Summary
Early in the pathogenesis of Type 2 diabetes mellitus (T2DM), dysregulated glucagon secretion from pancreatic α-cells occurs prior to impaired glucose stimulated insulin secretion (GSIS) from β-cells. However, whether hyperglucagonemia is causally linked to β-cell dysfunction remains unclear. Here we show that glucagon stimulates via cAMP-PKA-CREB signaling hepatic production of the neuropeptide kisspeptin1, which acts on β-cells to suppress GSIS. Synthetic kisspeptin suppresses GSIS in vivo in mice and from isolated islets in a kisspeptin1 receptor-dependent manner. Kisspeptin1 is increased in livers and in serum from humans with T2DM and from mouse models of diabetes mellitus. Importantly, liver Kiss1 knockdown in hyperglucagonemic, glucose intolerant high fat diet fed and Leprdb/db mice augments GSIS and improves glucose tolerance. These observations indicate a hormonal circuit between the liver and the endocrine pancreas in glycemia regulation and suggest in T2DM a sequential link between hyperglucagonemia via hepatic kisspeptin1 to impaired insulin secretion.
Introduction
Glucagon and insulin are secreted respectively, by pancreatic α- and β-cells to precisely control blood glucose homeostasis. An early hallmark of type 2 diabetes mellitus (T2DM) is dysregulated glucagon secretion by pancreatic α-cells. Non-diabetic humans exhibit postprandial suppression of blood glucagon, while individuals with T2DM lack this suppression and may even exhibit increased glucagon levels. In addition, studies in subsets of patients with T2DM suggest that elevated glucagon secretion occurs antecedent to β-cell dysfunction (D'Alessio, 2011) and references therein).
Upon binding to its receptor Gcgr, glucagon activates cellular adenosine-3’-5’-cyclic monophosphate (cAMP) - protein kinase A (PKA) signaling to stimulate hepatic glucose production (HGP) and cause hyperglycemia (Chen et al., 2005). While hyperglycemia stimulates insulin secretion from β-cells, transgenic upregulation of protein kinase A (PKA) activity in hepatocytes in mice results as expected in increased HGP and hyperglycemia but paradoxically in impaired GSIS (Niswender et al., 2005). Consistent with the idea that glucagon may be causally linked to β-cell dysfunction, are findings made during exogenous glucose infusion in rats, where insulin secretion only fails after blood glucagon levels rise, and recovers upon glucagon inactivation by neutralizing antiserum (Jamison et al., 2011).
Based on these considerations for hyperglucagonemia and β-cell dysfunction in T2DM, we reasoned that independent of HGP and hyperglycemia, glucagon signaling in the liver initiates a process, which impacts on GSIS. We tested this hypothesis by comparing a mouse model of liver-specific PKA disinhibition (L-ΔPrkar1a mice, see below) with a model of hyperglycemia resulting from intravenous glucose infusion (D-glucose mice) combined with array-based gene expression analysis for secreted hepatic peptides, and identified Kiss1, which encodes the neuropeptide kisspeptin1 to be upregulated in livers of L-ΔPrkar1a but not in D-glucose mice and also to be directly stimulated by glucagon action via Gcgr on hepatocytes.
Kisspeptin1, has been described to be synthesized in the central nervous system and to regulate hypothalamic gonadotropin releasing hormone (GnRH) neurons, and is processed to multiple biologically active N-terminally truncated fragments kisspeptin 54 (K54), K14, K13, K10, of which the latter exerts full biological effects (Seminara and Kaiser, 2005). In experimental conditions individual kisspeptin isoforms are reported to suppress GSIS at nanomolar concentrations but stimulate GSIS at micromolar concentrations (Hauge-Evans et al., 2006; Silvestre et al., 2008). However, a (patho-) physiologic context for circulating levels of kisspeptin1, its effects on β-cell function specifically via kisspeptin1 receptor (Kiss1R), and its interplay with gluco-regulatory hormones are not understood.
Immunometric assays for kisspeptin have provided conflicting information on kisspeptin levels in rodents and humans (Akinci et al., 2012; Cetkovic et al., 2012; Horikoshi et al., 2003; Logie et al., 2012), and mass-spectroscopy-based assays do not provide exact information on circulating concentrations and functional bioactivity of the complement of kisspeptin isoforms in biological samples (Liu et al., 2013). Therefore, we have used - in addition to immunoassays - a bioassay of kisspeptin1 action on GSIS from mouse islets, which either express Kiss1R or selectively lack Kiss1R to reliably measure functional kisspeptin concentrations.
Using this assay, we find that synthetic kisspeptin inhibits GSIS from cultured islets in a dose- and Kiss1R-dependent manner at nanomolar concentrations. Furthermore, mice rendered glucose intolerant by high fat-content diet (HFD) or leptin receptor defective diabetic (Leprdb/db) mice are hyperglucagonemic, exhibit increased liver kisspeptin1 and harbor in their circulation functional kisspeptin bioactivity equivalent to nanomolar concentrations of synthetic kisspeptin. In these mice, selective liver kisseptin1 knockdown derepresses GSIS and improves glucose tolerance (GT). Importantly, humans with T2DM also exhibit increased liver and plasma kisspeptin1 levels. Furthermore, mice selectively lacking pancreas Kiss1R, when fed a HFD, as compared to control counterparts, show improved GT owing to increased GSIS.
These observations identify the liver as a site of regulated kisspeptin1 synthesis, define a liver to islet endocrine circuit in glucoregulation, and suggest a pathogenic mechanism in T2DM, causally linking hyperglucagonemia via hepatic kisspeptin1 to insufficient insulin secretion. In addition, these findings extend a potential for kisspeptin1 antagonism as a therapeutic means to improve β-cell function in diabetes mellitus.
Results
Disinhibited PKA Activity in Liver Causes Impaired GSIS Independent of Hyperglycemia
To mimic upregulated glucagon-cAMP-PKA signaling in vivo in mouse liver independently of glucagon action in other tissues, we selectively disinhibited liver PKA catalytic (PKAc) activity by ablating hepatic protein kinase A regulatory subunit 1A (Prkar1a) using the CRE/LoxP method. Mice homozygous for floxed Prkar1a (Prkar1afl/fl mice) (Kirschner et al., 2005) were treated by tail vein injection with adenovirus driving CRE recombinase under control of the CMV promoter (Adv-CRE) to generate mice selectively lacking liver Prkar1a (L-ΔPrkar1a mice). Control mice received adenovirus expressing green fluorescent protein (Adv-GFP).
Liver extracts harvested four days after injection from Adv-CRE injected mice revealed a 90% reduction in Prkar1a protein (Fig 1A), while other Prkar isoforms and Pkac levels remained unaltered. As expected, L-ΔPrkar1a mice, as opposed to controls, exhibited increased hepatic phosphorylation of cAMP-response element binding protein (CREB) at serine 133 (pCREB), an established PKAc target (Gonzalez and Montminy, 1989) (Fig 1A). Adv-CRE treatment did not affect Prkar1a expression in islet, hypothalamus, adpose tissue and skeletal muscle (Fig. S1A). Liver-specific PKA disinhibition stimulated within 4 days hepatic expression of transcriptional co-activators (Ppargc1a, Src1) and rate-limiting enzymes (G6p, Pck1) of the gluconeogenic pathway (Louet et al., 2010), which resulted in fasting hyperglycemia and notably also in insufficient insulin secretion to correct glycemia during intraperitoneal glucose tolerance tests (ipGTT) (Fig 1C,D).
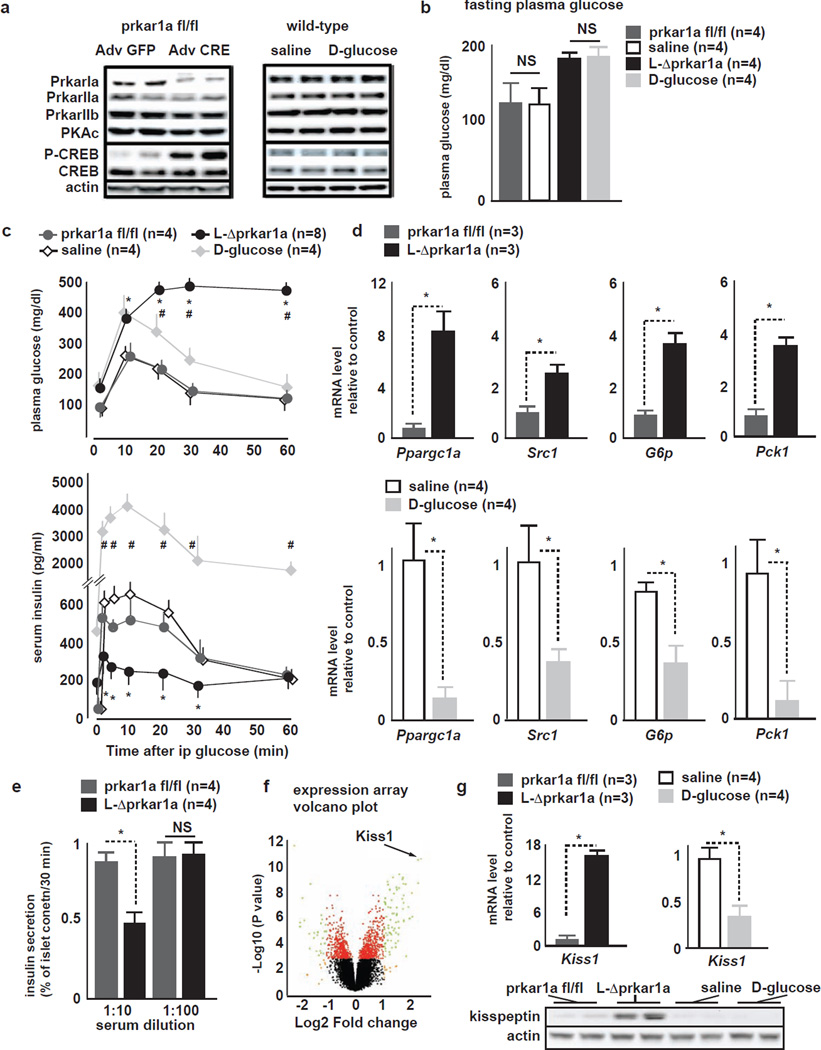
Figure 1. Comparison between L-Δprkar1a and D-glucose mice identifies Kiss1.
A (left) Representative liver IB of prkar1afl/fl and L-Δprkar1a 4 days after adenovirus treatment. L-Δprkar1a mice show Prkar1a ablation and increased pCREB (right) Liver IB from Sal- and D-glucose mice shows unaltered Prkar subtypes, Pkac, pCREB.
B Fasting glucose levels in prkar1afl/fl, L-Δprkar1a, Sal- and D-glucose mice. Prkar1afl/fl and Sal- mice have similar fasting glucose; D-glucose infusion achieves fasting glucose similar to L-Δprkar1a mice (mean±SEM, * P<0.05).
C (top) plasma glucose and (bottom) serum insulin during ipGTT in prkar1afl/fl, L-Δprkar1a, Sal- and D-glucose mice. L-Δprkar1a mice exhibit impaired GT (top) and GSIS (bottom). D-glucose mice have mildly impaired GT and robust GSIS. Prkar1a fl/fl and Sal-mice have similar GT and GSIS (mean±SEM, * P<0.05).
D qRT-PCR of indicated genes of gluconeogenic program in prkar1afl/fl, L-Δprkar1a, Sal- and D-glucose-mouse livers. (top) gluconeogenic program is upregulated in L-Δprkar1a as compared to prkar1afl/fl mice; (bottom) gluconeogenic program is downregulated in D-glucose as compared to saline-mice (mean±SEM, * P<0.05)..
E GSIS of WT mouse islets cultured in serum free media conditioned with plasma of prkar1afl/fl or L-Δprkar1a mice. Prkar1afl/fl plasma does not affect GSIS. L-Δprkar1a plasma at 1:10 but not at 1:100 dilution suppresses GSIS (mean±SEM, * P<0.05).
F Volcano plot of gene expression analysis of liver from prkar1afl/fl and L-Δprkar1a mice. Significant upregulation of Kiss1 transcript is detected in L-Δprkar1a mice.
G (top) qRT-PCR of Kiss1 transcript and (bottom) IB in liver tissue from mice with indicated liver genetic complement or intravenous infusion. L-Δprkar1a liver shows increased Kiss1 transcript and kisspeptin protein. D-glucose mice show Kiss1 downregulation as compared to controls (mean±SEM, * P<0.05).
To assess whether hyperglycemia during 4 days is directly associated with impaired GSIS, we generated a model of chronic hyperglycemia without hepatic PKA-CREB activation. Wild-type mice were intravenously infused during 4 days with D-glucose (D-glucose mice) to achieve fasting glucose levels to match those measured in L-ΔPrkar1a mice (Fig 1B). Mice infused with saline served as controls (Sal mice). D-glucose mice exhibited no change in liver pCREB (Fig 1A) and reduced gene expression of the gluconeogenic program (Fig 1D). In contrast to L-ΔPrkar1a mice, D-glucose mice showed increased GSIS and only mildly impaired GT (Fig 1C). Both L-ΔPrkar1a and D-glucose mice showed similar increases in β-cell proliferation, as assessed by Ki67 expression (Fig S1E); albeit, pancreas morphometric parameters or plasma glucagon levels in L-ΔPrkar1a and D-glucose infused mice did not change during the short 4-day protocols (Fig S1B-H), excluding differences in β-, or α-cell mass or in glucagon action to account for the differences in glucose homeostasis.
Selective PKA Disinhibition in Liver Induces Secreted Neuropeptide Kiss1
The liver secretes factors, which regulate pancreatic α- or β-cell growth, and which in part have been identified by liver gene expression analyses (El Ouaamari et al., 2013; Longuet et al., 2013; Yi et al., 2013). We reasoned that the liver may also secrete a factor(s) that regulates β-cell function and that may be altered in L-ΔPrkar1a mice. We therefore treated isolated WT mouse islets with serum-free media conditioned with plasma extracted from L-ΔPrkar1a or control mice and examined GSIS at 10 mM glucose. Plasma in 1:10 dilution from L-ΔPrkar1a but not from control mice suppressed GSIS (Fig 1E). 1:100 diluted L-ΔPrkar1a plasma did not suppress GSIS in this functional bioassay. These observations suggested that PKA signaling in the liver generates a secreted molecule(s), which suppresses β-cell GSIS in a dose-dependent fashion (Fig 1E).
To identify this PKA-regulated factor, we analyzed by microarray L-ΔPrkar1a and control mouse livers for differentially expressed genes encoding secreted proteins. We identified a single candidate transcript encoding kisspeptin1 (Kiss1) (Fig 1F) to be elevated in liver tissue of L-ΔPrkar1a mice (P<10−11, log2FC=2.17). Quantitative RT-PCR (qRT-PCR) and immunoblots (IB) of liver tissue confirmed increased hepatic kisspeptin1 production in L-ΔPrkar1a mice (Fig 1G). Conversely, liver Kiss1 was reduced by 50% in D-glucose mice (Fig 1G).
Glucagon Stimulates Hepatic Kiss1 Expression via Gcgr and cAMP-PKA-CREB Signaling
We next examined whether glucagon directly stimulates hepatic kisspeptin1 production. Glucagon treatment (200 pg/ml) of primary mouse hepatocytes stimulated within 2 hours Kiss1 expression and protein (Fig 2A,C). As expected, the gluconeogenic gene Pck1, an internal control, was also stimulated by glucagon (Fig 2A). Consistent with glucagon activated cAMP-PKA signaling, mouse hepatocytes treated for 2 hours with the adenylyl cyclase activator forskolin (fsk) plus the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) exhibited increased Kiss1 expression (Fig 2B).
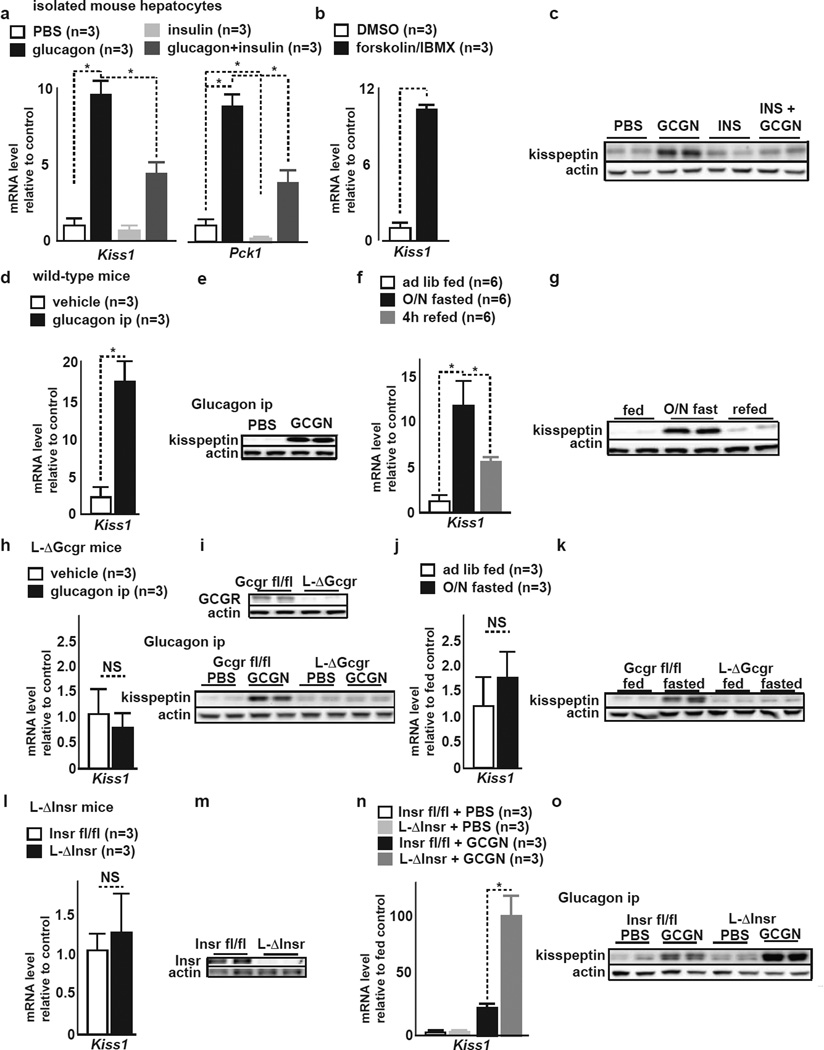
Figure 2. Glucagon and insulin counter-regulate liver Kiss1 expression.
A qRT-PCR of Kiss1 and Pck1 in isolated mouse hepatocytes exposed to indicated treatment. Glucagon stimulates, insulin suppresses both genes (mean±SEM, *P<0.05).
B qRT-PCR of Kiss1 in isolated mouse hepatocytes exposed to vehicle (DMSO) or fsk/IBMX. cAMP stimulation stimulates Kiss1 expression (mean±SEM, *P<0.05)..
C Representative IB of cultured mouse hepatocytes after treatment with PBS, glucagon, insulin or INS plus GCGN. Glucagon stimulates kisspeptin, insulin treatment has little effect on already low kisspeptin. Insulin counterregulates glucagon stimulation of kisspeptin.
D qRT-PCR of Kiss1 in liver tissue of WT mice after in vivo ip treatment with vehicle (PBS) or glucagon. Glucagon treatment stimulates Kiss1 in liver (mean±SEM, *P<0.05).
E Representative liver IB in WT mice after in vivo ip treatment with PBS or glucagon. Glucagon increases liver kisspeptin1 (mean±SEM, *P<0.05).
F qRT-PCR of Kiss1 in liver of ad lib fed, O/N fasted, and refed WT mice. Fasting stimulates liver Kiss1 expression, refeeding supresses elevated Kiss1 (mean±SEM, *P<0.05).
G Liver IB from ad lib fed, O/N fasted, and refed WT mice. Fasting stimulates liver kisspeptin1.
H qRT-PCR of Kiss1 in liver of L-ΔGcgr mice after ip PBS or glucagon. Glucagon does to stimulate Kiss1 in L-ΔGcgr mice (mean±SEM, * P<0.05).
I (top) Representative liver IB from Gcgrfl/fl and L-ΔGcgr mice. L-ΔGcgr lack GCGR. (bottom) Liver IB from Gcgrfl/fl and L-ΔGcgr mice after ip treatment with PBS or glucagon. Baseline kisspeptin1 is similar in Gcgrfl/fl and L-ΔGcgr mouse liver. Glucagon treatment stimulates kisspeptin1 in Gcgrfl/fl but not in L-ΔGcgr mice.
J qRT-PCR of Kiss1 in liver of ad lib fed and O/N fasted L-ΔGcgr mice. O/N fast does not stimulate Kiss1 in L-ΔGcgr mouse liver (mean±SEM, *P<0.05).
K Representative liver IB from ad lib fed and O/N fasted Gcgrfl/fl and L-ΔGcgr mice. Baseline kisspeptin1 is similar in Gcgrfl/fl and L-ΔGcgr livers. Fasting stimulates kisspeptin1 in Gcgr fl/fl but not in L-ΔGcgr liver.
L qRT-PCR of Kiss1 in liver of Insrfl/fl and L-ΔInsr mice. Liver Insr ablation does not affect Kiss1 expression (mean±SEM, *P<0.05).
M Representative liver IB of Insrfl/fl and L-ΔInsr mice. L-ΔInsr liver lacks insulin receptor immunoreactivity.
N qRT-PCR of Kiss1 in liver of Insrfl/fl and L-ΔInsr mice after ip treatment with vehicle PBS or glucagon. Glucagon stimulates Kiss1 in Insrfl/fl mice and more so in L-ΔInsr liver (mean±SEM, *P<0.05).
O Representative liver IB of Insrfl/fl and L-ΔInsr mice after ip PBS or glucagon. Glucagon stimulates stronger liver kisspeptin1 production in L-ΔInsr than in Insrfl/fl liver.
A luciferase reporter plasmid containing 1kb of the mouse Kiss1 promoter element transfected into mouse H2.35 hepatoma cells showed transcriptional activation in response to glucagon or to fsk/IBMX treatment (Fig. S2A). The mouse Kiss1 promoter contains two putative functional cAMP response element (CRE) half-sites (TGACT) (Zhang et al., 2005) located at −27 and −758 base pairs upstream of the Kiss1 transcription start site. Mutation of either of the CRE half-sites decreased the transcriptional responses to glucagon or to fsk/IBMX, and combined mutation of both CRE half-sites further decreased the responses (Fig. S2A).
In accordance with CREB mediating cAMP-stimulated Kiss1 transcription, co-transfection in H2.35 cells with constitutively active CREB Y134F stimulated Kiss1 reporter activity as long as CRE1 and 2 sites were intact, while dominant negative CREB inhibitor A-CREB blocked reporter stimulation by fsk/IBMX (Fig. S2B,C). Consistent with these findings is robust cAMP-PKA-CREB responsiveness of the human Kiss1 promoter, which contains a functional CRE half-site at −45 bp proximal to the transcription start site (Zhang et al., 2005). Chromatin of mouse liver extracts using non-specific versus CREB-specific antiserum combined with qPCR of CRE half-site sequences (in vivo ChIP), confirmed in vivo CREB occupancy of both CRE half-sites within the Kiss1 promoter (Fig. S2D).
Intraperitoneal glucagon (16 µg/kg) but not PBS treatment in mice increased hepatic kisspeptin1 production 30 minutes after injection (Fig. 2D,E). Physiologic endogenous glucagon secretion provoked by overnight fasting (Fig. S2E) resulted in increased hepatic kisspeptin1 production when compared to ad libitum fed mice (Fig. 2F,G). Fasted and subsequently refed mice exhibited a reduction in blood glucagon levels (Fig. S2D) and also of liver Kiss1 mRNA and kisspeptin1 protein (Fig. 2F,G). Kiss1 transcript was also detectable by qRT-PCR at low levels in spleen, kidney, skeletal muscle and epididymal fat tissue. In these tissues, Kiss1 expression remained unchanged during fasting (not shown) indicating that the liver is the main site where Kiss1 expression is regulated by metabolic cues.
To confirm the role of the liver glucagon receptor in mediating in vivo effects of glucagon on hepatic kisspeptin1 production, we generated floxed glucagon receptor (Gcgrfl/fl) mice (Fig. S3A), in which we conditionally ablated liver Gcgr by Adv-CRE delivery (L-ΔGcgr mice) (Fig. S3B). Adv-GFP injected Gcgrfl/fl mice served as controls. Adv-CRE treatment did not ablate Grgr in islet, hypothalamus, and adpose tissue (Fig. S3B). Deprivation of hepatic glucagon signaling in L-ΔGcgr mice resulted in reduced fasting glucose levels, improved GT and unchanged insulin tolerance (Fig. S3C,D). L-ΔGcgr mice showed, as compared to controls, slightly but not significantly reduced liver kisspeptin1 expression (Fig. 2H,I). Importantly, in L-ΔGcgr mice, kisspeptin production did not change in response to intraperitoneal glucagon treatment or after overnight fasting (Fig. 2J,K). Plasma kisspeptin levels in Gcgrfl/fl and L-ΔGcgr mice reflected respectively, changes in liver kisspeptin production in response to glucagon treatment or fasting (Fig. S2G,H). Furthermore, L-ΔGcgr mice showed, as compared to controls, significantly dampened liver CREB phosphorylation and CREB occupancy of the Kiss1 promoter CRE half-sites in response to glucagon treatment or to overnight fasting (Fig. S3E,F).
Glucagon and Insulin Counter-regulate Liver Kisspeptin1 Expression
In the liver, insulin counteracts glucagon action on cAMP-CREB regulated genes (He et al., 2009). Accordingly, in vitro insulin treatment (2000 pg/ml) of mouse hepatocytes reduced basal Kiss1 expression, and as expected, also reduced Pck1 expression (Fig. 2A). Insulin treatment dampened glucagon-stimulated Kiss1 and Pck1 expression in mouse hepatocytes (Fig. 2A). Changes in Kiss1 expression were qualitatively reflected in corresponding changes in kisspeptin1 protein levels in response to glucagon and insulin treatment, respectively (Fig. 2C).
To verify that in vivo insulin effects on liver Kiss1 expression are directly mediated by liver insulin receptors and to examine whether isolated hepatic insulin resistance may modulate Kiss1 expression, we generated mice with liver-specific insulin receptor deficiency by Adv-CRE treatment of floxed insulin receptor (Insrfl/fl) mice (Bruning et al., 1997) (L-ΔInsr mice; Fig. 2L). Importantly, Insr expression in islets, hypothalamus, adipose tissue and skeletal muscle was not different between Insrfl/fl and L-ΔInsr mice (Fig S2F). Hepatic ablation of insulin receptor by Adv-CRE in mice did not change liver Kiss1 mRNA or protein levels (Fig. 2M,O). Conversely, glucagon treatment of L-ΔInsr mice, as compared to Insrfl/fl mice, dramatically increased liver Kiss1 transcript and kisspeptin protein (Fig. 2N,O). Plasma kisspeptin1 levels in Insrfl/fl and L-ΔInsr mice, respectively, reflected the changes in liver kisspeptin production (Fig. S2I).
Consistent with these observations of insulin counter-regulation of PKA mediated Kiss1 stimulation, in vivo intraperitoneal insulin (1 IU/kg) administration in L-ΔPrkar1a mice to supplement relatively deficient endogenous serum insulin concentrations and to achieve blood glucose reduction, decreased hepatic Kiss1 mRNA and protein levels within 60 minutes (Fig. S1I-K).
These findings indicate that – as is established for cAMP-CREB responsive gluconeogenic genes- insulin at sufficiently high concentrations antagonizes glucagon stimulation of Kiss1 expression. Further, in vivo disruption of the hepatic insulin receptor (i.e. liver insulin resistance) alone does not de-repress liver kisspeptin1 production absent additional glucagon signaling. These findings also indicate that the liver is the predominant source of circulating kisspeptin1, which is subject to hormonal regulation.
Kisspeptin1 Knockdown in L-ΔPrkar1a Mice Ameliorates GSIS Despite Continued Gluconeogenesis
To verify that in L-ΔPrkar1a mice hepatic kisspeptin1 is directly linked to impaired GSIS, we knocked down hepatic kisspeptin1 in L-ΔPrkar1a mice by administering adenovirus expressing Kiss1-specific shRNA (Adv-Kiss1 shRNA) or a control scrambled shRNA adenovirus (Adv-scr shRNA). Within 3 days of treatment, Adv-Kiss1 shRNA treated mice showed reduced hepatic Kiss1 mRNA and plasma kisspeptin (Fig. 3A). In contrast, gluconeogenesis, as reflected by mRNA of the gluconeogenic genes (Fig. 3B) as well as functional conversion of intraperitoneally administered gluconeogenic precursor pyruvate to glucose in the fed (non-fasting) state (ip pyruvate conversion test = ipPCT) was similar between Adv-Kiss1 shRNA and control Adv-scr shRNA treated L-ΔPrkar1a mice and also significantly increased as compared to WT mice (Fig. 3D). Furthermore, liver CREB phosphorylation and CREB occupancy of the endogenous Kiss1 promoter was similar in Adv-scr shRNA and Adv-Kiss1 shRNA treated L-ΔPrkar1a mice (Fig. 3B,C), indicating that PKA signaling per se is not differentially affected by Kiss1-specific versus scr shRNA treatment.
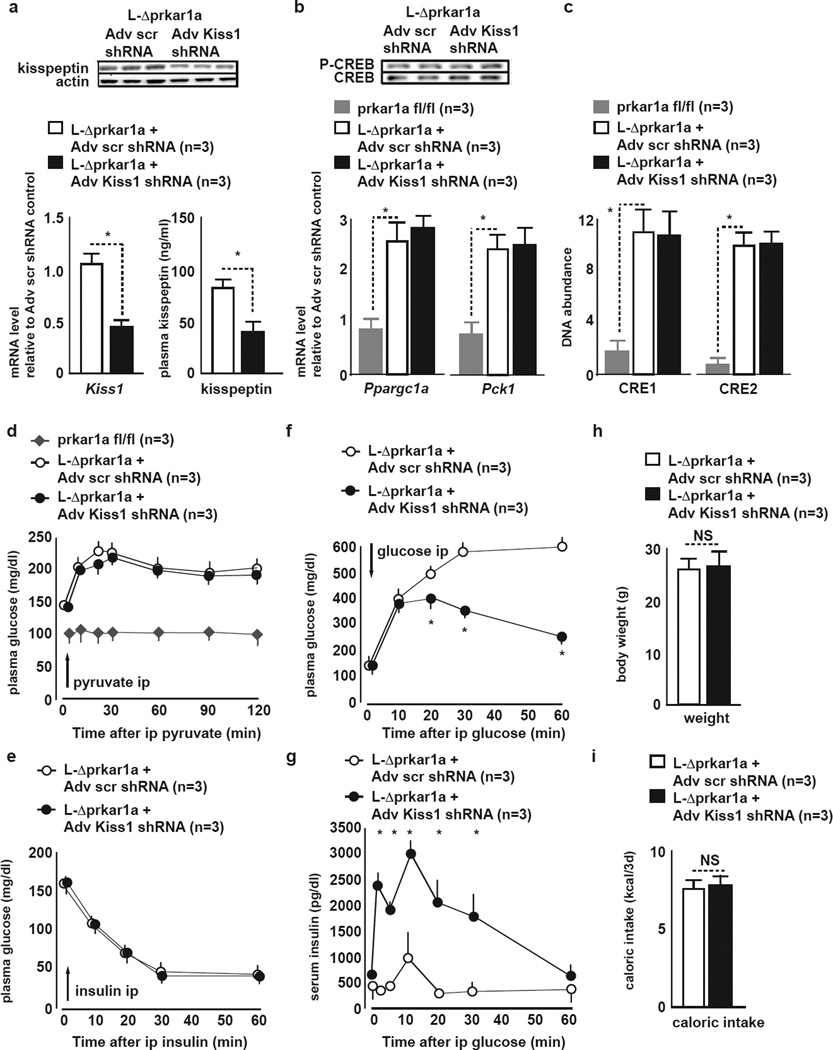
Figure 3. In L-Δprkar1a mice liver Kiss1 knockdown derepresses GSIS and ameliorates glucose tolerance despite continued upregulated gluconeogenesis.
A (top) Liver kisspeptin1 IB, (bottom left) liver qRT-PCR of Kiss1 mRNA, and (bottom right) plasma kisspeptin1 in L-Δprkar1a mice 3 days after Adv-scr or -Kiss1 shRNA treatment. Liver kisspeptin1 protein, Kiss1 mRNA and plasma kisspeptin1 are reduced after Kiss1 Kiss1 L-Δprkar1a mice (mean±SEM, *P<0.05).
B (top) pCREB and total CREB IB, (bottom) qRT-PCR of Ppargc1a and Pck1 in liver of L-Δprkar1a mice 3 days after Adv-scr shRNA or Adv-Kiss1 shRNA treatment. CREB phosphorylation and total CREB protein are unaffected by Kiss1 knockdown in L-Δprkar1a liver. Ppargc1a and Pck1 mRNA levels are upregulated in L-Δprkar1a as compared to prkar1afl/fl livers and are unaffected by liver Kiss1 knockdown in L-Δprkar1a mice (mean±SEM, *P<0.05).
C In vivo ChIP of CREB occupancy on CRE1 and CRE2 within the Kiss1 promoter in liver samples. CREB occupancy on Kiss1 CRE1 and 2 in L-Δprkar1a liver is increased as compared to prkar1afl/fl liver and unaffected by Kiss1 knockdown in L-Δprkar1a mice (mean±SEM, *P<0.05).
D ipPCT in fed prkar1afl/fl and L-Δprkar1a mice 3 days after Adv-scr or -Kiss1 shRNA treatment. Gluconeogensis activity is increased in L-Δprkar1a as compared to prkar1afl/fl mice. Gluconeogenesis activity in L-Δprkar1a mice is unaffected by Kiss1 knockdown (mean±SEM, *P<0.05).
E ipITT in L-Δprkar1a mice 3 days after treatment with Adv-scr or -Kiss1 shRNA. Peripheral insulin sensitivity in L-Δprkar1a mice is unaffected by Kiss1 knockdown (mean±SEM, *P<0.05).
F ipGTT in L-Δprkar1a mice 3 days after treatment with Adv-scr or -Kiss1 shRNA. L-Δprkar1a mice with Kiss1 knockdown show improved GT as compared to controls (mean±SEM, *P<0.05).
G Serum insulin during ipGTT in L-Δprkar1a mice 3 days after treatment with Adv-scr or -Kiss1 shRNA. GSIS is augmented in L-Δprkar1a mice after liver Kiss1 knockdown as compared to controls (mean±SEM, *P<0.05).
H Body weight in L-Δprkar1a mice 3 days after treatment with Adv-scr or -Kiss1 shRNA. Body weight in L-Δprkar1a mice is unaffected by Kiss1 knockdown.
I Caloric intake in L-Δprkar1a mice during 3 days after treatment with Adv-scr or -Kiss1 shRNA. Caloric intake in L-Δprkar1a mice is unaffected by Kiss1 knockdown.
Despite ongoing upregulated hepatic gluconeogenesis (Fig. 3B,D), Kiss1 knockdown in L-ΔPrkar1a mice increased in vivo GSIS and improved GT (Fig. 3F,G). ipITT (Fig. 3E), food intake and body weight (Fig. 3H,I), were similar in Adv-Kiss1 and -scr shRNA treated L-ΔPrkar1a mice excluding differences in insulin sensitivity or caloric intake in Adv-Kiss1 versus -scr shRNA treated animals as mechanisms, respectively, for improved GT or for a compensatory increase in insulin secretion in the face of altered insulin resistance.
Kisspeptin impairs GSIS at nanomolar concentrations via Interaction with its Receptor Kiss1R on Pancreatic β-cells
The kisspeptin1 receptor (Kiss1R) shares 82% homology between humans and mouse (Ohtaki et al., 2001). IB confirmed Kiss1R expression in protein extracts of mouse islets, in INS1 and Min6 insulinoma cells as well as in human islets (Fig. 4A). Immunohistochemistry combined with confocal imaging of mouse pancreas localized Kiss1R expression to insulin-producing pancreatic β-cells but not to α-cells (Fig. 4B), indicating that kisspeptin1-Kiss1R signaling likely occurs directly on β-cells.
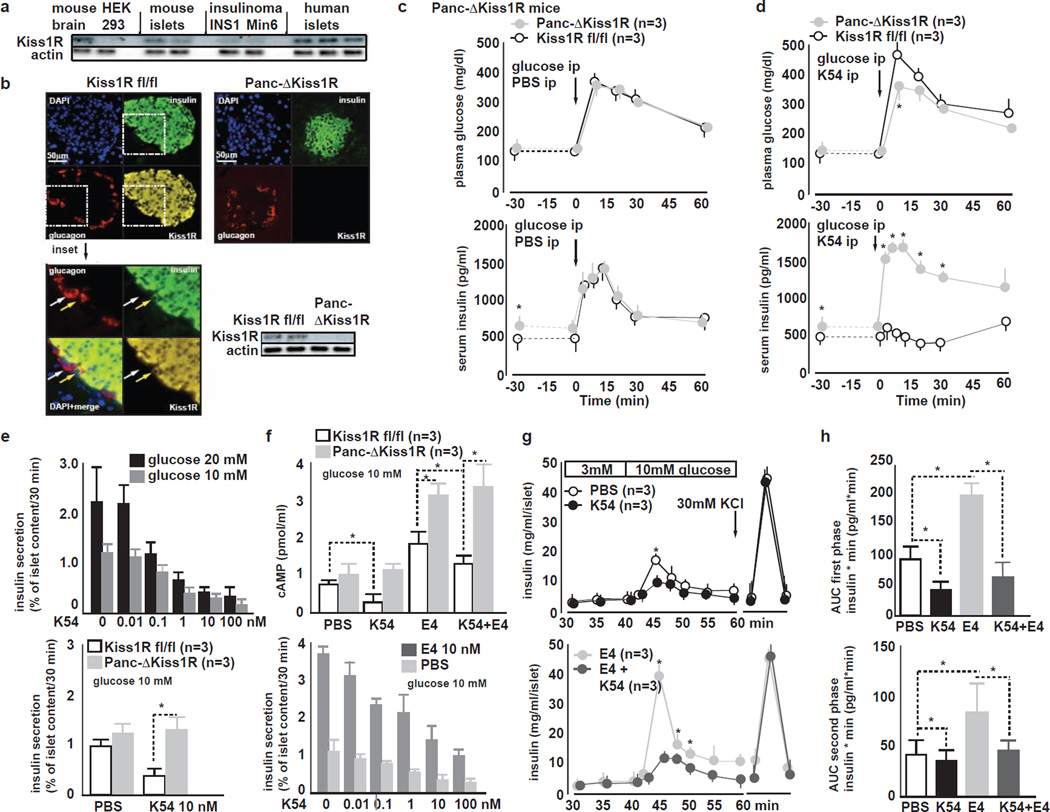
Figure 4. Kisspeptin1 at nanomolar concentrations inhibits GSIS in a Kiss1R-dependent manner. Pancreas Kiss1R is located in β-cells.
A Representative IB for Kiss1R mouse brain, HEK 293T cells, mouse islets, INS1 rat insulinoma cells, Min6 mouse insulinoma cells and human islets. Mouse brain, mouse islets, insulinoma cells and human islets express Kiss1R. HEK 293T cells do not expess Kiss1R.
B (left) Immunohistochemistry for insulin, glucagon and Kiss1R in pancreas from Kiss1Rfl/fl mice. Kiss1R immunoreactivity colocalizes with insulin-positive β-cells but not with glucagon-positive α-cells. 20× magnification. Pseudocoloring: red: glucagon, green: insulin, yellow: Kiss1R, blue: nucleus counter-stain with DAPI (left bottom) inset of previous image at 40× magnification.
(right top) Immunohistochemistry for insulin, glucagon and Kiss1R in pancreas from Panc-ΔKiss1R mice. Kiss1R immunoreactivity is lacking in Panc-ΔKiss1R islet.
(right bottom) Representative islet IB from Kiss1Rfl/fl and Panc-ΔKiss1R mice. Kiss1R is absent in Panc-ΔKiss1R islets.
C ipGTT in Kiss1Rfl/fl and Panc-ΔKiss1R mice during ip co-injection of PBS and glucose. (top) GT is similar in Kiss1Rfl/fl and Panc-ΔKiss1R mice. (bottom) baseline fasting glucose is slightly elevated in Panc-ΔKiss1R mice as compared to Kiss1Rfl/fl littermates. In vivo GSIS is similar in Kiss1Rfl/fl and Panc-ΔKiss1R mice (mean±SEM, *P<0.05).
D ipGTT in Kiss1Rfl/fl and Panc-ΔKiss1R mice during ip co-injection of 10 nM K54 and glucose. K54 impairs GT (top) and GSIS (bottom) in Kiss1Rfl/fl but not in Panc-ΔKiss1R mice (mean±SEM, *P<0.05).
E (top) Dose response curve of K54 and K10 inhibition of GSIS from WT mouse islets during static at 10 or 20 mM glucose. Both K54 and K10 inhibit GSIS in a dose-dependent manner from 0 to 100 nM at both 10 and 20 mM glucose; (bottom) GSIS from Kiss1R fl/fl and Panc-ΔKiss1R islets treated with PBS K10 or K54. K10 or K54 (both 10 nM) inhibit GSIS from Kiss1R fl/fl but not from Panc-ΔKiss1R islets.
F cAMP synthesis and GSIS in response to K54 and to incretin analogue exendin-4 (E4) in Kiss1R fl/fl and Panc-ΔKiss1R islets. (top) K54 impairs cAMP synthesis in Kiss1Rfl/fl but not in Panc-ΔKiss1R islets. E4 stimulates cAMP synthesis similarly in both Kiss1Rfl/fl and in Panc-ΔKiss1R islets. K10 reduces E4-stimulated cAMP levels in Kiss1Rfl/fl but not in Panc-ΔKiss1R islets. (bottom) During static incubation of mouse islets, K54 impairs GSIS and also E4 potentiation of GSIS from islets in a dose dependent manner (mean±SEM, *P<0.05).
G Islet perifusion assay in WT islets in response to K54 and to E4. (top) K54 impairs both first and second phasea of GSIS and (bottom) E4-potentiated first and second phase GSIS; End of perifusion shows similar insulin release upon KCL induced depolarization (mean±SEM, *P<0.05).
H Area under the curve (AUC) of (top) first and (bottom) second phase GSIS from WT mouse islets treated with PBS, K54, E4 or K54+E4. K54 inhibits both first and second phases of GSIS and E4 potentiated GSIS (mean±SEM, *P<0.05).
To specifically investigate the functional role of Kiss1R on β-cells in mediating kisspeptin1 action on GSIS, we generated mice lacking pancreatic Kiss1R by interbreeding PDX1-CRE (Lammert et al., 2001) and floxed Kiss1R (Kiss1rfl/fl) (Novaira et al., 2013) mice to yield Panc-ΔKiss1R mice (Fig. S4A). Analysis of pancreata revealed similar morphometric parameters and insulin content in control and Panc-ΔKiss1R mice (Fig. S4B). Panc-ΔKiss1R mice, as compared to Kiss1rfl/fl controls, exhibited similar plasma glucagon levels (Fig. S4B), slightly elevated fasting serum insulin levels, similar glucose levels, and similar GT during ipGTT (Fig. 4C). In contrast, treatment with K54 (10 nmol ip) suppressed GSIS in Kiss1rfl/fl mice, but did not affect GSIS in Panc-ΔKiss1R mice (Fig. 4D). Together with the confocal microscopic observation, which localized Kiss1R within the endocrine pancreas restricted to β-cells (Fig. 4B), these results indicate that kisspeptin1 suppresses GSIS by direct action via its receptor on β-cells.
Synthetic K54 suppressed GSIS in a dose-dependent manner and at concentrations as low as 0.1 nM from Kiss1Rfl/fl mouse islets that were cultured in serum free media containing either 10 or 20 mM glucose (Fig. 4E top), whereas Panc-ΔKiss1R mice were impervious to K54-mediated GSIS suppression (Fig. 4E bottom) K10 tested at concentrations equimolar to K54 was equally effective in suppressing GSIS in vivo and in vitro (not shown).
Kisspeptin at different concentrations has been reported to either suppress or stimulate GSIS. Kisspeptin isoforms at nM concentrations suppress GSIS (Silvestre et al., 2008; Vikman and Ahren, 2009). In contrast, GSIS stimulation has been reported only at kisspeptin concentrations in the range of 103 nM (= 1 µM) (Bowe et al., 2012; Hauge-Evans et al., 2006), which are unusually high for a hormone.
To examine the effects of such high kisspeptin concentrations on GSIS, we treated islets with 103 nM (= 1 µM) K54 or K10 and found GSIS stimulation from both Kiss1Rfl/fl and Panc-ΔKiss1R islets (Fig. S4C). These findings, using selective genetic Kiss1R ablation suggest that kisspeptin at very high concentrations may stimulate GSIS in a Kiss1R-independent mechanism raising the possibility of off-target effects on GSIS of supraphysiologic kisspeptin concentrations.
Kisspeptin Suppresses Islet cAMP Synthesis and Antagonizes Incretin Hormone Glucagon-like peptide-1 Receptor mediated GSIS Potentiation
GSIS is potentiated by increased cAMP concentrations in β-cells (Drucker, 2006). Kiss1R belongs to Class I/A of G-protein coupled receptors and shares structural similarities with the galanin receptor, activation of which suppresses cAMP synthesis in β-cells (Lee et al., 1999; Tang et al., 2012). We reasoned that kisspeptin1 may also modulate β-cell cAMP levels. WT mouse islets kept at 10 mM glucose and treated with K54 (10 nM) contained lower cAMP concentrations as compared to PBS treated islets (Fig. 4F). K54 also impaired islet cAMP production in response to the long-acting incretin hormone analogue exendin-4 (E4) (Fig. 4F) – a widely used antidiabetic agent that binds and activates on β-cells the receptor for the incretin hormone glucagon-like peptide-1 (GLP-1) and potentiates GSIS by stimulating β-cell cAMP synthesis (Drucker, 2006). Conversely, Panc-ΔKiss1R islets exhibited slightly increased baseline and E4-induced cAMP concentrations, which were not affected by K54 treatment (Fig. 4F). Consistent with an antagonism between K54 and E4, respectively on β-cell function, K54 dose-dependently suppressed E4-potentiated GSIS from cultured mouse islets cultured at 10 mM glucose (Fig. 4F bottom). Perifusion studies of isolated mouse islets revealed that both K54 and K10 suppressed first (0–10 min after glucose stimulation) and second phases of GSIS (Fig. 4G,H) as well as E4-potentiated GSIS (Fig. 4G,H). KCl-induced (30 mM) depolarization of Kiss1Rfl/fl and Panc-ΔKiss1R islets after perifusion stimulated release of equal amounts of insulin, indicating that insulin exocytosis mechanisms distal to the regulatory β-cell ATP-dependent potassium channel (KATP channel) are not impaired in Panc-ΔKiss1R islets (Fig 4G).
These observations indicate that kisspeptin1 reduces β-cell cAMP production and renders β-cells resistant to incretin action on cAMP synthesis and GSIS potentiation.
Mouse Models of Impaired GT and DM exhibit Increased Liver Kisspeptin1 Production
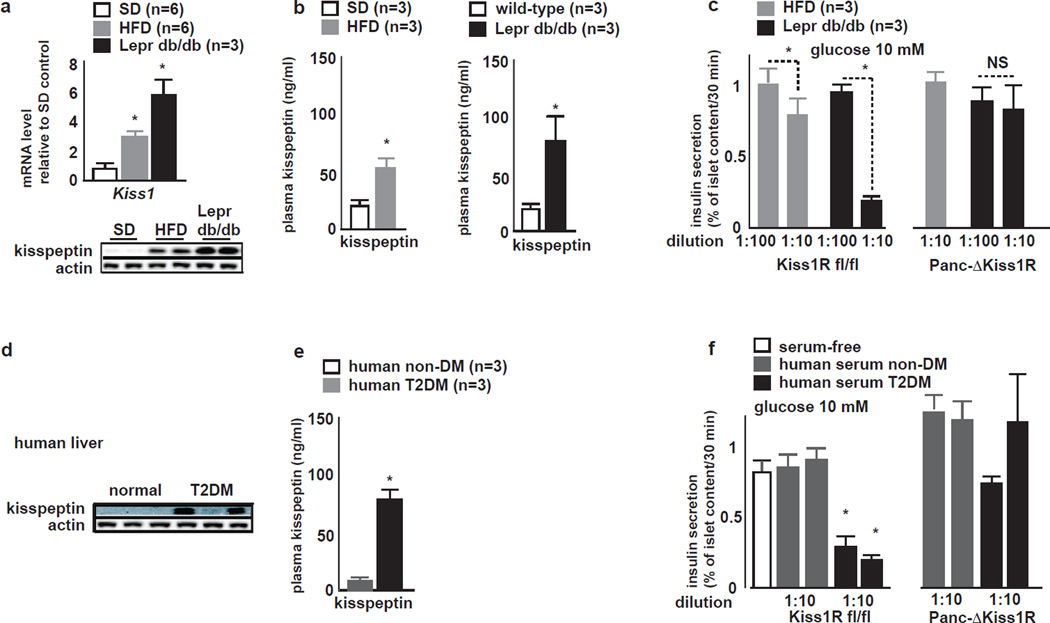
We next examined the relevance of glucagon and kisspeptin1 production in the context of DM. Mice receiving for 8 weeks a HFD developed glucose intolerance and insulin resistance as compared to standard diet (SD) fed controls (Fig. S5A,B) and importantly, exhibited increased plasma glucagon levels in the fed state (Fig. S5C) and an increase in liver Kiss1 expression (Fig. 5A)
Figure 5. Liver Kisspeptin1 expression and plasma kisspeptin levels are elevated in mouse models of DM and in humans with T2DM.
A (top) qRT-PCR for Kiss1 in liver tissue; and (bottom) liver IB for kisspeptin1 in SD, HFD fed and Leprdb/db mice. Both Kiss1 mRNA and kisspepetin1 protein are increased in HFD fed mice and found at higher levels in db/db mouse livers (mean±SEM, *P<0.05).
B Plasma kisspeptin1 in SD, HFD fed and Leprdb/db mice. Plasma kisspeptin1 is increased in (left) HFD fed and (right) in Leprdb/db mice as compared to SD fed littermates (mean±SEM, *P<0.05).
C GSIS from cultured (left) Kiss1Rfl/fl and (right) Panc-ΔKiss1R mouse islets in media conditioned with plasma from HFD fed and Leprdb/db mice. GSIS from Kiss1Rfl/fl is suppressed during culture in media conditioned with HFD fed or db/db plasma at 1:10 dilution but not at 1:100 dilution. GSIS from Panc-ΔKiss1R islets is unaffected by media conditioned with plasma of HFD fed or Leprdb/db mice (mean±SEM, *P<0.05).
D Representative liver IB for kisppeptin1 in humans without DM and humans with T2DM. Humans with T2DM exhibit varying degrees of kisspeptin immunoreactivity in liver tissue.
E Plasma kisspeptin1 in humans without DM and with T2DM. Plasma kisspeptin1 levels are elevated in humans with T2DM as compared to humans without diabetes (mean±SEM, *P<0.05).
F GSIS from cultured (left) Kiss1Rfl/fl and (right) Panc-ΔKiss1R mouse islets in media conditioned with plasma from humans with T2DM and without DM. GSIS from Kiss1Rfl/fl but not from Panc-ΔKiss1R islets is suppressed during culture in media conditioned with T2DM plasma (mean±SEM, *P<0.05).
In the hypothalamus, Kiss1 expression is modulated by leptin signaling (Smith et al., 2006). Furthermore, leptin inhibits GSIS via its receptor on β-cells (Kieffer et al., 1997). Therefore, to elucidate the relevance of hyperglucagonemia on liver Kiss1 and GSIS independent of leptin effects, we also examined mice homozygous for the inactivating leptin receptor db mutation (Leprdb/db mice).
Leprdb/db as compared to WT mice exhibited hyperglucagonemia in the fed state (Fig. S5D) and significantly increased liver Kiss1 expression (Fig. 5A). Both plasma glucagon and liver Kiss1 mRNA levels were greater in magnitude in Leprdb/db as compared to those found in HFD mice. Accordingly, kisspeptin1 immunoreactivity was detectable at low levels in liver of SD mice and was increased both in HFD and in db/db liver tissue (Fig. 5A). Both HFD fed mice and Leprdb/db liver tissue showed higher CREB phosphorylation (Fig S5F) and in vivo CREB occupancy on both CRE half-sites within the Kiss1 promoter (Fig. S5G).
Plasma from HFD fed and diabetic Leprdb/db mice - as compared to SD fed WT mice - exhibited higher kisspeptin1 concentrations as determined by ELISA (Fig. 5B). Kiss1Rfl/fl islets cultured in serum-free media conditioned with plasma from HFD or from Leprdb/db mice exhibited impaired GSIS. In contrast, Panc-ΔKiss1R islets resisted GSIS inhibition by plasma of HFD or Leprdb/db mice. GSIS suppression from WT islets cultured in serum-free media conditioned with HFD or Leprdb/db plasma (Fig. 5C) - when compared with GSIS suppression by synthetic K54 (Fig. 4H) - indicated that the functional plasma kisspeptin concentrations in HFD and Leprdb/db mice to be equivalent to 0.5–1 nM and 7–10 nM of K10, respectively.
We further examined the relevance of liver kisspeptin in the context of human T2DM. Kisspeptin1 immunoreactivity was detectable at variable intensity by immunoblot of liver samples from humans with T2DM but not from non-diabetic humans (Fig. 5D). Accordingly, circulating kisspeptin1 immunoreactivity was increased in serum from humans with T2DM, as compared to non-diabetic individuals (Fig. 5E). Media conditioned with serum from T2DM but not from non-diabetic individuals, suppressed GSIS from Kiss1Rfl/fl but not from Panc-ΔKiss1R islets (Fig 5F). These observations indicate that - akin to rodent models of DM - in human T2DM, liver kisspeptin1 expression is increased and that circulating kisspeptin suppresses GSIS.
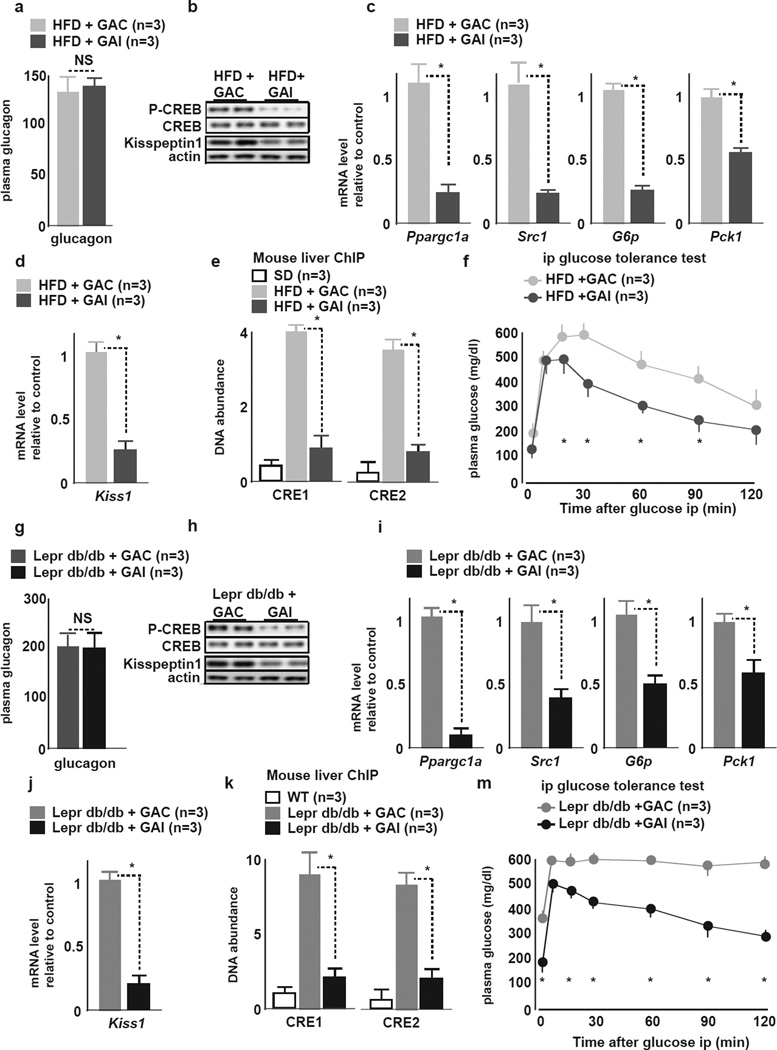
Liver Kiss1 Upregulation is Linked to Hyperglucagonemia in DM
We determined whether hyperglucagonemia is linked to liver kisspeptin production in HFD and Leprdb/db mice by administering a single dose of the selective Gcgr antagonist (GAI) or an inactive analog (GAC) (Qureshi et al., 2004) 60 minutes before an ipGTT. GAI - as compared to GAC - treated (Fig. 6F,M) mice exhibited improved basal glycemia as well as GT. GAI treatment led to a reduction in liver gluconeogenic gene (Fig 6C,I) and in Kiss1 expression (Fig 6D,J) and to corresponding reductions in liver pCREB (Fig 6B,H), and CREB occupancy of CRE 1 and 2 in the Kiss1 promoter (Fig. 6E,K). Circulating plasma glucagon levels remained unchanged after GAI or GAC treatment in both HFD fed (Fig. 6A) and Leprdb/db mice (Fig. 6G), respectively.
Figure 6. Hyperlucagonemia is linked to liver kisspeptin1 production in HFD fed and Leprdb/db mice.
HFD fed mice: Panels A–F
Leprdb/db mice: Panels G–M
A, G Plasma glucagon levels in the fed state 60 min after treatment with GAI or GAC. Plasma glucagon levels remain unchanged after GAI or GAC treament.
B, H Representative liver IB for pCREB, total CREB and kisspeptin1 in GAI and GAC treated mice. Phospho-CREB is reduced in mice treated with GAI but not GAC.
C, I qRT-PCR of indicated genes of the gluconeogenic program in livers of GAI or GAC treated mice. GAI but not GAC treatment downregulates Pparg1a, Src1, G6P and Pck1 mRNA (mean±SEM, *P<0.05).
D, J qRT-PCR of Kiss1 in livers of GAI and GAC treated mice. GAI but not GAC treatment downregulates liver Kiss1 mRNA (mean±SEM, *P<0.05).
E, K In vivo ChIP of CREB occupancy on (left) CRE1 and (right) CRE2 half-sites of the Kiss1 promoter in livers of GAI or GAC treated SD mice, HFD and Leprdb/db mice. GAI reduces CREB occupancy on Kiss1 CRE 1 & 2 to levels similar to those in control mice (mean±SEM, *P<0.05).
F, L ipGTT in GAI or GAC treated mice. GAI treatment improves GT as compared to GAC treatment (mean±SEM, *P<0.05).
Thus, in the context of glucose intolerance and DM, hyperglucagonemia significantly contributes to hepatic kisspeptin1 production. Further, as demonstrated in Leprdb/db mice, hepatic Kiss1 regulation occurs independently of leptin.
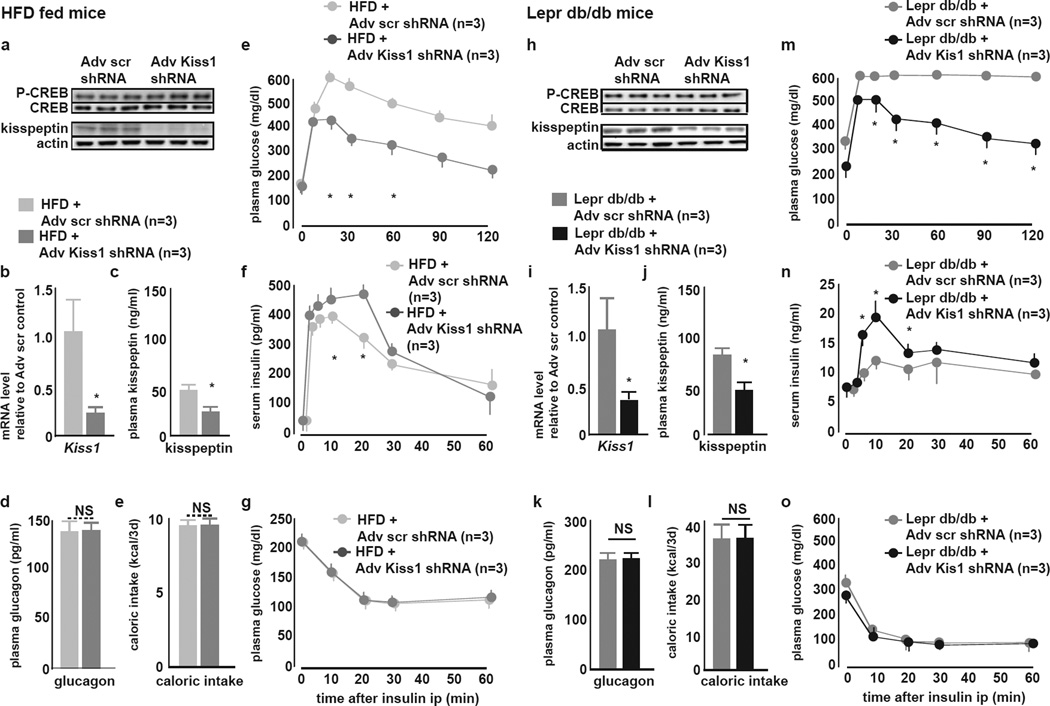
Liver Kisspeptin1 Knockdown in Diabetic Mice Ameliorates GSIS and GT
We next examined the contribution in DM of hepatic kisspeptin1 towards impaired GSIS by liver selective shRNA-mediated Kiss1 knockdown. Adv-Kiss1 but not -scr shRNA treatment in HFD or Leprdb/db mice (5–6 weeks of age), respectively, reduced within 3 days liver kisseptin1 production (Fig. 7A,H) and Kiss1 mRNA (Fig. 7B,I) as well as plasma kisspeptin1 (Fig. 7C,J). Plasma glucagon (Fig. 7D,K), liver pCREB (Fig. 7A,H), liver CREB occupancy on Kiss1 promoter CRE sites (Fig. S6A,C) remained similar in Adv-Kiss1 and -scr shRNA treated littermates. Liver kisspeptin1 knockdown in both HFD and Leprdb/db mice resulted in improved in vivo GT (Fig. 7E,M) and increased GSIS (Fig 7F,N). Caloric intake (Fig 7E,K), body weight (Fig. S6B,D) and insulin tolerance (Fig 7G,O), were not different in Adv-Kiss1 and -scr shRNA treated counterparts, ruling out changes in insulin sensitivity after Kiss1 knockdown as a mechanism for improvements in GSIS and GT.
Figure 7. Kiss1 shRNA knockdown in vivo in livers of in HFD and Leprdb/db mice de-represses GSIS and glucose tolerance.
HFD mice: Panels A–H
Leprdb/db mice: Panels I–P
A, I Representative liver IB of pCREB, total CREB and kisspeptin1 3 days after treatment with Adv-scr or -Kiss1 shRNA. Liver pCREB is not affected and liver kisspeptin1 protein is reduced by Kiss1 knockdown.
B, J qRT-PCR of Kiss1 in livers 3 days after treatment with Adv-scr or -Kiss1 shRNA. Adv-Kiss1 shRNA downregulates liver Kiss1 mRNA levels as compared to Adv-scr shRNA treatment (mean±SEM, *P<0.05).
C, K Plasma kisspeptin1 levels 3 days after treatment with Adv-scr or -Kiss1 shRNA. Liver Kiss1 knockdown reduces plasma kisspeptin1 (mean±SEM, *P<0.05).
D, L Plasma glucagon levels 3 days after treatment with Adv-scr or -Kiss1 shRNA. Liver Kiss1 knockdown does not change plasma glucagon levels (mean±SEM0.
E, M Caloric intake in during 3 days after treatment with Adv-scr or -Kiss1 shRNA. Caloric intake is unaffected by Kiss1 knockdown (mean±SEM).
F, N ipGTT 3 days after treatment with Adv-scr or -Kiss1 shRNA. GT is improved after Kiss1 knockdown (mean±SEM, *P<0.05).
G, O GSIS during ipGTT 3 days after treatment with Adv-scr or -Kiss1 shRNA. GSIS is improved after Kiss1 knockdown (mean±SEM, *P<0.05).
H, P ipITT 3 days after treatment with Adv-scr or -Kiss1 shRNA. Insulin tolerance is not different after Kiss1 knockdown.
These observations indicate that in DM, liver kisspeptin1 negatively impacts GSIS, which can be de-repressed by inhibiting hepatic kisspeptin1 production.
Selective Pancreas Kiss1R Ablation Ameliorates Insulin Secretion and GT in HFD Mice
To directly assess the relevance of kisspeptin-Kiss1R signaling on GSIS in the context of DM, we examined the effects of conditional pancreas Kiss1R ablation in HFD fed mice. Kiss1Rfl/fl and Panc-ΔKiss1R mice, placed on a HFD for 8 weeks, exhibited similar weight gain (Fig. S7). Liver kisspeptin1 immunoreactivity, plasma kisspeptin and glucagon levels were similar in HFD mice independent of pancreas Kiss1R status (Fig. S7). Importantly, Panc-ΔKiss1R showed, as compared to Kiss1Rfl/fl littermates, slightly lower fasting blood glucose and improved GT (Fig. S7). HFD fed Panc-ΔKiss1R showed relative to controls, both increased fasting insulin and in vivo GSIS. No differences were found in insulin tolerance, islet, β-cell or α-cell mass, or pancreas insulin content between Panc-ΔKiss1R and Kiss1Rfl/fl littermates (Fig. S7), excluding changes in peripheral insulin action or β-cell mass to account for improved GSIS and GT in HFD fed Panc-ΔKiss1R mice.
Discussion
The present findings using genetically defined mouse models suggest a tri-hormonal regulatory circuit between pancreatic α-cells, hepatocytes and β-cells, and assign kisspeptin1 an unexpected role in liver to islet endocrine signaling. In addition, the findings indicate in T2DM a sequential link between hyperglucagonemia and impaired β-cell function via liver-derived kisspeptin1.
Hyperglucagonemia, which occurs early during development of T2DM upregulates kisspeptin1 production by the liver (Fig. 2). Kisspeptin1 in turn functions as a hormone to suppress GSIS (Fig 4). Thus, in T2DM the β-cell is exposed to two counteracting stimuli elicited by glucagon action on the liver. Glucagon-induced HGP and hyperglycemia stimulate, whereas kisspeptin1 production inhibits GSIS.
The physiologic relevance of opposing actions of hepatic cAMP-CREB signaling on β-cell function remains to be fully explored. Teleological considerations render plausible a survival mechanism in that hepatic cAMP-CREB-induced kisspeptin1 serves as a (among other mechanisms) safeguard against insulin secretion and hypoglycemia during fight and flight reactions, should these occur during and interrupt prandial nutrient absorption – when insulin secretion otherwise would be elevated. To this end, it is likely that epinephrine, another fight and flight mediator, that activates liver cAMP signaling (Sherline et al., 1972), may also participate in liver Kiss1 regulation.
It is important to note that our studies using genetically defined Kiss1R deficient islets reveal that kisspeptin applied at supraphysiologic doses in the micromolar range stimulates GSIS, likely due to effects which are not mediated by the bona-fide kisspeptin1 receptor Kiss1R (Fig. S4C). This observation of Kiss1R-independent effects at high doses of kisspeptin, which are unusually high for a hormone, may in part explain the contradictory observations on GSIS between physiologic and supraphysiologic kisspeptin1 concentrations (Bowe et al., 2012; Hauge-Evans et al., 2006; Silvestre et al., 2008; Vikman and Ahren, 2009). Our studies suggest that Kiss1R signaling in β-cells suppresses cAMP and inhibits GSIS. An intravenous bolus K10 in the non-human primate Macaca mulatta is reported to stimulate GSIS, albeit circulating kisspeptin concentrations were not measured in that study (Wahab et al., 2011). Based on the studies herein, humans with T2DM exhibit increased liver kisspeptin1 immunoreactivity, increased circulating kisspeptin and their plasma suppresses GSIS from mouse islets in a Kiss1R-dependent manner (Fig 3, 4). Future studies on the interplay between glucagon and kisspeptin1 in humans will need to carefully examine the dose response of kisspeptin1 on GSIS combined with reliable measurements of circulating functional kisspeptin1 isoforms.
Our observations further suggest that in T2DM GSIS is insufficient to overcome the co-existing inhibition on β-cells exerted by kisspeptin1. This mechanism results in inadequate insulin secretion to meet metabolic demands and aggravates β-cell dysfunction and hyperglycemia. Clinical observations indicate that in T2DM, although incretin GLP-1 is normally secreted, endogenous GLP-1 is insufficient to achieve physiologic GSIS potentiation. Furthermore, treatment with dipeptidyl-peptidase IV inhibitors, which inhibit GLP-1 degradation and increase endogenous GLP-1 concentrations by two-fold, or treatment with long-acting GLP-1 analog E4 restore β-cell function in T2DM. These observations have led to the concept of reduced GLP-1 sensitivity of β-cells in T2DM (Meier and Nauck, 2008). Antagonism by K54 on E4 induced GSIS potentiation (Fig. 4E,F) suggests that increased circulating kisspeptin1 levels in T2DM may at least in part contribute to the diminished response to endogenous GLP-1 in type 2 diabetic subjects.
Thus, our findings uncover the liver as a site of regulated kisspeptin production and provide mechanistic and causal underpinnings for common observations made in clinical T2DM: a) relative hyperglucagonemia, b) insufficient insulin secretion to regulate glycemia, and c) diminished response to endogenously secreted GLP-1 and restoration of β-cell function by pharmacologic GLP-1 receptor agonism.
In a broader context, neutralizing circulating kisspeptin1 or antagonism of Kiss1R on β-cells are appealing avenues to augment GSIS and improve glucose homeostasis in T2DM. In this regard, Kiss1R antagonists, which would not cross the blood brain barrier and interfere with hypothalamic reproductive functions of kisspeptin1 would be particularly advantageous. Another important aspect is that plasma kisspeptin1 activity may serve as a biomarker to identify T2DM patients who would benefit most from aggressive β-cell-targeted therapy.
Experimental Procedures
Animal Studies
Animal studies were approved by the local Institutional Animal use and Care Committee, and were performed in 6–8 week old C57Bl/6 male mice. Leprdb/db (Leprdb/db; B6.BKS(D)-leprdb/J) mice were from Jackson Laboratories. Gcgrfl/fl were generated by homologous recombination technology (Supplemental information). Intravenous glucose infusions were performed as described (Alonso et al., 2007). Genotyping PCR primers and dynamic physiologic testing details are provided in Supplemental information.
Adenovirus Injection Studies
Adenovirus (Adv-CRE and Adv-GFP, University of Iowa) were injected into tail vein (109 plaque forming units/mouse in 1×PBS). Adenovirus expressing shRNA under U6 promoter was generated (Life Technologies) to target mouse Kiss1 sequence: GCTCTCTCTCTTTGACCTAGG.
Immunofluorescence Histology and Islet Morphometry
Pancreas immunofluorescence histology and morphometry and pancreas insulin content measurment were conducted as described (Song et al., 2011). Confocal imaging was performed on a Zeiss Axoivert. Antibodies are provided in Supplemental information.
Isolated Islet Studies
Islet isolation was performed by collagenase digestion, gradient centrifugation and three rounds of microscope-assisted manual picking of islets. Static incubation studies were conducted as previously described with 20 hand-picked equal-sized islets were studied in each group (Song et al., 2011).. After overnight culture (37 C, 5% CO2, 95% O2 in humid chamber) of isolated islets in RPMI 1640 (Mediatech) containing 5 mM glucose, 1% each Na-Pyruvate, HEPES, Penicillin/Streptomycin and 0.2% bovine serum albumin (BSA), islets were switched to either 10 or 20 mM glucose containing RPMI 1640. Where indicated, Kisspeptin-10 (0–100 nM, and 1 µM), E4 (10 nM) or vehicle (PBS) was added. After 30 min incubation glucose concentrations, supernatant was taken for insulin measurements and pelleted islets were taken in acid ethanol (0.18M HCl in 70% ethanol) for insulin measurements in islets (ELISA, Alpco). Islet protein concentration was measured using the BCA method (Thermo Fisher). Dose response curves of GSIS inhibition by Kisspeptin-54 or -10 (0–100 nM) to serve as a functional bioassay for plasma kisspeptin1 activity performed at 6 separate times provided intra-assay and inter-assay coefficient of variations of 7.3% and 9.2%, respectively.
Immunoblots
IB were performed as described (Song et al., 2011) in at least three different separately obtained experimental samples. Luminescence images of representative IBs are shown. Corresponding Actin IB show protein loading control.
Human samples
The Institutional Review Board at Johns Hopkins University approved studies of de-identified human samples. Human tissue and serum samples were obtained from National Disease Research Interchange (NDRI) and Origene. Information on samples is provided in Supplemental information.
Immunoassays
Kisspeptin1 ELISA for mouse (USCN Life Sciences) and human (Phoenix) were used according to manufacturers’ instructions. The mouse ELISA kit failed to recognize kisspeptin in human samples and vice versa.
Statistics
Results are presented as average ± standard error of the mean (SEM). Student’s T-test was used for single comparisons and ANOVA with Bonferroni adjustment for multiple to calculate differences between groups. A P value of <0.05 was considered significant and indicated with *.
Supplementary Material
Glucagon stimulates both hepatic kisspeptin1 production and gluconeogenesis.
Kisspeptin1 suppresses glucose stimulated insulin secretion (GSIS) from β-cells.
Hyperglucagonemia in diabetes impairs insulin secretion via hepatic kisspeptin1.
In diabetic mice, liver Kiss1 knockdown improves GSIS and glycemia
Aknowledgement
Supported through grants from the National Institutes of Health and from the Swami International Institute for Medical Education. We thank J. Brüning, C. Stratakis, L. Kirschner for sharing mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession codes. Microarray data are deposited in Gene Expression Omnibus (Barrett et al., 2005) and accessible through number GSE48815.
References
- Akinci A, Cetin D, Ilhan N. Plasma kisspeptin levels in girls with premature thelarche. J Clin Res Pediatr Endocrinol. 2012;4:61–65. doi: 10.4274/jcrpe.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O'Donnell CP, Garcia-Ocana A. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes. 2007;56:1792–1801. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T, Suzek TO, Troup DB, Wilhite SE, Ngau WC, Ledoux P, Rudnev D, Lash AE, Fujibuchi W, Edgar R. NCBI GEO: mining millions of expression profiles--database and tools. Nucleic Acids Res. 2005;33:D562–D566. doi: 10.1093/nar/gki022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe JE, Foot VL, Amiel SA, Huang GC, Lamb M, Lakey J, Jones PM, Persaud SJ. GPR54 peptide agonists stimulate insulin secretion from murine, porcine and human islets. Islets. 2012;4 doi: 10.4161/isl.18261. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- Cetkovic A, Miljic D, Ljubic A, Patterson M, Ghatei M, Stamenkovic J, Nikolic-Djurovic M, Pekic S, Doknic M, Glisic A, et al. Plasma kisspeptin levels in pregnancies with diabetes and hypertensive disease as a potential marker of placental dysfunction and adverse perinatal outcome. Endocr Res. 2012;37:78–88. doi: 10.3109/07435800.2011.639319. [DOI] [PubMed] [Google Scholar]
- Chen M, Gavrilova O, Zhao WQ, Nguyen A, Lorenzo J, Shen L, Nackers L, Pack S, Jou W, Weinstein LS. Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gs alpha deficiency. J Clin Invest. 2005;115:3217–3227. doi: 10.1172/JCI24196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes Metab. 2011;13(Suppl 1):126–132. doi: 10.1111/j.1463-1326.2011.01449.x. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- El Ouaamari A, Kawamori D, Dirice E, Liew CW, Shadrach JL, Hu J, Katsuta H, Hollister-Lock J, Qian WJ, Wagers AJ, et al. Liver-derived systemic factors drive beta cell hyperplasia in insulin-resistant states. Cell Rep. 2013;3:401–410. doi: 10.1016/j.celrep.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Hauge-Evans AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A role for kisspeptin in islet function. Diabetologia. 2006;49:2131–2135. doi: 10.1007/s00125-006-0343-z. [DOI] [PubMed] [Google Scholar]
- He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, Wondisford FE. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, Fujino M. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88:914–919. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- Jamison RA, Stark R, Dong J, Yonemitsu S, Zhang D, Shulman GI, Kibbey RG. Hyperglucagonemia precedes a decline in insulin secretion and causes hyperglycemia in chronically glucose-infused rats. Am J Physiol Endocrinol Metab. 2011;301:E1174–E1183. doi: 10.1152/ajpendo.00175.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TJ, Heller RS, Leech CA, Holz GG, Habener JF. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes. 1997;46:1087–1093. doi: 10.2337/diab.46.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner LS, Kusewitt DF, Matyakhina L, Towns WH, 2nd, Carney JA, Westphal H, Stratakis CA. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65:4506–4514. doi: 10.1158/0008-5472.CAN-05-0580. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Lee DK, Nguyen T, O'Neill GP, Cheng R, Liu Y, Howard AD, Coulombe N, Tan CP, Tang-Nguyen AT, George SR, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446:103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Liu Z, Ren C, Jones W, Chen P, Seminara SB, Chan YM, Smith NF, Covey JM, Wang J, Chan KK. LC-MS/MS quantification of a neuropeptide fragment kisspeptin-10 (NSC 741805) and characterization of its decomposition product and pharmacokinetics in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;926:1–8. doi: 10.1016/j.jchromb.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logie JJ, Denison FC, Riley SC, Ramaesh T, Forbes S, Norman JE, Reynolds RM. Evaluation of kisspeptin levels in obese pregnancy as a biomarker for pre-eclampsia. Clin Endocrinol (Oxf) 2012;76:887–893. doi: 10.1111/j.1365-2265.2011.04317.x. [DOI] [PubMed] [Google Scholar]
- Longuet C, Robledo AM, Dean ED, Dai C, Ali S, McGuinness I, de Chavez V, Vuguin PM, Charron MJ, Powers AC, et al. Liver-specific disruption of the murine glucagon receptor produces alpha-cell hyperplasia: evidence for a circulating alpha-cell growth factor. Diabetes. 2013;62:1196–1205. doi: 10.2337/db11-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louet JF, Chopra AR, Sagen JV, An J, York B, Tannour-Louet M, Saha PK, Stevens RD, Wenner BR, Ilkayeva OR, et al. The coactivator SRC-1 is an essential coordinator of hepatic glucose production. Cell Metab. 2010;12:606–618. doi: 10.1016/j.cmet.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ, Nauck MA. Is secretion of glucagon-like peptide-1 reduced in type 2 diabetes mellitus? Nat Clin Pract Endocrinol Metab. 2008;4:606–607. doi: 10.1038/ncpendmet0946. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Willis BS, Wallen A, Sweet IR, Jetton TL, Thompson BR, Wu C, Lange AJ, McKnight GS. Cre recombinase-dependent expression of a constitutively active mutant allele of the catalytic subunit of protein kinase A. Genesis. 2005;43:109–119. doi: 10.1002/gene.20159. [DOI] [PubMed] [Google Scholar]
- Novaira HJ, Sonko ML, Hoffman G, Koo Y, Ko C, Wolfe A, Radovick S. Disrupted kisspeptin signaling in GnRH neurons leads to reproductive abnormlities an HH. Mol Endocrinol. 2013 doi: 10.1210/me.2013-1319. early release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Qureshi SA, Rios Candelore M, Xie D, Yang X, Tota LM, Ding VD, Li Z, Bansal A, Miller C, Cohen SM, et al. A novel glucagon receptor antagonist inhibits glucagon-mediated biological effects. Diabetes. 2004;53:3267–3273. doi: 10.2337/diabetes.53.12.3267. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Kaiser UB. New gatekeepers of reproduction: GPR54 and its cognate ligand, KiSS-1. Endocrinology. 2005;146:1686–1688. doi: 10.1210/en.2005-0070. [DOI] [PubMed] [Google Scholar]
- Sherline P, Lynch A, Glinsmann WH. Cyclic AMP and adrenergic receptor control of rat liver glycogen metabolism. Endocrinology. 1972;91:680–690. doi: 10.1210/endo-91-3-680. [DOI] [PubMed] [Google Scholar]
- Silvestre RA, Egido EM, Hernandez R, Marco J. Kisspeptin-13 inhibits insulin secretion without affecting glucagon or somatostatin release: study in the perfused rat pancreas. J Endocrinol. 2008;196:283–290. doi: 10.1677/JOE-07-0454. [DOI] [PubMed] [Google Scholar]
- Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- Song WJ, Seshadri M, Ashraf U, Mdluli T, Mondal P, Keil M, Azevedo M, Kirschner LS, Stratakis CA, Hussain MA. Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab. 2011;13:308–319. doi: 10.1016/j.cmet.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Wang Y, Park S, Bajpayee NS, Vi D, Nagaoka Y, Birnbaumer L, Jiang M. Go2 G protein mediates galanin inhibitory effects on insulin release from pancreatic beta cells. Proc Natl Acad Sci U S A. 2012;109:2636–2641. doi: 10.1073/pnas.1200100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikman J, Ahren B. Inhibitory effect of kisspeptins on insulin secretion from isolated mouse islets. Diabetes Obes Metab. 2009;11(Suppl 4):197–201. doi: 10.1111/j.1463-1326.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Wahab F, Riaz T, Shahab M. Study on the effect of peripheral kisspeptin administration on basal and glucose-induced insulin secretion under fed and fasting conditions in the adult male rhesus monkey (Macaca mulatta) Horm Metab Res. 2011;43:37–42. doi: 10.1055/s-0030-1268458. [DOI] [PubMed] [Google Scholar]
- Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, Hu Y, Zhou Z, Yan X, Tian H, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- Yi P, Park JS, Melton DA. Betatrophin: A Hormone that Controls Pancreatic beta Cell Proliferation. Cell. 2013;153:747–758. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci U S A. 2005;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.