Abstract
Background
Gout is the most common inflammatory arthritis in men over 40 years and has an increasing prevalence among postmenopausal women. Lowering serum uric acid levels remains one of the primary goals in the treatment of chronic gout. In clinical trials, febuxostat has been shown to be effective in lowering serum uric acid levels to < 6.0 mg/dL.
Objectives
To evaluate the benefits and harms of febuxostat for chronic gout.
Search methods
We searched The Cochrane Library, MEDLINE, EMBASE, and International Pharmaceutical Abstracts from inception to July 2011. The ClinicalTrials.gov website was searched for references to trials of febuxostat. Our search did not include any restrictions.
Selection criteria
Two authors independently reviewed the search results and disagreements were resolved by discussion. We included any controlled clinical trial or open label trial (OLT) using febuxostat at any dose.
Data collection and analysis
Data and risk of bias were independently extracted by two authors and summarised in a meta‐analysis. Continuous data were expressed as mean difference and dichotomous data as risk ratio (RR).
Main results
Four randomised trials and two OLTs with 3978 patients were included. Risk of bias differed by outcome, ranging from low to high risk of bias. Included studies failed to report on five to six of the nine outcome measures recommended by OMERACT. Patients taking febuxostat 120 mg and 240 mg reported more frequent gout flares than in the placebo group at 4 to 28 weeks (RR 1.7; 95% CI 1.3 to 2.3, and RR 2.6; 95% CI 1.8 to 3.7 respectively). No statistically significant differences were observed at 40 mg and 80 mg. Compared to placebo, patients on febuxostat 40 mg were 40.1 times more likely to achieve serum uric acid levels < 6.0 mg/dL at 4 weeks (95% CI 2.5 to 639), with an absolute treatment benefit of 56% (95% CI 37% to 71%). For febuxostat 80 mg and 120 mg, patients were 68.9 and 80.7 times more likely to achieve serum uric acid levels < 6.0 mg/dL at their final visit compared to placebo (95% CI 13.8 to 343.9, 95% CI 16.0 to 405.5), respectively; with an absolute treatment benefit of 75% and 87% (95% CI 68 to 80% and 81 to 91%), respectively. Total discontinuation rates were significantly higher in the febuxostat 80 mg group compared to placebo (RR 1.4; 95% CI 1.0 to 2.0, absolute risk increase 11%; 95% CI 3 to 19%). No other differences were observed.
When comparing allopurinol to febuxostat at 24 to 52 weeks, the number of gout flares was not significantly different between the two groups, except for febuxostat 240 mg (RR 2.3; 95% CI 1.7 to 3.0). Patients on febuxostat 40 mg showed no statistically significant differences in benefits or harms. Patients on febuxostat 80 mg and 120 mg were 1.8 and 2.2 times more likely to achieve serum uric acid levels < 6.0 mg/dL at their final visit (95% CI 1.6 to 2.2, 95% CI 1.9 to 2.5) with an absolute treatment benefit of 29% and 44% (95% CI 25% to 33%, 95% CI 38% to 50%), respectively, at 24 to 52 weeks. Total discontinuation rates were higher for febuxostat 80 mg and 120 mg compared to allopurinol (RR 1.5; 95% CI 1.2 to 1.8, absolute risk increase 11%; 95% CI 6% to 16%; and RR 2.6; 95% CI 2.0 to 3.3, absolute risk increase 20%; 95% CI 3% to 14%, respectively). Discontinuations due to adverse events were similar across groups. Total adverse events were lower for febuxostat 80 mg and 120 mg compared with allopurinol (RR 0.93; 95% CI 0.87 to 0.99, absolute risk increase 6%; 95% CI 0.7% to 11%; and RR 0.90; 95% CI 0.84 to 0.96, absolute risk increase 8%; 95% CI 3% to 13%, respectively). No other relevant differences were noted.
After 3 years of follow‐up there were no statistically significant differences regarding effectiveness and harms between febuxostat 80 mg or 120 mg and allopurinol groups (adverse event rate per 100 patient‐years 227, 216, and 246, respectively).
Authors' conclusions
Although the incidence of gout flares requiring treatment may be increased in patients taking febuxostat compared to placebo or allopurinol during early treatment, no such increase in gout flares was observed in the long‐term follow‐up study when compared to allopurinol. Febuxostat at any dose was shown to be beneficial in achieving serum uric acid levels < 6.0 mg/dL and reducing serum uric acid levels in the period from baseline to final visit when compared to placebo and to allopurinol. However, the grade of evidence ranged from low to high, which indicates that further research is needed.
Keywords: Female, Humans, Male, Allopurinol, Allopurinol/adverse effects, Allopurinol/therapeutic use, Chronic Disease, Febuxostat, Gout, Gout/blood, Gout/drug therapy, Gout Suppressants, Gout Suppressants/adverse effects, Gout Suppressants/therapeutic use, Hyperuricemia, Hyperuricemia/drug therapy, Randomized Controlled Trials as Topic, Thiazoles, Thiazoles/adverse effects, Thiazoles/therapeutic use
Plain language summary
Febuxostat for treating chronic gout
This summary of a Cochrane review represents what we know from research about the effect of febuxostat for treating chronic gout.
From six studies including 3978 people with chronic gout, the review shows that,
In people with chronic gout:
‐ febuxostat probably reduces uric acid levels;
‐ febuxostat probably increases the incidence of gout flares during early treatment (while getting the uric acid levels down). The normalization of serum uric acid leads to mobilization of urate from tissue deposits, which in turn may increase the number of attacks;
‐ febuxostat probably shows similar benefits as allopurinol after three years of use.
We do not have information about febuxostat effects on joint imaging, musculoskeletal function, pain, overall assessment, and quality of life. Also, we do not have precise information about side effects and complications. This is particularly true for rare but serious side effects. Possible side effects may include liver enzyme elevations, high blood pressure and diarrhoea. Rare complications may include certain cardiovascular events (chest pain, coronary artery disease, myocardial infarction, or atrial fibrillation).
What is chronic gout and what is febuxostat?
Uric acid is a product normally present in the blood as a result of the breakdown of certain products called 'purines'. Gout is a disease caused by high uric acid levels in the blood leading to crystal formation in the joints, most commonly joints of the lower limbs such as the big toe, heels, ankles and knees. Gout usually presents as acute attacks causing joint swelling and pain, but also can lead to chronic arthritis. While there is no cure for the disease, treatment can prevent recurrent gout attacks and improve its chronic form.
Research shows that keeping uric acid levels below 6.0 mg/dL can reduce gout attacks over time. However, in the first months of therapy, there could be an increased number of gout attacks, due to the nature of the treatment.
Febuxostat is a new drug that can help lower uric acid levels in the blood in adults with gout.
Best estimate of what happens to people with chronic gout who take febuxostat:
Febuxostat was proven effective in lowering uric acid to less than 6.0 mg/dL.
In short‐term studies (one year or less), when febuxostat was compared with placebo (a sham or fake medication):
‐ 6 patients out of 100 had more gout attacks taking febuxostat 80 mg (6% absolute increase in attacks);
‐ 75 more patients out of 100 taking febuxostat 80 mg reached their goal of uric acid level below 6.0 mg/dL (75% absolute benefit).
When febuxostat was compared with allopurinol, another medicine often used to lower uric acid:
‐ 2 patients out of 100 had more gout attacks taking febuxostat 80 mg (2% absolute increase in attacks);
‐ 29 more patients out of 100 on febuxostat 80 mg reached an acid level below 6.0 mg/dL (29% absolute benefit).
In studies of more than three years:
febuxostat at any dose had the same effect as allopurinol in reaching a uric acid level of less than 6.0 mg/dL, and there was no observed increase in gout attacks.
Summary of findings
Summary of findings for the main comparison. Febuxostat 40 mg/day versus placebo.
| Febuxostat 40 mg/day compared to placebo for chronic gout | ||||||
| Patient or population: patients with chronic gout Settings: Primary care Intervention: Febuxostat 40 mg/day Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Febuxostat 40 mg/day | |||||
| Incidence of gout flares Follow‐up: 28 days | 368 per 1000 | 350 per 1000 (191 to 640) | RR 0.95 (0.52 to 1.7) | 75 (1 study) | ++OO low1,2 | Not statistically significant. |
| Serum uric acid <6.0 mg/dL at final visit Follow‐up: 28 days | 27 per 10004 | 1000 per 1000 (68 to 1000)4 | RR 40.1 (2.5 to 639.1) | 69 (1 study) | ++OO low1,3 | NNT = 2 (95% CI 1 to 3)4; ATB = 56% (95% CI 37 to 71%); RRR = 39% (637% to 1.5%)4. |
| Pain | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Patient global assessment | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Health Related Quality of Life | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Serious Adverse Events Follow‐up: 28 days | 26 per 10004 | 26 per 1000 (0 to 0)4 | RR 1.0 (0 to 0)4 | 75 (1 study) | +++O moderate1 | Not statistically significant . |
| Discontinuations due to adverse events Follow‐up: 28 days | 26 per 1000 | 27 per 1000 (2 to 416) | RR 1.0 (0.07 to 15.8) | 75 (1 study) | +++O moderate1 | Not statistically significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; NNT: Number needed to treat; ATB: Absolute treatment benefit; RRR: Relative risk reduction; NE: Not estimable. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
|
1 High risk of bias in 1 item (intention to treat was not performed)
2 Study include relatively few patients and few events and thus has wide confidence intervals around the estimate of the effect
3 Outcome is a substitute measurement (surrogate endpoint). 4 Numbers calculated adding a continuity correction value of 0.5 to each cell of the 2x2 table. | ||||||
Summary of findings 2. Febuxostat 80 mg/day versus placebo.
| Febuxostat 80 mg/day compared to placebo for chronic gout | ||||||
| Patient or population: patients with chronic gout Settings: Primary care Intervention: Febuxostat 80 mg/day Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Febuxostat 80 mg/day | |||||
| Incidence of gout flares Follow‐up: 4‐28 weeks | 238 per 1000 | 314 per 1000 (228 to 431) | RR 1.32 (0.96 to 1.8) | 474 (2 studies) | ++OO low1,2 | Not statistically significant. |
| Serum uric acid <6.0 mg/dL at final visit Follow‐up: 4‐28 weeks | 7 per 1000 | 482 per 1000 (97 to 1000) | RR 68.9 (13.8 to 343.9) | 332 (2 studies) | ++OO low1,3 | NNT = 1 (95% CI 1 to 1); ATB = 75% (95% CI 68 to 80%); RRR = 10,000% (95% CI 71,500 to 13,300%). |
| Pain | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Patient global assessment | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Health Related Quality of Life | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Serious Adverse Events Follow‐up: 4‐28 weeks | 12 per 1000 | 32 per 10005 (8 to 125) | RR 2.8 (0.72 to 10.7) | 479 (2 studies) | +++O moderate1 | Not statistically significant. |
| Discontinuations due to adverse events Follow‐up: 4‐28 weeks | 35 per 1000 | 63 per 1000 (26 to 156) | RR 1.8 (0.74 to 4.5) | 479 (2 studies) | +++O moderate | Not statistically significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; NNT: Number needed to treat; ATB: Absolute treatment benefit; RRR: Relative risk reduction; NE: Not estimable. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
|
1 High risk of bias in 1 item (intention to treat was not performed)
2 The Becker 2005b trial includes relatively few patients and few events and thus has wide confidence intervals around the estimate of the effect
3 Outcome is a substitute measurement (surrogate endpoint).
4 Heterogeneity exists across the studies 5 In the placebo group 12 people out of 1000 had Serious Adverse Events over 4 to 28 weeks, compared to 32 (95% CI 8 to 125) out of 1000 for the febuxostat group. The reported serious adverse events were cardiovascular disorders (chest pain, coronary artery disease, myocardial infarction and atrial fibrillation). | ||||||
Summary of findings 3. Febuxostat 120 mg/day versus placebo.
| Febuxostat 120 mg/day compared to placebo for chronic gout | ||||||
| Patient or population: patients with chronic cout Settings: Primary care Intervention: Febuxostat 120 mg/day Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Febuxostat 120 mg/day | |||||
| Incidence of gout flares Follow‐up: 4‐28 weeks | 238 per 1000 | 407 per 1000 (300 to 552) | RR 1.7 (1.3 to 2.3) | 479 (2 studies) | +++O moderate1 | NNT = 6 (95% CI 4 to 17); ATB = 15% (95% CI 6 to 23); RRR = 61%. |
| Serum uric acid <6.0 mg/dL at final visit Follow‐up: 4‐28 weeks | 7 per 1000 | 565 per 1000 (112 to 1000) | RR 80.7 (16.0 to 405.5) | 356 (2 studies) | ++OO low1,2,3 | NNT = NE; ATB = 87% (95% CI 81 to 91%); RRR = NE. |
| Pain | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Patient global assessment | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Health Related Quality of Life | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Serious Adverse events Follow‐up: 4‐28 weeks | 12 per 1000 | 31 per 1000 (8 to 19) | RR 2.7 (0.70 to 10.2) | 479 (2 studies) | +++O moderate1 | Not statistically significant. |
| Discontinuations due to adverse events Follow‐up: 4‐28 weeks | 35 per 1000 | 58 per 1000 (23 to 142) | RR 1.7 (0.67 to 4.1) | 479 (2 studies) | +++O moderate1 | Not statistically significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; NNT: Number needed to treat; ATB: Absolute treatment benefit; RRR: Relative risk reduction; NE: Not estimable. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
| 1 High risk of bias in 1 item (intention to treat was not performed) 2 Outcome is a substitute measurement (surrogate endpoint). 3 The Becker 2005b trial includes relatively few patients and few events and thus has wide confidence intervals around the estimate of the effect | ||||||
Summary of findings 4. Febuxostat 40 mg/day versus allopurinol.
| Febuxostat 40 mg/day compared to allopurinol for chronic gout | ||||||
| Patient or population: patients with chronic gout Settings: Primary care Intervention: Febuxostat 40 mg/day Comparison: Allopurinol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Allopurinol | Febuxostat 40 mg/day | |||||
| Incidence of gout flares Follow‐up: 24 weeks | 41 per 1000 | 40 per 1000 (23 to 68) | RR 0.97 (0.57 to 1.65) | 1324 (1 study) | ++++ high | Not statistically significant. |
| Serum uric acid <6.0 mg/dL at final visit Follow‐up: 24 weeks | 408 per 1000 | 432 per 1000 (384 to 494) | RR 1.1 (0.94 to 1.2) | 1324 (1 study) | +++O moderate1 | Not statistically significant. |
| Pain | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Patient global assessment | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Health Related Quality of Life | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Serious Adverse Events Follow‐up: 24 weeks | 41 per 1000 | 25 per 1000 (14 to 44) | RR 0.61 (0.35 to 1.07) | 1513 (1 study) | ++++ high | Not statistically significant. |
| Discontinuations due to adverse events Follow‐up: 24 weeks | 85 per 1000 | 104 per 1000 (76 to 143) | RR 1.2 (0.90 to 1.7) | 1513 (1 study) | ++++ high | Not statistically significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
| 1 Outcome is a substitute measurement (surrogate endpoint). | ||||||
Summary of findings 5. Febuxostat 80 mg/day versus allopurinol.
| Febuxostat 80 mg/day compared to allopurinol for chronic gout | ||||||
| Patient or population: patients with chronic gout Settings: Primary care Intervention: Febuxostat 80 mg/day Comparison: Allopurinol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Allopurinol | Febuxostat 80 mg/day | |||||
| Incidence of gout flares Follow‐up: 8, 26, & 52 weeks | 204 per 1000 | 228 per 1000 (200 to 259) | RR 1.1 (0.98 to 1.3) | 2325 (3 studies) | ++++ high | Not statistically significant. |
| Serum uric acid <6.0 mg/dL at final visit Follow‐up: 8, 26, & 52 weeks | 398 per 1000 | 716 per 1000 (617 to 832) | RR 1.8 (1.6 to 2.2) | 2193 (3 studies) | ++OO low1,2 | NNT= 3 (95%CI 3 to 5); ATB = 29% (95% CI 25 to 33%); RRR = 73%. |
| Pain | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Patient global assessment | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Health Related Quality of Life | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Serious Adverse Events Follow‐up: 24. 28. & 52 weeks | 50 per 1000 | 45 per 1000 (17 to 122) | RR 0.91 (0.34 to 2.4) | 1044 (3 studies) | +++O moderate1,4 | Not statistically significant. |
| Discontinuations due to adverse events Follow‐up: 24. 28. & 52 weeks | 50 per 1000 | 65 per 1000 (39 to 107) | RR 1.3 (0.79 to 2.1) | 1044 (3 studies) | +++O moderate4 | Not statistically significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; NNT: Number needed to treat; ATB: Absolute treatment benefit; RRR: Relative risk reduction; NE: Not estimable. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
|
1 Heterogeneity exists across the studies
2 Outcome is a substitute measurement (surrogate endpoint).
3 Pooled estimates are from 2 studies (Schumacher 2008 and Becker 2005a) 4 High risk of bias in 1 item (intention to treat was not performed) | ||||||
Summary of findings 6. Febuxostat 120 mg/day versus allopurinol.
| Febuxostat 120 mg/day compared to allopurinol for chronic gout | ||||||
| Patient or population: patients with chronic gout Settings: Primary care Intervention: Febuxostat 120 mg/day Comparison: Allopurinol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Allopurinol | Febuxostat 120 mg/day | |||||
| Incidence of gout flares Follow‐up: 28 & 52 weeks | 420 per 1000 | 542 per 1000 (365 to 802) | RR 1.3 (0.87 to 1.9) | 986 (2 studies) | ++++ high | Not statistically significant. |
| Serum uric acid <6.0 mg/dL at final visit Follow‐up: 28 & 52 weeks | 384 per 1000 | 829 per 1000 (733 to 941) | RR 2.2 (1.9 to 2.5) | 880 (2 studies) | +++O moderate1 | NNT= 3 (95%CI 2 to 3); ARR = 44% (95% CI 38 to 50%); RRR = NE. |
| Pain | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Patient global assessment | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Health Related Quality of Life | See comment | See comment | See comment | See comment | See comment | Not assessed |
| Serious Adverse Events Follow‐up: 28 & 52 weeks | 50 per 1000 | 58 per 1000 (35 to 96) | RR 1.2 (0.70 to 1.93) | 1041 (3 studies) | ++++ high | Not statistically significant. |
|
Discontinuations due to adverse events Follow‐up: 28 & 52 weeks |
50 per 1000 | 78 per 1000 (24 to 251) | RR 1.6 (0.49 to 5.0) | 1041 (3 studies) | ++++ high | Not statistically significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; NNT: Number needed to treat; ARR: Absolute risk reduction; ARI: Absolute risk increase; RRR: Relative risk reduction; RRI: Relative risk increase; NE: Not estimable. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
| 1 Outcome is a substitute measurement (surrogate endpoint). | ||||||
Background
Description of the condition
Gout is the most common inflammatory arthritis in men over 40 years and has an increasing prevalence among postmenopausal women (Chohan 2009). It results from the deposition of monosodium uric acid crystals in and around the joints and soft tissues (Schlesinger 2004). It has been recognized that the formation of such crystals requires the presence of hyperuricaemia, defined as a serum uric acid concentration (serum uric acid levels) above its solubility limit (6.8 mg/dL) supersaturating the body fluids (Schumacher 2005). The disease can evolve from an asymptomatic stage of hyperuricaemia to recurrent gout attacks with inter‐critical periods and subsequently chronic gouty arthritis. Formation of tophi and progression to chronic destructive arthritis can result from persistent monosodium uric acid crystal deposition that is left untreated. Furthermore, hyperuricaemia can be associated with renal damage secondary to interstitial monosodium uric acid crystal deposition and the formation of kidney stones (Schlesinger 2004).
Description of the intervention
Lowering serum uric acid levels below saturating levels, at a target < 6.0 mg/dL, remains one of the major goals in the treatment of chronic gout to reduce or reverse clinical events (Zhang 2006). The pharmacological methods currently employed for that purpose are i) reduction of uric acid production by use of the xanthine oxidase inhibitors; ii) enhancement of urinary uric acid excretion with uricosuric agents; and iii) promotion of the catabolism of uric acid with the pegylated recombinant uricase (pegloticase) (Anderson 2010). Among the xanthine oxidase inhibitors, allopurinol, a purine analogue has been the main drug available for decades. However, allopurinol’s side effects, although rare, can be serious (hypersensitivity syndrome) and are more common in patients with renal dysfunction (Arellano 1993; Dalbeth 2007).
Febuxostat, a novel non‐purine analogue xanthine oxidase inhibitor, at daily dosages of 40 mg to 240 mg has been shown in studies to be at least as good as allopurinol (dose ≤ 300 mg/day) in lowering serum uric acid levels to < 6.0 mg/dL, and may require fewer dose adjustments in patients with mild to moderate renal dysfunction (Bruce 2006; Edwards 2009). Approved doses in the US are 40 mg and 80 mg daily, and in Europe 80 mg and 120 mg.
How the intervention might work
The reversibility of monosodium uric acid crystal deposition by reducing the serum uric acid levels below saturation level has been recognized for years, making the reduction and maintenance of serum uric acid levels below 6.0 mg/dL a goal in managing chronic gout (Pascual 2007). Allopurinol has been the gold standard therapy for the past 40 years. However, there are patients who are refractory to allopurinol or with impaired renal function who may benefit from the newer alternative, febuxostat. The newer inhibitor of xanthine oxidase was approved for use in many countries in 2009. Individual studies found that it could be at least as effective as allopurinol. Furthermore, there were no reports of hypersensitivity reactions in patients on febuxostat, and it has been shown to be safe when used in patients with mild to moderate renal dysfunction (Becker 2010). A potential benefit with its uses could also be in allopurinol refractory patients.
Why it is important to do this review
The goal of this review was to systematically review the current data on febuxostat's benefit and harms in treating chronic gout (Bruce 2006; Stevenson 2011). Patients, clinicians and policy‐makers need to keep abreast with the current literature in terms of benefit and harms of febuxostat.
To our knowledge there are only two systematic reviews to date on febuxostat for the treatment of chronic gout. This is the first systematic review of the literature with a meta‐analysis of randomised controlled trials and it differs from other reviews in that it includes summary data on open label trials.
Objectives
To evaluate the benefits and harms of febuxostat alone or combined with non‐steroidal antiinflammatory drugs or colchicine, or both, in comparison to allopurinol or placebo for the treatment of chronic gout.
Methods
Criteria for considering studies for this review
Types of studies
Any randomised controlled trial (RCT), controlled clinical trial, or open label trial (OLT) comparing febuxostat (alone or combined with non‐steroidal antiinflammatory drugs or colchicine, or both) in patients with gout with any control or placebo, with a minimum duration of three months.
Types of participants
Patients at least 16 years of age meeting the preliminary American College of Rheumatology (ACR) criteria for acute arthritis of primary gout (Wallace 1977) or given a diagnosis of gout as described by the authors.
Types of interventions
The following comparisons were eligible for inclusion: febuxostat alone or in combination with colchicine or non‐steroidal antiinflammatory drugs (NSAIDs) or lifestyle changes versus placebo or any control alone or in combination with colchicine, NSAIDs or lifestyle changes. Any dosages were included.
Types of outcome measures
We used the primary outcome measures for response to gout treatment that were proposed by the American College of Rheumatology outcome measures for gout clinical trials and OMERACT 9 gout report (OMERACT 9).
Major outcomes
Benefit as assessed by the OMERACT 9 outcome domains for studies of acute and chronic gout (OMERACT 9).
1) Gout flares: we extracted data on frequency of recurrent attacks in all ways reported.
2) Serum urate: change in serum uric acid levels, and per cent change in serum uric acid levels from baseline at final visit. Evidence suggests that lowering serum uric acid levels to < 6.0 mg/dL increases crystal disappearance from synovial fluid (Edwards 1981; Li‐Yu 2001).
3) Harms as assessed by the incidence of patients with adverse events (total and serious adverse events, liver function test abnormalities, skin reactions, cardiovascular events, hypertension, and diarrhoea) and the withdrawal rates (total withdrawals, withdrawals due to adverse events, withdrawals due to gout flares, withdrawals due to lack of efficacy, withdrawals due to other reasons).
Minor outcomes
The following secondary outcomes were also considered when reported.
Tophus burden as measured by size measurement of individual tophus (regression of tophi), including disappearance of tophi and velocity of tophus regression.
Health‐related quality of life as assessed by measures: Short Form‐36 (SF‐36), Gout Assessment Questionnaire (GAQ), and the Gout Impact Scale (GIS).
Pain: on Likert scales as well as the visual analog scale (VAS), numeric rating scale (NRS), or qualitative scales.
Musculoskeletal function: function as assessed by activities of daily living scales including composite outcomes such as the Health Assessment Questionnaire (HAQ) or other activities of daily living scales (ADLs), and work productivity.
Patient global and physician global assessment.
Joint imaging: joint damage as assessed by the van der Heijde‐Sharp radiographic score modified for gout.
We used the GRADE software to generate the 'Summary of findings' table and reported outcomes include: 1) incidence of gout flares, 2) serum uric acid levels < 6.0 mg/dL at final visit, 3) pain, 4) patient global assessment, 5) health‐related quality of life, 6) total number of patients with serious adverse events, and 7) total number of withdrawals due to adverse events.
Search methods for identification of studies
Electronic searches
The following electronic databases were searched: The Cochrane Library (2011, Issue 6), including the Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), and Health Technology Assessments (HTA); MEDLINE (1950 to July 2011); EMBASE (1980 to July 2011); and International Pharmaceutical Abstracts Database (IPAD). The ClinicalTrials.gov website was searched for references to trials of febuxostat.
The search was not limited by language, year of publication or type of publication. The full search strategy in Appendix 1 was developed for MEDLINE and was adapted for the other electronic databases.
Searching other resources
The reference lists from comprehensive reviews and identified clinical trials were also manually searched.
Websites of the following regulatory agencies were searched for reported adverse events using the terms 'gout', 'febuxostat' and 'uloric':
FDA’s MedWatch, www.fda.gov/harms/medwatch/default.htm; National Guideline Clearing House, www.guideline.gov; National Institute for Health and Clinical Excellence, www.nice.org.uk/guidance/index.jsp?action=byID&o=11830; Agency for Healthcare Research and Quality (AHRQ), www.ahrq.gov/; Health Technology Assessment (HTAi), www.htai.org/; Turning Research Into Practice (TRIP), www.tripdatabase.com.
Data collection and analysis
EndNote X2 software was used to manage the records retrieved from searches of electronic databases. Results from handsearches were tracked on a Microsoft Excel spreadsheet. A data extraction form was created in Word to capture all the information available for each individual trial.
Selection of studies
Results of the various searches were independently reviewed by two authors (JT, MLO). Titles and abstracts were reviewed and if additional information was required, the full text was obtained. A record of reasons for excluding studies was kept. Disagreements were resolved by discussion. Inter‐rater agreement was calculated using Cohen's kappa.
Data extraction and management
Data were independently extracted from the included trials by two review authors (MLO, JT); then the collected data were entered into RevMan 5.0 using the double‐entry system.
Data included the following.
General study information such as title, authors, contact address, publication source, publication year, country, and study sponsor.
Characteristics of the study: design, study setting, inclusion and exclusion criteria, quality criteria (e.g. randomisation method; allocation procedure; blinding of patients, caregivers and outcome assessors; withdrawals and dropouts, intention‐to‐treat (ITT) analysis).
Characteristics of the study population (age, sex, duration of disease, treatment history, presence of co‐morbidity and peripheral disease, concurrent treatments).
Characteristics of the intervention, such as treatment comparators, dose, method of administration, frequency of administration, duration of treatment, and numbers in each intervention group.
Outcome measures as noted above.
Results for the ITT population (where possible); outcome measures at the end of the controlled phase; and any summary measures with standard deviations, confidence intervals and P values, where given; dropout rate and reasons for withdrawal.
Assessment of risk of bias in included studies
The risk of bias of the included studies was also assessed by two independent review authors. As recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008), the following methodological domains were assessed.
Random sequence generation.
Allocation sequence concealment.
Blinding of participants and personnel.
Blinding of outcome assessors.
Incomplete outcome data.
Selective reporting.
Other bias.
Each of these criteria were explicitly judged using: 'low risk of bias' ,'high risk of bias', or 'unclear' (uncertainty over the potential for bias).
Measures of treatment effect
The results of the studies were analysed using Review Manager 5.1. Data were summarised in a meta‐analysis when they were sufficiently homogeneous, both clinically and statistically. Continuous data were expressed as mean difference (MD) or standardized mean difference (SMD) depending on similarity of scales measuring an outcome. Dichotomous data were expressed as risk ratios (RR) or, in the case of rare events (< 10%), Peto odds ratios (Peto OR) were used.
To establish equivalence between febuxostat and allopurinol we used the Becker et al definition where non‐inferiority is a greater than 10% difference in achieving final serum uric acid levels < 6.0 mg/dL of the lower limit of the 95% confidence interval (CI) (Becker 2010).
Summary of findings tables were completed in order to improve the readability of the review. In addition to the absolute and relative magnitude of effect provided in the summary of findings table, the number needed to treat (NNT) was calculated from the control group event rate (unless the population event rate was known) and the risk ratio using the Visual Rx NNT calculator (Cates 2004). For continuous outcomes, the NNT was calculated using the Wells calculator available at the Cochrane Musculoskeletal Group editorial office. GRADE software was used to provide an overall grading of the quality of the evidence and to provide a comment on our confidence in the results. The possible grades of evidence are: 'high quality', 'moderate quality', 'low quality', 'very low quality'.
Unit of analysis issues
Treatment groups were analysed separately. No comparisons were added by combining all relevant experimental intervention groups of the study into a single group.
Dealing with missing data
We considered two strategies for handling withdrawals and dropouts. i. Accounting for the numbers: were the numbers of withdrawals and dropouts reported for both groups? ii. Accommodating withdrawals (ITT analysis, imputation): were the withdrawals and dropouts accounted for in the analysis (for example through ITT analysis using imputation methods) or the last observation carried forward (LOCF) was used.
Assessment of heterogeneity
Heterogeneity of the data was formally tested using the Chi2 test with a P value < 0.10 indicating significant heterogeneity. The I2 statistic (Higgins 2003) was also assessed. A value greater than 50% may indicate substantial heterogeneity. In the case of substantial heterogeneity, the data were further explored, including subgroup analyses, in an attempt to explain the heterogeneity.
Assessment of reporting biases
A funnel plot was not performed due to the limited number of publications retrieved.
Data synthesis
The fixed‐effect model of meta‐analysis was used to assess all outcomes. However, when significant heterogeneity was found and could not be explained, the random‐effects model was used.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were performed to explore possible effect size differences:
intervention (different dosage, duration of treatment), and
characteristics of participants (severity of baseline disease, age, disease duration, renal function).
Sensitivity analysis
We explored effect size differences and the robustness of conclusions by:
effect of study quality that is defined as adequate allocation concealment and outcome assessor blinding, and
effect of imputation of missing data or statistical transformations.
Results
Description of studies
Studies are reported according to the follow‐up duration. Characteristics of included studies are summarised in the tables section.
Results of the search
In our electronic search we retrieved a total of 271 citations: 93 from MEDLINE, 135 from EMBASE, 30 from IPAD, and 13 from web links. Searching other resources (described in the Methods paragraph) did not reveal additional records for review. In a first review based on abstracts and titles we excluded 72 citations. Of the remaining 199, 189 were excluded in a second step review mainly because of the type of citation (Figure 1). Of the 10 full texts then retrieved for our third and final review, two were excluded (see Characteristics of excluded studies for details) and a total of six met the inclusion criteria. The percentage of inter‐rater agreement for the study selection was 93% (κ = 0.80). Four RCTs (six publications) were included in our efficacy and harms analysis (Becker 2005a; Becker 2005b; Becker 2010; Schumacher 2008) and two open label trials (OLTs) were included in effectiveness and harms analysis (Becker 2009; Schumacher 2009). We have also included two more tables: Studies awaiting classification and Ongoing studies, which may be useful for future updates of this review.
1.
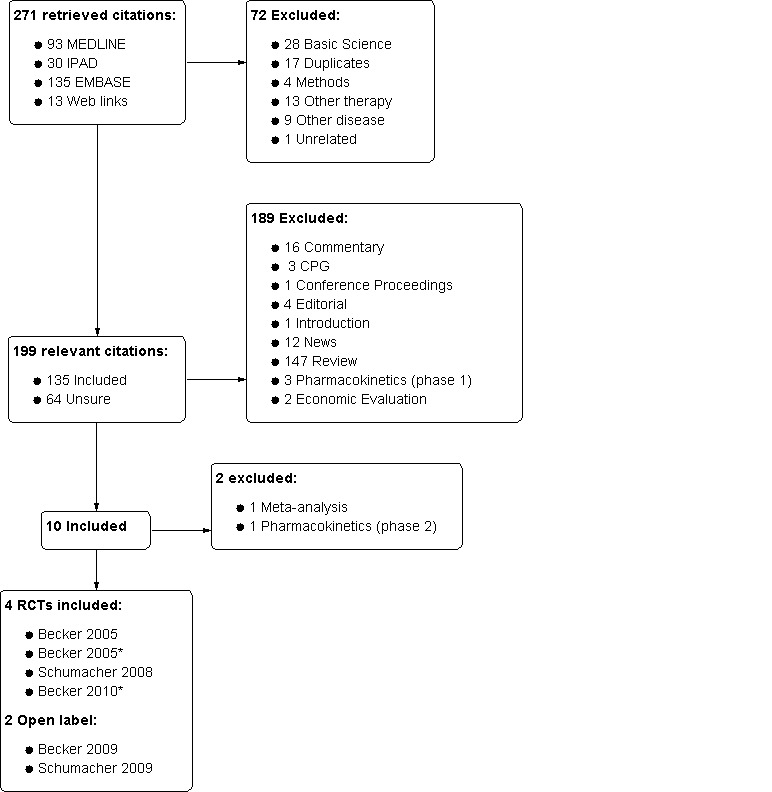
Diagram of study selection.
* RCT plus abstract (2 publications)
Included studies
Design
There were four randomised, double‐blind, placebo‐controlled trials: Becker 2005b, Becker 2010, Schumacher 2008, Becker 2005a of 4‐week, 26‐week, 28‐week, and 52‐week duration, respectively. From these four RCTs, two were combined (Becker 2005a; Schumacher 2008) and followed over a three‐year period in an OLT (Becker 2009), and one (Becker 2005b) had an OLT of five years (Schumacher 2009). Table 7 shows the characteristics of the two OLTs included in this review. In Becker 2010 patients completing either one of the OLTs were eligible to participate. Randomisation was reported in ratios of 1:1:1:1 for Becker 2005b, 1:1:1 for Becker 2010 and Becker 2005a, and 2:2:1:2:1 for Schumacher 2008. Schumacher 2008 had three febuxostat arms versus allopurinol versus placebo; Becker 2005b had three febuxostat arms versus placebo; and Becker 2010 and Becker 2005a had two febuxostat arms versus allopurinol.
1. Open label trials characteristics.
| Schumacher 2009 | Becker 2009 | |||||
| Name | Febuxostat in the treatment of gout: 5‐yr findings of the FOCUS efficacy and harms study | Clinical efficacy and harms of successful long term uric acid lowering with febuxostat or allopurinol in subjects with gout FACT and APEX open label trial |
||||
| Duration of RCT | 28 days | 52 weeks and 28 weeks | ||||
| Duration of open label trial | 60 months | 40 months | ||||
| Arms | Febuxostat 40mg | Febuxostat 80mg | Febuxostat 120mg | Febuxostat 80mg | Febuxostat 120mg | Allopurinol |
| Subjects enrolled in open label trial | 8 | 79 | 29 | 606 | 388 | 92 |
| Subjects completed open label trial | 6 | 41 | 11 | 412 | 217 | 35 |
| Primary endpoint | Proportion of subjects that achieved and maintained SUA < 6mg/dL | Proportion of subjects with sUA < 6mg/dL at each visit | ||||
| Secondary endpoints | Percentage reduction from baseline sUA; Proportion of subjects with sUA < 5mg/dL and 4 mg/dL;Proportion of subjects with flares requiring treatment; resolution of palpable tophi | Percentage reduction from baseline sUA; proportion of subjects with sUA decreasing to < 6mg/dL across treatment changes; reduction in the incidence of flares requiring treatment; percentage reduction in number of tophi; reduction in the size or disappearance of the index tophus | ||||
Sample sizes
Sample sizes ranged from 153 in Becker 2005b to 2269 in the Becker 2010.
Setting
All trials were reported as 'multicentre' trials, with no specific information regarding the setting. Becker 2005a enrolled participants from 112 centres in US and Canada. The remaining three studies were from US centres (324 centres for Becker 2010, 167 centres for Schumacher 2008, and 24 centres for Becker 2005b).
Participants
A total of 3978 patients were included in this analysis (4254 in total, but we subtracted 276 patients from Becker 2010 that took part in previous trials); 2619 participants were randomised to febuxostat (696 to 40 mg, 1231 to 80 mg, 558 to 120 mg, and 134 to 240 mg), 172 to placebo, and 1187 to allopurinol (up to 300 mg). Of these, 84% to 97% were men and 75% to 89% were white. The mean age of participants ranged from 51.6 to 56.2 years. Mean disease duration, when reported, ranged from 10 to 12.6 years. Becker 2005b did not report participants' mean disease duration. Participants were at least 18 years old and met the preliminary criteria of the American College of Rheumatologists (ACR) for acute gout arthritis and had serum uric acid levels of ≥ 8.0 mg/dL. Common exclusion criteria included: serum creatinine > 1.5 mg/dL (Becker 2010 and Schumacher 2008 included participants with moderate renal dysfunction); body mass index (BMI) higher than 50; history of xanthinuria; pregnancy or lactation; active liver disease; history of alcohol abuse; and use of uric acid‐lowering agents or medications that could interfere with the treatment.
Intervention
Febuxostat reported dosages were 40 mg/day, 80 mg/day, 120 mg/day, and 240 mg/day. If needed, patients underwent a washout period before the trial where only naproxen or colchicine or both were provided. After the washout period, patients were allowed to maintain naproxen or colchicine dosages for 2 to 26 weeks. Gout flares were treated at the investigator's discretion. No other gout medication was allowed. Becker 2010, Schumacher 2008, and Becker 2005a had a control group with allopurinol at 100, 200, or 300 mg/day. Becker 2005b and Schumacher 2008 included a placebo group.
Outcomes
All trials reported the proportion of participants with serum uric acid levels of < 6.0 mg/dL as a primary outcome measure. Becker 2005b and Becker 2010 measured the serum uric acid levels at the final visit. Schumacher 2008 and Becker 2005a required that the serum uric acid levels were maintained on the last three monthly measurements.
Secondary outcomes included the proportion of participants with a serum uric acid level of < 6.0 mg/dL at each visit, the per cent reduction of serum uric acid levels from baseline at each visit, the proportion of participants requiring treatment for a self reported gout flare between weeks 8 and 28, and the reduction in the number of tophi at each visit for participants with palpable tophi at baseline. Becker 2005b included the per cent reduction in daily urinary uric acid excretion from baseline to day 28. Becker 2005a also reported the per cent reduction from baseline in tophus area. Becker 2010 evaluated the proportion of participants with < 5.0 and < 4.0 mg/dL at each visit and stratified their results by renal function.
Reported adverse events were similar across trials. Trials reported any adverse event, serious adverse events, and those occurring in at least 2% to 5% of participants in any group. Only Schumacher 2008 reported liver function test results.
Funding
All trials were funded by TAP Pharmaceutical Products, Inc., which is now part of Takeda Global Research & Development Center, Inc.
Excluded studies
Two articles were excluded from the meta analysis. Becker 2008 was a meta‐analysis of individual patient data and Komoriya 2004 was a pharmacokinetics (phase 2) study with no outcome of interest for this review (see table Characteristics of excluded studies).
Risk of bias in included studies
Summary assessment of the risk of bias tables is presented in Figure 2 and Figure 3. The studies included in the meta‐analysis were rated as unclear in most domains due to a poor quality of reporting. High risk of bias was rated in Becker 2005b and Becker 2005a in terms of incomplete outcome data given the high dropout rates of participants and lack of ITT analyses. Additionally, all studies were sponsored by TAP Pharmaceutical Products, Inc.
2.
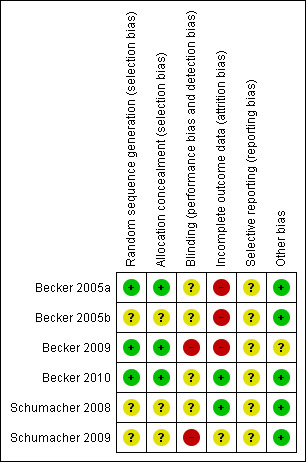
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
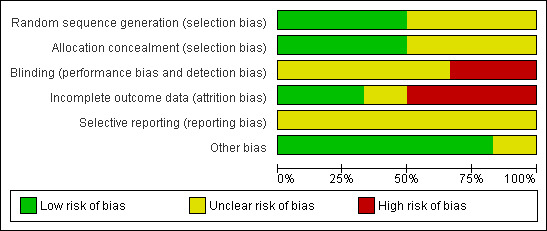
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Adequate sequence generation was reported in two trials (Becker 2005a; Becker 2010). Becker 2010 described an interactive voice response system and stratified by baseline renal function and prior completion of either of two open label extension trials. A computer‐generated central randomisation to allocate treatment was reported by Becker 2005a. For those reporting central randomisation we assumed that allocation was probably concealed. Methods of randomisation were not described by Schumacher 2008 and Becker 2005b.
Blinding
All trials were reported as 'double‐blind', but no further details were provided. Febuxostat and allopurinol were not provided by the same manufacturer.
Incomplete outcome data
We used the same judging criteria as Maxwell 2009, with a less than 80% completion rate in the treatment group considered as a high risk of bias, and reviewed how missing data were imputed and if the primary outcome was analysed on an ITT basis.
Although Becker 2005b reported ITT analysis, not all randomised patients were included in the efficacy analysis (13 patients out of 153 were excluded). Authors did not provide a description on how incomplete outcome data were analysed. In Becker 2010, only one participant (out of 2268 randomised) was not included in the efficacy analysis (baseline serum uric acid level was < 8.0 mg/dL). Also, in this study there was no report on how missing data were imputed. Schumacher 2008 was the only trial with a proper ITT analysis and considered participants to be non‐responders if they discontinued the study before ≥ 3 serum uric acid levels were obtained. Statistical analysis in Becker 2005a was based on completers: four participants were excluded because they had serum uric acid levels < 8.0 mg/dL at baseline and three because they did not receive the study treatment. Missing data for the primary efficacy endpoint were considered as non‐responses.
The completion rates ranged from 61% to 97% for the febuxostat group, 74% to 82% in the allopurinol‐treated group, and 75% to 95% in the placebo group. Lower completion rates were observed in trials with longer follow‐up periods and in higher dosage groups. Adverse events were the most common reason for discontinuation in all trials (not including gout flares).
Selective reporting
All trials assessed the expected outcomes. However, there was failure to report findings of some of the pre‐specified secondary outcomes.
Other potential sources of bias
All trials were sponsored and partially designed by TAP Pharmaceutical products, and the specific role of the study sponsor and the potential conflicts of interest for each author were not reported in three trials.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Comparisons were analysed by febuxostat dosage and by the control group used: i) febuxostat 40 mg/day, 80 mg/day, 120 mg/day, or 240 mg/day versus placebo; and ii) febuxostat 40 mg/day, 80 mg/day, 120 mg/day, or 240 mg/day versus allopurinol. The current approved dosages for febuxostat in the US are 40 and 80 mg daily (FDA 2009), and 80 and 120 mg in Europe (EMEA 2008).
Efficacy
Outcomes are described in the following order: gout flares, per cent of patients achieving serum uric acid levels < 6.0 mg/dL, and per cent reduction in serum uric acid levels from baseline to the final visit. Results for the following outcome measures were not reported in any of the included studies: joint imaging, musculoskeletal function, patient or physician global assessment, and pain. Tophi burden was measured differently in the trials, which prevented the analysis of this outcome. A summary of each trial result is provided at the end of this section. Mean serum uric acid levels at baseline ranged from 9.2 mg/dL to 9.9 mg/dL and the proportion of patients with a history or presence of tophi ranged from 16% to 33%.
Febuxostat versus placebo
1. Febuxostat 40 mg versus placebo
In Becker 2005b no statistically significant difference in the patients' self‐reported gout flares was observed between patients assigned to febuxostat compared to those in the placebo group at 28 days (RR 0.95; 95% CI 0.52 to 1.7). However, patients in the febuxostat group were more likely to achieve serum uric acid levels < 6.0 mg/dL at 28 days (RR 40.1; 95% CI 2.5 to 639.1) and had a statistically significant reduction in serum uric acid levels from baseline at 28 days (MD ‐5.2; 95% CI ‐5.8 to ‐4.7) compared to patients in the placebo group (Analysis 1.1, Analysis 1.2, and Analysis 1.3).
1.1. Analysis.
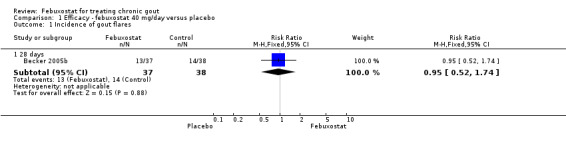
Comparison 1 Efficacy ‐ febuxostat 40 mg/day versus placebo, Outcome 1 Incidence of gout flares.
1.2. Analysis.
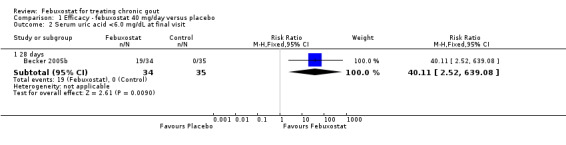
Comparison 1 Efficacy ‐ febuxostat 40 mg/day versus placebo, Outcome 2 Serum uric acid <6.0 mg/dL at final visit.
1.3. Analysis.
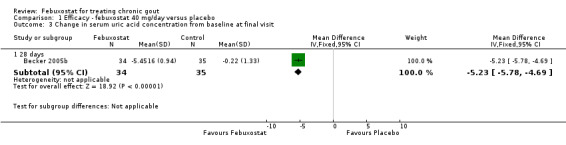
Comparison 1 Efficacy ‐ febuxostat 40 mg/day versus placebo, Outcome 3 Change in serum uric acid concentration from baseline at final visit.
2. Febuxostat 80 mg versus placebo
Two studies provided data on the incidence of gout flares at four to eight weeks (Becker 2005b; Schumacher 2008) with a pooled RR of 1.3 (95% CI 0.96 to 1.8). The combined RR for achieving serum uric acid levels < 6.0 mg/dL at the final visit was 68.9 (95% CI 13.8 to 343.9). The combined mean reduction observed in serum uric acid levels from baseline to the final visit of ‐5.0 was statistically significant in favour of febuxostat (95% CI ‐6.2 to ‐3.7) (Analysis 2.1, Analysis 2.2, and Analysis 2.3).
2.1. Analysis.
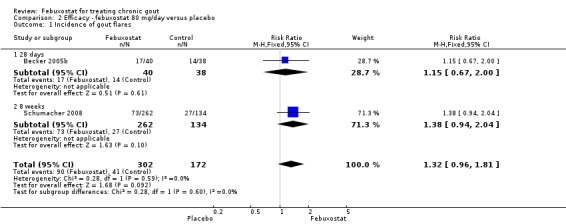
Comparison 2 Efficacy ‐ febuxostat 80 mg/day versus placebo, Outcome 1 Incidence of gout flares.
2.2. Analysis.
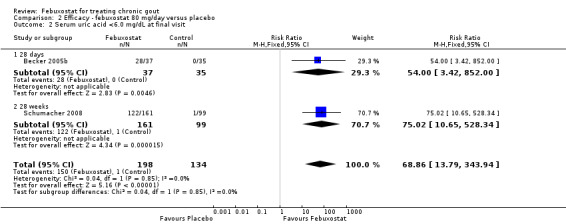
Comparison 2 Efficacy ‐ febuxostat 80 mg/day versus placebo, Outcome 2 Serum uric acid <6.0 mg/dL at final visit.
2.3. Analysis.
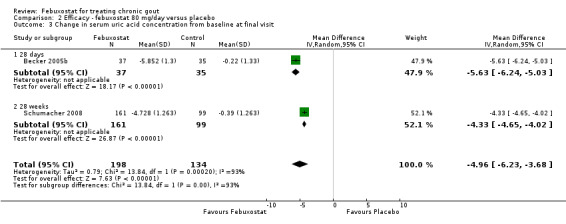
Comparison 2 Efficacy ‐ febuxostat 80 mg/day versus placebo, Outcome 3 Change in serum uric acid concentration from baseline at final visit.
3. Febuxostat 120 mg versus placebo
Becker 2005b and Schumacher 2008 provided data for this comparison at four to eight weeks. Patients in the febuxostat group reported more frequent gout flares than in the placebo group (pooled RR 1.7; 95% CI 1.3 to 2.3). However, serum uric acid levels < 6.0 mg/dL at the final visit were 80.7 times more likely to be achieved in the febuxostat group compared to the placebo group (95% CI 16.0 to 405.5). The combined mean reduction in serum uric acid levels from baseline to the final visit between the febuxostat group and the placebo group was ‐5.3 (95% CI ‐5.7 to ‐4.9) (Analysis 3.1, Analysis 3.2, and Analysis 3.3).
3.1. Analysis.
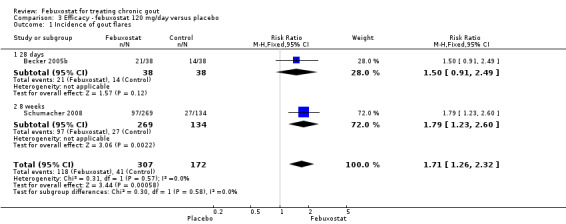
Comparison 3 Efficacy ‐ febuxostat 120 mg/day versus placebo, Outcome 1 Incidence of gout flares.
3.2. Analysis.
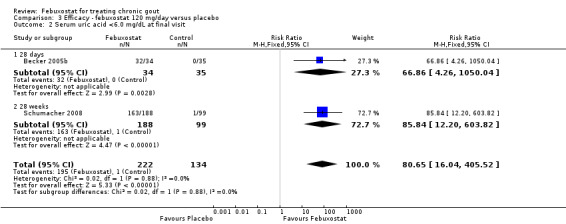
Comparison 3 Efficacy ‐ febuxostat 120 mg/day versus placebo, Outcome 2 Serum uric acid <6.0 mg/dL at final visit.
3.3. Analysis.
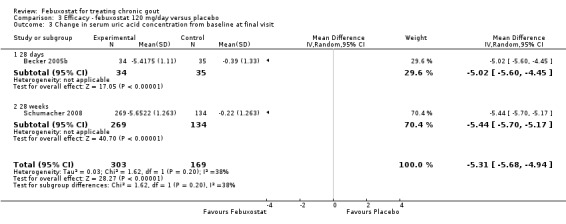
Comparison 3 Efficacy ‐ febuxostat 120 mg/day versus placebo, Outcome 3 Change in serum uric acid concentration from baseline at final visit.
4. Febuxostat 240 mg versus placebo
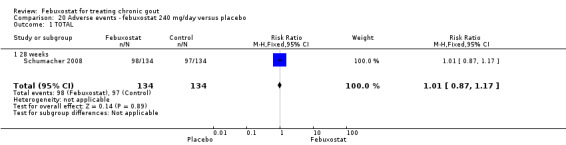
Only one study (Schumacher 2008) reported data on the incidence of gout flares for febuxostat 240 mg/day versus placebo with an observed RR at 28 weeks of 2.6 (95% CI 1.8 to 3.7). More febuxostat‐treated patients achieved serum uric acid levels < 6.0 mg/dL at the final visit compared to placebo (RR 93.4; 95% CI 13.2 to 654.5). Furthermore, they had a greater decrease in their MD in serum uric acid levels from baseline at 28 weeks (MD ‐6.3; 95% CI ‐6.6 to ‐6.0) (Analysis 4.1, Analysis 4.2, and Analysis 4.3).
4.1. Analysis.
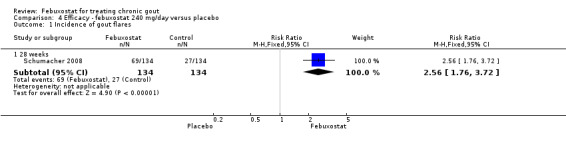
Comparison 4 Efficacy ‐ febuxostat 240 mg/day versus placebo, Outcome 1 Incidence of gout flares.
4.2. Analysis.
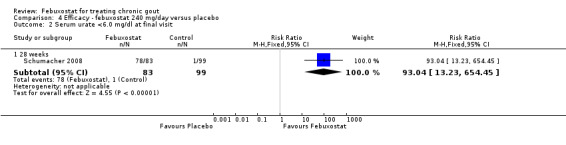
Comparison 4 Efficacy ‐ febuxostat 240 mg/day versus placebo, Outcome 2 Serum urate <6.0 mg/dl at final visit.
4.3. Analysis.
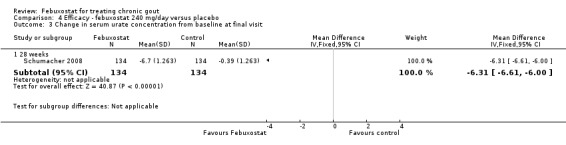
Comparison 4 Efficacy ‐ febuxostat 240 mg/day versus placebo, Outcome 3 Change in serum urate concentration from baseline at final visit.
Febuxostat versus allopurinol
5. Febuxostat 40 mg versus allopurinol
One study provided data on the incidence of gout flares at 24 weeks (Becker 2010). The number of gout flares was not significantly different between the two groups (RR 0.97; 95% CI 0.57 to 1.7). Also, the two groups did not differ in the per cent achieving serum uric acid levels < 6.0 mg/dL (RR 1.1; 95% CI 0.94 to 1.2). Data on per cent reduction in serum uric acid levels from baseline to final visit were not provided (Analysis 5.1, Analysis 5.2).
5.1. Analysis.
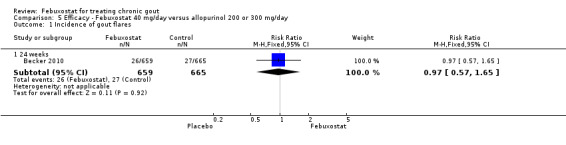
Comparison 5 Efficacy ‐ Febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 1 Incidence of gout flares.
5.2. Analysis.
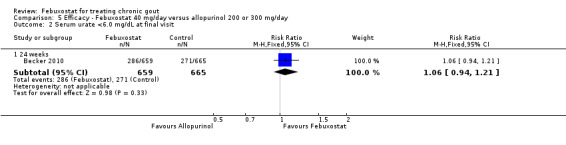
Comparison 5 Efficacy ‐ Febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 2 Serum urate <6.0 mg/dL at final visit.
6. Febuxostat 80 mg versus allopurinol
Three studies provided data on the efficacy of febuxostat 80 mg compared to allopurinol: Schumacher 2008 at 8 weeks, Becker 2010 at 26 weeks, and Becker 2005a at 52 weeks. The pooled RR for the incidence of gout flares was 1.1 (95% CI 0.98 to 1.3). Patients in the febuxostat group were 1.8 times more likely to achieve a serum uric acid level < 6.0 mg/dL at the final visit compared to those patients in the allopurinol group (95% CI 1.6 to 2.1). The pooled estimate for per cent reduction in serum uric acid levels from baseline to final visit did not reach statistical significance (MD ‐1.2; 95% CI ‐1.5 to ‐0.99) (Analysis 6.1, Analysis 6.2, and Analysis 6.3).
6.1. Analysis.
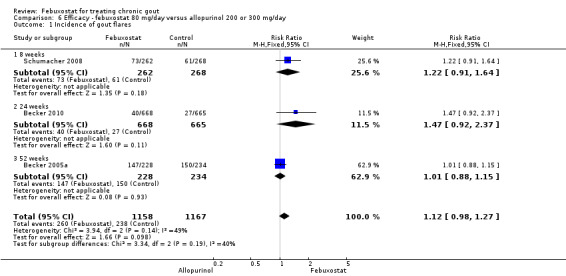
Comparison 6 Efficacy ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 1 Incidence of gout flares.
6.2. Analysis.
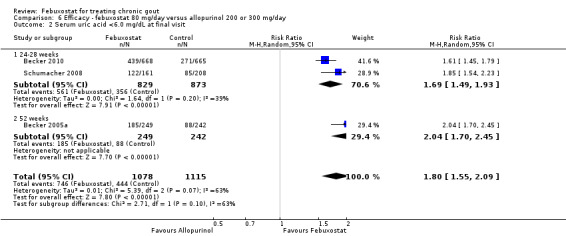
Comparison 6 Efficacy ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 2 Serum uric acid <6.0 mg/dL at final visit.
6.3. Analysis.
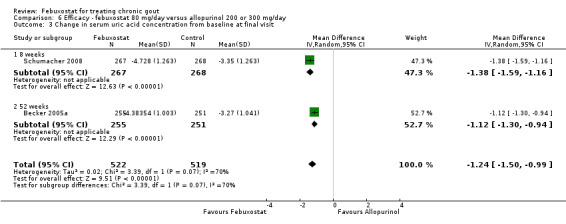
Comparison 6 Efficacy ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 3 Change in serum uric acid concentration from baseline at final visit.
7. Febuxostat 120 mg versus allopurinol
At 28 to 52 weeks, the pooled RR from Schumacher 2008 and Becker 2005a for having gout flares, with febuxostat 120 mg, was 1.29 (95% CI 0.87 to 1.91). On the other hand, the pooled RR for achieving serum uric acid levels < 6.0 mg/dL at the final visit was 2.2 (95% CI 1.91 to 2.45). Similarly, there was a statistically significant reduction in serum uric acid levels from baseline to final visit (MD ‐1.9; 95% CI ‐2.2 to ‐1.7) (Analysis 7.1, Analysis 7.2, and Analysis 7.3).
7.1. Analysis.
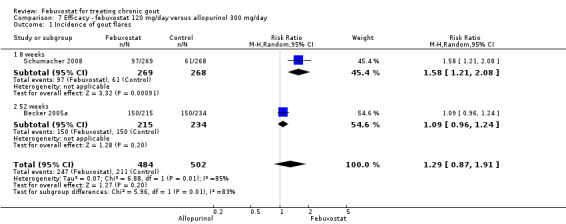
Comparison 7 Efficacy ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 1 Incidence of gout flares.
7.2. Analysis.
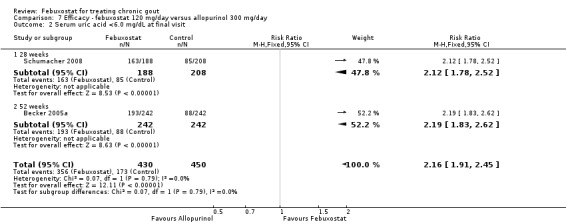
Comparison 7 Efficacy ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 2 Serum uric acid <6.0 mg/dL at final visit.
7.3. Analysis.
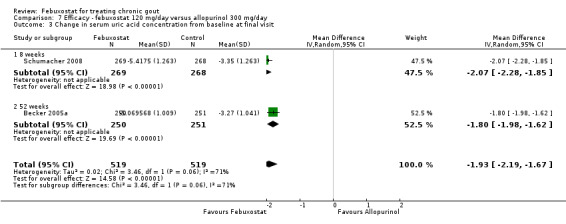
Comparison 7 Efficacy ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 3 Change in serum uric acid concentration from baseline at final visit.
8. Febuxostat 240 mg versus allopurinol
Patients on febuxostat 240 mg were 2.3 times more likely to report gout flares compared to those on allopurinol at 28 weeks (95% CI 1.7 to 3.0). They were also more likely to achieve serum uric acid levels < 6.0 mg/dL at the final visit (RR 93.0; 95% CI 13.2 to 654.5). Additionally, the reduction in serum uric acid levels from baseline to final visit was significantly greater in the febuxostat group compared to the allopurinol group at eight weeks (MD ‐3.4; 95% CI ‐3.6 to ‐3.1) (Analysis 8.1, Analysis 8.2, and Analysis 8.3).
8.1. Analysis.
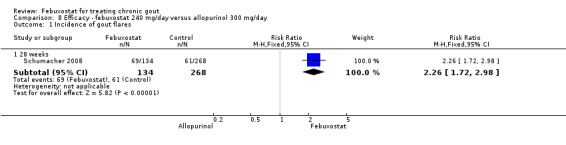
Comparison 8 Efficacy ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 1 Incidence of gout flares.
8.2. Analysis.
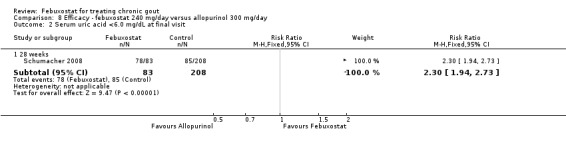
Comparison 8 Efficacy ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 2 Serum uric acid <6.0 mg/dL at final visit.
8.3. Analysis.
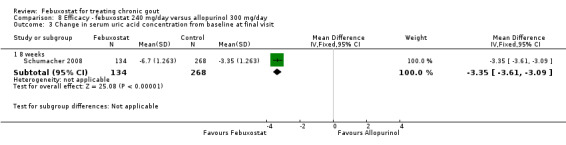
Comparison 8 Efficacy ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 3 Change in serum uric acid concentration from baseline at final visit.
Tophi burden
At 28 weeks, the mean per cent reduction in the number of tophi in the febuxostat 120 mg group was greater than in the placebo group (‐1.2 versus ‐0.3, P ≤ 0.05) (Schumacher 2008). The presence of tophi at baseline was associated with a lower proportion of patients achieving serum uric acid levels < 6.0 mg/dL at the final visit: 48% versus 35% for febuxostat 40 mg, and 45% versus 32% for allopurinol (Becker 2010).
At 52 weeks, the median per cent reduction in tophus area was greater for patients receiving febuxostat 80 mg or 120 mg compared to allopurinol (83%, 66% versus 50%). However, the proportion of patients with a reduction in the tophus area and a median reduction in the number of tophi were similar between groups (54%, 40% versus 48%; and 1%, 0% versus 0%, respectively). The presence of tophi at baseline was associated with a lower proportion of patients achieving serum uric acid levels < 6.0 mg/dL at final visit: 70% versus 57% for febuxostat; and 45% versus 32% for allopurinol (Becker 2010).
Effectiveness
Only one OLT had a control group (Becker 2009). During three years of follow‐up the authors did not find any differences across groups. The mean percentage of participants requiring treatment for an acute attack was 4.5% (0% to 10%) for the febuxostat 80 mg group, 6.6% (2% to 17%) for the febuxostat 120 mg group, compared to 5.7% (0% to 11%) for the allopurinol group (numbers based on completers). Ninety per cent (109/120) of participants taking febuxostat 80 mg maintained serum uric acid levels < 6.0 mg/dL, 91% (43/47) of those taking febuxostat 120 mg, and 90% (9/10) of participants in the allopurinol group. Mean percentage reductions in serum uric acid levels from baseline to final visit were 47% and 53% for patients on febuxostat 80 mg and 120 mg respectively compared to 32% for patients on allopurinol. See Table 8.
2. Effectiveness data from open label trials.
| 1year n/N (%) | 2year n/N (%) | 3year n/N (%) | 4year n/N (%) | 5year n/N (%) | ||
| Schumacher 2009 | Febuxostat 40 mg | 4/7 (57) | 5/8 (63) | 4/ 6 (67) | 5/ 6 (83) | 6/6 (100) |
| Febuxostat 80 mg | 47/55 (85) | 37/49 (76) | 38/ 45 (84) | 36/39 (92) | 38/41 (93) | |
| Febuxostat 120 mg | 12/18 (67) | 12/13 (92) | 12/13 (92) | 11/13 (85) | 10/11 (91) | |
| Becker 2009 | Febuxostat 80 mg | 375/422 (89) | 325/364 (89) | 109/120 (91) | ‐ | ‐ |
| Febuxostat 120 mg | 145/168 (86) | 123/141 (87) | 43/47 (92) | ‐ | ‐ | |
| Allopurinol 300 mg | 37/45 (82) | 33/42 (79) | 9/10 (90) | ‐ | ‐ | |
Proportion of subjects with serum uric acid < 6 mg/dL
In Becker 2009, the mean reduction in primary tophus size among participants with a history or the presence of tophi was ‐68%, ‐48%, and ‐52% for febuxostat 80 mg, 120 mg, and allopurinol, respectively. The mean reduction in total number of tophi was ‐60%, ‐58%, and ‐50%, respectively; and the proportion of participants with complete resolution of primary tophi at three years was 47%, 38%, and 30%, respectively. In Schumacher 2009, the incidence of gout flares was higher in participants with tophi compared with participants without tophi at one year (31% versus 10%). At four years only 10% of the participants with baseline tophi reported flares. Resolution of the tophi occurred in 18 of 26 (69%) of the participants at five years. However, the proportion of patients achieving serum urate levels < 6.0 mg/dL was similar between patients with or without a history or presence of tophi at baseline.
Withdrawals
We report five types of withdrawals: total withdrawals, due to adverse events, gout flares, lack of efficacy, and other reasons (as reported by authors).
Febuxostat versus placebo
9. Febuxostat 40 mg versus placebo
For the only study comparing febuxostat 40 mg versus placebo (Becker 2005b), withdrawals in both groups were mostly due to adverse events or gout flares, with no statistically significant differences between the two groups (RR 1.0; 95% CI 0.07 to 15.8, and RR 0.34; 95% CI 0.01 to 8.1, respectively). Also, looking at total withdrawals, there was no statistically significant difference between groups (RR 0.51; 95%CI 0.05 to 5.4) (Analysis 9.1, Analysis 9.2, Analysis 9.3, Analysis 9.4, and Analysis 9.5).
9.1. Analysis.
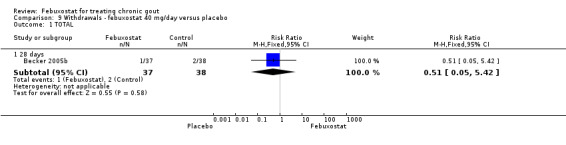
Comparison 9 Withdrawals ‐ febuxostat 40 mg/day versus placebo, Outcome 1 TOTAL.
9.2. Analysis.
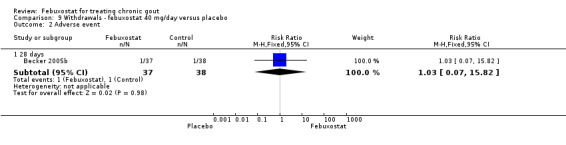
Comparison 9 Withdrawals ‐ febuxostat 40 mg/day versus placebo, Outcome 2 Adverse event.
9.3. Analysis.
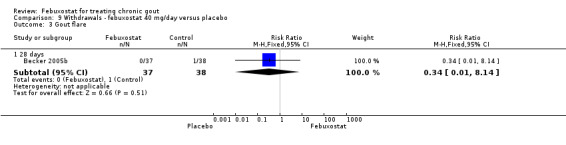
Comparison 9 Withdrawals ‐ febuxostat 40 mg/day versus placebo, Outcome 3 Gout flare.
9.4. Analysis.
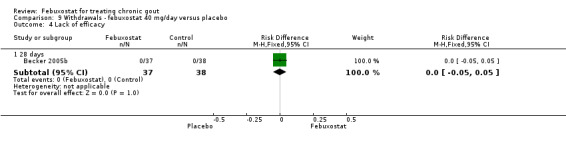
Comparison 9 Withdrawals ‐ febuxostat 40 mg/day versus placebo, Outcome 4 Lack of efficacy.
9.5. Analysis.
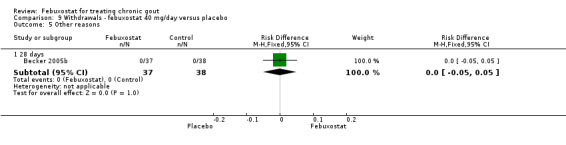
Comparison 9 Withdrawals ‐ febuxostat 40 mg/day versus placebo, Outcome 5 Other reasons.
10. Febuxostat 80 mg versus placebo
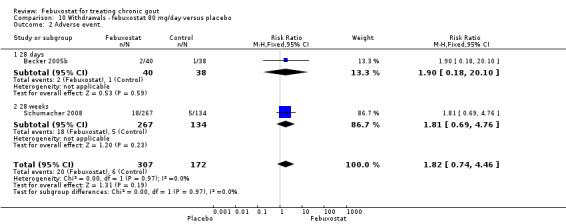
The pooled RR for total withdrawals was 1.4 (95% CI 1.0 to 2.0) for patients in the febuxostat group compared to placebo. Otherwise, withdrawals because of adverse events, gout flares, lack of efficacy, and other reasons were not statistically higher in the febuxostat group compared to placebo (Analysis 10.1, Analysis 10.2, Analysis 10.3, Analysis 10.4, and Analysis 10.5).
10.1. Analysis.
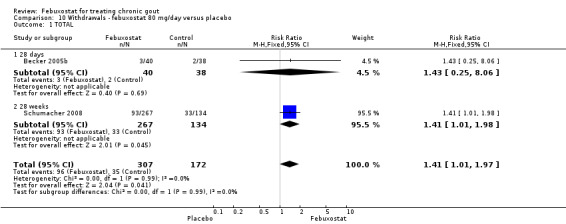
Comparison 10 Withdrawals ‐ febuxostat 80 mg/day versus placebo, Outcome 1 TOTAL.
10.2. Analysis.
Comparison 10 Withdrawals ‐ febuxostat 80 mg/day versus placebo, Outcome 2 Adverse event.
10.3. Analysis.
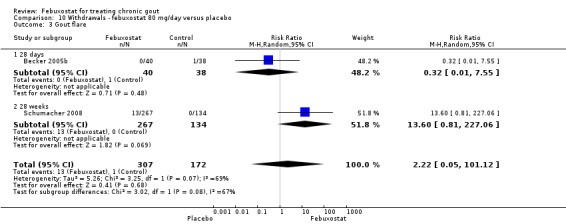
Comparison 10 Withdrawals ‐ febuxostat 80 mg/day versus placebo, Outcome 3 Gout flare.
10.4. Analysis.
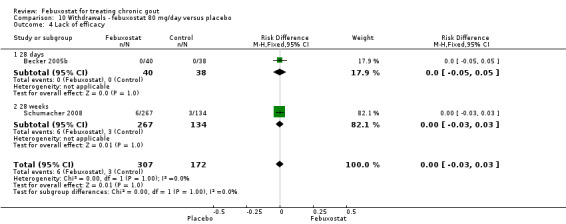
Comparison 10 Withdrawals ‐ febuxostat 80 mg/day versus placebo, Outcome 4 Lack of efficacy.
10.5. Analysis.
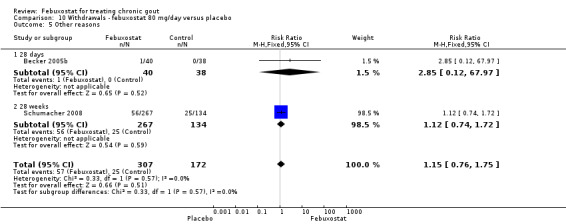
Comparison 10 Withdrawals ‐ febuxostat 80 mg/day versus placebo, Outcome 5 Other reasons.
11. Febuxostat 120 mg versus placebo
In the two trials included for this comparison (Becker 2005b; Schumacher 2008) there was no statistically significant difference between the groups for any withdrawal types (Analysis 11.1, Analysis 11.2, Analysis 11.3, Analysis 11.4, and Analysis 11.5).
11.1. Analysis.
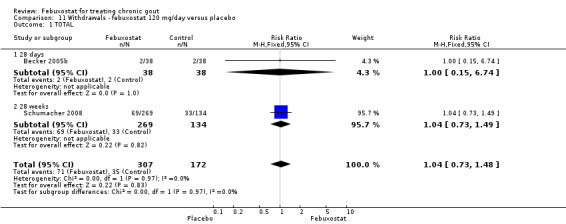
Comparison 11 Withdrawals ‐ febuxostat 120 mg/day versus placebo, Outcome 1 TOTAL.
11.2. Analysis.
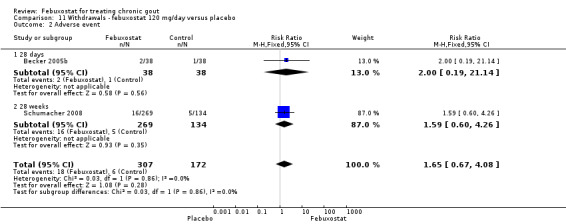
Comparison 11 Withdrawals ‐ febuxostat 120 mg/day versus placebo, Outcome 2 Adverse event.
11.3. Analysis.
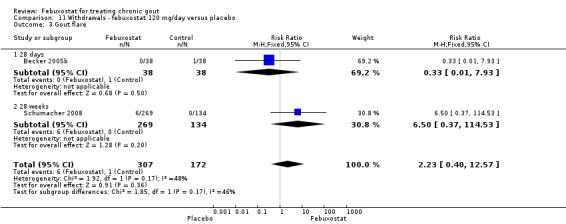
Comparison 11 Withdrawals ‐ febuxostat 120 mg/day versus placebo, Outcome 3 Gout flare.
11.4. Analysis.
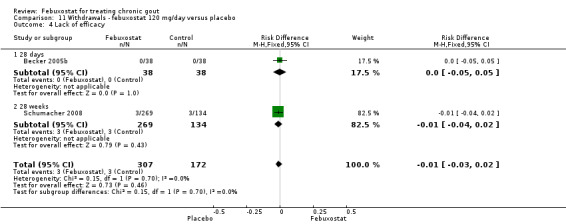
Comparison 11 Withdrawals ‐ febuxostat 120 mg/day versus placebo, Outcome 4 Lack of efficacy.
11.5. Analysis.
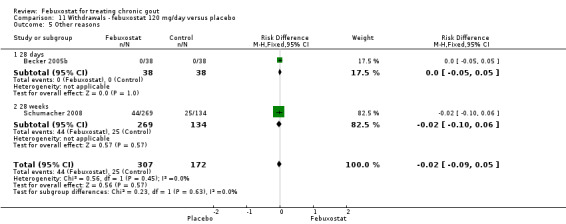
Comparison 11 Withdrawals ‐ febuxostat 120 mg/day versus placebo, Outcome 5 Other reasons.
12. Febuxostat 240 mg versus placebo
Patients in the febuxostat group were 1.5 times more likely to withdraw compared to placebo (95% CI 1.0 to 2.1). Also, there was a trend for a higher withdrawal rate due to gout flare (RR 17.0; 95% CI 0.99 to 291.6). Otherwise there were no observed differences (Analysis 12.1, Analysis 12.2,Analysis 12.3, Analysis 12.4, and Analysis 8.3).
12.1. Analysis.
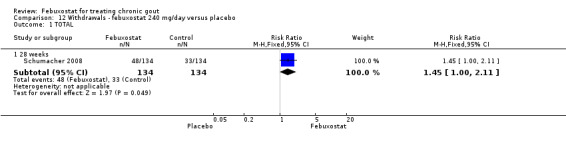
Comparison 12 Withdrawals ‐ febuxostat 240 mg/day versus placebo, Outcome 1 TOTAL.
12.2. Analysis.
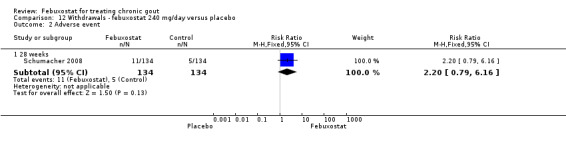
Comparison 12 Withdrawals ‐ febuxostat 240 mg/day versus placebo, Outcome 2 Adverse event.
12.3. Analysis.
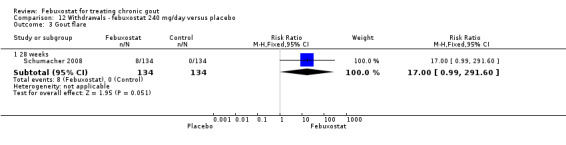
Comparison 12 Withdrawals ‐ febuxostat 240 mg/day versus placebo, Outcome 3 Gout flare.
12.4. Analysis.
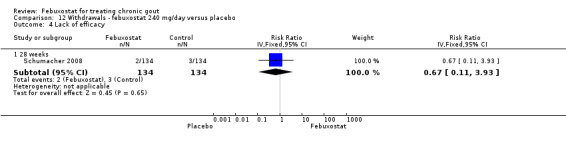
Comparison 12 Withdrawals ‐ febuxostat 240 mg/day versus placebo, Outcome 4 Lack of efficacy.
Febuxostat versus allopurinol
13. Febuxostat 40 mg versus allopurinol
In (Becker 2010), again there was no statistically significant difference across groups in any type of withdrawal (Analysis 13.1, Analysis 13.2, Analysis 13.3, Analysis 13.4, and Analysis 8.3).
13.1. Analysis.
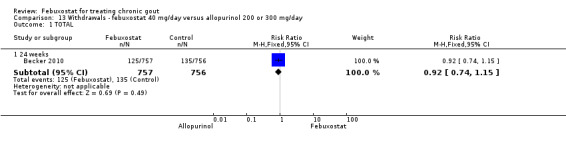
Comparison 13 Withdrawals ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 1 TOTAL.
13.2. Analysis.
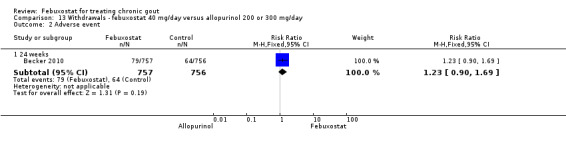
Comparison 13 Withdrawals ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 2 Adverse event.
13.3. Analysis.
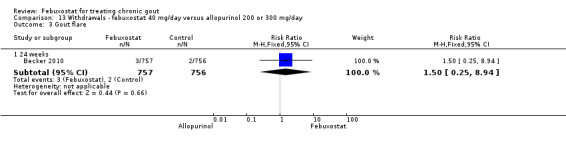
Comparison 13 Withdrawals ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 3 Gout flare.
13.4. Analysis.
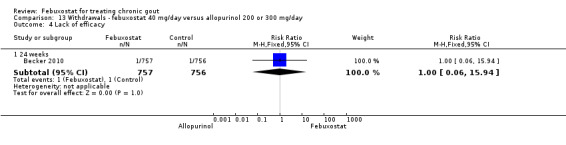
Comparison 13 Withdrawals ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 4 Lack of efficacy.
14. Febuxostat 80 mg versus allopurinol
Patients in the febuxostat group had a significantly higher total withdrawal rate than placebo with a pooled RR of 1.3 (95% CI 1.1 to 1.5) (Analysis 14.1). Also, patients in the febuxostat group were 1.3 times more likely to withdraw for reasons other than adverse events, gout flares, or lack of efficacy compared to allopurinol (95% CI 1.1 to 1.6) (Analysis 14.2, Analysis 14.3, Analysis 14.4, and Analysis 14.5).
14.1. Analysis.
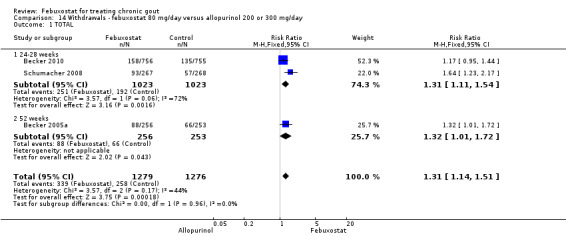
Comparison 14 Withdrawals ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 1 TOTAL.
14.2. Analysis.
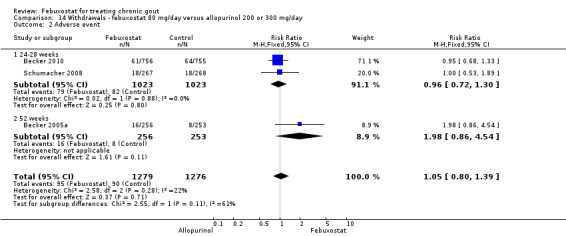
Comparison 14 Withdrawals ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 2 Adverse event.
14.3. Analysis.
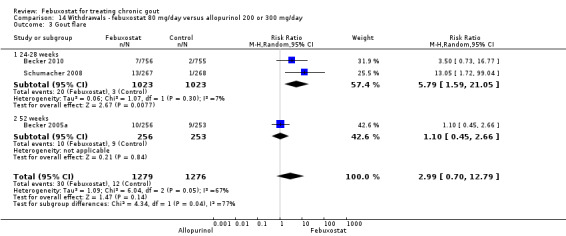
Comparison 14 Withdrawals ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 3 Gout flare.
14.4. Analysis.
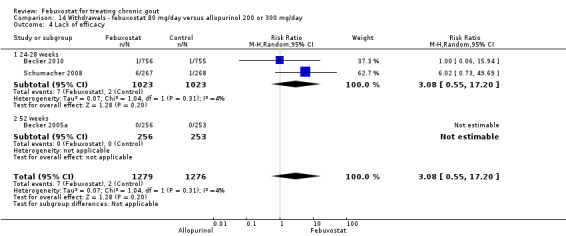
Comparison 14 Withdrawals ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 4 Lack of efficacy.
14.5. Analysis.
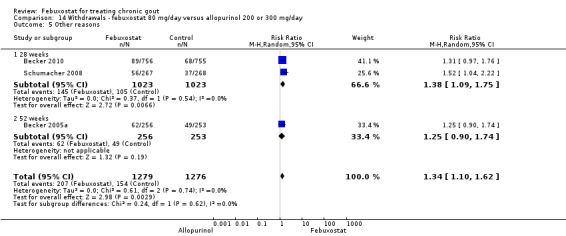
Comparison 14 Withdrawals ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 5 Other reasons.
15. Febuxostat 120 mg versus allopurinol
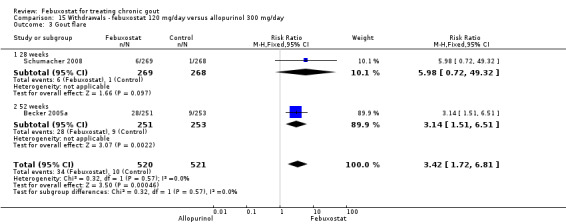
Trials included for this comparison were Schumacher 2008 and Becker 2005a. Patients in the febuxostat group were 1.4 times more likely to withdraw for any reason compared to the allopurinol group (95% CI 1.1 to 1.7) (Analysis 15.1). Furthermore, they were 3.4 times more likely to withdraw due to gout flares compared with allopurinol (95% CI 1.7 to 6.8). No other difference was found (Analysis 15.2, Analysis 15.3, Analysis 15.4, and Analysis 15.5).
15.1. Analysis.
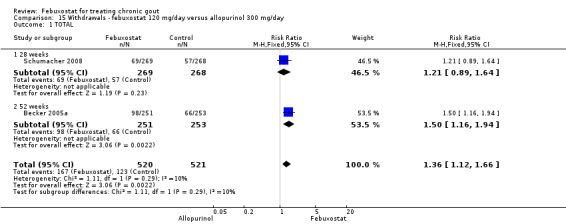
Comparison 15 Withdrawals ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 1 TOTAL.
15.2. Analysis.
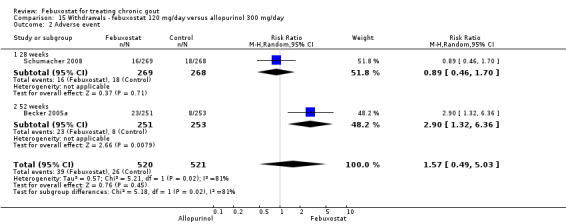
Comparison 15 Withdrawals ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 2 Adverse event.
15.3. Analysis.
Comparison 15 Withdrawals ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 3 Gout flare.
15.4. Analysis.
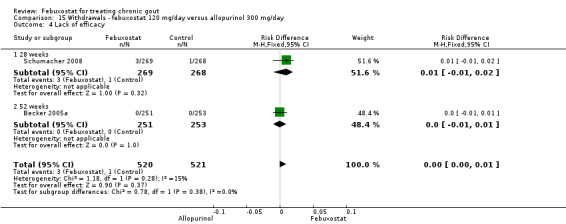
Comparison 15 Withdrawals ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 4 Lack of efficacy.
15.5. Analysis.
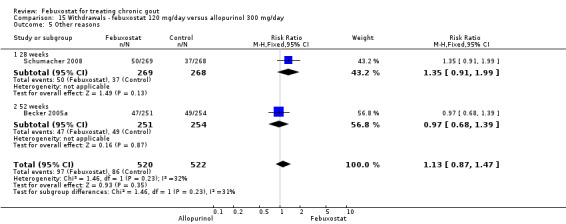
Comparison 15 Withdrawals ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 5 Other reasons.
16. Febuxostat 240 mg versus allopurinol
Similar to the 120 mg group, patients taking febuxostat 240 mg had increased total withdrawals and increased withdrawals due to gout flares when compared to allopurinol (RR 1.7; 95% CI 1.2 to 2.2, and RR 16.0; 95% CI 2.0 to 129.6, respectively). Again, no other differences were observed (Analysis 16.1, Analysis 16.2, Analysis 16.3, Analysis 16.4, and Analysis 16.5).
16.1. Analysis.
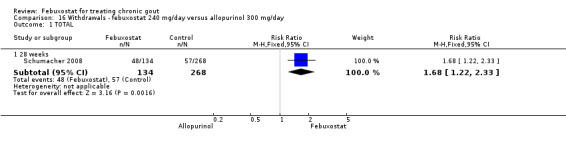
Comparison 16 Withdrawals ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 1 TOTAL.
16.2. Analysis.
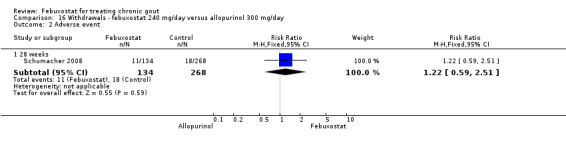
Comparison 16 Withdrawals ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 2 Adverse event.
16.3. Analysis.
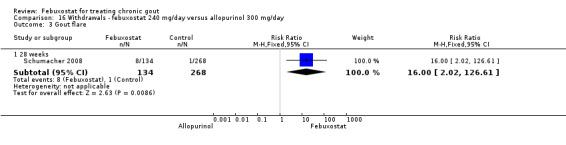
Comparison 16 Withdrawals ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 3 Gout flare.
16.4. Analysis.
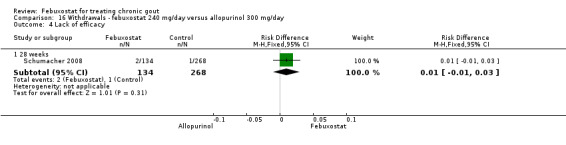
Comparison 16 Withdrawals ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 4 Lack of efficacy.
16.5. Analysis.
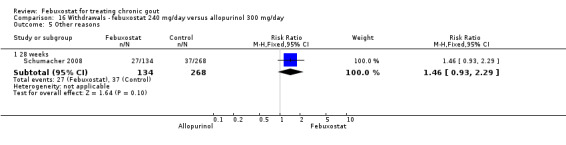
Comparison 16 Withdrawals ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 5 Other reasons.
Adverse events
The most clinically relevant adverse events reported were: any adverse events, serious adverse events, liver function test abnormalities, skin reaction, cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation), hypertension, and diarrhoea. Adverse events were generally reported by treatment group. Three studies classified the events according to the definitions in the MedRA (Becker 2005b; Becker 2010; Schumacher 2008).
Febuxostat versus placebo
17. Febuxostat 40 mg versus placebo
Comparing febuxostat 40 mg to placebo we did not find any statistically significant difference between the two groups in any of the above adverse events. Of note, there were no reports in either group of any cardiovascular event, skin reaction, or hypertension (Analysis 17.1, Analysis 17.3, Analysis 17.4, Analysis 17.5, Analysis 17.6, and Analysis 17.7).
17.1. Analysis.
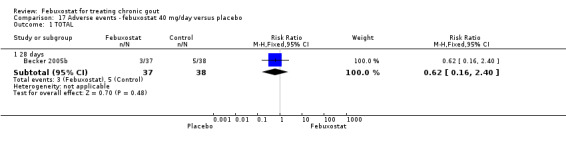
Comparison 17 Adverse events ‐ febuxostat 40 mg/day versus placebo, Outcome 1 TOTAL.
17.3. Analysis.
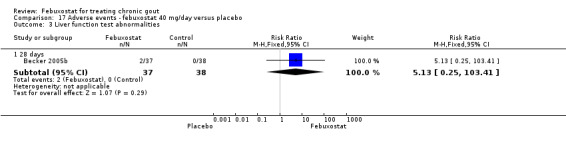
Comparison 17 Adverse events ‐ febuxostat 40 mg/day versus placebo, Outcome 3 Liver function test abnormalities.
17.4. Analysis.
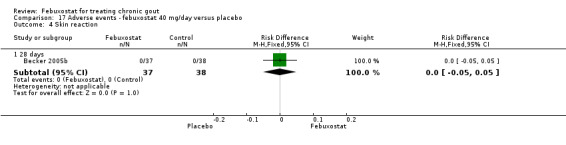
Comparison 17 Adverse events ‐ febuxostat 40 mg/day versus placebo, Outcome 4 Skin reaction.
17.5. Analysis.
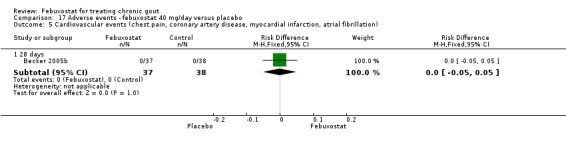
Comparison 17 Adverse events ‐ febuxostat 40 mg/day versus placebo, Outcome 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation).
17.6. Analysis.
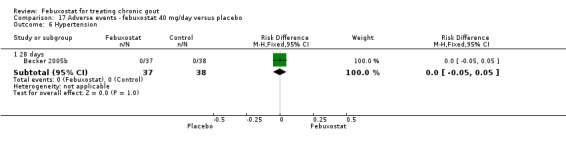
Comparison 17 Adverse events ‐ febuxostat 40 mg/day versus placebo, Outcome 6 Hypertension.
17.7. Analysis.
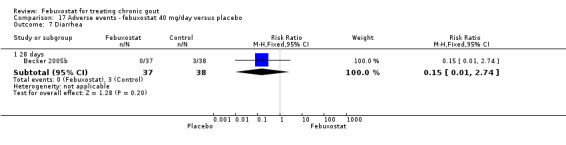
Comparison 17 Adverse events ‐ febuxostat 40 mg/day versus placebo, Outcome 7 Diarrhea.
18. Febuxostat 80 mg versus placebo
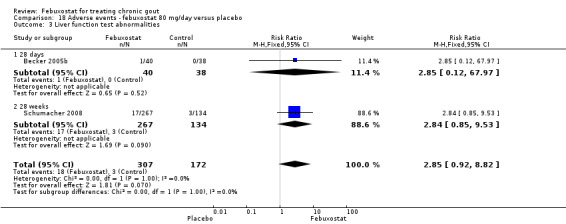
No significant difference was noted between the two groups for the measure of any adverse event (RR 0.95; 95% CI 0.83 to 1.1). Pooled data from the studies included in this analysis (Becker 2005b; Schumacher 2008) showed that the febuxostat group tended to have more liver enzyme abnormalities but without reaching statistical significance (RR 2.9; 95% CI 0.92 to 8.8). Otherwise there were no differences between both groups (Analysis 18.1, Analysis 18.3, Analysis 18.4, Analysis 18.5, Analysis 18.6, and Analysis 18.7).
18.1. Analysis.
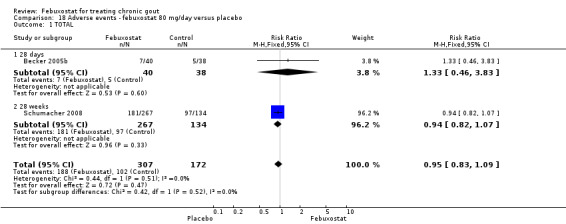
Comparison 18 Adverse events ‐ febuxostat 80 mg/day versus placebo, Outcome 1 TOTAL.
18.3. Analysis.
Comparison 18 Adverse events ‐ febuxostat 80 mg/day versus placebo, Outcome 3 Liver function test abnormalities.
18.4. Analysis.
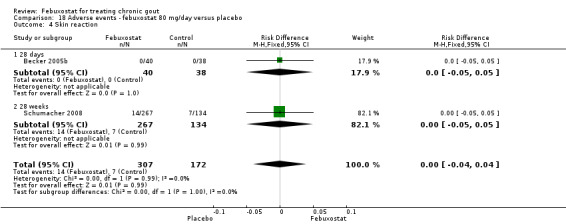
Comparison 18 Adverse events ‐ febuxostat 80 mg/day versus placebo, Outcome 4 Skin reaction.
18.5. Analysis.
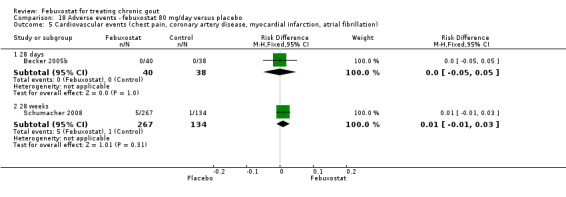
Comparison 18 Adverse events ‐ febuxostat 80 mg/day versus placebo, Outcome 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation).
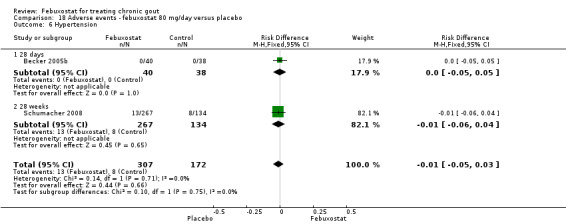
18.6. Analysis.
Comparison 18 Adverse events ‐ febuxostat 80 mg/day versus placebo, Outcome 6 Hypertension.
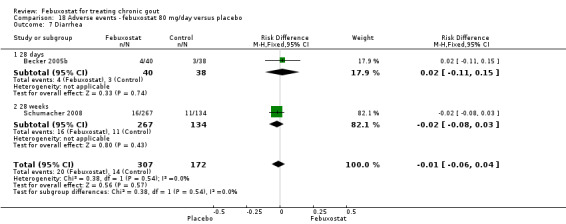
18.7. Analysis.
Comparison 18 Adverse events ‐ febuxostat 80 mg/day versus placebo, Outcome 7 Diarrhea.
19. Febuxostat 120 mg versus placebo
No statistically significant differences were noted between the two groups in any measure (Analysis 19.1, Analysis 19.3, Analysis 19.4, Analysis 19.5, Analysis 19.6, and Analysis 19.7).
19.1. Analysis.
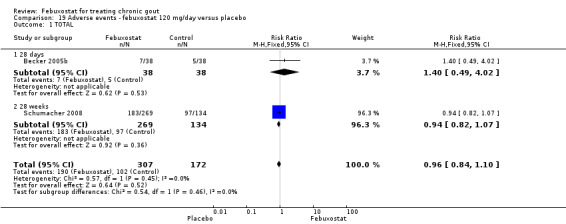
Comparison 19 Adverse events ‐ febuxostat 120 mg/day versus placebo, Outcome 1 TOTAL.
19.3. Analysis.
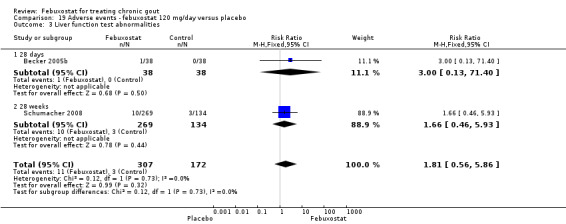
Comparison 19 Adverse events ‐ febuxostat 120 mg/day versus placebo, Outcome 3 Liver function test abnormalities.
19.4. Analysis.
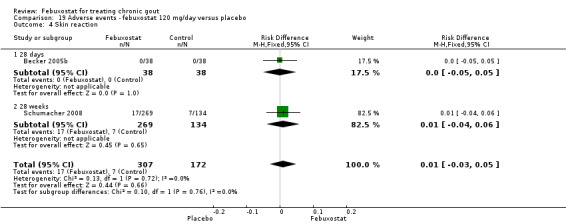
Comparison 19 Adverse events ‐ febuxostat 120 mg/day versus placebo, Outcome 4 Skin reaction.
19.5. Analysis.
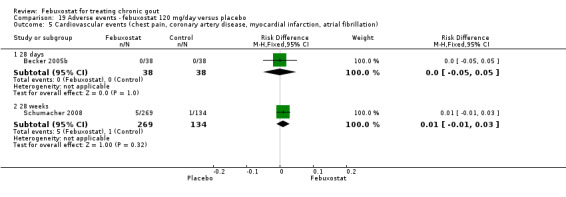
Comparison 19 Adverse events ‐ febuxostat 120 mg/day versus placebo, Outcome 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation).
19.6. Analysis.
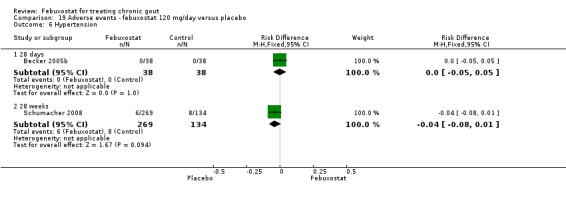
Comparison 19 Adverse events ‐ febuxostat 120 mg/day versus placebo, Outcome 6 Hypertension.
19.7. Analysis.
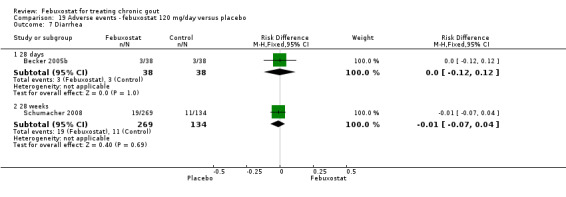
Comparison 19 Adverse events ‐ febuxostat 120 mg/day versus placebo, Outcome 7 Diarrhea.
20. Febuxostat 240 mg versus placebo
Similarly in this analysis there were no statistically significant differences noted between the two groups (Analysis 20.1, Analysis 20.3, Analysis 20.4, Analysis 20.5, Analysis 20.6, and Analysis 20.7).
20.1. Analysis.
Comparison 20 Adverse events ‐ febuxostat 240 mg/day versus placebo, Outcome 1 TOTAL.
20.3. Analysis.
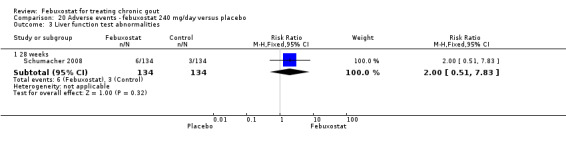
Comparison 20 Adverse events ‐ febuxostat 240 mg/day versus placebo, Outcome 3 Liver function test abnormalities.
20.4. Analysis.
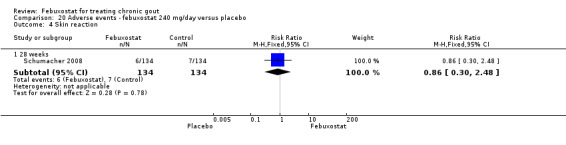
Comparison 20 Adverse events ‐ febuxostat 240 mg/day versus placebo, Outcome 4 Skin reaction.
20.5. Analysis.
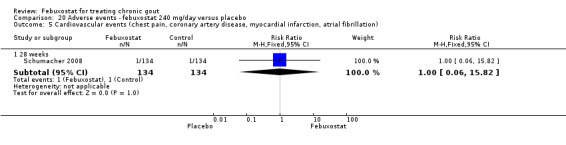
Comparison 20 Adverse events ‐ febuxostat 240 mg/day versus placebo, Outcome 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation).
20.6. Analysis.
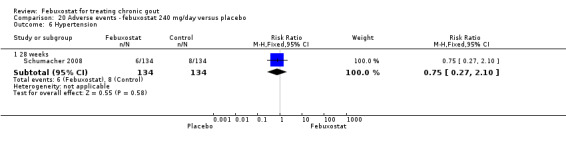
Comparison 20 Adverse events ‐ febuxostat 240 mg/day versus placebo, Outcome 6 Hypertension.
20.7. Analysis.
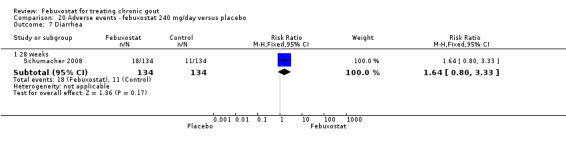
Comparison 20 Adverse events ‐ febuxostat 240 mg/day versus placebo, Outcome 7 Diarrhea.
Febuxostat versus allopurinol
21. Febuxostat 40 mg versus allopurinol
No statistically significant differences in any of the adverse events we reported were noted between these two groups (Analysis 21.1, Analysis 21.3, Analysis 21.4, Analysis 21.5, Analysis 21.6, and Analysis 21.7).
21.1. Analysis.
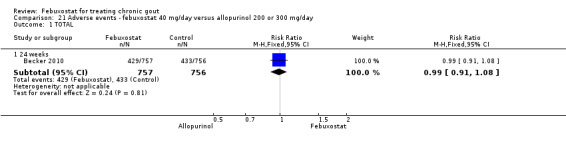
Comparison 21 Adverse events ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 1 TOTAL.
21.3. Analysis.
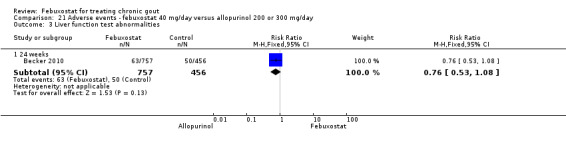
Comparison 21 Adverse events ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 3 Liver function test abnormalities.
21.4. Analysis.
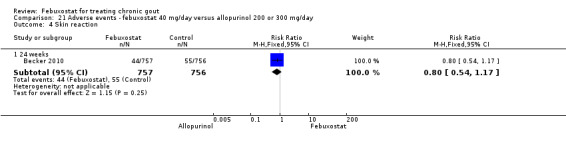
Comparison 21 Adverse events ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 4 Skin reaction.
21.5. Analysis.
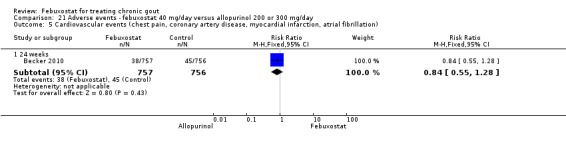
Comparison 21 Adverse events ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation).
21.6. Analysis.
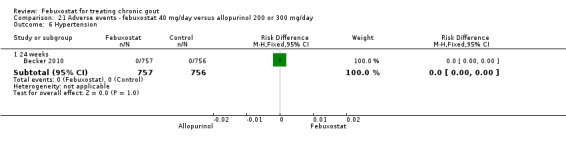
Comparison 21 Adverse events ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 6 Hypertension.
21.7. Analysis.
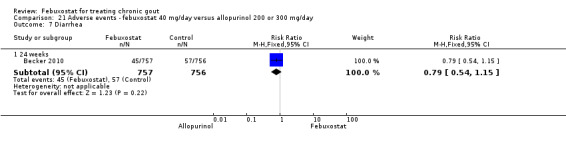
Comparison 21 Adverse events ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 7 Diarrhea.
22. Febuxostat 80 mg versus allopurinol
Three studies provided data on adverse events for this comparison (Becker 2005a; Becker 2010, Schumacher 2008). Total adverse events were less likely to be reported in the febuxostat group compared with patients in the allopurinol group (RR 0.94; 95% CI 0.89 to 0.99) (Analysis 22.1). In one study, hypertension was 4.4 times more likely to be reported in the febuxostat group compared to the allopurinol group (RR 4.4; 95% CI 1.3 to 15.1) (Analysis 22.6). No other statistically significant differences in adverse events were found between the two groups (Analysis 22.3, Analysis 22.4, Analysis 22.5, and Analysis 22.7).
22.1. Analysis.
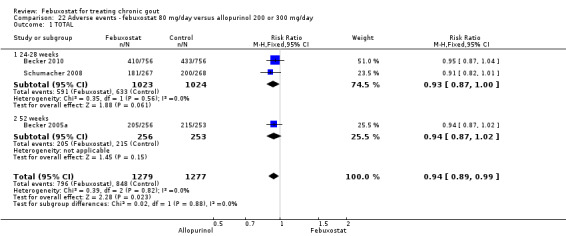
Comparison 22 Adverse events ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 1 TOTAL.
22.6. Analysis.
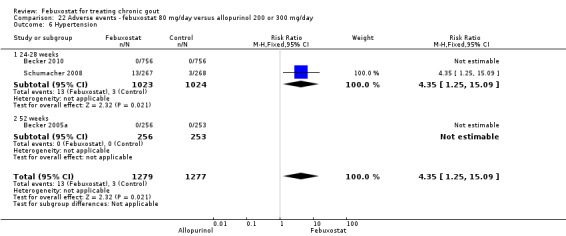
Comparison 22 Adverse events ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 6 Hypertension.
22.3. Analysis.
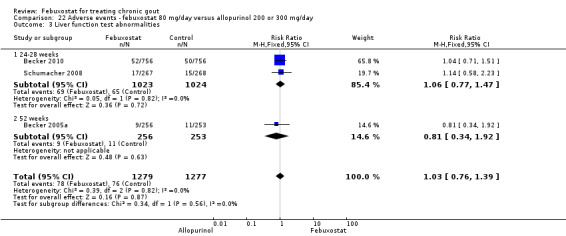
Comparison 22 Adverse events ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 3 Liver function test abnormalities.
22.4. Analysis.
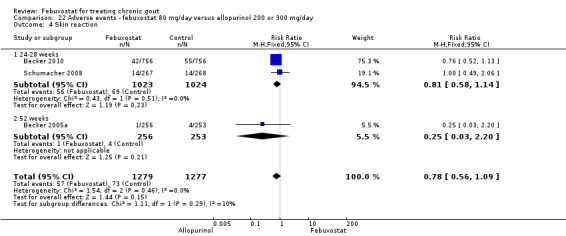
Comparison 22 Adverse events ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 4 Skin reaction.
22.5. Analysis.
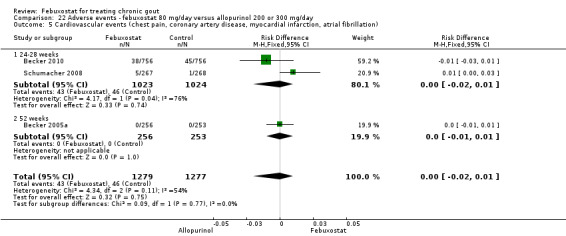
Comparison 22 Adverse events ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation).
22.7. Analysis.
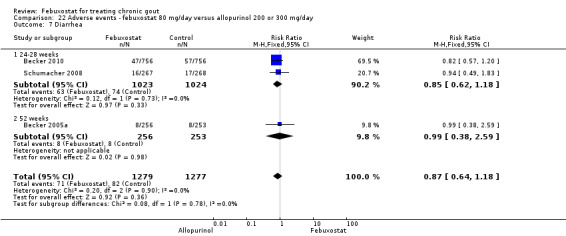
Comparison 22 Adverse events ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 7 Diarrhea.
23. Febuxostat 120 mg versus allopurinol
From data provided by Schumacher 2008 and Becker 2005a, febuxostat‐treated patients were 0.90 times less likely to report any adverse event compared to allopurinol‐treated patients (95% CI 0.84 to 0.96). No other statistically significant differences were noted between the two groups (Analysis 23.1,Analysis 23.3, Analysis 23.4, Analysis 23.5, Analysis 23.6, and Analysis 23.7).
23.1. Analysis.
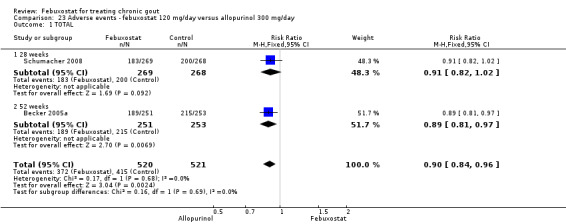
Comparison 23 Adverse events ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 1 TOTAL.
23.3. Analysis.
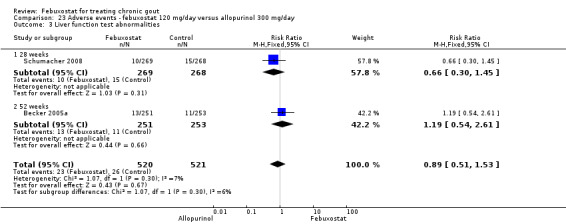
Comparison 23 Adverse events ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 3 Liver function test abnormalities.
23.4. Analysis.
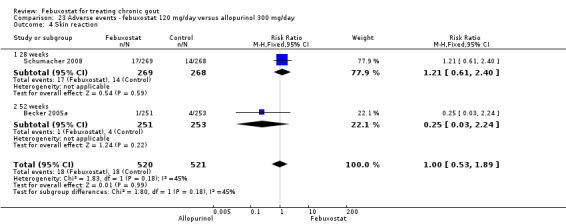
Comparison 23 Adverse events ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 4 Skin reaction.
23.5. Analysis.
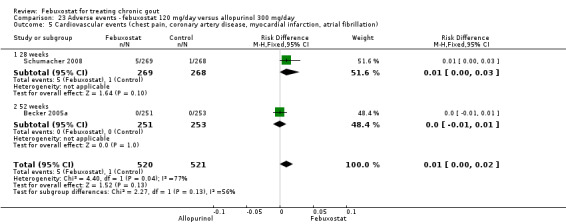
Comparison 23 Adverse events ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation).
23.6. Analysis.
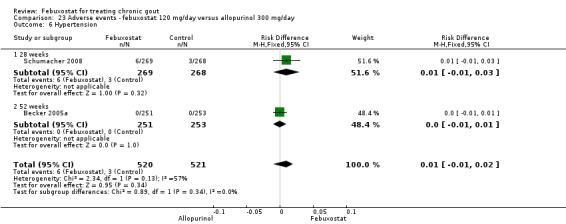
Comparison 23 Adverse events ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 6 Hypertension.
23.7. Analysis.
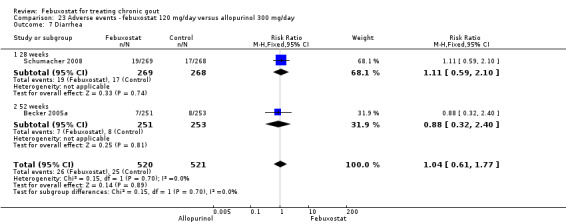
Comparison 23 Adverse events ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 7 Diarrhea.
24. Febuxostat 240 mg versus allopurinol
In Schumacher 2008, hypertension and diarrhoea were more likely to be reported in the febuxostat group compared to the allopurinol group, with hypertension being 4.0 times more likely to develop (95% CI 1.0 to 15.8) and diarrhoea twice as likely (RR 2.1; 95% CI 1.1 to 4.0) (Analysis 24.1, Analysis 24.3, Analysis 24.4, Analysis 24.5, Analysis 24.6, and Analysis 24.7).
24.1. Analysis.
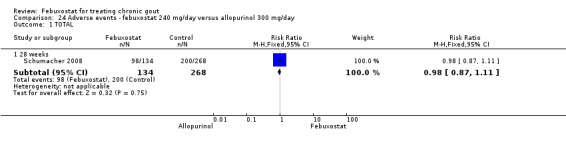
Comparison 24 Adverse events ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 1 TOTAL.
24.3. Analysis.
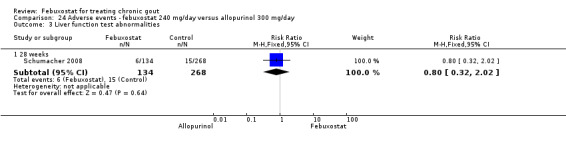
Comparison 24 Adverse events ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 3 Liver function test abnormalities.
24.4. Analysis.
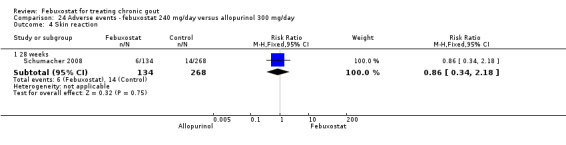
Comparison 24 Adverse events ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 4 Skin reaction.
24.5. Analysis.
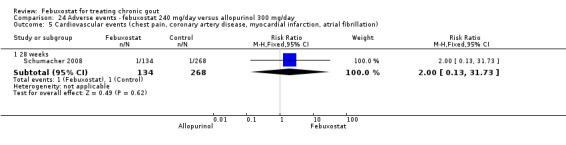
Comparison 24 Adverse events ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation).
24.6. Analysis.
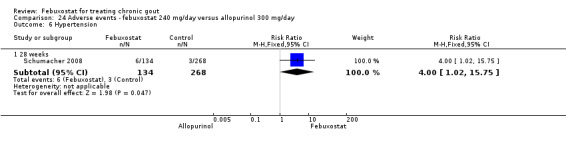
Comparison 24 Adverse events ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 6 Hypertension.
24.7. Analysis.
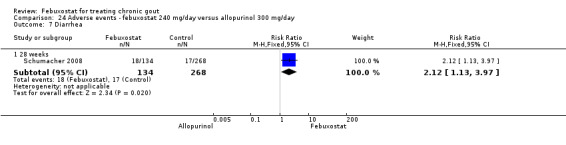
Comparison 24 Adverse events ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 7 Diarrhea.
Long‐term harms
There were no statistically significant differences regarding long‐term harms over three years between febuxostat 80 mg or 120 mg and the allopurinol group in the only OLT with a control group (Becker 2009). The adverse events rate per 100 patient years were 227, 216, and 246, respectively (Table 9).
3. Adverse events data from open label trials.
| AEs Rate per 100 patient‐year | ||
| Schumacher 2009 | Febuxostat 40 mg | 274 |
| Febuxostat 80 mg | 385 | |
| Febuxostat 120 mg | 259 | |
| Becker 2009 | Febuxostat 80 mg | 227 |
| Febuxostat 120 mg | 216 | |
| Allopurinol 300 mg | 245 |
Subgroup and sensitivity analysis
Analyses by different dosage, duration of treatment, and renal function did not result in different effect sizes. Subgroups based on severity of baseline disease, age, and disease duration were not performed because these characteristics were similar across groups. No statistically significant differences were observed when data were analysed by renal function or considering effect of study quality.
Discussion
This systematic review analysed and summarised evidence from all published RCTs of febuxostat for treating chronic gout. Four RCTs with 3978 participants were included, 2619 assigned to febuxostat, 172 to placebo, and 1187 to allopurinol. At the approved doses (40 mg, 80 mg, and 120 mg) febuxostat seemed to be beneficial in achieving serum uric acid levels < 6.0 mg/dL and reducing serum uric acid levels from baseline to the final visit. The benefit was noted for any dosage of febuxostat in comparison to placebo. When compared to allopurinol febuxostat showed benefit only at dosages of 80 and 120 mg. The incidence of gout flares requiring treatment may be increased in patients taking febuxostat compared to placebo or allopurinol during early treatment. However, no such increase in gout flares, compared to allopurinol, was observed in the long‐term follow‐up study. Additionally, trials did not report data on pain, functional assessment, quality of life, and patient and physician global assessments.
Summary of main results
For our primary outcome acute gout flares, although there was only a statistically significant increase when comparing febuxostat 120 mg versus placebo (absolute risk increase 15%; 95% CI 6% to 23%, and a number need to treat to harm of 6, 95% CI 4 to 17), there was a strong trend towards such higher rates when comparing febuxostat 80 mg to placebo and febuxostat 80 mg or 120 mg to allopurinol.
Compared to placebo, febuxostat 40 mg had an absolute treatment benefit of 55% and a number needed to treat (NNT) of 1.8 people to achieve serum uric acid levels < 6.0 mg/dL at the final visit. Thus, patients taking febuxostat were 40 times more likely to achieve serum uric acid levels < 6.0 mg/dL at the final visit (Table 1). For febuxostat 80 mg and 120 mg, the absolute treatment benefit was 75% and 87% and the NNT 2 and 1, respectively (Figure 4). Patients were 69 and 81 times more likely to achieve serum uric acid levels < 6.0 mg/dL at the final visit, respectively (Table 2, and Table 3).
4.
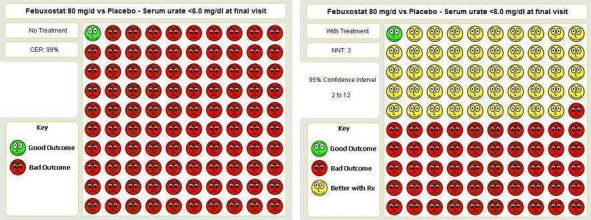
People not affected by a treatment (green faces for those with a good outcome and red for those with a bad outcome) People for which treatment changes their category from a bad outcome to a good outcome (yellow faces) People for which treatment causes an adverse event and changes their category from a good outcome to bad outcome (crossed out green faces)
Compared to allopurinol, febuxostat 40 mg showed no statistically significant difference between groups to achieve serum uric acid levels < 6.0 mg/dL at the final visit (Table 4). For febuxostat 80 mg and 120 mg, the absolute treatment benefit of achieving serum uric acid levels < 6.0 mg/dL at the final visit was 29% and 44% and the NNT was 3 people for both dosages (Figure 5). Patients were 1.8 and 1.2 times more likely to achieve serum uric acid levels < 6.0 mg/dL at the final visit (Table 5 and Table 6).
5.
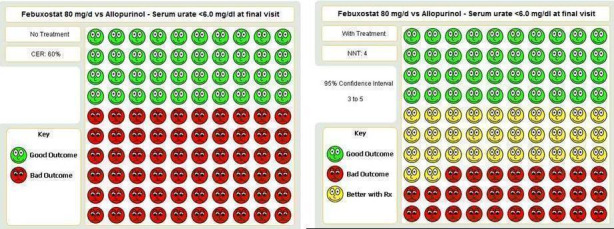
People not affected by a treatment (green faces for those with a good outcome and red for those with a bad outcome) People for which treatment changes their category from a bad outcome to a good outcome (yellow faces) People for which treatment causes an adverse event and changes their category from a good outcome to bad outcome (crossed out green faces)
For total discontinuation rates, compared to placebo only febuxostat 80 mg showed a significantly higher rate (11% absolute risk increase; 95% CI 6 to 16%, RR 1.4; 95% CI 1.2 to 1.8). Similarly, compared to allopurinol the rates were higher for febuxostat 80 mg and 120 mg (11% absolute risk increase; 95% CI 6 to 16%, RR 1.5; 95 % CI 1.2 to 1.8, and 20% absolute risk increase; 95% CI 3 to 14%, RR 2.6; 95% CI 2.0 to 3.3, respectively). There were no statistically significant differences between groups for discontinuation rates due to adverse events.
For total adverse events, there were no statistically significant differences except for a lower rate when comparing febuxostat 80 mg and 120 mg against allopurinol (ARR 6%; 95% CI 0.7 to 11%, RR 0.93; 95% CI 0.87 to 0.99, and ARR 8%; 95% CI 3 to 13%, RR 0.90; 95% CI 0.84 to 0.96, respectively). No differences were observed between groups for serious adverse events rates.
Overall completeness and applicability of evidence
We have included all febuxostat RCTs published to date. All outcomes reported by each trial were analysed. However, the studies only evaluated four of the efficacy outcome measures listed by OMERACT 9 (OMERACT 9). Five relevant outcomes were not assessed in any of the studies including pain, functional assessment, quality of life, and patient and physician global assessment.
Listed study inclusion criteria were broad and general. Participants in the majority of the included studies had chronic gout with normal renal function and mild to moderate renal dysfunction. Patients included in these trials may not represent typical gout patients seen in daily clinical practice, where alcohol intake can be higher than 14 drinks per week or concomitant medications can interfere with the metabolism of febuxostat. Furthermore, patients selected for RCTs generally have few major co‐morbidities.
On the other hand, included trials used a standard allopurinol dosage of 100 to 300 mg/day depending on renal function. This could contrast with what is considered best practice by major agencies guidelines (EULAR (Zhang 2006) and BSR (Jordan 2007)), which recommend dosage titration of allopurinol up to 900 mg daily when warranted to achieve the goal of a serum uric acid of < 6.0 mg/dL and if permitted by renal function. OMERACT 9 lists hyperuricaemia and incidence of gout flares as major outcome measures, among others, for RCTs including gout patients (OMERACT 9). In our review, all included studies had the goal of a serum uric acid of 6.0 mg/dL as the primary outcome and the incidence of gout flares, a more clinically relevant outcome, as a secondary one. While it is established that the target of uric lowering therapies in gout patients is maintaining uric acid levels at < 6.0 mg/dL, the indication to initiate such therapy is usually a clinical one, most commonly recurrent gout flares, and also uric acid renal stones or presence of tophi. Furthermore, all studies had a short‐term follow‐up, making the markedly higher incidence of gout flares in the febuxostat groups somewhat expected, especially when initiating uric lowering therapy with an increased pace of reducing uric acid levels. To be noted though, in the OLTs there were no observed differences in gout flares between the febuxostat and allopurinol groups.
Quality of the evidence
We used GRADEprofiler 3.6 to appraise the overall quality of evidence. Evidence quality ranged from low to high. There were two studies with high risk of bias in one item (ITT), one study included relatively few patients and few events providing wider confidence intervals, and all studies reported a surrogate endpoint as their primary outcome measure. Also, significant heterogeneity was noted in 20 out of the 59 analyses, ranging from 38% to 98%.
Regarding individual trial quality assessment, there were concerns about attrition bias given the dropout rates and some excluded participants from the efficacy analyses that were included in harms analyses. Of note, all studies failed to report on six of the nine outcome measures recommended by OMERACT, and were sponsored by the same pharmaceutical company; evidence shows that trials sponsored by industry may overestimate the treatment effect (Bhandari 2004).
A funnel plot was not created due to the limited number of studies included in this study.
Nonetheless, we concluded that the evidence provided in this review for short‐term serum uric acid levels is unlikely to have an important impact on our confidence in the estimate. For long‐term adverse events most trials that were included were not powered to detect adverse events, which can lead to type 2 error. Further studies are needed to assess long‐term harms.
Potential biases in the review process
This review included a comprehensive search performed by an experience information specialist. Two review authors independently reviewed all abstracts and titles, abstracted data, and performed bias and quality assessment. Consensus was reached by discussing any discrepancies. Therefore, errors in selection and abstraction are minimized. Published trial reports did not provide enough details to adequately assess risk of bias, and we are unable to determine fully if there is selective outcome reporting since we do not have access to the protocols for these studies. Additionally, since most studies are designed primarily for efficacy outcomes, analyses on harms are somewhat limited. A lack of differences in the harms outcomes may be due either to lack of power to detect differences or lack of difference in these outcomes.
A protocol was published for this review. All analyses were specified a priori. However, the presence of significant heterogeneity poses a threat to the combinability of the included studies. Also, only one of the included open label trials (OLT) had a comparison group, which prevented us from pooling long‐term data.
Agreements and disagreements with other studies or reviews
One health technology assessment, two reviews, one consensus statement, and one editorial have reviewed the evidence on the efficacy and harms of febuxostat for treating chronic gout.
Stevenson et al conducted a technology appraisal to evaluate the clinical and cost effectiveness of febuxostat (Stevenson 2011). The clinical evidence was derived from two RCTs. A simple pooled analysis of the individual patient‐level data from the two trials was undertaken. There was substantial uncertainty in the relationships reported by the manufacturer regarding serum uric acid levels and the incidence of gout flares, and underlying patient utility. The study concluded that febuxostat is an option for the management of chronic hyperuricaemia in gout only for people who are intolerant to allopurinol or for whom allopurinol is contraindicated. Jansen et al published a position statement based on three RCTs and one OLT and concluded that, based on the available data, febuxostat should not be considered as first line drug treatment in patients with gout and associated hyperuricaemia (Jansen 2010). In an editorial for Arthritis Research and Therapy, Dr Singh (Singh 2010), conducted a meta‐analysis to evaluate the cardiovascular risk with febuxostat treatment compared to allopurinol. Similar to our results, the author concluded that there were not statistically significant differences between febuxostat and allopurinol, although there was a non‐significant trend towards more serious adverse events with febuxostat compared to placebo. Furthermore, in a systematic review, Bruce et al found that febuxostat significantly reduces serum uric acid levels within two weeks after initiation of therapy, and up to 48% by the end of 104 weeks of therapy (Bruce 2006). Two thirds of the patients achieve serum uric acid levels < 6.0 mg/dL during the last three months following once‐daily administration of febuxostat 80 mg or 120 mg for at least 52 weeks. However, he listed abnormal function tests and diarrhoea as the most common adverse event. Similar to our findings he concluded that patients on febuxostat are at an increased risk of experiencing gout flares (up to 70%).
Authors' conclusions
Implications for practice.
Allopurinol is the first line treatment for chronic gout. However, both inadequate response and safety in patients with impaired renal function continue to be a concern. Febuxostat is approved at 40, 80, and 120 mg/day in the US and Europe. From our results, febuxostat has comparable efficacy as allopurinol. Thus, febuxostat is a promising alternative to allopurinol for the treatment of hyperuricaemia in chronic gout, especially in those who cannot tolerate allopurinol. Another benefit is the potential safety of febuxostat in patients with mild to moderate renal dysfunction. There are concerns about the initial increased rate of gout flares associated with the use of febuxostat, and the optimal length of prophylactic therapy is unclear.
Implications for research.
On‐going post‐marketing surveillance is required to determine the incidence of adverse events and sustainability of the treatment response. Also needed are studies comparing febuxostat to higher allopurinol doses, and its harms in patients with severe renal dysfunction.
History
Protocol first published: Issue 8, 2010 Review first published: Issue 11, 2012
| Date | Event | Description |
|---|---|---|
| 2 July 2010 | Amended | CMSG ID: C201‐R |
Notes
None
Acknowledgements
Stephanie Fulton, MSIS, AHIP, Assistant Director, Research Medical Library of The University of Texas M.D. Anderson Cancer Center, for providing comments on the search strategy. Elizabeth Ghogomu from the CMSG, for her assistance and valuable comments in the protocol development.
Appendices
Appendix 1. Search strategy
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1950 to Present> 201107.up.
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 gout/ (7747)
2 (gout or gout?).mp. (10434)
3 1 or 2 (10434)
4 Hyperuricemia/ (775)
5 hyperuricemi*.mp. (3483)
6 4 or 5 (3483)
7 3 or 6 (12596)
8 Febuxostat.af. (101)
9 144060‐53‐7.af. (60)
10 Uloric.af. (1)
11 "TMX‐67".af. (8)
12 "TEI 6720".af. (11)
13 Adenuric.af. (0)
14 UNII‐101V0R1N2E.af. (0)
15 "2‐3‐cyano‐4‐isobutoxyphenyl‐4‐methyl‐5‐thiazolecarboxylic acid".af. (5)
16 or/8‐15 (104)
17 16 and 3 (69)
18 16 and 6 (45)
19 18 not 17 (4)
20 18 or 17 (73)
Database: International Pharmaceutical Abstracts <1970 to July 2011> [201107.em]
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 gout/ (268)
2 (gout or gout?).mp. (412)
3 1 or 2 (412)
4 Hyperuricemia/ (33)
5 hyperuricemi*.mp. (216)
6 4 or 5 (216)
7 3 or 6 (536)
8 Febuxostat.af. (32)
9 144060‐53‐7.af. (32)
10 Uloric.af. (2)
11 "TMX‐67".af. (4)
12 "TEI 6720".af. (3)
13 Adenuric.af. (0)
14 UNII‐101V0R1N2E.af. (0)
15 "2‐3‐cyano‐4‐isobutoxyphenyl‐4‐methyl‐5‐thiazolecarboxylic acid".af. (0)
16 or/8‐15 (33)
17 16 and 3 (28)
18 16 and 6 (13)
19 18 not 17 (0)
20 18 or 17 (28)
21 from 20 keep 1‐28 (28)
Database: EMBASE <1980 to 2011 Week 31> [201131.up.]
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp gout/ (5306)
2 hyperuricemia/ (4546)
3 exp febuxostat/ (168)
4 144060‐53‐7.rn. (168)
5 Uloric.af. (7)
6 "TMX‐67".af. (19)
7 "TEI 6720".af. (19)
8 Adenuric.af. (11)
9 UNII‐101V0R1N2E.af. (0)
10 "2‐3‐cyano‐4‐isobutoxyphenyl‐4‐methyl‐5‐thiazolecarboxylic acid".af. (6)
11 or/3‐10 (169)
12 1 or 2 (8644)
13 11 and 12 (141)
14 from 13 keep 1‐141 (141)
Cochrane Database of Systematic Reviews, Cochrane Methodology Register, Database of Abstracts of Reviews of Effects, NHA Economic Evaluation Database, Health Technology Assessment Database
There are 0 results out of 5933 records for: "gout* or Hyperuricemi* and Febuxostat or 144060‐53‐7 or Uloric or TMX‐67 or TEI 6720 or Adenuric or UNII‐101V0R1N2E or "2‐3‐cyano‐4‐isobutoxyphenyl‐4‐methyl‐5‐thiazolecarboxylic acid" in Cochrane Database of Systematic Reviews"
There are 0 results out of 12200 records for: "gout* or Hyperuricemi* and Febuxostat or 144060‐53‐7 or Uloric or TMX67 or "TMX 67" or TMX‐67 or TEI6720 or "TEI 6720" or Adenuric or UNII‐101V0R1N2E or "2‐3‐cyano‐4‐isobutoxyphenyl‐4‐methyl‐5‐thiazolecarboxylic acid" in Cochrane Methodology Register"
There are 0 results out of 7596 records for: "gout* or Hyperuricemi* and Febuxostat or 144060‐53‐7 or Uloric or TMX67 or "TMX 67" or TMX‐67 or TEI6720 or "TEI 6720" or Adenuric or UNII‐101V0R1N2E or "2‐3‐cyano‐4‐isobutoxyphenyl‐4‐methyl‐5‐thiazolecarboxylic acid" in Record Title in Database of Abstacts of Reviews of Effects"
There are 0 results out of 27436 records for: "gout* or Hyperuricemi* and Febuxostat or 144060‐53‐7 or Uloric or TMX67 or "TMX 67" or TMX‐67 or TEI6720 or "TEI 6720" or Adenuric or UNII‐101V0R1N2E or "2‐3‐cyano‐4‐isobutoxyphenyl‐4‐methyl‐5‐thiazolecarboxylic acid" in NHS Economic Evaluation Database"
Cochrane Central Register of Controlled Trials
There are 5 results out of 600472 records for: "gout* or Hyperuricemi* and Febuxostat or 144060‐53‐7 or Uloric or TMX‐67 or TEI 6720 or Adenuric or UNII‐101V0R1N2E or "2‐3‐cyano‐4‐isobutoxyphenyl‐4‐
Health Technology Assessment
There are 3 results out of 7596 records for: "gout* or Hyperuricemi* and Febuxostat or 144060‐53‐7 or Uloric or TMX67 or "TMX 67" or TMX‐67 or TEI6720 or "TEI 6720" or Adenuric or UNII‐101V0R1N2E or "2‐3‐cyano‐4‐isobutoxyphenyl‐4‐methyl‐5‐thiazolecarboxylic acid" in Record Title in Health Technology Assessment Database
Data and analyses
Comparison 1. Efficacy ‐ febuxostat 40 mg/day versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of gout flares | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 28 days | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.52, 1.74] |
| 2 Serum uric acid <6.0 mg/dL at final visit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 28 days | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 40.11 [2.52, 639.08] |
| 3 Change in serum uric acid concentration from baseline at final visit | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 28 days | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐5.23 [‐5.78, ‐4.69] |
Comparison 2. Efficacy ‐ febuxostat 80 mg/day versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of gout flares | 2 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.96, 1.81] |
| 1.1 28 days | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.67, 2.00] |
| 1.2 8 weeks | 1 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.94, 2.04] |
| 2 Serum uric acid <6.0 mg/dL at final visit | 2 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 68.86 [13.79, 343.94] |
| 2.1 28 days | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 54.0 [3.42, 852.00] |
| 2.2 28 weeks | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 75.02 [10.65, 528.34] |
| 3 Change in serum uric acid concentration from baseline at final visit | 2 | 332 | Mean Difference (IV, Random, 95% CI) | ‐4.96 [‐6.23, ‐3.68] |
| 3.1 28 days | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐5.63 [‐6.24, ‐5.03] |
| 3.2 28 weeks | 1 | 260 | Mean Difference (IV, Random, 95% CI) | ‐4.33 [‐4.65, ‐4.02] |
Comparison 3. Efficacy ‐ febuxostat 120 mg/day versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of gout flares | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.26, 2.32] |
| 1.1 28 days | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.91, 2.49] |
| 1.2 8 weeks | 1 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.23, 2.60] |
| 2 Serum uric acid <6.0 mg/dL at final visit | 2 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 80.65 [16.04, 405.52] |
| 2.1 28 days | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 66.86 [4.26, 1050.04] |
| 2.2 28 weeks | 1 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 85.84 [12.20, 603.82] |
| 3 Change in serum uric acid concentration from baseline at final visit | 2 | 472 | Mean Difference (IV, Random, 95% CI) | ‐5.31 [‐5.68, ‐4.94] |
| 3.1 28 days | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐5.02 [‐5.60, ‐4.45] |
| 3.2 28 weeks | 1 | 403 | Mean Difference (IV, Random, 95% CI) | ‐5.44 [‐5.70, ‐5.17] |
Comparison 4. Efficacy ‐ febuxostat 240 mg/day versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of gout flares | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 28 weeks | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.76, 3.72] |
| 2 Serum urate <6.0 mg/dl at final visit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 28 weeks | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 93.04 [13.23, 654.45] |
| 3 Change in serum urate concentration from baseline at final visit | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 28 weeks | 1 | 268 | Mean Difference (IV, Fixed, 95% CI) | ‐6.31 [‐6.61, ‐6.00] |
Comparison 5. Efficacy ‐ Febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of gout flares | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 24 weeks | 1 | 1324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.57, 1.65] |
| 2 Serum urate <6.0 mg/dL at final visit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 24 weeks | 1 | 1324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.94, 1.21] |
Comparison 6. Efficacy ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of gout flares | 3 | 2325 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.98, 1.27] |
| 1.1 8 weeks | 1 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.91, 1.64] |
| 1.2 24 weeks | 1 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.92, 2.37] |
| 1.3 52 weeks | 1 | 462 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.15] |
| 2 Serum uric acid <6.0 mg/dL at final visit | 3 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [1.55, 2.09] |
| 2.1 24‐28 weeks | 2 | 1702 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.49, 1.93] |
| 2.2 52 weeks | 1 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [1.70, 2.45] |
| 3 Change in serum uric acid concentration from baseline at final visit | 2 | 1041 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐1.50, ‐0.99] |
| 3.1 8 weeks | 1 | 535 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐1.59, ‐1.16] |
| 3.2 52 weeks | 1 | 506 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.30, ‐0.94] |
Comparison 7. Efficacy ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of gout flares | 2 | 986 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.87, 1.91] |
| 1.1 8 weeks | 1 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.21, 2.08] |
| 1.2 52 weeks | 1 | 449 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.96, 1.24] |
| 2 Serum uric acid <6.0 mg/dL at final visit | 2 | 880 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.91, 2.45] |
| 2.1 28 weeks | 1 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.78, 2.52] |
| 2.2 52 weeks | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.83, 2.62] |
| 3 Change in serum uric acid concentration from baseline at final visit | 2 | 1038 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐2.19, ‐1.67] |
| 3.1 8 weeks | 1 | 537 | Mean Difference (IV, Random, 95% CI) | ‐2.07 [‐2.28, ‐1.85] |
| 3.2 52 weeks | 1 | 501 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐1.98, ‐1.62] |
Comparison 8. Efficacy ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of gout flares | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 28 weeks | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.72, 2.98] |
| 2 Serum uric acid <6.0 mg/dL at final visit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 28 weeks | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.94, 2.73] |
| 3 Change in serum uric acid concentration from baseline at final visit | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 8 weeks | 1 | 402 | Mean Difference (IV, Fixed, 95% CI) | ‐3.35 [‐3.61, ‐3.09] |
Comparison 9. Withdrawals ‐ febuxostat 40 mg/day versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 28 days | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.42] |
| 2 Adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 28 days | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.82] |
| 3 Gout flare | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 28 days | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.14] |
| 4 Lack of efficacy | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 28 days | 1 | 75 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 5 Other reasons | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 28 days | 1 | 75 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
Comparison 10. Withdrawals ‐ febuxostat 80 mg/day versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.01, 1.97] |
| 1.1 28 days | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.25, 8.06] |
| 1.2 28 weeks | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.01, 1.98] |
| 2 Adverse event | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.74, 4.46] |
| 2.1 28 days | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.9 [0.18, 20.10] |
| 2.2 28 weeks | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.69, 4.76] |
| 3 Gout flare | 2 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [0.05, 101.12] |
| 3.1 28 days | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.55] |
| 3.2 28 weeks | 1 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 13.60 [0.81, 227.06] |
| 4 Lack of efficacy | 2 | 479 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.03, 0.03] |
| 4.1 28 days | 1 | 78 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 4.2 28 weeks | 1 | 401 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.03, 0.03] |
| 5 Other reasons | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.76, 1.75] |
| 5.1 28 days | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 67.97] |
| 5.2 28 weeks | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.74, 1.72] |
Comparison 11. Withdrawals ‐ febuxostat 120 mg/day versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.48] |
| 1.1 28 days | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.74] |
| 1.2 28 weeks | 1 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.49] |
| 2 Adverse event | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.67, 4.08] |
| 2.1 28 days | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.14] |
| 2.2 28 weeks | 1 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.60, 4.26] |
| 3 Gout flare | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [0.40, 12.57] |
| 3.1 28 days | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.93] |
| 3.2 28 weeks | 1 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.5 [0.37, 114.53] |
| 4 Lack of efficacy | 2 | 479 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 4.1 28 days | 1 | 76 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 4.2 28 weeks | 1 | 403 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 5 Other reasons | 2 | 479 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.09, 0.05] |
| 5.1 28 days | 1 | 76 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 5.2 28 weeks | 1 | 403 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.06] |
Comparison 12. Withdrawals ‐ febuxostat 240 mg/day versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 28 weeks | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.00, 2.11] |
| 2 Adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 28 weeks | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.2 [0.79, 6.16] |
| 3 Gout flare | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 28 weeks | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.0 [0.99, 291.60] |
| 4 Lack of efficacy | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 28 weeks | 1 | 268 | Risk Ratio (IV, Fixed, 95% CI) | 0.67 [0.11, 3.93] |
| 5 Other reasons | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 28 weeks | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.66, 1.76] |
12.5. Analysis.
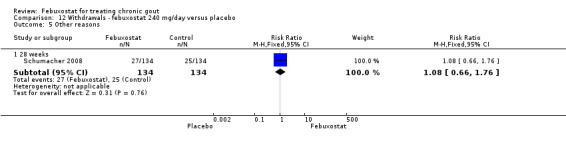
Comparison 12 Withdrawals ‐ febuxostat 240 mg/day versus placebo, Outcome 5 Other reasons.
Comparison 13. Withdrawals ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 24 weeks | 1 | 1513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.74, 1.15] |
| 2 Adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 24 weeks | 1 | 1513 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.90, 1.69] |
| 3 Gout flare | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 24 weeks | 1 | 1513 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.25, 8.94] |
| 4 Lack of efficacy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 24 weeks | 1 | 1513 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.94] |
| 5 Other reasons | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 24 weeks | 1 | 1512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.45] |
13.5. Analysis.
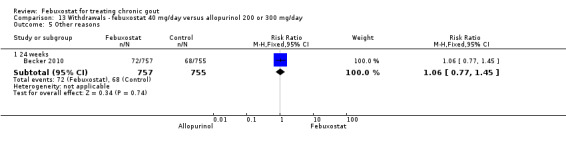
Comparison 13 Withdrawals ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 5 Other reasons.
Comparison 14. Withdrawals ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 3 | 2555 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.14, 1.51] |
| 1.1 24‐28 weeks | 2 | 2046 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.11, 1.54] |
| 1.2 52 weeks | 1 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.01, 1.72] |
| 2 Adverse event | 3 | 2555 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.80, 1.39] |
| 2.1 24‐28 weeks | 2 | 2046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.30] |
| 2.2 52 weeks | 1 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.86, 4.54] |
| 3 Gout flare | 3 | 2555 | Risk Ratio (M‐H, Random, 95% CI) | 2.99 [0.70, 12.79] |
| 3.1 24‐28 weeks | 2 | 2046 | Risk Ratio (M‐H, Random, 95% CI) | 5.79 [1.59, 21.05] |
| 3.2 52 weeks | 1 | 509 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.45, 2.66] |
| 4 Lack of efficacy | 3 | 2555 | Risk Ratio (M‐H, Random, 95% CI) | 3.08 [0.55, 17.20] |
| 4.1 24‐28 weeks | 2 | 2046 | Risk Ratio (M‐H, Random, 95% CI) | 3.08 [0.55, 17.20] |
| 4.2 52 weeks | 1 | 509 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Other reasons | 3 | 2555 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.10, 1.62] |
| 5.1 28 weeks | 2 | 2046 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.09, 1.75] |
| 5.2 52 weeks | 1 | 509 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.90, 1.74] |
Comparison 15. Withdrawals ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 2 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.12, 1.66] |
| 1.1 28 weeks | 1 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.89, 1.64] |
| 1.2 52 weeks | 1 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.16, 1.94] |
| 2 Adverse event | 2 | 1041 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.49, 5.03] |
| 2.1 28 weeks | 1 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.46, 1.70] |
| 2.2 52 weeks | 1 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 2.90 [1.32, 6.36] |
| 3 Gout flare | 2 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.42 [1.72, 6.81] |
| 3.1 28 weeks | 1 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.98 [0.72, 49.32] |
| 3.2 52 weeks | 1 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.14 [1.51, 6.51] |
| 4 Lack of efficacy | 2 | 1041 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.00, 0.01] |
| 4.1 28 weeks | 1 | 537 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.01, 0.02] |
| 4.2 52 weeks | 1 | 504 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 5 Other reasons | 2 | 1042 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.87, 1.47] |
| 5.1 28 weeks | 1 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.91, 1.99] |
| 5.2 52 weeks | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.68, 1.39] |
Comparison 16. Withdrawals ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 28 weeks | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.22, 2.33] |
| 2 Adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 28 weeks | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.59, 2.51] |
| 3 Gout flare | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 28 weeks | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.0 [2.02, 126.61] |
| 4 Lack of efficacy | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 28 weeks | 1 | 402 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 5 Other reasons | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 28 weeks | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.93, 2.29] |
Comparison 17. Adverse events ‐ febuxostat 40 mg/day versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 28 days | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.16, 2.40] |
| 2 Serious | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 28 days | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Liver function test abnormalities | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 28 days | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.13 [0.25, 103.41] |
| 4 Skin reaction | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 28 days | 1 | 75 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation) | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 28 days | 1 | 75 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 6 Hypertension | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 28 days | 1 | 75 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 7 Diarrhea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 28 days | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.74] |
17.2. Analysis.
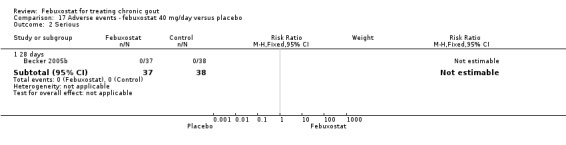
Comparison 17 Adverse events ‐ febuxostat 40 mg/day versus placebo, Outcome 2 Serious.
Comparison 18. Adverse events ‐ febuxostat 80 mg/day versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.09] |
| 1.1 28 days | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.46, 3.83] |
| 1.2 28 weeks | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.07] |
| 2 Serious | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [0.72, 10.72] |
| 2.1 28 days | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 67.97] |
| 2.2 28 weeks | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.62, 12.28] |
| 3 Liver function test abnormalities | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.92, 8.82] |
| 3.1 28 days | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 67.97] |
| 3.2 28 weeks | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.85, 9.53] |
| 4 Skin reaction | 2 | 479 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.04, 0.04] |
| 4.1 28 days | 1 | 78 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 4.2 28 weeks | 1 | 401 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.05, 0.05] |
| 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation) | 2 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 28 days | 1 | 78 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 5.2 28 weeks | 1 | 401 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 6 Hypertension | 2 | 479 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 6.1 28 days | 1 | 78 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 6.2 28 weeks | 1 | 401 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 7 Diarrhea | 2 | 479 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 7.1 28 days | 1 | 78 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.11, 0.15] |
| 7.2 28 weeks | 1 | 401 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.08, 0.03] |
18.2. Analysis.
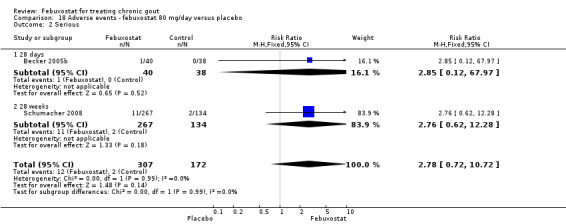
Comparison 18 Adverse events ‐ febuxostat 80 mg/day versus placebo, Outcome 2 Serious.
Comparison 19. Adverse events ‐ febuxostat 120 mg/day versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.10] |
| 1.1 28 days | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.49, 4.02] |
| 1.2 28 weeks | 1 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.07] |
| 2 Serious | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [0.70, 10.20] |
| 2.1 28 days | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.80] |
| 2.2 28 weeks | 1 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.49, 10.23] |
| 3 Liver function test abnormalities | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.56, 5.86] |
| 3.1 28 days | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.40] |
| 3.2 28 weeks | 1 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.46, 5.93] |
| 4 Skin reaction | 2 | 479 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 4.1 28 days | 1 | 76 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 4.2 28 weeks | 1 | 403 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation) | 2 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 28 days | 1 | 76 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 5.2 28 weeks | 1 | 403 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 6 Hypertension | 2 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 28 days | 1 | 76 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 6.2 28 weeks | 1 | 403 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.08, 0.01] |
| 7 Diarrhea | 2 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 28 days | 1 | 76 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.12, 0.12] |
| 7.2 28 weeks | 1 | 403 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.07, 0.04] |
19.2. Analysis.
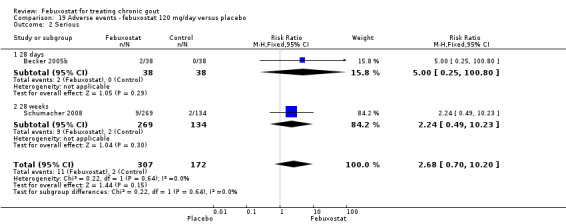
Comparison 19 Adverse events ‐ febuxostat 120 mg/day versus placebo, Outcome 2 Serious.
Comparison 20. Adverse events ‐ febuxostat 240 mg/day versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.87, 1.17] |
| 1.1 28 weeks | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.87, 1.17] |
| 2 Serious | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.49, 12.66] |
| 2.1 28 weeks | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.49, 12.66] |
| 3 Liver function test abnormalities | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 28 weeks | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.51, 7.83] |
| 4 Skin reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 28 weeks | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.48] |
| 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 28 weeks | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.82] |
| 6 Hypertension | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 28 weeks | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.27, 2.10] |
| 7 Diarrhea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 28 weeks | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.80, 3.33] |
20.2. Analysis.
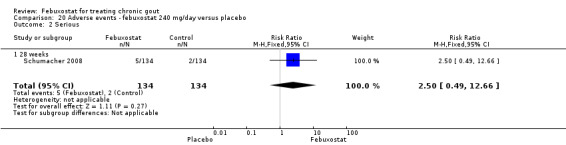
Comparison 20 Adverse events ‐ febuxostat 240 mg/day versus placebo, Outcome 2 Serious.
Comparison 21. Adverse events ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 24 weeks | 1 | 1513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.08] |
| 2 Serious | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 24 weeks | 1 | 1513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.35, 1.07] |
| 3 Liver function test abnormalities | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 24 weeks | 1 | 1213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.53, 1.08] |
| 4 Skin reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 24 weeks | 1 | 1513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.54, 1.17] |
| 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 24 weeks | 1 | 1513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.28] |
| 6 Hypertension | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 24 weeks | 1 | 1513 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.00, 0.00] |
| 7 Diarrhea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 24 weeks | 1 | 1513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.54, 1.15] |
21.2. Analysis.
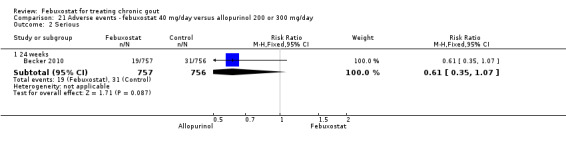
Comparison 21 Adverse events ‐ febuxostat 40 mg/day versus allopurinol 200 or 300 mg/day, Outcome 2 Serious.
Comparison 22. Adverse events ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 3 | 2556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.89, 0.99] |
| 1.1 24‐28 weeks | 2 | 2047 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| 1.2 52 weeks | 1 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 2 Serious | 3 | 2556 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.55, 1.42] |
| 2.1 24‐28 weeks | 2 | 2047 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.65, 1.65] |
| 2.2 52 weeks | 1 | 509 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.28, 1.18] |
| 3 Liver function test abnormalities | 3 | 2556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.76, 1.39] |
| 3.1 24‐28 weeks | 2 | 2047 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.47] |
| 3.2 52 weeks | 1 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.34, 1.92] |
| 4 Skin reaction | 3 | 2556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.09] |
| 4.1 24‐28 weeks | 2 | 2047 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.58, 1.14] |
| 4.2 52 weeks | 1 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.20] |
| 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation) | 3 | 2556 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 5.1 24‐28 weeks | 2 | 2047 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 5.2 52 weeks | 1 | 509 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 6 Hypertension | 3 | 2556 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.35 [1.25, 15.09] |
| 6.1 24‐28 weeks | 2 | 2047 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.35 [1.25, 15.09] |
| 6.2 52 weeks | 1 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Diarrhea | 3 | 2556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.64, 1.18] |
| 7.1 24‐28 weeks | 2 | 2047 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.18] |
| 7.2 52 weeks | 1 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.38, 2.59] |
22.2. Analysis.
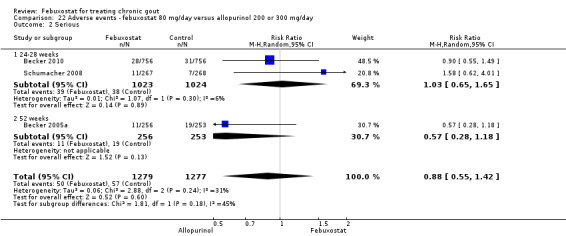
Comparison 22 Adverse events ‐ febuxostat 80 mg/day versus allopurinol 200 or 300 mg/day, Outcome 2 Serious.
Comparison 23. Adverse events ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 2 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.96] |
| 1.1 28 weeks | 1 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.82, 1.02] |
| 1.2 52 weeks | 1 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.81, 0.97] |
| 2 Serious | 2 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.70, 1.93] |
| 2.1 28 weeks | 1 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.48, 3.39] |
| 2.2 52 weeks | 1 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.61, 2.02] |
| 3 Liver function test abnormalities | 2 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.51, 1.53] |
| 3.1 28 weeks | 1 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.30, 1.45] |
| 3.2 52 weeks | 1 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.54, 2.61] |
| 4 Skin reaction | 2 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.53, 1.89] |
| 4.1 28 weeks | 1 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.61, 2.40] |
| 4.2 52 weeks | 1 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.24] |
| 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation) | 2 | 1041 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.00, 0.02] |
| 5.1 28 weeks | 1 | 537 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.00, 0.03] |
| 5.2 52 weeks | 1 | 504 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 6 Hypertension | 2 | 1041 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.01, 0.02] |
| 6.1 28 weeks | 1 | 537 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 6.2 52 weeks | 1 | 504 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 7 Diarrhea | 2 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.61, 1.77] |
| 7.1 28 weeks | 1 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.59, 2.10] |
| 7.2 52 weeks | 1 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.32, 2.40] |
23.2. Analysis.
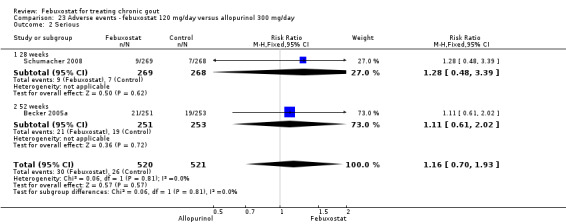
Comparison 23 Adverse events ‐ febuxostat 120 mg/day versus allopurinol 300 mg/day, Outcome 2 Serious.
Comparison 24. Adverse events ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TOTAL | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 28 weeks | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.87, 1.11] |
| 2 Serious | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 28 weeks | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.46, 4.42] |
| 3 Liver function test abnormalities | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 28 weeks | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.32, 2.02] |
| 4 Skin reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 28 weeks | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.34, 2.18] |
| 5 Cardiovascular events (chest pain, coronary artery disease, myocardial infarction, atrial fibrillation) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 28 weeks | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.13, 31.73] |
| 6 Hypertension | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 28 weeks | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [1.02, 15.75] |
| 7 Diarrhea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 28 weeks | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.13, 3.97] |
24.2. Analysis.
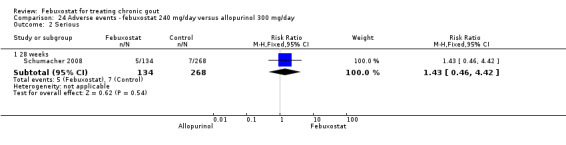
Comparison 24 Adverse events ‐ febuxostat 240 mg/day versus allopurinol 300 mg/day, Outcome 2 Serious.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Becker 2005a.
| Methods | Phase 3, randomised, double‐blind, allopurinol‐controlled trial. 52‐week, multicentre study. No of patients randomised = 762. No of patients analysed = 756 (2 withdrew without receiving study drug, 4 excluded because baseline serum uric acid was < 8.0mg/dL). |
|
| Participants | Inclusion criteria: preliminary criteria of the ACR for gout and serum uric acid ≥ 8.0 mg/dL. Adults. Exclusion criteria: serum creatinine > 1.5 mg/dL or estimated creatinine clearance rate < 50 ml/min; pregnancy or lactation; use of uric acid‐lowering agents, azathioprine, 6‐mercaptopurine, thiazide diuretics, or medications containing aspirin (> 325 mg/day) or other salicylates; BMI > 50; history of xanthinuria, active liver disease, or hepatic dysfunction; use of prednisone at > 10 mg/day; change in hormone‐replacement therapy or oral‐contraceptive therapy within the previous 3 months; and history of alcohol abuse or alcohol intake of more than 14 drinks/week. Location: Centers in the USA and Canada. |
|
| Interventions | Patient randomised to 3 groups 1. Febuxostat 80 mg/day N = 257(256 received ≥1dose) 2. Febuxostat 120 mg/day N = 251 3. Allopurinol 300 mg/day N = 254 (253 received ≥1dose) Two‐weeks washout period before randomisation for subjects already on uric acid‐lowering therapy. Prophylaxis with naproxen (250 mg BID) or colchicine (0.6 mg daily) given to all subjects during the washout period and the first 8 weeks of the trial. Subsequent gout flares treated at the investigators' discretion. Duration: 52 weeks. |
|
| Outcomes |
Primary efficacy endpoint: ‐ serum uric acid < 6.0 mg/dL at each of the last three monthly measurements. Secondary efficacy endpoints: ‐ proportion of subjects with serum uric acid < 6.0 mg/dL at each visit ‐ percentage reduction from baseline in the serum uric acid concentration at each visit. ‐ percentage reduction from baseline in tophus area. ‐ change in the number of tophi at each visit. ‐ proportion of subjects requiring treatment for gout flares from weeks 9 through 52. Adverse events. |
|
| Notes | Source of funding: TAP Pharmaceutical Products, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated central randomisation schedule with a block size of three was used to assign each subject to one of the three groups. |
| Allocation concealment (selection bias) | Low risk | Not described, but considered probable due to central randomisation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details provided other than stating that this is a double‐blind trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some study participants were not accounted for in the efficacy analysis: 4 subjects excluded because they had sUA < 8mg/dL at baseline and 3 because they did not receive the study treatment. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, but the authors did not report on 5 of the 9 outcomes recommended by OMERACT (OMERACT 9). |
| Other bias | Low risk | Representatives of TAP Pharmaceutical Products collected the data, and statisticians at TAP conducted all statistical analyses. |
Becker 2005b.
| Methods | Phase 2, randomised, double‐blind, placebo‐controlled, dose‐response trial. 28‐day multicentre study. No of patients randomised = 153. No of patients analysed for efficacy = 140 ( 13 excluded because baseline serum uric acid was collected outside the day ‐2 window). No of patients analysed for harms and gout flares = 153. |
|
| Participants | Inclusion criteria: preliminary criteria of the ACR for gout and serum uric acid ≥ 8.0 mg/dL. Exclusion criteria: serum creatinine > 1.5 mg/dL or estimated creatinine clearance (eCLcr) < 50 ml/min; pregnancy or lactation; use of uric acid‐lowering agents, azathioprine, 6‐mercaptopurine, or medications containing aspirin (> 325 mg/day) or other salicylates; BMI > 50; history of xanthinuria, active liver disease, or hepatic dysfunction; use of prednisone at > 10 mg/day; change in thiazide or steroid therapy (within 1 month of study) or hormone‐replacement therapy or oral‐contraceptive therapy (within 3 months of study); and history of alcohol abuse or alcohol intake of ≥ 14 drinks/week. Location: centres in the USA. |
|
| Interventions | Patient randomised to 4 groups: 1. Febuxostat 40 mg/day N = 37 2. Febuxostat 80 mg/day N = 40 3. Febuxostat 120 mg/day N = 38 4.Placebo N = 38 Two‐weeks washout period before randomisation for subjects already on uric acid‐lowering therapy. Prophylaxis with colchicine (0.6 mg BID) given to all subjects during the washout period and the first 2 weeks of the trial. Subsequent gout flares treated at the investigators' discretion. |
|
| Outcomes |
The primary efficacy endpoint: ‐ serum uric acid < 6.0 mg/dL at day 28. Secondary efficacy endpoints: ‐ proportion of subjects with serum uric acid < 6.0 mg/dL at each visit. ‐ percentage reduction from baseline in the serum uric acid concentration at day 28. ‐ incidence of gout flares. Adverse events. |
|
| Notes | Source of funding: TAP Pharmaceutical Products, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Reported as a double‐blind trial, but methods of masking were not described (only 2 weeks of double blind treatment). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Efficacy analyses were based on a modified intention‐to‐treat population (only those that received treatment). There is no description on how incomplete outcome data was analysed |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, but the authors did not report on 6 of the 9 outcomes recommended by OMERACT(OMERACT 9). |
| Other bias | Low risk | Drs Becker, Schumacher, and Wortmann have received consulting fees from TAP Pharmaceutical Products, but role of the sponsor was not stated. 4 of the 7 authors are from TAP Pharmaceutical Products. |
Becker 2009.
| Methods | Open label extension study (3 years findings). No of patients randomised =1280 subjects who previously completed one of the 2 Phase III double‐blind trials (Becker 2005a; Schumacher 2008). No. of patients completing the study = 1086. No. of patients analysed for harms and gout flares = 664 |
|
| Participants | Inclusion criteria: preliminary criteria of the ACR for gout. Exclusion criteria: pregnancy or lactation; serious drug‐related AE in the prior study; other significant medical conditions that would interfere with treatment harms or compliance; or known intolerance to allopurinol.. Location: 174 centres in the USA and Canada. |
|
| Interventions | 3 groups: 1. Febuxostat 80 mg/day N = 606 2. Febuxostat 120 mg/day N = 388 3.Allopurinol N = 92 During the first six months of treatment, subjects could switch their febuxostat doses if necessary. Subsequent gout flares treated at the investigators' discretion. |
|
| Outcomes |
The primary efficacy endpoint: ‐ serum uric acid < 6.0 mg/dL at each visit. Secondary efficacy endpoints: ‐ proportion of subjects with serum uric acid < 6.0 mg/dL at each visit. ‐ percentage reduction from baseline in the serum uric acid concentration at each visit. ‐ incidence of gout flares. ‐ reduction in number of tophi ‐ reduction in the size or disappearance of the index tophus Adverse events. |
|
| Notes | Source of funding: TAP Pharmaceutical Products, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Open label extension from FACT and APEX studies |
| Allocation concealment (selection bias) | Low risk | Open label extension from FACT and APEX studies |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label extension study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Efficacy analyses were based on a modified intention‐to‐treat population (only those that received treatment). There is no description on how incomplete outcome data was analysed |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, but the authors did not report on 5 of the 9 outcomes recommended by OMERACT(OMERACT 9). |
| Other bias | Unclear risk | Source of funding: TAP Pharmaceutical Products, Inc. |
Becker 2010.
| Methods | Randomized, double‐blind, allopurinol‐controlled. 6‐month multicentre study. Stratification by renal function ( normal, mildly impaired = eCLcr 60 to 89 ml/min, or moderately impaired = eCLcr 30 to 59 ml/min) and prior completion of either of two open‐label febuxostat or febuxostat/allopurinol extension trials. No of patients randomised = 2269. No of patients analysed (modified intent‐to‐treat cohort) = 2268 ( one subject randomised to allopurinol was excluded from the efficacy analyses because baseline sUA was < 8.0 mg/dL). |
|
| Participants | Inclusion criteria: preliminary criteria of the ACR for gout, serum uric acid of ≥ 8.0 mg/dL, age 18‐85. Subjects successfully completing either of two prior open label extension studies were eligible (subjects from FACT, FOCUS, APEX were eligible for one of these two open label studies) . Exclusion criteria: secondary hyperuricaemia; xanthinuria; severe renal dysfunction (eCLcr < 30 ml/min);hepatic dysfunction (ALT and AST > 1.5 times upper limit of normal);consumption of more than 14 alcoholic drinks per week or a history of alcoholism or drug abuse within five years;or a medical condition that, in the investigator's opinion, would interfere with treatment, harms, or adherence to the protocol. Location: centres in the USA. |
|
| Interventions | Patient randomised to 3 groups: 1. Febuxostat 40 mg/day N = 757 2. Febuxosatat 80 mg/day N = 756 3. Allopurinol 200/300 mg (for moderately impaired/ normal to mildly impaired renal function respectively) N = 755 (145/610) [modified intent‐to‐treat cohort]. 30‐day washout period before randomisation for subjects already on uric acid‐lowering therapy. Prophylaxis with naproxen (250 mg BID) or colchicine (0.6 mg daily) given to all subjects during the washout period and the study period (6 months). All subjects receiving naproxen prophylaxis also received lansoprazole 15 mg daily. Subjects with eCLcr < 50 ml/min were not to receive naproxen. Gout flares were regarded as expected gout manifestations rather than as AEs. Serum uric acid was blinded after baseline determination at Day‐4. |
|
| Outcomes |
The primary efficacy endpoint: ‐ serum uric acid < 6.0 mg/dL at the final visit. Treatment with febuxostat 40 mg was compared with allopurinol with regard to non‐inferiority in uric acid lowering. If non‐inferiority of febuxostat 40 mg was established, superiority to allopurinol was to be assessed. Treatment with febuxostat 80 mg was compared with the allopurinol and febuxostat 40 mg for superiority. "In subgroup analyses, the primary endpoint was stratified by baseline serum uric acid, renal functional status, presence of tophi at baseline, and prior participation in a ULT trial" Secondary efficacy endpoints: ‐ patients with renal impairment and serum uric acid < 6.0 mg/dL at the final visit. ‐ patients with serum uric acid < 6.0mg/dL, < 5.0mg/dL, < 4.0mg/dL at each visit. Pairwise comparisons were made between treatment groups. Adverse events. |
|
| Notes | Source of funding: TAP Pharmaceutical Products, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors stated that "An Interactive Voice Response System was utilized by site personnel during screening visits to initiate double blind randomisation. Subjects were randomised 1:1:1 on Day 1 to receive daily febuxostat 40 mg, febuxostat 80 mg, or allopurinol". |
| Allocation concealment (selection bias) | Low risk | No details provided, but considered probable due to randomisation type. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details provided other than stating that this is a double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one subject out of 2268 was not included in the efficacy analysis because his serum uric acid was < 8 mg/dL; the rest included in a modified ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, but the authors did not report on 5 of the 9 outcomes recommended by OMERACT (OMERACT 9). |
| Other bias | Low risk | Source of funding: TAP Pharmaceutical Products, Inc. |
Schumacher 2008.
| Methods | Phase 3, randomised, double‐blind, allopurinol‐ and placebo‐controlled, parallel‐group trial. 28‐week multicentre study. 2:2:1:2:1 febuxostat 80 mg, febuxostat 120 mg, febuxostat 240 mg, allopurinol, or placebo of random assignment. Stratification by renal function ( serum creatinine ≤ 1.5mg/dL or >1.5mg/dL to ≤ 2mg/dL). No of patients randomised = 1072. No of patients analysed = 1072. |
|
| Participants | Inclusion criteria: preliminary criteria of the ACR for gout, serum uric acid concentrations of ≥ 8.0 mg/dL, age 18‐85, serum creatinine ≤ 2mg/dL. Exclusion criteria: intolerance to allopurinol, naproxen, or colchicine; history of renal calculi; alcohol intake of ≥ 14 drinks/week; hepatic dysfunction (ALT and AST > 1.5 times upper limit of normal); any other significant medical conditions. Location: centres in the USA. |
|
| Interventions | Patient randomised to 5 groups: 1. Febuxostat 80 mg/day N = 267 2. Febuxostat 120 mg/day N = 269 3. Febuxostat 240 mg/day N = 134 4. Allopurinol 100 mg/day or 300 mg/day ( for serum creatinine > 1.5 mg/dL to ≤ 2 mg/dL or ≤ 1.5 mg/dL respectively) N = 268 5. Placebo N = 134 Two‐weeks washout period before randomisation for subjects already on uric acid‐lowering therapy. Prophylaxis with naproxen (250 mg BID) or colchicine (0.6 mg daily) given to all subjects during the washout period and the first 8 weeks of the trial. Subsequent gout flares treated at the investigators' discretion. |
|
| Outcomes |
The primary efficacy endpoint: ‐ serum uric acid < 6.0 mg/dL at each of the last three monthly measurements. Secondary efficacy endpoints: ‐ proportion of subjects with serum uric acid < 6.0 mg/dL at each visit. ‐ percentage reduction from baseline in the serum uric acid concentration at each visit. ‐ percentage reduction from baseline in tophus area.. ‐ proportion of subjects requiring treatment for gout flares from weeks 8 through 28. ‐ change in the number of tophi at each visit ‐ percent reduction in primary tophus size at each visit. Adverse events. |
|
| Notes | Study supported by Takeda Global Research & Development Center, Inc. (of which TAP Pharmaceutical Products, Inc., is a subsidiary). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated that “subjects were randomised in a 2:2:1:2:1 ratio to once‐daily febuxostat 80 mg, febuxostat 120 mg, febuxostat 240 mg, allopurinol, or placebo".The randomisation was stratified by renal function, but not further details about the sequence generation were given. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details were provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All efficacy analyses were performed on the intention‐to‐treat population. If a subject discontinued the study before ≥ 3 serum uric acid levels were obtained, the subject was considered a non‐responder. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, but the authors did not report on 5 of the 9 outcomes recommended by OMERACT(OMERACT 9). |
| Other bias | Low risk | Study supported by a pharmaceutical company. Representatives of Takeda Global Research & Development Center, Inc., collected the data and statisticians at Takeda Global Research & Development Center, Inc., conducted all statistical analyses |
Schumacher 2009.
| Methods | Open label extension study (5 years findings). No. of patients randomised =145 subjects who previously completed one of the 1 Phase II double‐blind trial (Becker 2005b). No. of patients completing the study.=.116. No. of patients analysed for harms and gout flares.= 116 |
|
| Participants | Inclusion criteria: enrolment and completion of the 28‐day Phase II study. Exclusion criteria: pregnancy or lactation;use of uric acid‐lowering agents, azathioprine, 6‐mercaptopurine, or medications containing aspirin (> 325 mg/day) or other salicylates; BMI > 50; history of xanthinuria, active liver disease, or hepatic dysfunction; use of prednisone at > 10 mg/day; change in thiazide or steroid therapy (within 1 month of study) or hormone‐replacement therapy or oral‐contraceptive therapy (within 3 months of study); and history of alcohol abuse or alcohol intake of ≥ 14 drinks/week. Location: centres in the USA. |
|
| Interventions | 3 groups: 1. Febuxostat 40 mg/day N = 8 2. Febuxostat 80 mg/day N = 79 3. Febuxostat 120 mg/day N = 29 Subsequent gout flares treated at the investigators' discretion. |
|
| Outcomes |
The primary efficacy endpoint: ‐ proportion of subjects with serum uric acid < 6.0 mg/dL at each visit. Secondary efficacy endpoints: ‐ percentage reduction from baseline in the serum uric acid concentration at each visit. ‐ incidence of gout flares. ‐ disappearance of the index tophus Adverse events. |
|
| Notes | Source of funding: TAP Pharmaceutical Products, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Open label extension from FOCUS study |
| Allocation concealment (selection bias) | Unclear risk | Open label extension from FOCUS study |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label extension study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Efficacy analyses were based on a modified intent‐to‐treat population (only those that received treatment). There is no description on how incomplete outcome data was analysed |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, but the authors did not report on 5 of the 9 outcomes recommended by OMERACT(OMERACT 9). |
| Other bias | Low risk | Source of funding: TAP Pharmaceutical Products, Inc. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Becker 2008 | Meta‐analysis of individual data |
| Komoriya 2004 | Pharmacokinetics (Phase 2) |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00821392.
| Methods | Phase 3, randomised, double‐blind, allopurinol‐ and placebo‐controlled, parallel‐group trial. No. randomised = 181 |
| Participants | Inclusion criteria:18 Years to 85 Years; female: either postmenopausal for at least 2 years, surgically sterile, or using a medically accepted means of contraception; negative serum pregnancy test; satisfy ARA; serum creatinine ≤ 1.5 mg/dL <Day ‐1>; serum uric acid level ≥ 8.0mg/dL Exclusion Criteria: women who are breast‐feeding or pregnant; xanthinuria; allopurinol intolerance; thiazide diuretic therapy; secondary hyperuricaemia; required > 10mg/day of prednisone during the study; therapy containing aspirin or other salicylates (stable low doses aspirin allowed); change in hormone replacement therapy or oral contraceptive therapy within 3 months of the screening visit; alcohol abuse within 5 years prior to the Screening; more than14 alcoholic beverages per week;therapy with any uric acid‐lowering therapy; active liver disease or hepatic dysfunction; unable to take colchicine; rheumatoid arthritis or any active arthritis; cancer (other than basal cell carcinoma of the skin) within 5 years prior to the screening visit; participated in another investigational trial within the 30days prior to the screening visit; significant medical condition |
| Interventions | Patients randomised to 5 groups: 1. Febuxostat 40 mg/day 2. Febuxostat 80 mg/day 3. Febuxostat 120 mg/day 4. Allopurinol 300mg/day 5. Placebo Colchicine 0.6 mg QD is given to minimize the risk of gout flares during the washout/run‐in period and during the study period |
| Outcomes | harms/Efficacy Study |
| Notes | No Contacts or Locations Provided Responsible Party: SK Chemicals Co.,Ltd Health Authority: Korea: Food and Drug Administration |
Characteristics of ongoing studies [ordered by study ID]
NCT01078389.
| Trial name or title | A Multicenter, Randomized, Double‐Blind, Phase 2 Study to Evaluate the Effect of Febuxostat Versus Placebo in Joint Damage in Hyperuricemic Subjects With Early Gout |
| Methods | Phase 2, randomised, double‐blind, parallel assignment efficacy trial. 24 months multicentre study Estimated enrolment 240 |
| Participants | Inclusion criteria: ≥18 years old; preliminary ACR criteria for gout and have experienced only one gout flare; hyperuricaemia. Exclusion criterias: secondary hyperuricaemia; Previously on uric acid‐lowering therapy; xanthinuria; known hypersensitivity to any component of the febuxostat formulation; rheumatoid arthritis; cancer, except basal cell carcinoma of the skin, not in remission for at least 5 years prior to the first dose of study medication; MI or stroke within 90 days prior to the Screening visit; ALT and/or AST > 2.0 times the upper limit of normal;significant medical condition and/or conditions; eCLcr ≥ 60 mL/min; serum creatinine at Screening > 2.0 mg/dL; known history of hepatitis B, hepatitis C or HIV; hypersensitivity to gadolinium; severe asthma; electronically, magnetically or mechanically activated implanted device; object as potential hazard or interfere with MRI interpretation |
| Interventions | Patients will be randomised to 2 groups: 1. Febuxostat 40 mg/day or 80 mg/day (based on serum uric acid levels) 2. Placebo |
| Outcomes |
Primary Outcome Measures: ‐ Mean Change from Baseline to Month 24 in a modified Sharp/van der Heijde Erosion Score of the single affected joint Secondary Outcome Measures: ‐ Mean Change from Baseline to Month 24 in the modified Sharp/van der Heijde Total Scores from full hand and foot radiographs. ‐ Mean Change from Baseline to Month 24 in the modified Sharp/van der Heijde Erosion Scores from full hand and foot radiographs. ‐ Mean change from baseline to Month 24 in the Rheumatoid Arthritis MRI Scoring System (RAMRIS) score. ‐ Mean change from baseline to Month 24 in a modified Sharp/van der Heijde Total Score of the single affected joint. |
| Starting date | March 2010 |
| Contact information | Takeda Study Registration Call Center 800‐778‐2860 medicalinformation@tpna.com |
| Notes |
NCT01082640.
| Trial name or title | A Multicenter, Randomized, Double‐Blind, Phase 2 Study to Evaluate the Effect of Febuxostat Versus Placebo on Renal Function in Gout Subjects With Hyperuricemia and Moderate to Severe Renal Impairment |
| Methods | Phase 2, randomised, double‐blind,parallel assignment efficacy trial. 12 months multicentre study. Estimated Enrollment 7500. |
| Participants | Inclusion criteria: ≥18 years old; inadequate response to treatment with allopurinol, defined as:current use of allopurinol and a serum uric acid greater than 7.0 mg/dL OR a past history of allopurinol use that was discontinued due to lack of therapeutic effect or due to adverse effects and a serum uric acid greater than 7.0 mg/dL; history or presence of gout defined as having one or more of the American Rheumatism Association criteria for the diagnosis of gout (the criteria related to tophi have been excluded for the purpose of the study); estimated Glomerular Filtration Rate (eGFR) > 20 and ≤ 45 mL/min, AND a serum creatinine > 1.5 mg/dL at all 3 Screening Visits (Day ‐49//35, Day ‐14 and Day ‐7). Exclusion criteria: secondary hyperuricaemia; tophaceous gout; history of xanthinuria; received aspirin > 325 mg/day within 35 days prior to Day 1/Randomization Visit; known hypersensitivity or allergy to allopurinol or febuxostat or colchicine or any component in their formulation; myocardial infarction or stroke within the 90 days prior to the Screening Visit; ALT and/or AST > 2.0 times the upper limit of normal; end stage renal disease or is likely to be a candidate for dialysis over the 1 year study period; eCLcr ≤ 20 mL/min or > 45 mL/min; serum creatinine ≤ 1.5 mg/dL, or an eGFR at any of the screening visits ≥ 20 mL/min or > 45 mL/min; required to take excluded medications |
| Interventions | Patients will be randomised to 3 groups: 1. Febuxostat 40 mg/day or 80 mg/day (based on serum uric acid levels) 2. Febuxostat 30 mg BID 3. Placebo |
| Outcomes |
Primary Outcome Measures: ‐ Change from Baseline to Month 12 in Serum Creatinine Secondary Outcome Measures: ‐ Change from Baseline to Month 12 in 24‐HR measured creatinine clearance (mCLcr). ‐ Mean Clearance (CL/F) of febuxostat during the study. ‐ Mean Area Under the Concentration‐Time Curve (AUC) of Febuxostat during the study. ‐ Percentage of subjects with Serum uric acid (sUA) < 6 mg/dL at Month 12. ‐ Change from baseline to Month 12 in estimated Glomerular Filtration Rate (eGFR) using the Modification of Diet in Renal Disease (MDRD) formula (as calculated by the central laboratory). |
| Starting date | April 2010 |
| Contact information | Takeda Study Registration Call Center 800‐778‐2860 medicalinformation@tpna.com |
| Notes |
NCT01101035.
| Trial name or title | A Multicenter, Randomized, Active‐Control, Phase 3B Study to Evaluate the Cardiovascular harms of Febuxostat and Allopurinol in Subjects With Gout and Cardiovascular Comorbidities |
| Methods | Phase 3, randomised, double‐blind, parallel assignment harms trial. 60 months multicentre study. Estimated Enrollment 240. |
| Participants | Inclusion criteria: ≥ 50 years old; a major cardiovascular or cerebrovascular disease (MI, unstable angina, cardiac or cerebrovascular revascularization procedure, stroke, hospitalised for transient Ischaemic attack, peripheral vascular disease, diabetes mellitus with evidence of micro‐ or macrovascular disease); ACR criteria for gout; serum uric acid level ≥ 7.0 mg/dL at the Day ‐7 Visit OR ≥ 6.0 mg/dL at the Day ‐7 Visit AND inadequately controlled gout Exclusion criteria: secondary hyperuricaemia; on uric acid‐lowering therapy; xanthinuria; known hypersensitivity to any component of the febuxostat or allopurinol formulation; active peptic ulcer disease; cancer within 5 years prior to the first dose of study medication; MI or stroke within 60 days prior to the Screening visit; ALT and/or AST > 2.0 times the upper limit of normal; eCLcr < 30 mL/min; known history of hepatitis B, hepatitis C or HIV; history of drug abuse or a history of alcohol abuse within 5 years prior to the Screening; more than14 alcoholic beverages per week; received any investigational medicinal product within the 30 days prior to the Screening Visit and throughout the study; required to take excluded medications |
| Interventions | Patients will be randomised to 2 groups: 1. Febuxostat 40 mg/day or 80 mg/day (dependent on serum uric acid levels) 2. Allopurinol 200 mg/day to 600 mg/day (dependent on renal function) |
| Outcomes |
Primary Outcome Measures: ‐ First occurrence of any event in the predefined Major Adverse Cardiovascular Events Composite (Cardiovascular death, Non‐fatal Myocardial Infarction, Nonfatal Stroke and Unstable Angina with Urgent Coronary Revascularization) Secondary Outcome Measures: ‐ First occurrence of any Antiplatelet Trialists' Collaborative Event (Cardiovascular Death, Non‐fatal Myocardial Infarction or Non‐fatal Stroke) ‐ First occurrence of Cardiovascular Death death ‐ First occurrence of Non‐fatal Myocardial Infarction ‐ First occurrence of Non‐fatal stroke ‐ First occurrence of Unstable Angina with Urgent Coronary Revascularization |
| Starting date | May 2010 |
| Contact information | Takeda Study Registration Call Center 800‐778‐2860 medicalinformation@tpna.com |
| Notes |
Differences between protocol and review
None
Contributions of authors
Link with editorial base and co‐ordinate contributions from co‐authors (MSA) Draft protocol (JT, MLO, MSA) Run search (SF) Identify relevant titles and abstracts from searches (JT, MLO) Obtain copies of trials (MLO) Selection of trials (JT, MLO, MSA) Extract data from trials (JT, MLO) Enter data into RevMan (MLO) Carry out analysis (MLO, MSA) Interpret data (JT, MLO, MSA) Draft final review (MSA with contributions from all) Update review (JT, MLO, MSA)
Sources of support
Internal sources
-
The University of Texas M.D. Anderson Cancer Center, Not specified.
Computer Access and Office Space
External sources
No sources of support supplied
Declarations of interest
None known. This systematic review did not receive specific funding.
New
References
References to studies included in this review
Becker 2005a {published data only}
- Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. New England Journal of Medicine 2005;353(23):2450‐61. [PUBMED: 16339094] [DOI] [PubMed] [Google Scholar]
Becker 2005b {published data only}
- Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Palo WA, Eustace D, et alt L, Joseph‐Ridge N. Febuxostat, a Novel Nonpurine Selective Inhibitor ofXanthine Oxidase:a twenty‐eight‐day, multicenter, phase II, randomized, double‐blind, placebo‐controlled, dose‐response clinical trial examining safety and efficacy in patients with gout. Arthritis and Rheumatism 2005;52(3):916–23. [PUBMED: 15751090] [DOI] [PubMed] [Google Scholar]
- Goldfarb, D. S.MacDonald, P.Hunt, B.Gunawardhana, L. [Febuxostat in Gout: Serum Urate Responses in Uric Acid Overproducers vs. Underexcretors]. American Journal of Kidney Diseases. 2010; Vol. 55:B59. [DOI] [PubMed]
Becker 2009 {published data only}
- Becker MA, Schumacher HR, MacDonald PA, Lloyd E, Lademacher C. Clinical efficacy and safety of successful long‐term urate lowering with febuxostat or allopurinol in subjects with gout. Journal of Rheumatology 2009;36(6):1273‐82. [DOI] [PubMed] [Google Scholar]
Becker 2010 {published data only}
- Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, Lademacher C. The urate‐lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Research and Therapy 2010;12(2):R63 Apr 6. [PUBMED: 20370912 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton A, Becker MA, MacDonald P, Hunt B, Jackson RL. [Gout subjects with hyperuricemia and renal impairment treated withfebuxostat or allopurinol for 6 months]. International Journal of Rheumatic Diseases. 2010; Vol. 13:172‐7.
Schumacher 2008 {published data only}
- Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28‐week, phase III, randomized, double‐blind, parallel‐group trial. Arthritis and Rheumatism 2008;59(11):1540‐8. [PUBMED: 18975369] [DOI] [PubMed] [Google Scholar]
Schumacher 2009 {published data only}
- Schumacher HR, Becker MA, Lloyd E, MacDonald PA, Lademacher C. Febuxostat in the treatment of gout: 5‐yr findings of the FOCUS efficacy and safety study. Rheumatology 2009;48:188‐94. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Becker 2008 {published data only}
- Becker MA, MacDonald PA, Hunt BJ, Lademacher C, Joseph‐Ridge N. Determinants of the clinical outcomes of gout during the first year of urate‐lowering therapy. Nucleosides, Nucleotides & Nucleic Acids 2008;27(6):585‐91. [DOI] [PubMed] [Google Scholar]
Komoriya 2004 {published data only}
- Komoriya K, Hoshide S, Takeda K, Kobayashi H, Kubo J, Tsuchimoto M, et al. Pharmacokinetics and pharmacodynamics of febuxostat (TMX‐67), a non‐purine selective inhibitor of xanthine oxidase/xanthine dehydrogenase (NPSIXO) in patients with gout and/or hyperuricemia. Nucleosides, Nucleotides & Nucleic Acids 2004;23(8‐9):1119‐22. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00821392 {unpublished data only}
References to ongoing studies
NCT01078389 {unpublished data only}
- A Multicenter, Randomized, Double‐Blind, Phase 2 Study to Evaluate the Effect of Febuxostat Versus Placebo in Joint Damage in Hyperuricemic Subjects With Early Gout. Ongoing study March 2010.
NCT01082640 {unpublished data only}
- A Multicenter, Randomized, Double‐Blind, Phase 2 Study to Evaluate the Effect of Febuxostat Versus Placebo on Renal Function in Gout Subjects With Hyperuricemia and Moderate to Severe Renal Impairment. Ongoing study April 2010.
NCT01101035 {unpublished data only}
- A Multicenter, Randomized, Active‐Control, Phase 3B Study to Evaluate the Cardiovascular harms of Febuxostat and Allopurinol in Subjects With Gout and Cardiovascular Comorbidities. Ongoing study May 2010.
Additional references
Anderson 2010
- Anderson A, Singh JA. Pegloticase for chronic gout. Cochrane Database of Systematic Reviews 2010;Issue 3(Art. No.:CD008335):DOI: 10.1002/14651858.CD008335.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arellano 1993
- Arellano F, Sacristán JA. Allopurinol hypersensitivity syndrome: a review. Annals of Pharmacotherapy 1993;27(3):337‐43. [PUBMED: 8453174] [DOI] [PubMed] [Google Scholar]
Bhandari 2004
- Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, et al. Association between industry funding and statistically significant pro‐industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004;170(4):477‐80. [14970094] [PMC free article] [PubMed] [Google Scholar]
Bruce 2006
- Bruce SP. Febuxostat: a selective xanthine oxidase inhibitor for the treatment of hyperuricemia and gout. Annals of Pharmacotherapy 2006;40(12):2187‐94. [PUBMED: 17132810] [DOI] [PubMed] [Google Scholar]
Cates 2004
- Cates C. Visual Rx 2.0 NNT Calculator [Computer program]. Dr Chris Cates EBM Website www.nntonline.net 2004.
Chohan 2009
- Chohan S, Becker MA. Update on emerging urate‐lowering therapies. Current Opinion in Rheumatology 2009;21(2):143‐9. [PUBMED: 19339925] [DOI] [PubMed] [Google Scholar]
Dalbeth 2007
- Dalbeth N, Stamp L. Allopurinol dosing in renal impairment: Walking the tightrope between adequate urate lowering and adverse events. Seminars in Dialysis September–October 2007;20(5):391–5. [DOI] [PubMed] [Google Scholar]
Edwards 1981
- Edwards NL, Recker D, Airozo D, Fox IH. Enhanced purine salvage during allopurinol therapy: an important pharmacologic property in humans. Journal of Laboratory and Clinical Medicine 1981;98(5):673‐83. [PUBMED: 7299239] [PubMed] [Google Scholar]
Edwards 2009
- Edwards NL. Febuxostat: a new treatment for hyperuricaemia in gout. Rheumatology (Oxford) 2009;48 Suppl 2:ii15‐9. [PUBMED: 19447778] [DOI] [PubMed] [Google Scholar]
EMEA 2008
- European Medicines Agency. European Public Assessment Report. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for_the_public/human/000777/WC500021813.pdf April 21, 2012.
FDA 2009
- US Food, Drug Administration. Summary Review for Regulatory Action. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/021856s000_SumR.pdf February 13, 2009.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jansen 2010
- Jansen TL, Richette P, Perez‐Ruiz F, Tausche AK, Guerne PA, Punzi L, et al. International position paper on febuxostat. Clinical Rheumatology 2010;29:835‐40. [PUBMED: 20401506] [DOI] [PubMed] [Google Scholar]
Jordan 2007
- Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J, et al. British Society for Rheumatology and British Health Professionals in Rheumatology Standards, Guidelines and Audit Working Group (SGAWG). British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford) 2007;46(8):1372‐4. [PUBMED: 17522099] [DOI] [PubMed] [Google Scholar]
Li‐Yu 2001
- Li‐Yu J, Clayburne G, Sieck M, Beutler A, Rull M, Eisner E, Schumacher HR Jr. Treatment of chronic gout. Can we determine when urate stores are depleted enough to prevent attacks of gout?. Journal of Rheumatology 2001;28(3):577‐80. [PUBMED: 11296962] [PubMed] [Google Scholar]
Maxwell 2009
- Maxwell LJ, Singh JA. Abatacept for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007277.pub2; PUBMED: 19821401] [DOI] [PMC free article] [PubMed] [Google Scholar]
OMERACT 9
- Schumacher HR, Taylor W, Edwards L, Grainger R, Schlesinger N, Dalbeth N, et al. Outcome domains for studies of acute and chronic gout. Journal of Rheumatology October 2008;36(10):2342‐5. [DOI] [PubMed] [Google Scholar]
Pascual 2007
- Pascual E, Sivera F. Time required for disappearance of urate crystals from synovial fluid after successful hypouricaemic treatment relates to the duration of gout. Annals of the Rheumatic Diseases 2007;66(8):1056‐8. [PUBMED: 17223663] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schlesinger 2004
- Schlesinger N. Management of acute and chronic gouty arthritis: present state‐of‐the‐art. Drugs 2004;64(21):2399‐416. [PUBMED: 15481999] [DOI] [PubMed] [Google Scholar]
Schumacher 2005
- Schumacher HR Jr, Edwards LN, Perez RF, Becker M, Chen LX, Furst DE, et al. Outcome measures for acute and chronic gout. Journal of Rheumatology 2005;32:2452–5. [PubMed] [Google Scholar]
Singh 2010
- Singh JA. Advances in gout: some answers, more questions. Arthritis Research and Therapy 2010;12(5):136. [PUBMED: 20959031] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevenson 2011
- Stevenson M, Pandor A. Febuxostat for the management of hyperuricaemia in patients with gout: a NICE single technology appraisal. Pharmacoeconomics 2011;29(2):133‐40. [PUBMED: 21155617] [DOI] [PubMed] [Google Scholar]
Wallace 1977
- Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis and Rheumatism 1977;20(3):895‐900. [PUBMED: 856219] [DOI] [PubMed] [Google Scholar]
Zhang 2006
- Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Annals of the Rheumatic Diseases 2006;65(10):1312‐24. [PUBMED: 16707532] [DOI] [PMC free article] [PubMed] [Google Scholar]


