Abstract
Rationale
Vulnerability to alcoholism is determined by many factors, including the balance of pleasurable vs. aversive alcohol-induced sensations: pleasurable sensations increase drinking, aversive sensations reduce it. Both female sex and adolescent age are associated with fewer use-limiting effects of ethanol and more rapid development of alcohol abuse.
Objectives
This study assessed voluntary drinking and the aversive effects of alcohol in adolescent and adult male and female rats, to determine whether these measures are inversely related across the sexes and development.
Methods
Voluntary drinking of 20% ethanol in an every-other-day (EOD) availability pattern and the full dose-response relationship of ethanol CTA were assessed in male and female adolescent and adult rats.
Results
CTA was sex-specific in adult but not adolescent rats, with adult females exhibiting less aversion. Voluntary ethanol consumption varied according to age and interindividual differences in consumption patterns but was not sex-specific. Adolescents initially drank more than adults, exhibited greater day-to-day variation in consumption, were more susceptible to the alcohol deprivation effect, and took longer to establish individual differences in consumption patterns.
Conclusions
These results show that the evolution of drinking patterns differs in adolescents and adults. While a small cohort of adults establish high consumption patterns quickly, most adolescents drink at high levels initially and show marked deprivation-induced increases, but a significant percentage reduce intake as they become adult. High drinking adolescents do not ramp up like adults, but maintain adolescent drinking patterns into adulthood. Sex differences were not observed in EOD drinking during either adolescence or adulthood.
Introduction
It is well-established that most problematic drug use begins during adolescence (Chen and Kandel 1995; Robins and Przybeck 1985). However, the mechanisms (both biological and sociological) underlying this observation are poorly understood. It is also well-established that the likelihood of repeatedly consuming an addictive substance is at least partly determined by the balance of rewarding and aversive effects within each user (Schuckit et al. 2006; Schuckit et al. 2009; Schuckit et al. 1997; Verendeev and Riley 2012). Sex also influences the development of drug abuse. More men than women suffer from substance use disorders, but women who become addicted have a more rapid transition from initial use to addiction than their male counterparts, a phenomenon known as “telescoping” (Randall et al. 1999). Evidence suggests that age, sex, and sensitivity to rewarding and aversive effects all interact to promote and/or discourage the development of substance use disorders within each individual. In this study, we examined these three factors in a rodent model.
Factors Determining Aversive effects of Drugs of Abuse
Previous studies have shown that adolescent rodents are less sensitive than adult rodents to the aversive effects of drugs of abuse. This observation applies to both conditioned and unconditioned aversive effects. Adolescents exhibit reduced conditioned taste aversion (CTA) to cocaine (Schramm-Sapyta et al. 2006), nicotine (Shram et al. 2006; Wilmouth and Spear 2004), THC (Schramm-Sapyta et al. 2007), amphetamine (Infurna and Spear 1979), and ethanol (Holstein et al. 2011; Schramm-Sapyta et al. 2010; Schramm-Sapyta et al. 2008; Vetter-O’Hagen et al. 2009). Adolescents also exhibit reduced unconditioned “use-limiting” effects of ethanol, such as motor incoordination and sedation (Little et al. 1996), hangover-related anxiety, and social and exploratory behaviors (Doremus-Fitzwater and Spear 2007; Varlinskaya and Spear 2004a; b).
Sex differences in aversive effects have also been examined in response to many drugs of abuse. Generally, males exhibit stronger aversive reactions than females, though there are exceptions. Males show greater aversion to ethanol (Cailhol and Mormede 2002; Lucas and McMillen 2002; Sherrill et al. 2011), but this effect may be strain- (Roma et al. 2007; Roma et al. 2006) and age-dependent (Vetter-O’Hagen et al. 2009). Males are also more averse to cocaine (injected subcutaneously but not intraperitoneally) (Busse et al. 2005), THC (Chambers and Sengstake 1976), and the non-addictive emetic lithium chloride (Chambers et al. 1981; Choleris et al. 2000; Foy and Foy 2003). Females, however, seem to be more averse to amphetamine (Roma et al. 2008) and a magnetic field (Cason et al. 2006). No sex difference was observed in response to nicotine (Rinker et al. 2008) and morphine (Randall-Thompson and Riley 2003). Thus, sex differences in aversion to addictive drugs are substance-specific and susceptible to other experimental manipulations.
Impact of Aversiveness on Voluntary Alcohol Consumption
Prior studies have examined the relationship between conditioned aversive effects and voluntary alcohol consumption. Across an array of both inbred and outbred mouse and rat strains, CTA is negatively correlated with voluntary alcohol drinking (Green and Grahame 2008). Rats that are selectively bred to prefer alcohol have reduced ethanol CTA compared to their non-preferring counterparts (Brunetti et al. 2002; Froehlich et al. 1988). Within a population of outbred rats, CTA is negatively correlated with voluntary consumption in adolescent but not adult male rats (Schramm-Sapyta et al. 2010). These studies suggested that alcohol consumption should decline in male rats as they mature from adolescence to adulthood and their aversion increases. In contrast, consumption in females should increase or remain high as they mature, reflecting retention of adolescent levels of low aversiveness. One goal of the present study was to test that hypothesis.
Sex Differences in Voluntary Alcohol Consumption
The examination of sex differences in voluntary ethanol drinking in rodents has produced complex results. Male rats drink more ethanol when ethanol concentrations are high or when ethanol access is schedule-induced (Bell et al. 2004; van Haaren and Anderson 1994). Female mice drink more in an every-other day pattern of availability (Hwa et al. 2011). In at least one study, male and female mice consumed different amounts of ethanol but achieved comparable blood ethanol concentrations (Rhodes et al. 2007), a pattern that has also been well-documented in humans (York et al. 2003; York and Welte 1994).
In female rats, ovarian hormones play a role in determining the level of alcohol consumption. Ovariectomy decreases voluntary consumption levels (Becker et al. 1985; El-Mas and Abdel-Rahman 2000) and estrogen replacement restores these levels (Becker et al. 1985; Ford et al. 2002; Li and Lumeng 1984). Drinking levels of male rats were not compared in these studies, though exogenous estradiol has been shown to increase drinking in males (Hilakivi-Clarke 1996).
These sex differences emerge during adolescence in both humans and rodents (Schulte et al. 2009), suggesting that the onset of pubertal hormones may affect ethanol intake. However, the role of pubertal hormones in this change has been questioned in at least one study (Vetter-O’Hagen and Spear 2011) which reported an effect of gonadectomy in males only.
Age Differences in Voluntary Consumption
A handful of studies have compared adolescent and adult rodents in voluntary ethanol intake. These studies suggest that, like sex effects, age effects also depend on the pattern of ethanol availability. For example, in a 2-hour limited access model (sweetened 20% ethanol vs. water available for 2 hours per day in water-restricted rats), adolescent males consumed more than adult males (Vetter-O’Hagen and Spear 2011). Adolescent rats also consumed more than adult rats in a three-days per week (Monday, Wednesday, Friday), 3-bottle choice model (5%, 20%, tap water) (Daoura et al. 2011). In contrast, in an overnight access model (8% ethanol vs. water available for 16 hours per night), we observed no differences in voluntary consumption between adolescent rats except when there was a 2-day ethanol deprivation period (Schramm-Sapyta et al. 2010). It is possible, therefore, that age differences in voluntary consumption depend on the presence of breaks in availability or “alcohol deprivation.” This possibility has been explored further in the current report.
The present study assessed the relationship of developmental and sex differences in alcohol CTA and voluntary consumption by examining adolescent and adult male and female rats. In CTA, we observed sex differences in adult but not adolescent rats. In voluntary consumption, we observed age differences and interindividual differences, but no sex differences.
Methods
Animals
Male and female CD rats (an outbred, Sprague-Dawley-derived strain) were obtained from Charles River Laboratories, Raleigh, NC. Adolescent rats were received at postnatal day 21 and adult rats were received at postnatal day 58 and allowed to acclimate to the housing facility for one week before the start of testing. Rats were maintained in a temperature and humidity-controlled vivarium on a standard 12:12 light-dark cycle during all experiments (lights on at 7am). Food and water were available ad libitum except as indicated below. Separate batches of rats were used for Conditioned Taste Aversion and Ethanol Drinking experiments. All procedures were approved by Duke University’s Animal Care and Use committee.
Conditioned Taste Aversion
Ethanol conditioned taste aversion (CTA) was performed as previously described (Schramm-Sapyta et al. 2010). A total of 196 adult and 144 adolescent experimentally naïve rats were used for this experiment, divided into 10 adult and 7 adolescent cohorts. Each cohort contained both male and female rats, but adolescents and adults were tested in separate cohorts for the sake of maintaining manageable group sizes.
As previously reported, rats that consumed less than 1mL of total fluid on either the water day, saccharin day, or test day were excluded from further analysis. In the adult group, a total of 38 adult rats were excluded for this reason. An additional 8 adult rats were excluded due to illness. In the adolescent group, 2 rats were excluded for drinking less than 1 mL of fluid, 3 were excluded due to illness, and an additional 4 were excluded due to experimenter error.
Adults were tested in CTA at doses of 0, 0.5, 1, and 3.5 g/kg ethanol. Adolescents were tested at 0, 0.5, 1 and 2 g/kg ethanol. The lower maximum dose was used in adolescents based on preliminary experiments in which several adolescents died after receiving 3.5 g/kg. Percent saccharin choice was calculated as the ratio of saccharin consumed on test day to the total fluid consumption.
Determination of Estrous Stage
Vaginal lavage was performed to assess estrous stage in the adult female rats as previously described (Walker et al. 2001; Walker et al. 2002). The tip of a medicine dropper (3 mm outside diameter) was gently inserted into the vagina and 0.25-ml saline at room temperature was washed in and out several times. This lavage fluid was then placed on a microscope slide and allowed to dry. Slides were then fixed with ethanol and stained with toluidine blue. Identification of cell types was made microscopically according to published methods (Long and Evans 1922).
Every-Other-Day drinking
A total of 70 experimentally naïve rats were tested in the drinking experiment, divided into 4 cohorts. Each cohort contained both male and female rats. Adolescent and adult rats were tested separately to maintain manageable group sizes. Adolescent rats were allowed access to ethanol or water from 28 to 65 days of age; adult rats were allowed access from 65 to 102 days of age, resulting in 17 sessions of access for each group.
Rats were allowed to consume either tap water or 20% ethanol (in tap water) in an every-other-day pattern. They were given the two bottle choice on Mondays, Wednesdays, and Fridays, beginning at 4pm. Access was provided in the rats’ home cages (standard plastic cages with wire racks for food and water access, Allentown Caging, 24 × 26 × 45 cm). On Tuesdays, Thursdays, and Saturdays, the two bottles were removed at 4pm and replaced with a single bottle of tap water. The volume consumed was assessed by weighing bottles before and after each drinking opportunity. Rats were weighed on ethanol presentation days, immediately prior to placement of ethanol and water bottles, for calculation of g/kg ethanol consumption. Rats were housed individually throughout testing, and food was available ad libitum. This procedure was adapted from published methods (Simms et al. 2008). Blood alcohol content was not assessed in these rats to avoid the association of ethanol with the potentially aversive experience of a needle-stick to withdraw blood. Previous citations have shown that the EOD drinking method achieves blood alcohol levels in the range of 5–80 mg/dL in Sprague-Dawley rats, and that intake correlates well with blood alcohol level (Li et al. 2011). Another study with Sprague-Dawley rats has similarly demonstrated that intake is related to BEC (higher intake associated with higher BEC) (Vetter-O’Hagen and Spear 2011). Similar results have been documented in an ethanol-preferring strain (Sabino et al. 2013).
Ethanol g/kg/day was calculated based on the change in ethanol bottle weight, percentage ethanol (20%) and density of ethanol (0.789 g/mL) and the rat’s weights. Water consumption was calculated based on the change in water bottle weight and rat’s daily weights. Preference was calculated as % of total fluid consumed (change in ethanol bottle weight divided by the sum of change in ethanol + water bottle weights). Careful observers will note a slight “bump” in water consumption in the adolescent animals on day 12. This occurred in one of the cohorts over a holiday weekend, and does not substantively change our conclusions, so we have opted to retain data from all subjects.
Statistics
Conditioned taste aversion data were analyzed using a two-way ANOVA (sex × dose) in each age group. To assess whether the current study replicated previous studies comparing adolescent vs. adult males, these 2 groups were combined and analyzed using a two-way ANOVA (age × dose). Age differences, sex differences, dose effects, and interaction effects were considered significant at p<0.05.
Every-other-day drinking data were analyzed using repeated measures ANOVA with drinking day as the within-subjects factor and sex and age as the between-subjects factors. Age and sex differences and interaction effects were considered significant at p<0.05. Classification of rats as high or low drinkers was performed by averaging the g/kg consumed on the last 5 drinking sessions and then taking the top (high) and bottom (low) thirds of each age group.
Results
Conditioned Taste Aversion in Adolescents
As shown in Figure 1A, we obtained a significant dose effect in adolescent male and female rats: higher doses created stronger aversions as indicated by lower percent saccharin choice values. In contrast, we observed no effect of sex on ethanol-conditioned taste aversion in the adolescent group. (Dose effect, F(3, 127)=14.6, p<0.0001; Sex effect, ns.)
Figure 1.
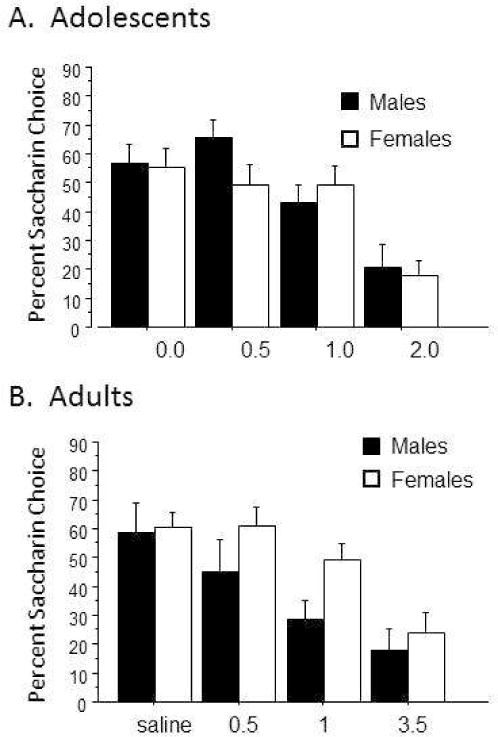
Conditioned Taste Aversion. Rats were tested in Ethanol Conditioned Taste Aversion at the doses indicated. Bars indicate average percent saccharin choice +/− SEM. Panel A: Adolescent Rats. Males: closed bars; females: open bars. Main effect of dose, p<0.0001; effect of sex, ns. N=16–18/dose. Panel B: Adult rats. Males: Closed bars; females: open bars. Main effect of dose: p<0.0001; main effect of sex: p=0.048; sex × dose interaction, ns. Males, N=10–13/dose; Females, N=16–36 per dose. Because of our interest in examining the effect of estrus stage, experimenters were blinded to estrus state until after CTA testing. Thus there are higher numbers of females in some groups and a wide range in number of females per dose.
Conditioned Taste Aversion in Adults
As shown in Figure 1B, conditioned taste aversion to ethanol was also dose-sensitive in adult rats, with increasing dose again leading to stronger aversions (dose effect, F(3, 142)=9.3; p<0.0001). In addition, we observed a significant effect of sex on ethanol CTA in adult rats. Males showed stronger aversions (effect of sex, F(1, 142)=3.97, p=0.048); (sex × dose interaction, ns).
There was a non-significant trend for an effect of estrus stage in the female rats (data not shown: proestrus females vs. non-proestrus females, F(1,96)=3.32; p=0.07; estrus stage × dose interaction, ns). A similar analysis on a subset of the animals examining the effect of estrous stage on the choice day, rather than the saccharin-association day, revealed that the estrous stage on choice day also did not affect taste aversion (F(2,101)=1.79, p=0.17), data not shown.
CTA in adolescent vs. adult male rats
To determine whether the current results replicated previously published observations that adolescent males experience less aversion than adult males at moderate doses of ethanol, we compared the adolescent and adult males given 0.5 and 1 g/kg ethanol (despite the fact that they were tested in different cohorts by different experimenters). In this analysis, there was a significant effect of dose (F(2,79)=4.6; p=0.01), and a significant effect of age (F(1,51)=5.5, p=0.02). Thus, the present results replicated the previously published age difference in ethanol CTA (data not shown, (Schramm-Sapyta et al. 2010)).
Every-Other-Day Drinking
As shown in Figure 2, rats consumed significant amounts of ethanol in 24-hour exposure periods in the EOD protocol. Average consumption ranged from 2–7 g/kg/session. Drinking varied significantly across the 18 days of availability (RMANOVA, effect of day: F(16, 1056)=7.1, p<0.0001). Adolescent and adult rats altered their drinking in different patterns across the drinking schedule (age × day interaction, F(16, 1056)=9.4, p<0.0001), as discussed in detail below. Most of the age differences in drinking occurred early in the ethanol exposure schedule, so that by the end of the schedule, adolescents (now young adults) consumed at the same level as adults (now older adults).
Figure 2.
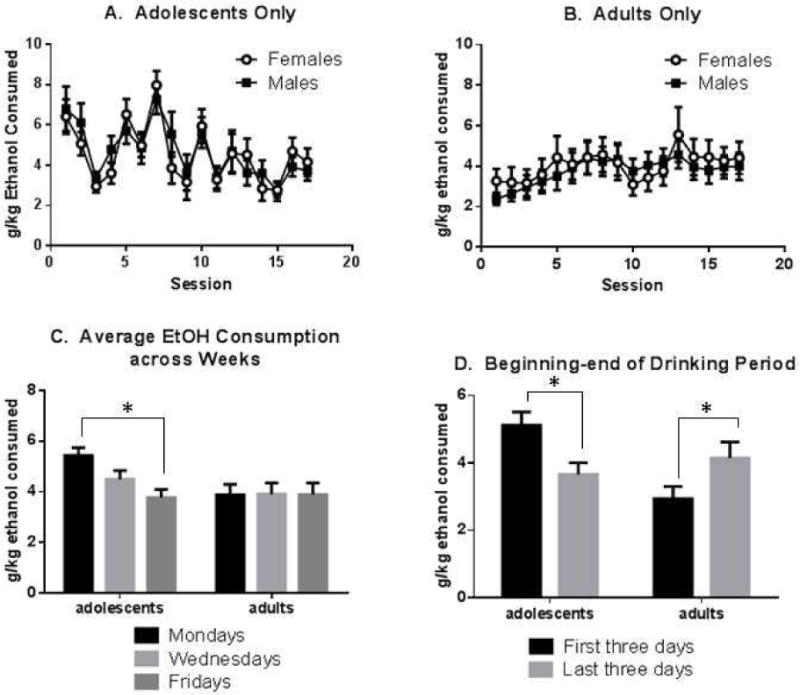
Voluntary Drinking in EOD Availability Schedule. Panel A: g/kg ethanol consumed on ethanol-available sessions for male and female adolescents. Panel B: g/kg ethanol consumed by male and female adults. Panel C: average g/kg ethanol consumed by adolescent and adult rats (both sexes combined) across the 3 sessions of ethanol availability each week. Panel D: average g/kg ethanol consumed on the first 3 sessions vs. the last 3 sessions for adolescent and adult rats (both sexes combined). *, indicated groupings are significantly different, p<0.05; see text for detailed statistics. N=17–18 per age/sex grouping. Bars indicate average g/kg consumed +/− SEM.
There was no main effect of sex on voluntary ethanol consumption in this exposure schedule, and also no sex interaction (age × sex × day interaction, ns and sex × day interaction, ns). The drinking curves for male and female adolescents completely overlap (Figure 2A), as do the curves for male and female adults (Figure 2B), although the shape of adolescent curve differs significantly from the adult curve, as discussed below.
The drinking pattern across the full availability period differs in two important ways between adolescents and adults. First, adolescents show more day-to-day variation, as evident in Figure 2A. These data are summarized in Figure 2C, which shows that adolescents demonstrate a regular pattern of change within each week of drinking. On average, adolescents consumed more on Mondays, then decreased consumption on Wednesdays and Fridays. In contrast, adults’ consumption did not vary from Monday to Wednesday to Friday. Thus, the effect of the “weekend day off” was to increase subsequent drinking in adolescents only (age × day interaction: F(2, 136)=21.0, p<0.0001; Day effect in adolescents only, F(2, 66)=27.4, p<0.0001; Day effect in adults only, ns).
The two age groups also exhibited differing changes in consumption from beginning to end of the drinking period. As shown in Figure 2D, adolescents experienced a net decrease in consumption from beginning to end of ethanol availability (comparing the average of the first three drinking days to the last 3 drinking days), whereas adults experienced a net increase in consumption. (Age × time interaction, F(1, 68)=18.8, p<0.0001; time effect in adults, F(1,35)=9.1, p=0.0047; time effect in adolescents, F(1,33)=9.6, p=0.0039).
Adolescents and adults also differed in terms of the establishment of high vs. low individual drinking levels, as shown in Figure 3A and B. Rats were categorized as high or low drinkers based on their average g/kg consumed on the last 5 drinking sessions. Regardless of their final drinking level, all adolescents initially drank at a high level; the high-drinker vs. low-drinker categorization did not emerge until about halfway through the drinking period. (Adolescent high drinkers were not different from adolescent low drinkers on days 1, 5, 6, 7, and 8. Adolescent high and low drinkers were statistically different from each other on the last 9 days of the drinking period. (Day 2, (p=0.009), 3 (p=0.01), 4 (p=0.049), 9 (p=0.009), 10 (p=0.004), 11 (p=0.004), 12 (p<0.0001), 13 (p=0.0002), 14 (p<0.0001), 15, (p<0.0001), 16 (p=0.0004), and 17 (p<0.0001) by Fisher’s PLSD.)) In contrast, high-drinking adults differentiated themselves from low-drinking adults immediately. Adult high drinkers were significantly different from adult low drinkers on Day 1 and throughout the drinking period (Figure 3B, p<0.01 for all days. P<0.0001 for most days). Thus, consistent drinking levels were not established within adolescent individuals until the 3rd–4th week of drinking, whereas they were established immediately in adults. These patterns were evident and overlapping in both sexes, and equivalent numbers of male and female rats were categorized as high and low drinkers in both age groups (data not shown).
Figure 3.
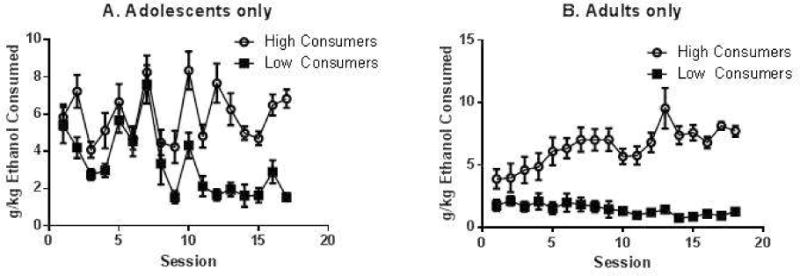
High vs. low-drinking levels in adolescence (Panel A) and adulthood (Panel B). Rats from Figure 3A and B were split into groups based on their ethanol consumption on the last 5 drinking days (males and females combined). The highest and lowest third of each group are plotted. N=11–12/age group per high vs. low grouping (males and females combined). Bars indicate average g/kg consumed +/− SEM. See text for detailed statistics.
Ethanol Preference
As shown in Figure 4A, ethanol preference increased across the availability period in the highdrinking animals (the same animals which were identified as being in the top third of drinkers on a g/kg basis in Figure 3), but not the low-drinking animals (RMANOVA, age × drinking level interaction, F(1,42)=33.5, p<0.0001; age × drinking level × session interaction, F(16,672)=3.13, p<0.0001). Preference was greater in high-drinking adults than in high-drinking adolescents (RMANOVA, high drinkers only: effect of age, F(1,19)=34.2, p<0.0001). Low drinking animals, in contrast, showed a consistently low preference (10% or below) for ethanol, regardless of age (RMANOVA, low drinkers only: effect of age, ns). The age difference in preference in the high drinkers is likely driven by differences in water intake, as indicated in Figure 4B. Adolescent rats consumed more water than adult rats, regardless of their ethanol consumption level (RMOANVA, effect of age: F(1,44)=95.0, p<0.0001; effect of drinking level, ns). The water consumption curves for high and low-drinking adolescents completely overlap, as do the curves for high and low-drinking adults. Comparing figure 3 with Figure 4, it is evident that high-consuming adolescents and adults consumed similar g/kg ethanol, but differing amounts of water, thereby resulting in differing preference. There was no effect of sex on preference.
Figure 4.
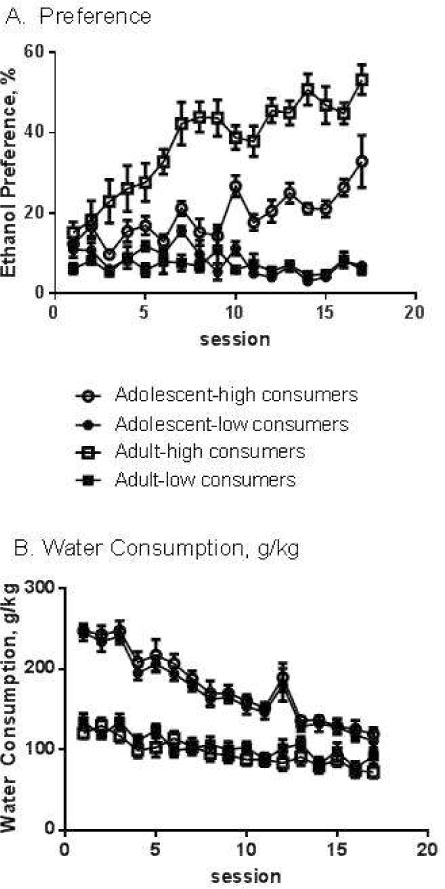
Ethanol preference (Panel A) and water consumption (Panel B) in high- and low-consuming adolescent and adult rats. Bars indicate average preference (Panel A) and average water g/kg consumed (Panel B) +/−SEM. See text for detailed statistics.
Discussion
These experiments reveal age differences in patterns of drinking across a six-week period of every-other-day ethanol availability. Adolescents were more susceptible to days off in the availability schedule but showed a net decline from beginning to end of the drinking period, whereas adults showed a net increase across the drinking period. High-and low-drinking individuals were observed in both age groups, and these individual differences were established more quickly in adults than in adolescents. Adults exhibited greater ethanol preference than adolescents, likely attributable to lower water consumption.
Voluntary drinking was not sex-dependent in either age group. In contrast, ethanol conditioned taste aversion was sex-dependent in adults, with females exhibiting less aversion than males. These results also confirmed our previous finding of stronger aversion in adult males compared to adolescent males. Taken together, these results suggest a disconnect between aversion and voluntary drinking in adult rats: adult females found ethanol less aversive but did not consume more ethanol on a g/kg basis or exhibit stronger preference. As mentioned in the introduction, results from previous experiments comparing voluntary drinking in male vs. female rodents have obtained varying results depending upon the availability pattern and other factors. The model used here approximates the common human pattern in which men and women consume similar amounts on a g/kg basis (York et al. 2003; York and Welte 1994).
The adolescent drinking pattern observed here, in which intake declines with maturation, is typical of the pattern exhibited by most human adolescents. Consumption typically peaks in the late teens and declines to a steady-state adult level (Chen and Kandel 1995). The adult drinking pattern observed here, involving a steady increase in consumption followed by a plateau, could reflect the development of tolerance and habituation to the ethanol consumption patterns.
The “weekend effect” we observed, in which adolescent rats increased consumption after a day off in ethanol availability, is reminiscent of previous results from our laboratory in which adolescents but not adults increased consumption after a 2-day break in availability (Schramm-Sapyta et al. 2010). This “alcohol deprivation effect” (Sinclair and Senter 1968) is thought to model human alcoholics who abstain and then relapse to consume higher levels than they had before abstinence (Rodd et al. 2004). Thus, two experiments from our laboratory, using different availability schedules, suggest that adolescents are more vulnerable than adults to the alcohol deprivation effect, which may be an indicator of increased vulnerability to alcoholism (Spanagel and Holter 1999). Similar observations have been described for stress-induced reinstatement of alcohol drinking (Fullgrabe et al. 2007; Siegmund et al. 2005). The age difference in the alcohol deprivation effect could result from age differences in ethanol withdrawal, however, adolescents are known to have reduced ethanol withdrawal symptoms compared to adults (Doremus-Fitzwater and Spear 2007; Doremus et al. 2003; Ristuccia and Spear 2005; Varlinskaya and Spear 2004a), which would predict results in the opposite direction.
The difference in establishment of individual drinking levels between adolescent-onset and adult-onset drinkers suggests that different factors determine drinking level in these two developmental periods within each individual. Adults quickly sort into high vs. low-drinkers and maintain these differences. In contrast, all adolescents began drinking at a high level, then bounced up and down in drinking level before eventually establishing consistent high vs. low levels toward the end of the ethanol availability period. As indicated previously, drinking in adolescents but not adults is partially predicted by conditioned aversive effects (Schramm-Sapyta et al. 2010). We speculate that developmental differences in specific neural circuits, perhaps those mediating responses to aversive experiences, contribute to the initially high drinking levels observed in all adolescents. These data as a whole demonstrate that, in the every-other-day drinking protocol, both individual factors (alcohol avoidance or acceptance) and developmental factors contribute to alcohol consumption patterns as adolescents mature into adulthood.
Relationship of Aversion to Voluntary Drinking
In adolescent animals, the lack of a sex difference in CTA was paralleled by a lack of sex difference in voluntary drinking. Although a simultaneous lack of effect is not evidence of a correlation between the two measures, these results parallel our previous finding that individual differences in Ethanol-CTA in adolescent males are predictive of subsequent voluntary ethanol consumption (Schramm-Sapyta et al. 2010). In adults, the interpretation is more complicated. We observed a sex difference in CTA, but no sex difference in voluntary drinking. A recent report (Vetter-O’Hagen et al. 2009) describes a sex difference in voluntary drinking but no sex difference in CTA in adult rats. These two differing results lead to the same conclusion: CTA does not predict sex differences in voluntary drinking in outbred adult rats.
Across ages, an understanding of the relationship between aversion and voluntary drinking requires careful examination of the phase of drinking. Adult males exhibited stronger aversion than adolescent males, and adults initially consumed less ethanol than adolescents. However, after extended ethanol availability, adolescents decreased while adults increased consumption. In contrast, ethanol preference was consistently greater in adults than in adolescents. The complexity of these results underscores the importance of taking into account interindividual differences rather than group differences in such analyses as well as careful examination of drinking history. Future work will examine the relationship of aversion to consumption across individuals within these sex and age groups.
It is also possible that the age difference in CTA is attributable to other factors, such as differential water deprivation (adolescents need more water due to their growth spurt), differences in neophobia (adolescents are known to be more novelty-seeking) and differences in sweet preference (adolescents are known to be more attracted to sweet taste). However, it has not yet been established whether these differences apply to males and females equally, and the current experiments were not designed to fully test these effects. Such determinations will be the subject of future studies.
References
- Becker HC, Anton RF, De Trana C, Randall CL. Sensitivity to ethanol in female mice: effects of ovariectomy and strain. Life Sci. 1985;37:1293–300. doi: 10.1016/0024-3205(85)90244-9. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Hsu CC, Lumeng L, Li TK, Murphy JM, McBride WJ. Effects of concurrent access to a single concentration or multiple concentrations of ethanol on ethanol intake by periadolescent high-alcohol-drinking rats. Alcohol. 2004;33:107–15. doi: 10.1016/j.alcohol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Brunetti G, Carai MA, Lobina C, Melis S, Serra S, Vacca G, Gessa GL, Colombo G. Differences in ethanol-induced conditioned taste aversion in Sardinian alcohol-preferring and Sardinian alcohol-nonpreferring rats. Alcohol. 2002;26:167–72. doi: 10.1016/s0741-8329(02)00195-7. [DOI] [PubMed] [Google Scholar]
- Busse GD, Freeman KB, Riley AL. The interaction of sex and route of drug administration in cocaine-induced conditioned taste aversions. Pharmacol Biochem Behav. 2005;81:814–20. doi: 10.1016/j.pbb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Conditioned taste aversion and alcohol drinking: strain and gender differences. J Stud Alcohol. 2002;63:91–9. [PubMed] [Google Scholar]
- Cason AM, Denbleyker M, Ferrence K, Smith JC, Houpt TA. Sex and estrous cycle differences in the behavioral effects of high-strength static magnetic fields: role of ovarian steroids. Am J Physiol Regul Integr Comp Physiol. 2006;290:R659–67. doi: 10.1152/ajpregu.00305.2005. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Sengstake CB. Sexually dimorphic extinction of a conditioned taste aversion in rats. Animal learning & behavior. 1976;4:181–5. doi: 10.3758/bf03214032. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Sengstake CB, Yoder RL, Thornton JE. Sexually dimorphic acquisition of a conditioned taste aversion in rats: effects of gonadectomy, testosterone replacement and water deprivation. Physiol Behav. 1981;27:83–8. doi: 10.1016/0031-9384(81)90303-6. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–7. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Ossenkopp K, Kavaliers M, Valsecchi P, Prato FS. Sex differences in conditioned taste aversion and in the effects of exposure to a specific pulsed magnetic field in deer mice Peromyscus maniculatus. Physiol Behav. 2000;71:237–49. doi: 10.1016/s0031-9384(00)00323-1. [DOI] [PubMed] [Google Scholar]
- Daoura L, Haaker J, Nylander I. Early environmental factors differentially affect voluntary ethanol consumption in adolescent and adult male rats. Alcohol Clin Exp Res. 2011;35:506–15. doi: 10.1111/j.1530-0277.2010.01367.x. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2007;31:1516–27. doi: 10.1111/j.1530-0277.2007.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–8. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Ovariectomy alters the chronic hemodynamic and sympathetic effects of ethanol in radiotelemetered female rats. Clin Exp Hypertens. 2000;22:109–26. doi: 10.1081/ceh-100100066. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Ethanol consumption in the female Long-Evans rat: a modulatory role of estradiol. Alcohol. 2002;26:103–13. doi: 10.1016/s0741-8329(01)00203-8. [DOI] [PubMed] [Google Scholar]
- Foy MR, Foy JG. Reversal of long-delay conditioned taste aversion learning in rats by sex hormone manipulation. Integr Physiol Behav Sci. 2003;38:203–13. doi: 10.1007/BF02688854. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li TK. Differences in response to the aversive properties of ethanol in rats selectively bred for oral ethanol preference. Pharmacol Biochem Behav. 1988;31:215–22. doi: 10.1016/0091-3057(88)90336-x. [DOI] [PubMed] [Google Scholar]
- Fullgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86:320–6. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi-Clarke L. Role of estradiol in alcohol intake and alcohol-related behaviors. J Stud Alcohol. 1996;57:162–70. doi: 10.15288/jsa.1996.57.162. [DOI] [PubMed] [Google Scholar]
- Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcohol Clin Exp Res. 2011;35:1842–51. doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–47. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversions. Pharmacol Biochem Behav. 1979;11:31–5. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- Li J, Zou Y, Ye JH. Low frequency electroacupuncture selectively decreases voluntarily ethanol intake in rats. Brain Res Bull. 2011;86:428–34. doi: 10.1016/j.brainresbull.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Lumeng L. Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcohol Clin Exp Res. 1984;8:485–6. doi: 10.1111/j.1530-0277.1984.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–51. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Long J, Evans H. The Oestrous Cycle in the Rat and Its Associated Phenomena. University of California Press; Berkeley, CA: 1922. [Google Scholar]
- Lucas LA, McMillen BA. Conditioned taste aversion and the Myers’ high-ethanol-preferring rat. Alcohol Alcohol. 2002;37:427–31. doi: 10.1093/alcalc/37.5.427. [DOI] [PubMed] [Google Scholar]
- Randall-Thompson JF, Riley AL. Morphine-induced conditioned taste aversions: assessment of sexual dimorphism. Pharmacol Biochem Behav. 2003;76:373–81. doi: 10.1016/j.pbb.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60:252–60. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rinker JA, Busse GD, Riley AL. An assessment of sex differences in nicotine-induced conditioned taste aversions. Pharmacol Biochem Behav. 2008;88:427–31. doi: 10.1016/j.pbb.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Sensitivity and tolerance to autonomic effects of ethanol in adolescent and adult rats during repeated vapor inhalation sessions. Alcohol Clin Exp Res. 2005;29:1809–20. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- Robins LN, Przybeck TR. Age of onset of drug use as a factor in drug and other disorders. NIDA Res Monogr. 1985;56:178–92. [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79:439–50. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Roma PG, Chen SA, Barr CS, Riley AL. Dissociation between the aversive and pharmacokinetic effects of ethanol in female Fischer and Lewis rats. Behav Brain Res. 2007;182:51–6. doi: 10.1016/j.bbr.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roma PG, Davis CM, Kohut SJ, Huntsberry ME, Riley AL. Early maternal separation and sex differences in the aversive effects of amphetamine in adult rats. Physiol Behav. 2008;93:897–904. doi: 10.1016/j.physbeh.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Roma PG, Flint WW, Higley JD, Riley AL. Assessment of the aversive and rewarding effects of alcohol in Fischer and Lewis rats. Psychopharmacology (Berl) 2006;189:187–99. doi: 10.1007/s00213-006-0553-6. [DOI] [PubMed] [Google Scholar]
- Sabino V, Kwak J, Rice KC, Cottone P. Pharmacological characterization of the 20% alcohol intermittent access model in sardinian alcohol-preferring rats: a model of binge-like drinking. Alcohol Clin Exp Res. 2013;37:635–43. doi: 10.1111/acer.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology (Berl) 2007;191:867–77. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Difeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, Zhou C, Kuhn CM. Aversive Effects of Ethanol in Adolescent Versus Adult Rats: Potential Causes and Implication for Future Drinking. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Kingsley MA, Rezvani AH, Propst K, Swartzwelder HS, Kuhn CM. Early ethanol consumption predicts relapse-like behavior in adolescent male rats. Alcohol Clin Exp Res. 2008;32:754–62. doi: 10.1111/j.1530-0277.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84:344–52. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit M, Smith T, Pierson J, Danko G, Beltran IA. Relationships among the level of response to alcohol and the number of alcoholic relatives in predicting alcohol-related outcomes. Alcohol Clin Exp Res. 2006;30:1308–14. doi: 10.1111/j.1530-0277.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Trim R, Bucholz KK, Edenberg HJ, Hesselbrock V, Kramer JJ, Dick DM. An evaluation of the full level of response to alcohol model of heavy drinking and problems in COGA offspring. J Stud Alcohol Drugs. 2009;70:436–45. doi: 10.15288/jsad.2009.70.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Wiesbeck GA, Kalmijn J. The relationship between Self-Rating of the Effects of alcohol and alcohol challenge results in ninety-eight young men. J Stud Alcohol. 1997;58:397–404. doi: 10.15288/jsa.1997.58.397. [DOI] [PubMed] [Google Scholar]
- Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev. 2009;29:535–47. doi: 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behav Brain Res. 2011;225:104–9. doi: 10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–8. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–45. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–23. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–7. [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34:231–43. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Anderson K. Sex differences in schedule-induced alcohol consumption. Alcohol. 1994;11:35–40. doi: 10.1016/0741-8329(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004a;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Changes in sensitivity to ethanol-induced social facilitation and social inhibition from early to late adolescence. Ann N Y Acad Sci. 2004b;1021:459–61. doi: 10.1196/annals.1308.064. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. Conditioned taste aversion and drugs of abuse: history and interpretation. Neurosci Biobehav Rev. 2012;36:2193–205. doi: 10.1016/j.neubiorev.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–54. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on age- and sex-typical patterns of ethanol consumption in Sprague-Dawley rats. Alcohol Clin Exp Res. 2011;35:2039–49. doi: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25:118–30. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–52. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Ann N Y Acad Sci. 2004;1021:462–4. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- York JL, Welte J, Hirsch J. Gender comparison of alcohol exposure on drinking occasions. J Stud Alcohol. 2003;64:790–801. doi: 10.15288/jsa.2003.64.790. [DOI] [PubMed] [Google Scholar]
- York JL, Welte JW. Gender comparisons of alcohol consumption in alcoholic and nonalcoholic populations. J Stud Alcohol. 1994;55:743–50. doi: 10.15288/jsa.1994.55.743. [DOI] [PubMed] [Google Scholar]


