Introduction
Implantable devices for electrical stimulation of the brain have been in routine clinical use since 1997, when the first commercial deep brain stimulation (DBS) system was approved for the treatment of tremor1. These DBS devices provide an invariant train of stimulatory pulses at a fixed frequency. This “open-loop” mode (meaning unidirectional signal generated from the device and delivered to the brain) of DBS therapy has proven to be effective for treatment of essential tremor2, Parkinson’s disease3,4, and dystonia5,6. As we expand our understanding of the neurophysiological mechanisms of both DBS and movement disorders, the shortcomings of open loop therapy DBS are evident and will be discussed in this review. The design of a “closed-loop” implantable pulse generator (IPG) to sense and respond to physiological signals (“closed-loop” meaning bidirectional signals moving in both sensing and responding directions, allowing for the use of sensor signals to provide feedback modulation of stimulation) within or outside the brain is considered the next frontier in brain stimulation research and will likely broaden the field to include new applications for neuromodulation.
Implantable closed loop stimulation systems are well established in the treatment of cardiac arrhythmias. Cardiac pacemaker devices capable of sensing and responding to atrial activity are closed loop mode cardiac stimulation devices and have been in clinical use since 19637. Despite the precedent for an IPG with dual sensing and stimulating functionality set 50 years ago, efforts to bring similar concepts to DBS devices8,9 have been delayed 50-years in part due to the complexity of brain signals. Whereas cardiac pacemakers detect the P-wave signal of the atrial pacemaker, brain-generated signals are statistically complex. Perhaps more importantly, the clinical meaningfulness of recordable brain signals is not immediately obvious.
Strategy development for interpreting neuronal signals in closed-loop neurostimulation application is underway. In broadest terms, an understanding of the relationship between a patient’s clinical state and a neuronal signal under the influence of external stimulation is fundamental to any future utilization of the signal as a surrogate marker for clinical states. Clinical states are disease specific but collectively can be categorized by pathological expressions of the disease (e.g. the magnitude of tremor) and behavioral intentions (i.e. attempting a task at hand such as walking, talking or writing). Therefore, closed-loop neurostimulation relates available neuronal recording to meaningful clinical states and uses the surrogate measurements to update neurostimulation as the device is operating.
Recordable neurophysiological signals are available from multiple levels of the brain, including a single neuron, multiple individual neurons, a localized population of neurons, or a large-scale population of neurons. Single neuron recordings have been shown to be related to certain specific aspects of movement10 and cognition11. Technical challenges of chronic recording from single neurons exist, such as increased sampling rate requirements, difficulty maintaining recordings from the same neuron for extended periods of time, and degradation at the neuron-electrode interface. These challenges contribute to the overall difficulty in maintaining sustained recordings from a single neuron. Recording from large populations of neurons, or local field potential (LFP) recordings, are much more stable over time.
Oscillatory components of LFP recordings from highly specialized cortex, such as motor cortex or visual cortex, have been successfully related to clinical states such as movement and visual percepts12–14. However, recordings from these specialized cortical regions of the brain are limited because these regions are not typically accessed during routine surgery for neurostimulation.
This review article will present current developments in closed loop neurostimulation and strategies for manipulation of recordable signals in order to relate this information to a patient’s clinical state (Figure 1). Specifically, this review will cover the rationale for closed loop stimulation, meaningful categories of clinical patient states, brain signals available for recording, signal processing for prediction of patient states, and interventional DBS patterns aimed at restoring a desired state, or facilitating a desired state. Parkinson’s Disease will be a primary focus; however, principles can be applied to other movement disorders, as well as epilepsy, mood disorders, and other neuropsychiatric diseases. This review article is intended as an introduction of engineering issues for clinicians and of clinical issues for engineers.
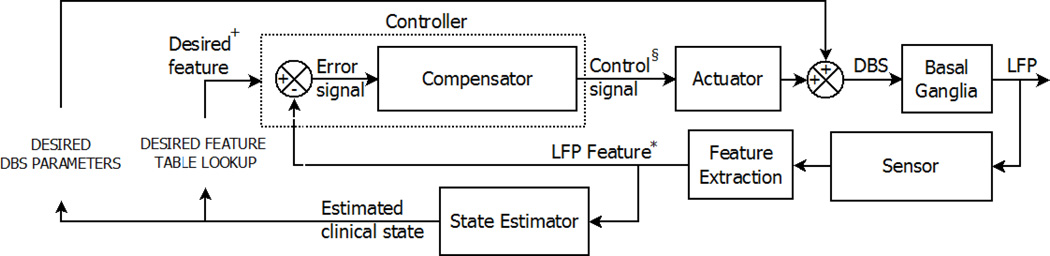
Figure 1.
General control system diagram representing a conceptualized closed loop neurostimulation system for DBS. In this diagram, the reference signal (* LFP Feature) is the feature extracted from the basal ganglia local field potential. This reference signal* is used to predict a meaningful clinical state of the patient (estimated or predicted clinical state) and these states subsequently direct the selection of desired DBS parameters and appropriate reference values († Desired feature). The controller compares (and calculates an error signal) the reference signal* and value†, and calculates the controlled variable (§ Control signal). The control signal§ and actuator, together with the selection of desired DBS parameters, influence the DBS feedback to the basal ganglia, ultimately impacting the LFP and extracted features*. This impact on the LFP serves as a surrogate marker for the beneficial effect of DBS on the patient, bringing the reference signal* and value† closer together.
Rationale for closed loop stimulation: Parkinson’s Disease
Open-loop DBS is effective for treating the motor signs of Parkinson’s disease, but side effects of this therapy and its inefficiencies may be diminished within a closed loop system. Side effects of open-loop DBS experienced by some patients include impaired cognition, speech, gait and balance15. Open loop DBS could potentially disrupt decision making, learning, and cognitive association through its effect on LFP oscillations of the brain. Open-loop DBS therapy was developed prior to an understanding of how LFP oscillations influence the precise timing of neuronal action potentials (integral to the mechanism of Hebbian learning16,17), and influence communication between distant neuronal ensembles (the basis of cognitive association18,19). Studies have demonstrated that open-loop DBS impairs learning and object-naming during stimulation of the pulvinar20, and impairs verbal fluency and reactive inhibition during stimulation of the subthalamic nucleus. These impairments may be due to disruption of cortico-cortical or cortico-subcortical oscillatory synchronization between LFPs of connected brain regions20.
As a result of dopaminergic depletion in Parkinson’s disease, the basal ganglia are characterized by radical changes at the level of a single neuron and above. Neuronal firing patterns change significantly in animal models and in humans with Parkinson’s disease. In the pathological state, neuronal pairs within and across boundaries of basal ganglia nuclei fire together with increased synchrony21. This phenomenon is reflected in the abnormal oscillatory activity revealed by LFPs within and between those structures.
The method by which high frequency stimulation influences cortico-basal ganglia circuits and leads to a therapeutic outcome is under debate22,23. DBS effects are likely a combination of a local inhibitory effect and an excitatory effect on distal connected nuclei. The local inhibitory effect of DBS may be evident by the fact that both stimulation and lesioning of the globus pallidus lead to similar therapeutic effects in the clinical treatment of Parkinson’s disease. For the treatment of essential tremor, clinical effects of stimulation and lesioning of the thalamus are roughly equivalent. The distal excitatory effect of subthalamic nucleus DBS is evident by its observed effect of increasing the firing rate of neurons in the globus pallidus, a distal connected nucleus24.
Though the mechanism of DBS therapeutic benefit is poorly understood, an end-result of open-loop DBS may be a neuronal network that is able to retain partial functionality despite insufficient dopaminergic innervation. If this is true, the present therapeutic methods may not be restoring basal ganglia functionality to the fullest extent possible, due to limitations of the static approach of open-loop DBS in an inherently dynamic system. It is therefore compelling to seek a treatment that could enhance “surviving” functionality in the basal ganglia. Dynamic restoration of neuronal rhythms by the integration of responsive signal processing might be an advantage of a closed-loop system. Conversely, damage to the basal ganglia may be the result of irreversible loss of neuronal circuitry involved in highly specialized information processing25. In this case, dynamic and responsive signal processing may not present an advantage compared to current open-loop DBS, especially if a damaged neuronal network generates faulty signal outputs.
Restoring the desired state: A role for closed-loop stimulation
A central challenge for closed loop therapy is the definition of a therapeutic or optimal state that neurostimulation attempts to maintain or restore. Therefore, closed-loop systems incorporate a single or multiple set points, that is, reference values corresponding to the desired state. Returning to the example of optimizing behavioral goals, each of these set points could correspond to a different behavioral intention, such as walking, talking, or writing.
The goal of closed-loop DBS in Parkinson’s disease is to restore lost functionality by stimulating the target nucleus. Stimulation of the basal ganglia is based on a “state-space model” of the basal ganglia dynamics. Stimulation is intended to force a certain feature that leads to some desired LFP reference value associated with the desired therapeutic state (Figure 1). The compensator attempts to bring the controlled variable closer to the desired reference value. The “state-space model” relies on the assumption that a disease symptom is attributable to one or more features (often just one) that is constant at some reference value level in a non-diseased physiological state. A second assumption is that restoring the value of the LFP output feature to the desired value is sufficient to restore basal ganglia functionality. These assumptions may be implausible if the pathological etiology of Parkinson’s disease is characterized more by loss of neuronal organization (precluding signal processing of normal neuronal signals) than by a disturbance of LFP oscillations.
A model for a closed-loop system that contains multiple modes of compensation could be useful in an attempt to replicate the non-linear characteristics of the basal-ganglia system and subcortical motor network facilitating a wide range of motor and cognitive tasks26. Such a model would address the need to generate a variety of concurrent signal patterns corresponding to multiple desired reference values. For example, prior to movement initiation, a desired reference value could constitute high suppression of the beta (13–30Hz) oscillatory power of the LFP signal, while continuous execution of movement might call for concurrent beta suppression with an increase in high frequency gamma (>30Hz) oscillatory power. The advantage of multiple modes of compensation compared to a single set-point closed-loop compensation system is that therapy could be tailored to an individual patient’s goals. First, desired reference values for normal cortico-basal ganglia activity must be defined in order to later dynamically adjust the controlled variables. Those values could be obtainable by recording from motor-planning cortical areas10 or sites in the basal ganglia, including those at the stimulation site27.
Available signals
The availability of reliably detectable bio-signals capable of driving feedback is essential to a closed-loop neuromodulation system. In current open-loop systems, the patient’s clinical status and the provider’s assessment of the clinical status via physical examination provides the feedback to regulate neuromodulation. While effective and the basis for newer neuromodulation models, this approach may be overly subjective/observer-dependent, time-intensive, overly consumptive of battery power, and most importantly, may not provide patients with optimal clinical benefit. Ideally, bio-signals for closed-loop neuromodulation would be easily detectable in a biocompatible manner, reliably recorded with limited noise and error, over an extended time (i.e. years), rich in content, and dynamically and accurately related to clinical states. For example, while functional magnetic resonance imaging (fMRI) is used extensively to study brain function, brain organization, and neural connectivity, fMRI is an impractical resource for measurable neuronal activity on an ongoing basis. On the other hand, invasive and implantable electrophysiological sensors that can detect neuronal signals on an ongoing basis would improve signal detection and feedback within closed-loop systems. Current research is focused primarily on neuroelectrophysiological signal processing; however, biochemical, optical, electromyographic, and mechanical signals are other potentially useful resources.
Depending on the location of neuroelectrophysiological signal recording, signals may represent the activity of one neuron or an aggregate of cortical or subcortical neurons28. In general, the further away parenchymal recordings are from the brain, the poorer the temporal and spatial resolution and the higher the noise content in the measurements, but the better the perceived safety of each signal source.
Single-unit recordings, performed with invasive high-impedance (0.4–1.0 MΩ) penetrating microelectrodes such as those used for microelectrode recording in DBS surgery or those used with the Utah microelectrode array, detect action potentials from neighboring neurons (single or multiple). The action potential is considered the core unit of communication between neurons and therefore is extremely appealing as a potential data source for driving closed-loop systems. Recordings of single- and multi-unit neuronal activity have provided insight into patterned activity within the subthalamic nucleus and globus pallidus29, and for cognitive processing and memory30. Despite the potential appeal of microelectrode recording, the practicality of recording action potentials for long-term use are hindered by requirement for repeated re-calibration and difficulty in maintaining the fidelity of recordings over extended periods31,32. Moreover, because the measurement of unit activity requires penetrating microelectrodes, spatial sampling is inherently limited (although spatial resolution is outstanding), and necessitates precision when selecting a cortical or subcortical region for recording. Nevertheless, an ongoing trial, the BrainGate trial, is making use of Utah arrays as a signal source for prosthetic control, underscoring the potential of single neuron recordings33,34.
Unlike measurement of individual action potentials, LFP provide a measure of integrated population level activity, which is thought to be a combination of action potential activity, sub-threshold membrane voltage changes, and changes in glial potentials. LFP can be measured with an array of electrodes, including the same microelectrodes that are used for detection of unit activity as well as standard DBS leads and subcortical electrocorticographic strips and grids35–37. LFPs are less susceptible to drift over time and therefore provide higher fidelity and more reliable long-term recordings, which is a desirable characteristic for a control signal. Moreover, because LFPs measure population level oscillatory activity, the data is very rich in content, both in the temporal as well as the frequency domain, providing several potential bands of interest (e.g., alpha, theta, beta, gamma, high-gamma). Because signals are recorded using invasive probes, spectral content includes very high frequencies. These high frequency bands may be critical to the control of closed-loop systems38,39. For example, within subthalamic nuclei and globus pallidus, very high frequency bands in the 200–300 Hz range and greater than 300 Hz range have been described and correlate with degree of Parkinson’s disease motor states38,39. Investigations of LFP measured via electrocorticographic arrays in patients with Parkinson’s disease have noted increased beta band activity and synchronization within the motor cortex in patients with Parkinson’s disease compared to other patients35. These studies have also shown that therapeutic stimulation in patients with Parkinson’s diseases specifically modulates aberrant beta band activity40. Adaptations of DBS devices already in clinical use for invasive recording or stimulation, have simplified development of biocompatible probes for a closed-loop system8. The only closed-loop neuromodulation trial to date (NeuroPace) utilized recordings made by penetrating macroelectrodes and subdural electrocorticographic arrays41. Though there have been concerns regarding potential limitations of spatial resolution, studies using LFPs have so far concluded LFP recording allows for sufficient specificity and functional localization, suggesting LFP recording is usable in closed-loop systems13,27.
Although measurement of unit activity and LFPs involves invasive recording approaches, non-invasive measurement of neuroelectrophysiological activity using electroencephalography (EEG) is also a consideration. A model of the brain-computer interface, the P300 speller, utilizes EEG detection of evoked cerebral electrophysiological activity to restore communication to locked-in patients42. In patients with Parkinson’s disease, EEG has detected aberrant patterns of cortical activity that are potential biomarkers of the disease state43. EEG essentially measures the same neuronal signals measured by electrocorticography. However, because EEG signals are measured noninvasively, the recording electrode is further away from the electrical source and much of the higher frequency spectra (i.e., >70–100Hz) is filtered by the scalp and skull. Both of these factors contribute to a significant loss of spatial resolution. Additionally, because EEG sensors are not implanted, EEG sensors must be affixed to the scalp or skull and this may or may not be acceptable to the patient. Care must be taken in the sampling and recording process to avoid inadvertent creation of artifacts (such as aliasing)44.
Aside from recording neuronal electrophysiological signals, a closed-loop system might also utilize non-neuronal biological signals. For example, the biochemical state of various deep brain regions may be detected in real time using cyclic voltometry45. Given the dopaminergic-related origins of Parkinson’s disease and biochemical basis of other neuropsychiatric diseases, the concept of integrating real-time biochemical assessments may be useful for managing dynamic fluctuations in medication effects. The groundwork for such models has begun with efforts to develop a wireless non-neuronal feedback system46. This approach would have potential limitations related to sampling error and would require additional implantation of penetrating electrodes, which might impart greater risk than other approaches. Other non-neuronal biosignals given serious consideration are recording of peripheral signals by EMG and non-invasive accelerometers, because such signals indicate patient movement and clinical status in real-time. Hilliard and colleagues have reported the use of accelerometers attached to patients’ wrists for determining effective stimulation sites within the subthalamic nucleus for treatment of tremor, bradykinesia, and gait disturbance47. Such kinesthetically-rich information derived from noninvasive peripheral measurement of clinical states might soon drive a successful closed loop system.
The various biosignals available for incorporation within a closed-loop system each have distinct advantages and disadvantages with respect to invasiveness, resolution, signal content and clinical relevance (Table 1). It is unlikely that any single signal source will be appropriate for all closed-loop neuromodulation approaches. The selection of appropriate source will ultimately depend on the design of the entire system and is intimately related to the feature extraction and signal classification, as described in the subsequent sections.
Table 1.
Available Signals for signal based neurostimulation: The following table summarizes recent studies, experiments and device development regarding brain signal based neurostimulation.
| Feedback Signal | Stimulation Type | Feedback mechanism | Application | Subject | Reference |
|---|---|---|---|---|---|
| Arm and hand EMG and acceleration sensors |
DBS (STN, VIM) | Features of EMG and acceleration signals are used to predict tremor onset, regulating DBS not implemented. |
ET, PD | Human | 48 |
| EMG | DBS (VIM) | Features of EMG signals measure tremor and regulate DBS system via remote programmer. |
Intention Tremor |
Human | 49 |
| Cortical ECoG/LFP |
Cortical stimulation via cortical depth electrodes or ECoG |
Abnormal phase synchrony of cortical signals triggers stimulation. |
Epilepsy, seizure detection |
Animal model (on-line) and human (off-line) |
50 |
| Skull mounted EEG |
Cortical stimulation via skull mounted EEG (F3) |
Stimulation triggered by reduced EEG phase synchronization across multiple electrodes. |
Epilepsy | Rats | 51 |
| ECoG | Pharmacological | Voltage window detectors and frequency analysis parameters matching patient’s specific seizures trigger drug delivery system. |
Epilepsy | Rat model and offline human ECoG |
52,53 |
| Single unit recording of spinal dorsal horn neurons |
DBS Periaqueductal Grey |
Stimulation triggered by user- defined clustering of inter spike intervals used to classify physical stimuli. |
Pain | Rats | 54 |
| Neuronal firing rate |
DBS | Adaptive algorithm adjusts DBS to maintain a stimulus to firing frequency ratio. |
Epilepsy | Computational Model |
55 |
| Skull mounted EEG over frontal barrel cortex |
DBS (Zona Incerta) |
Linear least squares was utilized to classify seizures based on entropy and spectral power. |
Epilepsy | Rats | 56 |
| Single unit recording of primary motor cortex |
DBS (GPi) | DBS pulse train delivered at fixed latency following M1 spike detection. |
PD | Primate model of PD |
57 |
| LFP recording (Hippocampus) |
DBS (Hippocampus) |
DBS and LFP recording via distinct contacts on macroelectrode. Seizures detected during stimulation with machine learning. Seizure modeled by afterdischarge potentials induced by hippocampa stimulation. |
Seizure | Sheep | 58 |
EMG: Electromyography, DBS: Deep Brain Stimulation, STN: Subthalamic Nucleus, VIM: Ventral Intermedius Nucleus of the Thalamus, ET: Essential Tremor, PD: Parkinson’s disease, ECoG: Electrocorticography, GPi: Globus Pallidus pars internus, LFP: Local Field Potential.
Prediction Methods
Feature Extraction
The purpose of feature extraction is to transform this time series data for successive processing and/or improved computational efficiency. For example, time series data could be transformed from the time domain into the frequency domain, thus changing the meaning of the data stream from “when an event occurs” to “how frequently an event occurs.” Neuronal ensembles may use frequency coding to communicate, and therefore transformation of data into the frequency domain can be considered translation of data into the language of neuronal networks.
To improve computational efficiency and increase processing speed, data is compressed, transformed or otherwise reduced in dimension to remove redundancy and condense overall data size. After signal features are extracted, an additional processing component or feature selection/dimensionality reduction may be required to further reduce the number of features and/or dimensions, or remove noise/outlier features. As a result, the features that are meaningful in the learning and classification stage are identified and chosen, while outliers and artifacts are excluded from the data.
Time domain
Time domain features of EEG or LFP signals such as event related potentials (ERP) may be extracted by splicing long (e.g. 10 minutes) time series data recordings into shorter time segments/epochs (e.g. 500 milliseconds), before and after an event. By averaging many event-aligned epochs, features of the data related to the event are emphasized and those features unrelated to the event cancel out through averaging. This technique is used to identify visual evoked potentials (VEP), somatosensory evoked potentials (SSEPs), and the cognitive oddball response, the P300.
Quantified temporal information derived from action potentials includes neuronal firing rates, peri-stimulus time histograms (PSTH), and interspike time intervals (ISI). These measures are useful for interpreting neural information from cerebral neurons that are responding to external influences such as visuospatial memory59, visual presentation of faces60, or passive joint movements61. Firing rates are modulated by neurons responding to these external influences and also encode motor plans. An example of this is evidenced in the motor cortex by the cosine tuning of neuronal firing rates in a manner corresponding to the direction of arm reach62. The timing of an electrophysiological spike relative to the previous spike provides other specific information useful for decoding neural information63. The timing of the neuronal spikes is further influenced by the phase of low frequency LFPs, as demonstrated by spike clustering at the trough of beta frequency LFP oscillations in the subthalamic nucleus64.
Time-Frequency Domain
Several power spectral estimation methods are used to transform recorded time domain data into time-frequency domain data and allow quantification of neuronal electrical oscillations. Common techniques to estimate spectral power include variations of short time Fourier transform, wavelet transformation, and autoregressive models65–67. Frequency bands of interest within neuronal signals have historically been defined by their visual appearance and spatial distribution on EEG tracings. These include delta (<4Hz), theta (4–8Hz), alpha (8–13Hz), mu (7–11Hz), beta (13–30Hz), and gamma rhythms (>30Hz)68. These definition terms are also applied to equivalent frequency signal ranges recorded invasively by electrocorticography (ECoG) and LFP. Unlike synchronous processes which are band frequency limited, asynchronous processes have been shown to produce broad band spectral power, whereby power exponentially decreases at increasing frequencies69. Such changes in relative power within frequency bands have been correlated to movement and speech in both cortical and subcortical recordings13,27, and thus show promise for use in neural decoding within closed loop neurostimulation paradigms.
Phase Domain
Features of amplitude, frequency, and phase can describe a sinusoidal function infinite in time. Phase can be interpreted as a shift or offset in time of that wave from some arbitrary reference time point. Valid phase values vary from −π to +π, or −λ/2 to λ/2, where λ is the wavelength (λ = 1/frequency). Simple signal processing techniques such as filtering can change the phase of a signal in a frequency dependent fashion. Phase analysis is inherently more difficult than simple signal processing due to the absence of a clear zero-phase reference point and the presence of artifacts resulting from signal processing techniques. Neural oscillations are finite in time and statistically non-stationary signals. For these reasons, no definitive method has been demonstrated to measure the phase of neural oscillations70–72. Furthermore, there is no clear interpretation for the instantaneous phase of a neural signal72. Despite these limitations, phases of neural oscillations have been shown to influence spike timing64, and are particularly relevant to mechanisms of cognitive processes like memory18.
Oscillations in the motor network are related to each other through non-linear interactions. In particular, the phase of slower oscillations in the theta, alpha, and beta bands modulates the amplitude of faster rhythms in the beta, gamma and very high gamma ranges38,73,74, a phenomenon referred to as phase-to-amplitude cross-frequency coupling (PA-CFC). PA-CFC indicates the extent to which the phase of one oscillation extracted from a LFP determines the amplitude of another oscillation. Hierarchical relationships between oscillations might play a significant role in a variety of cognitive and motor tasks73,74 and could be applied as parameters in the design of a closed-loop DBS system. Despite accumulating evidence for PA-CFC’s ubiquity and functional importance in the nervous system, PA-CFC has not received much attention as a potential closed-loop system parameter for indicating motor task selection and planning.
Studies have shown that PA-CFC is correlated with the pathological state of the basal ganglia in movement disorders. For example, PA-CFC in the motor cortex (beta-gamma) is exaggerated in Parkinson’s disease patients compared to patients with epilepsy who do not have a movement disorder35. Therapeutic stimulation of the STN and dopaminergic therapy reduce the magnitude of coupling between beta-phase and gamma-amplitude35,38, highlighting the potential of PA-CFC as a biomarker of disease.
A current goal in the development of closed-loop DBS systems is to discover reference points to which a controlled variable converges and thereby leads to enhancement of motor capacity. Feature extraction is truly limitless in its potential for abstract manipulation of raw data. Table 2 highlights techniques and approaches for a subset of major approaches.
Table 2.
Feature Extraction: Feature Extraction: This table summarizes the signal processing techniques for feature extraction which appear in a number of recent publications.
| Domain | Method | Reference |
|---|---|---|
| Time- Domain |
Linear filtering | 75–78 |
| Linear combination and regression | 76,77 | |
| Blind source separation (BSS) | 76,77 | |
| Matched filter | 76,79,80 | |
| Autoregressive model parameters | 76,80–84 | |
| Independent component analysis (ICA) | 76,80,83,85,86 | |
| Karhunen-Loeve transform (KLT) | 87,88 | |
| Kalman filtering, Unscented Kalman Filter | 76,80,89 | |
| Correlation of temporal average of stimulus locked response with template |
76,79,80,90 | |
| Size of temporal average of stimulus locked response | 76,80 | |
| Covariate shift adaptation | 91 | |
| Neural time series prediction preprocessing | 92 | |
| Barlow- and Hjorth-based feature | 76,92 | |
| Neural firing rate | 62,76,80,93,94 | |
| Signal amplitude differences | 76,80 | |
| Event Related Potential P300, Steady-state visual evoked potential (SSVEP) |
80,93–105 | |
| Lateralized readiness potential (LRP) | 99 | |
| Slow cortical potentials (SCP) | 94,96,99,100 | |
| Cross-Correlation | 90 | |
| Frequency Domain |
Spectral parameters, frequency band power | 27,75,76,80,83,87,88,93,96,97,106–110 |
| Event-related (de)synchronization (ERD, ERS) | 27,75–77,80,81,95,98,99,102 | |
| Sensorimotor activity PSD | 94 | |
| Motor imagery | 84,93 | |
| Neural time series prediction preprocessing | 92 | |
| Time- Frequency Domain |
Wavelet transform | 76,80,83,111 |
| STFT | 27,107 | |
| Matching pursuit decomposition | 112 | |
| Spatial Domain |
Common spatial patterns (CSP) | 76,83,106,113 |
| Beamforming | 114 | |
| Surface Laplacian derivation | 75,81 | |
| Linear minimum mean squared error (LMMSE) spatial filter | 115 | |
| Spatial filtering | 75 | |
| Subspace Domain |
Principle component analysis (PCA) | 76,77 |
| Phase Domain |
Coherence or phase calculation | 76,80 |
Pattern Classification
The objective of pattern classification is to learn a mathematical model (a classifier) that can recognize and segregate novel patterns. Pattern classification algorithms are powerful in that they can be applied naively to data. These “machine learning” algorithms may be applied to recordings of the human voice for speech recognition,116 images of handwriting for association with personality,117 functional MRI to decode emotion evoked by facial expressions118 and scalp EEG to allow communication119. For instance, in the case of brain computer interfaces, a classifier given a segment of recorded brain signal is able to associate the neural signal with a given state, such as emotion, thought, behavior, or intention (a “class label”). During the training phase, subjects perform activities for which the corresponding class label is known (e.g., “motor activities” class label) and the algorithm “learns” the corresponding pattern. Table 3 summarizes several classification methods used for brain signal classification, including Support Vector Machines (SVMs), Artificial Neural Networks (ANNs), K-Nearest Neighbor (KNN), Bayesian Classification, and Hidden Markov Model (HMM). We will describe two well-known techniques for pattern classification, KNN (a non-linear classifier), and SVM (a linear classifier), also presented in Figure 3.
Table 3.
Prediction Methods: This table summarizes several feature processing techniques, including feature modeling, detection and classification reported in a number of recent publications.
| Method | Reference |
|---|---|
| Support Vector Machines | 76,80,82,86,95,102,105,111 |
| K-Nearest Neighbors (KNN) | 76,80,87,95 |
| Thresholding | 75,76,79,80,90 |
| Bayesian Classification | 76,80,104,106 |
| Linear Discriminant Analysis (LDA), Fisher Linear Discriminant (FLD), Mahalanobis Linear Distance (MLD) |
76,80,82,84,87,92,95,102–105,109,115 |
| Quadratic Discriminant Analysis (QDA) | 82,84 |
| Hidden Markov Model (HMM) | 76,80 |
| Linear/Non-linear Continuous Transformation | 76,80 |
| Classifier Adaptation | 91 |
| Gaussian Process | 110 |
| Decision Tree | 81,97 |
| Gaussian Mixture Model | 78,84 |
| Genetic Algorithm | 88,102 |
| Logistic Regression Linear Classifier | 114 |
| Neural Network | 82,92 |
| Common Spatial Patterns (CSP), Multiple-class CSP | 84,106,113 |
| Filter Bank Common Spatial Patterns (FBCSP) | 84 |
| Iterative Spatio-Spectral Patterns Learning (ISSPL) |
108 |
| Fuzzy Inference | 131 |
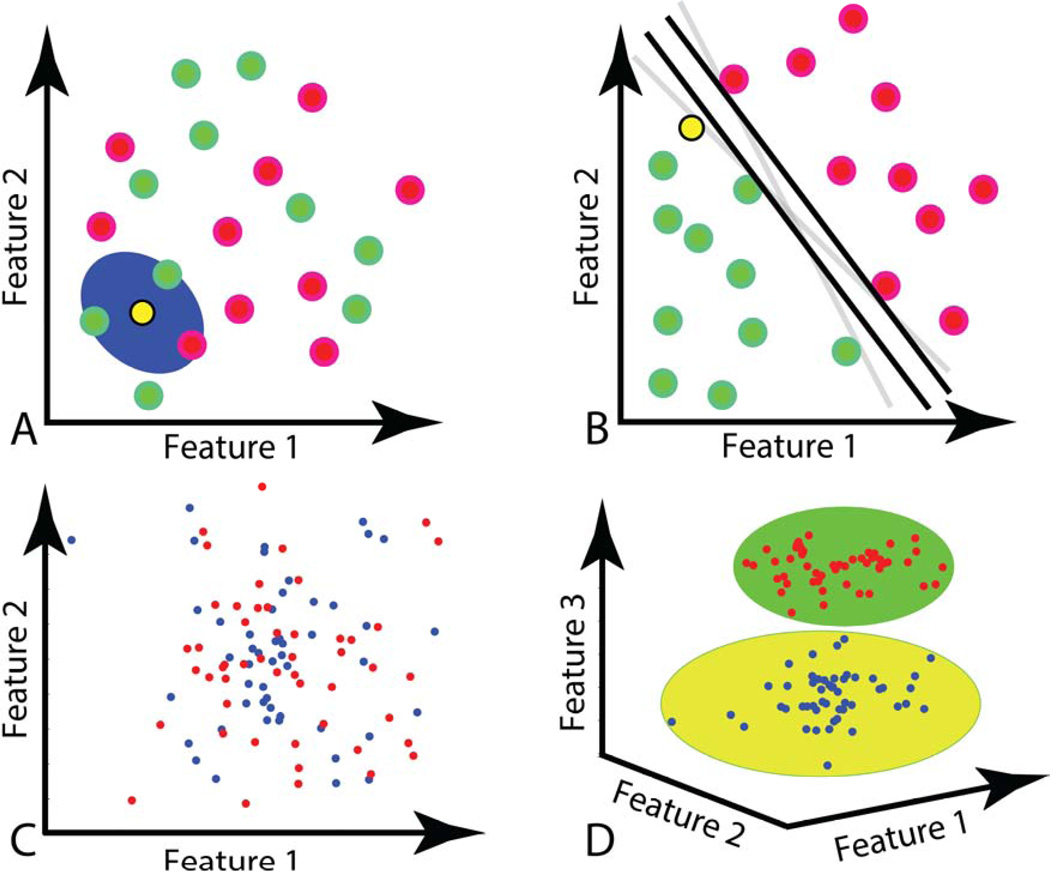
Figure 3.
Graphical representations of pattern classification algorithms. A: k-nearest neighbor (KNN). In this case, the yellow (unknown) sample will be classified by polling its 3 nearest neighbors, and will be classified as a member of the green group. B: Support Vector Machine (SVM). If two groups of data can be separated by a line or plane in feature space, SVM will provide the plane that defines the widest gap between the 2 data sets. The group members that lie on (support) the parallel planes are called support vectors. C: In this example, the two classes of data are not linearly separable in the depicted feature space. D: Here, kernel functions add additional dimensions, or features to data sets. By adding a third feature to the data set in C, the two groups are now separable and a SVM algorithm can classify new data.
KNN120 is a simple but effective non-linear classification technique. After a training phase using samples with known corresponding class labels in the feature space, KNN predicts the class label of a novel sample based on the label of its K closest (“neighbor”) samples. For example, if K is set to 3, KNN first finds the 3 neighbor samples closest to the test sample and then chooses the label of the novel sample based on the majority label of those 3 neighbor samples (Figure 3A). Techniques for finding the nearest neighbors include calculations for Euclidean distance and Manhattan distance. The parameter K is usually selected empirically and can affect the sample recognition rate. In this KNN algorithm, the training data sets do not need to be linearly separable, as illustrated in Figure 3.
Support Vector Machine (SVM) is another common method for pattern classification and regression121 introduced by Cortes and Vapnik122. SVMs are used in many applications including isolated handwritten digit recognition122,123, brain signal classification124,125, face recognition in images126, and text categorization127. A linear binary SVM classifier technique uses two parallel hyper-planes to separate the margin between two different classes of data in the feature space (Figure 3B). The SVM selects directions for the hyper-planes via an optimization problem algorithm where the objective is to maximize the margin between the separating hyper-planes. SVM solves the optimization problem using training samples, called support vectors, that lie on the margin. Two classes of data are often not able to be separated by a plane in the original space (Figure 3C) and require mapping into a higher dimensional space to become linearly separable (Figure 3D). SVM uses kernel functions to project feature vectors onto a more discriminative space by representing data with a higher dimensionality, allowing the separation of data groups using non-linear functions (e.g., separating curves). The use of several different types of kernel mappings, such as radial basis function (RBF)128 and the polynomial function129, is reported in the literature.
Classification techniques such KNN and SVM are directly applicable to closed loop neurostimulation, and DBS devices with these algorithms embedded within the system hardware is in the near future130. With the appropriate choice of neural signals and feature extraction techniques, machine learning algorithms have the potential to classify behavior and would allow the DBS system to adapt to dynamic patient requirements.
Closing the Loop: DBS Parameter Interventions
Current DBS system neurostimulation consists of a delivering a train of biphasic pulses with adjustable parameters (amplitude, pulse width, and frequency). This train is spatially applied across a cathode and an anode that are adjusted according to the size of the stimulation electrode array. The charge is deposited at the cathode, the negative pole, and the current flows from the cathode to the anode132. Safety of the DBS is ensured because of a net-zero current application across the biphasic waveform of each pulse of the train of stimulation133–135, use of platinum-iridium electrode material136, and limiting the charge density and charge per phase to 26µC/cm2/phase and 0.018µC/phase respectively136. The exact threshold for neural injury is unknown, however neural injury has been noted at charge density of 50µC/cm2/phase and charge per phase of 1.0µC/phase with platinum electrodes137, and at considerably lower values for stainless steel electrodes136. Although the spatial and pulse parameters of stimulation may be variably controlled in a closed loop system, early closed loop systems have used only an on/off control49 for these parameters, or have used variable control of amplitude only138.
There are a limited number of published studies demonstrating mature closed loop stimulation systems for essential tremor, Parkinson’s disease, and epilepsy. Therefore, individual reports of human and animal closed loop neurostimulation systems will be reviewed.
Case Study 148: Pathological tremor prediction using surface electromyogram and acceleration
Population: Humans with Parkinson’s disease (PD) and essential tremor (ET), open label feasibility trial
Objective: To develop a closed loop neurostimulation system to switch DBS on/off using a tremor prediction algorithm. Tremor was predicted using surface electromyogram (sEMG) and acceleration from tremor-affected extremities.
Approach: Signal features were extracted from recorded EMG and acceleration signals. These features included spectral parameters from Fourier and wavelet transforms, and nonlinear time series, such as sample entropy and recurrence quantification analysis (RQA). Features are used to classify and predict PD and ET states using a simple thresholding classifier, to turn the DBS on and off.
Main results: The resulting algorithm predicted tremor onset for all 91 trials recorded in 4 Parkinson’s disease patients and for all 91 trials recorded in 4 essential tremor patients. The predictor achieved a 100% sensitivity for all trials considered, with an overall accuracy of 80.2% for PD and 85.7% for ET.
Case Study 2130: Validation of a bi-directional pulse generator for neural state classification
Study design: Ovine model of acute epilepsy, feasibility trial
Objective: To determine the feasibility of the use of an implantable bidirectional sensing and recording pulse generator to create a closed loop neurostimulation system. Specific objectives were to measure disease related neural data to detect a desired neural state and to update neurostimulation parameters in real time based on the detection state of the embedded algorithm.
Approach: Hippocampal stimulation-induced seizures were induced with concurrent sensing and recording. SVM pattern classification was used to categorize the 30s stimulation train induced as a seizure, visible as after discharges on raw LFP tracing and visible in the frequency domain as increased low frequency power in the 5–20Hz band.
Main results: It is feasible to use LFPs recorded during ongoing stimulation to classify neural states such as an acute seizure. Attention must be given to the stimulation and recording electrode selection, as well as the stimulation frequency in order to minimize contamination of LFP with stimulation artifact.
Case Study 357: Spike triggered closed-loop DBS for Parkinson’s disease
Study design: Non-human primate MPTP Parkinsonian model, feasibility trial
Objective: To compare the effectiveness of open loop and closed loop neurostimulation paradigms for Globus Pallidus pars internus (GPi) DBS.
Approach: A single pulse or a short pulse train (7 pulses at 130 Hz, biphasic, 80 µA, 200 µsec) was delivered through a pair of GPi electrodes at a predetermined and fixed latency following action potential detection in either primary motor cortex or GPi. The open loop paradigm consisted of 130Hz continuous stimulation.
Main results. 7-pulse train after an M1 spike significantly reduced GPi firing rate and eliminated oscillatory firing patterns characteristic of PD and improved limb akinesia. Standard DBS had a lesser effect on GPi neuronal firing, with greater maintenance of oscillatory properties and less improvement in limb akinesia.
Case Study 441: Prediction of seizure likelihood with an implanted seizure advisory system
Study design: Humans with medically refractory epilepsy, open label feasibility trial
Objective: To demonstrate feasibility of implanting devices to predict an oncoming seizure, and advise the patient using a handheld device.
Approach: Sixteen channels of electrocorticography recordings surrounding known seizure onset zones were transmitted wirelessly to a hand held device, which advised the patient of the likelihood of an upcoming seizure based on a proprietary algorithm based on patient-specific data. These algorithms were subsequently installed in the patient’s hand held advisory device.
Main Results: 11 of 15 patients were able to train an algorithm for seizure prediction and advisory, and 8 of the 11 patients had a stable prediction algorithm over time. Five patients achieved a seizure prediction sensitivity of > 60%.
Case Study 5139–141: Responsive cortical stimulation for the treatment of epilepsy (NeuroPace Trial)
Study design: Humans with medically refractory epilepsy, randomized pivotal clinical trial with a 12 week blinded, randomized on- or off-stimulation period
Objective: To demonstrate effectiveness of a closed loop neurostimulation system to detect and react to seizures. The trial included 191 patients; 50% had mesial temporal lobe onset, and 73% had bilateral temporal lobe epilepsy.
Approach: The research group implanted a responsive neurostimulator with intracranial depth and surface electrodes at known seizure onset zones. The device was capable of three seizure detection algorithms: line length142, half wave decomposition143, and integrated area under the ECoG signal in a sliding time window. Responsive stimulation parameters included frequency (1–333Hz), current amplitude (0.5–12mA), pulse width per phase (40–1000 (µs), and burst duration (10 to 5000ms). Initial programming recommendations were provided. Maximum charge density was hardware limited at 25 µC/cm2/phase141. Responders were defined as patients with 50% or greater reduction in seizure frequency.
Main Results: In the randomized pivotal study, there was a statistically significant difference in mean seizure count per month between sham and stimulation groups. During the blinded period, the reduction in seizures in stimulation arm was 38%, compared to a 17% reduction in sham group (p=0.012). The responder rate was 43% at one year.
Alternate Applications
While this chapter has focused on movement disorders, the principles involved in system design, signal sources, feature extraction, signal classification and effector control is applicable to virtually any neuropsychiatric disease neuromodulation system under development.
The most work in the closed loop neurostimulation field has been done in the area of epilepsy and signal classification algorithm design for prediction of seizure onset144. Such work has led to the first human implanted seizure prediction system for treatment of medically refractory epilepsy41. The challenges of developing closed-loop neuromodulation systems for epilepsy, as it is with movement disorders, is identification of appropriate patient state variables to drive neuromodulatory therapy; development of informed models that accurately determine the optimal pattern of stimulation in response to patient states; and detection of reliable biomarkers indicating response to therapy. While the NeuroPace trial is seen as a success by some, the inability to precisely address all of these challenges of closed-loop systems may have, in part, contributed to sub-optimal results140.
One can foresee the application of closed loop systems to other neuropsychiatric diseases, particularly to diseases with cyclical symptom patterns, such as depression. Identification of neurophysiological biomarkers indicative of a diseased or pathological state could theoretically drive therapy parameters “on-demand.” Similarly, one could envision a system for closed-loop neuromodulation that delivers therapies coincident with memory acquisition states so as to reinforce learning.
Closing remarks
The field of closed loop neurostimulation is an interdisciplinary science incorporating disciplines of clinical neurosciences and electrical engineering. Given the importance of neuronal oscillations in the cooperative functioning of brain ensembles, the appeal for a neurostimulation system with a small electrical footprint is evident. Thus, closed loop stimulation is preferable over open loop stimulation for its less disruptive impact on cognitive processes that depend on coordinated neuronal oscillations.
Fundamentally, closed loop stimulation strategies must target certain desirable neural states that can be restored, such as an optimal state for walking, talking, or writing. Closed loop neurostimulation systems may use physiological signals available at the site of the stimulation (e.g. LFP data), or signals that are geographically removed from the site of stimulation (e.g. ECoG or EEG data). Non-central nervous system signals are also available for use in closed loop systems, such as EMG signals, or signals from internal and external sensors (e.g. accelerometers, timing devices, or audio devices). Once these signals are measured and digitized, they must be compressed or transformed into features suitably efficient for processing by pattern classification algorithms for accurate prediction of neural patient states. These algorithms may be pre-embedded in the device130, or first explored within a high power computer cluster before downloading a lightweight patient-customized version into the device’s memory41. Finally, the algorithm must intelligently manipulate stimulation parameters in the domains of time, frequency, or space to optimize the patient’s neurological condition.
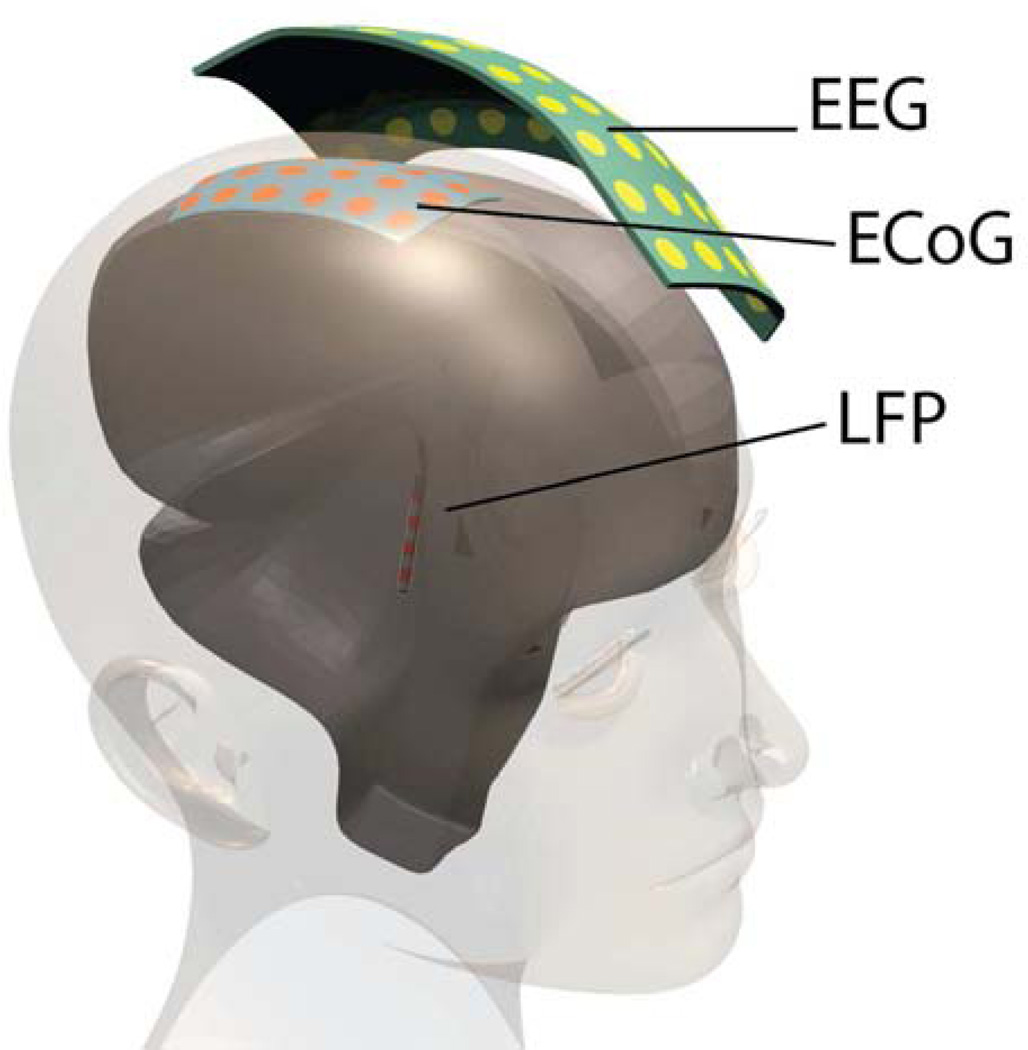
Figure 2.
Candidate neural signals for closed loop neurostimulation systems include non-invasive electroencephalography (EEG), or invasive electrocorticography (ECoG) or local field potentials (LFP). In addition to these brain signals, the system could use electromyography (EMG), or physicals sensors such as accelerometry (not shown).
Highlights.
Closed-loop stimulation may be superior to open loop therapy by reducing the impact of DBS on cognitive processes that depend on coordinated neuronal oscillations.
Understanding the relationship between the gross patient behavior (or severity of disease) and a neuronal signal that is under the influence of external stimulation is fundamental to using the signal in a control system.
A closed loop system extracts a particular feature of a biological signal that has a desired reference value associated with a desired therapeutic state. The system attempts to bring the feature closer to the desired reference value to induce the desired therapeutic state.
Reference values for biosignal features are expected to vary over time in the same patient, and vary over behavioral goals of the patient. Thus, systems must be designed to update with time and cover a range of behavioral situations, such as walking, talking, or writing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.FDA. [Cited 2003 Nov 21];Medtronic Activa Tremor Control System P960009. 1997 Available from http://www.accessdata.fda.gov/cdrh_docs/pdf/p960009.pdf.
- 2.Pahwa R, Lyons KE, Wilkinson SB, Simpson RK, Jr, Ondo WG, Tarsy D, et al. Long-term evaluation of deep brain stimulation of the thalamus. J. Neurosurg. 2006;104:506–512. doi: 10.3171/jns.2006.104.4.506. [DOI] [PubMed] [Google Scholar]
- 3.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 4.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease. Jama J. Am. Med. Assoc. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiss ZHT, Doig-Beyaert K, Eliasziw M, Tsui J, Haffenden A, Suchowersky O, et al. The Canadian multicentre study of deep brain stimulation for cervical dystonia. Brain. 2007;130:2879–2886. doi: 10.1093/brain/awm229. [DOI] [PubMed] [Google Scholar]
- 6.Vidailhet M, Vercueil L, Houeto J-L, Krystkowiak P, Lagrange C, Yelnik J, et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 2007;6:223–229. doi: 10.1016/S1474-4422(07)70035-2. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DA, Center S, Wu C, Keller W. An implantable synchronous pacemaker for the long term correction of complete heart block. Am. J. Cardiol. 1963;11:362–567. doi: 10.1016/0002-9149(63)90130-9. [DOI] [PubMed] [Google Scholar]
- 8.Rouse AG, Stanslaski SR, Cong P, Jensen RM, Afshar P, Ullestad D, et al. A chronic generalized bi-directional brain–machine interface. J. Neural Eng. 2011;8:036018. doi: 10.1088/1741-2560/8/3/036018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Priori A, Foffani G, Rossi L, Marceglia S. Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp. Neurol. 2013;245:77–86. doi: 10.1016/j.expneurol.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Shanechi MM, Hu RC, Powers M, Wornell GW, Brown EN, Williams ZM. Neural population partitioning and a concurrent brain-machine interface for sequential motor function. Nat. Neurosci. 2012;15:1715–1722. doi: 10.1038/nn.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ojemann GA, Schoenfield-McNeill J, Corina D. Different neurons in different regions of human temporal lobe distinguish correct from incorrect identification or memory. Neuropsychologia. 2004;42:1383–1393. doi: 10.1016/j.neuropsychologia.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Flint RD, Lindberg EW, Jordan LR, Miller LE, Slutzky MW. Accurate decoding of reaching movements from field potentials in the absence of spikes. J. Neural Eng. 2012;9:046006. doi: 10.1088/1741-2560/9/4/046006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller KJ, Zanos S, Fetz EE, den Nijs M, Ojemann JG. Decoupling the Cortical Power Spectrum Reveals Real-Time Representation of Individual Finger Movements in Humans. J. Neurosci. 2009;29:3132–3137. doi: 10.1523/JNEUROSCI.5506-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Logothetis NK, Liang H. Decoding a bistable percept with integrated time-frequency representation of single-trial local field potential. J. Neural Eng. 2008;5:433–442. doi: 10.1088/1741-2560/5/4/008. [DOI] [PubMed] [Google Scholar]
- 15.Hariz MI, Rehncrona S, Quinn NP, Speelman JD, Wensing C Multicentre Advanced Parkinson’s Disease Deep Brain Stimulation Group. Multicenter study on deep brain stimulation in Parkinson’s disease: An independent assessment of reported adverse events at 4 years. Mov. Disord. 2008;23:416–421. doi: 10.1002/mds.21888. [DOI] [PubMed] [Google Scholar]
- 16.Johnson LA, Blakely T, Hermes D, Hakimian S, Ramsey NF, Ojemann JG. Sleep spindles are locally modulated by training on a brain-computer interface. Proc. Natl. Acad. Sci. U. S. A. 2012;109:18583–18588. doi: 10.1073/pnas.1207532109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masquelier T, Hugues E, Deco G, Thorpe SJ. Oscillations, Phase-of-Firing Coding, and Spike Timing-Dependent Plasticity: An Efficient Learning Scheme. J. Neurosci. 2009;29:13484–13493. doi: 10.1523/JNEUROSCI.2207-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 2011;12:105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- 19.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Hebb AO, Ojemann GA. The thalamus and language revisited. Brain Lang. 2013;126:99–108. doi: 10.1016/j.bandl.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Quiroga-Varela A, Walters JR, Brazhnik E, Marin C, Obeso JA. What basal ganglia changes underlie the parkinsonian state? The significance of neuronal oscillatory activity. Neurobiol. Dis. 2013 doi: 10.1016/j.nbd.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miocinovic S, McIntyre CC, Savasta M, Vitek J. Mechnisms of Deep Brain Stimulation. In: Tarsy D, editor. Deep brain stimulation in neurological and psychiatric disorders. Totowa, NJ: Humana Press; 2008. pp. 151–177. [Google Scholar]
- 23.Montgomery EB, Gale JT. Mechanisms of action of deep brain stimulation (DBS) Neurosci. Biobehav. Rev. 2008;32:388–407. doi: 10.1016/j.neubiorev.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J. Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Temel Y, Blokland A, Steinbusch HWM, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog. Neurobiol. 2005;76:393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Hebb AO, Darvas F, Miller KJ. Transient and state modulation of beta power in human subthalamic nucleus during speech production and finger movement. Neuroscience. 2012;202:218–233. doi: 10.1016/j.neuroscience.2011.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makeig S, Kothe C, Mullen T, Bigdely-Shamlo N, Zhilin Zhang, Kreutz-Delgado K. Evolving Signal Processing for Brain Computer Interfaces. Proc. Ieee. 2012;100:1567–1584. [Google Scholar]
- 29.Lettieri C, Rinaldo S, Devigili G, Pauletto G, Verriello L, Budai R, et al. Deep brain stimulation: Subthalamic nucleus electrophysiological activity in awake and anesthetized patients. Clin. Neurophysiol. 2012;123:2406–2413. doi: 10.1016/j.clinph.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Suthana N, Fried I. Percepts to recollections: insights from single neuron recordings in the human brain. Trends Cogn. Sci. 2012;16:427–436. doi: 10.1016/j.tics.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perge JA, Homer ML, Malik WQ, Cash S, Eskandar E, Friehs G, et al. Intra-day signal instabilities affect decoding performance in an intracortical neural interface system. J. Neural Eng. 2013;10:036004. doi: 10.1088/1741-2560/10/3/036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simeral JD, Kim S-P, Black MJ, Donoghue JP, Hochberg LR. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J. Neural Eng. 2011;8:025027. doi: 10.1088/1741-2560/8/2/025027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.BrainGate2: Feasibility Study of an Intracortical Neural Interface System for Persons With Tetraplegia. [Cited 2013 Jul 13]; Available from http://clinicaltrials.gov/ct2/show/NCT00912041.
- 34.Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc. Natl. Acad. Sci. 2013;110:4780–4785. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ince NF, Gupta R, Arica S, Tewfik AH, Ashe J, Pellizzer G. High Accuracy Decoding of Movement Target Direction in Non-Human Primates Based on Common Spatial Patterns of Local Field Potentials. Plos One. 2010;5:e14384. doi: 10.1371/journal.pone.0014384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wander JD, Blakely T, Miller KJ, Weaver KE, Johnson LA, Olson JD, et al. Distributed cortical adaptation during learning of a brain-computer interface task. [Cited 2013 Jun 25];Proc. Natl. Acad. Sci. 2013 doi: 10.1073/pnas.1221127110. Available from http://www.pnas.org/cgi/doi/10.1073/pnas.1221127110. [DOI] [PMC free article] [PubMed]
- 38.Lopez-Azcarate J, Tainta M, Rodriguez-Oroz MC, Valencia M, Gonzalez R, Guridi J, et al. Coupling between Beta and High-Frequency Activity in the Human Subthalamic Nucleus May Be a Pathophysiological Mechanism in Parkinson’s Disease. J. Neurosci. 2010;30:6667–6677. doi: 10.1523/JNEUROSCI.5459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsiokos C, Hu X, Pouratian N. 200–300Hz movement modulated oscillations in the internal globus pallidus of patients with Parkinson’s Disease. Neurobiol. Dis. 2013;54:464–474. doi: 10.1016/j.nbd.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. [Cited 2013 Jun 25];Front. Hum. Neurosci. 2012 6 doi: 10.3389/fnhum.2012.00155. Available from http://www.frontiersin.org/Human_Neuroscience/10.3389/fnhum.2012.00155/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook MJ, O’Brien TJ, Berkovic SF, Murphy M, Morokoff A, Fabinyi G, et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. [Cited 2013 Jun 25];Lancet Neurol. 2013 doi: 10.1016/S1474-4422(13)70075-9. Available from http://www.sciencedirect.com/science/article/pii/S1474442213700759. [DOI] [PubMed]
- 42.Mak JN, McFarland DJ, Vaughan TM, McCane LM, Tsui PZ, Zeitlin DJ, et al. EEG correlates of P300-based brain-computer interface (BCI) performance in people with amyotrophic lateral sclerosis. J. Neural Eng. 2012;9:026014. doi: 10.1088/1741-2560/9/2/026014. [DOI] [PubMed] [Google Scholar]
- 43.Sarnthein J, Jeanmonod D. High Thalamocortical Theta Coherence in Patients with Parkinson’s Disease. J. Neurosci. 2007;27:124–131. doi: 10.1523/JNEUROSCI.2411-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matlack C, Moritz C, Chizeck HJ. Applying Best Practices from Digital Control Systems to BMI Implementation. Engineering in Medicine and Biology Society, 2012. EMBC 2012; Annual International Conference of the IEEE; 2012. [DOI] [PubMed] [Google Scholar]
- 45.Lee KH, Chang S-Y, Jang D-P, Kim I, Goerss S, Van Gompel J, et al. Emerging techniques for elucidating mechanism of action of deep brain stimulation. Engineering in Medicine and Biology Society, EMBC, 2011; Annual International Conference of the IEEE; 2011. [Cited 2013 Jun 25]. pp. 677–680. [Cited 2013 Jun 25] Available from http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=6090152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griessenauer CJ, Chang S-Y, Tye SJ, Kimble CJ, Bennet KE, Garris PA, et al. Wireless Instantaneous Neurotransmitter Concentration System: electrochemical monitoring of serotonin using fast-scan cyclic voltammetry-a proof-of-principle study: Laboratory investigation. J. Neurosurg. 2010;113:656–665. doi: 10.3171/2010.3.JNS091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hilliard JD, Frysinger RC, Elias WJ. Effective Subthalamic Nucleus Deep Brain Stimulation Sites May Differ for Tremor, Bradykinesia and Gait Disturbances in Parkinson’s Disease. Stereotact. Funct. Neurosurg. 2011;89:357–364. doi: 10.1159/000331269. [DOI] [PubMed] [Google Scholar]
- 48.Basu I, Graupe D, Tuninetti D, Shukla P, Slavin KV, Metman LV, et al. Pathological tremor prediction using surface electromyogram and acceleration: potential use in “ON- OFF” demand driven deep brain stimulator design. J. Neural Eng. 2013;10:036019. doi: 10.1088/1741-2560/10/3/036019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto T, Katayama Y, Ushiba J, Yoshino H, Obuchi T, Kobayashi K, et al. On-Demand Control System for Deep Brain Stimulation for Treatment of Intention Tremor. Neuromodulation Technol. Neural Interface. 2013;16:230–235. doi: 10.1111/j.1525-1403.2012.00521.x. [DOI] [PubMed] [Google Scholar]
- 50.Abdelhalim K, Jafari HM, Kokarovtseva L, Velazquez JLP, Genov R. 64-Channel UWB wireless neural vector analyzer and phase synchrony-triggered stimulator SoC. [Cited 2013 Jun 22];ESSCIRC (ESSCIRC), 2012 Proceedings of the. 2012 :281–284. [Cited 2013 Jun 22] Available from http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=6341340.
- 51.Wang L, Guo H, Yu X, Wang S, Xu C, Fu F, et al. Responsive Electrical Stimulation Suppresses Epileptic Seizures in Rats. Plos One. 2012;7:e38141. doi: 10.1371/journal.pone.0038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salam MT, Mirzaei M, Ly MS, Dang Khoa Nguyen, Sawan M. An Implantable Closedloop Asynchronous Drug Delivery System for the Treatment of Refractory Epilepsy. Ieee Trans. Neural Syst. Rehabil. Eng. 2012;20:432–442. doi: 10.1109/TNSRE.2012.2189020. [DOI] [PubMed] [Google Scholar]
- 53.Salam MT, Mounaïm F, Nguyen DK, Sawan M. Low-Power Circuit Techniques for Epileptic Seizures Detection and Subsequent Neurostimulation. J. Low Power Electron. 2012;8:133–145. [Google Scholar]
- 54.Farajidavar A, Hagains CE, Peng YB, Chiao J-C. A Closed Loop Feedback System for Automatic Detection and Inhibition of Mechano-Nociceptive Neural Activity. Ieee Trans. Neural Syst. Rehabil. Eng. 2012;20:478–487. doi: 10.1109/TNSRE.2012.2197220. [DOI] [PubMed] [Google Scholar]
- 55.Beverlin IIB, Netoff TI. Dynamic control of modeled tonic-clonic seizure states with closed-loop stimulation. [Cited 2013 Jun 22];Front. Neural Circuits. 2013 6 doi: 10.3389/fncir.2012.00126. Available from http://www.frontiersin.org/Neural_Circuits/10.3389/fncir.2012.00126/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang S-F, Liao Y-C, Shaw F-Z, Chang D-W, Young C-P, Chiueh H. Closed-loop seizure control on epileptic rat models. J. Neural Eng. 2011;8:045001. doi: 10.1088/1741-2560/8/4/045001. [DOI] [PubMed] [Google Scholar]
- 57.Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, et al. Closed-Loop Deep Brain Stimulation Is Superior in Ameliorating Parkinsonism. Neuron. 2011;72:370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 58.Stanslaski S, Cong P, Carlson D, Santa W, Jensen R, Molnar G, et al. An implantable bidirectional brain-machine interface system for chronic neuroprosthesis research. Engineering in Medicine and Biology Society, 2009. EMBC 2009; Annual International Conference of the IEEE; 2009. [Cited 2013 Jun 22]. pp. 5494–5497. [Cited 2013 Jun 22] Available from http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=5334562. [DOI] [PubMed] [Google Scholar]
- 59.Holmes MD, Ojemann GA, Lettich E. Neuronal activity in human right lateral temporal cortex related to visuospatial memory and perception. Brain Res. 1996;711:44–49. doi: 10.1016/0006-8993(95)01351-2. [DOI] [PubMed] [Google Scholar]
- 60.Fried I, Cameron KA, Yashar S, Fong R, Morrow JW. Inhibitory and excitatory responses of single neurons in the human medial temporal lobe during recognition of faces and objects. Cereb. Cortex New York N 1991. 2002;12:575–584. doi: 10.1093/cercor/12.6.575. [DOI] [PubMed] [Google Scholar]
- 61.Sierens D, Kutz S, Pilitsis J, Bakay RAE. Stereotactic Surgery with Microelectrode Recordings. In: Bakay RAE, editor. Movement disorder surgery: the essentials. New York: Thieme; 2008. pp. 83–114. [Google Scholar]
- 62.Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J. Neurosci. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reich DS, Mechler F, Purpura KP, Victor JD. Interspike intervals, receptive fields, and information encoding in primary visual cortex. J. Neurosci. 2000;20:1964–1974. doi: 10.1523/JNEUROSCI.20-05-01964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kühn AA, Trottenberg T, Kivi A, Kupsch A, Schneider G-H, Brown P. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson’s disease. Exp. Neurol. 2005;194:212–220. doi: 10.1016/j.expneurol.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Avestruz A-T, Santa W, Carlson D, Jensen R, Stanslaski S, Helfenstine A, et al. A 5 uW/Channel Spectral Analysis IC for Chronic Bidirectional Brain Machine Interfaces. Ieee J. Solid-State Circuits. 2008;43:3006–3024. [Google Scholar]
- 66.Costa AH, Hengstler S. Adaptive time-frequency analysis based on autoregressive modeling. Signal Process. 2011;91:740–749. [Google Scholar]
- 67.Semmlow JL. Biosignal and biomedical image processing: MATLAB-based applications. New York: London: Marcel Dekker Taylor & Francis; 2004. [Google Scholar]
- 68.Chatrian G, Bergamini L, Dondey M, Klass D, Lennox-Buchthal M, Petersen I. A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr. Clin. Neurophysiol. 1974;37:538–548. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- 69.Miller KJ, Sorensen LB, Ojemann JG, den Nijs M. Power-law scaling in the brain surface electric potential. Plos Comput. Biol. 2009;5:e1000609. doi: 10.1371/journal.pcbi.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le Van Quyen M, Foucher J, Lachaux J-P, Rodriguez E, Lutz A, Martinerie J, et al. Comparison of Hilbert transform and wavelet methods for the analysis of neuronal synchrony. J. Neurosci. Methods. 2001;111:83–98. doi: 10.1016/s0165-0270(01)00372-7. [DOI] [PubMed] [Google Scholar]
- 71.Le Van Quyen M, Bragin A. Analysis of dynamic brain oscillations: methodological advances. Trends Neurosci. 2007;30:365–373. doi: 10.1016/j.tins.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Denker M, Roux S, Linden H, Diesmann M, Riehle A, Grun S. The Local Field Potential Reflects Surplus Spike Synchrony. Cereb. Cortex. 2011;21:2681–2695. doi: 10.1093/cercor/bhr040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. High gamma power is phase-locked to theta oscillations in human neocortex. science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, et al. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc. Natl. Acad. Sci. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bai O, Lin P, Vorbach S, Floeter MK, Hattori N, Hallett M. A high performance sensorimotor beta rhythm-based brain-computer interface associated with human natural motor behavior. J. Neural Eng. 2008;5:24–35. doi: 10.1088/1741-2560/5/1/003. [DOI] [PubMed] [Google Scholar]
- 76.Bashashati A, Fatourechi M, Ward RK, Birch GE. A survey of signal processing algorithms in brain-computer interfaces based on electrical brain signals. J. Neural Eng. 2007;4:R32–R57. doi: 10.1088/1741-2560/4/2/R03. [DOI] [PubMed] [Google Scholar]
- 77.Fatourechi M, Bashashati A, Ward RK, Birch GE. EMG and EOG artifacts in brain computer interface systems: A survey. Clin. Neurophysiol. 2007;118:480–494. doi: 10.1016/j.clinph.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 78.Schalk G, Brunner P, Gerhardt LA, Bischof H, Wolpaw JR. Brain-computer interfaces (BCIs): Detection instead of classification. J. Neurosci. Methods. 2008;167:51–62. doi: 10.1016/j.jneumeth.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 79.Levine SP, Huggins JE, BeMent SL, Kushwaha RK, Schuh LA, Passaro EA, et al. Identification of electrocorticogram patterns as the basis for a direct brain interface. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 1999;16:439–447. doi: 10.1097/00004691-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Mason SG, Bashashati A, Fatourechi M, Navarro KF, Birch GE. A Comprehensive Survey of Brain Interface Technology Designs. Ann. Biomed. Eng. 2006;35:137–169. doi: 10.1007/s10439-006-9170-0. [DOI] [PubMed] [Google Scholar]
- 81.Bento VA, Cunha JP, Silva FM. Towards a human-robot interface based on the electrical activity of the brain. Humanoid Robots, 2008. Humanoids 2008; 8th IEEE-RAS International Conference on; 2008. [Cited 2013 Jun 22]. pp. 85–90. [Cited 2013 Jun 22] Available from http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=4755936. [Google Scholar]
- 82.Faradji F, Ward RK, Birch GE. Plausibility assessment of a 2-state self-paced mental task-based BCI using the no-control performance analysis. J. Neurosci. Methods. 2009;180:330–339. doi: 10.1016/j.jneumeth.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 83.Hoffmann U, Vesin J-M, Ebrahimi T. Recent advances in brain-computer interfaces. [Cited 2013 Jun 22];IEEE 9th Workshop on Multimedia Signal Processing (MMSP’2007) 2007 :17–19. [Cited 2013 Jun 22] Available from http://www.researchgate.net/publication/37452383_Recent_Advances_in_Brain-Computer_Interfaces/file/79e4150ed5c483a00e.pdf.
- 84.Lotte F, Guan C, Ang KK. Comparison of designs towards a subject-independent brain-computer interface based on motor imagery. Engineering in Medicine and Biology Society, 2009. EMBC 2009; Annual International Conference of the IEEE; 2009. [Cited 2013 Jun 22]. pp. 4543–4546. [Cited 2013 Jun 22] Available from http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=5334126. [DOI] [PubMed] [Google Scholar]
- 85.Kachenoura A, Albera L, Senhadji L, Comon P. ICA: a potential tool for BCI systems. Signal Process. Mag. Ieee. 2008;25:57–68. [Google Scholar]
- 86.Ming D, Sun C, Cheng L, Bai Y, Liu X, An X, et al. ICA-SVM combination algorithm for identification of motor imagery potentials. Computational Intelligence for Measurement Systems and Applications (CIMSA), 2010; IEEE International Conference on; 2010. [Cited 2013 Jun 22]. pp. 92–96. [Cited 2013 Jun 22] Available from http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=5611755. [Google Scholar]
- 87.Bashashati A, Ward RK, Birch GE. Towards Development of a 3-State Self-Paced Brain-Computer Interface. Comput. Intell. Neurosci. 2007;2007:1–8. doi: 10.1155/2007/84386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fatourechi M, Bashashati A, Ward RK, Birch GE. A hybrid genetic algorithm approach for improving the performance of the LF-ASD brain computer interface. Acoustics, Speech, and Signal Processing, 2005. Proceedings.(ICASSP’05); IEEE International Conference on; 2005. [Cited 2013 Jun 22]. pp. v–345. [Cited 2013 Jun 22] Available from http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=1416311. [Google Scholar]
- 89.Li Z, O’Doherty JE, Hanson TL, Lebedev MA, Henriquez CS, Nicolelis MAL. Unscented Kalman Filter for Brain-Machine Interfaces. Plos One. 2009;4:e6243. doi: 10.1371/journal.pone.0006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huggins JE, Levine SP, BeMent SL, Kushwaha RK, Schuh LA, Passaro EA, et al. Detection of event-related potentials for development of a direct brain interface. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 1999;16:448–455. doi: 10.1097/00004691-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 91.Krusienski DJ, Grosse-Wentrup M, Galán F, Coyle D, Miller KJ, Forney E, et al. Critical issues in state-of-the-art brain-computer interface signal processing. J. Neural Eng. 2011;8:025002. doi: 10.1088/1741-2560/8/2/025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coyle D, Prasad G, McGinnity TM. Faster Self-Organizing Fuzzy Neural Network Training and a Hyperparameter Analysis for a Brain–Computer Interface. Ieee Trans. Syst. Man Cybern. Part B Cybern. 2009;39:1458–1471. doi: 10.1109/TSMCB.2009.2018469. [DOI] [PubMed] [Google Scholar]
- 93.Graimann B, Allison B, Pfurtscheller G. Brain–Computer Interfaces: A Gentle Introduction. In: Graimann B, Pfurtscheller G, Allison B, editors. Brain-Computer Interfaces. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. [Cited 2013 Jun 22]. pp. 1–27. [Cited 2013 Jun 22] Available from http://www.springerlink.com/index/10.1007/978-3-642-02091-9_1. [Google Scholar]
- 94.Molina GG, Tsoneva T, Nijholt A. Emotional brain-computer interfaces. Affective Computing and Intelligent Interaction and Workshops, 2009. ACII 2009; 3rd International Conference on; 2009. [Cited 2013 Jun 22]. pp. 1–9. [Cited 2013 Jun 22] Available from http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=5349478. [Google Scholar]
- 95.Besserve M, Jerbi K, Laurent F, Baillet S, Martinerie J, Garnero L. Classification methods for ongoing EEG and MEG signals. Biol Res. 2007;40:415–437. [PubMed] [Google Scholar]
- 96.Carabalona R, Castiglioni P, Gramatica F. Brain-computer interfaces and neurorehabilitation. Stud. Health Technol. Inform. 2009;145:160–176. [PubMed] [Google Scholar]
- 97.Cecotti H. A Self-Paced and Calibration-Less SSVEP-Based Brain Computer Interface Speller. Ieee Trans. Neural Syst. Rehabil. Eng. 2010;18:127–133. doi: 10.1109/TNSRE.2009.2039594. [DOI] [PubMed] [Google Scholar]
- 98.Pfurtscheller G, Allison BZ, Brunner C, Bauernfeind G, Solis-Escalante T, Scherer R, et al. The hybrid BCI. Front. Neurosci. 2010;4:30. doi: 10.3389/fnpro.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Plass-Oude Bos D, Reuderink B, Laar B, Gürkök H, Mühl C, Poel M, et al. Brain-Computer Interfacing and Games. In: Tan DS, Nijholt A, editors. Brain-Computer Interfaces. London: Springer London; 2010. [Cited 2013 Jun 22]. pp. 149–178. [Cited 2013 Jun 22] Available from http://link.springer.com/10.1007/978-1-84996-272-8_10. [Google Scholar]
- 100.Stamps K, Hamam Y. Brain Informatics. Springer; 2010. [Cited 2013 Jun 22]. Towards inexpensive bci control for wheelchair navigation in the enabled environment–a hardware survey; pp. 336–345. [Cited 2013 Jun 22] Available from http://link.springer.com/chapter/10.1007/978-3-642-15314-3_32. [Google Scholar]
- 101.Aloise F, Schettini F, Aricò P, Bianchi L, Riccio A, Mecella M, et al. Advanced brain computer interface for communication and control; Proceedings of the International Conference on Advanced Visual Interfaces; 2010. [Cited 2013 Jun 22]. pp. 399–400. [Cited 2013 Jun 22] Available from http://dl.acm.org/citation.cfm?id=1843076. [Google Scholar]
- 102.Bai O, Lin P, Huang D, Fei D-Y, Floeter MK. Towards a user-friendly brain-computer interface: Initial tests in ALS and PLS patients. Clin. Neurophysiol. 2010;121:1293–1303. doi: 10.1016/j.clinph.2010.02.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ikegami S, Takano K, Saeki N, Kansaku K. Operation of a P300-based brain-computer interface by individuals with cervical spinal cord injury. Clin. Neurophysiol. 2011;122:991–996. doi: 10.1016/j.clinph.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 104.Panicker RC, Puthusserypady S, Ying Sun Adaptation in P300 Brain Computer Interfaces: A Two-Classifier Cotraining Approach. Ieee Trans. Biomed. Eng. 2010;57:2927–2935. doi: 10.1109/TBME.2010.2058804. [DOI] [PubMed] [Google Scholar]
- 105.Salvaris M, Sepulveda F. Visual modifications on the P300 speller BCI paradigm. J. Neural Eng. 2009;6:046011. doi: 10.1088/1741-2560/6/4/046011. [DOI] [PubMed] [Google Scholar]
- 106.Bobrov P, Frolov A, Cantor C, Fedulova I, Bakhnyan M, Zhavoronkov A. Brain-Computer Interface Based on Generation of Visual Images. Plos One. 2011;6:e20674. doi: 10.1371/journal.pone.0020674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kronegg J, Chanel G, Voloshynovskiy S, Pun T. EEG-Based Synchronized Brain-Computer Interfaces: A Model for Optimizing the Number of Mental Tasks. Ieee Trans. Neural Syst. Rehabil. Eng. 2007;15:50–58. doi: 10.1109/TNSRE.2007.891389. [DOI] [PubMed] [Google Scholar]
- 108.Wei Wu, Xiaorong Gao, Bo Hong, Shangkai Gao. Classifying Single-Trial EEG During Motor Imagery by Iterative Spatio-Spectral Patterns Learning (ISSPL) Ieee Trans. Biomed. Eng. 2008;55:1733–1743. doi: 10.1109/tbme.2008.919125. [DOI] [PubMed] [Google Scholar]
- 109.Zhang L, He W, He C, Wang P. Improving Mental Task Classification by Adding High Frequency Band Information. J. Med. Syst. 2008;34:51–60. doi: 10.1007/s10916-008-9215-z. [DOI] [PubMed] [Google Scholar]
- 110.Zhong M, Lotte F, Girolami M, Lécuyer A. Classifying EEG for brain computer interfaces using Gaussian processes. Pattern Recognit. Lett. 2008;29:354–359. [Google Scholar]
- 111.Sherwood J, Derakhshani R. On classifiability of wavelet features for EEG-based brain-computer interfaces. Neural Networks, 2009. IJCNN 2009; International Joint Conference on; 2009. [Cited 2013 Jun 22]. pp. 2895–2902. [Cited 2013 Jun 22] Available from http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=5178939. [Google Scholar]
- 112.Jiang H, Zhang J, Hebb AO, Mahoor M. Time-frequency Analysis of Brain Electrical Signals for Behaviour Recognition in Patients with Parkinson’s Disease; 47th Asilomar Conference on Signals, Systems and Computers; Pacific Grove, CA. 2013. [Google Scholar]
- 113.Haixian Wang, Wenming Zheng. Local Temporal Common Spatial Patterns for Robust Single-Trial EEG Classification. Ieee Trans. Neural Syst. Rehabil. Eng. 2008;16:131–139. doi: 10.1109/TNSRE.2007.914468. [DOI] [PubMed] [Google Scholar]
- 114.Grosse-Wentrup M, Liefhold C, Gramann K, Buss M. Beamforming in Noninvasive Brain Computer Interfaces. Ieee Trans. Biomed. Eng. 2009;56:1209–1219. doi: 10.1109/TBME.2008.2009768. [DOI] [PubMed] [Google Scholar]
- 115.Gutiérrez D, Escalona-Vargas DI. EEG data classification through signal spatial redistribution and optimized linear discriminants. Comput. Methods Programs Biomed. 2010;97:39–47. doi: 10.1016/j.cmpb.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 116.Han K, Wang D. A classification based approach to speech segregation. J. Acoust. Soc. Am. 2012;132:3475–3483. doi: 10.1121/1.4754541. [DOI] [PubMed] [Google Scholar]
- 117.Górska Z, Janicki A. Recognition of extraversion level based on handwriting and support vector machines. Percept. Mot. Skills. 2012;114:857–869. doi: 10.2466/03.09.28.PMS.114.3.857-869. [DOI] [PubMed] [Google Scholar]
- 118.Mourão-Miranda J, Hardoon DR, Hahn T, Marquand AF, Williams SCR, Shawe-Taylor J, et al. Patient classification as an outlier detection problem: an application of the One-Class Support Vector Machine. Neuroimage. 2011;58:793–804. doi: 10.1016/j.neuroimage.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Furdea A, Ruf CA, Halder S, De Massari D, Bogdan M, Rosenstiel W, et al. A new (semantic) reflexive brain-computer interface: in search for a suitable classifier. J. Neurosci. Methods. 2012;203:233–240. doi: 10.1016/j.jneumeth.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 120.Duda RO, Hart PE, Stork DG. Pattern classification. 2nd edition. New York: Wiley; 2000. [Google Scholar]
- 121.Drucker H, Burges CJC, Kaufman L, Smola A, Vapnik V. Support vector regression machines. In: Mozer MC, Jordan MI, Petsche T, editors. Advances in Neural Information Processing Systems 9. Cambridge, MA: MIT Press; 1997. pp. 155–161. [Google Scholar]
- 122.Cortes C, Vapnik V. Support-Vector Networks. Mach. Learn. 1995;20:273–297. [Google Scholar]
- 123.Schölkopf B, Burges C, Vapnik V. Proceedings, First International Conference on Knowledge Discovery & Data Mining. Menlo Park: AAAI Press; 1995. Extracting Support Data for a Given Task; pp. 252–257. http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.42.1588. [Google Scholar]
- 124.Garrett D, Peterson DA, Anderson CW, Thaut MH. Comparison of linear, nonlinear, and feature selection methods for EEG signal classification. Ieee Trans. Neural Syst. Rehabil. Eng. 2003;11:141–144. doi: 10.1109/TNSRE.2003.814441. [DOI] [PubMed] [Google Scholar]
- 125.Hill NJ, Lal TN, Schröder M, Hinterberger T, Widman G, Elger CE, et al. Classifying Event-Related Desynchronization in EEG, ECoG and MEG signals. DAGM. Berlin, Heidelberg: Springer-Verlag; 2006. [Google Scholar]
- 126.Jennifer H, Volker B, Bernd H. Face Recognition Using Component-Based SVM Classification and Morphable Models. Springer-Verlag; 2002. [Google Scholar]
- 127.Joachims T. Text categorization with Support Vector Machines: Learning with many relevant features. In: Nédellec C, Rouveirol C, editors. Machine Learning: ECML-98. Springer Berlin Heidelberg; 1998. pp. 137–142. [Cited 2013 Oct 6]. [Cited 2013 Oct 6] Available from http://link.springer.com/chapter/10.1007/BFb0026683. [Google Scholar]
- 128.Chapelle O, Haffner P, Vapnik VN. Support vector machines for histogram-based image classification. Neural Networks Ieee Trans. 1999;10:1055–1064. doi: 10.1109/72.788646. [DOI] [PubMed] [Google Scholar]
- 129.Scholkopf B, Smola AJ. Learning with Kernels: Support Vector Machines, Regularization, Optimization, and Beyond. Cambridge, MA, USA: MIT Press; 2001. [Google Scholar]
- 130.Stanslaski S, Afshar P, Peng Cong, Giftakis J, Stypulkowski P, Carlson D, et al. Design and Validation of a Fully Implantable, Chronic, Closed-Loop Neuromodulation Device With Concurrent Sensing and Stimulation. Ieee Trans. Neural Syst. Rehabil. Eng. 2012;20:410–421. doi: 10.1109/TNSRE.2012.2183617. [DOI] [PubMed] [Google Scholar]
- 131.Lotte F. [Cited 2013 Jul 7];Study of electroencephalographic signal processing and classification techniques towards the use of brain-computer interfaces in virtual reality applications. 2008 Available from http://hal.archives-ouvertes.fr/tel-00356346/
- 132.Montgomery EB. Deep brain stimulation programming: principles and practice. Oxford [UK]; New York: Oxford University Press; 2010. [Google Scholar]
- 133.Butson CR, McIntyre CC. Differences among implanted pulse generator waveforms cause variations in the neural response to deep brain stimulation. Clin. Neurophysiol. 2007;118:1889–1894. doi: 10.1016/j.clinph.2007.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lilly JC, Hughes JR, Alvord EC, Jr, Galkin TW. Brief, noninjurious electric waveform for stimulation of the brain. Science. 1955;121:468–469. doi: 10.1126/science.121.3144.468. [DOI] [PubMed] [Google Scholar]
- 135.Pudenz RH, Bullara LA, Dru D, Talalla A. Electrical stimulation of the brain. II. Effects on the blood-brain barrier. Surg. Neurol. 1975;4:265–270. [PubMed] [Google Scholar]
- 136.Harnack D, Winter C, Meissner W, Reum T, Kupsch A, Morgenstern R. The effects of electrode material, charge density and stimulation duration on the safety of high-frequency stimulation of the subthalamic nucleus in rats. J. Neurosci. Methods. 2004;138:207–216. doi: 10.1016/j.jneumeth.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 137.McCreery DB, Agnew WF, Yuen TG, Bullara L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans. Biomed. Eng. 1990;37:996–1001. doi: 10.1109/10.102812. [DOI] [PubMed] [Google Scholar]
- 138.Santaniello S, Fiengo G, Glielmo L, Grill WM. Closed-Loop Control of Deep Brain Stimulation: A Simulation Study. Ieee Trans. Neural Syst. Rehabil. Eng. 2011;19:15–24. doi: 10.1109/TNSRE.2010.2081377. [DOI] [PubMed] [Google Scholar]
- 139.Neuropace, Inc. [Cited 2013 Jul 7];RNS® System for Epilepsy. 2013 Available from http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/NeurologicalDevicesPanel/UCM340257.pdf.
- 140.Morrell MJ On behalf of the RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 141.Fountas KN, Smith JR. A novel closed-loop stimulation system in the control of focal, medically refractory epilepsy. In: Sakas DE, Simpson BA, editors. Operative Neuromodulation. Vienna: Springer Vienna; [Cited 2013 Jul 13]. pp. 357–362. [Cited 2013 Jul 13] Available from http://www.springerlink.com/index/10.1007/978-3-211-33081-4_41. [Google Scholar]
- 142.Esteller R, Echauz J, Tcheng T, Litt B, Pless B. Line length: an efficient feature for seizure onset detection. Engineering in Medicine and Biology Society, 2001; Proceedings of the 23rd Annual International Conference of the IEEE; 2001. [Cited 2013 Jul 13]. pp. 1707–1710. [Cited 2013 Jul 13] Available from http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=1020545. [Google Scholar]
- 143.Gotman J. Automatic recognition of epileptic seizures in the EEG. Electroencephalogr. Clin. Neurophysiol. 1982;54:530–540. doi: 10.1016/0013-4694(82)90038-4. [DOI] [PubMed] [Google Scholar]
- 144.Carney PR, Myers S, Geyer JD. Seizure prediction: Methods. Epilepsy Behav. 2011;22:S94–S101. doi: 10.1016/j.yebeh.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]