Abstract
Summary
To determine whether there are race/ethnic differences in bone mineral density (BMD) by fracture history in men aged 65 and older, we performed cross-sectional analysis in five large independent cohorts. Low BMD was associated with a higher prevalence of fracture in all cohorts and the magnitude of the BMD differences by fracture status was similar across groups.
Introduction
We aimed to determine whether there are race/ethnic and geographic differences in bone mineral density (BMD) by fracture history in men aged 65 and older.
Method
The datasets included the Osteoporotic Fractures in Men (MrOS) Study (5,342 White, 243 African-American, 190 Asian, and 126 Hispanic), MrOS Hong Kong (1,968 Hong Kong Chinese), Tobago Bone Health Study (641 Afro-Caribbean), Namwon Study (1,834 Korean), and Dong-gu Study (2,057 Korean). The 2 Korean cohorts were combined.
Results
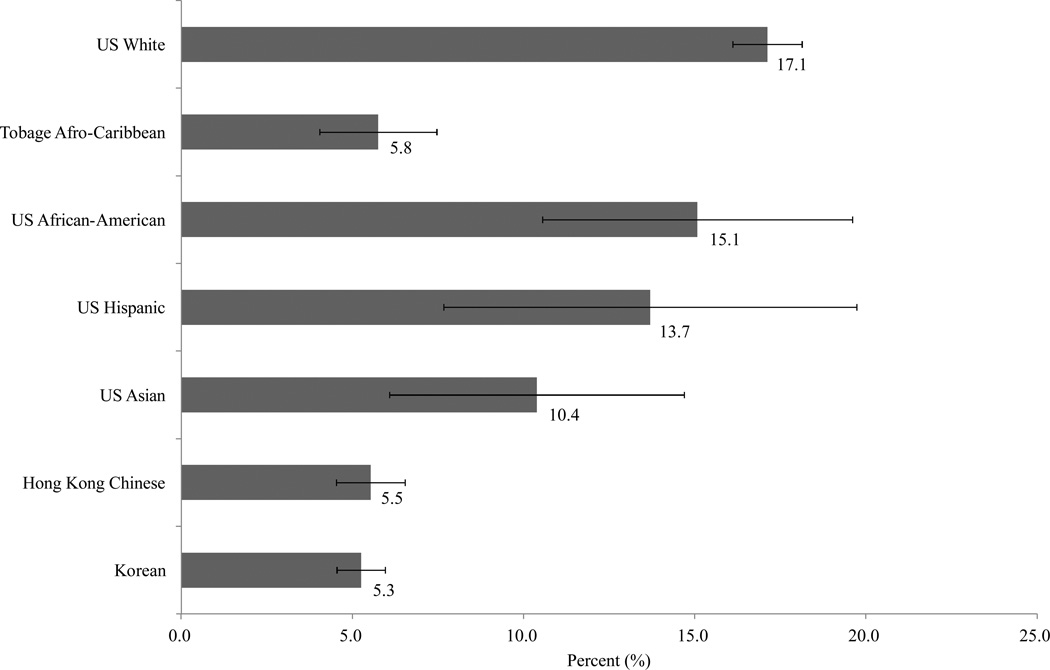
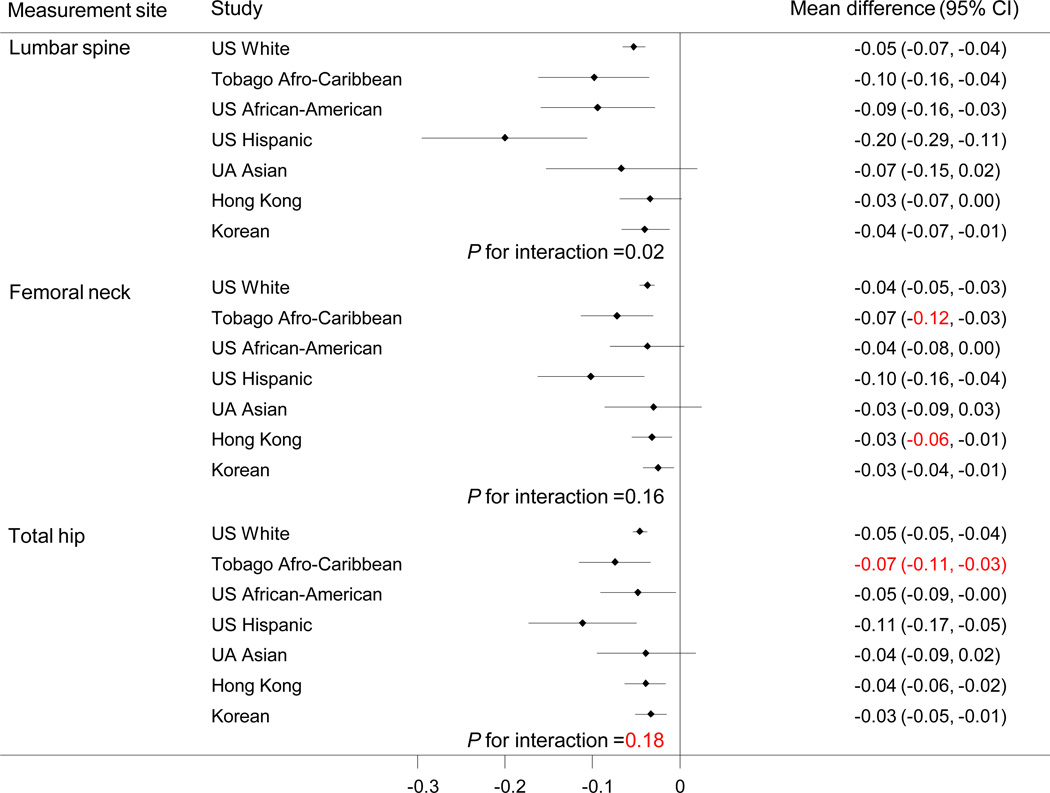
The prevalence of self-reported non-traumatic fracture was US white, 17.1%; Afro-Caribbean, 5.5%; US African-American, 15.1%; US Hispanic, 13.7%; US Asian, 10.5%; Hong Kong Chinese, 5.6% and Korean, 5.1%. The mean differences in hip and lumbar spine BMD between subjects with fracture and without fracture were statistically significant in all cohorts except US African American and US Asian men. There was a significant race/ethnic interaction for lumbar spine BMD by fracture status (p for interaction=0.02), which was driven by the small number of Hispanic men. There was no interaction for femoral neck or total hip BMD. There were no significant race/ethnic differences in the odds ratio of fracture by BMD.
Conclusions
Low BMD was associated with a higher prevalence of fracture in all cohorts and the magnitude of the BMD differences by fracture status was similar across groups suggesting homogeneity in the BMD-fracture relationship among older men.
Keywords: Fracture, Bone mineral density, Epidemiology, Men, Race/ethnicity
Introduction
Osteoporotic fractures are an important public health problem in older men. Previous research has shown that there are considerable geographic variations in fracture rates [1–3]. Many studies in the US also report that hip fracture rates among older African-American, Asian, and Hispanic men were lower than rates among Caucasian men [4–11]. The exact reasons for these geographic and race/ethnic variations are not understood.
There are also substantial differences in age-adjusted BMD across race/ethnic groups and countries [1, 12, 13]. Understanding race/ethnic and geographic differences may help inform the design of clinical and public health interventions and policies to reduce disparities in fracture. However, there is insufficient data regarding the relationship between BMD and fracture risk across race/ethnic groups in men. Most studies have been conducted on Caucasian men and few have been conducted in Asian men or men of African ancestry. The aim of our study was to determine whether there are race/ethnic differences in BMD by fracture history in men aged 65 and older. We hypothesized that the magnitude by the differences in BMD among men by fracture status will be similar across race/ethnic and geographic area. This hypothesis is consistent with the assumption in the Fracture Risk assessment tool (FRAX) that the relationship between risk factors and fracture is similar irrespective of race/ethnicity and country [14, 15].
Materials and Methods
Subjects
We used a cross-sectional design. The datasets included the Osteoporotic Fractures in Men (MrOS) Study, MrOS Hong Kong, Tobago Bone Health Study, Namwon Study, and Dong-gu Study. For all race/ethnic groups, we restricted analyses to men aged 65 and older.
The MrOS Study enrolled 5,994 men aged 65 or older at six US clinical settings in Birmingham, AL; Minneapolis, MN; the Monongahela Valley near Pittsburgh, PA; Palo Alto, CA; Portland, OR; and San Diego, CA from March 2000 to April 2002 [16, 17]. Of those, 5,362 were self-reported as White, 244 as African-American, 191 as Asian, 126 as Hispanic, and 71 men as other ethnicity. The MrOS Hong Kong Study enrolled 2,000 Chinese men aged 65–92 between August 2001 and February 2003 [18]. All were Hong Kong residents of Chinese ethnicity.
In the Tobago Bone Health Study, we used the second visit survey data, because data about fracture was not obtained at the baseline survey. 2,652 men aged 40 or older were enrolled on the Caribbean Island of Tobago in the baseline survey during 2000–2004 [19]. 2,476 men (1,748 revisitors and 451 new participants) were enrolled at a second visit during 2004–2007. Of those, 720 Afro-Caribbean men were aged 65 and were of African ancestry (all four grandparents of African ancestry).
The Dong-gu Study is an ongoing prospective study designed to investigate the prevalence, incidence, and risk factors for chronic disease in an urban population. From 2007 to 2010, 34,040 eligible subjects aged ≥50 years, and who resided in the Dong-gu district of Gwangju Metropolitan City in South Korea, were invited to participate by telephone. A total of 9,260 subjects were enrolled (response rate: 27.2%; 3,713 male and 5,547 female). Of those, 2,094 men aged 65 and older were 65 and older with BMD and included in the analysis.
The Namwon Study enrolled 10,667 subjects (4,201 men and 6,466 women) in the baseline survey from 2004 to 2007 and 8157 subjects (3231 men and 4926 women) were studied in a follow-up examination from October 2007 to February 2012. 6135 subjects had BMD measurements in the baseline survey and 7926 subjects at the follow-up survey. We used the follow-up survey data (BMD, fracture history, covariates) if there was no BMD data in the baseline survey. Of those, 1,910 men were aged 65 and older with BMD; 990 from baseline survey data and 853 from follow-up survey data.
We restricted analyses to men aged 65 and older who had BMD at the femoral neck, hip, and lumbar spine with complete age, weight, height, smoking and alcohol intake data. The final dataset included 5,342 US White, 243 African-American, 190 US Asian, 126 US Hispanic men from the MrOS Study, 641 Afro-Caribbean men, 1,968 Hong Kong Chinese men, 2,057 Korean men from Dong-gu Study, and 1,834 Korean men from Namwon Study.
Determination of bone mineral density
Femoral neck, total hip, and total lumbar spine BMD (gram per square centimeter) were measured using Hologic QDR 4,500-W densitometer (Hologic Inc, Bedford, MA) in the MrOS Study, the MrOS Hong Kong Study, and the Tobago Bone Health Study. The Lunar Prodigy (GE, Madison, WI) was used in the Dong-gu Study and the Namwon Study. Dong-gu Study and Namwon Study used the same machine and the same procedure.
Details on the cross-calibration procedure have been published [12]. Briefly, from 2002 to 2005, the Musculoskeletal and Quantitative Imaging Research Group at the University of California, San Francisco (UCSF), carried out cross-calibration studies using the Hologic spine, femur, and block phantoms for the scanners used in the MrOS Study (US sites; 2000), the MrOS Hong Kong Study (2002), and the Tobago Bone Health Study (2004). UCSF also carried out a cross-calibration procedure using the same phantoms for the scanner of the Namwon Study and Dong-gu Study. Since the sites included Lunar and Hologic scanners, BMD parameters were standardized (converted to sBMD) according to the formulas published by Hui et al. [20] and Lu et al. [21]. Corrections for any statistically significant differences across scanners were then applied to participant spine, total hip, and femoral neck BMD values.
Assessment of fractures
In the MrOS Study, MrOS Hong Kong Study and the Tobago Bone Health Study, participants were asked if they had a fracture since age 50. In the Korean cohorts, participants were asked if they had ever had a non-trauma fracture. Because we had information on the age the fracture occurred, we considered a fracture history positive if they had a fracture since age 50 in the Korean cohorts. All fracture sites (hip, wrist, skull/face, cervical, thoracic and lumbar vertebrae; shoulder; arm; hand/finger; rib/chest/sternum; pelvis/tailbone; leg; and ankle/foot/heel/toe) were included in this study. We only considered prevalent fractures that occurred with minimal or no trauma (fall from standing height or less).
Other measurement
Information on covariates was collected at the same time as the BMD and fracture history assessment. Smoking status was categorized as never smoker, former, or current smoker. Current alcohol consumption was categorized as nondrinker and current drinker. Body weight was measured in subjects wearing indoor clothing or light gown without shoes using a calibrated Inbody 520 (Biospace Co. Korea) in Dong-gu Study, a calibrated Inbody 3.0 (Biospace Co. Korea) in the Namwon Study, a calibrated digital scale in one site (Portland) of the MrOS Study and calibrated balanced beam scales in the other five sites of MrOS Study, the MrOS Hong Kong Study and the Tobago Bone Health Study. Standing height was measured using a stadiometer in each study.
Statistical analysis
We compared baseline characteristics of men with and without a history of fracture by Student's t-test and chi-square test. We combined the 2 Korean cohorts because there was no significant heterogeneity in the results. We performed analyses in the pooled dataset. The age-adjusted prevalence of previous fracture was calculated based on a logistic regression model. Linear regression analysis was used to estimate the difference in BMD between subjects with a positive fracture history and those without a fracture within each race/ethnic group. Logistic regression was used to examine the association of BMD with fracture within each race/ethnic group. The odds ratio was presented for a 1-SD decrement in BMD. We used the SD of US white men in MrOS to compare the ethnic difference using the same SD. Standard deviations for lumbar spine, femoral neck, and total hip were 0.19, 0.13 and 0.14 g/cm2, respectively. In all models, we adjusted for age, body weight, height, smoking status (ever smoker/never smoker) and alcohol use (nondrinker/current drinker). We added interaction terms between ethnicity and fracture status in linear regression models and between ethnicity and BMD in logistic models. Discrimination of the three BMD measurement sites for fracture was assessed using receiver operating characteristic (ROC) analysis. ROC curve was based on the fitted logistic regression model including only BMD and BMD and covariates (age, weight, height, history of smoking and current alcohol consumption). Area under curve (AUC) for three BMD measurement sites was computed using the method of Hanley and McNeil[22]. All statistical analyses were conducted with STATA 12 software (STATA Corp, Texas, US).
Results
The age adjusted proportion of men with a history of non-traumatic fracture was US white, 17.1%; Afro-Caribbean, 5.5%; US African-American, 15.1%; US Hispanic, 13.7%; US Asian, 10.5%; Hong Kong Chinese, 5.6% and Korean, 5.1% (Fig. 1). Table 1 shows baseline characteristics of men with and without history of fracture. Mean age (years) of the US white, Afro-Caribbean, US African-American, US Hispanic, US Asian, Hong Kong Chinese, and Korean was similar (71.2 to 73.8). Men with a history of fracture tended to be older than those without a fracture history but the difference was statistically significant only for US Whites and Koreans. Body weight varied markedly across cohorts, ranging from a low of about 63 kgs in the Koreans and Chinese from Hong Kong to a high of 85–90 kgs in African Americans. Of interest, African American men who reported a fracture had significantly higher body weight and greater height than men who did not report a fracture but there were no differences in body weight or height by fracture status in the other cohorts. US White, African American and Afro-Caribbean men tended to be taller than the Asian or Hispanic men.
Figure 1.
Age-adjusted proportion (%) and 95% confidence intervals of men reporting a prevalent fracture according to race/ethnic groups
Table 1.
Baseline characteristics by fracture history within each of the race/ethnic groups.
| US White | Tobago Afro- Caribbean |
US African- American |
US Hispanic | US Asian | Hong Kong Chinese |
Korean | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fx(−) | Fx(+) | Fx(−) | Fx(+) | Fx(−) | Fx(+) | Fx(−) | Fx(+) | Fx(−) | Fx(+) | Fx(−) | Fx(+) | Fx(−) | Fx(+) | |
| N=4401 | N=941 | N=606 | N=35 | N=207 | N=36 | N=109 | N=17 | N=170 | N=20 | N=1859 | N=109 | N=3702 | N=189 | |
| Age(years) | 73.7±5.9 | 74.6±6.1* | 72.2±5.7 | 73.2±6 | 71.7±5.2 | 71.5±4.9 | 71.8±4.6 | 73.2±5.5 | 72.9±4.9 | 73.4±6.6 | 72.3±5.0 | 73.3±5.2 | 71.1±4.3 | 71.9±4.4* |
| Weight(kg) | 83.6±12. 9 | 83.1±13.7 | 79.9±14. 5 | 79.6±12.7 | 85.3±14. 9 | 91.8±18.6 * | 81.2±13. 2 | 87.9±11.9 | 69.8±9.2 | 72.3±10 | 62.5±9.3 | 61.6±9.3 | 63.4±8.9 | 62.8±9.1 |
| Height(cm) | 174.5±6. 6 | 174.4±6.8 | 172.2±6. 7 | 172.5±6.1 | 173.6±7. 1 | 176.4±7.4* | 170.2±6. 1 | 171.8±7.2 | 166.7±6 | 168.1±5. 4 | 163.1±5. 7 | 162.9±5.6 | 164.2±5. 8 | 164.4±5.7 |
| Lumbar BMD | 1.15±0.1 9 | 1.10±0.19* | 1.24±0.21 | 1.14±0.17* | 1.22±0.22 | 1.15±0.22 | 1.13±0.19 | 0.97±0.13* | 1.12±0.18 | 1.07±0.2 1 | 1.03±0.19 | 0.99±0.18* | 1.09±0.2 | 1.05±0.21* |
| Femoral neck BMD | 0.87±0.13 | 0.83± 0.13* | 1.03± 0.17 | 0.95± 0.15* | 0.98±0.1 6 | 0.96±0.19 | 0.90±0.1 3 | 0.83±0.09* | 0.84±0.1 2 | 0.82±0.1 3 | 0.82±0.1 2 | 0.78±0.12* | 0.89±0.1 2 | 0.85±0.13* |
| Total femur BMD | 0.98±0.1 4 | 0.93±0.14* | 1.12±0.1 6 | 1.04±0.14* | 1.05±0.1 5 | 1.03±0.20 | 0.99±0.1 4 | 0.91±0.06* | 0.92±0.1 1 | 0.89±0.1 4 | 0.90±0.1 3 | 0.86±0.12* | 0.85±0.1 2 | 0.82±0.13* |
| Ever smoker (%) | 62.0 | 65.1* | 32.7 | 51.4* | 65.2 | 75.0 | 62.4 | 58.8 | 54.7 | 60.0 | 63.5 | 67.0 | 77.3 | 84.7* |
| Current smoker (%) | 3.1 | 3.2 | 6.4 | 5.7 | 11.1 | 11.1 | 0.9 | 11.8* | 2.4 | 5.0 | 11.6 | 14.7 | 23.3 | 28.0 |
| Alcohol consumptio n (%) | 66.0 | 65.5 | 51.2 | 42.9 | 50.7 | 41.7 | 73.4 | 70.6 | 44.7 | 50.0 | 24.0 | 19.3 | 61.7 | 60.3 |
Data are presented as mean � standard deviation or percent.
p value <0.05 for comparison of fracture versus non-fracture groups.
Unadjusted lumbar spine BMD was greatest in men of African origin, followed by Caucasian, Asian and Hispanic men. Korean and Chinese men had the lowest spine BMD. Similar patterns were observed for total hip BMD except US Asian men had BMD more similar to Korean and Chinese men. Femoral neck BMD was almost 1SD higher in Afro Caribbean men compared with African American men. Femoral neck BMD was similar in US Caucasian, Hispanic and Korean men with lowest values in US Asian and Hong Kong Chinese men. BMD was significantly lower in men who had a history of fracture among US white, Afro-Caribbean, US Hispanic, Hong Kong Chinese, and Korean. The prevalence of smoking varied from a low of about 30% in Afro Caribbean men to a high of >80% in Korean men and smoking tended to be more common among men with a positive fracture history. Alcohol consumption also varied with the lowest prevalence among Hong Kong Chinese and highest among US Hispanic men, but with little difference across the cohorts by fracture status.
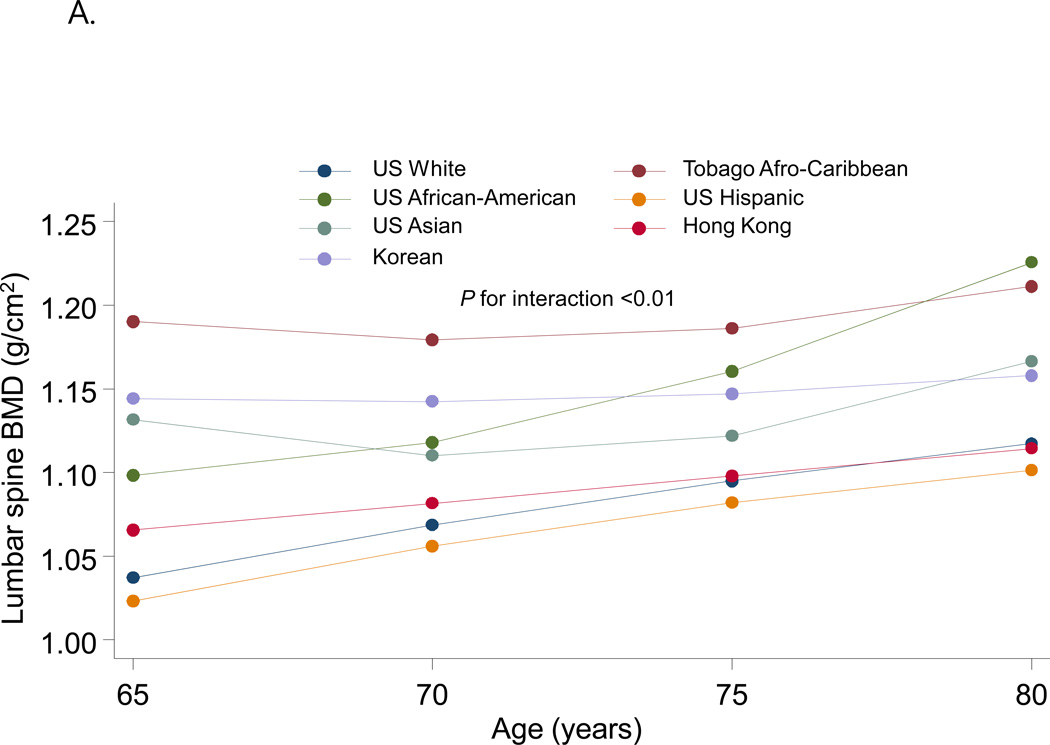
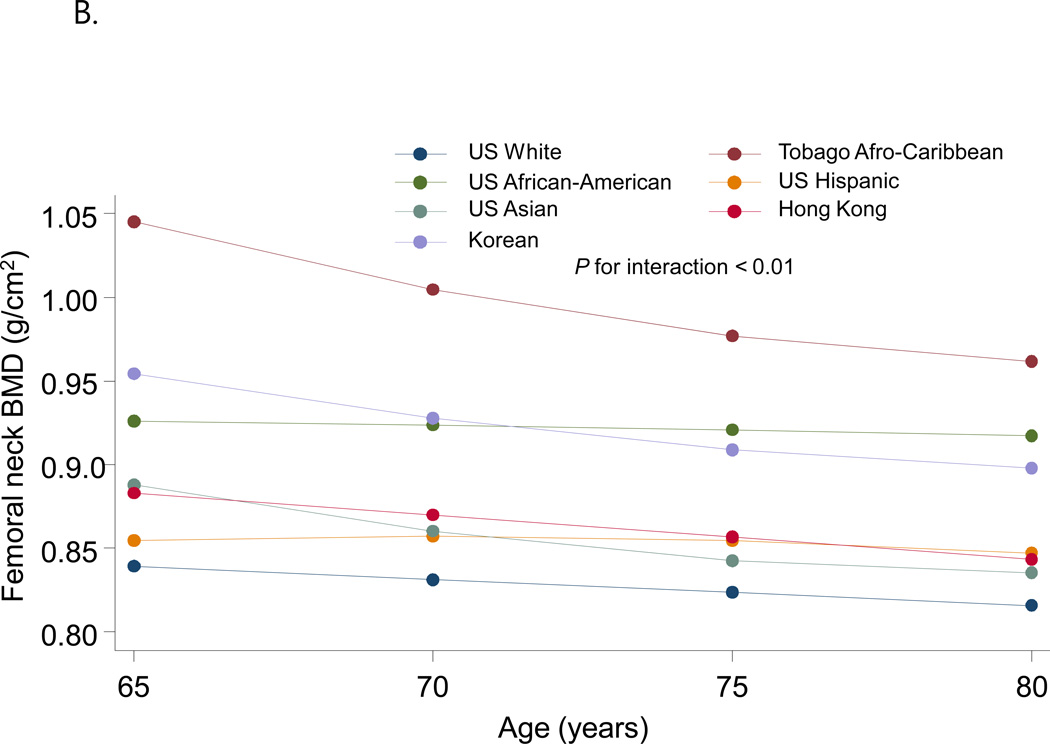
Figure 2 shows the fitted quadratic regression line between age and BMD by race/ethnic groups. There were significant ethnic differences with respect to the pattern of the difference in BMD with increasing age. Lumbar spine BMD increased with age with highest slope in US African-Americans and lowest slope in Koreans while femoral neck BMD decreased with age with highest slope in Tobago Afro-Caribbean and lowest slope in US African-American.
Figure 2.
Quadratic regression line between age and BMD according to race/ethnic groups. A Lumbar spine BMD; B Femoral neck BMD. Data were adjusted for weight, height, history of smoking, current alcohol consumption and interaction term between age and ethnicity.
Multiple adjusted BMD differences between subjects with and without fracture are shown in Figure 3 (for age, weight, height, smoking and alcohol).
Figure 3.
Differences in mean BMD (g/cm2) (95% confidence interval) between men with and without prevalent fracture adjusted for age, weight, height, history of smoking, current alcohol consumption and interaction term between ethnicity and fracture status.
The mean differences in hip and lumbar spine BMD between subjects with and without fracture were significant in all cohorts except US African American and US Asian men with the largest differences for US Hispanic and Afro-Caribbean men. There was a significant race/ethnic interaction for lumbar spine BMD by fracture status (p for interaction=0.02), but not for femoral neck (p for interaction=0.16) or total hip BMD (p for interaction=0.20). We excluded the US Hispanic men from this analysis and the interaction term was no longer significant.
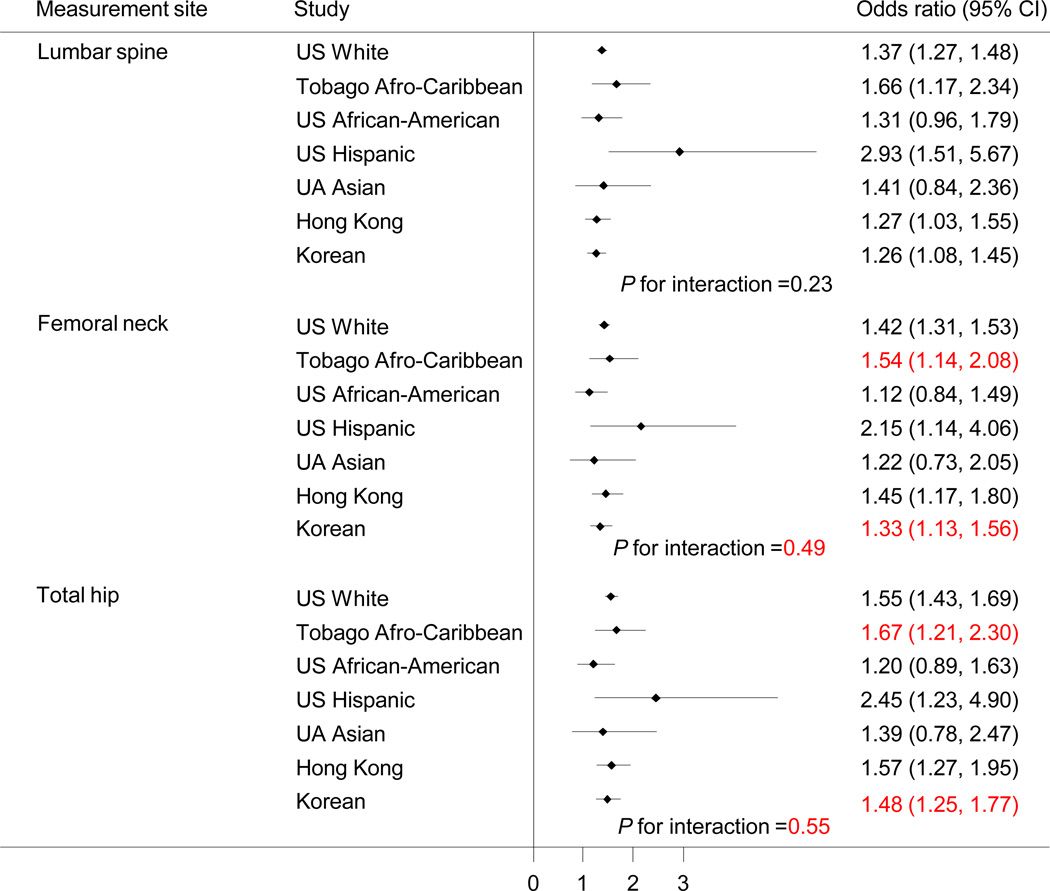
The odds ratio of fracture for 1 SD decrease in BMD is shown in Figure 4. US Hispanic and Afro-Caribbean men seemed to have a higher odds of fracture per SD decrease in BMD than the other groups but the interaction terms were not significant. Similar findings were observed for the total hip and femoral neck.
Figure 4.
Odds ratio (95% confidence interval) of prevalent fracture for 1 standard deviation decrease in bone mineral density adjusted for age, weight, height, history of smoking, current alcohol consumption and interaction term between ethnicity and BMD. Standard deviations for lumbar spine, femoral neck, and total hip were 0.19, 0.13 and 0.14 g/cm2, respectively.
Discussion
Fracture history is an important and consistent risk factor for future fracture. A meta-analysis reported that a previous fracture was associated with a significantly increased risk of an osteoporotic fracture (RR=1.86; 95% CI = 1.72 – 2.01) [23]. These consistent associations have led to the incorporation of fracture history as a risk factor in the WHO fracture risk assessment (FRAX) tool.
Kanis et al. [3] reported that there was approximately a 10-fold range in hip fracture incidence worldwide. The factors that may contribute to the variation are unknown. In our study, there was a substantial difference in prevalence of fracture across race/ethnic groups. The highest age-adjusted prevalence of self-reported fracture was observed in US Whites, followed by US African-Americans, US Hispanics, US Asians, Tobago Afro-Caribbean, Hong Kong Chinese, and Koreans. These results are consistent with previous reports showing that older white men have higher hip fracture rates than other ethnic groups within the United States [4–11]. In this study, US Asians had a higher prevalence of fracture than Koreans or Hong Kong Chinese. This may reflect a change in risk in immigrant populations or reflect the heterogeneity of Asian ancestry.
We previously reported that Afro-Caribbean men had the highest level of total hip BMD, followed by African-American men, US Hispanic, US Caucasian, US Asian, Hong Kong Chinese, and Korean men [12]. In the present study, we extended these findings to test whether the magnitude of the differences in BMD between men with a previous fracture compared with men without a previous fracture were similar across race/ethnicity. We found that BMD differences by fracture status were similar across race/ethnic. Thus, despite large differences in the prevalence of fracture and in the characteristics of the men, the associations between BMD and fracture status were remarkably similar. Our study is limited to areal BMD measures. It’s possible that ethnic differences in volumetric BMD, bone geometry or microarchitecture could differentiate fracture risk by ethnicity to a larger degree. Indeed, several studies have reported major race/ethnic differences in bone strength and structure [24–28]. In a cross-sectional analysis of 1,190 randomly-chosen black, Hispanic and white community-dwelling men, Travison et al. reported that Hispanic men tended to have lower hip strength compared with White or Black men and experienced the sharpest age-related declines [28]. However, confidence intervals were wide reflecting the small sample size of Hispanic and black men. In MrOS, Asian men had similar areal BMD to Caucasian men but had significantly higher volumetric BMD [29].
The odds of previous fracture for 1 SD decrease in BMD ranged 1.26–2.93 at the lumbar BMD and 1.12–2.15 at the femoral neck BMD. In a meta-analysis from 11 prospective cohort studies in women, Marshall et al [29] reported that the relative risk of all fracture for 1 SD was 1.5 (1.4–1.7) and 1.6 (1.4–1.8) for lumbar spine and hip, respectively. In another meta-analysis from 12 prospective cohort studies, half of which included men, Johnell et al [30] reported that the risk of osteoporotic fractures increased similarly in men (1.60 per SD decrease in femoral neck BMD (95% CI = 1.43–1.79)) and in women by 1.53 per SD (95% CI = 1.46–1.62) and the gradient of risk increased with age. They did not provide study-specific risk ratios although they reported there was a low grade of heterogeneity for hip fracture, but heterogeneity was marked for other fracture outcome. Most of the studies included in these meta-analyses were conducted in Caucasians, with only one study in Asians.
To our knowledge, there were three multiethnic cohort studies to evaluate race/ethnicity difference in the BMD fracture relationship in women. In these studies, there was no a significant race/ethnicity difference in the BMD fracture relationship. The National Osteoporosis Risk Assessment (NORA) study, observational study of postmenopausal women in the United States, reported that the relative risk of all fracture for 1 SD in T score was 1.54 (1.48–1.61) [31]. However, this study had a limitation in that they used peripheral BMD or ultrasound measurements. In the Study of Osteoporotic Fracture (SOF), in Black women and White women, respectively, the relative risk of nonspinal fracture for 1 SD decrease in BMD was 1.23 (0.92–1.65) and 1.42 (1.33–1.52) for the total hip and 1.20 (0.93–1.55) and 1.42 (1.32–1.52) for the femoral neck [32]. In the Women’s Health Initiative study, age-adjusted relative risk for fracture for 1 SD decrease in BMD was 1.42 (1.34–1.51), 1.24 (1.05–1.46), and 1.46 (1.11–1.92) for the total hip and 1.30 (1.24–1.37), 1.31 (1.11–1.54) and 1.16 (0.90–1.50) for lumbar spine in White, Black, and Hispanic women, respectively [33]. Although we studied prevalent fractures, the odds ratios for most of the groups except Hispanics were within the range of relative risks reported in these meta-analyses.
The magnitude of the SD can influence the gradient of risk. The meta-analysis by Marshall et al. [29] used the 1 SD decrease in bone density below the age adjusted mean, but, did not present the value of 1 SD. Another meta-analysis by Johnell et al. [30] used the SD of the young female reference range aged 20–29 years (femoral neck, 0.137; total hip, 0.144) which are similar to our SDs for femoral neck (0.13 g/cm2), and total hip (0.14 g/cm2). However, our SD for lumbar spine (0.19 g/cm2) is higher than SD of the young female reference range aged 20–29 years of NHANES 2005–2008 (0.115 g/cm2). Thus, in present study, the estimated gradient of risk of lumbar spine is likely to be overestimated, compared with the meta-analysis by Marshall et al.
There were marked differences in several characteristics across the race/ethnic groups, especially body weight. The mean body weight in the Hong Kong Chinese and Koreans was 20–30 kg less than US white and Black men. These differences could contribute to differences in BMD. In addition, smoking prevalence also varied across the cohorts with a higher prevalence of current smoking in the Asian cohorts. Of importance, however, we adjusted for these factors and the observed differences in BMD were independent of these lifestyle differences.
Our study has several potential limitations. First, because of our cross-sectional study design, we cannot establish causal associations between low BMD and fracture. Second, because of the relatively small sample size and low prevalence of fracture, our statistical power was low and we were not adequately powered to assess interaction in the logistic regression model. Third, we relied on self-reported information on fracture and the validity of this measure in our study cohorts is not known. However, several studies have suggested moderate to excellent agreement between self-reported fracture information and medical records [34, 35]. Fourth, we were unable to examine specific sites of fracture. This could lead to a bias if there was a significant difference in the proportions of specific sites of fracture across the cohorts. Fifth, information on alcohol consumption varied in each cohort. To harmonize across the cohorts, we limited our alcohol information to note if they drank alcohol or not. Sixth, all densitometers used in this study were the fan beam scanners, but the BMD results were converted to sBMD using the equations for pencil beam scanner. Fan et al. [36] reported that the sBMD values using the equation for pencil beam scanner had a significant difference of 3–4% in the spine between GE-lunar and Hologic fan-beam systems but there is no evidence that this difference would differ by race/ethnicity. Finally, we had no information on specific ethnic subgroups of Asians or Hispanics in the US.
In conclusion, our data show that the prevalence of fracture varies greatly among older men with the highest prevalence in US White men and lowest observed in Afro-Caribbean and Asian men. Low BMD was associated with a higher prevalence of fracture in all cohorts and the magnitude of the BMD differences by fracture status was similar across groups suggesting homogeneity in the BMD-fracture relationship among older men. Prospective studies are needed to further our understanding of the race/ethnic differences in fracture causation.
Table 2.
Area under the curve (95% CI) from ROC analysis for prevalent fracture by bone mineral density
| Lumbar spine | Femoral neck | Total hip | ||||
|---|---|---|---|---|---|---|
| BMD* | BMD + covariates† | BMD | BMD + covariates† | BMD | BMD + covariates† | |
| US White | 0.58(0.56–0.60) | 0.59(0.57–0.61) | 0.59(0.57–0.61) | 0.60(0.58–0.62) | 0.60(0.58–0.62) | 0.61(0.59–0.63) |
| Tobago Afro-Caribbean | 0.64(0.55–0.73) | 0.67(0.58–0.76) | 0.64(0.55–0.73) | 0.65(0.55–0.74) | 0.65(0.56–0.73) | 0.66(0.57–0.75) |
| US African-American | 0.59(0.49–0.69) | 0.64(0.55–0.73) | 0.50(0.40–0.60) | 0.65(0.55–0.75) | 0.51(0.41–0.62) | 0.67(0.57–0.77) |
| US Hispanic | 0.75(0.64–0.86) | 0.78(0.68–0.88) | 0.67(0.55–0.80) | 0.69(0.56–0.81) | 0.69(0.58–0.79) | 0.74(0.63–0.84) |
| US Asian | 0.59(0.45–0.72) | 0.63(0.48–0.78) | 0.58(0.46–0.70) | 0.61(0.47–0.74) | 0.61(0.48–0.74) | 0.65(0.52–0.78) |
| Hong Kong Chinese | 0.59(0.46–0.72) | 0.58(0.52–0.64) | 0.59(0.54–0.65) | 0.60(0.54–0.66) | 0.61(0.56–0.66) | 0.62(0.56–0.67) |
| Korean | 0.56(0.50–0.61) | 0.59(0.55–0.63) | 0.57(0.53–0.62) | 0.59(0.55–0.63) | 0.59(0.54–0.63) | 0.60(0.56–0.64) |
ROC curve based on the fitted logistic regression model including BMD and interaction term between ethnicity and BMD.
ROC curve based on the fitted multivariate logistic regression model including BMD, covariates (age, weight, height, history of smoking and current alcohol consumption) and interaction term between ethnicity and BMD.
Acknowledgments
This study was supported by Research Institute of Medical Sciences, Chonnam National University. Dong-gu study was partially supported by the Health Promotion Fund, Ministry of Health &Welfare, Republic of Korea (07-27). Namwon Study was also partially supported by the Korea Centers for Disease Control and Prevention (2004-E71004-00, 2005-E71011-00, 2006-E71009-00, 2007-E71002-00). The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140. The Tobago Bone Health Study is supported by NIAMS grant R01-AR049747.
Footnotes
Conflicts of interest None
References
- 1.Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clinical orthopaedics and related research. 2011;469:1891–1899. doi: 10.1007/s11999-011-1863-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhanwal DK, Cooper C, Dennison EM. Geographic variation in osteoporotic hip fracture incidence: the growing importance of asian influences in coming decades. J Osteoporos. 2010;2010:757102. doi: 10.4061/2010/757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C Epidemiology IOFWGo, Quality of L. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23:2239–2256. doi: 10.1007/s00198-012-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kellie SE, Brody JA. Sex-specific and race-specific hip fracture rates. American journal of public health. 1990;80:326–328. doi: 10.2105/ajph.80.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. American journal of public health. 1990;80:871–873. doi: 10.2105/ajph.80.7.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross PD, Norimatsu H, Davis JW, Yano K, Wasnich RD, Fujiwara S, Hosoda Y, Melton LJ., 3rd A comparison of hip fracture incidence among native Japanese, Japanese Americans, and American Caucasians. American journal of epidemiology. 1991;133:801–809. doi: 10.1093/oxfordjournals.aje.a115959. [DOI] [PubMed] [Google Scholar]
- 7.Lauderdale DS, Jacobsen SJ, Furner SE, Levy PS, Brody JA, Goldberg J. Hip fracture incidence among elderly Asian-American populations. American journal of epidemiology. 1997;146:502–509. doi: 10.1093/oxfordjournals.aje.a009304. [DOI] [PubMed] [Google Scholar]
- 8.Lauderdale DS, Jacobsen SJ, Furner SE, Levy PS, Brody JA, Goldberg J. Hip fracture incidence among elderly Hispanics. American journal of public health. 1998;88:1245–1247. doi: 10.2105/ajph.88.8.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang J, Freeman R, Jeganathan R, Alderman MH. Variations in hip fracture hospitalization rates among different race/ethnicity groups in New York City. Ethn Dis. 2004;14:280–284. [PubMed] [Google Scholar]
- 10.Silverman SL, Madison RE. Decreased incidence of hip fracture in Hispanics, Asians, and blacks: California Hospital Discharge Data. American journal of public health. 1988;78:1482–1483. doi: 10.2105/ajph.78.11.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright NC, Saag KG, Curtis JR, Smith WK, Kilgore ML, Morrisey MA, Yun H, Zhang J, Delzell ES. Recent trends in hip fracture rates by race/ethnicity among older US adults. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27:2325–2332. doi: 10.1002/jbmr.1684. [DOI] [PubMed] [Google Scholar]
- 12.Nam HS, Shin MH, Zmuda JM, Leung PC, Barrett-Connor E, Orwoll ES, Cauley JA. Race/ethnic differences in bone mineral densities in older men. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21:2115–2123. doi: 10.1007/s00198-010-1188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkelstein JS, Lee ML, Sowers M, Ettinger B, Neer RM, Kelsey JL, Cauley JA, Huang MH, Greendale GA. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. The Journal of clinical endocrinology and metabolism. 2002;87:3057–3067. doi: 10.1210/jcem.87.7.8654. [DOI] [PubMed] [Google Scholar]
- 14.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44:734–743. doi: 10.1016/j.bone.2009.01.373. [DOI] [PubMed] [Google Scholar]
- 16.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemporary clinical trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Lau EM, Leung PC, Kwok T, Woo J, Lynn H, Orwoll E, Cummings S, Cauley J. The determinants of bone mineral density in Chinese men--results from Mr. Os (Hong Kong), the first cohort study on osteoporosis in Asian men. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2006;17:297–303. doi: 10.1007/s00198-005-2019-9. [DOI] [PubMed] [Google Scholar]
- 19.Hill DD, Cauley JA, Sheu Y, Bunker CH, Patrick AL, Baker CE, Beckles GL, Wheeler VW, Zmuda JM. Correlates of bone mineral density in men of African ancestry: the Tobago bone health study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19:227–234. doi: 10.1007/s00198-007-0450-9. [DOI] [PubMed] [Google Scholar]
- 20.Hui SL, Gao S, Zhou XH, Johnston CC, Jr, Lu Y, Gluer CC, Grampp S, Genant H. Universal standardization of bone density measurements: a method with optimal properties for calibration among several instruments. J Bone Miner Res. 1997;12:1463–1470. doi: 10.1359/jbmr.1997.12.9.1463. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Fuerst T, Hui S, Genant HK. Standardization of bone mineral density at femoral neck, trochanter and Ward's triangle. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2001;12:438–444. doi: 10.1007/s001980170087. [DOI] [PubMed] [Google Scholar]
- 22.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 23.Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Danielson ME, Beck TJ, Lian Y, Karlamangla AS, Greendale GA, Ruppert K, Lo J, Greenspan S, Vuga M, Cauley JA. Ethnic variability in bone geometry as assessed by hip structure analysis: Findings from the hip strength across the menopausal transition study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012 doi: 10.1002/jbmr.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wetzsteon RJ, Hughes JM, Kaufman BC, Vazquez G, Stoffregen TA, Stovitz SD, Petit MA. Ethnic differences in bone geometry and strength are apparent in childhood. Bone. 2009;44:970–975. doi: 10.1016/j.bone.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Ishii S, Cauley JA, Greendale GA, Danielson ME, Safaei Nili N, Karlamangla A. Ethnic differences in composite indices of femoral neck strength. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23:1381–1390. doi: 10.1007/s00198-011-1723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson DA, Beck TJ, Wu G, Lewis CE, Bassford T, Cauley JA, LeBoff MS, Going SB, Chen Z. Ethnic differences in femur geometry in the women's health initiative observational study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22:1377–1388. doi: 10.1007/s00198-010-1349-4. [DOI] [PubMed] [Google Scholar]
- 28.Travison TG, Beck TJ, Esche GR, Araujo AB, McKinlay JB. Age trends in proximal femur geometry in men: variation by race and ethnicity. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19:277–287. doi: 10.1007/s00198-007-0497-7. [DOI] [PubMed] [Google Scholar]
- 29.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Bmj. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ, 3rd, O'Neill T, Pols H, Reeve J, Silman A, Tenenhouse A. Predictive value of BMD for hip and other fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 31.Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 32.Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA : the journal of the American Medical Association. 2005;293:2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 33.Cauley JA, Wu L, Wampler NS, Barnhart JM, Allison M, Chen Z, Jackson R, Robbins J. Clinical risk factors for fractures in multi-ethnic women: the Women's Health Initiative. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22:1816–1826. doi: 10.1359/jbmr.070713. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, Kooperberg C, Pettinger MB, Bassford T, Cauley JA, LaCroix AZ, Lewis CE, Kipersztok S, Borne C, Jackson RD. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women's Health Initiative observational study and clinical trials. Menopause. 2004;11:264–274. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 35.Nevitt MC, Cummings SR, Browner WS, Seeley DG, Cauley JA, Vogt TM, Black DM. The accuracy of self-report of fractures in elderly women: evidence from a prospective study. Am J Epidemiol. 1992;135:490–499. doi: 10.1093/oxfordjournals.aje.a116315. [DOI] [PubMed] [Google Scholar]
- 36.Fan B, Lu Y, Genant H, Fuerst T, Shepherd J. Does standardized BMD still remove differences between Hologic and GE-Lunar state-of-the-art DXA systems? Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21:1227–1236. doi: 10.1007/s00198-009-1062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]