Abstract
Background
Previous studies have linked prenatal influenza exposure to increased risk of schizophrenia; however, no study has examined the neurodevelopmental sequelae of this prenatal insult before the onset of psychotic symptoms using serological evidence of infection. This study sought to examine the contribution of prenatal influenza A and B exposure to cognitive performance among children who developed psychoses in adulthood versus nonpsychiatric control children.
Methods
Subjects were 111 cases (70 with schizophrenia and 41 with affective psychoses) and 333 matched control subjects followed from gestation until age 7 through the Collaborative Perinatal Project. The Wechsler Intelligence Scale for Children (age 7) was administered and adult psychiatric morbidity was assessed by medical records review and confirmed by a validation study. Assays were conducted from archived prenatal maternal sera collected at birth, and influenza infection was determined by immunoglobulin G (IgG) antibody titers >75th percentile.
Results
Significant decreases in verbal IQ and the information subtest, as well as similar nonsignificant reductions in full scale IQ scores and vocabulary, comprehension, digit span, and picture arrangement subtests were found among cases who were prenatally exposed to influenza B versus cases who were not exposed. Fetal exposure to influenza B did not lead to any significant differences in cognitive performance among control children.
Conclusions
Cumulatively, these findings suggest that a genetic and/or an environmental factor associated with psychosis rendered the fetal brain particularly vulnerable to the effects of influenza B, leading to poorer cognitive performance even before symptom onset.
Keywords: Collaborative Perinatal Project, cognitive outcomes, infection, influenza, IQ, obstetric complications, premorbid, psychosis, schizophrenia, serological
Abnormalities in cognitive performance have been documented as a common occurrence in the premorbid period of schizophrenia, including decreased IQs, delayed language development, and reductions in scores on the Wechsler Intelligence Scale for Children (WISC) subtests picture arrangement, vocabulary, and coding (1–5). Despite these findings, it remains unclear whether premorbid cognitive difficulties are a result of a genetic vulnerability for schizophrenia, environmental risk factors, and/or a combination of genetic and environmental risk factors.
Of the potential environmental contributors, fetal exposures to infectious agents, especially the influenza virus, have been prominent risk factors for schizophrenia (6 –11). Some, but not all, studies have reported that women who were pregnant during influenza epidemics were more likely to give birth to offspring who later developed psychosis, although in these epidemiological studies it was not known whether pregnant women were actually infected (7–9). However, a serological study by Brown et al. (6) found an association between influenza infection during the first trimester of pregnancy and increased risk of schizophrenia spectrum disorders (SSDs). Nevertheless, no study has explored whether fetal exposure to influenza (using serological indicators of infection) is associated with cognitive abnormalities in the premorbid period of psychosis. Examining individuals before the onset of psychotic symptoms not only is key in identifying targets for early intervention/prevention strategies, it also provides an opportunity to parse apart genetic and environmental contributors to cognitive difficulties among individuals with psychotic disorders without the potential confounding effects of medications and symptoms.
The primary aim of this study was to examine whether third trimester prenatal influenza exposure was associated with decreased performance on WISC IQ measures among 7-year-old children who developed psychosis in adulthood versus control children. In keeping with the gene-environment interaction model of the role of obstetric complications (OCs) in the etiology of schizophrenia, children who later developed psychoses were expected to show a differential sensitivity to fetal exposure to influenza compared with control subjects, as evidenced by worsened performance on WISC measures at age 7. Further, the present study sought to determine whether fetal exposure to influenza in the third trimester of pregnancy led to increased incidence of psychosis, as opposed to the first trimester, which was observed in the Brown et al. study (6).
Methods and Materials
Cohort Formation
The Collaborative Perinatal Project (CPP) was a prospective study aimed at investigating prenatal and perinatal contributors to adverse infant and child development. From 1959 to 1966, over 50,000 women were followed during the course of pregnancy at multiple sites throughout the United States (12). Cohort offspring underwent a series of physical, cognitive, and psychosocial evaluations from birth to age 7. The present study used cohort members from the Philadelphia site of the CPP, which consisted of 9236 offspring of 6753 mothers who delivered at two inner-city hospitals—the Pennsylvania Hospital and the Children's Hospital of Philadelphia (90% of deliveries at hospitals) (12). The hospitals for the Philadelphia cohort were chosen to ensure a predominantly African American sample (88%), thereby permitting ethnic balance across the CPP sites.
Identification of Psychotic Offspring
In 1996 (CPP members ages 30–37), a search of the Penn Longitudinal Database was conducted to identify CPP participants (13). This database contained information pertaining to patient contacts with public mental health facilities in Philadelphia from 1985 to 1995. The search yielded 1197 individuals, and of these individuals 339 had a previous diagnosis of a psychotic disorder and 858 individuals received other psychiatric diagnoses (for specific diagnoses, see 14). To verify psychotic disorder register diagnoses, a validation study was conducted using psychiatric medical records.
Records for 144 patients were obtained. Using a standard coding form based on DSM-IV criteria, six raters (two psychiatrists, two clinical psychologists, and two advanced clinical psychology graduate students) conducted chart reviews. There were no exclusion criteria for cases, except those specified in the DSM-IV (15). Ten charts were used for training and 56 cases were selected randomly for interrater reliability (Kappa = .85; 93% simple agreement). To confirm chart diagnoses, 14 individuals diagnosed with a psychotic disorder were interviewed (confirmed in 13 cases). There was only moderate agreement between chart-based and register-based diagnoses (Kappa = .63); thus, only chart-based diagnoses are used in this study. Out of 144 chart reviews, 72 received a diagnosis of schizophrenia or schizoaffective disorder-depressed type (.8% of cohort), 41 were diagnosed with a psychotic form of major depression or bipolar disorder (.4 % of cohort), and 31 were diagnosed with other diagnoses (for specific diagnoses, see [14]).
Three control subjects were matched to each case to maximize the detection of abnormalities with potentially small effect sizes. Control subjects were selected by matching them to cases based on the following criteria: 1) from the same hospital as cases; 2) same sex and race as cases; 3) no sibling in the analytic sample; and 4) being the next 3 CPP births (i.e., closest in date of birth) that match on these criteria. Specifically, 111 subjects who were diagnosed with psychoses in adulthood (70 with schizophrenia and 41 with affective psychoses) and 333 matched control subjects had available prenatal maternal sera and were used in the present study. Demographic characteristics for the sample are displayed in Table 1. Socioeconomic status reflects the occupation and income of the primary wage earner on a scale of 1 (unemployed, on public assistance) to 9 (professional, upper middle class).
Table 1. Demographic Characteristics of the Sample.
| Characteristic | Category | Psychotic Disorders (n = 111) | No Diagnosis (n = 33) | Analysis | |
|---|---|---|---|---|---|
|
| |||||
| F, df, p | χ2, df, p | ||||
| % Female Children | N (%) | 47 (42.34%) | 141 (42.34%) | .0, 1, 1.0 | |
| Birth Weight (Grams) | Mean (SD) | 3074.6 (523.19) | 3130.7 (515.39) | .98, 1, .323 | |
| Gestational Length (Weeks)a | Mean (SD) | 38.77 (4.81) | 39.01 (3.65) | .28, 1, .595 | |
| Mother's Agea | Mean (SD) | 24.17 (6.90) | 24.17 (6.14) | .00, 1, .994 | |
| Previous Pregnanciesa | Mean (SD) | 2.71 (2.66) | 2.61 (2.64) | .13, 1, .717 | |
| Earlier Deliveriesa | Mean (SD) | 3.40 (2.15) | 2.98 (2.15) | 2.23, 1, .136 | |
| Maternal Social Statusa | Mean (SD) | 3.61 (1.86) | 4.07 (1.55) | 6.37, 1, .01 | |
| Mother's Education (Years)a | Mean (SD) | 9.97 (2.02) | 10.33 (2.18) | 2.25, 1, .13 | |
| % African American Children | N (%) | 103 (92.79%) | 308 (92.49%) | .01, 1, .917 | |
| Right Handedness (Child)a | N (%) | 82 (79.61%) | 264 (89.49%) | 6.56, 1, .01 | |
| Influenza A (75th) | N (%) | 28 (25.23%) | 84 (25.23%) | .00, 1, 1.00 | |
| Influenza B (75th) | N (%) | 35 (31.53%) | 80 (24.02%) | 2.44, 1, .118 | |
Data incomplete for noted variables.
Childhood Variables
Three hundred seventy children (96 cases, 60 diagnosed with schizophrenia) were evaluated at age 7 (mean = 7.54, SD = 1.46) with 7 of the 10 subtests of the WISC (information, vocabulary, comprehension, digit span, picture completion, block design, and coding) (16). Verbal IQ (VIQ), performance IQ (PIQ), full scale IQ (FIQ), and scaled scores for subtests were calculated using published norms (16). Lateral dominance was measured at ages 4 and 7 by recording which hand the child used while writing and manipulating objects. Ambiguous cases were identified as left-handed (Table 1).
Processing of Serum and Antibody Measurements
Maternal blood samples were collected at the time of birth and serum samples were stored at the National Institutes of Health repository. All assays were carried out in the Stanley Laboratory of Developmental Neurovirology at Johns Hopkins University Medical Center under the supervision of Dr. Robert Yolken.
Levels of specific immunoglobulin G (IgG) class antibodies to influenza A and influenza B were measured by solid-phase enzyme immunoassays. Reagents were obtained from IBL Corp (Hamburg, Germany) (enzyme-linked immunosorbent assay [ELISA], kits RE56541 and RE5651). Wells of microtiter plates coated with target antigens for influenza A and B were reacted with 100 μL of test serum diluted 1:101 with diluent buffer containing phosphate-buffered saline (PBS) buffer, bovine serum albumin (BSA), <.1 % sodium azide (NaN3). Plates were covered with adhesive foil and incubated for 60 minutes at 18°C to 25°C. Adhesive foil was removed and incubation solution was discarded. Plates were washed three times with 300 μL of diluted wash buffer (PBS buffer, Tween 20). Excess solution was removed by tapping the inverted plate on a paper towel. A total of 100 μL of anti-human IgG, conjugated to peroxidase, protein-containing buffer, stabilizers was pipetted into each well. Plates were covered with new adhesive foil and then wells were incubated for 30 minutes at 18°C to 25°C. Adhesive foil was removed, incubation solution was discarded, and plates were washed three times with 300 μL of diluted wash buffer (PBS buffer, Tween 20). Excess solution was removed by tapping the inverted plate on a paper towel. TMB Substrate Solution (100 μL) was pipetted into each well and then incubated for 20 minutes at 18°C to 25°C in the dark (without adhesive foil). Substrate reactions were stopped by adding 100 μL of TMB Stop Solution into each well (.5 mol/L sulfuric acid [H2SO4]). Contents were mixed by gently shaking the plate. Optical densities were measured with a photometer at 450 nm (reference-wavelength: 600–650 nm) within 60 minutes after pipetting of the Stop Solution.
Most individuals have been exposed to both influenza A and B and therefore are seropositive (carry antibodies) for both. The present study used IgG antibody levels, as measured by optical density values, of greater than the 75th percentile to indicate the probability of an active infection. An increase in IgG antibodies occurs in response to influenza if an individual has been previously exposed to influenza (17). This elevation in levels of IgG persists for approximately 7 to 21 days in healthy adults (17) and data suggest that the IgG response to infection in pregnant women does not differ from nonpregnant women (18). Women with IgG antibodies greater than the 75th percentile had an increased likelihood of having an active influenza infection at some point during the third trimester, given that the samples were collected at birth. All samples were analyzed under code, with the laboratory performing the studies blind to clinical statuses of participants.
Statistical Analyses
Given limited cases exposed to infection (Table 1), individuals who were diagnosed with schizophrenia, schizoaffective disorder, and affective psychoses in adulthood were combined to maximize power to detect significant results. Nevertheless, supplementary analyses were conducted to determine if results differed by diagnostic category, although these results were viewed as preliminary. All statistical analyses were conducted in SAS 9.1 (SAS, Inc., Cary, North Carolina) and all analyses controlled for maternal social status, infant sex, and child's race, being that many of these variables are known to affect the risk of OCs and have associations with schizophrenia (15,19–21). Further, maternal social status can be used as a proxy for postnatal adversity to help parse apart pre- versus postnatal influences on key dependent variables.
To determine if influenza A and/or B significantly predicted case status, logistic regressions were conducted. Coefficients in logistic regression analyses were exponentiated to determine odds ratios and Wald tests were used to test the significance of each coefficient. Analyses of covariance (ANCOVAs) were conducted to determine the relationship between case status and influenza A and B on cognitive variables. All models included influenza by case status interaction terms to determine whether this interaction improved the fit of the models. Three post hoc analyses were conducted: 1) unexposed control subjects were compared with exposed control subjects to determine whether fetal exposure to influenza led to decreases in cognitive performance among children at presumed low genetic risk for psychosis; 2) unexposed cases were compared with unexposed control subjects to determine whether there were significant differences in performance on cognitive measures in the absence of exposure to influenza; and 3) unexposed cases were compared with exposed cases to determine whether cases were preferentially sensitive to influenza exposure. All analyses were conducted separately for influenza A and influenza B due to potential differences in the severity of the strains. Handedness also was controlled for in cognitive analyses due to a significant difference in handedness between case and control children and the potential for handedness to affect cognitive performance (22) (Table 1). Least square mean scores were computed for all of the dependent variables and Cohen's d effect sizes were computed for all post hoc comparisons, such that effect sizes of .0 to .2 were considered small, .3 to .5 were considered medium, and greater than .5 were considered large (23). Any effect size greater than or equal to .25 was considered of importance in interpretations. Analyses were viewed as exploratory and therefore unadjusted for multiple comparisons with p values less than .05 considered significant.
Results
There were no significant associations of fetal exposure to influenza A with any of the dependent variables; therefore, influenza B results will be discussed herein. Results indicated that while controlling for social status, sex, and race, prenatal exposure to influenza B was associated with a 1.5 times increase in the odds of being diagnosed with a psychotic disorder versus no diagnosis, although this association did not reach significance (χ2 = 2.798, df = 1, p = .09; 95% confidence interval [CI] = .932, 2.446). Influenza B exposure was associated with a 1.702 times increases in the odds of a schizophrenia diagnosis compared with control status (χ2 = 2.896, df = 1, p = .089; 95% CI = .922, 3.141), whereas there was no significant relationship between influenza B exposure and affective psychosis status (χ2 = .127, df = 1, p = .722; 95% CI = .515, 2.604).
Intelligence Quotient Results
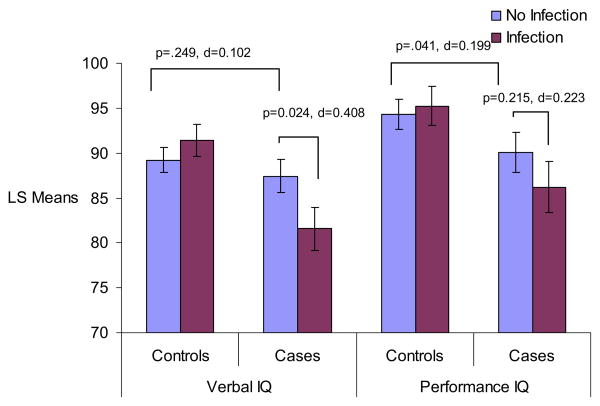
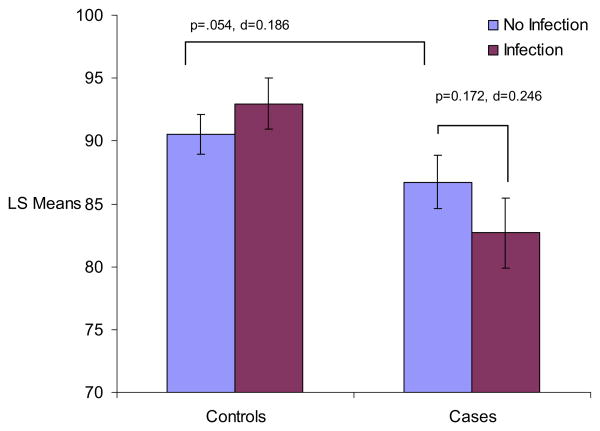
Results from ANCOVA analyses are presented in Table 2 and Figures 1 and 2. There were nonsignificant interactions between case status and fetal exposure to influenza B for FIQ scores (F = 3.34, df = 1, p = .068) and a significant interaction for VIQ scores (F = 6.75, df = 1, p = .010). There was not a significant interaction for PIQ scores (F = 1.66, df = 1, p = .198). Post hoc analyses indicated stepwise decreases on all IQ scores according to genetic loading and influenza exposure, with unexposed cases performing worse than unexposed control subjects and exposed cases performing worse than unexposed cases, although some of these differences did not reach significance. Specifically, there were only nonsignificant decreases in FIQ scores (p = .054, Cohen's d = .186) and VIQ scores (p = .294, Cohen's d = .102) and a significant difference in PIQ between unexposed cases and unexposed control subjects (p = .041, Cohen's d = .199). Further, results indicated nonsignificant decreases in FIQ scores (p = .172, Cohen's d = .246), significant decreases in VIQ scores (p = .024, Cohen's d = .408), and no differences in PIQ scores (p = .2146, Cohen's d = .223) among cases who were exposed prenatally to influenza B compared with cases unexposed. Interestingly, fetal exposure to influenza B led to no significant differences in IQ scores in control subjects.
Table 2. Post Hoc Comparisons from WISC ANCOVA Analyses of Influenza B by Case Status.
| Test | Unexposed Control Subjects vs. Exposed Control Subjects | Unexposed Cases vs. Unexposed Control Subjects | Unexposed Cases vs. Exposed Cases | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| t, df, p | Cohen's d | t, df, p | Cohen's d | t, df, p | Cohen's d | |
| FIQ | − 1.249, 1, .213 | .121 | 1.933, 1, .054 | .186 | 1.369, 1, .172 | .246 |
| PIQ | − .463, 1, .644 | .045 | −2.051, 1, .041 | .199 | 1.243, 1, .215 | .223 |
| VIQ | − 1.287, 1, .199 | .125 | − 1.050, 1, .294 | .102 | 2.270, 1, .024 | .408 |
| Information | − .677, 1, .499 | .067 | −.690, 1, .491 | .068 | 2.061, 1, .04 | .370 |
| Comprehension | − 1.015, 1, .311 | .100 | −.355, 1, .723 | .035 | 1.097, 1, .274 | .197 |
| Vocabulary | − .813, 1, .417 | .100 | −2.257, 1, .025 | .221 | 1.637, 1, .103 | .294 |
| Digit Span | − 1.412, 1, .159 | .139 | .059, 1, .953 | − .006 | 1.757, 1, .0799 | .315 |
| Picture Arrangement | − 1.208, 1, .228 | .119 | −.782, 1, .435 | .077 | 1.459, 1, .146 | .262 |
| Block Design | .753, 1, .452 | − .074 | −2.320, 1, .021 | .227 | .726, 1, .469 | .130 |
| Coding | − .328, 1, .743 | .032 | − 1.458, 1, .146 | .143 | .500, 1, .618 | .090 |
All analyses controlled for child's handedness, child's sex, child's race, and maternal social status.
ANCOVA, analysis of covariance; FIQ, full scale IQ; PIQ, performance IQ; VIQ, verbal IQ; WISC, Wechsler Intelligence Scale for Children.
Figure 1.
ANCOVA age 7 WISC performance and verbal IQ scores by influenza B and group status. Figure displays least square mean values for WISC VIQ and PIQ scores by influenza B and case status. p values and Cohen's d effect sizes are presented for significant post hoc comparisons, as well as post hoc comparisons that approached significance. All analyses controlled for child's handedness, maternal social class, child's race, and child's sex. ANCOVA, analysis of covariance; VIQ, verbal IQ; PIQ, performance IQ; WISC, Wechsler Intelligence Scale for Children.
Figure 2.
ANCOVA analyses for age 7 full scale WISCIQ scores by influenza B and case status. Figure displays least square mean values for WISC FIQ scores by influenza B and case status. p values and Cohen's d effect sizes are presented for significant post hoc comparisons, as well as post hoc comparisons that approached significance. All analyses controlled for child's handedness, maternal social class, child's race, and child's sex. ANCOVA, analysis of covariance; FIQ, full scale IQ; WISC, Wechsler Intelligence Scale for Children.
WISC Subtest Results
ANCOVA analyses (Table 2 and Figures 3 and 4) m indicated that there were significant interactions between case status and fetal exposure to influenza B for the WISC subscales information (F = 8.05, df = 1, p = .005) and digit span (F = 5.01, df = 1, p = .026) and interactions approached significance for vocabulary (F = 3.28, df = 1, p = .071) and picture arrangement (F = 3.53, df = 1, p = .06). In contrast, there were no significant interactions between case status and fetal exposure to influenza B for the subscales comprehension (F = 2.17, df = 1, p = .142), block design (F = .04, df = 1, p = .851), and coding (F = .36, df = 1, p = .551).
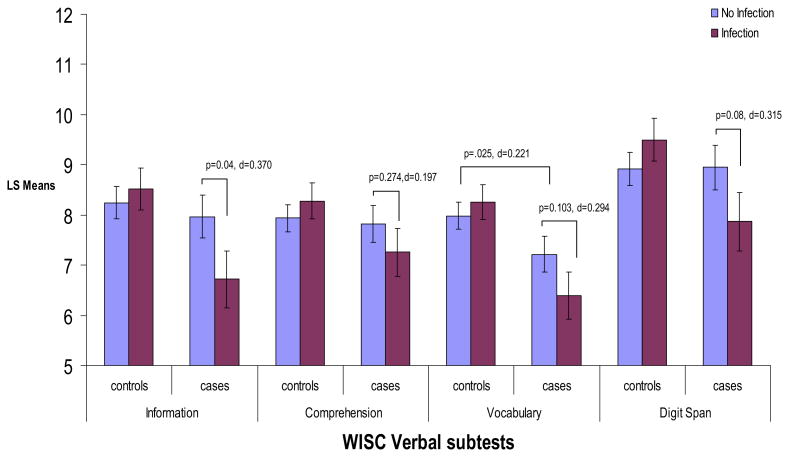
Figure 3.
ANCOVA analyses for age 7WISC verbal subscales by influenza B and case status. Figure displays least square mean values for verbal subscales of the WISC by influenza B and case status. p values and Cohen's d effect sizes are presented for significant post hoc comparisons, as well as post hoc comparisons that approached significance. All analyses controlled for child's handedness, maternal social class, child's race, and child's sex. ANCOVA, analysis of covariance; WISC, Wechsler Intelligence Scale for Children.
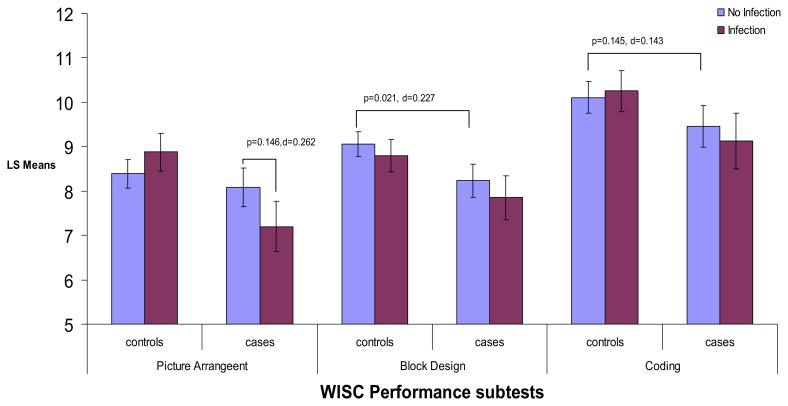
Figure 4.
ANCOVA analyses for age 7 WISC performance subscales by influenza B and case status. Figure displays least square mean values for performance subscales of the WISC by influenza B and case status. p values and Cohen's d effect sizes are presented for significant post hoc comparisons, as well as post hoc comparisons that approached significance. All analyses controlled for child's handedness, maternal social class, child's race, and child's sex. ANCOVA, analysis of covariance; WISC, Wechsler Intelligence Scale for Children.
Post hoc comparisons indicated significant decreases among exposed cases compared with unexposed cases on information (p = .040, Cohen's d = .370) and nonsignificant decreases on digit span (p = .080, Cohen's d = .315), vocabulary (p = .103, Cohen's d = .294), and picture arrangement (p = .146, Cohen's d = .262). Interestingly, after controlling for the other variables in the model, unexposed cases compared with unexposed control subjects only showed significant differences in scores on vocabulary (p = .025, Cohen's d = .221) and block design (p = .021, Cohen's d = .227). There were no significant differences between unexposed cases and unexposed control subjects on information, comprehension, digit span, and picture arrangement. In addition, there were no significant differences on any of the subtests between unexposed and exposed control subjects.
Diagnostic Specificity
Supplementary analyses indicated that the cases who developed schizophrenia in adulthood appeared to primarily contribute to the decreases in IQ scores, with these cases being preferentially sensitive to fetal exposure to influenza B (Table 3). The two exceptions were on the subtests digit span, where fetal exposure to influenza B only led to decreases in scores among cases who developed affective psychosis in adulthood (small effect size), and the subtest vocabulary, where nonsignificant decreases in scores were observed among exposed cases who developed both affective psychosis and schizophrenia in adulthood compared with respective unexposed cases (small effect sizes). After controlling for the other variables in the model, unexposed children who developed schizophrenia in adulthood compared with unexposed control children showed nonsignificant decreases in FIQ, vocabulary, and block design; however, unexposed children who developed affective psychosis in adulthood compared with unexposed control subjects showed nonsignificant decreases on PIQ, block design, and coding.
Table 3. Post Hoc Comparisons for WISC ANCOVA Analyses for Influenza B by Affective Psychosis and Schizophrenia/Schizoaffective Disorder Statuses.
| Test | Affective Psychosis | Schizophrenia/Schizoaffective | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Unexposed Control Subjects vs. Unexposed Cases | Unexposed Cases vs. Exposed Cases | Unexposed Control Subjects vs. Unexposed Cases | Unexposed Cases vs. Exposed Cases | |||||
|
|
|
|
|
|||||
| t, df, p | Cohen's d | t, df, p | Cohen's d | t, df, p | Cohen's d | t, df, p | Cohen's d | |
| FIQ | .625, 1, .533 | .070 | .809, 1, .420 | .159 | 1.888, 1, .060 | .134 | .988, 1, .324 | .148 |
| PIQ | 1.901, 1, .060 | .213 | .071, 1, .944 | .014 | 1.144, 1, .254 | .083 | 1.308, 1, .192 | .196 |
| VIQ | .151, 1, .880 | .017 | 1.372, 1, .173 | .269 | 1.156, 1, .249 | .084 | 1.725, 1, .086 | .258 |
| Information | −.523, 1, .602 | − .059 | .752, 1, .453 | .147 | 1.161, 1, .247 | .085 | 1.904, 1, .058 | .285 |
| Comprehension | .116, 1, .908 | .013 | .511, 1, .610 | .100 | .378, 1, .706 | .028 | 1.098, 1, .273 | .164 |
| Vocabulary | 1.447, 1, .151 | .164 | .666, 1, .507 | .131 | 1.658, 1, .099 | .121 | 1.549, 1, .123 | .232 |
| Digit Span | −.432, 1, .666 | − .049 | 1.738, 1, .085 | .341 | .297, 1, .767 | .022 | .655, 1, .513 | .098 |
| Picture Arrangement | .770, 1, .443 | .087 | 1.144, 1, .255 | .224 | .381, 1, .703 | .028 | .746, 1, .457 | .112 |
| Block Design | 1.607, 1, .111 | .182 | −.174, 1, .862 | −.034 | 1.672, 1, .096 | .122 | .745, 1, .457 | .112 |
| Coding | 1.534, 1, .128 | .174 | −.746, 1, .457 | −.146 | .633, 1, .527 | .046 | 1.290, 1, .198 | .193 |
All analyses controlled for handedness, child's sex, child's race, and maternal social status.
ANCOVA, analysis of covariance; FIQ, full scale IQ; PIQ, performance IQ; VIQ, verbal IQ; WISC, Wechsler Intelligence Scale for Children.
Discussion
The results of this study provide the first evidence that fetal exposure to influenza is associated with preferential decreases in cognitive performance among children who develop psychosis in adulthood compared with unexposed cases. Interestingly, fetal exposure to influenza did not result in any decrements in cognitive performance among control children at presumed low genetic risk for psychosis. These findings suggest that a genetic and/or an additional environmental factor associated with psychosis likely rendered the fetal brain vulnerable to the effects of influenza.
Decreases in cognitive performance among influenza exposed cases were primarily restricted to verbal tasks, with significant decreases in VIQ scores and the subtest information and nonsignificant decreases in FIQ, comprehension, vocabulary, digit span, and picture arrangement scores. During this period of childhood (i.e., around age 7), verbal tasks primarily tap into the child's acquired knowledge, verbal ability, and verbal comprehension, but also involve attention, working memory, and multiple other cognitive domains (24). The results from the present study raise the possibility that early risk factors, such as fetal exposure to influenza B, may partially explain the occurrence of deficits in language acquisition and decreased performance on verbal IQ tasks during the premorbid period of schizophrenia (5,25). The findings from the present study also are likely specific to fetal infection exposure, given that hypoxia-associated OCs were found to be unrelated to language acquisition deficits in the premorbid period (5).
In addition, verbal subtests measure the child's acquired knowledge in word meaning and a variety of disciplines (24), which can provide insight into the child's ability to store and retrieve acquired knowledge (26). Findings from animal studies (mice and rats) suggest that although the influenza virus rarely crosses the placenta, the maternal antiviral responses to infection, such as increases in proinflammatory cytokines, can lead to morphological changes in the hippocampus (e.g., shrinkage of pyramidal cells) and correlated problems in learning and memory, which may partially explain the findings related to verbal scales involving acquired knowledge (27–30). In addition to hippocampal and learning abnormalities, offspring of pregnant rodents injected with polyinosinic:polycytidylic acid (poly I:C), a nonviral inducer of interferon, show similar neuronal and cognitive abnormalities in adulthood as those found among schizophrenic patients, such as increased dopamine turnover and decreased receptor binding of D2-like receptors (but not D1) in the striatum; increased sensitivity to the locomotor-stimulating effects of an N-methyl-D-aspartate (NMDA) receptor antagonist, MK-801, implicating alterations of the dopaminergic and/or glutamatergic systems; deficits in prepulse inhibition (PPI); excessive behavioral switching; loss of latent inhibition; and rapid reversal learning (28,30–32). Moreover, treatment with the anti-psychotic, clozapine, ameliorated most of these deficits, including rapid reversal learning, latent inhibition, and PPI (28,31). Cumulatively, these findings suggest that prenatal exposure to influenza can have long-lasting neurobehavioral effects that persist until adulthood.
Contrary to previous case-control differences on the subtests picture arrangement and coding from the Philadelphia site of the CPP, the present study found that unexposed cases compared with unexposed control subjects only demonstrated significantly decreased scores on PIQ and the vocabulary subtest (1). Although there were fewer matching criteria for control subjects in the present study compared with previous investigations, leading to a slightly different control group, the results from the present study suggest that previous findings partially may have been due to fetal exposure to infection. What was previously thought of as an intermediate endophenotype (i.e., poorer premorbid verbal performance) likely represents a combination of environmental and genetic risk factors, which should be considered in future studies and prevention strategies.
Fetal exposure to influenza A did not result in a similar pattern of results as with influenza B, which raises the possibility that exposure to infection is not sufficient to cause neurodevelopmental sequelae. Although we do not have information on the exact strains of influenza A and B that women were exposed to, the present study suggests that the virulence of the influenza strain, as well as the associated maternal antiviral responses to the infection, likely determine the degree of teratogenicity. Differences in virulence of infections may explain conflicting findings linking various prenatal infections to risk of schizophrenia, but further research with information pertaining to the virulence of the infection is necessary (33,34).
Similar to previous findings (6), the present study found a relationship between fetal exposure to influenza B and psychotic outcome in offspring, although this relationship fell just short of significance. It is possible that the timing of exposure to infection plays a role in determining increased risk for psychosis, given that the Brown et al. study (6) only found an association between first trimester influenza exposure and increased risk for SSD, whereas the present study measured infection during the third trimester of pregnancy, possibly decreasing our power to detect a significant effect (17).
Of particular note was that results seemed to be restricted to cases who developed schizophrenia in adulthood versus those who developed affective psychosis, although these findings should be tentatively interpreted given that there were only 13 cases of affective psychosis who had been exposure to influenza B. Future research is necessary to examine whether fetal exposure to influenza only increases risk for schizophrenia and/or increases risk for many related psychological and neurodevelopmental sequelae.
There are multiple limitations of this study that future studies should address. Specifically, due to the possibility of type 1 errors and the limited sample size, only the gene-environment/environment-environment interaction model was possible to test in the present study; whereas, it is possible that prenatal exposure to influenza additively influences risk for schizophrenia and premorbid abnormalities among cases. Moreover, multiple tests were conducted without adjusting for multiple comparisons, which increases the probability of type 1 errors. Future studies with larger sample sizes are necessary to test whether our results are resilient under more conservative p values and whether fetal exposure to influenza acts additively, interactively, epigenetically (or as a combination of these possibilities) within the neurodevelopmental course of the disorder.
Although there have been multiple studies using the CPP cohort linking cognitive deficits in the premorbid period of schizophrenia to schizophrenic outcome (1,4), it is important to consider that the measures used were designed to assess general intellectual functioning and were not intended to tap into specific cognitive domains associated with OCs or psychiatric diagnoses. There could be many contributors to cognitive functioning besides a genetic vulnerability for schizophrenia and/or OCs, such as sex, race, handedness, socioeconomic status, education, and other life experiences (1,4). Nevertheless, many of these environmental contributors were controlled for in the present study and differences in cognitive performance were still observed. Even so, our study included predominantly African American participants, who have been previously shown to be at increased risk for schizophrenia (21). The present results may have reduced generalizability to other ethnic/racial groups but provide potential clues about the contributors to racial disparities in schizophrenia that should be examined by future investigations. Further, when conducting analyses stratifying by maternal social status (data not shown), results were more pronounced for cases in lower SES strata, supporting theories that there is likely an interaction between prenatal exposures and later environmental adversity (35). Despite these findings, results were in the same direction for cases in higher SES strata, suggesting that prenatal influenza exposure has an independent influence on premorbid childhood cognitive functioning.
There also is a possibility of psychiatric morbidity among control subjects that were not ascertained by screening for reasons such as not being able to identify female patients whose last name changed after marriage, not being able to identify patients who sought treatment during a period other than the ascertainment period, not being able to identify patients who moved, and so on. Although the extent of misclassification cannot be determined precisely, the prevalence of schizophrenia was .8% within the Philadelphia cohort, which is reasonably high given that the most common prevalence estimates of schizophrenia are around 1% (36). Misclassification of some control subjects creates the possibility that the results of the present study are an underestimation of the association between fetal exposure to influenza and premorbid cognitive functioning.
An additional caveat is that serum samples used in this study have been frozen for more than 40 years, raising concerns about the stability of IgG antibodies over time. Careful procedures were used to ensure that samples remained frozen and undisturbed, which considerably protects against the breakdown of proteins. Further, all of the sera were handled uniformly for cases and control subjects; therefore, differences found among these groups should not be attributable to decay over time. In addition, multiple antibodies to infections have been assayed from maternal sera from the CPP and have shown significant relationships with psychotic outcome in offspring, suggesting that the maternal sera are in good condition (34,37).
The present study is the first study to suggest that previously determined premorbid endophenotypes for schizophrenia may be partially explained by exposure to environmental risk factors. Moreover, the effects of these exposures likely vary depending on the severity and mechanisms of action of the given teratogen. Efforts to delineate the causes of neuropsychological disruption in schizophrenia, as well as efforts to adopt early treatment and prevention strategies, would benefit greatly from consideration of additive, interactive, or epigenetic effects of prenatal exposures in genetically susceptible individuals.
Acknowledgments
This study was supported by Grants from the Stanley Medical Research Institute and a gift from Garen and Shari Staglin to TDC; a National Institute of Mental Health (NIMH) fellowship to LME as part of the University of California, Los Angeles (UCLA) Health Psychology Doctoral Program (MH15750); and a postdoctoral NIMH fellowship to LME as part of Columbia University's schizophrenia research fellowship (5 T32 MH018870-20).
We thank Nashid Chaudhury, Anna Plociak, and Katherine Karlsgodt for their contributions to edits on the manuscript. We also would like to thank Bob Bilder, Julie Bower, Chris Dunkel Schetter, Sarosh Motivala, and Elizabeth Breen for their input on this study.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Lauren M. Ellman, Department of Psychiatry, Columbia University, New York, New York
Robert H. Yolken, Stanley Division of Developmental Neurovirology, Johns Hopkins University School of Medicine, Baltimore, Maryland
Stephen L. Buka, Department of Community Health, Brown University Division of Biology and Medicine, Providence, Rhode Island
E. Fuller Torrey, Stanley Medical Research Institute, Chevy Chase, Maryland.
Tyrone D. Cannon, Departments of Psychology, Psychiatry and Biobehavioral Sciences, University of California-Los Angeles, Los Angeles, California
References
- 1.Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, Cannon TD. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- 2.Reichenberg A, Weiser M, Caspi A, Knobler HY, Lubin G, Harvey PD, et al. Premorbid intellectual functioning and risk of schizophrenia and spectrum disorders. J Clin Exp Neuropsychol. 2006;28:193–207. doi: 10.1080/13803390500360372. [DOI] [PubMed] [Google Scholar]
- 3.Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: A meta-analytic review. Am J Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- 4.Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: A prospective cohort study. Schizophr Bull. 2000;26:379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- 5.Bearden CE, Rosso IM, Hollister JM, Sanchez LE, Hadley T, Cannon TD. A prospective cohort study of childhood behavioral deviance and language abnormalities as predictors of adult schizophrenia. Schizophr Bull. 2000;26:395–410. doi: 10.1093/oxfordjournals.schbul.a033461. [DOI] [PubMed] [Google Scholar]
- 6.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 7.McGrath JJ, Pemberton MR, Welham JL, Murray RM. Schizophrenia and the influenza epidemics of 1954, 1957 and 1959: A southern hemisphere study. Schizophr Res. 1994;14:1–8. doi: 10.1016/0920-9964(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 8.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 9.Morgan V, Castle D, Page A, Fazio S, Gurrin L, Burton P, et al. Influenza epidemics and incidence of schizophrenia, affective disorders and mental retardation in Western Australia: No evidence of a major effect. Schizophr Res. 1997;26:25–39. doi: 10.1016/S0920-9964(97)00033-9. [DOI] [PubMed] [Google Scholar]
- 10.Yolken RH, Torrey EF. Viruses, schizophrenia, and bipolar disorder. Clin Microbiol Rev. 1995;8:131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niswader KR, Gordon M. The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke: The Women and Their Pregnancies. Philadelphia: W.B. Saunders; 1972. [Google Scholar]
- 13.Rothbard AB, Schinnar AP, Hadley TR, Rovi JI. Integration of mental health data on hospital and community services. Adm Policy Ment Health. 1990;18:91–99. [Google Scholar]
- 14.Cannon TD, Rosso IM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of neurodevelopmental processes in the genesis and epigenesis of schizophrenia. Dev Psychopathol. 1999;11:467–485. doi: 10.1017/s0954579499002163. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 16.Weschler D. Weschler Intelligence Scale for Children. San Antonio, TX: The Psychological Corporation; 1949. [Google Scholar]
- 17.Janeway CA, Travers P, Walport M, Schlomchik MJ. Immunobiology: The Immune System in Health and Disease. 6th. New York & London: Garland Science; 2005. [Google Scholar]
- 18.Fernandez-Sesma A, Marcell N, Steren C, Sperling R, Moran T. Evaluation of the maternal IgG response to influenza vaccine during pregnancy. Am J Obstet Gynecol. 2005;193(suppl 1):S84. [Google Scholar]
- 19.Feldman PJ, Dunkel-Schetter C, Sandman CA, Wadhwa PD. Maternal social support predicts birth weight and fetal growth in human pregnancy. Psychosom Med. 2000;62:715–725. doi: 10.1097/00006842-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: A life-course perspective. Matern Child Health J. 2003;7:13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 21.Bresnahan M, Begg MD, Brown A, Schaefer C, Sohler N, Insel B, et al. Race and risk of schizophrenia in a US birth cohort: Another example of health disparity? Int J Epidemiol. 2007;36:751–758. doi: 10.1093/ije/dym041. [DOI] [PubMed] [Google Scholar]
- 22.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th. Oxford, England: Oxford University Press; 2004. [Google Scholar]
- 23.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 24.Sattler JS. Assessment of Children: Cognitive Applications. 4th. San Diego: Jerome M. Sattler, Publisher, Inc; 2001. [Google Scholar]
- 25.Niendam TA, Bearden CE, Johnson JK, McKinley M, Loewy R, O'Brien M, et al. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr Res. 2006;84:100–111. doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman AS, Lichtenberger EO. Essentials of WISC-III and WPPSI-R Assessment. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 27.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: The virus is not detected in the fetus. Int J Dev Neurosci. 2005;23:299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Golan HM, Lev V, Hallak M, Sorokin Y, Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48:903–917. doi: 10.1016/j.neuropharm.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: A neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 32.Wolff AR, Bilkey DK. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav Brain Res. 2008;190:156–159. doi: 10.1016/j.bbr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Brown AS, Schaefer CA, Quesenberry CP, Jr, Shen L, Susser ES. No evidence of relation between maternal exposure to herpes simplex virus type 2 and risk of schizophrenia? Am J Psychiatry. 2006;163:2178–2180. doi: 10.1176/ajp.2006.163.12.2178. [DOI] [PubMed] [Google Scholar]
- 34.Buka SL, Cannon TD, Torrey EF, Yolken RH. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol Psychiatry. 2008;63:809–815. doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 35.March D, Hatch SL, Morgan C, Kirkbride JB, Bresnahan M, Fearon P, Susser E. Psychosis and place. Epidemiol Rev. 2008;30:84–100. doi: 10.1093/epirev/mxn006. [DOI] [PubMed] [Google Scholar]
- 36.Cannon TD, Kaprio J, Lonnqvist J, Huttunen M, Koskenvuo M. The genetic epidemiology of schizophrenia in a Finnish twin cohort: A population-based modeling study. Arch Gen Psychiatry. 1998;55:67–74. doi: 10.1001/archpsyc.55.1.67. [DOI] [PubMed] [Google Scholar]
- 37.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]