Abstract
Objective
To examine whether interference with FRNK targeting to focal adhesions (FAs) affects its inhibitory activity and tyrosine phosphorylation.
Methods and Results
Focal adhesion kinase and its autonomously expressed C-terminal inhibitor, focal adhesion kinase–related nonkinase (FRNK), regulate vascular smooth muscle cell (VSMC) signaling and migration. FRNK-paxillin binding was reduced by a point mutation in its FA targeting domain (L341S-FRNK). Green fluorescent protein–tagged wild type and L341S-FRNK were then adenovirally expressed in VSMCs. L341S-FRNK targeted to VSMC FAs, despite previous studies in other cell types. L341S-FRNK affected FA binding kinetics (assessed by total internal reflection fluorescnece [TIRF] microscopy and fluorescence recovery after photobleaching [FRAP]) and reduced its steady-state paxillin interaction (determined by coimmunoprecipitation). Both wt-FRNK and L341S-FRNK lowered basal and angiotensin II–stimulated focal adhesion kinase, paxillin, and extracellular signal–regulated kinase 1/2 phosphorylation. However, the degree of inhibition was significantly reduced by L341S-FRNK. L341S-FRNK also demonstrated significantly greater migratory activity compared with wt-FRNK–expressing VSMCs. Angiotensin II–induced Y168 phosphorylation was Src dependent, as evident by a significant reduction in Y168 phosphorylation by the Src family kinase inhibitor PP2 is 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2). Surprisingly, Y168 phosphorylation was unaffected by its targeting. Furthermore, Y232 phosphorylation increased approximately 3-fold in L341S-FRNK, which was less sensitive to PP2.
Conclusion
FRNK inhibition of VSMC migration requires both FA targeting and Y168 phosphorylation by Src family kinases. FRNK-Y232 phosphorylation occurs outside of FAs, probably by a PP2-insensitive kinase.
Keywords: vascular biology, vascular muscle, Src, cell migration, mechanotransduction, tyrosine phosphorylation, vascular remodeling
Vascular remodeling requires a complex interaction between growth factor receptors, extracellular matrix components, and integrins. Key proteins are involved in integrating extracellular signals and promoting the intracellular signal transduction required for vascular remodeling. One of these intracellular proteins is focal adhesion kinase (FAK), which is activated by growth factor receptors and integrin clustering, and is critical for the assembly of a variety of signaling complexes within focal adhesions (FAs).1 FAK possesses autocatalytic tyrosine kinase activity but also functions as an activatable scaffolding protein by providing phosphotyrosine and phosphoserine binding sites for docking additional signaling proteins.2 The phosphorylation status of specific residues in FAK appears to regulate specific cellular processes. For instance, FAK-Y397 phosphorylation provides a docking site for Src family protein tyrosine kinases (PTKs) to bind to and to phosphorylate FAK and other cytoskeletal substrates.3 Phosphorylation of Y861 may be involved in FAK-dependent cell migration signaling events,4–6 whereas phosphorylation of FAK-Y925 is thought to mediate FAK-dependent extracellular signal–regulated kinase (ERK) activation and cellular proliferation.7–10
In addition to FAK, FAK-related nonkinase (FRNK) is also a product of the PTK2 gene, but is autonomously expressed under control of an alternative, intronic promoter.11 FRNK is composed of the noncatalytic C-terminal region of FAK, containing the FA targeting sequence and proline-rich domains important for adaptor protein binding. FRNK is selectively expressed in vascular smooth muscle cells (VSMCs), with high levels found after arterial injury.12–14 FRNK is thought to act as a dominant-negative inhibitor of full-length FAK in cultured cells. FRNK inhibits cell spreading of chick embryo fibroblasts,15 inhibits migration of endothelial and rat aortic smooth muscle cells (RASMs),12,14,16 and reduces proliferation of VSMCs.12,17 Previous laboratory studies14,18–21 have demonstrated that FRNK inhibits FAK-dependent signaling in both cardiomyocytes and VSMCs.
It is possible that FRNK functions not only as an inhibitor of FAK but may also initiate alternative signaling cascades. FRNK retains the C-terminal region containing the Y861 and Y925 phosphorylation sites present on FAK. Because the Y861 and Y925 residues function in FAK-dependent cell migration and proliferation, the corresponding residues on FRNK (Y168 and Y232) may be necessary for the phenotype seen with FRNK overexpression. However, FRNK’s inhibitory function in vascular remodeling and the importance of specific signaling residues on FRNK are not fully understood.
In a previous report,14 the inhibitory effects of FRNK on VSMC spreading and migration were highly dependent on its phosphorylation at Y168 but not at Y232. The overexpression of a green fluorescent protein (GFP)–tagged nonphosphorylatable mutant (Y168F-FRNK) markedly abrogated FRNK’s inhibition of cell spreading after cell attachment and also restored VSMC migration in a 3D Boyden chamber assay. In contrast, Y168 phosphorylation was dispensable for FRNK’s targeting to FAs and for its ability to inhibit FAK-Y397 and paxillin phosphorylation. These results suggested that FRNK requires both FA targeting and phosphorylation at Y168 to inhibit VSMC spreading and migration. Therefore, in the present study, we tested the hypothesis that abrogation of FRNK binding to paxillin within VSMC FAs would substantially reduce the inhibition of FAK-dependent signaling, restore cell migration, and reduce FRNK tyrosine phosphorylation at Y168 (and perhaps Y232).
To this end, we used a previously described point mutation within the FA targeting domain of FAK and FRNK. Studies in cultured fibroblasts demonstrated that the mutation of leucine to serine at position 341 on FRNK (analogous to L1034 on FAK) disrupted its association with paxillin in vitro and exhibited a cytoplasmic localization that did not lead to the inhibition of FAK autophosphorylation at Y397.22,23 In this report, data are presented to indicate that, in contradistinction to our hypothesis, FRNK targeting to FAs is not necessary for FRNK phosphorylation at Y168; in fact, it reduces basal- and agonist-stimulated phosphorylation at Y232.
Methods
Rats were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited University Animal Care Facility; all experimental procedures were approved by Loyola University Medical Center’s Animal Care and Use Committee, Maywood, Ill. A complete description of the materials and reagents, cell culture, adenoviral constructs, TIRF microscopy and FRAP analysis, and general methods used for these studies is given in the supplemental data (available online at http://atvb.ahajournals.org).
The results were expressed as mean ± SEM. Data for multiple groups were compared by 2- and 3-way ANOVA, followed by the Holm-Sidak test; and 1-way ANOVA or 1-way ANOVA on ranks, followed by the Student-Newman-Keuls test. Data for 2 groups were compared by the unpaired t test or the Mann-Whitney rank sum test, where appropriate. Differences among means were considered significant at P<0.05. Data were analyzed using computer software (SigmaStat Statistical Software Package, Version 3.1; Systat Software, San Jose, Calif).
Results
TIRF Microscopy and FRAP Analysis of Wild-Type and Mutant GFP-FRNK
TIRF microscopy was used first to examine the localization of GFP-tagged wild-type (wt) FRNK and L341S-FRNK in living RASMs and to compare their localization to cells expressing only GFP. TIRF microscopy has the advantage of detecting fluorescent proteins localized only to the cell-substratum interface of living cells, eliminating out-of-focus fluorescence and providing an approximately 100-nm-thick high-resolution optical section at this site. As seen in Figure 1A, GFP was diffusely localized throughout the basilar cytoplasm, whereas wtFRNK was localized to discrete linear structures typical of FAs. Surprisingly, the L341S-FRNK mutant showed a similar distribution to wtFRNK, despite the fact that this mutation was previously shown to completely disrupt FAK and FRNK localization to FAs by conventional epifluorescent microscopy in other cell types.22–25
Figure 1.
TIRF microscopy and FRAP analysis of wt and mutant GFP-FRNK. A, Live TIRF microscopic images of RASMs infected (100 multiplicity of infection, 24 hours) with Adv-GFP, Adv-GFP–wtFRNK, or Adv-GFP–L341S–FRNK. B, Representative FRAP analyses of similarly infected cells. The position of the laser beam is represented by the dashed circle. Images were acquired every 22.9 milliseconds (sec) before, during, and up to 20 sec after the photobleaching flash. C, Fluorescence intensity within the region of interest (ROI) was recorded for each of 20 TIRF-FRAP experiments for each cell type. Fluorescence within the ROI (F) was normalized to the average fluorescence intensity recorded before the flash (F0), which lasted approximately 115 milliseconds. Data are given as mean values for GFP, L341S-FRNK, and wtFRNK, expressing RASMs plotted vs time (sec), where time 0 was the first data point after the flash. D, Average kFRAP values for the same 20 individual TIRF-FRAP recordings were compared. E, Best-fitting values for k1 and k2 were compared. Data are given as the mean ± SEM. *P<0.05 for wt vs mutant FRNK.
In light of these findings, we investigated the kinetics of wt and mutant FRNK binding to FAs using FRAP. Figure 1B depicts a representative TIRF-FRAP experiment. The average normalized fluorescence intensity findings from 20 TIRF-FRAP recordings derived from 20 cells, each expressing GFP, wtFRNK, or L341S-FRNK, are depicted in Figure 1C. As is evident from Figure 1, the recovery of GFP fluorescence was rapid compared with the localized fluorescence of both wt and mutant GFP-FRNK. However, a double-exponential curve-fitting analysis of wtFRNK and L341S-FRNK revealed a significant difference in the value for average kFRAP (Figure 1D). Although the magnitude of the k1 rate constant was not significantly different, the value of the k2 rate constant was approximately 50% greater for L341S-FRNK (Figure 1E). We conclude that the difference in average kFRAP was the result of an increase in the slow component of fluorescence recovery. Additional results of the TIRF-FRAP experiments and curve fitting are presented in the supplemental data (supplemental Table I and supplemental Table II). The recovery of fluorescence within the region of interest in each TIRF-FRAP experiment was not the result of a redistribution of fluorescence from adjacent unbleached proteins within the same FA. Rather, the unbleached regions maintained the same level of fluorescence, whereas the region of interest fluorescence appeared to be restored from a rapidly diffusible cytoplasmic pool (supplemental Figure). Overall, these results demonstrate the dynamic exchange of FRNK between the cytoplasm and binding partners within VSMC FAs. They also demonstrate that the L341S mutation reduced the binding affinity of FRNK by approximately 50%, most likely as the result of an increase in the rate of unbinding (ie, kOFF). However, the L341S mutation did not completely eliminate FRNK targeting to VSMC FAs.
wtFRNK and L341S-FRNK Both Bind Paxillin
Our kinetic data demonstrated that FRNK binding to paxillin was reduced, but not eliminated, by the L341S mutation. To verify these results, RASMs were infected (100 multiplicity of infection, 24 hours) with Adv-GFP, Adv-GFP–wtFRNK, or adenovirus (Adv)-GFP–L341S–FRNK; fixed; permeabilized; counterstained with antipaxillin monoclonal antibody; and imaged by confocal microscopy. As seen in Figure 2A, both wt and mutant FRNK colocalized with paxillin in FAs. To quantify the FRNK-paxillin interaction, we performed coimmunoprecipitation and Western blotting experiments of similarly infected cells. Figure 2B depicts a representative experiment in which RASM extracts were immunoprecipitated with antipaxillin monoclonal antibody and the resulting coimmunoprecipitates were probed with anti-GFP (to detect wtFRNK and L341S-FRNK). Paxillin alone was present in immunoprecipitates derived from control cells expressing only GFP, whereas both wtFRNK and L341S-FRNK formed a complex with paxillin. Western blots of cell extracts before coimmunoprecipitation indicated equal expression of both transgenes. However, quantitative analysis of 6 coimmunoprecipitations revealed a significant reduction in the amount of L341S-FRNK pulled down with the paxillin monoclonal antibody (Figure 2C). Interestingly, the same coimmunoprecipitates were analyzed for FAK binding to paxillin and demonstrated a significantly greater displacement of FAK by wtFRNK versus L341S-FRNK (Figure 2D).
Figure 2.
wtFRNK and L341S-FRNK are targeted to FAs and colocalize with paxillin. A, RASMs were infected (100 multiplicity of infection [moi], 48 hours) with Adv-GFP, Adv-GFP–wtFRNK, or Adv-GFP–L341S–FRNK (green). Cells were then fixed, permeabilized, counterstained with anti–paxillin monoclonal antibody (mAb), and viewed by confocal microscopy. Colocalization of GFP fluorescence (green) and paxillin (red) in these approximate 1-µm optical sections at the cell-substratum interface was represented in the merged image (yellow). The bar indicates 15 µm. B, RASMs were infected (300 moi, 48 hours) with Adv-GFP, Adv-GFP–wtFRNK, and Adv-GFP–L341S–FRNK. Cell extracts (500-µg total protein) were coimmunoprecipitated with anti–paxillin mAb, and immunoblots were probed with anti–GFP mAb (to detect GFP-wtFRNK and GFP-L341S-FRNK) and anti–N-terminal FAK mAb. The position of molecular weight markers is indicated to the left of each blot. C, Quantitative analysis of GFP-wtFRNK and GFP-L341S-FRNK coimmunoprecipitated with paxillin. Data are given as the mean ± SEM (n=6 experiments). *P<0.05 vs wtFRNK. D, Quantitative analysis of FAK coimmunoprecipitated with paxillin in the same pull downs. *P<0.05 vs GFP control, and +P<0.05 for wtFRNK vs L341S-FRNK. IP indicates insoluble fraction; WB, Western blot.
Mutation of FRNK’s Paxillin Binding Domain Enhanced FAK-Dependent Signaling
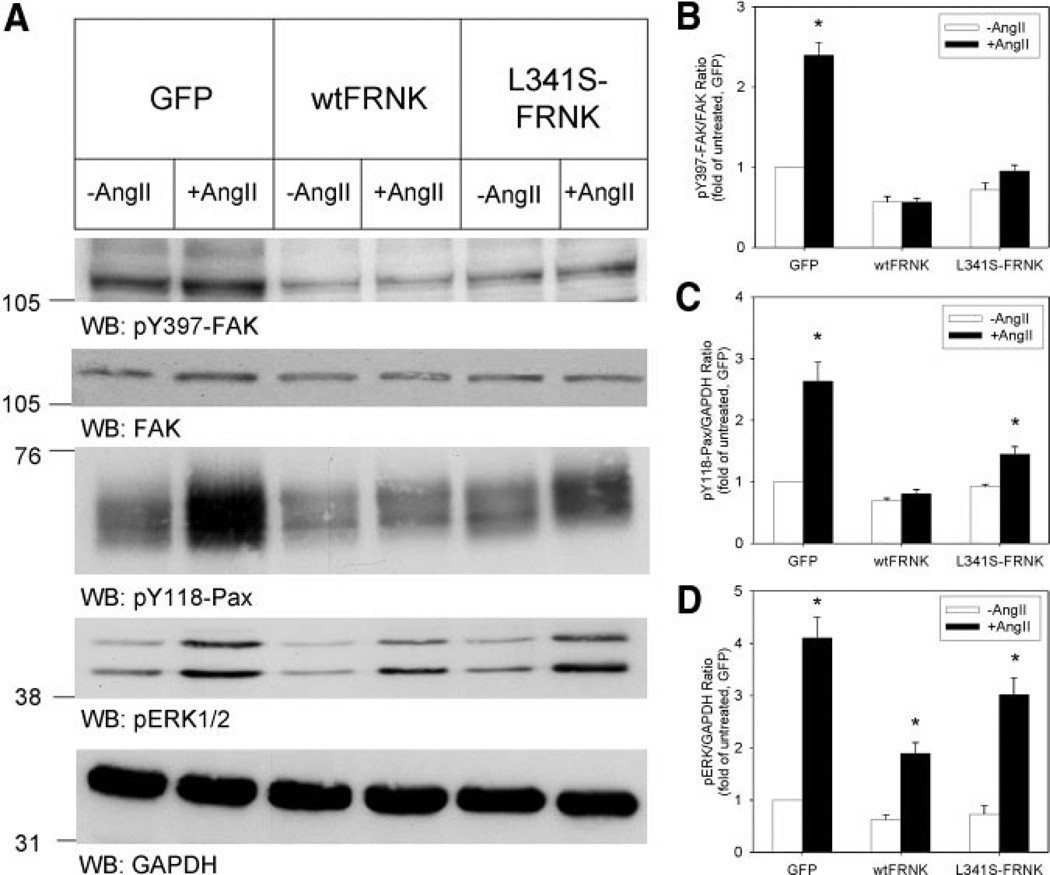
To determine whether wtFRNK and L341S-FRNK inhibited FAK autophosphorylation at Y397, and downstream signaling to paxillin and ERK1/2, RASMs were infected with Adv-GFP, Adv-GFP–wtFRNK, or Adv-GFP–L341S–FRNK (100 multiplicity of infection, 48 hours); and then stimulated with angiotensin II (AngII), 100 nmol/L, for 10 minutes. Cell extracts were analyzed by SDS-PAGE and Western blotting with total and phosphospecific antibodies. Representative Western blots are shown in Figure 3A, and the average results of 5 experiments are summarized in Figures 3B, 3C and 3D. As is evident from Figure 3, RASM contained substantial amounts of phosphorylated FAK, paxillin, and ERK1/2 under basal conditions. Both wt and mutant FRNK lowered basal FAK, paxillin, and ERK1/2 phosphorylation compared with Adv-GFP–infected cells, although there was a small, but statistically significant, difference for the degree of inhibition of basal phosphorylation between wtFRNK and L341-FRNK. AngII stimulation of Adv-GFP–infected RASMs resulted in an approximate 2-fold increase in phosphorylated FAK-Y397, an approximate 3-fold increase in phosporylated Pax-Y118, and an approximate 5-fold increase in phosphorylated ERK1/2 (T183/Y185). In contrast, AngII stimulation of cells expressing wtFRNK or L341S-FRNK did not result in a significant increase in phosphorylated FAK-Y397. Furthermore, AngII significantly increased paxillin and ERK1/2 phosphorylation in cells expressing L341S-FRNK, although the resulting increases were significantly lower than in control GFP-expressing cells. The reduction in basal and AngII-induced FAK phosphorylation was not because of a reduction in the total amount of FAK in either wtFRNK– or L341S-FRNK–expressing cells.
Figure 3.
Mutation of FRNK’s paxillin binding domain enhanced FAK-dependent signaling. A, RASMs were infected (300 multiplicity of infection, 48 hours) with Adv-GFP, Adv-GFP–wtFRNK, and Adv-GFP–L341S–FRNK. Paired cultures were then stimulated with AngII, 100 nmol/L, for 10 minutes. FAK-Y397, paxillin-Y118, and ERK1/2 T183/Y185 phosphorylations were analyzed by Western blotting with phosphospecific antibodies. B, Quantitative analysis of pY397-FAK relative to total FAK. C, Quantitative analysis of pY118-paxillin relative to GAPDH. D, Quantitative analysis of phosphorylated ERK1/2 relative to GAPDH. Data are given as the mean ± SEM (n=5 experiments). Data were analyzed by 2-way ANOVA, which revealed that the type of virus, AngII stimulation, and their interaction were all statistically significant factors. *P<0.05 for unstimulated vs AngII. WB indicates Western blot.
Mutation of FRNK’s Paxillin Binding Domain Enhanced FAK-Dependent Cell Migration
RASMs were infected (100 multiplicity of infection, 24 hours) with Adv-GFP, Adv-GFP–wtFRNK, and Adv-GFP–L341S-FRNK; and cell migration was assayed in Matrigel-coated Boyden chambers. Results of representative fluorescent images, and the average results of 20 images per group from 2 separate experiments using platelet-derived growth factor, 10 ng/mL, as chemoattractant, are depicted in Figure 4A. Another set of experiments using AngII, 100 nmol/L, is shown in Figure 4B. As is evident from Figure 4, wtFRNK markedly inhibited cell migration, confirming previous results.14 However, RASM-expressing L341S-FRNK demonstrated significantly greater migratory activity compared with wtFRNK-expressing cells for both chemoattractants.
Figure 4.
A and B, Mutation of FRNK’s paxillin binding domain enhanced FAK-dependent cell migration. RASMs were infected (100 multiplicity of infection, 24 hours) with Adv-GFP, Adv-GFP–wtFRNK, and Adv-GFP–L341S–FRNK. Equal numbers of Adv-infected cells were suspended in serum-free medium and placed in the upper chamber of Matrigel-coated Boyden chambers. Platelet-derived growth factor–BB, 10 ng/mL (A), or AngII, 100 nmol/L (B), was placed in the lower chamber. Cells were allowed to migrate for 2 hours, and fluorescently labeled cells were visualized and counted. Representative fluorescent images from single experiments are shown to the left, and a quantitative analysis of 20 images per group from 2 separate experiments for each chemoattractant is depicted to the right of each panel. *P<0.05 vs GFP control, and +P<0.05 for wtFRNK vs L341S-FRNK.
Mutation of FRNK’s Paxillin Binding Domain Enhanced FAK C-Terminal Tyrosine Phosphorylation
Next, we examined whether reduced paxillin binding affinity of FRNK affected FAK tyrosine phosphorylation at Y861 and Y925. FAK-Y397, paxillin, and ERK1/2 phosphorylation in the same cell extracts was also examined, as were the effects of AngII and the Src family PTK inhibitor, PP2, 20 µmol/L, for 1-hour pretreatment. As seen in Figure 5A and B, basal and AngII-induced FAK-Y397 phosphorylation was only modestly reduced by PP2 in GFP-expressing control cells. In contrast, FAK-Y861 (Figure 5A and C), FAK-Y925 (Figure 5A and D), paxillin (Figure 5A), and ERK1/2 (Figure 5A) phosphorylations were markedly reduced by the Src inhibitor. The overexpression of wtFRNK significantly reduced basal FAK phosphorylation at all sites, suggesting that FAK N- and C-terminal phosphorylations and downstream signaling to paxillin and ERK1/2 require FAK localization within VSMC FAs. The overexpression of L341S-FRNK also significantly lowered FAK phosphorylation at all 3 sites, but the degree of inhibition was significantly reduced by the mutation.
Figure 5.
Mutation of FRNK’s paxillin binding domain enhanced FAK tyrosine phosphorylation and downstream signaling. A, RASMs were infected (100 multiplicity of infection, 24 hours) with Adv-GFP, Adv-GFP–wtFRNK, and Adv-GFP–L341S–FRNK. Paired cultures were stimulated with AngII, 100 nmol/L, for 30 minutes in the absence or presence of PP2, 20 µmol/L (1-hour pretreatment). FAK-Y397, FAK-Y861, FAK-Y925, paxillin-Y118, and ERK1/2 phosphorylations were analyzed by Western blotting with phosphospecific antibodies. B through D, Quantitative analyses of pY397-FAK (B), pY861-FAK (C), and pY925-FAK (D), relative to total FAK, are depicted. Data are given as the mean ± SEM (n=5 experiments). Data were compared by 3-way ANOVA, as described in the “Results” section. UT indicates untreated; WB, Western blot.
Reduced FRNK Targeting to FAs Had No Effect on FRNK-Y168 Phosphorylation and Enhanced FRNK-Y232 Phosphorylation
The same cell extracts were then analyzed for FRNK tyrosine phosphorylation. As seen in Figure 6A and B, basal FRNK-Y168 phosphorylation was not affected by the L341S mutation (P=0.50, 3-way ANOVA). Furthermore, AngII stimulation increased FRNK-Y168 phosphorylation to the same degree in both wtFRNK and L341S-FRNK (P=0.65, 3-way ANOVA), which was clearly different from the effect of the FRNK mutation on AngII-induced FAK phosphorylation (Figure 5A and C). We interpret these results to indicate that FRNK phosphorylation at Y168 was not dependent on its localization within VSMC FAs, in contradistinction to the requirement for FA localization of FAK-Y861 phosphorylation. Basal and AngII-induced FRNK-Y168 phosphorylation was also PP2 sensitive (P<0.001, 3-way ANOVA), indicating that both FAK and FRNK are phosphorylated by a Src family PTK at this residue.
Figure 6.
Reduced FRNK targeting to FAs has no effect on FRNK-Y168 phosphorylation and enhances FRNK-Y232 phosphorylation. A, RASMs were infected (100 multiplicity of infection [moi], 24 hours) with Adv-GFP–wtFRNK and Adv-GFP–L341S–FRNK. Paired cultures were stimulated with AngII, 100 nmol/L, for 30 minutes in the absence or presence of PP2, 20 µmol/L (1-hour pretreatment). FRNK Y168 and Y232 phosphorylations were analyzed by Western blotting with phosphospecific antibodies. B and C, Quantitative analysis of pY168-FRNK (B) and pY232-FRNK (C) relative to total GFP-FRNK are depicted. Data are given as the mean ± SEM (n=4 experiments). Data were compared by 3-way ANOVA as described in the text. D, Representative subcellular fractionation experiment of RASMs infected (100 moi, 24 hours) with Adv-GFP–wtFRNK and Adv-GFP–L341S–FRNK. Cells were left untreated (UT) or stimulated with AngII, 100 nmol/L, for 30 minutes. Cell homogenates were separated into insoluble (IF) and soluble (SF) fractions by differential centrifugation. E, Quantitative analysis of 4 experiments, comparing the percentage of total wtFRNK with L341S-FRNK in the IF of UT and AngIIstimulated cultures. *P<0.05 for UT vs AngII-stimulated RASMs. WB indicates Western blot.
In contrast, reduced FA targeting profoundly affected FRNK-Y232 phosphorylation. As seen in Figure 6A and C, the L341S mutation significantly increased both basal and AngII-stimulated Y232 phosphorylation (P<0.001, 3-way ANOVA), which was relatively insensitive to PP2 (P=0.42, 3-way ANOVA). We interpret these results to indicate that FRNK-Y232 phosphorylation occurred outside of VSMC FAs, probably by a PP2-insensitive kinase. These results are also in contradistinction to the requirement for FA localization and Src for FAK-Y925 phosphorylation (Figure 5A and D). Subcellular fractionation of similarly prepared cell extracts confirmed that mutation of FRNK’s paxillin binding domain markedly increased the amount of Y232-phosphorylated FRNK in the soluble fraction (Figure 6D). Consistent with both the TIRF-FRAP analysis (Figure 1) and paxillin coimmunoprecipitation experiments (Figure 2), there was a significant difference in the basal distribution of wtFRNK versus L341S-FRNK (P<0.001, 1-way ANOVA). AngII stimulation did not cause a significant redistribution of wtFRNK (P=0.61). However, the agonist did significantly increase L341S-FRNK in the insoluble fraction (P=0.01) (Figure 6E).
Discussion
In this study, we examined whether FRNK targeting to FAs was necessary for FRNK phosphorylation at Y168, which is required for its inhibitory activity on VSMC spreading and migration.14 We used a previously described point mutationin the FA targeting domain of FAK to interfere with the paxillin-FRNK interaction. This mutation is located within the fourth α-helix of the FA targeting α-helical bundle of FRNK, precisely within residues that compose the second of 2 hydrophobic patches (HP) (ie, HP1 and HP2) involved in paxillin binding.26 The introduction of a hydrophilic serine in place of a hydrophobic leucine was predicted to interfere with FAK binding to the 4th LD motif in paxillin (LD4) domain within the N-terminal domain of paxillin.27,28 Surprisingly, we found that L341S-FRNK still localized to VSMC FAs, as assessed by TIRF and confocal microscopy. However, FRAP analysis revealed that both wtFRNK and L341S-FRNK were dynamically assembled and disassembled within these cells, but the exchange rate of the FRNK mutant differed significantly from that of wtFRNK. The rapid exchange of wtFRNK within FAs was quite similar to the kinetics of dissociation of other rapidly exchanging FA proteins, including FAK.29 The L341S mutation produced a substantial increase in average kFRAP, which is most consistent with a substantial increase in its rate of dissociation (kOFF).30 These kinetic results were confirmed by steady-state evaluation of paxillin binding, FAK displacement, and subcellular fractionation; and indicated that FA targeting was reduced, but not eliminated, by this point mutation.
Therefore, L341S-FRNK provided a mechanism to evaluate the requirement of FRNK tyrosine phosphorylation for targeting to VSMC FAs. Our results indicate that the paxillin-FRNK interaction is not required for efficient FRNK-Y168 phosphorylation. These results are similar to those of Scheswohl et al,31 who showed that phosphorylation of FAK-Y861 was not dependent on paxillin binding because mutants that disrupted FAK binding to paxillin had no effect on FAK phosphorylation at Y861. However, we showed that FRNK overexpression was required to displace FAK from VSMC FAs, which subsequently reduced FAK phosphorylation at this and other sites. Our data also indicate that, unlike FAK, FRNK-Y168 phosphorylation occurred independently of FA localization because both wtFRNK and L341S-FRNK were equally phosphorylated at Y168 within the soluble and insoluble fractions. The converse situation was also true (ie, FRNK-Y168 phosphorylation was not necessary for FA targeting, as demonstrated previously).14
In contrast, FRNK-Y232 phosphorylation was highly dependent on FRNK localization, but FRNK displacement from FAs substantially increased its phosphorylation at this site. Our results are consistent with a previously described model, suggesting the existence of an intermediate folding state for the FA targeting domain within FAK.32 Dixon et al32 demonstrated that the FAK FA targeting domain in solution partially unfolds, causing helix-1 to lose its helical character while separating from the helix bundle. This conformational dynamic likely facilitated FAK-Y925 phosphorylation while interfering with paxillin binding. We now demonstrate that reducing the paxillin-FRNK interaction within VSMC FAs increased FRNK-Y232 phosphorylation. However, FRNK translocation into FAs increased somewhat after AngII stimulation, but only with L341S-FRNK. It is conceivable that AngII increased the phosphorylation of paxillin and other binding partners that facilitated the FRNK-paxillin interaction despite the L341S mutation. In fact, there are likely multiple paxillin binding sites within the C-terminal domain of FAK and, by inference, within FRNK.26,31,32
L341S-FRNK also provided a tool to begin to identify which kinases are responsible for FRNK phosphorylation. Both FAK and FRNK were phosphorylated at Y861 and Y168, respectively, by a PP2-sensitive Src family PTK. However, unlike FAK phosphorylation at Y861 and Y925, PP2 only partially inhibited basal- and AngII-induced FRNK-Y168 phosphorylation and had less of an effect on FRNK-Y232 phosphorylation. Thus, FRNK phosphorylation at Y168 and Y232 appears to be executed differently than FAK phosphorylation at the same residues. A previous study14 indicated that VSMC FRNK is a naturally occurring protein that, along with FAK, was upregulated after arterial injury. FRNK-Y168 phosphorylation appeared to have a distinct function in inhibiting cell migration, which was unrelated to its ability to inhibit FAK-dependent downstream signaling. We show that the PP2 sensitivity of Y168 and Y232 phosphorylation is also distinct from FAK, suggesting that FRNK phosphorylation may require both Src-dependent and Src-independent pathways. As seen in Figure 6B, FRNK-Y168 phosphorylation was independent of FA targeting because both the wt and mutant FRNK were phosphorylated by AngII stimulation and inhibited by PP2 to the same extent. This observation was different than FAK-Y861 phosphorylation (Figure 5C), in which basal-and AngII-stimulated phosphorylations were highly dependent on FAK localization and Src activity. Furthermore, FRNK-Y232 phosphorylation was highly dependent on FA targeting. Reducing the binding affinity of FRNK for paxillin increased the off rate of FRNK from FAs and resulted in hyperphosphorylation at the Y232 site, probably by a Src-independent pathway. Again, these results are quite distinct from the effects of PP2 on FAK-Y925 phosphorylation, which was highly dependent on both FA targeting and Src activity. We believe that these results provide additional evidence to support our contention that FRNK has independent signaling functions in VSMCs. These independent functions may explain the difference in phenotype exhibited by FRNK-overexpressing cells compared with FAK−/− cells. FAK−/− cells have increased immature FAs that result in reduced cell migration. However, the migration defect is thought to be because of their inability to release FAs. In addition to decreased migratory capacity, FRNK overexpression results in the loss of FAs, cell rounding, decreased proliferation, and anoikis.12,16,20 The future identification of the specific PTKs responsible for both FAK and FRNK phosphorylation may be useful for the development of treatment strategies to limit the process of vasculogenesis and neointimal development by inhibiting FAK, but not FRNK, tyrosine phosphorylation.
Supplementary Material
Acknowledgments
We thank Daniel Blackwell, BS, for assistance with TIRF microscopy and FRAP analysis.
Sources of Funding
This study was supported in part by a grant from the Dr. Ralph and Marian Falk Medical Research Trust. While these studies were performed, Dr Koshman was an American Heart Association post-doctoral fellow and Dr Engman was an American Heart Association predoctoral fellow.
Footnotes
Disclosures
None.
References
- 1.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 3.Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker CE, Parsons JT. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene. 2001;20:1152–1163. doi: 10.1038/sj.onc.1204208. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Ghazaleh R, Kabir J, Jia H, Lobo M, Zachary I. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem J. 2001;360:255–264. doi: 10.1042/0264-6021:3600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin αvβ5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 8.Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JG, Miyazu M, Matsushita E, Sokabe M, Naruse K. Uniaxial cyclic stretch induces focal adhesion kinase (FAK) tyrosine phosphorylation followed by mitogen-activated protein kinase (MAPK) activation. Biochem Biophys Res Commun. 2001;288:356–361. doi: 10.1006/bbrc.2001.5775. [DOI] [PubMed] [Google Scholar]
- 10.Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase: 2 tyrosine sites involved in ERK activation. J Biol Chem. 2004;279:30588–30599. doi: 10.1074/jbc.M313244200. [DOI] [PubMed] [Google Scholar]
- 11.Schaller MD, Borgman CA, Parsons JT. Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK. Mol Cell Biol. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor JM, Mack CP, Nolan K, Regan CP, Owens GK, Parsons JT. Selective expression of an endogenous inhibitor of FAK regulates proliferation and migration of vascular smooth muscle cells. Mol Cell Biol. 2001;21:1565–1572. doi: 10.1128/MCB.21.5.1565-1572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayers RL, Sundberg-Smith LJ, Rojas M, Hayasaka H, Parsons JT, Mack CP, Taylor JM. FRNK expression promotes smooth muscle cell maturation during vascular development and after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:2115–2122. doi: 10.1161/ATVBAHA.108.175455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koshman YE, Engman SJ, Kim T, Iyengar R, Henderson KK, Samarel AM. Role of FRNK tyrosine phosphorylation in vascular smooth muscle spreading and migration. Cardiovasc Res. 2010;85:571–581. doi: 10.1093/cvr/cvp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson A, Parsons T. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK. Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauck CR, Hsia DA, Schlaepfer DD. Focal adhesion kinase facilitates platelet-derived growth factor-BB-stimulated ERK2 activation required for chemotaxis migration of vascular smooth muscle cells. J Biol Chem. 2000;275:41092–41099. doi: 10.1074/jbc.M005450200. [DOI] [PubMed] [Google Scholar]
- 18.Eble DM, Strait JB, Govindarajan G, Lou J, Byron KL, Samarel AM. Endothelin-induced cardiac myocyte hypertrophy: role for focal adhesion kinase. Am J Physiol Heart Circ Physiol. 2000;278:H1695–H1707. doi: 10.1152/ajpheart.2000.278.5.H1695. [DOI] [PubMed] [Google Scholar]
- 19.Govindarajan G, Eble DM, Lucchesi PA, Samarel AM. Focal adhesion kinase is involved in angiotensin II-mediated protein synthesis in cultured vascular smooth muscle cells. Circ Res. 2000;87:710–716. doi: 10.1161/01.res.87.8.710. [DOI] [PubMed] [Google Scholar]
- 20.Heidkamp MC, Bayer AL, Kalina JA, Eble DM, Samarel AM. GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes. Circ Res. 2002;90:1282–1289. doi: 10.1161/01.res.0000023201.41774.ea. [DOI] [PubMed] [Google Scholar]
- 21.Brewster LP, Ucusian AA, Brey EM, Liwanag M, Samarel AM, Greisler HP. FRNK overexpression limits the depth and frequency of vascular smooth muscle cell invasion in a three-dimensional fibrin matrix. J Cell Physiol. 2010;225:562–568. doi: 10.1002/jcp.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 23.Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Tachibana K, Sato T, D’Avirro N, Morimoto C. Direct association of pp125FAK with paxillin, the focal adhesion-targeting mechanism of pp125FAK. J Exp Med. 1995;182:1089–1099. doi: 10.1084/jem.182.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson A, Malik RK, Hildebrand JD, Parsons JT. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi I, Vuori K, Liddington RC. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat Struct Biol. 2002;9:101–106. doi: 10.1038/nsb755. [DOI] [PubMed] [Google Scholar]
- 27.Brown MC, Perrotta JA, Turner CE. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J Cell Biol. 1996;135:1109–1123. doi: 10.1083/jcb.135.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown MC, Curtis MS, Turner CE. Paxillin LD motifs may define a new family of protein recognition domains. Nat Struct Biol. 1998;5:677–678. doi: 10.1038/1370. [DOI] [PubMed] [Google Scholar]
- 29.Lele TP, Thodeti CK, Pendse J, Ingber DE. Investigating complexity of protein-protein interactions in focal adhesions. Biochem Biophys Res Commun. 2008;369:929–934. doi: 10.1016/j.bbrc.2008.02.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sprague BL, Pego RL, Stavreva DA, McNally JG. Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys J. 2004;86:3473–3495. doi: 10.1529/biophysj.103.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheswohl DM, Harrell JR, Rajfur Z, Gao G, Campbell SL, Schaller MD. Multiple paxillin binding sites regulate FAK function. J Mol Signal. 2008;3:1. doi: 10.1186/1750-2187-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixon RD, Chen Y, Ding F, Khare SD, Prutzman KC, Schaller MD, Campbell SL, Dokholyan NV. New insights into FAK signaling and localization based on detection of a FAT domain folding intermediate. Structure. 2004;12:2161–2171. doi: 10.1016/j.str.2004.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.