Abstract
We used the Utah Population Database to examine risk of cancer in relatives of 4,482 pediatric cancer cases (≤ 18 years old) diagnosed from 1966 to 2009 compared to matched population controls. We quantified cancer risk in relatives of children with cancer to determine evidence of familial aggregation and to inform risk assessment and counseling for families. Odds ratios that reflect risk were obtained using conditional logistic regression models adjusting for number of biological relatives, their degree of genetic relatedness and their person-years at risk. First-degree relatives (primarily siblings) of pediatric cases faced a twofold increased risk of a cancer diagnosis before age 19, which extended to their second-degree relatives (p < 10–, respectively). Furthermore, first-degree relatives of children diagnosed before age 5 had a 3.6-fold increased risk of developing pediatric cancer (p < 10–), second-degree relatives of very young (under age 5) cases were at 2.5-fold risk (p < 10–) and third-degree relatives were at twofold risk (P < 1023) of childhood cancer. Although first-degree relatives of pediatric cases have a slight increased risk of adult tumors, when they do develop cancer they have a 1.7-fold risk of developing a tumor in the Li-Fraumeni spectrum. Our findings support the hypothesis of familial aggregation in pediatric cancer and suggest that a higher percent of childhood cancers may be related to hereditary syndromes than are adult cancers. We encourage the collection of a family medical history that is routinely updated for all pediatric cancer patients, and that families with early-onset adult cancers or clusters of several cancers are referred for genetic counseling.
Keywords: pediatric cancers, Li-Fraumeni, familial risk, family history, genetic counseling
Family history is a well-established risk factor for most types of cancer. However, risk of cancer in family members of children diagnosed with a malignancy remains understudied at the population level. Family history of cancer (FHC) changes significantly with time, but accurate and ongoing assessment remains clinically challenging as it relies on self-report and many pediatric cancer survivors in the United States are lost to follow-up as they enter adulthood, compounded by a lack of centralized medical record system. Nevertheless, collection of accurate and comprehensive family history is crucial in the continuum of care1 both in the United States and in other countries where there is less follow-up loss (e.g., the United Kingdom and Canada). Prior studies have evaluated FHC in the setting of pediatric cancers, and increased risks for both childhood and adult-onset cancers have been noted among close relatives.2–5 The magnitude of risk may vary by the type of cancer presenting in the child, indicating that some cancers may have a greater genetic contribution.6 However, because of challenges of collecting and updating FHC, reports of the risk of developing cancer to other relatives in families of pediatric cancer probands are few. Limitations of these investigations include that the analyses are often restricted to first-degree relatives, and most rely on parent-reported family histories.
We examined the risk of childhood and adult cancer in first-, second- and third-degree family members of 4,482 pediatric subjects with cancer diagnosed before age 19 in Utah between 1966 and 2009 compared to matched population controls. Our study uses the unique resource of the Utah Population Database (UPDB), in which an extensive set of family histories containing millions of individuals are record-linked to comprehensive and routinely updated cancer information. The investigation we describe here is novel in that it is the first, to our knowledge, to use this large and unique genealogic resource linked to comprehensive cancer data to broadly examine the risk of all types of cancer in relatives (diagnosed as children themselves or diagnosed in adulthood) of pediatric cancer cases compared to controls in the population who also have pedigree information. Unlike other clinic-based investigations or cancer registry settings,1,3,4 the assessment of family history in our study does not rely in whole or in part on self- or family-reported medical history.
Hereditary syndromes such as Li-Fraumeni syndrome (LFS), an autosomal dominant disorder caused by germline TP53 mutations, are thought to be a rare cause of pediatric cancers. Although most cancer types have been reported to occur in LFS families, Chompret and colleagues have designated the following cancers to be specifically associated with LFS: soft tissue sarcomas, osteosarcomas, brain tumors, premenopausal breast cancer, adrenocortical carcinoma, leukemia and lung cancer. Current clinical recommendations for genetic testing for TP53 mutations are based on the Chompret criteria and include: (i) an individual diagnosed with an LFS-related cancer <46 years of age who has at least one first-degree relative with an LFS-related cancer before age 56 and (ii) an individual with multiple tumors (except for multiple breast cancers), two of which belong to the LFS spectrum and the first having occurred at <46 years of age.7 The large population available in UPDB allowed us to evaluate the LFS spectrum of cancers separately. Our findings provide further evidence of a familial aggregation of childhood cancer and adult early-onset tumors in the LFS spectrum in pediatric cancer patients. This information can guide protocols for identifying families who may benefit from genetic counseling and testing.
Material and Methods
Utah Population Database
The UPDB is a dynamic, shared resource located at the University of Utah and consists of computerized data records for nearly 7 million individuals. It is the only database of its kind in the United States and one of a few in the world; most families living in Utah are represented in the UPDB. For example, when considering all individuals born in Utah in 1950, 79% have grandparent information available in the UPDB and 67% have five or more previous generations documented. The UPDB includes statewide vital records for births and deaths, driver licenses and voter registration records, and statewide hospital inpatient records that are linked concurrently to individuals in existing multigenerational pedigrees, or used to create new pedigrees. Comprehensive cancer records from the Utah Cancer Registry (UCR) are record-linked to the UPDB. The UCR is a registry of statewide cancer records beginning in 1966, and became a Surveillance Epidemiology and End Results (SEER) registry in 1973. For over 40 years, researchers have used the UPDB and linked UCR information to identify and study individuals and their family members that have higher than expected incidence of cancer. Given an ongoing and accurate assessment of FHC that does not depend on self-report, the UPDB provides a valuable resource for a thorough analysis of the familial nature of both childhood and adult cancers in pediatric pro-bands. Approvals were received from the University of Utah's Institutional Review Board (IRB) and Resource for Genetic Epidemiologic Research (the body that reviews potential projects that use the UPDB) to conduct our study. As this non-contact, retrospective-cohort study was designed to pose minimal risk to individuals, we obtained a waiver of informed consent from our IRB.
Statistical analysis
Using a software suite developed specifically for the UPDB,8 we determined the risk of cancer to child and adult relatives of pediatric cancer cases compared to matched, population-based controls within categories of relationship: first-degree, including parents and siblings; second-degree, including grandparents, aunts/uncles, nieces/nephews and third-degree (first cousins). The magnitude of familial risk was estimated by calculating odds ratios (ORs) using conditional logistic regression, adjusting for number of biological relatives, their degree of relatedness and their person-years at risk as described elsewhere.9 Randomly selected controls with a follow-up year in Utah equal to or greater than the case year of diagnosis were matched 5:1 to cases on sex, year of birth and place of birth (in Utah or outside of Utah) and no history of childhood cancer. All cases and matched controls were required to link to a UPDB pedigree comprised of at least two generations to provide family relationships. All relatives of pediatric cases and of matched controls were systematically included in the calculations, even if that relative had been counted previously. For example, in sibships that contain multiple pediatric cancer patients, each case was included as a separate proband and risk among all siblings of each case calculated separately. This approach has been shown to lead to unbiased estimates of familial risk.10 As observations within families are nonindependent, a robust variance estimator for clustered data was incorporated.11 In addition to our analysis of risk of cancer in relatives, we investigated multigeneration families of pediatric cancer cases available in the UPDB to determine if excess clustering of cancers among family members exists within these pedigrees. Familial aggregation of cancer in extended UPDB pedigrees of pediatric probands was assessed using UPDB software to determine the familial standardized incidence ratio (FSIR) of observed cancers in comparison to the number of expected cancers.12
Follow-up procedure
In population-based cohort studies, an accurate assessment of follow-up times in both cases and unaffected controls merits careful consideration. Valid case–control comparisons using retrospective cohort data depend on appropriate matching of exposure periods and longitudinal tracking of individuals. The UPDB records the date that each person in the database was last known to be residing in Utah. However, assessing length of follow-up for selection of population-based pediatric controls can be problematic, as healthy children and adolescents often do not have a statewide record (other than a birth certificate) until they obtain a driver's license, register to vote or marry. We developed an algorithm for determining a date of last residence in children based on the date of an event indicating their mother or adoptive mother was known to be living in Utah, if this date is more recent (Supporting Information Appendix 1). This approach results in a larger, and we suggest a more representative, statewide pool of children with adequate follow-up for potential selection as controls. Of 297,659 children in the UPDB born in Utah between 2000 and 2005, 92% have follow-up information determined by this approach compared to 8% as determined by using the records of the child only. To better capture follow-up periods for healthy adults and to avoid any potential bias in selecting controls for recently diagnosed cases, we developed a secondary procedure to modify the “last residence in Utah” date. If an individual's “last residence” date is based on a driver's license renewal, we assume that an individual who renews a Utah driver's license as required (once every 5 years) is still living in Utah until she or he becomes licensed outside of Utah, surrenders a license or dies (Supporting Information Appendix 1).
Results
There were 6,399 first-primary cancers (the original site at which a tumor becomes clinically detectable) in 6,338 pediatric probands diagnosed before age 19 in Utah identified through the UCR from 1966 through 2009 (Table 1, first column). The majority (80%) of first-primary pediatric tumors were comprised of the following International Classification of Childhood Cancer Third edition (ICCC-3) groups13: leukemias, myeloproliferative and myelodisplastic diseases (26.3%); central nervous system (CNS) and intracranial and intraspinal neoplasms (19.8%); lymphomas and reticuloendothelial neoplasms (12.4%); other malignant epithelial neoplasms and malignant melanomas (9.1%); soft tissue and other extraosseous sarcomas (6.6%) and malignant bone tumors (6.3%). These cancer frequencies reflect the same frequencies observed across the United States.
Table 1.
Characteristics of pediatric cancer cases ages 0–18 in Utah
| All pediatric cases |
Cases with family history available |
Cases without family history available |
|||||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | p 1 | ||||
| Total | 6,338 | 100.0 | 4,482 | 70.7 | 1,856 | 29.3 | |
| Place of birth | |||||||
| In Utah | 3,724 | 58.8 | 3,673 | 82.0 | 51 | 2.7 | |
| Outside of Utah | 2,614 | 41.2 | 809 | 18.0 | 1,805 | 97.3 | <0.0001 |
| Gender | |||||||
| Female | 2,962 | 46.7 | 2,130 | 47.5 | 832 | 44.8 | |
| Male | 3,376 | 53.3 | 2,352 | 52.5 | 1,024 | 55.2 | |
| Year of diagnosis | |||||||
| 1966–1979 | 1,332 | 21.0 | 930 | 20.7 | 402 | 21.7 | |
| 1980–1989 | 1,407 | 22.2 | 973 | 21.7 | 434 | 23.4 | |
| 1990–1999 | 1,697 | 26.8 | 1,225 | 27.3 | 472 | 25.4 | |
| 2000–2009 | 1,902 | 30.0 | 1,354 | 30.2 | 548 | 29.5 | |
| Age at diagnosis | |||||||
| 0–4 | 2,247 | 35.5 | 1,552 | 34.6 | 695 | 37.4 | |
| 5–18 | 4,091 | 64.5 | 2,930 | 65.4 | 1,161 | 62.6 | |
| Last follow-up year in Utah | |||||||
| No Utah follow-up | 1,530 | 24.1 | 104 | 2.3 | 1,426 | 76.8 | |
| Same as birth year | 94 | 1.5 | 87 | 1.9 | 7 | 0.4 | |
| 1–4 years > birth year | 517 | 8.2 | 439 | 9.8 | 78 | 4.2 | |
| 5+ years > birth year | 4,197 | 66.2 | 3,852 | 85.9 | 345 | 18.6 | <0.0001 |
| Vital status | |||||||
| Alive at last follow-up | 4,307 | 68.0 | 3,020 | 67.4 | 1,287 | 69.3 | |
| Deceased, cancer | 1,185 | 18.7 | 1,052 | 23.5 | 133 | 7.2 | |
| Deceased, other/unknown | 846 | 13.3 | 410 | 9.1 | 436 | 23.5 | <0.0001 |
| Multiple primary cancers | |||||||
| First primary only | 6,167 | 97.3 | 4,340 | 96.8 | 1,827 | 98.4 | |
| Multiple primaries | 171 | 2.7 | 142 | 3.2 | 29 | 1.6 | <0.001 |
| Total pediatric tumors (ICCC-3) | 6,399 | 100.0 | 4,528 | 71.4 | 1,871 | 29.5 | |
| Leukemias, myeloproliferative, myelodisplastic diseases | 1,686 | 26.3 | 1,150 | 25.4 | 536 | 28.6 | <0.01 |
| CNS, misc. intracranial and intraspinal neoplasms | 1,270 | 19.8 | 870 | 19.2 | 400 | 21.4 | |
| Lymphomas, reticuloendothelial neoplasms | 795 | 12.4 | 578 | 12.8 | 217 | 11.6 | |
| Other malignant epithelial neoplasms, malignant melanomas | 584 | 9.1 | 480 | 10.6 | 104 | 5.6 | <0.0001 |
| Soft tissue and other extraosseous sarcomas | 425 | 6.6 | 304 | 6.7 | 121 | 6.5 | |
| Malignant bone tumors | 403 | 6.3 | 258 | 5.7 | 145 | 7.7 | <0.01 |
| Neuroblastoma, other peripheral nervous cell tumors | 377 | 5.9 | 254 | 5.6 | 123 | 6.6 | |
| Germ cell tumors, trophoblastic tumors, neoplasms of gonads | 337 | 5.3 | 262 | 5.8 | 75 | 4.0 | <0.01 |
| Renal tumors | 291 | 4.5 | 203 | 4.5 | 88 | 4.7 | |
| Retinoblastomas | 125 | 2.0 | 87 | 1.9 | 38 | 2.0 | |
| Hepatic tumors | 77 | 1.2 | 60 | 1.3 | 17 | 0.9 | |
| Not classified or in situ | 29 | 0.5 | 22 | 0.5 | 7 | 0.4 | |
Chi-squared test of association between cases with and without genealogy; p-values > 0.01 are not displayed.
Abbreviations: ICCC-3: International Classification of Childhood Cancer, Third edition13; CNS: central nervous system.
Of the 6,338 pediatric cancer cases in Utah, 4,482 children (1,552 diagnosed under age 5) were members of at least a two-generation pedigree identified in the UPDB, and thus available for inclusion in our study of familial cancer risk (Table 1, second column). As expected, probands with genealogy information in UPDB were more likely to have been born in Utah, have longer follow-up in Utah, have (when appropriate) death certificate information and have a second primary cancer diagnosed in Utah. Given that Utah is home to a major regional pediatric research and teaching hospital (Primary Children's Medical Center) and a National Cancer Institute-designated Cancer Center (The Huntsman Cancer Institute), it is not surprising that many pediatric cancer patients travel to Utah for treatment from surrounding inter-mountain western states including Idaho, Montana, Nevada and Wyoming and therefore are less likely to have extensive genealogical information, a unique feature of the UPDB in which pedigrees often span several generations. If a pedigree is defined as having no more than three generations (in which pediatric cases within families can be first-, second- or third-degree relatives) 4,377 families had a single pediatric proband diagnosed before age 19; 95 families had two pro-bands per family; eight families had three probands per family and two families had four pediatric probands each. Total person-years at risk (crude incidence) for childhood cancer in relatives of pediatric cases were as follows: first-degree, 227,804 (22 per 100,000); second-degree, 445,390 (14 per 100,000) and third-degree, 590,449 (18 per 100,000). Although not directly comparable to crude incidence, the average age-adjusted incidence (1975–2009) of childhood cancer in Utah (ages 0–19) was 16 per 100,000.14
The numbers of childhood cancer index cases and matched controls are shown alongside the numbers of their first- (Table 2) and second-degree relatives (Table 3) who were diagnosed with pediatric cancer or who were undiag-nosed. Family members who are first-degree relatives (predominantly siblings) of pediatric cancer probands have a twofold increased risk (OR 5 2.0, 95% CI 1.4–2.7; p < 10–) of being diagnosed with any pediatric cancer themselves compared to first-degree relatives of population-based matched controls (Table 2). The increased pediatric cancer risk in first-degree relatives (mainly siblings) of very young cases diagnosed before age 5 is larger in magnitude (OR = 5 3.6, 95% CI 2.3–5.5; p < 107). Second-degree relatives of pediatric cases, who are less likely than siblings to have been raised in the same household, also exhibit a near doubling of risk of a childhood cancer diagnosis compared to second-degree relatives of controls (Table 3). Likewise, second-degree relatives of very young cases experienced a 2.5-fold significant increased risk of a pediatric cancer (OR = 2.5, 95% CI 1.6– 3.9; p < 10–), a risk also observed in first cousins of very young cases (OR = 1.9, 95% CI 1.3–2.6; p ≤ 10–3, data not shown). Within ICCC-3 classifications that comprise 80% of pediatric cancers in Utah, we observed a significant risk of a childhood cancer diagnosis (any site) in first- and second-degree relatives of probands diagnosed with: leukemias, myeloproliferative and myelodisplastic diseases; CNS and intracranial and intraspinal neoplasms and in first-degree relatives only, malignant bone tumors (Tables 2 and 3). Conversely, we did not observe an increased risk of pediatric cancer in relatives of childhood lymphoma patients (Tables 2 and 3).
Table 2.
Risk of childhood cancer in first-degree relatives of 4,482 pediatric cases diagnosed in Utah from 1966 to 2009
| Index cases | Matched controls | First-degree relatives, any cancer at ages 0–18 |
||||||
|---|---|---|---|---|---|---|---|---|
| Relatives of cases |
Relatives of controls |
|||||||
| Diagnosed | Undiagnosed | Diagnosed | Undiagnosed | |||||
| N | N | OR (95% CI) | p | |||||
| Cancer of pediatric case | ||||||||
| Any cancer, ages 0–4 | 1,552 | 7,760 | 32 | 6,226 | 52 | 33,460 | 3.6 (2.3–5.5) | <10–7 |
| Any cancer, ages 0–18 | 4,482 | 22,410 | 49 | 18,476 | 148 | 102,803 | 2.0 (1.4–2.7) | <10–4 |
| ICCC-3 classification, ages 0–18 | ||||||||
| Leukemias, myeloproliferative | 1,150 | 5,750 | 12 | 4,455 | 33 | 25,615 | 2.3 (1.2–4.6) | 0.01 |
| CNS and intracranial/intraspinal | 870 | 4,350 | 20 | 3,607 | 29 | 19,843 | 4.6 (2.6–8.1) | <10–6 |
| Lymphomas, reticuloendothelial | 578 | 2,890 | 4 | 2,426 | 25 | 14,060 | 1.0 (0.4–2.9) | 0.99 |
| Other epithelial and melanomas | 480 | 2,400 | 9 | 2,172 | 24 | 11,344 | 2.0 (0.9–4.3) | 0.08 |
| Soft tissue, other sarcomas | 304 | 1,520 | 3 | 1,205 | 12 | 6,993 | 1.7 (0.5–6.2) | 0.39 |
| Malignant bone tumors | 258 | 1,290 | 6 | 1,047 | 10 | 6,340 | 4.7 (1.7–13.0) | <0.01 |
| Neuroblastoma, nervous cell | 254 | 1,270 | 3 | 1,030 | 11 | 5,750 | 1.8 (0.5–6.3) | 0.38 |
| Germ cell tumors | 262 | 1,310 | 2 | 1,118 | 7 | 5,996 | 1.5 (0.3–7.3) | 0.60 |
| Renal tumors | 203 | 1,015 | 6 | 897 | 0 | 4,989 | Undetermined | – |
| Retinoblastomas | 87 | 435 | 2 | 347 | 2 | 1,928 | 5.7 (0.8–39.0) | 0.08 |
| Hepatic tumors | 60 | 300 | 0 | 235 | 1 | 1,231 | Undetermined | – |
Cases compared to controls matched 5:1 on sex, birth year and birthplace (in Utah or outside of Utah).
Abbreviations: OR: odds ratio from conditional logistic regression; ICCC-3: International Classification of Childhood Cancer, Third edition13; CNS: central nervous system.
Table 3.
Risk of childhood cancer in second-degree relatives of 4,482 pediatric cases diagnosed in Utah from 1966 to 2009
| Index cases | Matched controls | Second-degree relatives, any cancer at ages 0–18 |
||||||
|---|---|---|---|---|---|---|---|---|
| Relatives of cases |
Relatives of controls |
|||||||
| Diagnosed | Undiagnosed | Diagnosed | Undiagnosed | |||||
| Cancer of pediatric case | N | N | OR (95% CI) | p | ||||
| Any cancer, ages 0–4 | 1,552 | 7,760 | 31 | 11,320 | 60 | 53,493 | 2.5 (1.6–3.9) | <10–4 |
| Any cancer, ages 0–18 | 4,482 | 22,410 | 61 | 35,603 | 170 | 172,459 | 1.8 (1.3–2.4) | <10–4 |
| ICCC-3 classification, ages 0–18 | ||||||||
| Leukemias, myeloproliferative | 1,150 | 5,750 | 19 | 8,903 | 46 | 41,256 | 2.0 (1.2–3.3) | 0.01 |
| CNS and intracranial/intraspinal | 870 | 4,350 | 13 | 6,719 | 33 | 32,776 | 1.9 (1.0–3.7) | 0.05 |
| Lymphomas, reticuloendothelial | 578 | 2,890 | 4 | 4,776 | 21 | 23,690 | 1.0 (0.3–2.3) | 0.92 |
| Other epithelial and melanomas | 480 | 2,400 | 11 | 3,791 | 22 | 18,990 | 2.5 (1.2–5.2) | 0.01 |
| Soft tissue, other sarcomas | 304 | 1,520 | 4 | 2,245 | 13 | 11,959 | 1.7 (0.6–5.2) | 0.36 |
| Malignant bone tumors | 258 | 1,290 | 3 | 2,262 | 11 | 10,927 | 1.3 (0.4–4.8) | 0.65 |
| Neuroblastoma, nervous cell | 254 | 1,270 | 7 | 1,959 | 8 | 9,186 | 4.1 (1.5–11.1) | 0.01 |
| Germ cell tumors | 262 | 1,310 | 4 | 2,091 | 11 | 10,230 | 1.8 (0.6–5.8) | 0.30 |
| Renal tumors | 203 | 1,015 | 6 | 1,707 | 0 | 8,155 | Undetermined | – |
| Retinoblastomas | 87 | 435 | 1 | 659 | 4 | 3,017 | 1.3 (0.1–11.2) | 0.84 |
| Hepatic tumors | 60 | 300 | 1 | 459 | 3 | 1,811 | 1.3 (0.1–12.2) | 0.85 |
Cases compared to controls matched 5:1 on sex, birth year and birthplace (in Utah or outside of Utah).
Abbreviations: OR: odds ratio from conditional logistic regression; ICCC-3: International Classification of Childhood Cancer, Third edition13; CNS: central nervous system.
We also examined risk to relatives of probands ages 5–18 at diagnosis, and found an elevated childhood cancer risk (first-degree relatives: OR = 2.2, 95% CI 1.3–3.7, p = 0.003 and second-degree relatives: OR = 1.6, 95% CI 1.1–2.3, p = 0.01;data not shown). Thus, increased risk in relatives was observed whether or not the proband had a diagnosis at a very early age. In contrast to an increased risk of childhood cancer in families of pediatric probands, pediatric relatives of adults diagnosed with cancer (any site) between ages 19 and 79 years old (i.e., the probands are adults with cancer) exhibit only a borderline 1.2-fold increased risk of cancer for first-degree relationships (OR = 1.2, 95% CI 1.0–1.5; p = 0.08; data not shown). In the reverse comparison, first-degree adult relatives of pediatric cancer probands also have a very modest overall risk for developing any type of cancer between the ages of 19 and 79 (OR = 1.2, 95% CI 1.1–1.3; p < 0.001; data not shown).
As noted above, LFS is associated with significantly increased risk of developing several types of early-onset cancers. Although first-degree relatives of pediatric cases in our cohort were found to have a minimally increased risk for developing adult-onset cancer (ages 19–79), when they do develop cancer as an adult they appear more likely to be diagnosed with an LFS-related cancer (OR = 1.7, 95% CI 1.3–2.1; p < 10–3), although the confidence interval overlaps with that of non-LFS tumors diagnosed before age 56 (OR = 1.3, 95% CI 1.1–1.5; p < 0.01) (Table 4). The increased risk of LFS-related cancers was more pronounced in adult relatives (OR = 2.2, 95% CI 1.4–3.5; p < 10–) of very young probands diagnosed with any cancer before age 5. In families of pediatric probands diagnosed specifically with CNS tumors or soft tissue sarcomas, their first-degree adult relatives exhibited an increased risk of LFS-spectrum cancers compared to adult relatives diagnosed with tumors not in the LFS spectrum. Although the number of probands subsequently diagnosed with additional primary cancers in childhood or as adults was small, there was suggestive evidence that their relatives may be at somewhat greater risk of early-onset LFS-spectrum cancers (OR = 2.5, 95% CI 1.1–5.6; p = 0.03, data not shown).
Table 4.
Risk of adult cancer in relatives of 4,482 pediatric cases diagnosed in Utah from 1966 to 2009
| Index cases |
Matched controls |
First-degree relatives, LFS cancer at ages 19–55 |
First-degree relatives, non-LFS cancer at ages 19–55 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relatives of cases |
Relatives of controls |
Relatives of cases |
Relatives of controls |
|||||||||||
| Diagnosed | Undiagnosed | Diagnosed | Undiagnosed | Diagnosed | Undiagnosed | Diagnosed | Undiagnosed | |||||||
| Cancer of pediatric case |
N | N | OR (95% CI) |
p | N | OR (95% CI) |
p | |||||||
| Any cancer, ages 0–4 | 1,552 | 7,760 | 26 | 2,858 | 63 | 16,053 | 2.2 (1.4–3.5) | <10–3 | 52 | 2,832 | 212 | 15,904 | 1.3 (1.0–1.8) | 0.10 |
| Any cancer, ages 0–18 | 4,482 | 22,410 | 75 | 8,778 | 244 | 50,702 | 1.7 (1.3–2.1) | <10–3 | 166 | 8,687 | 711 | 50,235 | 1.3 (1.1–1.5) | <0.01 |
| ICCC-3 classification, ages 0–18 | ||||||||||||||
| Leukemias, myeloproliferative | 1,150 | 5,750 | 22 | 2,040 | 56 | 12,607 | 2.2 (1.3–3.5) | <10–3 | 35 | 2,027 | 155 | 12,508 | 1.2 (0.9–1.8) | 0.28 |
| CNS and intracranial/spinal | 870 | 4,350 | 13 | 1,805 | 37 | 9,796 | 1.8 (1.0–3.5) | 0.06 | 29 | 1,789 | 139 | 9,694 | 1.1 (0.7–1.6) | 0.72 |
| Lymphomas, reticuloendothelial | 578 | 2,890 | 9 | 1,076 | 34 | 6,688 | 1.5 (0.7–3.1) | 0.29 | 25 | 1,060 | 105 | 6,617 | 1.4 (0.9–2.1) | 0.17 |
| Other epithelial, melanomas | 480 | 2,400 | 11 | 1,002 | 34 | 5,522 | 1.7 (0.9–3.5) | 0.12 | 20 | 993 | 66 | 5,490 | 1.6 (1.0–2.7) | 0.06 |
| Soft tissue, other sarcomas | 304 | 1,520 | 6 | 625 | 20 | 3,561 | 1.6 (0.6–3.9) | 0.35 | 8 | 623 | 54 | 3,527 | 0.8 (0.4–1.6) | 0.84 |
| Malignant bone tumors | 258 | 1,290 | 8 | 534 | 24 | 3,107 | 1.6 (0.7–3.6) | 0.25 | 16 | 526 | 60 | 3,071 | 1.3 (0.7–2.3) | 0.38 |
| Neuroblastoma, nervous cell | 254 | 1,270 | 4 | 432 | 19 | 2,700 | 1.4 (0.5–4.1) | 0.59 | 12 | 424 | 25 | 2,694 | 3.1 (1.5–6.4) | <0.01 |
| Germ cell tumors | 262 | 1,310 | 1 | 493 | 16 | 2,890 | 0.4 (0.1–2.7) | 0.31 | 8 | 486 | 47 | 2,859 | 1.0 (0.5–2.1) | 0.94 |
| Renal tumors | 203 | 1,015 | 1 | 487 | 17 | 2,410 | 0.2 (0.0–1.8) | 0.17 | 10 | 478 | 36 | 2,391 | 1.2 (0.6–2.4) | 0.64 |
| Retinoblastomas | 87 | 435 | 1 | 173 | 7 | 998 | 0.7 (0.1–5.7) | 0.71 | 2 | 172 | 10 | 995 | 1.0 (0.2–4.5) | 0.97 |
| Hepatic tumors | 60 | 300 | 0 | 100 | 1 | 560 | Undetermined | – | 3 | 97 | 8 | 553 | 1.8 (0.5–7.1) | 0.39 |
Cases compared to controls matched 5:1 on sex, birth year and birthplace (in Utah or outside of Utah).
Abbreviations: OR: odds ratio from conditional logistic regression; ICCC-3: International Classification of Childhood Cancer, Third edition13; CNS: central nervous system.
As early-onset breast cancer occurs more frequently than other LFS-spectrum cancers we examined risk of breast cancer (diagnosed before age 56) in relatives of pediatric pro-bands. A nonstatistically significant 1.2-fold risk was observed in first-degree family members (OR = 1.2, 95% CI 1.0–1.5, p = 0.13; data not shown). If adult relatives with early-onset breast tumors (a subset who potentially carry non-TP53 gene mutations, e.g., BRCA1 or BRCA2) are excluded from LFS-related cancers, the increased risk to first-degree adult relatives of pediatric probands is threefold (OR = 3.0, 95% CI 1.9–4.5; p < 10–6), whereas increased risk of non-LFS tumors (excluding postmenopausal breast) is similar to non-LFS tumors overall (OR = 1.3, 95% CI 1.1–1.6; p < 0.01, data not shown).
When restricted to non-LFS spectrum tumors only, increased risk of childhood cancer in relatives of pediatric probands (first-degree: OR = 2.1, 95% CI 1.2–4.0, p = 0.02; second-degree: OR = 2.4, 95% CI 1.2–4.8, p = 0.01; data not shown) was consistent with a twofold increased risk of any childhood cancer in first-degree relatives of pediatric pro-bands (any cancer site) in Table 2 or 1.8-fold increased risk in second-degree relatives in Table 3. Unlike cancers diagnosed in adult relatives of childhood patients, which are more likely to be LFS-spectrum tumors, increased risk of pediatric cancer in family members of childhood patients appears to be independent of LFS-spectrum tumors.
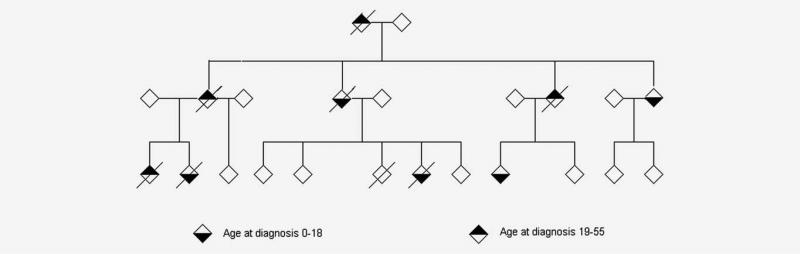
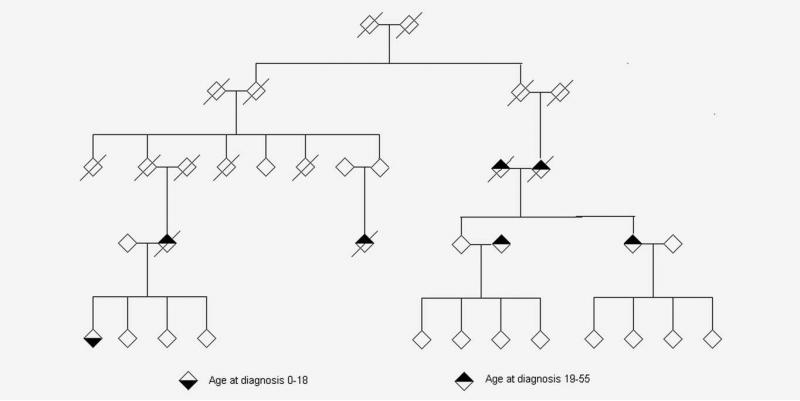
Given our finding that first-degree adult relatives of pediatric cancer cases are at increased risk of early-onset cancers in the LFS spectrum (Table 4), we identified 36 multigeneration UPDB families of pediatric cancer cases in which an excess of tumors were diagnosed in the pedigree (FSIR p < 10–4) suggesting a possible underlying diagnosis of LFS. Two examples of trimmed Utah pedigrees containing at least one pediatric proband and a greater than expected number of LFS-spectrum cancers among their relatives are shown in Figures 1 and 2. Figure 1 shows the pedigree of a family with a history of Li-Fraumeni cancers seen in the Huntsman Cancer Institute’s cancer genetics clinic with highly transmitted disease in which cancers occur in each generation. Figure 2 is an extended pedigree identified in the UPDB as having an excess of LFS-type tumors compared to the number expected in the population, which may harbor a less-penetrant genetic susceptibility in which cancers do not occur in each generation of the pedigree. This second family is not known to have been previously referred for genetic testing.
Figure 1.
Example of a Li-Fraumeni syndrome family containing three pediatric probands (two children diagnosed with brain cancer, a child diagnosed with bone cancer and a child diagnosed with adrenal gland carcinoma) and an excess of Li-Fraumeni syndrome tumors (brain, breast and lung) in adult family members. The pedigree founders were born between 1935 and 1940. All first-primary cancers diagnosed before age 56 are indicated in adults and children; three individuals in the pedigree had multiple primary tumors.
Figure 2.
Example of an extended UPDB pedigree containing a pediatric proband with brain cancer and an excess of Li-Fraumeni spectrum tumors (brain and breast) in adult family members. The pedigree founders were born between 1860 and 1865. All first-primary cancers diagnosed before age 56 are indicated in adults and children.
Discussion
This population-based study assesses cancer risk in relatives of pediatric cancer cases diagnosed in Utah over a more than 40-year period. We observed a general trend of increased familial risk of childhood cancer overall in relatives of pediatric probands who had cancer (any site) or who had a more commonly diagnosed cancer (leukemia, brain and bone tumors). A notable exception is the lack of elevated familial risk in probands diagnosed with lymphoma, the third largest ICCC-3 group in terms of number of cases. For less-often diagnosed childhood cancers (neuroblastomas, germ cell tumors, renal tumors, retinoblastomas and hepatic tumors) results are imprecise; however, there is a suggestion that relatives of probands with neuroblastomas are at elevated risk for childhood cancer. Importantly, our study provides support that childhood cancer risk in relatives (primarily siblings) is especially pronounced when the proband is diagnosed at an early age.
The risk of childhood and adult tumors in first-degree relatives of pediatric probands has been examined in a number of registry- and survey-based studies that have reported elevated risks in siblings.2–5,15 However, few large investigations have explored familial risk in relatives beyond first-degree, a strength of our study. In Swedish childhood cancer patients 18 years or younger, researchers examined the incidence of childhood and adult tumors across tumor sites in families using family history obtained through questionnaires and registry data,16 observing the incidence of childhood tumors among first- to third-degree relatives was 2.5-fold higher than expected; they reported that 14 of 194 (7%) childhood cancer patients also had a first- through third-degree relative with a childhood tumor. In our investigation of 4,482 pediatric patients with linked genealogy and cancer data, a somewhat lower proportion of cases (215 subjects or 5%) had a relative (first- through third-degree) with a childhood tumor. In the Nationwide Swedish Family-Cancer Database, in which children born in 1932 and later are registered with their biological parents as families and subsequently linked to cancer records, the highest familial risks of cancer (diagnosed from birth to age 80) were seen in offspring of parents who were diagnosed at an early age.17 Previous investigations of familial risk in pediatric probands have generally been confined to specific cancer sites. Increased site-specific cancer risks (central nervous system) among relatives of children with lymphoma18 and soft tissue sarcoma (cervix and stomach) have been reported,19 as well as cancer incidence among parents of children with solid tumors.20 In these studies, family histories were obtained from self-reports confirmed by medical records where available. Although we did not observe elevated cancer risk in relatives of probands with lymphoma, it is possible that tumors in specific tissues may cluster in their families. Relative risk of sarcomas in relatives of pediatric and adult sarcoma probands has been examined using the UPDB. A strong familiarity among complex genotype sarcomas independent from known cancer predisposition syndromes was identified.21
To date, LFS has been considered a very rare syndrome. However, the association between pediatric cancers and LFS-related cancers demonstrated in our study may indicate that this condition is more common than previously thought or that there are other genetic pathways that result in a similar cancer spectrum. A recent study that involved careful review of medical and family history by a genetic counselor found that up to 29% of children with cancer meet current guidelines for further genetic evaluation. The majority of children (61%) met criteria for cancer genetics evaluation based on additional FHC.1 Prior studies have also noted greater magnitude of risks in relatives of children diagnosed with cancers within the LFS spectrum, suggesting that this syndrome may drive some of the familial clustering.6 However, Searles Nielsen et al. found little evidence to support a relationship between children with brain tumors and family history of brain or other cancers in an international case–control study.22 Surprisingly, we found that 2% of our 4,482 pediatric probands with family relationships in the UPDB met the current Chompret criteria for LFS.7,23 We observed more generally that 542 cases (excluding cancers associated with other hereditary syndromes such as retinoblastoma) of 4,482 pediatric probands (12%) had relatives diagnosed with an LFS-type tumor including both first-degree family members as well as more distant relations (second- or third-degree).
Our study supports that a higher percentage of pediatric cancers appear to be related to hereditary syndromes than the often quoted 5–10% risk in adults for cancer due to inherited syndromes.1 It should be noted that genetic testing has not been performed to confirm whether these families have mutations in the TP53 gene. Therefore, we cannot report the true incidence of LFS-associated TP53 mutations in this unselected pediatric cancer population. Nevertheless, it is noteworthy that we observed such a high rate of families meeting the Chompret criteria for LFS testing based on pediatric probands. Because of the rarity of de novo childhood cancers and limited screening options, there are no recommendations other than routine pediatric visits for screening of pediatric cancer in children. However, recent studies in LFS suggest that aggressive screening approaches for early cancer surveillance may help to improve early cancer detection and survival in this high-risk population of patients.24 Continued research on the role of LFS in childhood cancers and optimal strategies for identifying those at risk is needed to optimize benefit from early targeted cancer surveillance. As follow-up to our findings, planned sequencing studies of pediatric cancers are underway.
The strengths of our study include a unique genealogical and medical database collected without bias. The UPDB is linked to statewide vital records and a comprehensive SEER registry in which cancer history of close and more distantly related family members for more than 40 years has been determined without reliance on self-reported data. Utah is represented by a relatively residentially stable population and the highest total fertility rate in the nation, with long-term follow-up available for both pediatric cancer cases and matched randomly selected controls. Using the UPDB, we are now investigating prognosis in pediatric cases with FHC compared to cases without FHC. In a clinic cohort from Stanford University (N = 263), Eichstadt et al. reported that pediatric cancer patients with positive FHC appeared to have a higher risk of relapse compared to those without FHC, and patients with a first-degree relative with cancer had an increased risk for death compared to first-degree relatives without cancer.25 Using the current pediatric cohort assembled in the UPDB, similar types of studies now can be replicated with much larger sample sizes of nearly 4,500 cases with linked genealogy data.
As all relatives of probands were systematically included in the analysis, in families that contained multiple pediatric cancer patients (e.g., an affected sibling pair) each case was included as a proband and risk among all siblings of each case calculated separately. We acknowledge that this may lead to an ascertainment bias. However, we believe that any such bias is minimal as diagnoses of pediatric cases were obtained from a comprehensive, statewide registry with near complete ascertainment. We acknowledge that the cases and controls linked to UPDB genealogies used to assess cancer risk in relatives may differ from subjects without pedigree information; individuals that link to pedigrees are more likely to be born in Utah and to relocate outside of Utah less often, which may result in a potential for bias due to differential attrition. We did not adjust for multiple comparisons and present nominal p-values for specific study hypotheses, as we performed a limited number of analyses in pediatric patient subgroups. Our observations of increased risk in relatives of pediatric probands overall and in very young probands were significant at the ≥0.001 level and unlikely to represent chance findings.
We report that children with a pediatric cancer have an increased risk for positive FHC compared to population-based controls than adults with cancer, and that this cancer risk is more pronounced in pediatric relatives. These findings have potential clinical implications that merit careful consideration. In particular, we observed a highly significant, near quadrupling of risk of a childhood cancer diagnosis at any tumor site in siblings of probands diagnosed before age 5. This risk is independent of LFS-spectrum tumors and extends beyond first-degree relatives, supporting the hypothesis that earlier age of onset may indicate a greater genetic contribution to cancer risk in these families. Although relatively few databases exist worldwide with features comparable to the UPDB to perform a similar study, future replication is important to validate our findings and those of other research groups in a variety of settings.
On the basis of our findings, we recommend that a three generation family history be collected and routinely updated for all pediatric cancer patients—even after the pediatric patients have completed their treatment. Family members (children and adults) of children presenting with earlier than average onset for their cancer type or with a positive FHC of early-onset adult cancers or multiple adult cancers should be considered for referral for genetic counseling. Although the annual incidence of childhood cancer in the United States is rare26 and poses a very low absolute risk in families, parents of children diagnosed with an early childhood cancer (before age 5) with a positive FHC should be cautiously advised of the potential for increased risk to other children in the family, even in the absence of an identified genetic syndrome such as LFS.
Supplementary Material
What’s new?
Childhood cancer, although relatively rare compared to adulthood cancers, is the leading cause of death in children up to 14 years of age. Little is known about the hereditary components of the disease. Using a large population-based genealogical database in the US state Utah, Curtin and colleagues report that children with cancer have an increased risk for positive cancer family history as compared to controls. Siblings of patients diagnosed before age five have a 3.6-fold childhood cancer risk. Notably, a second report in this issue of IJC also investigated risks associated with childhood cancer and shows an increased risk of adulthood cancer in relatives of children with cancer (Neale et al.). A resulting clinical recommendation is that a three-generation family history be collected and updated for all pediatric patients, and that families with clusters of several cancers are referred to genetic counseling.
ACKNOWLEDGEMENTS
This study was funded by an Alex’s Lemonade Stand Foundation Epidemiology Award (to JDS). JDS is a Damon Runyon Clinical Investigator supported by the Damon Runyon Cancer Research Foundation (CI#56-11). The authors thank the Pedigree and Population Resource (funded by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance and support of the Utah Population Database. They express their gratitude to the Genetic Counseling Shared Resource supported by National Institutes of Health P30 CA042014 awarded to Huntsman Cancer Institute. This research was also supported by the Utah Cancer Registry, which is funded by Contract No. HHSN261201000026C from the National Cancer Institute's SEER Program with additional support from the Utah State Department of Health and the University of Utah. They gratefully acknowledge Carole Schaeffer for her programming assistance and drawing of pedigrees for this study.
Grant sponsor: Alex's Lemonade Stand Foundation Epidemiology Award; Grant sponsor: Utah State Department of Health; Grant sponsor: University of Utah; Grant sponsor: Huntsman Cancer Foundation; Grant sponsor: National Institutes of Health; Grant number: P30 CA042014; Grant sponsor: National Cancer Institute’s SEER Program; Grant number: HHSN261201000026C
Footnotes
Conflicts of interest: Nothing to report
Additional Supporting Information may be found in the online version of this article.
References
- 1.Knapke S, Nagarajan R, Correll J, et al. Hereditary cancer risk assessment in a pediatric oncology follow-up clinic. Pediatr Blood Cancer. 2012;58:85–9. doi: 10.1002/pbc.23283. [DOI] [PubMed] [Google Scholar]
- 2.Draper GJ, Heaf MM, Kinnier Wilson LM. Occurrence of childhood cancers among sibs and estimation of familial risks. J Med Genet. 1977;14:81–90. doi: 10.1136/jmg.14.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draper GJ, Sanders BM, Lennox EL, et al. Patterns of childhood cancer among siblings. Br J Cancer. 1996;74:152–8. doi: 10.1038/bjc.1996.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green DM. Childhood cancer in siblings. Pediatr Hematol Oncol. 1986;3:229–39. doi: 10.3109/08880018609031222. [DOI] [PubMed] [Google Scholar]
- 5.Miller RW. Deaths from childhood leukemia and solid tumors among twins and other sibs in the United States, 1960–67. J Natl Cancer Inst. 1971;46:203–9. [PubMed] [Google Scholar]
- 6.Pang D, Evans G, Birch J. Elevated breast cancer risk among mothers of a population-based series of 2668 children with cancer. Ecancermedicalscience. 2008;2:57. doi: 10.3332/ecancer.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tinat J, Bougeard G, Baert-Desurmont S, et al. 2009 version of the Chompret criteria for Li Fraumeni syndrome. J Clin Oncol. 2009;27:e108–e109. doi: 10.1200/JCO.2009.22.7967. author reply e10. [DOI] [PubMed] [Google Scholar]
- 8.Kerber RA, O'Brien E. A cohort study of cancer risk in relation to family histories of cancer in the Utah population database. Cancer. 2005;103:1906–15. doi: 10.1002/cncr.20989. [DOI] [PubMed] [Google Scholar]
- 9.Guthery SL, Mineau G, Pimentel R, et al. Inflammatory bowel disease aggregation in Utah kindreds. Inflamm Bowel Dis. 2011;17:823–30. doi: 10.1002/ibd.21390. [DOI] [PubMed] [Google Scholar]
- 10.Bai Y, Sherman S, Khoury MJ, et al. Bias associated with study protocols in epidemiologic studies of disease familial aggregation. Am J Epidemiol. 2000;151:927–37. doi: 10.1093/oxfordjournals.aje.a010297. [DOI] [PubMed] [Google Scholar]
- 11.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–6. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 12.Kerber RA. Method for calculating risk associated with family history of a disease. Genet Epidemiol. 1995;12:291–301. doi: 10.1002/gepi.1370120306. [DOI] [PubMed] [Google Scholar]
- 13.Steliarova-Foucher E, Stiller C, Lacour B, et al. International classification of childhood cancer. Cancer. (third edition) 2005;103:1457–67. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute [January 21, 2013];State Cancer Profiles. Incidence rates provided by the Surveillance, Epidemiology and End-Resuts (SEER) program. Available at: http://statecancerprofiles.cancer.gov/index.html.
- 15.Winther JF, Sankila R, Boice JD, et al. Cancer in siblings of children with cancer in the Nordic countries: a population-based cohort study. Lancet. 2001;358:711–17. doi: 10.1016/S0140-6736(01)05838-X. [DOI] [PubMed] [Google Scholar]
- 16.Magnusson S, Wiebe T, Kristoffersson U, et al. Increased incidence of childhood, prostate and breast cancers in relatives of childhood cancer patients. Fam Cancer. 2012;11:145–55. doi: 10.1007/s10689-011-9493-3. [DOI] [PubMed] [Google Scholar]
- 17.Kharazmi E, Fallah M, Sundquist K, et al. Familial risk of early and late onset cancer: nationwide prospective cohort study. BMJ. 2012;345:e8076. doi: 10.1136/bmj.e8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang D, Alston RD, Eden TO, et al. Cancer risks among relatives of children with Hodgkin and non-Hodgkin lymphoma. Int J Cancer. 2008;123:1407–10. doi: 10.1002/ijc.23651. [DOI] [PubMed] [Google Scholar]
- 19.Hartley AL, Birch JM, Teare MD, et al. Cancer risk in second degree relatives of children with soft tissue sarcoma. Br J Cancer. 1991;63:959–62. doi: 10.1038/bjc.1991.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pang D, McNally R, Kelsey A, et al. Cancer incidence and mortality among the parents of a population-based series of 2604 children with cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:538–44. [PubMed] [Google Scholar]
- 21.Jones KB, Schiffman JD, Kohlmann W, et al. Complex genotype sarcomas display familial inheritance independent of known cancer predisposition syndromes. Cancer Epidemiol Biomarkers Prev. 2011;20:751–7. doi: 10.1158/1055-9965.EPI-10-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Searles Nielsen S, Mueller BA, Preston-Martin S, et al. Family cancer history and risk of brain tumors in children: results of the SEARCH international brain tumor study. Cancer Causes Control. 2008;19:641–8. doi: 10.1007/s10552-008-9128-7. [DOI] [PubMed] [Google Scholar]
- 23.Ruijs MW, Verhoef S, Rookus MA, et al. TP53 germline mutation testing in 180 families suspected of Li-Fraumeni syndrome: mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet. 2010;47:421–8. doi: 10.1136/jmg.2009.073429. [DOI] [PubMed] [Google Scholar]
- 24.Villani A, Tabori U, Schiffman J, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: a prospective observational study. Lancet Oncol. 2011;12:559–67. doi: 10.1016/S1470-2045(11)70119-X. [DOI] [PubMed] [Google Scholar]
- 25.Eichstadt SL, Dahl GV, Fisher PG, et al. Correlation of a family history of cancer with risk of relapse and death in pediatric cancer patients. J Clin Oncol. 2009;27:15s. (suppl; abstr 10029) [Google Scholar]
- 26.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.