Abstract
Mutation of a single copy of the adenomatous polyposis coli (APC) gene results in familial adenomatous polyposis (FAP), which confers an extremely high risk for colon cancer. ApcMin/+ mice exhibit multiple intestinal neoplasia (MIN) that causes anemia and death from bleeding by 6 months. Mechanistic target of rapamycin complex 1 (mTORC1) inhibitors were shown to improve ApcMin/+ mouse survival when administered by oral gavage or added directly to the chow, but these mice still died from neoplasia well short of a natural life span. The National Institute of Aging Intervention Testing Program showed that enterically targeted rapamycin (eRapa) extended life span for wild type genetically heterogeneous mice in part by inhibiting age-associated cancer. We hypothesized that eRapa would be effective in preventing neoplasia and extend survival of ApcMin/+ mice. We show that eRapa improved survival for ApcMin/+ mice in a dose-dependent manner. Remarkably, and in contrast to previous reports, most of the ApcMin/+ mice fed 42 ppm eRapa lived beyond the median life span reported for wild type syngeneic mice. Furthermore, chronic eRapa did not cause detrimental immune effects in mouse models of cancer, infection or autoimmunity; thus, assuaging concerns that chronic rapamycin treatment suppresses immunity. Our studies suggest that a novel formulation (enteric targeting) of a well-known and widely used drug (rapamycin) can dramatically improve its efficacy in targeted settings. eRapa or other mTORC1 inhibitors could serve as effective cancer preventatives for people with FAP without suppressing the immune system, thus reducing the dependency on surgery as standard therapy.
INTRODUCTION
Familial adenomatous polyposis (FAP) is an autosomal dominant disease caused by mutation of the adenomatous polyposis coli (APC) gene, located on chromosome 5. APC inhibits the pro-growth WNT signaling pathway by regulating β-catenin nuclear localization and its mutation results in the development of numerous adenomatous colorectal polyps at a young age. Polyposis inevitably progresses to colorectal cancer if left untreated. Given the predictable development of colorectal cancer in patients with FAP, the safest preventative strategy is surgical resection of the colon before cancer develops. Genetic screening and endoscopy in concert with prophylactic total colectomy significantly improve the overall survival of FAP patients. Unfortunately, colonoscopies identify only 56% and 77% of tumors on the right and left side of the colon, respectively (1), and total colectomy is morbid and life altering. Further, the second leading cause of death in FAP is duodenal adenocarcinoma. Ninety percent of FAP patients develop duodenal polyps, the precursor lesions of duodenal adenocarcinoma and of these 5% will develop duodenal adenocarcinoma in their lifetime (2). Duodenal surgery is currently indicated for FAP patients with severe duodenal polyposis or duodenal carcinoma at the expense of significant morbidity. Thus, this patient population has a strong need for non-surgical treatments to prevent or reduce polyp formation and carcinogenesis in the gastrointestinal track with the possibility of reducing the need for life altering surgery.
Hence, the goal of our study was to test enteric-targeted rapamycin (eRapa) in the ApcMin/+ mouse model and assess whether this intervention delayed or prevented hemorrhaging intestinal neoplasia that lead to anemia and mortality. Notably, the etiology for intestinal tumors in ApcMin/+ mice is the same as most FAP lesions, so interventions that prevent neoplasia in the ApcMin/+ mouse model are also likely to work for FAP.
Rapamycin has been proposed to be a cancer preventative agent. It allosterically inhibits mTORC1 when bound to FKBP12. mTORC1 promotes cell growth (mass) by coordinating numerous cellular processes including macromolecule biosynthesis in response to nutrient, energy and growth factor stimuli (3). Upregulation of mTOR contributes to the development and growth of cancer, including intestinal tumors, making the mTOR pathway an attractive candidate for anti-cancer therapy (4). Recent evidence suggests that mTORC1 inhibition delays or prevents cancer in human kidney transplant patients treated with rapamycin (5) and mouse cancer models (6, 7). Thus, rapamycin could be an effective anti-cancer prophylactic agent.
Two previous studies support the use of rapamycin in delaying intestinal neoplasias in Apc–mutated mouse models. In one study, a rapamycin derivative, RAD001 (everolimus) was administered by oral gavage (3 or 10 mg/kg body weight/day, 5 times a week) (8) and the other study fed non-encapsulated rapamycin (sirolimus) in high fat food pellets (40 mg/kg) (9). Both studies showed that these agents improved survival in Apc-mutant mice although they died from intestinal neoplasias before the median life span for wild type syngenic C57BL/6J mice (median life spans for ApcMin/+ and ApcΔ716 mice between 420 – 550 days as compared to a range of 692–866 days for wild type mice (10, 11)).
We tested if enteric rapamycin delivery could improve efficacy to prevent intestinal polyposis in ApcMin/+ mice. eRapa prevented or significantly delayed intestinal neoplasia in ApcMin/+ mice and improved survival and other health span indicators without eliciting undesirable side-effects like immune suppression. Survival was prolonged beyond that of the median life span for wild type mice supporting the possibility that enteric targeting of mTORC1 inhibitors could serve as safe and effective preventatives to complement cancer surveillance procedures indicated for patients with a high risk for intestinal cancers.
MATERIALS and METHODS
Rapamycin diets
Preparation of rapamycin diets was described previously (6). The concentration of rapamycin in food was expressed as ng/mg food (parts per million).
Mice, eRapa chow and rapamycin blood levels
We treated and housed mice according to IACUC standards. Cohorts of ApcMin/+ mice (Jackson Laboratories, C57BL/6-ApcMin/J) or C57BL/6 wild type mice were fed microencapsulated rapamycin-containing diets either containing a concentration of 14 mg/kg food, (14 ppm), which provided a dose of ~2.24 mg of rapamycin/kg body weight/day (12, 13) or 42 mg/kg food (42 ppm) (14) which provided a dose of ~6.72 mg of rapamycin/kg body weight/day. Control diet was the same but with empty capsules. We housed mice in accordance with the NIH guide for the care and use of lab animals.
In our longevity studies, mice were allowed to live out their life span, i.e., there was no censoring due to morbidity in the groups of mice used to measure lifespan. Mice were euthanized only if they were either (1) unable to eat or drink, (2) bleeding from a tumor or other condition, or (3) when they were laterally recumbent, i.e., they failed to move when prodded or were unable to right themselves. Rapamycin blood levels were done as previously described (6).
Measurement of Rapamycin in Intestine Tissue
Reagents and our HPLC/MS system were previously described (6). Rapamycin was quantified in mouse small intestine tissue according to the following protocol. Briefly, 100 mg of calibrator, control, and unknown tissue samples were mixed by sonication (three 5 sec bursts) with 10 μL of 0.5 μg/mL ASCO (internal standard) and 300 μL of a solution containing 0.1% formic acid and 10 mM ammonium formate dissolved in 95% HPLC grade methanol. After sonication, the samples were vortexed vigorously for 2 min, and then centrifuged at 15,000 g for 5 min at 23°C (subsequent centrifugations were performed under the same conditions). Supernatants were transferred to 1.5 mL microfilterfuge tubes and spun at 15,000 g for 1 minute and then 40 μL of the final extracts were injected into the LC/MS/MS. The ratio of the peak area of rapamycin to that of the internal standard ASCO (response ratio) for each unknown sample was compared against a linear regression of calibrator response ratios at 0, 1.78, 3.13, 6.25, 12.5, 50, and 100 μg/g to quantify rapamycin. The concentration of rapamycin was expressed as μg/g of tissue (parts per million).
Pathology
The severity of neoplastic lesions was assessed using the grading system previously described (15). Two pathologists separately examined all of the samples without knowledge of thegenotype or diet.
Immunoblots
Because Western blotting of intestinal tissue is difficult, we optimized a procedure as follows. Mouse small intestine (~15–35 mg) was ground to a powder with a mortar and pestle after cryofracture. Powdered tissue was lysed by homogenization in 5 X dry weight RIPA (modified) buffer (150 mM sodium chloride, 50 mM Tris-HCl, pH 7.4, 1 mM ethylenediaminetetraacetic acid, 1 mM phenylmethylsulfonyl flouride, 1% Triton X-100, 1% sodium deoxycholic acid, 0.1% sodium dodecyl sulfate, and one Pierce protease and phosphatase inhibitor mini tablet/10 ml). Debris was cleared by centrifugation. Bio-Rad Protein Assay was used to determine protein concentrations and ~40–50 μg of each sample was mixed with SDS loading dye, boiled, and separated on a gel before transfer to nitrocellulose (35 min at 20 V, Bio-Rad Semi Dry transfer) using Towbin buffer containing 0.0375% SDS. Blots were dried, cut into strips and blocked for 1 hour at room temperature in Odyssey blocking buffer. Individual strips were incubated overnight at 4° C in appropriate primary antibodies and then for 1 hour at room temperature in corresponding IRDye secondary antibodies. Blots were washed 4 times (5 minutes each) in TBS-T after each antibody incubation with a final wash in TBS before scanning and quantification on an Odyssey Imager. We quantified fluorescent signals as integrated intensities (I.I. K counts) using the Odyssey Infrared Imaging System, Application version 3.0 software. We used a local background subtraction method to subtract independent background values from each box: the median background function with a 3 pixel width border above and below each box was subtracted from individual counts. We calculated ratios for each antibody against the pan-actin loading control using I.I. K counts. The respective antibody to pan-actin ratio was then used to calculate phosphorylated protein to total protein ratio. Prism 5 (GraphPad Software, Inc.) was used to analyze and graph the data. We used an unpaired two-tailed t-test or Mann Whitney test (nonparametric for rpS6) to obtain p values. P values below 0.05 were considered significant. All Odyssey products and IRDye secondary antibodies were obtained from LI-COR Biosciences. All antibodies were diluted into Odyssey Blocking Buffer + 0.2% Tween 20. Actin, pan Ab-5 (clone ACTN05) mouse monoclonal primary antibody was obtained from Thermo Fisher and all other primary antibodies were obtained from Cell Signaling Technologies:phospho rpS6 (Ser240/244) rabbit polyclonal and rpS6 (5G10) mouse monoclonal, phospho AKT (Ser473) rabbit polyclonal, and AKT rabbit polyclonal.
Activity assay
Mice were individually housed in clear, Plexiglas cages (38 × 20 × 11.5 cm) for 36 hours (two light and one dark phase) to assess spontaneous activity as described (16).
Hematocrit
Red blood cells were packed using centrifugation in the presence of anti-coagulant and percentage of pellet (% packed cell volume) to supernatant was reported. Blood was either collected at time of sacrifice or from the tail vein.
Cell lines
We obtained authenticated mycoplasma free cell lines from the ATCC for use in our studies. We tested cells periodically for mycoplasma according to ATTC recommendations.
B16 tumor model
B16F10 melanoma cells were cultivated and injected subcutaneously (125,000 cells/flank), growth was measured with Vernier calipers and immunity was studied as we previously described (17).
Toxoplasma gondii challenge
Tachyzoites of T. gondii (Me49 strain expressing OVA) were cultivated and used to challenge mice, and immunity was studied all as we described (18–20). Mice were fed eRapa 14 ppm or Eudragit control for 30 days before, and during the infection with 10,000 tachyzoites by intraperitoneal inoculation. Animals were sacrificed 11 days after inoculation for immune studies.
Bm12 autoimmunity model
Autoimmune kidney disease was induced by transfer of bm12 splenocytes into BL6 mice, and kidney disease was monitored by albuminuria as described (21). Mice were fed eRapa 14 ppm or Eudragit control for 30 days before, and during the post-splenocyte transfer monitoring period of 12 weeks. Immune events were analyzed as for tumor and T. gondii-challenged mice.
Regulatory T cell and myeloid derived suppressor cell suppression assays
These assays were performed as described (17). Briefly, CD4+CD25− naïve T cells from naïve C57Bl/6 mice were incubated with carboxyfluoroscein succinimidyl ester, a fluorescent dye. Anti-CD3/anti-CD28 beads were added to induce T cell proliferation, and flow cytometry sorted CD4+CD25hi regulatory T cells or CD11b+Gr-1hi myeloid derived suppressor cells were added at indicated ratios. Suppression was measured by dye dilution in indicator T cells by flow cytometry 4 days later.
Results and Discussion
We tested the ability of eRapa to inhibit mTORC1 signaling in the small intestine where polyps primarily develop in ApcMin/+ mice. Eudragit S-100, the excipient in our eRapa microencapsulated formulation, dissolves at pH greater than 7 and is designed for colonic drug delivery in humans (22). Eudragit S-100 also provides rapamycin stability in mouse chow and improves rapamycin blood levels (12–14), which is ideal for long-term disease prevention studies. The mTORC1 substrate S6 kinase 1 phosphorylates the small ribosomal subunit protein S6 (rpS6) in response to nutrient and growth factor stimuli (23). Therefore, we measured rpS6 phosphorylation in 607–627 day old female C57BL/6 mice fed eRapa for 42 days using the same doses as the NIA Intervention Testing Program (14 and 42 ppm) (12–14). Chronic eRapa treatment inhibited rpS6 Ser240/244 phosphorylation in the proximal and distal segments of the small intestine demonstrating mTORC1 inhibition (Figure 1A). These results were reproduced in the distal intestine from multiple mice (Figure 1A, right panel, Figure 1B). Because rpS6 is encoded by a 5′ TOP-containing mRNA (24) and since mTORC1 regulates translation of 5′ TOP-containing mRNAs (25), we also assayed rpS6 content in lysates. There were no differences in the levels of rpS6 relative to actin, encoded by a non-5′ TOP mRNA (Figure 1C). We found blood levels from treated mice averaged 37 ng/ml and 170 ng/ml for the 14 and 42 ppm group, respectively (a 4.6 fold increase in response to 3 fold higher concentration in the food), Figure 1D. These blood concentrations are higher than the therapeutic range used for organ transplant recipients (26). We also observed a dose response in proximal and distal small intestine tissue levels of rapamycin (Figure 1E). Note the increase in rapamycin levels in distal intestine compared to proximal, which is consistent with the pH gradient approaching neutrality thereby resulting in an increase drug release by Eudragit. Since phosphorylation of rpS6 by S6 kinase plays a role in ribosome biogenesis (27), eRapa-mediated depression of rpS6 phosphorylation indicates decrease ribosome biogenesis, which would inhibit cell growth needed for polyp formation. Thus, eRapa is an effective and convenient method to deliver rapamycin to both the small intestine and blood indicating eRapa could have both local and systemic affects.
Figure 1.
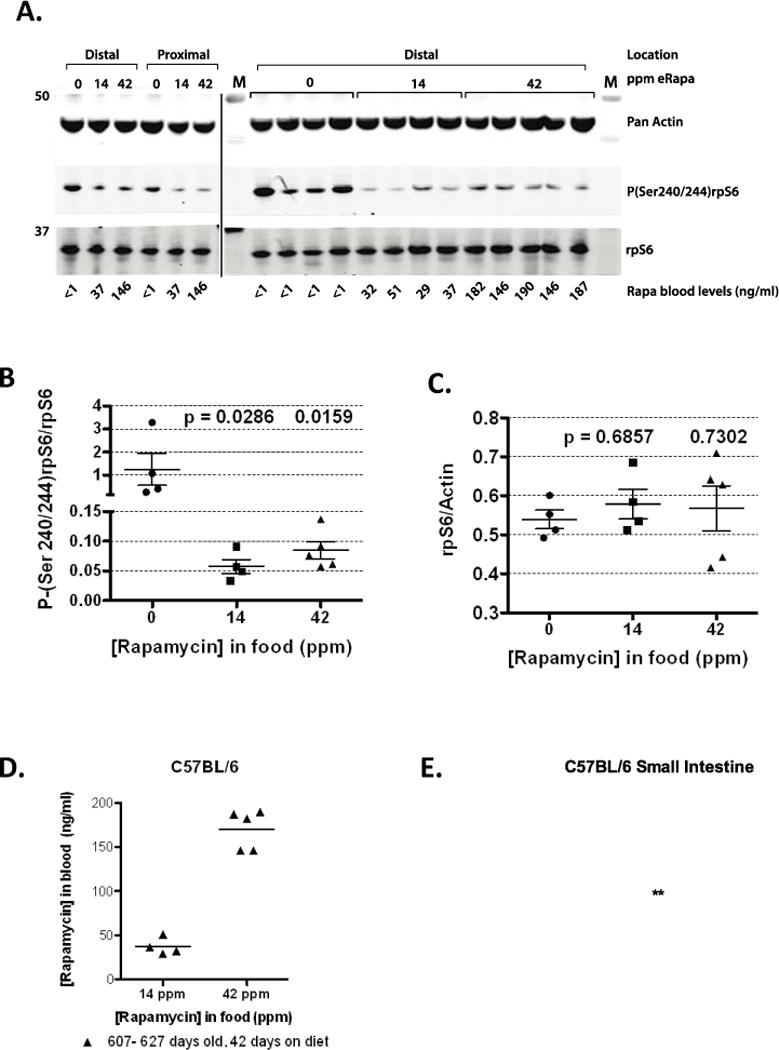
Pharmacodynamic data for C57BL/6 mice fed eRapa. (A) Western blots developed with indicated antibodies. C57Bl/6 mice (607–627 days old) were fed eRapa for 42 days, intestine dissected and immunoassayed as described in methods. Segments of the small intestine (proximal and distal) were assayed as indicated. Graphs showing the quantitation of the immunoblot data as measured by the ratio of intensity values for the phosphorylation state-dependent signal (P(240/244)rpS6) to phosphorylation state-independent (rpS6) signal (B)and rpS6 to actin antibody signal (C). (D) Rapamycin blood levels of C57BL/6 mice at 607–627 days of age (42 days on eRapa diets, which averaged 37 ng/ml and 170 ng/ml for the 14 and 42 ppm group, respectively). (E) Rapamycin tissue levels in proximal and distal small intestine of C57BL/6 mice (607–627 days of age and 42 days on diet). Mean levels (μg/g) are: proximal 14 ppm 91.62 ± 14.91, 42 ppm 266.7 ± 26.35; distal 14 ppm 627.3 ± 135.5, 42 ppm 1488 ± 141.6. P values are: 0.001 (proximal 14 ppm compared to 42 ppm); 0.035 (distal 14 ppm compared to 42 ppm); 0.0077 (proximal 14 ppm compared to distal 14 ppm); <0.0001 (proximal 42 ppm compared to distal 42 ppm).
We also examined the status of the other major arm of mTORC1 effectors, the Cap-binding translation initiation factor, eIF4E and its binding repressor, 4E-BP1, which are implicated in cancer growth (28). We detected no significant effects on this arm of the pathway including no alterations in the ratio eIF4E to its repressor 4E-BP1 (data not shown). Thus, the major effect of chronic rapamycin treatment directed to the small intestine appears to be a reduction of S6K1 activity manifested by decreased rpS6 phosphorylation.
Chronic rapamycin improved survival for ApcMin/+ mice (n=5 females/group) in a dose dependent manner (Figures. 2A, p<0.005, logrank test, and 2B for rapamycin blood levels). The ApcMin/+ cohort fed control chow lived 164–182 days (median 174) versus the 14 ppm eRapa cohort that lived 570–685 days (median 668). The 42 ppm eRapa fed ApcMin/+ mice lived 559–1093 days (median 937, approximately a 5.4-fold increase in life span over our control cohort). Our Supplementary video 1 at 902 days of age shows that three surviving mice were active and appeared healthy. Previous studies have shown the median life span for C567BL/6J mice is in the range of 692–866 days (e.g., see (10, 11))(shaded area in the graph in Figure 2A). The shaded area of the graph also includes female C57BL/6 survival results reported by Zhang et al., (16), and is representative of the colony in Nathan Shock Aging Animal and Longevity Assessment Core in the Barshop Center for Longevity and Aging Studies, the facility used for housing our ApcMin/+ mice. Thus, the 14 ppm eRapa-fed mice live close to this range, while four out of five of the 42 ppm eRapa fed mice lived within this range and three surpassed it. In addition, the life span of eRapa fed ApcMin/+ mice mimics the longevity of wild type C57BL/6Nia mice fed a 14 ppm eRapa diet beginning at 19 months of age (16). The blood levels of rapamycin achieved by 14 ppm and 42 ppm diets in the ApcMin/+ mice (Figure 2B) were lower than those observed for wild type C57BL/6 mice (Figure 1D). Although the exact reason for this difference is unknown, possibilities include variation in lots of eRapa-containing foods, differential drug absorption in wild type mice compared to ApcMin/+, and/or slower drug clearance in older mice (607–627 days for wild type, compare to 217 and 308 days ApcMin/+).
Figure 2.
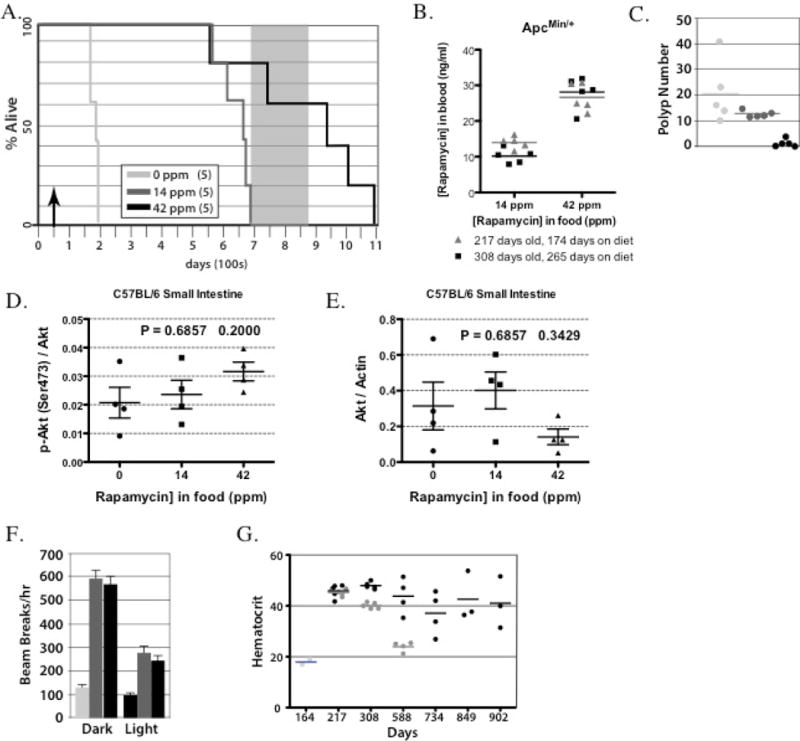
eRapa increases life and health span for ApcMin/+ mice. (A) eRapa increases life span for ApcMin/+ mice. The grey box represents a range of median life spans for C57BL/6J female mice taken from previous studies {Wijnhoven et al., 2005, #5122; Yuan et al., 2009, #78681; Zhang et al., 2013, #44400}, which is also representative of syngeneic colonies at the Barshop Institute animal facility used in our study. Note that 60% of the 42 ppm eRapa fed ApcMin/+ mice have lived beyond this range. (B) Rapamycin blood levels in ApcMin/+ mice at 217 days (174 days on eRapa diets, which averaged 14 ng/ml or 26.6 ng/ml on 14 ppm or 42 ppm diets, respectively) or at 308 days of age (265 days on eRapa diet, which averaged 10.2 ng/ml or 28.1 ng/ml on 14 ppm or 42 ppm diets, respectively) (C) Polyp count for ApcMin/+ mice at the time of death. Note the first and third mouse that died from the 42 ppm cohort had no polyps. (D&E) Graph showing quantification of phosphorylation state-dependent signal P(Ser473)Akt to phosphorylation state-independent signal (D) and Akt to actin antibody signal (E). (F) eRapa improves physical activity in ApcMin/+ mice. Graphed is the mean number of beam breaks (activity) for light (=inactive) and dark (=active) phases of the day. The food area of the cage is excluded. (G) eRapa maintains normal hematocrits in ApcMin/+ mice. Note the dose response. Also note the normal hematocrit as compared to wild type C57Bl/6 mice (~40%) in the high dose group even at late time points when many wild type C57Bl/6 mice would die from natural causes.
Necropsies were performed on ApcMin/+ mice that were sacrificed as they became moribund (unable to move and severely dehydrated) or recently after death. At necropsy, there was a reduction in the number of intestinal neoplasms for the 42 ppm cohort as compared to the control and 14 ppm eRapa dose cohorts (Table 1 and Figure 2C). The first mouse to die in the 42 ppm eRapa dose cohort (#15, Table 1) was found soon after death with an intact intestine. There were no gross pathological lesions including intestinal adenomas. Therefore, this mouse appeared to die from something other than intestinal neoplasms. The second mouse to die in this cohort (#13) was sacrificed when she was no longer able to move. Intestinal cancer likely contributed to her death since she had multiple grade 5 adenocarcinomas (Table 1). There was also a large skin ulcer (hyperplasia of squamous epithelium and amyloidosis) on her right lateral side that may have contributed to her death in addition to the intestinal neoplasia. Pathology on the third 42 ppm mouse to die (#11, 937 days of age) revealed one area of focal hyperplasia (mucosal epithelium) in the proximal small intestine and a necrotic liver with grade 4 (moderately severe) lymphoma (Table 1). Thus, this mouse died from a common age-related disease in mice, which is unrelated to the usual cause of death in ApcMin/+ mice. The fourth mouse to die (# 12, 995 days of age) had one intestinal tumor (moderately severe adenocarcinoma, Table 1). During manuscript review and revision, one ApcMin/+ mouse was still alive at 1087 days (October 3, 2013, Supplementary video 2), and she appeared to be a healthy old mouse. This mouse died 6 days later with a diagnosis of one grade 2 adenocarcinoma located in the distal segment of the small intestine and a thrombus located in the atrium of the heart (Table 1). Our results suggest that eRapa improves survival of Apc-mutant mice better than the delivery methods used in previous studies. eRapa’s superior performance was not due to higher rapamycin doses since the 14 ppm dose (2.24 mg/kg/day) was lower than all other reported doses [3 and 10 mg/kg/day by oral gavage (8) and 40 mg/kg food pellet (9)], yet exerted a greater improvement in survival. Analyses of eRapa effects on intestines and tumors of ApcMin/+ mice were not done in our survival studies since we find necropsy materials unsuitable for immunoassays of mTORC1 status. As mice approach death, they stop eating and drug levels likely drop significantly. Thus, off-tumor target effects of higher doses of rapamycin could also explain the dose responses we observed and require additional investigation.
Table 1.
Pathology 42 ppm ApcMin/+
| Mouse | Age at Death (days) | Diagnosis | Diagnosis Grade | Location |
|---|---|---|---|---|
| #11 | 937 | focal hyperplasia lymphoma necrosis |
1 4 3 |
proximal small intestine Liver |
| #12 | 995 | 1 adenocarcinoma | 4 | distal small intestine |
| #13 | 750 | 3 adenocarcinomas 1 adenocarcinoma 1adenoma |
5 5 5 |
distal small intestine proximal small intestine middle small intestine |
| #14 | 1093 | 1 adenocarcinoma thrombus |
2 2 |
distal small intestine atrium |
| #15 | 559 | not determined | NA | no intestinal polyps |
Grades: 0 = normal; 1 = minimal; 2 = mild; 3 = moderate; 4 = moderately severe; 5 = severe
NA: not applicable
As observed by Fujishita et al. study of ApcΔ716 mice (8), ApcMin/+ mice treated with both low and high dose eRapa also developed adenocarcinoma in the intestine, albeit in lower number in the high doses group (Table 1). One possibility for resistance of these tumors to chronic rapamycin is due to resistance of the mTORC1-4E-BP1 axis resulting from prolonged treatment (29–31). In agreement, our cross sectional analysis of mouse organs show no significant effects of chronic rapamycin treatment of 4E-BP1 phosphorylation. However, we do see a trend toward an increase in the ratio of eIF4E to 4E-BP1 levels (data not shown), which has been reported to cause resistance of cancer cells to active-site mTOR inhibitors (28). Finally, treatments with rapamycin or rapalogs results in the activation of a negative feedback loop resulting in activation of mTORC2 that phosphorylates and activates Akt (32, 33), which is a pro-growth, pro-survival pathway suggested to cause rapamycin resistance (34). In our analysis of tissues, we observe no significant effects on Akt Ser473 phosphorylation and a small decrease in Akt levels in distal small intestine (Figure 2D & E). This suggests that rebound activation of this pro-growth signaling pathway is less likely to be a factor in resistant tumors.
We measured the activity of ApcMin/+ mice as a surrogate for health. At 164–167 days, the eRapa-fed mice (both doses) exhibited a higher level of activity than control-fed mice during both the light (inactive) and dark (active) phases (Figure 2F, p<0.001). eRapa fed mice at both doses showed greater activity during the dark phaseas compared to the light phase (p<0.001), whereas control-fed mice show no difference between these phases (p = 0.415). Thus, ApcMin/+ mice on eRapa maintained diurnal rhythm and activity levels similar to wild type C57BL/6 mice suggesting better health versus ApcMin/+ mice on control chow.
Hematocrits were measured (Figure 2G) to determine the severity of intestinal bleeding that causes death. A normal hematocrit for wild type C57BL/6 mice is 44 ± 0.4 (35). At 164 days, two of the control mice exhibited hematocrits of 17% and 19% consistent with their early demise. The 14 ppm eRapa-fed cohort exhibited an average hematocrit of 45% at 217 days (n=5), 40% at 308 days (n=5) and 24% at 588 days (n=4), consistent with adenomas developing much later as verified by end-of-life necropsy. The 42 ppm eRapa-fed cohort exhibited average hematocrits of 46% at 217 days (n=5), 48% at 308 days (n=5), 44% at 588 days (n=4), 37% at 734 days (n=4), 43% (n=3) at 849 days and 40% (n=3) at 902 days. For the 734-day time point, one of the 42 ppm eRapa-fed cohort had an abnormally low hematocrit (27%), suggesting she had intestinal adenomas by this time. This mouse died at 750 days with adenocarcinomas (Table 1). The other mice in the 42ppm eRapa-fed cohort had normal or near normal hematocrits as late as 902 days suggesting a low adenoma burden. Thus, our study supports the possibility that eRapa could be administered prophylactically to FAP or similar patients to delay or prevent intestinal neoplasia and surgical intervention. Rapamycin targeted for release in the upper GI tract might also be an effective preventive for patients who have had colectomies but are at high risk for duodenal polyps and adenocarcinoma.
Rapamycin would need to have few harmful side effects in healthy adults if it is to be used as a prophylactic. One study found rapamycin caused glucose intolerance and insulin insensitivity in C57BLBL/6 mice (36) but these problems were not found in another study on the HET3 mice used by the Intervention Testing Program) (37) suggesting a difference in strain background (C57Bl/6 versus HET3), environment and/or delivery method (IP injections versus intestinal release by eRapa). Another study on C57BL/6 mice found that eRapa extended the life span of male C57BL/6 mice but also caused nephrotoxicity and testicular degeneration (38). However, nephrotoxicity was not found in other studies on C57BL/6 mice (16) or HET-3 mice (14). Thus, the only common toxicity associated with chronic rapamycin treatment is testicular degeneration (39).
Our observation that chronic eRapa exposure extends life span and ameliorates many age-dependent changes supports the notion that eRapa is safe, since toxic compounds would predictably shorten life span, not extend it. Furthermore, eRapa-fed ApcMin/+ mice with blood levels of 10–28 ng/ml (Figure 1) (often exceeding human therapeutic levels) live longer than wild type controls is inconsistent with clinically significant immunosuppression or other toxicities. Yet, a criticism of mouse studies is that mice are housed in a pathogen free environment that could mask potential problems with long-term rapamycin treatments.
To test for the possibility that eRapa is immunosuppressive in our setting, we evaluated its effects in several additional, relevant models. In a mouse melanoma model, four month-old mice were fed 14 ppm eRapa for 3 months and then challenged with B16 tumor cells. Tumor growth was similar between the eRapa and control mice (Figure 3A) as previously reported (40). Regulatory T cells and myeloid derived suppressor cells inhibit anti-tumor immunity generally and specifically in the B16 tumor model (17). However, 14 ppm eRapa did not increase regulatory T cell or myeloid derived suppressor cell numbers or suppression in the B16 tumor model (Figure 3B, C). Thus, in this tumor model we found no detrimental immune effects of chronic eRapa and specifically no increase in tumor-mediated immune suppression. In a T. gondii challenge model in which we and others have shown that survival following infection with this parasite depends on antigen-specific immunity and interferon-γ production (18, 20), eRapa at 14 ppm did not alter the numbers of antigen-specific CD8+ T cells or their interferon-γ production (Figure 4). In the well-established bm12 transfer model of autoimmunity, eRapa at 14 ppm did not accelerate autoimmune kidney disease, affect Treg numbers or function or alter host T cell numbers or activation versus Eudragit controls (not shown). Similarly, recent publications specifically examining rapamycin immune effects demonstrate that rapamycin boosts immunity to infection (41) including in a transplant model without worsening graft-versus-host disease (42), all underscoring the fact that rapamycin at clinically relevant concentrations that prolong life are not apparently immunosuppressive. When the optimal dose for increasing FAP survival is established, additional work will be required to assess specific immune effects at that dose.
Figure 3.
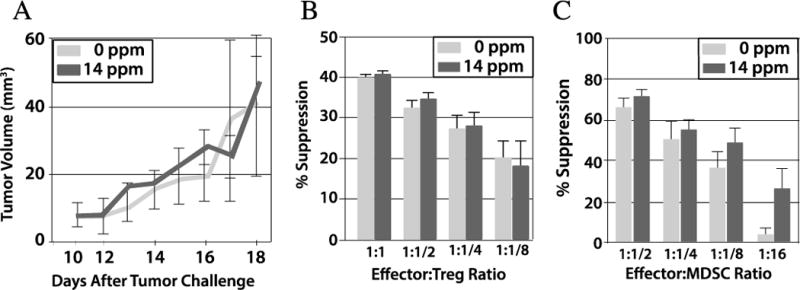
eRapa does not boost tumor growth or tumor-mediated immune suppression. C57BL/6J mice (n=6) were given either Eudragit or 14 ppm eRapa for 3 months and challenged with B16 melanoma. A. Tumor volume. B. Spleen Treg suppression assessed at various CD4+responder: Treg ratios. C. Spleen myloid derived suppressor cell (MDSC) suppression assessed at various CD4+responder:MDSC ratios. P>0.05 for all in B and C. Treg, regulatory T cell; MDSC, myeloid derived suppressor cell.
Figure 4.
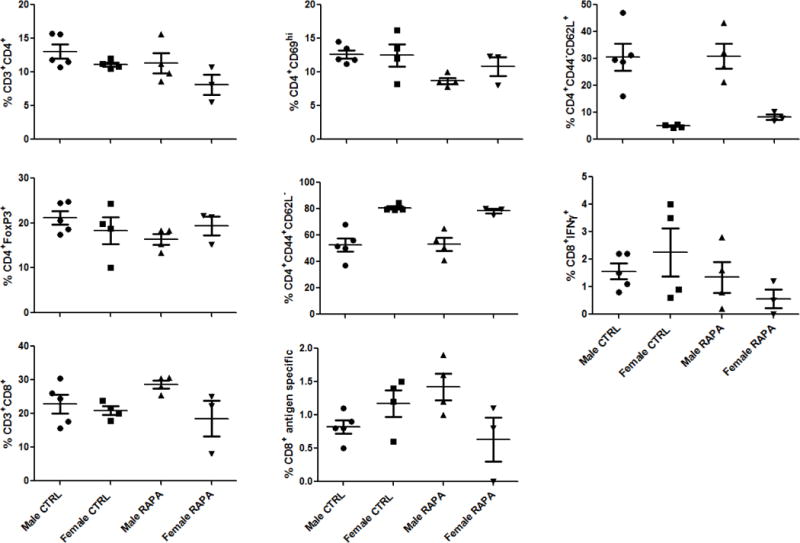
eRapa does not reduce mediators of T. gondii immunity during active infection. BL6 mice were infected with 10,000 Me49 strain T. gondii tachyzoites expressing an OVA reporter antigen by intraperitoneal challenge and mice were sacrificed 11 days later. Immune events were acquired from single-cell spleen suspensions on a Becton-Dickinson LSRII. Individual symbols represent individual mice and the bar is the median value. CD69 is an activation marker. Foxp3 is a regulatory T cell marker. CD44+CD62L− cells are memory T cells. CD44−CD62L+ cells are naive T cells. CTRL, mice fed Eudragit control chow. RAPA, mice fed chow with rapamycin 14 ppm. IFNγ, interferon-γ. “Antigen specific” refers to CD8+ T cells recognizing the SIINFEKL sequence of the OVA antigen engineered into T. gondii tachyzoites. No differences were statistically significant.
Non-steroidal anti-inflammatory drugs (NSAIDs) and various dietary supplements have been studied as potential chemoprevention agents. A large aspirin study showed daily consumption for five years or longer reduced cancer death in humans including deaths from gastrointestinal cancers (43). Aspirin inhibits mTOR and activates AMPK. However, in contrast to eRapa, aspirin extended median lifespan only in males (44). Aspirin can also cause significant side effects including gastrointestinal bleeding. Sulindac can be given to delay the progression of polyposis in the retained rectum among patients after colectomy with ileorectal anastomosis in conjunction with a strict endoscopic surveillance regimen (45) while the cyclooxygenase inhibitor, celecoxib reduced colorectal adenomas in a 6-month trial in FAP adults (46). Despite apparent effectiveness, neither sulindac nor celecoxib are recommended as primary prevention agents since reports suggest potential cardiovascular toxicity with COX-2 inhibitors in FAP. Furthermore, non-selective (COX-1 and COX-2) NSAIDs, including sulindac and naproxen, were suggested to increase cardiovascular thrombotic events. Thus, these agents are not ideal for cancer prevention.
Chemoprevention should ideally be well-tolerated, non-toxic, effective and inexpensive, for long-term use. As shown in Figure 2, enteric targeting of mTORC1 inhibitors demonstrates significant potential in meeting these first criteria and cost will decrease significantly when the rapamycin US patent (#5,100,899) expires in 2013.
We now need to determine the optimal eRapa dose to extend life span without toxic side effects and unravel its mechanism(s) of action. In this regard, it is interesting that mTORC1 mediates caloric restriction and rapamycin effects on intestinal crypt stem cell function and renewal (47), and identified as the cells-of-origin of intestinal cancer in ApcMin/+ mice (48). In sum, this report shows that targeted delivery of rapamycin (which is demonstrated here and elsewhere to be safe and to extend maximum lifespan in preclinical models) could be an effective prevention strategy for FAP patients and others predisposed to intestinal cancer. Further work is required to assess potential benefits of chronic eRapa treatment versus possible risks associated with chronic mTOR inhibition, which experience in transplant and cancers patients clearly demonstrate are manageable (49, 50), before moving into cancer prevention clinical trials.
Supplementary Material
Acknowledgments
We thank Ms. Charnae Williams, Vanessa Soto and Vanessa Martinez for technical assistance. Vivian Diaz and her staff in the Nathan Shock (P30AG013319) Aging Animal and Longevity Core directed by Dr. Jim Nelson provided expert care of our animals. Colton Allen in the Barshop Histology Core prepared tissues for pathology.
Grant Support
This work was supported by the following grants from the NIH: CA123203 and AG017242 to Paul Hasty. RC2AG036613 to Zelton D. Sharp, Paul Hasty, Randy Strong, Tyler J. Curiel. CA170491, Holly Beach Public Library and the Voelcker Fund supported Tyler J. Curiel. We would also like to thank the CTRC and colleagues in the Barshop Institute for Longevity and Aging Studies.
Footnotes
Co-first authors: PH, CBL
Conflicts of Interest Disclosure.
ZDS and RS are unpaid Scientific Advisory Board members for Rapamycin Holdings, Inc. The remaining authors do not have any relationships that could be construed as resulting in an actual, potential, or perceived conflict of interest with regard to the submitted manuscript.
References
- 1.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Annals of Internal Medicine. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Wallace MH, Phillips RK. Upper gastrointestinal disease in patients with familial adenomatous polyposis. The British journal of surgery. 1998;85:742–50. doi: 10.1046/j.1365-2168.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 3.Laplante M, Sabatini DM. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasty P. Rapamycin: The Cure for all that Ails. J Mol Cell Biol. 2010;2:17–9. doi: 10.1093/jmcb/mjp033. [DOI] [PubMed] [Google Scholar]
- 5.Piselli P, Serraino D, Segoloni GP, Sandrini S, Piredda GB, Scolari MP, et al. Risk of de novo cancers after transplantation: Results from a cohort of 7217 kidney transplant recipients, Italy 1997–2009. European Journal of Cancer. 2012 doi: 10.1016/j.ejca.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Livi CB, Hardman RL, Christy BA, Dodds SG, Jones D, Williams C, et al. Rapamycin extends life span of Rb1+/− mice by inhibiting neuroendocrine tumors. Aging (Albany NY) 2013;5:100–10. doi: 10.18632/aging.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, et al. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/− mice. Aging (Albany NY) 2012;4:709–14. doi: 10.18632/aging.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcΔ716 mice. Proc Natl Acad Sci U S A. 2008;105:13544–9. doi: 10.1073/pnas.0800041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koehl GE, Spitzner M, Ousingsawat J, Schreiber R, Geissler EK, Kunzelmann K. Rapamycin inhibits oncogenic intestinal ion channels and neoplasia in APCMin/+ mice. Oncogene. 2010;29:1553–60. doi: 10.1038/onc.2009.435. [DOI] [PubMed] [Google Scholar]
- 10.Wijnhoven SW, Beems RB, Roodbergen M, van den Berg J, Lohman PH, Diderich K, et al. Accelerated aging pathology in ad libitum fed Xpd(TTD) mice is accompanied by features suggestive of caloric restriction. DNA Repair (Amst) 2005;4:1314–24. doi: 10.1016/j.dnarep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–87. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. Rapamycin, But Not Resveratrol or Simvastatin, Extends Life Span of Genetically Heterogeneous Mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–82. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp ZD, Lee WH, Nikitin AY, Flesken-Nikitin A, Ikeno Y, Reddick R, et al. Minimal effects of dietary restriction on neuroendocrine carcinogenesis in Rb+/− mice. Carcinogenesis. 2003;24:179–83. doi: 10.1093/carcin/24.2.179. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, et al. Rapamycin Extends Life and Health in C57BL/6 Mice. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurez V, Daniel BJ, Sun L, Liu AJ, Ludwig SM, Kious MJ, et al. Mitigating age-related immune dysfunction heightens the efficacy of tumor immunotherapy in aged mice. Cancer Research. 2012;72:2089–99. doi: 10.1158/0008-5472.CAN-11-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei S, Marches F, Borvak J, Zou W, Channon J, White M, et al. Toxoplasma gondii-infected human myeloid dendritic cells induce T-lymphocyte dysfunction and contact-dependent apoptosis. Infect Immun. 2002;70:1750–60. doi: 10.1128/IAI.70.4.1750-1760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei S, Marches F, Daniel B, Sonda S, Heidenreich K, Curiel T. Pyridinylimidazole p38 mitogen-activated protein kinase inhibitors block intracellular Toxoplasma gondii replication. Int J Parasitol. 2002;32:969–77. doi: 10.1016/s0020-7519(02)00061-9. [DOI] [PubMed] [Google Scholar]
- 20.Daniel BJ, Pandeswara S, Brumlik MJ, Liu A, Thibodeaux SR, Ludwig SM, et al. A simple method to detect Toxoplasma gondii-specific cytotoxic T cells in vivo. J Immunol Methods. 2010;355:86–90. doi: 10.1016/j.jim.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Fueyo A, Sandner S, Habicht A, Mariat C, Kenny J, Degauque N, et al. Specificity of CD4+CD25+ regulatory T cell function in alloimmunity. J Immunol. 2006;176:329–34. doi: 10.4049/jimmunol.176.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan MZ, Prebeg Z, Kurjakovic N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation Of drug release using Eudragit L100-55 and Eudragit S100 combinations. Journal of controlled release: official journal of the Controlled Release Society. 1999;58:215–22. doi: 10.1016/s0168-3659(98)00151-5. [DOI] [PubMed] [Google Scholar]
- 23.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. The Biochemical journal. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 24.Meyuhas O, Dreazen A. Ribosomal protein S6 kinase from TOP mRNAs to cell size. Prog Mol Biol Transl Sci. 2009;90:109–53. doi: 10.1016/S1877-1173(09)90003-5. [DOI] [PubMed] [Google Scholar]
- 25.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–13. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trepanier DJ, Gallant H, Legatt DF, Yatscoff RW. Rapamycin: distribution, pharmacokinetics and therapeutic range investigations: an update. Clinical biochemistry. 1998;31:345–51. doi: 10.1016/s0009-9120(98)00048-4. [DOI] [PubMed] [Google Scholar]
- 27.Chauvin C, Koka V, Nouschi A, Mieulet V, Hoareau-Aveilla C, Dreazen A, et al. Ribosomal protein S6 kinase activity controls the ribosome biogenesis transcriptional program. Oncogene. 2013 doi: 10.1038/onc.2012.606. [DOI] [PubMed] [Google Scholar]
- 28.Alain T, Morita M, Fonseca BD, Yanagiya A, Siddiqui N, Bhat M, et al. eIF4E/4E-BP ratio predicts the efficacy of mTOR targeted therapies. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-2395. [DOI] [PubMed] [Google Scholar]
- 29.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–9. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-Site Inhibitors of mTOR Target Rapamycin-Resistant Outputs of mTORC1 and mTORC2. PLoS Biol. 2009 doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive Mammalian Target of Rapamycin Inhibitor Reveals Rapamycin-resistant Functions of mTORC1. Journal of Biological Chemistry. 2009;284:8023–32. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 34.Efeyan A, Sabatini DM. mTOR and cancer: many loops in one pathway. Curr Opin Cell Biol. 2010;22:169–76. doi: 10.1016/j.ceb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell ES, Neufeld EF, Higgins CT. Comparison of normal blood picture of young adults from 18 inbred strains of mice. Proc Soc Exp Biol Med. 1951;78:761–6. doi: 10.3181/00379727-78-19210. [DOI] [PubMed] [Google Scholar]
- 36.Lamming DW, Ye L, Astle MC, Baur JA, Sabatini DM, Harrison DE. Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive. Aging Cell. 2013;12:712–8. doi: 10.1111/acel.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–43. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–91. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson A. Rapamycin, anti-aging, and avoiding the fate of Tithonus. The Journal of Clinical Investigation J Clin Invest. 2013;123:3204–6. doi: 10.1172/JCI70800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marone R, Erhart D, Mertz AC, Bohnacker T, Schnell C, Cmiljanovic V, et al. Targeting melanoma with dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitors. Mol Cancer Res. 2009;7:601–13. doi: 10.1158/1541-7786.MCR-08-0366. [DOI] [PubMed] [Google Scholar]
- 41.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–12. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, et al. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. Journal of immunology. 2010;185:2004–8. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothwell PM, Wilson M, Price JF, Belch JFF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. The Lancet. 2012;379:1591–601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 44.Strong R, Miller R, Astle C, Floyd R, Flurkey K, Hensley K, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–50. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rigau J, Pique JM, Rubio E, Planas R, Tarrech JM, Bordas JM. Effects of long-term sulindac therapy on colonic polyposis. Annals of Internal Medicine. 1991;115:952–4. doi: 10.7326/0003-4819-115-12-952. [DOI] [PubMed] [Google Scholar]
- 46.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. The New England journal of medicine. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 47.Yilmaz OH, Katajisto P, Lamming DW, Gultekin Y, Bauer-Rowe KE, Sengupta S, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–5. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 49.Ma WW, Hidalgo M. Exploiting novel molecular targets in gastrointestinal cancers. World Journal of Gastroenterology. 2007;13:5845. doi: 10.3748/wjg.v13.i44.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soefje SA, Karnad A, Brenner AJ. Common toxicities of mammalian target of rapamycin inhibitors. Target Oncol. 2011;6:125–9. doi: 10.1007/s11523-011-0174-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


