Abstract
Purpose:
To evaluate and compare the performance of shear-wave elastography (SWE) for breast masses using the local shear wave speed (m/sec) vs. Young modulus (kPa).
Methods:
A total of 130 breast lesions in 123 women who underwent SWE before ultrasound- guided core needle biopsy or surgical excision were included. With the region-of-interest placed over the stiffest areas of the lesion on SWE, the quantitative mean, maximum, and standard deviation (SD) of the elasticity values were measured in kPa and m/sec for each lesion. The SD was also measured with the region-of-interest including the whole breast lesion (wSD). The area under the receiver operating characteristic curve (AUC), sensitivity, and specificity of each elasticity value measured in kPa and m/sec were compared.
Results:
Of the 130 lesions, 49 (37.7%) were malignant and 81 (62.3%) were benign. The AUCs for the mean, maximum, and SD of the elasticity values using kPa and m/sec did not differ significantly: mean, 0.974 vs. 0.974; maximum, 0.960 vs. 0.976; SD, 0.916 vs. 0.916. However, the AUC for wSD showed a significant difference: 0.964 (kPa) vs. 0.960 (m/sec) (P=0.036). There was no significant difference in the sensitivity and specificity of the mean, maximum, and wSD of the elasticity values. However, the specificity of the SD was significantly different between the two different measurements: 95.1% (kPa) vs. 87.7% (m/sec) (P=0.031).
Conclusion:
The quantitative elasticity values measured in kPa and m/sec on SWE showed good diagnostic performance. The specificity of the SD and AUC of the wSD measured in kPa were significantly higher than those measured in m/sec.
Keywords: Breast; Ultrasonography, mammary; Sonoelastography
Introduction
Breast elastography as a method of imaging tissue stiffness has been used to improve diagnostic confidence and increase the specificity of ultrasound interpretation. The recently developed shear-wave elastography (SWE; SuperSonic Imagine, Aix-en-Provence, France) uses the radiation force induced by the ultrasound push pulse generated by the ultrasound transducer [1]. This force induces mechanical waves, including shear waves, which propagate transversely in the tissue. The production of the radiation force by the probe rather than the operator means that the SWE is more operator-independent, reproducible, and quantitative [2,3]. The SWE allows measurement of the propagation speed of shear waves within the tissue to locally quantify its stiffness in kilopascals (kPa) or meters per second (m/sec). Within a given region- of-interest (ROI), a variety of stiffness parameters can be measured, including the mean stiffness (Emean), maximum stiffness (Emax), and standard deviation (SD).
Recently, several studies have shown that those quantitative parameters using Young’s modulus of elasticity (kPa) in SWE improved the diagnostic accuracy of breast ultrasound [2,4-9]. However, there has been no study on SWE locally quantifying tissue stiffness in m/sec. Although there are other commercial ultrasound systems that can measure shear-wave speed in m/sec, the type of shear-wave measurement (point SWE) is different from the ultrasound system in our study [10].
This study was performed to evaluate and compare the performance of SWE for breast masses using local shear wave speed (m/sec) and Young modulus (kPa).
Materials and Methods
Patients and Lesions
From August 2012 to September 2012, 128 consecutive patients underwent SWE before ultrasound-guided core needle biopsy or surgical excision for breast lesions visible on ultrasound. Among these patients, 123 women aged 20-82 years (mean, 46.7±11.2 years) with a total of 130 breast lesions were enrolled in this study. The remaining five women who underwent neoadjuvant chemotherapy at the time of SWE were excluded from this study.
Ultrasound Examinations and Biopsy
The breast ultrasound examinations were performed with the Aixplorer ultrasound system (SuperSonic Imagine) equipped with a 4- to 15-MHz linear-array transducer, by one of four radiologists with 5-10 years of experience in performing breast ultrasounds. The investigator knew the results of the clinical examination and mammography at the time of the ultrasound examination. After a B-mode ultrasound, SWE images were obtained for the breast lesions that were scheduled to be biopsied or excised surgically. The recommended technique for SWE was to image the lesion with no pressure induced by the transducer. After a few seconds of immobilization to allow the SWE image to stabilize, the SWE image was frozen and saved. The built-in ROI (Q-box; SuperSonic Imagine) of the system was set to include the lesion and the surrounding normal tissue, which was demonstrated on a semitransparent color map of tissue stiffness overlaid on the B-mode image with a range from dark blue, indicating the lowest stiffness, to red, indicating the highest stiffness (0-180 kPa; 0-7.7 m/sec). Areas of black on the SWE images represented tissue in which no shear wave was detected. Fixed ROIs 2×2 mm in size were placed by an investigator over the stiffest part of the lesion, including the immediately adjacent stiff tissue or halo. The system calculated the Emax, Emean, and SD of the elasticity value of the lesion in kPa and m/sec (Fig. 1). For the SD of the elasticity value of the whole breast lesion (wSD), the ROI for measuring the elasticity value was placed to include the whole breast lesion and stiffest part of the lesion [11] (Fig. 1).
Fig. 1. B-mode and shear-wave elastography (SWE) images in a 46-year-old woman with a pathologically proven invasive ductal carcinoma.
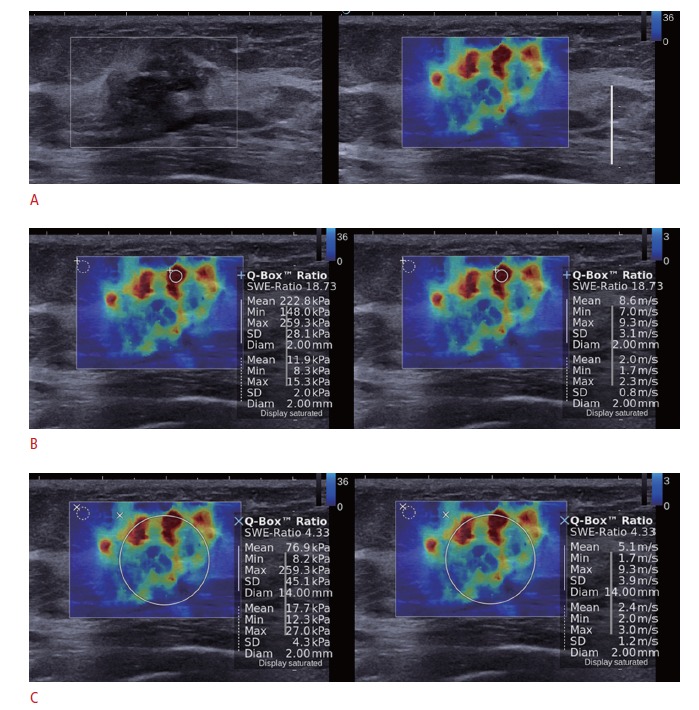
A. SWE (right) and B-mode images (left) on split-screen mode show a 20-mm, irregular, spiculated mass with red, heterogeneous elasticity. B. The mean, maximum, and standard deviation of elasticity values were measured in kPa (left) and in m/sec (right) by placing the region-ofinterest (ROI) over the stiffest part of the lesion. C. The ROI for measuring the elasticity value (wSD) was placed to include the whole breast lesion and the stiffest part of the lesion.
Ultrasound-guided core needle biopsy was performed using a freehand technique and the high-resolution ultrasound unit mentioned above. A 14-gauge dual-action semiautomatic core biopsy needle with a 22-mm throw (Stericut with coaxial; TSK Laboratory, Tochigi, Japan) or a vacuum-assisted device (Mammotome; Ethicon-Endosurgery, Cincinnati, OH, USA) with an 8-gauge or an 11-gauge probe were used, depending on the physician’s and patient’s preferences.
Data and Statistical Analysis
The Emax, Emean, SD, and wSD of the lesion in kPa and m/sec were collected, and their means were compared between benign and malignant lesions using the two-sample t-test. To evaluate the diagnostic performance of each elasticity value, we calculated and compared the area under the receiver operating characteristic curve (AUC) and generated a confidence interval (CI) by using the DeLong method [12]. The optimal cutoff value for the elasticity values was calculated. The sensitivity and specificity for each elasticity value were compared between kPa and m/sec by using the McNemar test. Statistical analysis was performed with statistical software programs (IBM SPSS ver. 20.0.0, IBM Co., Armonk, NY, USA; MedCalc ver. 12.2.1.0, MedCalc Software, Mariakerke, Belgium). Differences were considered to be statistically significant at P<0.05.
Results
Of the 130 lesions, 49 (37.7%; 10 for core biopsy and 39 for surgery) were malignant and 81 (62.3%; 66 for core biopsy and 15 for surgery) were benign. The lesion diameter on the B-mode ultrasound ranged from 4 mm to 38 mm (mean, 13.0±7.7 mm).
Table 1 summarizes the means of the Emax, Emean, SD, and wSD of the lesions in kPa and m/sec. The means of each elasticity value for the malignant lesions were significantly higher than those for the benign lesions (P<0.001). Table 2 demonstrates the sensitivity, specificity, and AUC of the Emax, Emean, SD, and wSD in kPa and m/sec for the diagnosis of breast cancer. In the receiver operating characteristic curve analysis, the AUC for the Emax, Emean, and SD were not significantly different using kPa and m/sec. However, the AUC for the wSD showed a significant difference between kPa and m/sec (0.964 vs. 0.960, P=0.036) (Fig. 2). The estimated cutoff values for the elasticity values were as follows: Emax, 63.4 kPa and 4.6 m/sec; Emean, 63.7 kPa and 4.8 m/sec; SD, 7.5 and 1.3; wSD, 11.4 and 1.9. There was no significant difference in the sensitivity and specificity of the Emean, Emax, and wSD of the elasticity values. However, the specificity of the SD using kPa and m/sec was significantly different (95.1% vs. 87.7%, P=0.031).
Table 1.
Elasticity values in benign and malignant breast lesions
| Variable | kPa | m/sec | ||||||
|---|---|---|---|---|---|---|---|---|
| Emax | Emean | SD | wSD | Emax | Emean | SD | wSD | |
| Benign | 40.6±19.5 | 34.8±17.7 | 3.6±2.0 | 6.7±3.6 | 3.6±0.9 | 3.3±0.8 | 1.1±0.3 | 1.5±0.4 |
| Malignant | 155.1±67.2 | 140.7±58.5 | 14.1±9.6 | 30.8±16.1 | 7.1±1.5 | 6.7±1.5 | 2.1±0.7 | 3.1±0.9 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Values are presented as mean±SD.
Emax, maximum elasticity; Emean, mean elasticity value; wSD, SD of elasticity value of the whole breast lesion.
Table 2.
Diagnostic performance of elasticity values for breast cancer
| Variable | Sensitivity (%) | Specificity (%) | AUC (95% CI) | |
|---|---|---|---|---|
| Emax | kPa | 91.8 | 88.9 | 0.960 (0.911 to 0.987) |
| m/s | 93.9 | 88.9 | 0.976 (0.933 to 0.995) | |
| P-value | >0.990 | >0.990 | 0.345 | |
| Emean | kPa | 89.8 | 93.8 | 0.974 (0.930 to 0.994) |
| m/s | 83.7 | 98.8 | 0.974 (0.929 to 0.994) | |
| P-value | 0.250 | 0.125 | 0.834 | |
| SD | kPa | 79.6 | 95.1 | 0.916 (0.854 to 0.957) |
| m/s | 85.7 | 87.7 | 0.916 (0.854 to 0.957) | |
| P-value | 0.250 | 0.031 | 0.932 | |
| wSD | kPa | 89.4 | 92.5 | 0.964 (0.915 to 0.989) |
| m/s | 89.4 | 91.3 | 0.960 (0.909 to 0.987) | |
| P-value | >0.990 | >0.990 | 0.036 |
AUC, area under the receiver operating characteristic curve; CI, confidence interval; Emax, maximum elasticity, SD, standard deviation; Emean, mean elasticity value; wSD, SD of elasticity value of the whole breast lesion.
Fig. 2. Receiver operating characteristic curves for the standard deviation of the elasticity value of the whole breast lesion (wSD) in kPa and m/sec.
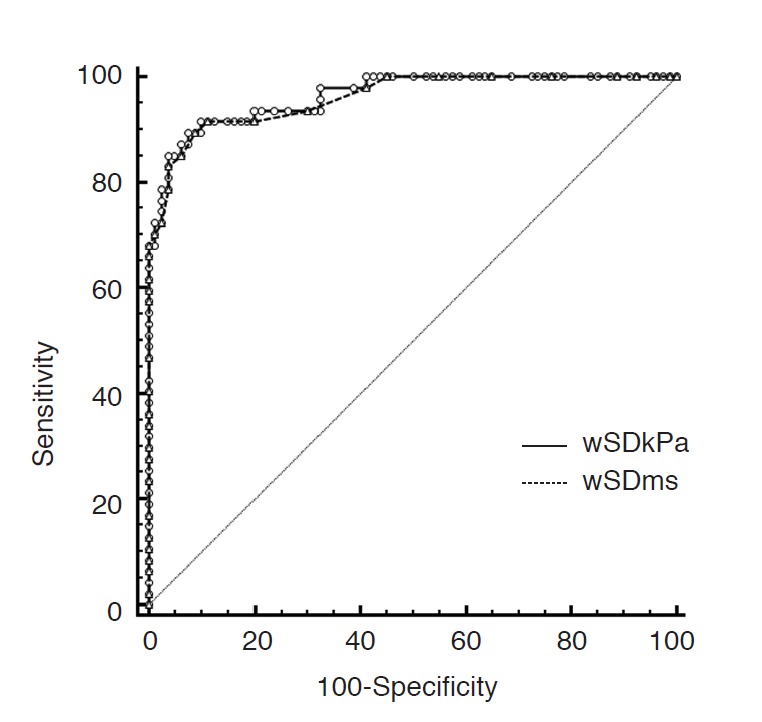
The areas under the curves were significantly different using kPa and m/sec (0.964 vs. 0.960, P=0.036).
Discussion
Tissue stiffness can be measured by a physical quantity called Young’s modulus and expressed in pressure units--pascals, or more commonly, kilopascals (kPa). The relationship between stress and strain is expressed by the Young modulus and is defined simply as the ratio of the applied stress to the induced strain. In SWE, shear waves generated by the ultrasound system propagate in tissue at speeds of 1-10 mm/sec (corresponding to a tissue elasticity of 1-300 kPa). The stiffer the tissue, the faster the shear wave propagates [13]. In fact, Young modulus or elasticity (E) and the shear wave propagation speed (c) are directly linked through the simple formula: E=3pc2, where p is the density of tissue expressed in kg/m3. Given that the density of tissues is well known (1,000 kg/m3), the elasticity of the tissue can be determined when the shear wave propagation velocity c can be measured. In this manner, SWE can provide quantitative elastic information in both kPa and m/sec.
The mean of each elasticity value for the malignant lesions were significantly higher than those for the benign lesions (P<0.001) (Table 1), which was compatible with previous studies of SWE in kPa [2,4-9,11]. In m/sec, no data for breast lesions have been reported for SWE. Virtual touch tissue quantification (VTQ) values, a different type of shear-wave measurement (point SWE) than the ultrasound system used in our study, were investigated in some studies to differentiate between benign and malignant breast lesions [14-16]. The reported values ranged from 2.25 to 3.2 m/sec for benign lesions and from 4.49 to 8.2 m/sec for malignant lesions, which was compatible with the Emean of our results (Table 1).
The diagnostic performance of SWE in both kPa and m/sec was good, ranging from 0.916 to 0.976 of the AUC (Table 2). The AUC showed no significant difference between kPa and m/sec for the Emax, Emean, and SD; nor was there any significant difference in the sensitivity or specificity of the Emax, Emean, or wSD. Considering the direct link between Young modulus and the shear wave propagation speed, these results could be expected. However, the AUC for the wSD showed a significant difference using kPa vs. m/sec (0.964 vs. 0.960, P=0.036), and the specificity of the SD was also significantly different (95.1% vs. 87.7%, P=0.031). On SWE, benign lesions tend to be homogeneously soft (blue), whereas malignant lesions have a heterogeneous hard (red) appearance. For hard lesions such as invasive cancers, the zone of stiffness surrounding the mass on SWE may in part correspond to the echogenic halo seen on B-mode images with many malignancies [6,17]. If the lesion is too hard for the shear wave to propagate normally into it, the interior area of the lesion has no results and appears without a color code (black) [1]. Gweon et al. [11] reported that qualitative color overlay features can be quantified as the wSD in kPa to assess breast mass heterogeneity, and they found that the wSD in kPa was significantly different in benign (6.7±6.9) and malignant (26.6±12.4) breast lesions (P<0.001) and showed a good diagnostic performance (AUC, 0.944), which was compatible with our results. For the SD, Evans et al. [4] reported that the SD values in kPa showed diagnostic accuracy (sensitivity 83%, specificity 96%, and accuracy 89%) and reproducibility almost as good as the mean stiffness, and they suggested that the SD is likely to be of value in benign/malignant differentiation because it is a measure of lesion heterogeneity, which is more common and more marked in malignant lesions than in benign lesions. Lee et al. [18] reported a sensitivity of 72%, specificity of 88%, and AUC of 0.850 for the SD in kPa. The reason for the difference in diagnostic performance between kPa and m/sec for the wSD or SD on SWE could not be explained clearly, but it might be related to the difference in the range of elasticity values that is expressed. The range of elasticity values on SWE is wider in kPa (0 to 300.0 kPa) than in m/sec (0 to 10.0 m/sec). However, the performance of SWE in m/sec itself is good enough to differentiate between benign and malignant breast lesions, and those differences in diagnostic performance between kPa and m/sec may have little significance in clinical practice.
This study has some limitations. Owing to its retrospective nature, there might have been unavoidable selection bias because the patients included in this study were scheduled for biopsy or excision of known breast lesions. Long-term follow-up data were not available in concordant benign lesions after core needle biopsy. Elasticity values according to histologic differentiation, individual lesion size, histologic grade, and the surrounding fibrotic component and internal microcalcification were not statistically analyzed and considered, although these factors are known to be able to influence diagnostic performance.
In conclusion, the quantitative elasticity values measured in kPa and m/sec showed good diagnostic performance. The specificity of the SD and AUC of the wSD measured in kPa were significantly higher than those measured in m/sec.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0007602).
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Barr RG. Sonographic breast elastography: a primer. J Ultrasound Med. 2012;31:773–783. doi: 10.7863/jum.2012.31.5.773. [DOI] [PubMed] [Google Scholar]
- 2.Athanasiou A, Tardivon A, Tanter M, Sigal-Zafrani B, Bercoff J, Deffieux T, et al. Breast lesions: quantitative elastography with supersonic shear imaging--preliminary results. Radiology. 2010;256:297–303. doi: 10.1148/radiol.10090385. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove DO, Berg WA, Doré CJ, Skyba DM, Henry JP, Gay J, et al. Shear wave elastography for breast masses is highly reproducible. Eur Radiol. 2012;22:1023–1032. doi: 10.1007/s00330-011-2340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, et al. Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res. 2010;12:R104. doi: 10.1186/bcr2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JM, Moon WK, Cho N, Yi A, Koo HR, Han W, et al. Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res Treat. 2011;129:89–97. doi: 10.1007/s10549-011-1627-7. [DOI] [PubMed] [Google Scholar]
- 6.Berg WA, Cosgrove DO, Dore CJ, Schafer FK, Svensson WE, Hooley RJ, et al. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology. 2012;262:435–449. doi: 10.1148/radiol.11110640. [DOI] [PubMed] [Google Scholar]
- 7.Evans A, Whelehan P, Thomson K, Brauer K, Jordan L, Purdie C, et al. Differentiating benign from malignant solid breast masses: value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI-RADS classification. Br J Cancer. 2012;107:224–229. doi: 10.1038/bjc.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youk JH, Gweon HM, Son EJ, Chung J, Kim JA, Kim EK. Threedimensional shear-wave elastography for differentiating benign and malignant breast lesions: comparison with two-dimensional shearwave elastography. Eur Radiol. 2013;23:1519–1527. doi: 10.1007/s00330-012-2736-3. [DOI] [PubMed] [Google Scholar]
- 9.Youk JH, Gweon HM, Son EJ, Han KH, Kim JA. Diagnostic value of commercially available shear-wave elastography for breast cancers: integration into BI-RADS classification with subcategories of category 4. Eur Radiol. 2013;23:2695–2704. doi: 10.1007/s00330-013-2873-3. [DOI] [PubMed] [Google Scholar]
- 10.Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169–184. doi: 10.1055/s-0033-1335205. [DOI] [PubMed] [Google Scholar]
- 11.Gweon HM, Youk JH, Son EJ, Kim JA. Visually assessed colour overlay features in shear-wave elastography for breast masses: quantification and diagnostic performance. Eur Radiol. 2013;23:658–663. doi: 10.1007/s00330-012-2647-3. [DOI] [PubMed] [Google Scholar]
- 12.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 13.Comstock C. Ultrasound-based technologies including elastography and automated whole breast scanning. In: Gaertner R, editor. Breast imaging. Philadelphia, PA: Elsevier Saunders; 2011. pp. 754–757. [Google Scholar]
- 14.Meng W, Zhang G, Wu C, Wu G, Song Y, Lu Z. Preliminary results of acoustic radiation force impulse (ARFI) ultrasound imaging of breast lesions. Ultrasound Med Biol. 2011;37:1436–1443. doi: 10.1016/j.ultrasmedbio.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Bai M, Du L, Gu J, Li F, Jia X. Virtual touch tissue quantification using acoustic radiation force impulse technology: initial clinical experience with solid breast masses. J Ultrasound Med. 2012;31:289–294. doi: 10.7863/jum.2012.31.2.289. [DOI] [PubMed] [Google Scholar]
- 16.Tozaki M, Isobe S, Fukuma E. Preliminary study of ultrasonographic tissue quantification of the breast using the acoustic radiation force impulse (ARFI) technology. Eur J Radiol. 2011;80:e182–e187. doi: 10.1016/j.ejrad.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Tozaki M, Fukuma E. Pattern classification of ShearWave Elastography images for differential diagnosis between benign and malignant solid breast masses. Acta Radiol. 2011;52:1069–1075. doi: 10.1258/ar.2011.110276. [DOI] [PubMed] [Google Scholar]
- 18.Lee EJ, Jung HK, Ko KH, Lee JT, Yoon JH. Diagnostic performances of shear wave elastography: which parameter to use in differential diagnosis of solid breast masses? Eur Radiol. 2013;23:1803–1811. doi: 10.1007/s00330-013-2782-5. [DOI] [PubMed] [Google Scholar]


