rapid fluctuations in TSH and T4 levels and sunitinib-induced transient hyperthyroidism.
The mechanism for sunitinib-induced thyroid dysfunction is unclear, but may be due to atrophy as a result of decreased capillary blood flow induced by VEGF inhibition 21 or anti-peroxidase activity22. Besides the direct effect on the thyroid gland, other mechanisms23 may be responsible for the increased requirements of L-thyroxine in our patient. The major mechanisms leading to an increased need for thyroxine are predominantly drug induced6 and can include decreased absorption of L-thyroxine, altered transport of T3 and T4 in the serum or altered metabolism of T3 and T4. The induction of microsomal enzymes that increase the clearance of fT4 and fT3 is proposed as a possible mechanism of persistent hypothyroidism in patients on imatinib and sunitnib already receiving replacement L-thyroxine9, 16, 12, 21. Postthyroidectomy patients on L-thyroxine have been reported to develop symptoms of hypothyroidism during imatinib24 or motesanib therapy 25
While studies suggest there may be a survival advantage for patients with renal cancer on TKI's who experience hypothyroidism, our patient with papillary renal cell cancer died only 6 weeks after discontinuing sunitinib. Progression free survival and overall survival of patients with subclinical (elevated TSH only) or clinical hypothyroidism (elevated TSH, decreased T4 andT3) on sunitinib are reported to be better, 26,16 however, it should be emphasized that the histologic subtype in these studies is almost exclusively renal clear cell .The survival benefit persists even in those receiving L-thyroxine replacement27, 28 perhaps because many remain in a persistent hypothyroid state despite replacement therapy24. Even patients with head and neck cancer not on sunitinib are reported to have improved survival associated with hypothyroidism, possibly due to a modulating effect on proliferation and apoptosis.29 Recommendations by some authors23 suggest TSH should be measured in all patients on sunitinib to validate TSH as a potential prognostic marker. Our patient's symptoms of overt hypothyroidism and poor prognosis suggest that patients with extreme hypothyroidism might have worse outcomes than their counterparts with mildly elevated TSH levels or subclinical hypothyroidism. Future studies should determine whether the relationship between survival and TSH is affected by severe hypothyroid symptoms, the degree of elevated TSH levels or by histologic subtype. Our patient's FH gene mutation- associated papillary renal cell cancer is known to be associated with worse prognosis and may have increased her susceptibility to clinical toxicities and thyroid abnormalities via an unexplained mechanism. Kidney cancer is a heterogeneous disease with markedly different clinical courses, is caused by different genes, and may have different responses to therapies30.
Even though our case of extreme hypothyroidism appears to be associated with sunitinib we cannot exclude the possibility that combination anti-angiogenic therapy (sunitinib plus AMG-386) may have contributed to the pronounced thyroid abnormalities.31. Although fatigue is reported as the most common side-effect of AMG-386, thyroid abnormalities have not been identified in animal studies or preliminary phase I and phase II trials, even when AMG-386 is combined with other small molecule TKI's such as sorafenib32 .
Ultimately, the patient's poor response and marked thyroid dysfunction may have been multifactorial including her genetic FH mutation, papillary renal cell cancer histologic subtype33, 34, or possibly combination therapy with another anti-angiogenic agent.
In summary, the case presented illustrates the potential for TKI's to cause extreme hypothyroidism, and unusually high serum TSH levels even while patients are on thyroxine replacement. Severe clinical toxicity is not necessarily accompanied by prolonged survival, and can contribute to substantial symptom burden which abates after TKI treatment is withdrawn In addition the profound hypothyroidism may be complicated by thyroiditis and transiently elevated thyroxine levels. Clinicians should monitor serum levels and consider early referral to their endocrinology colleagues if thyroid replacement is considered.
References
- 1.Kim TD, Schwarz M, Nogai H, Grille P, Westermann J, Plöckinger U, Braun D, Schweizer U, Arnold R, Dörken B, le Coutre P. Thyroid dysfunction caused by second-generation tyrosine kinase inhibitors in Philadelphia chromosome-positive chronic myeloid leukemia. Thyroid. 2010;11:1209–14. doi: 10.1089/thy.2010.0251. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg CN, Davis ID, Mardiak J. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 3.Miyake H, Kurahashi T, Yamanaka K, Kondo Y, Muramaki M, Takenaka A, Inoue TA, Fujisawa M. Abnormalities of thyroid function in Japanese patients with metastatic renal cell carcinoma treated with sorafenib: a prospective evaluation. Urol Oncol. 2010;5:515–9. doi: 10.1016/j.urolonc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Shinohara N, Takahashi M, Kamishima T, Ikushima H, Otsuka N, Ishizu A, Shimizu C, Kanayama H, Nonomura K. The incidence and mechanism of sunitinib-induced thyroid atrophy in patients with metastatic renal cell carcinoma. Br J Cancer. 2011;104:241–7. doi: 10.1038/sj.bjc.6606029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogiers A, Wolter P, Op de Beeck K, Thijs M, Decallonne B, Schöffski P. Shrinkage of thyroid volume in sunitinib-treated patients with renal-cell carcinoma: a potential marker of irreversible thyroid dysfunction? Thyroid. 2010;20:317–22. doi: 10.1089/thy.2009.0125. [DOI] [PubMed] [Google Scholar]
- 6.Torino F, Corsello SM, Longo R, Barnabei A, Gasparini G. Is hypothyroidism a clinically relevant toxicity of tyrosine kinase inhibitors? Thyroid. 2009;19:539–40. doi: 10.1089/thy.2008.0367. [DOI] [PubMed] [Google Scholar]
- 7.Makita N, Miyakawa M, Fujita T, Iiri T. Sunitinib induces hypothyroidism with a markedly reduced vascularity. Thyroid. 2010;20:323–6. doi: 10.1089/thy.2009.0414. [DOI] [PubMed] [Google Scholar]
- 8.Mannavola D, Coco P, Vannucchi G, Bertuelli R, Carletto M, Casali PG, Beck-Peccoz P, Fugazzola L. A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab. 2007;92:3531–4. doi: 10.1210/jc.2007-0586. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999 Aug;17(8):2530–40. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 10.Lehtonen HJ. Hereditary leiomyomatosis and renal cell cancer: update on clinical and molecular characteristics. Fam Cancer. 2011 doi: 10.1007/s10689-011-9428-z. [DOI] [PubMed] [Google Scholar]
- 11.Herbst RS, Hong D, Chap L, Kurzrock R, Jackson E, Silverman JM, Rasmussen E, Sun YN, Zhong D, Hwang YC, Evelhoch JL, Oliner JD, Le N, Rosen LS. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;27:3557–65. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- 12.Di Lorenzo G, Porta C, Bellmunt J, Sternberg C, Kirkali Z, Staehler M, Joniau S, Montorsi F, Buonerba C. Toxicities of targeted therapy and their management in kidney cancer. Eur Urol. 2011;59:526–40. doi: 10.1016/j.eururo.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Torino F, Corsello SM, Longo R, Barnabei A, Gasparini G. Hypothyroidism related to tyrosine kinase inhibitors: an emerging toxic effect of targeted therapy. Nat Rev Clin Oncol. 2009;6:219–28. doi: 10.1038/nrclinonc.2009.4. [DOI] [PubMed] [Google Scholar]
- 14.Di Fiore F, Rigal O, Ménager C, Michel P, Pfister C. Severe clinical toxicities are correlated with survival in patients with advanced renal cell carcinoma treated with sunitinib and sorafenib. Br J Cancer. 2011 doi: 10.1038/bjc.2011.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolter P, Stefan C, Decallonne B, Dumez H, Bex M, Carmeliet P, Schöffski P. The clinical implications of sunitinib-induced hypothyroidism: a prospective evaluation. Br J Cancer. 2008;99:448–54. doi: 10.1038/sj.bjc.6604497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai J, Yassa L, Marqusee E, George S, Frates MC, Chen MH, Morgan JA, Dychter SS, Larsen PR, Demetri GD, Alexander EK. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med. 2006;145:660–4. doi: 10.7326/0003-4819-145-9-200611070-00008. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 18.Baldazzi V, Tassi R, Lapini A, Santomaggio C, Carini M, Mazzanti R. The impact of sunitinib-induced hypothyroidism on progression-free survival of metastatic renal cancer patients: A prospective single-center study. Urol Oncol. 2010;28 doi: 10.1016/j.urolonc.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Rini BI, Tamaskar I, Shaheen P, Salas R, Garcia J, Wood L, Reddy S, Dreicer R, Bukowski RM. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007;99:81–3. doi: 10.1093/jnci/djk008. [DOI] [PubMed] [Google Scholar]
- 20.Grossmann M, Premaratne E, Desai J, Davis ID. Thyrotoxicosis during sunitinib treatment for renal cell carcinoma. Clin Endocrinol (Oxf) 2008;69:669–72. doi: 10.1111/j.1365-2265.2008.03253.x. [DOI] [PubMed] [Google Scholar]
- 21.Sato S, Muraishi K, Tani J, Sasaki Y, Tokubuchi I, Tajiri Y, Yamada K, Suekane S, Miyajima J, Matsuoka K. Clinical characteristics of thyroid abnormalities induced by sunitinib treatment in Japanese patients with renal cell carcinoma. Endocr J. 2010;57:873–80. doi: 10.1507/endocrj.k10e-130. [DOI] [PubMed] [Google Scholar]
- 22.Wong E, Rosen LS, Mulay M, Vanvugt A, Dinolfo M, Tomoda C, Sugawara M, Hershman JM. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. 2007;17:351–5. doi: 10.1089/thy.2006.0308. [DOI] [PubMed] [Google Scholar]
- 23.Kappers MH, van Esch JH, Smedts FM, de Krijger RR, Eechoute K, Mathijssen RH, Sleijfer S, Leijten F, Danser AH, van den Meiracker AH, Visser TJ. Sunitinib-induced hypothyroidism is due to induction of type 3 deiodinase activity and thyroidal capillary regression. J Clin Endocrinol Metab. 2011;96:3087–94. doi: 10.1210/jc.2011-1172. [DOI] [PubMed] [Google Scholar]
- 24.Jan Willem B, de Groot MD, Bernard A, Zonnenberg MD, John TM, Plukker MD, et al. Imatinib induces hypothyroidism in patients receiving levothyroxine. Clinical Pharmacology & Therapeutics. 2005;(78):433–438. doi: 10.1016/j.clpt.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Schlumberger MJ, Elisei R, Bastholt L, Wirth LJ, Martins RG, Locati LD, Jarzab B, Pacini F, Daumerie C, Droz JP, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Sherman SI. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009;27:3794–801. doi: 10.1200/JCO.2008.18.7815. [DOI] [PubMed] [Google Scholar]
- 26.Garfield DH, Wolter P, Schöffski P, Hercbergs A, Davis P. Documentation of thyroid function in clinical studies with sunitinib: why does it matter? J Clin Oncol. 2008;26:5131–2. doi: 10.1200/JCO.2008.18.8680. [DOI] [PubMed] [Google Scholar]
- 27.Schmidinger M, Vogl UM, Bojic M, Lamm W, Heinzl H, Haitel A, Clodi M, Kramer G, Zielinski CC. Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer. 2011;117:534–44. doi: 10.1002/cncr.25422. [DOI] [PubMed] [Google Scholar]
- 28.Riesenbeck LM, Bierer S, Hoffmeister I, Köpke T, Papavassilis P, Hertle L, Thielen B, Herrmann E. Hypothyroidism correlates with a better prognosis in metastatic renal cancer patients treated with sorafenib or sunitinib. World J Urol. 2010 doi: 10.1007/s00345-010-0627-2. [DOI] [PubMed] [Google Scholar]
- 29.Nelson M, Hercbergs A, Rybicki L, Strome M. Association between development of hypothyroidism and improved survival in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:1041–6. doi: 10.1001/archotol.132.10.1041. [DOI] [PubMed] [Google Scholar]
- 30.Rosner I, Bratslavsky G, Pinto PA, Linehan WM. The clinical implications of the genetics of renal cell carcinoma. Urol Oncol. 2009;27:131–6. doi: 10.1016/j.urolonc.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neal J, Wakelee H. AMG-386, a selective angiopoietin-1/-2-neutralizing peptibody for the potential treatment of cancer. Curr Opin Mol Ther. 2010;12:487–95. [PubMed] [Google Scholar]
- 32.Hong D, Gordon M, Appleman L, Kurzrock R, Sun Y, Rasmussen E, Zhong D, Le N, L R. Interim results from a phase 1b study of safety, pharmacokinetics (PK) and tumor response of the angiopoietin 1/2-neutralizing peptibody AMG 386 in combinations with AMG 706, bevacizumab (B) or sorafenib (S) in advanced solid tumors. Ann Oncol. 2008;19:154. [Google Scholar]
- 33.Ronnen EA, Kondagunta GV, Ishill N, Spodek L, Russo P, Reuter V, Bacik J, Motzer RJ. Treatment outcome for metastatic papillary renal cell carcinoma patients. Cancer. 2006 Dec 1;107(11):2617–21. doi: 10.1002/cncr.22340. [DOI] [PubMed] [Google Scholar]
- 34.Choueiri TK, Plantade A, Elson P, Negrier S, Ravaud A, Oudard S, Zhou M, Rini BI, Bukowski RM, Escudier B. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008 Jan 1;26(1):127–31. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]


