Abstract
IMPORTANCE
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare malignant neoplasm with cutaneous manifestations and a rapidly progressive clinical course. The diagnosis relies on characteristic clinicopathologic and immunopathologic features. However, the overlap of immunophenotypic features with other cancers, as well as newly discovered interpersonal and intrapersonal phenotypic variations, renders the identification of BPDCN challenging. A greater understanding of the proteins expressed by BPDCN might facilitate its recognition and provide insights into its clinical behavior.
OBSERVATIONS
In 7 of 9 patients at 4 tertiary care institutions, immunohistochemical analysis demonstrated strong CD31/PECAM-1 (platelet endothelial cell adhesion molecule 1) expression by neoplastic cells. Combined with similar findings observed in 1 former patient, 8 of 10 cases of BPDCN were CD31/PECAM-1 positive.
CONCLUSIONS AND RELEVANCE
Expression of CD31/PECAM-1 by BPDCN adds new information about the antigenic profile of this unusual neoplasm. CD31/PECAM-1 influences multiple cell functions including adhesion, apoptosis, coagulation, host response, and protein synthesis that might affect clinical features of BPDCN such as hemorrhage, aggressive tumor growth, and resistance to therapy. Therefore, the potential role of this molecule in the tumor formation and progression of BPDCN warrants additional exploration.
Blastic plasmacytoid dendritic cell neoplasm (BPDCN), formerly known as CD4+/CD56+ hematodermic neoplasm or blastic natural killer (NK) cell lymphoma, is an exceedingly rare and highly aggressive malignant neoplasm, recently reclassified as a distinct entity.1,2 It shows a strong predilection for elderly men but may affect any age group, including neonates.2,3 Because the skin is usually the site of initial presentation, dermatologists play a key role in timely recognition and treatment.2,4,5 Cutaneous involvement is invariably followed by rapidly progressing systemic dissemination, which may culminate in fulminant leukemia.2-5 The response to high-dose chemotherapy and/or radio-therapy is initially favorable but ultimately short lived, and median survival rarely exceeds 14 months.2-6 Occasionally, allogeneic bone marrow transplantation has achieved sustained remission in younger patients.2,4-6
The diagnosis of BPDCN is rendered difficult by the heterogeneity of clinical and immunophenotypic features.1-6 Generally, the following clinical, histopathologic, and immunopathologic criteria are used to establish the diagnosis. Skin manifestations range from nonspecific reddish-brown to violaceous patches, plaques, or nodules, which may appear bruise-like and tend to generalize as the malignancy progresses, to atypical eruptions mimicking lichen planus or lupus erythematosus.2,4-6 Flat solitary lesions occur in up to 30% of cases, and B-type symptoms are commonly absent, mandating a high level of suspicion.5 Histopathologic analysis demonstrates a nonepidermotropic dermal infiltrate of monomorphous, medium-sized lymphoid-appearing cells that display a slightly irregular-shaped nucleus with finely dispersed chromatin and scant cytoplasm.4-6 Definitive diagnosis relies on adequate immunophenotypic analysis, which shows CD4, CD56, and/or CD123 in approximately 95% of cases and TCL-1, CD2–associated protein (CD2AP), and/or BDCA2/CD303 in 75% to 90% of cases. There is absence of common B-cell, T-cell, and myeloid or myelomonocytic cell lineage markers such as CD3, 11c, 20, 34, 79a, 163, lysozyme, and myeloperoxidase.1-6 However, this profile overlaps substantially with those of other hematolymphoid malignant neoplasms, predominantly NK cell lymphoma and/or leukemia and myeloid leukemias with skin involvement.2,4-7 Moreover, the immunophenotypic diversity of BPDCN is greater than previously recognized.2,5,7 Although the exact proportion of aberrant phenotypes remains unknown, negativity of at least 1 of the 4 markers CD4, CD56, CD123, and TCL-1 was demonstrated in 34% to 36% of cases in 2 large studies, emphasizing the need to disclose additional immunophenotypic features of this lethal neoplasm.5,7
Herein we report the expression of CD31/PECAM-1 (platelet endothelial cell adhesion molecule 1) as an additional antigenic characteristic of BPDCN.
Report of Cases
Our study was initiated by the results of the immunohisto-chemical (IHC) analysis of a violaceous nodule with surrounding golden contusiform rim on the shoulder of an otherwise asymptomatic 80-year-old man (Figure 1A). Histopathologic examination revealed a dense dermal infiltrate of uniform, mononuclear cells in the absence of other inflammatory cells, necrosis, or angioinvasion (Figure 1B). Differential diagnostic considerations included hematopoietic and lymphoid malignant neoplasms, as well as vascular tumors, necessitating a broad panel of antibody tests. Samples stained for IHC analysis were positive for CD4, CD56, CD123, and CD31 (Figure 2) and negative for CD2, CD3, CD5, CD7, CD8, T-cell–restricted intra-cellular antigen 1 (TIA-1), CD20, PAX-5, lysozyme, myeloper oxidase, CD30, CD1a, CD68, and terminal deoxynucleotidyl transferase. A complete blood cell count with differential, comprehensive metabolic panel and serum as well as urine protein electrophoresis had normal results. A computed tomo-graphic scan showed a 5 × 2-cm lesion of the right side of the chest and a 1.7-cm-diameter splenic mass. The clinical and IHC findings were consistent with BPDCN. This case was reminiscent of that of a former patient of ours with BPDCN, whose tumor cells were also CD31 positive.8
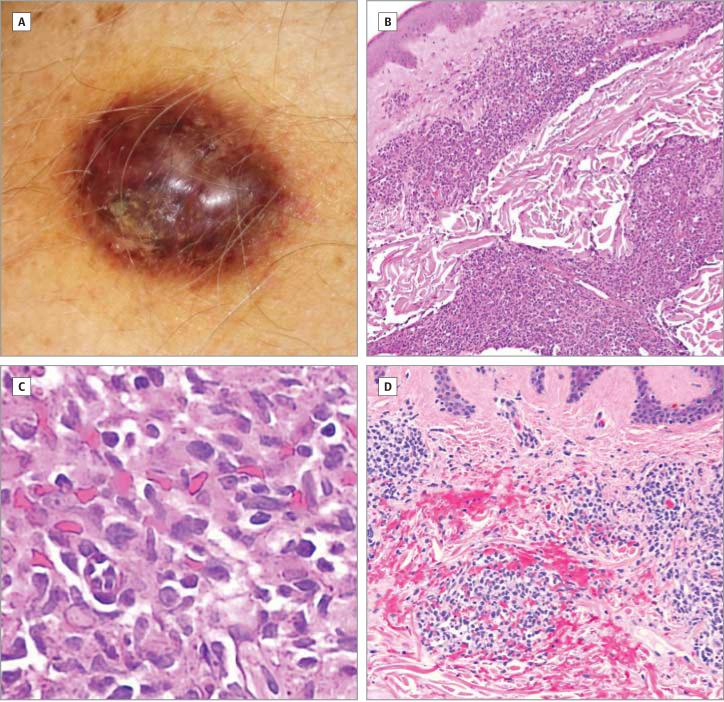
Figure 1. Clinical and Microscopic Images of the Illustrative Case.
A, Shiny 2 × 3-cm violaceous nodule displaying a golden contusiform rim, located on the left deltoid. B, Corresponding histopathologic image (hematoxylin-eosin [H&E], original magnification, ×100): dense infiltrate of tumor cells in the upper and lower dermis. C, A ×400 magnification of the infiltrate shows monomorphous medium-sized cells with slightly irregular nuclei, finely dispersed chromatin, and scant cytoplasm. D, Tumor cells infiltrating upper dermis are associated extensive extravasation of erythrocytes (H&E, original magnification, ×200).
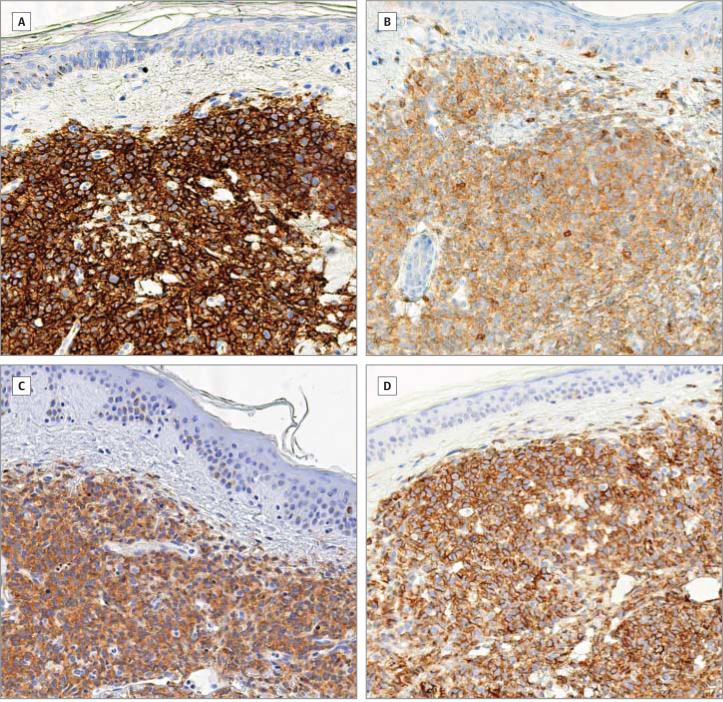
Figure 2. Immunohistochemical Staining of Formalin-Fixed Paraffin-Embedded Sections of the Lesion Shown in Figure 1.
Diaminobenzidine, original magnification, ×200: CD56 (A), CD4 (B), CD123 (C), and CD31/PECAM-1 (platelet endothelial cell adhesion molecule 1) (D).
The aforementioned observations prompted us to investigate the expression of CD31 by BPDCN cells. In collaboration with 3 other tertiary care institutions, 8 additional patients with BPDCN were identified and paraffin-embedded lesional tissue sections were subjected to immunoperoxidase IHC analysis for the CD31 protein in accordance with manufacturer-recommended protocols, using monoclonal mouse anti–human CD31 antibodies (clone JC/70A, isotype IgG1κ) from Biocare Medical, Dako North America, and Thermo Scientific. Tumor cells demonstrated strong immunoreactivity in 6 cases, paralleling the IHC results seen in Figure 2, whereas 2 cases had negative results. To exclude low-level protein expression undetectable by IHC analysis, the negative biopsy samples were subjected to multispectral image analysis, which has been demonstrated to be more sensitive than routine IHC analysis in the assessment of low-level protein expression by lymphoma cells.9 This method confirmed the absence of CD31 in 1 case, whereas the other case showed small clusters of weakly positive cells, which most likely represented admixed macrophages. The expression of CD31 by intratumoral macrophages has been described as a potential diagnostic pitfall in the evaluation of tumor tissue sections for the presence of this antigen.10 In view of this fact, and because more than 95% of the tumor cell infiltrate in this biopsy sample was nonreactive, we designated it CD31 negative without further investigation.
Taken together with our previously published report,8 IHC analysis detected strong, widespread expression of CD31 by neoplastic cells in 8 of 10 BPDCN cases (80%).
Discussion
Two major subtypes of circulating dendritic cell (DC) populations have been identified to date: myeloid DCs (mDCs) and plasmacytoid DCs (pDCs). Formerly thought to originate from NK cells, BPDCN is now believed to derive from pDC precursors, as evidenced by the expression of pDC antigens TCL1, CD123, and BDCA-2.2,4 Production of interferon α by BPDCN cells demonstrates that the tumor cells maintain some of the functional characteristics of pDCs.4
CD31 is best known as a marker for vascular components of tissues (both normal and diseased) because it is expressed on early and mature endothelial cells and related neoplasms. However, it is also present on platelets and some plasma cells, monocytes, immature mDCs, neutrophils, NK cells, and lymphocytes.11 In addition, CD31 expression has been observed commonly in some nonendothelial cancers, predominantly in leukemias and lymphomas such as acute myeloid leukemia, acute lymphoblastic leukemia, chronic lymphocytic leukemia, chronic myeloid leukemia, mantle cell lymphoma, hairy cell leukemia, and multiple myeloma but not in cutaneous T-cell lymphomas.11-13 Approximately one-third of uterine cervical carcinomas are CD31+, and it is also expressed occasionally by a wide variety of other carcinomas and sarcomas.11,12 We are contributing an additional type of neoplasm, BPDCN, to the list of nonendothelial malignancies that express CD31 frequently. However, because of the rarity of this neoplasm, it will be important to study additional cases to determine whether the true prevalence of CD31 expression is 80% (8 of 10) as we report here.
CD31 is able to engage in heterophilic as well as in homo-philic binding, allowing complex adhesive interactions, which represent a basis for the role of this molecule in vascular biology.11 Our observation raises the question of whether CD31 on tumor cells might bind to CD31 on platelets, thereby inhibiting their activation. Similarly, CD31 elaborated by BPDCN could bind to CD31 on endothelial cells, resulting in localized secretion of prostacyclin, which in turn would inhibit coagulation. These events, individually or in combination, might contribute to intralesional hemorrhage and the bruiselike appearance noted in some BPDCN cases. Adhesive interactions between tumoral and endothelial CD31 could also contribute to the aggressive dissemination characteristic of BPDCN.
Recent work has uncovered new functions of CD31 as a cytoprotective cell signaling molecule in a variety of physiological and pathological states; eg, it inhibits the intrinsic apoptotic pathway both in vitro and in vivo.11,14 Therefore, CD31 expression may play a role in the chemoresistance of BPDCN, and decreasing its expression in lymphoid malignant neoplasms could render them more susceptible to chemotherapy-induced apoptosis.11 One might also speculate that the capa bility of CD31 to activate regulatory T cells and to attenuate T-cell chemokinesis fosters a local microenvironment that suppresses antitumor T-cell responses.15
Alternatively spliced, functionally distinct CD31 iso-forms exist, and the ones expressed by BPDCN remain to be investigated. Future studies with larger case numbers are needed to evaluate the potential of CD31 as a biomarker for BPDCN and to clarify its role in the pathogenesis of this lethal disorder. Interpersonal and intrapersonal immunophenotypic variations, such as CD4/CD56 positivity followed by negativity for both markers in subsequent biopsy specimens from a single patient, have been reported.5,7 These findings underscore the importance of adding new antigens to the known repertoire of proteins expressed by BPDCN because their assessment may aid in early recognition of this tumor or help explain its biology.
Acknowledgments
Funding/Support: This study was supported in part by Merit Review funding from the Department of Veterans Affairs (to Dr Wood) and National Institutes of Health grant T32AR055893 (to Dr Salva). Dr Haemel is supported by the Dermatology Foundation Medical Dermatology Career Development Award.
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Salva and Wood had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Salva, Haemel, Liu, Wood.
Acquisition of data: All authors.
Analysis and interpretation of data: Salva, Haemel, Pincus, Sundram, Longley, Wood.
Drafting of the manuscript: Salva, Haemel, Pincus, Liu, Wood.
Critical revision of the manuscript for important intellectual content: Salva, Haemel, Sundram, Guitart, Longley, Wood.
Statistical analysis: Pincus.
Administrative, technical, or material support: Salva, Haemel, Guitart, Longley.
Study supervision: Wood.
Additional Contributions: The valuable efforts of Janyana Deonizio, MD (Northwestern University), contributed to the collection of patient data. Dr Deonizio was not compensated for her contribution.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Assaf C, Gellrich S, Whittaker S, et al. CD56-positive haematological neoplasms of the skin: a multicentre study of the Cutaneous Lymphoma Project Group of the European Organisation for Research and Treatment of Cancer. J Clin Pathol. 2007;60(9):981–989. doi: 10.1136/jcp.2006.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kharfan-Dabaja MA, Lazarus HM, Nishihori T, Mahfouz RA, Hamadani M. Diagnostic and therapeutic advances in blastic plasmacytoid dendritic cell neoplasm: a focus on hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(7):1006–1012. doi: 10.1016/j.bbmt.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Yang CS, Wang J, Chang TK. Congenital blastic plasmacytoid dendritic cell neoplasm. Pediatr Blood Cancer. 2012;58(1):109–110. doi: 10.1002/pbc.23208. [DOI] [PubMed] [Google Scholar]
- 4.Pilichowska ME, Fleming MD, Pinkus JL, Pinkus GS. CD4+/CD56+ hematodermic neoplasm (“blastic natural killer cell lymphoma”): neoplastic cells express the immature dendritic cell marker BDCA-2 and produce interferon. Am J Clin Pathol. 2007;128(3):445–453. doi: 10.1309/W9Q5AGYDE5LANN39. [DOI] [PubMed] [Google Scholar]
- 5.Cota C, Vale E, Viana I, et al. Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm—morphologic and phenotypic variability in a series of 33 patients. Am J Surg Pathol. 2010;34(1):75–87. doi: 10.1097/PAS.0b013e3181c5e26b. [DOI] [PubMed] [Google Scholar]
- 6.Petrella T, Bagot M, Willemze R, et al. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. Am J Clin Pathol. 2005;123(5):662–675. [PubMed] [Google Scholar]
- 7.Montes-Moreno S, Ramos-Medina R, Martínez-López A, et al. SPIB, a novel immunohistochemical marker for human blastic plasmacytoid dendritic cell neoplasms: characterization of its expression in major hematolymphoid neoplasms. Blood. 2013;121(4):643–647. doi: 10.1182/blood-2012-08-447599. [DOI] [PubMed] [Google Scholar]
- 8.Sale T, Wood GS, Longley BJ. Epstein-Barr virus–positive blastoid NK-cell lymphoma. In: Burg W, Kempf G, editors. Cutaneous Lymphomas: Unusual Cases. Vol. 2. Steinkopf; Darmstadt, Germany: 2006. pp. 2–55. [Google Scholar]
- 9.Salva K, Sundram U, Krathen M, et al. Analysis of protein expression in situ using multi-spectral imaging is superior to conventional immunohistochemistry (IHC): a new paradigm for patient selection for targeted therapy. J Invest Dermatol. 2013;133(S):169. [Google Scholar]
- 10.McKenney JK, Weiss SW, Folpe AL. CD31 expression in intratumoral macrophages: a potential diagnostic pitfall. Am J Surg Pathol. 2001;25(9):1167–1173. doi: 10.1097/00000478-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Bergom C, Gao C, Newman PJ. Mechanisms of PECAM-1–mediated cytoprotection and implications for cancer cell survival. Leuk Lymphoma. 2005;46(10):1409–1421. doi: 10.1080/10428190500126091. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson SA, McDermott MB, DeYoung BR, Swanson PE. CD31 immunoreactivity in small round cell tumors. Appl Immunohistochem Mol Morphol. 2000;8(1):19–24. doi: 10.1097/00129039-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Karpova MB, Fujii K, Jenni D, Dummer R, Urosevic-Maiwald M. Evaluation of lymphangiogenic markers in Sézary syndrome. Leuk Lymphoma. 2011;52(3):491–501. doi: 10.3109/10428194.2010.517877. [DOI] [PubMed] [Google Scholar]
- 14.Gao C, Sun W, Christofidou-Solomidou M, et al. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood. 2003;102(1):169–179. doi: 10.1182/blood-2003-01-0003. [DOI] [PubMed] [Google Scholar]
- 15.Kishore M, Ma L, Cornish G, Nourshargh S, Marelli-Berg FM. Primed T cell responses to chemokines are regulated by the immunoglobulin-like molecule CD31. PLoS One. 2012;7(6):e39433. doi: 10.1371/journal.pone.0039433. [DOI] [PMC free article] [PubMed] [Google Scholar]