Abstract
Background
Clinical observations indicate that mothers commonly perceive a reduction in, or absence of, the baby’s movements for some days preceding a baby’s death. For this reason, fetal movement monitoring is advised by caregivers and used spontaneously by mothers to assess the baby’s well-being. However, it is possible that the harmful effects of interventions may outweigh the benefits of such testing. Evidence of effectiveness of fetal movement screening to improve outcomes is limited, though indirect evidence suggests a potential benefit. A secondary question is whether any specific management response to perceived decreased fetal movements (DFM) improves clinical outcome.
Objectives
To determine, from the best available evidence, the effectiveness of various management strategies for DFM.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (28 February 2012) and bibliographies of included studies.
Selection criteria
Randomised clinical trials comparing various management strategies for DFM, including delivery, expectant management, cardiotocography (visual and computerised), ultrasound examination including Doppler ultrasound, and fetal arousal tests (cardiotocographic or clinical).
Data collection and analysis
Two assessors evaluated potentially eligible trials for inclusion, and extracted data onto a purpose-designed form. Where DFM was one among a number of inclusion criteria for the trial, we contacted trial authors for information on outcomes specific to the DFM subgroups.
Main results
No randomised trials of management of DFM were found. Of 13 randomised trials of management strategies for pregnancies with risk factors for fetal compromise including DFM, data on the DFM subgroups could only be provided by the authors of one trial. The numbers were too small for meaningful analysis (there were 28 cases of DFM).
Authors’ conclusions
There are insufficient data from randomised trials to guide practice regarding the management of DFM. Based on the results of other systematic reviews of management strategies for women whose babies are thought to be at risk of compromise for various reasons, the following strategies show promise and may be prioritised for further research: Doppler ultrasound studies, computerised cardiotocography, and fetal arousal to facilitate cardiotocography.
For settings where electronic fetal assessment methods are not available, clinical fetal arousal tests should be investigated.
Medical Subject Headings (MeSH): *Fetal Movement, *Pregnancy Outcome, Fetal Distress [*therapy]; Fetal Monitoring
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
Description of the condition
Unborn babies exhibit profound physiological adaptation to adverse intrauterine conditions which serve to reduce their energy consumption (Martin 2008). These include redistribution of blood flow away from non-essential organs and reduction in breathing movements and general body movements. Decreased fetal movements (DFM) may represent a state of chronic placental insufficiency (Warrander 2011). Intrauterine death of a baby is commonly preceded by a reduction or absence of perceived movements for some days. This observation has led to the concept that monitoring of fetal movements may be used to detect a deterioration in the baby’s condition.
Fetal movement assessment is widely used as a method of routine surveillance of the well-being of unborn babies (Frøen 2008; O’Sullivan 2009). It has the advantage that it does not require specialised equipment, and can be performed on a daily basis by the mother at home. DFM have been shown to be associated with adverse pregnancy outcomes such as intrauterine growth restriction, intrauterine fetal death, neonatal intensive care unit admission, low Apgar scores, and less common developmental anomalies such as those affecting the neuromuscular system (Frøen 2001; Heazell 2008a; Sinha 2007; Skornick-Rapaport 2010). Women’s perception of DFM is decreased with an anterior placenta, cigarette smoking and maternal obesity (Tuffnell 1991).
The effectiveness of fetal movement assessment is not well established (Frøen 2008; Tveit 2009). The largest trial to date failed to demonstrate improved perinatal outcome when routine fetal movement counting was compared with normal practice (which included informal fetal movement assessment). There was the possibility of contamination of the control group: during the study period, the rate of stillbirth in both groups was considerably lower than in the pre-trial period. In the routine counting group, significantly more women with subsequent stillbirths reached the hospital with the baby still alive, but subsequent management did not achieve the goal of reduced stillbirths.
There is also disagreement as to whether monitoring of fetal movements, if used at all, should be advised for all women or only those considered to be at increased risk of complications, and whether monitoring should be based on a formal counting method such as the Cardiff ‘count to 10’ chart, or derivatives, or on the mother’s subjective impression of movements.
Whether or not fetal movement monitoring is advised, women may report concern over a reduction in or absence of movements. This raises the important question of what is the appropriate management for women who report DFM, and can this achieve an improvement in perinatal outcome?
The literature on DFM has recently been extensively reviewed (Preston 2010).
Description of the intervention
Several interventions have been suggested for the management of reported DFM, which are directed to delivery of baby (induction of labour, caesarean section) or expectant management with close surveillance of the baby (Skornick-Rapaport 2010). Wide variations in clinicians’ management of DFM, and the need for guidelines have been highlighted (Flenady 2009; Heazell 2008b; Unterscheider 2010).
There is a striking dearth of guidelines for the management of women who report DFM. A Royal College of Obstetricians and Gynaecologists (RCOG) guideline was published in February 2011 (RCOG 2011). A guideline developed by The Australian and New Zealand Stillbirth Alliance (ANZSA) has been endorsed by the Perinatal Society of Australia and New Zealand (PSANZ); Australian College of Midwives (ACM); Stillbirth Foundation Australia; Australian National Council for Stillbirth and Neonatal Death Support (SANDS); National SIDS Council of Australia Ltd (SIDS and Kids), Bonnie Babes Foundation and Mater Health Services, Brisbane (Preston 2010). The group emphasise the lack of strong evidence, and the fact that the guidelines are mainly based on consensus. The guidelines are as follows.
All pregnant women should be routinely provided with verbal and written information regarding normal fetal movements during the antenatal period. This information should include a description of the changing patterns of movement as the fetus develops, normal wake/sleep cycles and factors which may modify the mother’s perception of movements such as maternal weight and placental position.
All women should be advised to contact their healthcare provider if they have any concern about decreased or absent fetal movements and be advised not to wait until the next day to report DFM.
- After discussion, women who remain unsure whether movements are decreased or not should be given guidance on counting fetal movements; i.e. to count while lying down on her side and concentrating on fetal movements. As a rule, when the baby is awake, if there are less than 10 movements felt in two hours she should contact her healthcare provider.
- Maternal concern of DFM overrides any definition of DFM based on numbers of fetal movements and women with a concern about DFM should be encouraged to contact their healthcare provider.
- Clinicians should emphasise the importance of maternal awareness of fetal movements at every routine antenatal visit.
- The use of kick-charts can currently not be recommended as part of routine antenatal care.
- When a woman presents with DFM, assessment of the woman and her fetus should be undertaken as soon as possible.
- This assessment should preferably be undertaken within two hours if fetal movements are absent and within 12 hours if they are reported as decreased.
- Women who report DFM should be assessed for the presence of other risk factors associated with an increased risk of stillbirth (i.e. fetal growth restriction, hypertension, diabetes, advanced maternal age, etc).
- Women with DFM in combination with other risk factors should be managed as a high-risk pregnancy.
Clinical assessment of a woman with DFM should always include review of fetal growth as noted by symphysis-fundal height measurements in the pregnancy record.
- A cardiotocograph (CTG) should be performed to exclude fetal compromise.
- Further evaluation is recommended for women with any abnormal CTG pattern.
Ultrasound scan assessment for fetal biometry and amniotic fluid volume should be considered as part of the preliminary investigation of a woman presenting with DFM where maternal perception of DFM persists despite a normal CTG or in the circumstance of suspected fetal growth restriction.
Ultrasound scan assessment should include assessment of fetal morphology if this has not already been performed.
Where, in the presence of DFM, an ultrasound scan assessment is indicated, this should be performed within 24 hours.
Testing for fetomaternal haemorrhage should be considered in the preliminary investigation of women with DFM where a CTG abnormality is detected, in the presence of an ultrasound scan showing a normally grown fetus.
Where, after further discussion and in the presence of a normal clinical assessment (including a CTG and ultrasound), maternal concern still remains about DFM, further management should be individualised.
How the intervention might work
Delivery of the baby aims to avoid the death of the unborn baby in utero or deterioration of the baby’s health.
Expectant management with various algorithms of surveillance of the baby’s condition aims to allow the pregnancy to continue and the baby to mature with a view to delivery if evidence arises of a deterioration in the baby’s condition, or once maturity has been achieved or spontaneous labour occurs.
Why it is important to do this review
Various algorithms are used for the management of women who report DFM. However, the benefits and risks of such algorithms are not clear. It is intuitive to expect that knowledge of the baby’s movement pattern will enhance the caregiver’s ability to provide the correct care. However, as with any screening test, ‘false positive’ results may provoke unnecessary interventions and adverse outcomes which outweigh the benefits of interventions for true positive tests, as well as causing maternal anxiety.
OBJECTIVES
To determine the effect of various methods of managing reported DFM on maternal, perinatal and childhood outcomes.
METHODS
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials as well as cluster-randomised trials in this review. We did not include quasi-randomised trials and trials using a cross-over design. We included studies reported only as abstracts only if adequate information to evaluate the study quality was available.
Types of participants
Women who report DFM. In the case of trials which have several entry criteria including DFM, we tabulated the trial data, indicating the proportion of the participants with DFM as the entry point. We limited results to our review primary outcomes and perinatal mortality, or if not reported, the main outcomes reported in the trials. We contacted trial authors to determine whether data on those participants with DFM were available, for inclusion in the review analysis.
Types of interventions
Any intervention, including:
induction of labour;
caesarean section;
fetal surveillance (e.g. cardiotocography; ultrasound assessment including Doppler studies; clinical and cardiotocographic fetal arousal tests; and various combinations of tests);
algorithms for assessment and management;
expectant management.
We included all valid comparisons of alternative management strategies.
Types of outcome measures
Primary outcomes
Perinatal mortality and severe morbidity
Caesarean section
Secondary outcomes
Perinatal mortality
Individual components of severe perinatal morbidity (neonatal intensive care unit (NICU) admission for more than 24 hours, organ failure, encephalopathy)
Labour induction
Maternal death or severe morbidity
Individual components of severe maternal morbidity (intensive care unit (ICU) admission for more than 24 hours, organ failure)
Maternal hospital admission for more than seven days
Disability in childhood
Women’s satisfaction
Caregiver satisfaction
Cost
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co-ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (28 February 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We reviewed the bibliographies of included studies to search for additional trials eligible for inclusion.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third person.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non-random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non-opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re-included missing data in the analyses which we undertook. We assessed methods as:
low risk of bias;
high risk of bias;
unclear risk of bias.
A cut-off point of 20% or less of missing data was used to assess the study as adequate.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre-specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre-specified outcomes have been reported; one or more reported primary outcomes were not pre-specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of bias;
high risk of bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses - see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
This review does not contain continuous data. In future updates, should we identify continuous data for inclusion, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster-randomised trials
We did not identify any cluster-randomised trials for inclusion. In future updates of this review, if we identify any cluster-randomised trials, we will include them in the analyses along with individually-randomised trials. We will adjust their sample size using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using an estimate of the intracluster correlation co-efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster-randomised trials and individually-randomised trials, we will synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. For all outcomes, we carried out analyses, as far as possible, on an intention-to-treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta-analysis using the T2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if the I2 was greater than 30% and either T2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta-analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes, we will use the test proposed by Egger 1997, and for dichotomous outcomes, we will use the test proposed by Harbord 2006. If asymmetry is detected in any of these tests or is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). As more data become available, we will use used fixed-effect meta-analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differs between trials, or if we detect substantial statistical heterogeneity, we will use random-effects meta-analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. We will treat the random-effects summary as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
Where we use random-effects analyses, we will present the results as the average treatment effect with its 95% confidence interval, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity in future updates of this review, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random-effects analysis to produce it. We will carry out the following prespecified subgroup analyses.
DFM based on a. formal counting; b. qualitative assessment; or c. mixed or unclear method.
Fetal movement counting used a. routinely; b. selectively for high-risk pregnancies; or c. mixed or unclear use.
Settings with a. high perinatal mortality (=/> 20 per 1000); b. low perinatal mortality (< 20 per 1000); or c. perinatal mortality mixed or unclear.
The following outcomes will be used in subgroup analysis.
Perinatal mortality and severe morbidity.
Caesarean section.
Womens’ satisfaction.
Cost.
For fixed-effect inverse variance meta-analyses, we will assess differences between subgroups by interaction tests. For random-effects and fixed-effect meta-analyses using methods other than inverse variance, we will assess differences between subgroups by inspection of the subgroups’ confidence intervals; non-overlapping confidence intervals indicate a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
As more data are added to this review, we will perform sensitivity analyses for aspects of the review that might affect the results, for example, where there is a risk of bias associated with the quality of some of the included trials; or to explore the effects of fixed-effect or random-effects analyses for outcomes with statistical heterogeneity; and to explore the effects of any assumptions made, such as the value of the ICC used for cluster-randomised trials. We will use the following outcomes in sensitivity analyses.
Perinatal mortality and severe morbidity.
Caesarean section.
Women’s satisfaction.
Cost.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
We contacted authors of potentially relevant papers, where there was mention of a subgroup of women with DFM, to ascertain whether data were available specifically relating to those women. Data for such a subgroup were available from only one trial, a prospective randomised trial comparing biophysical profile following vibroacoustic stimulation (VAS/mFBP) with mock stimulation (mFBP) (Sood 2007). Data from a subgroup of 28 cases with DFM, from the total sample of 214 singleton high-risk pregnancies, were kindly provided by Colonel Sood.
Results of the search
The search identified the following sixteen papers as of potential relevance to the review: Bracero 1999; Burke 1992; Eglinton 1984; Hofmeyr 1991; Jamal 2007; Johnstone 1993; Manning 1984; Newnham 1990; Platt 1985; Richardson 1983; Sleutel 1990; Smith 1986; Sood 2007; Tongsong 1994; Trungtawatchai 1999; Williams 2003. Thirteen of the sixteen studies met the inclusion criteria and three were excluded.
Included studies
No randomised trials specifically considering management strategies for DFM were obtained but the 13 included trials reported on a range of risk factors in which DFM were included. However, data specific to the DFM subgroup of 28 cases were available from only one study (Sood 2007). Data for the subgroup of cases with DFM were also sought from authors of the remaining 12 papers but were unavailable. See Characteristics of included studies for details of all included studies.
Excluded studies
Three studies were excluded because they were quasi-randomised trials (Eglinton 1984; Platt 1985; Richardson 1983), see Characteristics of excluded studies.
Risk of bias in included studies
The ‘Risk of bias’ assessments are reported below and relate to the whole sample rather than specifically to the cases with DFM. Only the trial which contributed data will be discussed further (Sood 2007).
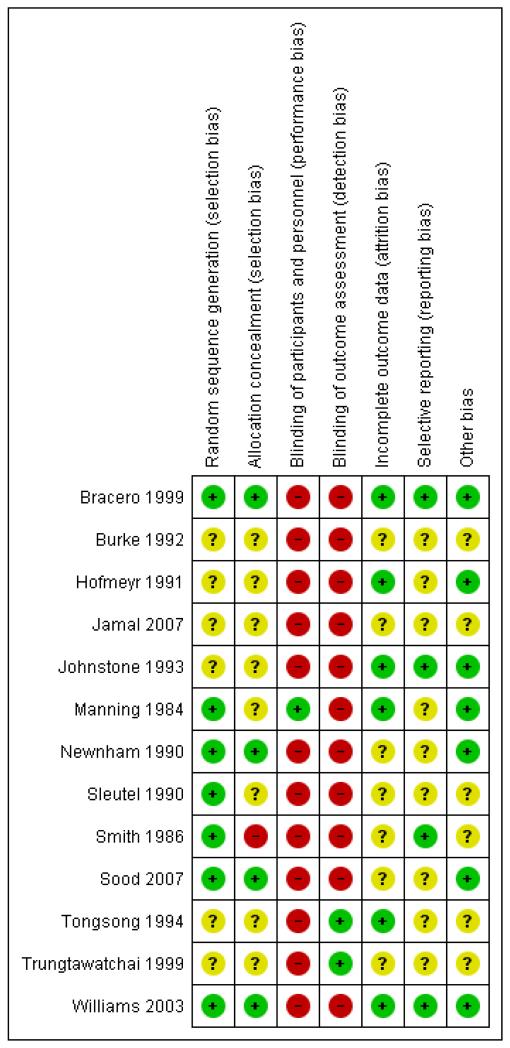
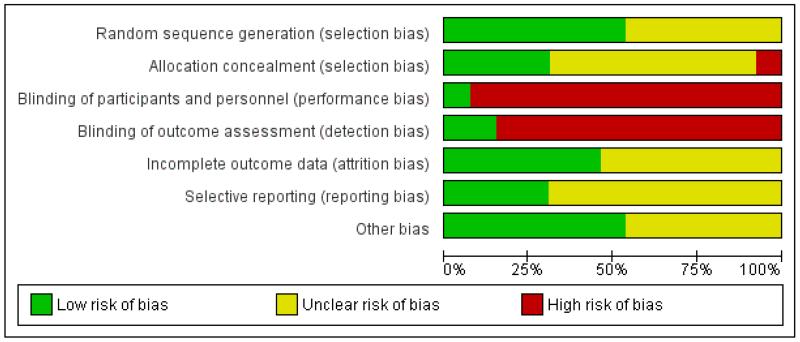
For risk of bias assessments for all trials see Figure 1; Figure 2.
Figure 1. Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Figure 2. Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The risk of bias in relation to allocation was judged to be low. The trial used a computer-generated random sequence with allocation concealment using sealed envelopes.
Blinding
The trial was not blinded.
Incomplete outcome data
There was no reported indication of incomplete outcome data.
Selective reporting
There was no reported indication of selective reporting.
Other potential sources of bias
At the outset of monitoring in the 214 singleton high-risk pregnancies, no significant differences were detected between the biophysical profile following vibroacoustic stimulation (VAS/mFBP) and mock stimulation (mFBP) groups with respect to the maternal age, parity, gestational age and high-risk factors (intrauterine growth retardation, pregnancy-induced hypertension, adverse obstetric history, DFM, postdated pregnancy, diabetes mellitus and antepartum haemorrhage).
Effects of interventions
Vibro-acoustic stimulation
In the Sood 2007 study there were only 28 cases in the subgroup with DFM and these are included in Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8. In none of these analyses were there significant differences between modified biophysical profile following vibroacoustic stimulation (15 cases) versus mock stimulation (13 cases). The following outcomes were not assessed in Sood 2007: disability in childhood, women’s satisfaction, care giver satisfaction and cost.
Cardiotocography (CTG)
No studies or subset data from studies of CTG for DFM were included.
Fetal Doppler ultrasound studies
No studies or subset data from studies of biophysical profile for DFM were included in this review.
Biophysical profile
No studies or subset data from studies of biophysical profile for DFM were included.
DISCUSSION
Vibro-acoustic stimulation
In the Sood 2007 study there were only 28 cases in the subgroup with DFM and these are included in Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8. As expected with such small numbers, in none of these analyses were there significant differences between modified biophysical profile following vibroacoustic stimulation (15 cases) versus mock stimulation (13 cases).
The Cochrane systematic review of studies of vibroacoustic stimulation for high-risk pregnancies included nine studies enrolling women undergoing cardiotocography including post-term pregnancy, hypertension, diabetes, growth impairment and DFM (Tan 2001). Overall, fetal vibroacoustic stimulation reduced the incidence of non-reactive antenatal cardiotocography test (seven trials; risk ratio (RR) 0.62, 95% confidence interval (CI) random-effects 0.52 to 0.74).
Cardiotocography (CTG)
The Cochrane systematic review of CTG for high-risk pregnancies included six studies enrolling women with post-term pregnancy, hypertension, diabetes, cardiac disease, antepartum haemorrhage, preterm labour, growth impairment, obstetric complications, ‘increased risk’ and social reasons (Grivell 2010). DFM was not specified as an inclusion criterion in any of the trials. Clinically interpreted CTG was associated with no improvement in perinatal outcome. Computerised compared with clinically interpreted CTG showed a reduction in overall perinatal mortality (RR 0.20, 95% CI 0.04 to 0.88, two studies, 0.9% versus 4.2%, 469 women).
Fetal Doppler ultrasound studies
The Cochrane systematic review of Doppler studies for high-risk pregnancies included 18 studies enrolling women with high-risk pregnancies including post-term pregnancy, preterm rupture of membranes, hypertension, diabetes, previous stillbirth, antepartum haemorrhage, preterm labour, growth impairment, DFM and previous low birthweight, antepartum haemorrhage or perinatal death (Alfirevic 2010). The use of Doppler ultrasound in high-risk pregnancy was associated with fewer perinatal deaths (RR 0.71, 95% CI 0.52 to 0.98, 16 studies, 10,225 babies); inductions of labour (average RR 0.89, 95% CI 0.80 to 0.99, 10 studies, 5633 women, random-effects) and fewer caesarean sections (RR 0.90, 95% CI 0.84 to 0.97, 14 studies, 7918 women). DFM was specified as a criterion for enrolment in only one study (Williams 2003; 22% of participants). On the other hand, non-randomised studies have suggested that Doppler velocimetry is not a useful predictor of outcome over and above CTG (Frøen 2008).
Biophysical profile
The Cochrane systematic review of biophysical profile for high-risk pregnancies included five studies enrolling women with post-term pregnancy, preterm rupture of membranes, hypertension, diabetes, previous stillbirth, antepartum haemorrhage, preterm labour, rhesus disease, abnormal ‘modified’ biophysical profile, growth impairment and DFM (Lalor 2008). In only two studies women with DFM were enrolled (Manning 1984: 4%; Platt 1985a: 7%). Overall, biophysical profile testing was associated with a probable increase in caesarean section and no improvement in neonatal outcome.
Summary of main results
No data on randomised comparisons of management strategies specifically for DFM were found. Several studies reported on a mixed group of risk factors including DFM (see Table 1), but to date, we have been able to obtain data on the DFM subgroup from only one study (Sood 2007). The numbers were too small for meaningful deductions.
Overall completeness and applicability of evidence
Very little evidence is available to guide practice.
Quality of the evidence
Unsure quality.
Potential biases in the review process
None known.
Agreements and disagreements with other studies or reviews
Too little data to comment.
AUTHORS’ CONCLUSIONS
Implications for practice
Current guidelines for the management of DFM lack evidence of effectiveness. Clinical observations that DFM commonly precede fetal death have led to recommendations that pregnant women be routinely advised to report a perceived decrease in fetal movements, that DFM be investigated with tests of fetal well-being such as cardiotocography and ultrasound examination including Doppler ultrasound, with a view to early delivery when the risk of fetal death or morbidity is thought to outweigh the risk of intervention. It is remarkable that there is so little evidence to support such a widespread practice. Given the high rate of false positive results for both fetal movement assessment and follow-up tests, there is a real possibility that risks of such a strategy (unnecessary interventions, iatrogenic prematurity and anxiety) may outweigh benefits.
Implications for research
There is a need for research to evaluate the benefits and risks of strategies to manage DFM. Such studies should include:
high-quality randomised control trials of sufficient size;
distinction between women with pre-existing risk factors and those without;
attention to strategies in settings where electronic fetal heart rate monitoring and ultrasound are not available.
Based on data from systematic reviews of various antepartum fetal assessment strategies for high-risk pregnancies in general, such research should give priority to Doppler ultrasound studies, computerised cardiotocography, and fetal arousal to facilitate cardiotocography.
For settings where electronic fetal assessment methods are not available, clinical fetal arousal tests (clinical assessment of fetal movements and/or fetal heart rate acceleration following stimulation) should be investigated.
PLAIN LANGUAGE SUMMARY.
Management of reported decreased fetal movements during pregnancy
Decreased fetal movements can indicate deterioration in the baby’s condition, for example, because of chronic placental insufficiency. Clinical observations indicate that mothers commonly perceive an absence or reduction in the baby’s movements for some days before a baby’s death. For this reason, fetal movement monitoring is advised by caregivers and is used spontaneously by mothers to assess the baby’s well-being. Women’s perception of decreased fetal movement is decreased with cigarette smoking, maternal obesity and if the placenta is at the front of the womb. Management strategies in response to perceived decreased fetal movements include early delivery, expectant management with close surveillance of the baby, cardiotocography (visual or analysed by computer to follow the baby’s heart beat with uterine activity), ultrasound examination including Doppler ultrasound, and fetal arousal tests (either cardiotocographic or clinical observation where electronic fetal assessment methods are not available) to assess the baby’s well-being. Evidence on the effectiveness of monitoring fetal movements and the subsequent management strategies in improving outcomes is limited. Given the high rate of false positive results for both fetal movement assessment and follow-up tests, there is a real possibility of risks with unnecessary interventions or the baby being born premature and increased anxiety for the mother.
Thirteen studies met the inclusion criteria. This review found that very little is known from high-quality randomised clinical trials to guide the management of reported decreased fetal movements. No randomised trials of management of decreased fetal movement alone were found. However, other systematic reviews indicate that Doppler ultrasound, computerised cardiotocography, and fetal arousal to facilitate cardiotocography show promise in high-risk pregnancies.
ACKNOWLEDGEMENTS
We are especially indebted to Col AK Sood for providing additional data from the study Sood 2007 specific to the subgroup of cases with DFM.
Stephen Milan for technical support; Sonja Henderson and the Cochrane Pregnancy and Childbirth team for administrative and technical support; Leanne Jones for making final edits on behalf of the review team.
As part of the pre-publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser.
SOURCES OF SUPPORT
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
UK NIHR Programme of centrally-managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS: 10/4001/02
Appendix
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial. | |
| Participants | Outpatients referred for fetal heart monitoring. | |
| Interventions | Visual or computerised interpretation of FHR monitoring recording | |
| Outcomes | Number of additional fetal surveillance tests, amount of time spent per FHR test, perinatal morbidity and mortality, NICU admission for more than 2 days, and total NICU days. Perinatal morbidity was defined as caesarean delivery for fetal distress, hypocalcaemia (total serum calcium level < 7 mg/dL), hyperbilirubinaemia (indirect bilirubin level > 12 mg/dL), hypoglycaemia (glucose serum level < 30 mg/dL), respiratory distress syndrome, transient tachypnoea, and meconium aspiration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Five pregnancies were excluded from the outcome analysis because infants were diagnosed with congenital anomalies: four of these appear to be from the visual interpretation group (n=201) and one from the computerised interpretation group (n=204) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes appear to have been reported upon. |
| Other bias | Low risk | “The two randomized groups were similar at baseline, but the computerized interpretation group has significantly fewer biophysical profiles compared with the visual interpretation group” |
| Methods | Randomised controlled trial. | |
| Participants | 476 women with high-risk singleton pregnancies referred for fetal assessment | |
| Interventions | Study group had Doppler ultrasound and control group was managed without Doppler ultrasound | |
| Outcomes | Induction of labour, elective and emergency caesarean section, preterm delivery, perinatal death | |
| Notes | Published in abstract form only. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly assigned. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Unclear - limited data as only abstract form available. |
| Selective reporting (reporting bias) | Unclear risk | Unclear - limited data as only abstract form available. |
| Other bias | Unclear risk | Unclear - limited data as only abstract form available. |
| Methods | Randomized feasibility study | |
| Participants | 897 women undergoing evaluation for fetal well-being. | |
| Interventions | Doppler (umbilical artery resistance index) or FHR testing (computerised FHR monitoring and numerical analysis on-line) | |
| Outcomes | Reasons for delivery, method of delivery, gestational age, birthweight, 5-minute Apgar score < 7, perinatal mortality, special care nursery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer-generated algorithm based on hospital number. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Appears to be no loss to follow-up according to data presented in table 2 and 3 - patient numbers add up to 438 for Doppler group and 459 for FHR group - so all patients accounted for from 897 initially referred |
| Selective reporting (reporting bias) | Unclear risk | Unclear - not clearly documented which outcomes were to be collected as part of the study |
| Other bias | Low risk | “The two groups were well-matched for the baseline variables measured.” |
| Methods | Randomised. | |
| Participants | 200 patients with high-risk pregnancies. | |
| Interventions | Biophysical profile protocol and modified biophysical profile protocol | |
| Outcomes | Abnormal test, perinatal mortality, meconium passage, caesarean section for abnormal test, Apgar score < 7, overall adverse outcome | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Unclear - limited data as only abstract available. |
| Selective reporting (reporting bias) | Unclear risk | Unclear - limited data as only abstract available. |
| Other bias | Unclear risk | Unclear - limited data as only abstract available. |
| Methods | Randomised. | |
| Participants | 2289 women referred for Dopplers studies and for antenatal fetal monitoring | |
| Interventions | Continuous wave Doppler studies of umbilical artery. | |
| Outcomes | “Fetal outcome: perinatal mortality, Apgar score and admission to the neonatal unit. Obstetric intervention: admission to hospital, induction of labour and caesarean section. Use of tests of fetal well being: cardiotocography, biophysical profile and ultrasound biometry.” | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Data appears to have been analysed according to the intention-to-treat principle. It states that 24 women randomised to Doppler didn’t have it carried out and 3 randomised to no Doppler, actually had Dopoler. Data on outcomes in table 3 suggest analysis was based on randomised groups Loss to follow-up - one patient appears to be missing from non Doppler group - table 4 - so minimal loss |
| Selective reporting (reporting bias) | Low risk | All outcomes specified within the abstract are reported upon in the paper |
| Other bias | Low risk | Baseline characteristics similar. “The treatment and control groups were comparable in age, parity, gestation at point of entry and risk features.” |
| Methods | Randomised. | |
| Participants | 735 women with high-risk pregnancies referred for fetal well-being testing | |
| Interventions | Fetal biophysical profile versus non-stress test. | |
| Outcomes | Apgar score, positive and negative predictive value of the test | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned -using flip of a coin. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes |
Low risk | “Neither patient nor referring physician was aware of which testing scheme had been assigned.” Study also described as “prospective blind study” - blinding only broken in women in whom a major lethal fetal anomaly was detected |
| Blinding of outcome assessment (detection bias) All outcomes |
High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All patients appear to be accounted for within the results tables: scheme A, N=360 scheme B, N=375, total number patients referred = 735 |
| Selective reporting (reporting bias) | Unclear risk | Unclear - not clearly documented which outcomes were to be collected as part of the study |
| Other bias | Low risk | “Obstetric risk factors at initial referral did not vary significantly between groups.” |
| Methods | Randomised. | |
| Participants | 172 pregnant women with at least 34 weeks’ gestation and with no contraindications to contraction stress test | |
| Interventions | Non-stress test versus fetal acoustic stimulation. | |
| Outcomes | Mode of delivery, Apgar scores, perinatal deaths, time to perform the test, abnormal test | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by draw of sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Unclear - does not report any withdrawals, but not enough information to tell if results are based on the whole sample randomised |
| Selective reporting (reporting bias) | Unclear risk | Unclear - not clearly documented which outcomes were to be collected as part of the study |
| Other bias | Low risk | “These two groups were comparable for maternal ages, parities, gestational ages, modes of delivery, and Apgar scores.” |
| Methods | Randomised. | |
| Participants | 60 pregnant women scheduled for non-stress test. Excluded if < 30 weeks | |
| Interventions | 1 group receiving a single-5-second stimuli, 2 group - receiving intermittent stimuli, 3 - control group | |
| Outcomes | Fetal heart tracing characteristics. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | High risk. |
| Blinding of outcome assessment (detection bias) All outcomes |
High risk | High risk. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Unclear from tables whether data from all participants included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Unclear - not clearly documented which outcomes were to be collected as part of the study |
| Other bias | Unclear risk | Differences existed between the groups in fetal gestational age and number of cigarette smokers |
| Methods | Randomised. | |
| Participants | 897 patients presenting for antenatal fetal monitoring. | |
| Interventions | Non-stress test compared with FAS test (monitoring for 5 minutes) | |
| Outcomes | Number of reactive tests, number of stimuli needed to achieve reactive test, antenatal fetal death | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by lottery. |
| Allocation concealment (selection bias) | High risk | None. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | None. |
| Blinding of outcome assessment (detection bias) All outcomes |
High risk | None. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Unclear from tables whether data from all participants included in the analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported upon. |
| Other bias | Unclear risk | No baseline characteristics table - mean parity of each group was similar |
| Methods | “Prospective randomised controlled study.” | |
| Participants | “214 singleton high risk pregnancies.” | |
| Interventions | “Modified biophysical profile following vibroacoustic stimulation (VAS/mFBP) versus mock stimulation (mFBP).” | |
| Outcomes | Data were provided for the subgroup of 28 cases with decreased fetal movements on the following outcomes Primary outcomes
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment using sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes |
High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | No indication ofincomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Low risk | In the total sample of 214 singleton high-risk pregnancies at the outset of monitoring there were no significant differences between the 2 groups with respect to the maternal age, parity, gestational age and high-risk factors (intrauterine growth retardation, pregnancy-induced hypertension, adverse obstetric history, decreased fetal movements, postdated pregnancy, diabetes mellitus and antepartum haemorrhage) |
| Methods | Randomised. | |
| Participants | “A total of 640 patients underwent a total of 1300 tests. 650 tests were randomised to nonstress group and 650 randomised to vibroacoustic test group.” High-risk patients with indication for fetal surveillance, e.g. postterm, intrauterine growth restriction, chronic hypertension, pregnancy-induced hypertension, decreased fetal movements, diabetes mellitus |
|
| Interventions | Non-stress test compared with vibroacoustic stimulation test | |
| Outcomes | Reactive and non-reactive test, the mean testing time to achieve a reactive test | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not reported, so presumably not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Test were interpreted blindly by an independent perinatologist |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 12 non-stress tests out of 650 and 15 vibroacoustic tests out of 650 were excluded due to poor quality of tracing - minimal loss |
| Selective reporting (reporting bias) | Unclear risk | Unclear - not clearly documented which outcomes were to be collected as part of the study |
| Other bias | Unclear risk | Groups appear to be well balanced for maternal age, gestational age and indications for antepartum fetal heart rate testing |
| Methods | Randomised controlled trial. | |
| Participants | 308 women with high-risk pregnancies (540 tests). | |
| Interventions | 270 non-stress tests and 270 manual stimulation tests. | |
| Outcomes | Incidence of non-reactive tests and time needed to produce a reactive test | |
| Notes | Report in only Abstract form. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | All fetal heart tracings were blindly reported by the same perinatologist who did not have information about the patients |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Unclear - data limited as report of trial in form of an Abstract |
| Selective reporting (reporting bias) | Unclear risk | Unclear - data limited as report of trial in form of an Abstract |
| Other bias | Unclear risk | Unclear - data limited as report of trial in form of an Abstract |
| Methods | Randomised. | |
| Participants | 1360 patients. | |
| Interventions | Doppler testing versus non-stress testing. | |
| Outcomes | Incidence of caesarean delivery for fetal distress, neonatal morbidity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Consenting patients were assigned randomly.” “Each envelope contained an allocation that had been determined with the use of a random number table with a variable block size of 4 and 6”. |
| Allocation concealment (selection bias) | Low risk | “Randomization was performed by opening sequentially numbered opaque envelopes.” “The nurse/sonaographer approached the unit clerk to obtain the next envelope in sequence. Envelopes were kept in a locked drawer that was accessible only to the unit clerk.” |
| Blinding of participants and personnel (performance bias) All outcomes |
High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes |
High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 16 patients were lost to follow up - reasons not reported. 4 patients were assigned randomly in error and did not have the identified high-risk condition and were removed from further analysis. Analysis occurred for 1340 women out of 1360 enrolled - minimal loss |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes appear to have been fully reported |
| Other bias | Low risk | There were no significant differences that were identified in the maternal demographic data - apart from a higher incidence of induction in the Doppler group for abnormal testing (31 women vs 13 women) |
FHR: fetal heart rate
ICU: intensive care unit
NICU: neonatal intensive care unit
VAS/mFBP: vibroacoustic stimulation with mock stimulation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Eglinton 1984 | This is a quasi-randomised trial. |
| Platt 1985 | This is a quasi-randomised trial. |
| Richardson 1983 | This is a quasi-randomised trial. |
DATA AND ANALYSES
Comparison 1. VAS/mFBP versus mFBP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal mortality and severe morbidity | 1 | 28 | Risk Ratio (M-H, Fixed, 95% CI) | 0.43 [0.04, 4.25] |
| 2 Caesarean section | 1 | 28 | Risk Ratio (M-H, Fixed, 95% CI) | 0.87 [0.27, 2.79] |
| 3 Perinatal mortality | 1 | 28 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Components of severe perinatal morbidity | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 4.1 NICU admission > 24 hours | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Organ failure | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Encephalopathy | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Labour induction | 1 | 28 | Risk Ratio (M-H, Fixed, 95% CI) | 0.69 [0.23, 2.05] |
| 6 Maternal death or severe maternal morbidity | 1 | 28 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Components of severe maternal morbidity | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 7.1 ICU admission > 24 hours | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Organ failure | 1 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Maternal hospital admission > 7 days | 1 | 28 | Risk Ratio (M-H, Fixed, 95% CI) | 0.87 [0.06, 12.52] |
Analysis 1.1. Comparison 1 VAS/mFBP versus mFBP, Outcome 1 Perinatal mortality and severe morbidity.
Review: Management of reported decreased fetal movements for improving pregnancy outcomes
Comparison: 1 VAS/mFBP versus mFBP
Outcome: 1 Perinatal mortality and severe morbidity
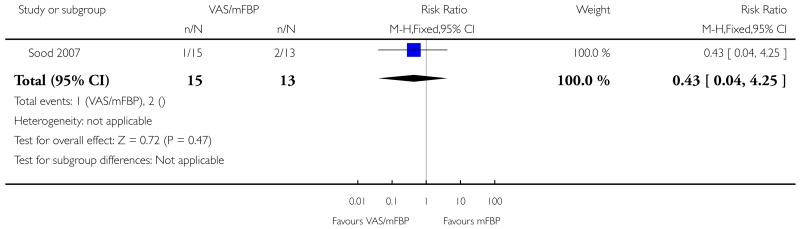 |
Analysis 1.2. Comparison 1 VAS/mFBP versus mFBP, Outcome 2 Caesarean section.
Review: Management of reported decreased fetal movements for improving pregnancy outcomes
Comparison: 1 VAS/mFBP versus mFBP
Outcome: 2 Caesarean section
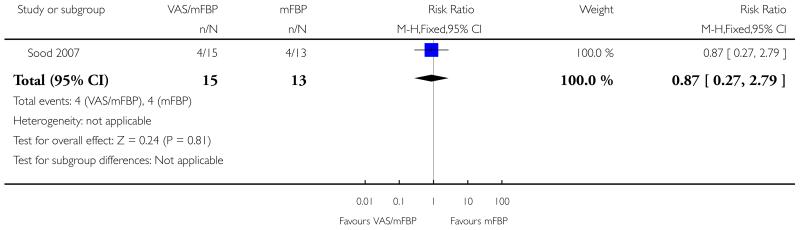 |
Analysis 1.3. Comparison 1 VAS/mFBP versus mFBP, Outcome 3 Perinatal mortality.
Review: Management of reported decreased fetal movements for improving pregnancy outcomes
Comparison: 1 VAS/mFBP versus mFBP
Outcome: 3 Perinatal mortality
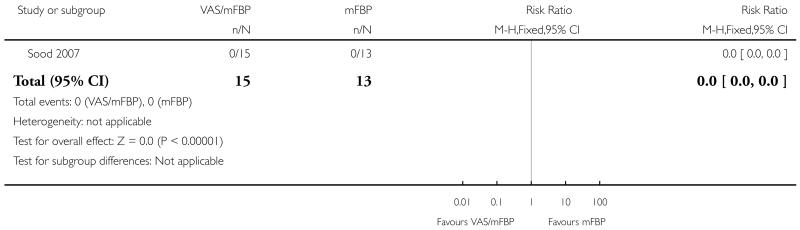 |
Analysis 1.4. Comparison 1 VAS/mFBP versus mFBP, Outcome 4 Components of severe perinatal morbidity.
Review: Management of reported decreased fetal movements for improving pregnancy outcomes
Comparison: 1 VAS/mFBP versus mFBP
Outcome: 4 Components of severe perinatal morbidity
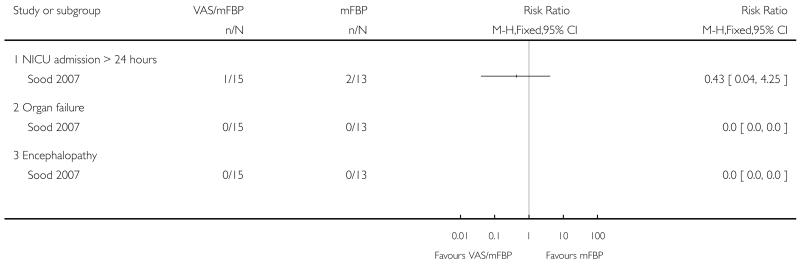 |
Analysis 1.5. Comparison 1 VAS/mFBP versus mFBP, Outcome 5 Labour induction.
Review: Management of reported decreased fetal movements for improving pregnancy outcomes
Comparison: 1 VAS/mFBP versus mFBP
Outcome: 5 Labour induction
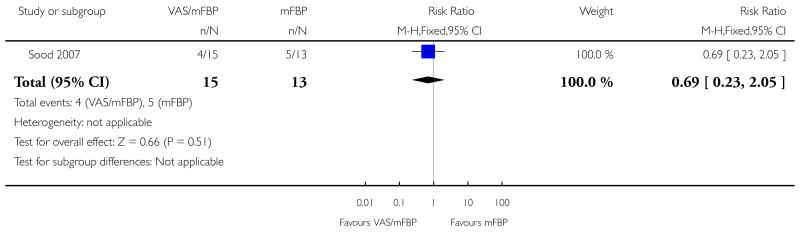 |
Analysis 1.6. Comparison 1 VAS/mFBP versus mFBP, Outcome 6 Maternal death or severe maternal morbidity.
Review: Management of reported decreased fetal movements for improving pregnancy outcomes
Comparison: 1 VAS/mFBP versus mFBP
Outcome: 6 Maternal death or severe maternal morbidity
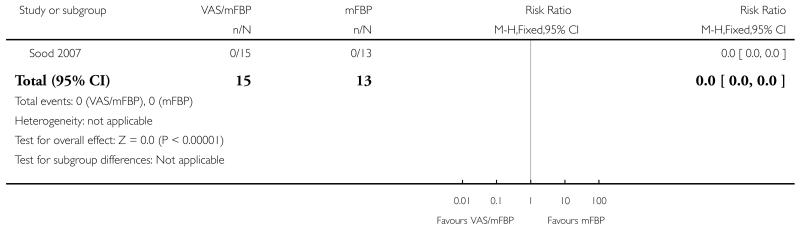 |
Analysis 1.7. Comparison 1 VAS/mFBP versus mFBP, Outcome 7 Components of severe maternal morbidity.
Review: Management of reported decreased fetal movements for improving pregnancy outcomes
Comparison: 1 VAS/mFBP versus mFBP
Outcome: 7 Components of severe maternal morbidity
 |
Analysis 1.8. Comparison 1 VAS/mFBP versus mFBP, Outcome 8 Maternal hospital admission > 7 days.
Review: Management of reported decreased fetal movements for improving pregnancy outcomes
Comparison: 1 VAS/mFBP versus mFBP
Outcome: 8 Maternal hospital admission > 7 days
 |
ADDITIONAL TABLES
Table 1. Management of reported decreased fetal movements.
| Study | Intervention A | Subgroup with decreased fetal movements/whole group n/N (%) | Intervention B | Subgroup with decreased fetal movements/whole group n/N (%) | Outcome | Main outcomes for whole group | ||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | RR (95% CI) | ||||||
| Computerised interpretation | ||||||||
| Bracero 1999 | Visual interpretation |
21/205 (10%) | Computerised interpretation |
23/205 (11%) | Perinatal mortality and severe morbidity |
20/201 | 11/204 | 1.85 [0.91 to 3.75] |
| Caesarean section |
NA | NA | ||||||
| Perinatal mortality alone |
1/201 | 0/204 | 3.04 [0.12 to 74.30] | |||||
| Doppler | ||||||||
| Burke 1992 | Doppler | 9/241 (4%) | Control | 16/235(7%) | Perinatal mortality and severe morbidity |
NA | NA | |
| Caesarean section |
58/241 | 51/235 | 1.11 [0.80 to 1.54] | |||||
| Perinatal mortality alone |
4/241 | 3/235 | 1.11 [0.80 to 1.54] | |||||
| Hofmeyr 1991 | Doppler | 49/438 (11%) | Fetal heart rate | 65/459 (14%) | Perinatal mortality and severe morbidity |
70/438 | 77/459 | 0.95 [0.71 to 1.28] |
| Caesarean section |
107/438 | 123/459 | 0.91 [0.73 to 1.14] | |||||
| Perinatal mortality alone |
4/438 | 8/459 | 0.52 [0.16 to 1.73] | |||||
| Johnstone 1993 | Doppler group | 157/1114 (14%) | Non-Doppler group | 181/1175 (15%) | Perinatal mortality and severe morbidity |
NA | NA | |
| Caesarean section |
170/1114 | 198/1175 | 0.91 [0.75 to 1.09] | |||||
| Perinatal mortality alone |
NA | NA | ||||||
| Williams 2003 | Doppler group | 147/649 (23%) | Non-stress test group | 162/691 (23%) | Perinatal mortality and severe morbidity |
16/649 | 24/691 | 0.71 [0.38 to 1.32] |
| Caesarean section |
183/649 | 223/691 | 0.87 [0.74 to 1.03] | |||||
| Perinatal mortality alone |
0/649 | 1/691 | 0.35 [0.01 to 8.70] | |||||
| Biophysical profile | ||||||||
| Jamal 2007 | Modified biophysical profile | 18/96 (19%) | Biophysical profile | 8/104 (8%) | Perinatal mortality and severe morbidity |
NA | NA | |
| Caesarean section |
NA | NA | ||||||
| Perinatal mortality alone |
1/96 | 1/104 | 1.08 [0.07 to 17.08] | |||||
| Abnormal test results |
30/96 | 24/104 | 1.52 [0.81 to 2.84] | |||||
| Manning 1984 | Biophysical profile scoring group, | 16/375 (4%) | Non-stress testing scheme group | 13/360(4%) | Perinatal mortality and severe morbidity |
NA | NA | |
| Caesarean section |
NA | NA | ||||||
| Perinatal mortality alone |
4/375 | 4/360 | 0.96 [0.24 to 3.81] | |||||
| Abnormal test result |
23/375 | 61/360 | 0.32 [0.19 to 0.53] | |||||
| Sood 2007 | Modified biophysical profile following vibroacoustic stimulation group | 15/110 (14%) | Mock stimulation group | 13/104 (13%) | Perinatal mortality and morbidity |
4/110 | 8/104 | 0.47 [0.15 to 1.52] |
| Caesarean section |
NA | NA | ||||||
| Perinatal mortality alone |
2/110 | 3/104 | 0.63 [0.11 to 3.70] | |||||
| Abnormal test result |
3/110 | 7/104 | 0.41 [0.11 to 1.53] first) | |||||
| Acoustic stimulation | ||||||||
| Newnham 1990 | Fetal acoustic stimulation group | 17/90 (19%) | Non-stress testing scheme group | 13/82 (16%) | Perinatal mortality and severe morbidity |
NA | NA | |
| Caesarean section |
NA | NA | ||||||
| Perinatal mortality alone |
0/90 | 0/82 | Not estimable | |||||
| Non-reactive CTG | 17/150* | 25/150* | 0.64 [0.33 to 1.24] | |||||
| Sleutel 1990 | Single 5 second vibroacoustic stimulus group | 1/23 (4%) | Intermittent vibroacoustic stimuli group | 5/22 (23%) | Perinatal mortality and severe morbidity |
NA | NA | |
| Caesarean section |
NA | NA | ||||||
| Perinatal mortality alone |
NA | NA | ||||||
| Smith 1986 | Fetal acoustic stimulation group | 63/366 (17%) | Non-stress test group | 49/349 (14%) | Perinatal mortality and severe morbidity |
NA | NA | |
| Caesarean section |
NA | NA | ||||||
| Perinatal mortality alone |
0/366 | 1/349 | 0.32 [0.01 to 7.78] | |||||
| Non-reactive test | 78/858* | 122/869* | 0.61 [0.45 to 0.83] | |||||
| Tongsong 1994 | Fetal acoustic stimulation group | 128/635 (20%) | Non-stress test group | 129/638 (20%) | Perinatal mortality and severe morbidity |
NA | NA | |
| Caesarean section |
NA | NA | ||||||
| Perinatal mortality alone |
NA | NA | ||||||
| Non-reactive CTG |
43/635* | 88/638* | 0.49 [0.35 to 0.70] | |||||
In May 2011 the lead authors on each of the following trials were contacted regarding the availability of outcomes specifically for cases admitted to the trial with decreased fetal movements. Data for this subgroup were available only for the 28 cases in Sood 2007. The data above refers to the whole sample in each trial.
NB refers to number of tests, not women
HISTORY
Protocol first published: Issue 5, 2011
Review first published: Issue 4, 2012
Footnotes
DECLARATIONS OF INTEREST
G Justus Hofmeyr was an author of an included study (Hofmeyr 1991a).
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
Added under ‘Types of participants’: “In the case of trials which have several entry criteria including DFM, we tabulated the trial data, indicating the proportion of the participants with DFM as the entry point. We limited results to our review primary outcomes and perinatal mortality, or if not reported, the main outcomes reported in the trials. We contacted trial authors to determine whether data on those participants with DFM were available, for inclusion in the review analysis”.
References to studies included in this review
* Indicates the major publication for the study
- Bracero 1999.Bracero LA, Morgan S, Byrne DW. Comparison of visual and computerized interpretation of nonstress test results in a randomized controlled trial. American Journal of Obstetrics and Gynecology. 1999;181(5 Pt 1):1254–8. doi: 10.1016/s0002-9378(99)70118-3. [published data only] [DOI] [PubMed] [Google Scholar]
- Burke 1992.*; Burke G, Stuart B, Crowley P, Ni Scanaill S, Drumm J. Does Doppler ultrasound alter the management of high-risk pregnancy?. Care Concern and Cure in Perinatal Medicine; 13th European Congress of Perinatal Medicine; Amsterdam, The Netherlands. Parthenon. 1992.May, pp. 597–604. [Google Scholar]; Burke G, Stuart B, Crowley P, Ni Scanaill S, Drumm J. Does Doppler ultrasound alter the management of high-risk pregnancy? Journal of Perinatal Medicine. 1992;20(Suppl 1):266. [published data only] [Google Scholar]
- Hofmeyr 1991.Hofmeyr GJ, Pattinson R, Buckley D, Jennings J, Redman CWG. Umbilical artery resistance index as a screening test for fetal well-being. II. Randomized feasibility study. Obstetrics & Gynecology. 1991;78:359–62. [published data only] [PubMed] [Google Scholar]
- Jamal 2007.Jamal A. A prospective trial of the fetal biophysical profile vs the modified biophysical in the management of high-risk pregnancies. Ultrasound in Obstetrics and Gynecology. 2005;26:376–471. [Google Scholar]; *; Jamal A, Marsoosi V, Eslamian L, Noori K. A prospective trial of the fetal biophysical profile versus modified biophysical profile in the management of high risk pregnancies. Acta Medica Iranica. 2007;45(3):204–8. [published data only] [Google Scholar]
- Johnstone 1993.Johnstone FD, Prescott R, Hoskins P, Greer IA, McGlew T, Compton M. The effect of introduction of umbilical Doppler recordings to obstetric practice. British Journal of Obstetrics and Gynaecology. 1993;100:733–41. doi: 10.1111/j.1471-0528.1993.tb14264.x. [published data only] [DOI] [PubMed] [Google Scholar]
- Manning 1984.Manning FA, Lange IR, Morrison I, Harman CR. Fetal biophysical profile score and the nonstress test: a comparative trial. Obstetrics & Gynecology. 1984;64:326–31. [published data only] [PubMed] [Google Scholar]
- Newnham 1990.Newnham JP, Burns SE, Roberman BD. Effect of vibratory acoustic stimulation on the duration of fetal heart rate monitoring tests. American Journal of Perinatology. 1990;7:232–4. doi: 10.1055/s-2007-999489. [published data only] [DOI] [PubMed] [Google Scholar]
- Sleutel 1990.Sleutel MR. Vibroacoustic stimulation and fetal heart rate in nonstress tests. Journal of Obstetric Gynecologic and Neonatal Nursing. 1990;19(3):199–204. doi: 10.1111/j.1552-6909.1990.tb01637.x. [published data only] [DOI] [PubMed] [Google Scholar]
- Smith 1986.Smith CV, Phelan JP, Platt LD, Broussard P, Paul RH. Fetal acoustic stimulation testing. II. A randomized clinical comparison with the nonstress test. American Journal of Obstetrics and Gynecology. 1986;155:131–4. doi: 10.1016/0002-9378(86)90095-5. [published data only] [DOI] [PubMed] [Google Scholar]
- Sood 2007.Sood AK. Vibroacoustic stimulation and modified fetal biophysical profile in high risk pregnancy. Journal of Obstetrics and Gynaecology of India. 2007;57(1):27–36. [published data only] [Google Scholar]
- Tongsong 1994.Tongsong T, Piyamongkol W. Comparison of the acoustic stimulation test with nonstress test. Journal of Reproductive Medicine. 1994;39:17–20. [published data only] [PubMed] [Google Scholar]
- Trungtawatchai 1999.Trungtawatchai S, Chanpraparp P. Comparison of the nonstress test (NST) and NST with manual stimulation test (MST) Thai Journal of Obstetrics and Gynaecology. 1999;11(4):273. [published data only] [Google Scholar]
- Williams 2003.Williams KP, Farquharson DF, Bebbington M, Dansereau J, Galerneau F, Wilson RD, et al. Screening for fetal well-being in a high-risk pregnant population comparing the nonstress test with umbilical artery doppler velocimetry: a randomized controlled clinical trial. American Journal of Obstetrics and Gynecology. 2003;188(5):1366–71. doi: 10.1067/mob.2003.305. [published data only] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Eglinton 1984.Eglinton GS, Paul RH, Broussard PM, Walla CA, Platt LD. Antepartum fetal heart rate testing. XI. Stimulation with orange juice. American Journal of Obstetrics and Gynecology. 1984;150:97–9. doi: 10.1016/s0002-9378(84)80116-7. [published data only] [DOI] [PubMed] [Google Scholar]
- Platt 1985.Platt LD, Walla CA, Paul RH, Trujillo ME, Loesser CV, Jacobs ND, et al. A prospective trial of the fetal biophysical profile vs the nonstress test in the management of high-risk pregnancies. American Journal of Obstetrics and Gynecology. 1985;153:624–33. [published data only] [PubMed] [Google Scholar]
- Richardson 1983.Richardson B, Briggs ML, Toomey C, Burry KJ, O’Grady JP. The effect of maternal glucose administration on the specificity of the nonstress test. American Journal of Obstetrics and Gynecology. 1983;145:141–6. doi: 10.1016/0002-9378(83)90480-5. [published data only] [DOI] [PubMed] [Google Scholar]
Additional references
- Alfirevic 2010.Alfirevic Z, Stampalija T, Gyte GML. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database of Systematic Reviews. 2010;(1) doi: 10.1002/14651858.CD007529.pub2. DOI: 10.1002/14651858.CD007529.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger 1997.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flenady 2009.Flenady V, MacPhail J, Gardener G, Chadha Y, Mahomed K, Heazell A, et al. Detection and management of decreased fetal movements in Australia and New Zealand: a survey of obstetric practice. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2009;49(4):358–63. doi: 10.1111/j.1479-828X.2009.01026.x. [DOI] [PubMed] [Google Scholar]
- Frøen 2001.Frøen JF, Arnestad M, Frey K, Vege Å , Saugstad OD, Stray-Pedersen B. Risk factors for sudden intrauterine unexplained death: epidemiologic characteristics of singleton cases in Oslo, Norway, 1986-1995. American Journal of Obstetrics and Gynecology. 2001;184:694–702. doi: 10.1067/mob.2001.110697. [DOI] [PubMed] [Google Scholar]
- Frøen 2008.Frøen JF, Tveit JV, Saastad E, Børdahl P, Stray-Pedersen B, Heazell AE, et al. Management of decreased fetal movements. Seminars in Perinatalogy. 2008;32:307–11. doi: 10.1053/j.semperi.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Grivell 2010.Grivell RM, Alfirevic Z, Gyte GML, Devane D. Antenatal cardiotocography for fetal assessment. Cochrane Database of Systematic Reviews. 2010;(1) doi: 10.1002/14651858.CD007863.pub2. DOI: 10.1002/14651858.CD007863.pub2. [DOI] [PubMed] [Google Scholar]
- Harbord 2006.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine. 2006;25(20):3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- Heazell 2008a.Heazell AE, Frøen JF. Methods of fetal movement counting and the detection of fetal compromise. Journal of Obstetrics and Gynaecology. 2008;28:147–54. doi: 10.1080/01443610801912618. [DOI] [PubMed] [Google Scholar]
- Heazell 2008b.Heazell AE, Green M, Wright C, Flenady V, Frøen JF. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica Scandinavica. 2008;87(3):331–9. doi: 10.1080/00016340801902034. [DOI] [PubMed] [Google Scholar]
- Higgins 2011.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Lalor 2008.Lalor JG, Fawole B, Alfirevic Z, Devane D. Biophysical profile for fetal assessment in high risk pregnancies. Cochrane Database of Systematic Reviews. 2008;(1) doi: 10.1002/14651858.CD000038.pub2. DOI: 10.1002/14651858.CD000038.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin 2008.Martin CB., Jr. Normal fetal physiology and behavior, and adaptive responses with hypoxemia. Seminars in Perinatology. 2008;32(4):239–42. doi: 10.1053/j.semperi.2008.04.003. [DOI] [PubMed] [Google Scholar]
- O’Sullivan 2009.O’Sullivan PO, Stephen G, Martindale E, Heazell AEP. Predicting poor perinatal outcome in women who present with decreased fetal movements. Journal of Obstetrics and Gynaecology. 2009;29:705–10. doi: 10.3109/01443610903229598. [DOI] [PubMed] [Google Scholar]
- Preston 2010.Preston S, Mahomed K, Chadha Y, Flenady V, Gardener G, MacPhail J, et al. Clinical Practice Guideline for the Management of Women who Report Decreased Fetal Movements. The Australian and New Zealand Stillbirth Alliance; Brisbane: Jul, 2010. [Google Scholar]
- RCOG 2011.Royal College of Obstetricians and Gynaecologists [accessed February 2012];Reduced Fetal Movements. http://www.rcog.org.uk/womens-health/clinical-guidance/reduced-fetal-movements-green-top-57. RCOG Green-top Guideline No. 57.
- RevMan 2011.The Nordic Cochrane Centre. The Cochrane Collaboration . Review Manager (RevMan). 5.1. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- Sinha 2007.Sinha D, Sharma A, Nallaswamy V, Jayagopal N, Bhatti N. Obstetric outcome in women complaining of reduced fetal movements. Journal of Obstetrics and Gynaecology. 2007;27:41–3. doi: 10.1080/01443610601016909. [DOI] [PubMed] [Google Scholar]
- Skornick-Rapaport 2010.Skornick-Rapaport A, Maslovitz S, Kupferminc M, Lessing JB, Many A. Proposed management for reduced fetal movements: five years’ experience in one medical center. Journal of Maternal-Fetal Medicine. 2010 Sep 9; doi: 10.3109/14767058.2010.511338. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Tan 2001.Tan KH, Smyth RMD. Fetal vibroacoustic stimulation for facilitation of tests of fetal well-being. Cochrane Database of Systematic Reviews. 2001;(1) doi: 10.1002/14651858.CD002963. DOI: 10.1002/14651858.CD002963. [DOI] [PubMed] [Google Scholar]
- Tuffnell 1991.Tuffnell DJ, Cartmill RS, Lilford RJ. Fetal movements; factors affecting their perception. European Journal of Obstetrics & Gynecology, and Reproductive Biology. 1991;39(3):165–7. doi: 10.1016/0028-2243(91)90052-m. [DOI] [PubMed] [Google Scholar]
- Tveit 2009.Tveit JV, Saastad E, Stray-Pedersen B, Børdahl PE, Flenady V, Fretts R, et al. Reduction of late stillbirth with the introduction of fetal movement information and guidelines - a clinical quality improvement. BMC Pregnancy and Childbirth. 2009;9:32. doi: 10.1186/1471-2393-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterscheider 2010.Unterscheider J, Horgan RP, Greene RA, Higgins JR. The management of reduced fetal movements in an uncomplicated pregnancy at term: results from an anonymous national online survey in the Republic of Ireland. Journal of Obstetrics and Gynaecology. 2010;30(6):578–82. doi: 10.3109/01443615.2010.481733. [DOI] [PubMed] [Google Scholar]
- Warrander 2011.Warrander LK, Heazell AE. Identifying placental dysfunction in women with reduced fetal movements can be used to predict patients at increased risk of pregnancy complications. Medical Hypotheses. 2011;76(1):17–20. doi: 10.1016/j.mehy.2010.08.020. [DOI] [PubMed] [Google Scholar]