Abstract
Silicosis, a fibrotic granulomatous lung disease, may occur through accidental high-dose or occupational inhalation of silica, leading to acute/accelerated and chronic silicosis, respectively. While chronic silicosis has a long asymptomatic latency, lung inflammation and apoptosis are hallmarks of acute silicosis. In animal models, histiocytic granulomas develop within days after high-dose intratracheal (IT) silica instillation. However, following chronic inhalation of occupationally relevant doses of silica, discrete granulomas resembling human silicosis arise months after the final exposure without significant lung inflammation/apoptosis. To identify molecular events associated with chronic silicosis, lung RNAs from controls or sub-chronic silica-exposed rats were analyzed by Affymetrix at 28 weeks after silica exposures. Results suggested a significant upregulation of 144 genes and downregulation of 7 genes. The upregulated genes included complement cascade, chemokines/chemokine receptors, G-protein signaling components, metalloproteases, and genes associated with oxidative stress. To examine the kinetics of gene expression relevant to silicosis, qPCR, ELISA, Luminex-bead assays, Western blotting, and/or zymography were performed on lung tissues from 4 day, 28 week, and intermediate times after sub-chronic silica exposure and compared with 14 day acute silicosis samples. Results indicated that genes regulating fibrosis (secreted phosphoprotein-1, Ccl2, and Ccl7), redox enzymes (superoxide dismutase-2 and arginase-1), and the enzymatic activities of matrix metalloproteinases 2 and 9 were upregulated in acute and chronic silicosis models. However, proinflammatory cytokines were strongly upregulated only in acute silicosis. Thus, inflammatory cytokines are associated with acute but not chronic silicosis. Data suggest that genes regulating fibrosis, oxidative stress, and metalloproteases may contribute to both acute and chronic silicosis.
Keywords: Silicosis, Fibrosis, Inflammation, Spp1, Ccl2, Ccl7, Toxicogenomic
Introduction
Epidemiological studies indicate that chronic silicosis is the most common form of silicosis. Chronic silicosis is generally associated with occupational exposure to crystalline silica and mainly prevalent in stone dressers, miners, and quarry and foundry workers (Shi et al. 1998; Hnizdo and Sluis-Cremer 1993; Chong et al. 2006). Although in animals, both high-dose acute and low-dose chronic silica exposures induce granulomatous changes in the lung (Borges et al. 2002; Langley et al. 2004; 2010; Castranova et al. 2002), the granuloma-like structures in acute silicosis are loosely aggregated foamy histiocytes and lymphocytes. On the other hand, chronic silicotic granulomas in humans (Hnizdo and Vallyathan 2003) and animals (Langley et al. 2004; 2010) are small, well-organized nodules consisting of epithelioid macrophages and multinucleated giant cells (MGC), and generally, in humans arise after decades of asymptomatic latency (Chong et al. 2006). Silicosis is a progressive disease that is resistant to all treatment, including corticosteroid therapy (Chong et al. 2006).
In humans, acute (accelerated) silicosis is rare and usually a result of accidental inhalation of a high dose of silica. Acute silicosis is characterized by severe alveolitis, alveolar lipoproteinosis, and a progressive clinical course that often results in death within a few years (Chong et al. 2006). In animal models, acute silicosis is induced by intratracheal (IT) instillation of large doses of silica (Borges et al. 2002; Zhang et al. 2002) as well as high dose daily inhalation exposure to silica (Porter et al. 2001), resulting in severe lung inflammation, alveolar lipoproteinosis, apoptosis, induction of reactive oxygen species (ROS) production of proinflammatory cytokine/chemokine, and tissue destruction (Borges et al. 2002; Zhang et al. 2002). Apoptosis-defective FasL−/− gld mice do not develop acute silicosis (Borges et al. 2001), which has led to the belief that apoptosis is critical in silicosis. The accelerated acute inhalation model developed by Porter et al. (2001, 2002a), also leads to the development of acute-like injury (e.g. apoptosis, inflammation, tissue destruction) after a long continuous exposure (≥70 days) day exposure and may be related to total deposition of silica within the lungs. On the other hand, sub-chronic inhalation exposures of rats to occupationally relevant doses of silica not only failed to promote significant apoptosis and inflammation in the lung, but on the contrary, the lungs exhibited anti-apoptotic markers during granuloma formation (Langley et al. 2010). Similarly, rats receiving weekly small doses of silica via IT instillation do not show lung injury or cell death, and only a quantitative increase in polymorphonuclear cells in bronchoalveolar (BAL) fluids (Porter et al. 2002a). Moreover, recent evidence suggests that fibrosis in acute silicosis is independent of proinflammatory cytokines such as IL-17A and type I interferon (Giordano et al. 2010; Lo Re et al. 2010). Oxidative stress is associated with the development of acute and chronic silicosis (Castranova 2004; Shi et al. 1998) and, interestingly, oxidative burden progressively rises even when silica exposures are terminated and lungs have cleared most of the silica (Fubini and Hubbard 2003; Rimal et al. 2005). In this study, a toxicogenomics approach was used to identify molecular events associated with chronic silicosis.
MATERIALS AND METHODS
ANIMALS
Six-to 8 week-old, pathogen-free, male Lewis rats weighing 150–175 g were purchased from Charles River Laboratories (Raleigh, NC). During the 2 week conditioning and 6 week silica exposure, the animals were housed in H2000 whole-body exposure chambers (Lab Products, Inc., Maywood, NJ). After the exposure, the animals were transferred to class-100 air quality rooms in shoebox cages with hardwood chip bedding. Food and water were provided ad libitum throughout the experimental period; animals were periodically monitored for common rat infections. All experiments were approved by the Lovelace Respiratory Research Institute’s Animal Care and Use Committee.
Animal Exposures
Rats were sub-chronically exposed to silica by inhalation as described (Langley et al. 2004). Briefly, exposure chambers were maintained with an airflow rate of approximately 15 cubic feet /m and temperature range of 22–26°C. Animals were exposed to 6.2 mg/m3 aerosolized silica (Min-U-Sil 5; U.S. Silica, Mill Creek, OK) with an average particle size of 1.75 ± 0.05 µm (mass median aerodynamic diameter) 6 hr/day, 5 days/week (Monday–Friday) for 6 weeks (Langley et al. 2004). Control animals received filtered air under similar inhalation conditions. Silica exposure level of these animals was within the range of human occupational exposure (Hnizdo and Sluis-Cremer 1993). As previously described, other than an increased number of foamy (e.g., silica-containing) macrophages, there were no apparent signs of lung inflammation between 4 and 28 day post-silica exposure (Langley et al. 2004). Animals were sacrificed at 4 day, or 7, 14 and 28 weeks after the last silica exposure (Langley et al. 2010). Macrophage aggregation (an early indication of granuloma formation) was first noted at 7 weeks post-silica exposure, but frank granulomas did not develop until 14 weeks after silica exposure and was well established by 28 weeks (Langley et al. 2004; 2010). To distinguish between acute and chronic silicosis, some rats received a single high-dose IT instillation of 35 mg silica in 750 µl of saline or saline alone (control). These animals were sacrificed 14 days post-silica exposure; the amount of silica and time of sacrifice were based on reports for induction of acute silicosis (Borges et al. 2001; Barbarin et al. 2005; Barbaro et al. 2002).
Whole Lung and Bronchoalveolar Lavage Collection
As described previously (Langley et al. 2010), the lungs were removed from the animals at sacrifice. The left lung lobe was clamped off at the bronchi with clips, removed, minced and split equally for RNA analysis or protein analysis in separate cryovials. The vials were snap frozen in liquid nitrogen and stored at −80°C. The right lobe was lavaged twice with 3 ml aliquots of sterile saline solution through a tracheal cannula. The lavage was pooled and centrifuged (500g, 5 min), and BAL fluid was collected.
cRNA Synthesis and Microarray Hybridizations
Briefly, RNA was isolated from non-lavaged whole lung from 3 controls and 3, 28 week postsilica-exposed rats as described (Razani-Boroujerdi and Sopori 2007) using Tri-Reagent (Molecular Research Center, Cincinnati, OH). RNA samples were processed individually. The microarray analysis was performed at the Keck-UNM Genomics Resource (University of New Mexico Cancer Research Center, Albuquerque, NM). Total RNA (1 µg) was used to generate double-stranded cDNA using an oligo dT-primer containing the T7 RNA polymerase promoter site and the One-Cycle Target Labeling Kit (Affymetrix). cDNA was purified via column purification using the GeneChip Sample Cleanup Module (Affymetrix); biotinylated cRNA was synthesized by in vitro transcription using the GeneChip IVT Labeling kit (Affymetrix). Biotin-labeled cRNA was purified (GeneChip Sample Cleanup Module, Affymetrix), and 20 µg of the labeled cRNA was fragmented. The cRNA and fragmented cRNA quality was assessed by the Agilent 2100 Bioanalyzer (Agilent; Foster City, CA) and the RNA 6000 Nano LabChip kit (Agilent). The labeled fragmented cRNA was hybridized to Affymetrix GeneChip Rat 230 2.0 arrays for 16 hr at 45°C following the Affymetrix protocol specific to this array type. Washing and staining were performed on the Affymetrix fluidics (450) station according to the antibody amplification protocol (Fluidics script: EukGE-WS2v5). The Gene Chips were scanned using the GeneChip Scanner 3000 (Affymetrix). The analysis was performed with GeneSpring software package (Agilent Technologies, Santa Clara, CA). All samples were subject to per-chip normalization. Data were filtered twice, a fold change filter (2 fold up or down), and the Statistical Analysis of Microarray software (http://www-stat.stanford.edu/~tibs/SAM/), to find all genes with a < 10% false positive q-score. The raw data have been deposited in NCBI’s Gene Expression Omnibus (Edgar et al. 2002) (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE29110).
Real-time PCR
Real-time PCR (qPCR) was performed per manufacturer’s suggestions on the Prism 7900 HT Sequence Detection System (ABI, Foster City, CA) using standard protocols. Primers and probes for arginase-1 (Arg1), β-Actin, interferon-γ (Ifnγ), interleukin-1β (Il1β), Ccl7, matrix metalloproteinase 12 (Mmp12), superoxide dismutase-2 (Sod2) and secreted phosphoprotien 1 (Spp1) were designed with Primer Express 2.0 software (Table 1; ABI). PCR was performed for 40 cycles with denaturation (95°C for 15 sec) and annealing temp (58°C for 60 sec). Relative expression is presented as fold change to control.
TABLE 1.
qPCR RAT PRIMER AND PROBE SETS
| Gene | Probe | Forward Primer | Reverse Primer |
|---|---|---|---|
| Arg1 | CAAGGACATCGTGTACATCGGCTTGC | GGTGACCCCCTGCATATCTG | TTCCCCAGGGTCCACATCT |
| β-Actin | CGTAGCCATCCAGGCTGTGTTGTCC | TTCAACACCCCAGCCATGT | GTGGTACGACCAGAGGCATACA |
| Ifnγ | TGAGCATCGCCAAGTTCGAGG | CAGTAAAGCAAAAAAGGATGCATTC | TGCTGGATCTGTGGGTTGTTC |
| Il1β | CCCCAGGACATGCTAGGGAGCCC | GATGGCTGCACTATTCCTAATGC | AGACTGCCCATTCTCGACAAG |
| Ccl7 | TGAGGAATTTTGCTTCTTGACTTCAGGGTTG | CAGCACAGTTCTCAGAAAAGACAAG | TGAAGACAGATGCCTGAACAGAAA |
| Mmp12 | AGCGAATTTGCTGAATGGTACTTG | GGCTGCTCCCATGAACGA | TCCCCTTGATAGTCAAAAAATCTTG |
| Sod2 | ACTATGGCGCGCTGGAGCCG | GCCTCCCTGACCTGCCTTAC | GCATGATCTGCGCGTTAATG |
| Spp1 | AGGAGTCCGATGAGGCTCTCAAGGTCA | CTCACCTCCCGCATGAAGAG | TCAGACGCTGGGCAACTG |
ELISA Assays for Cytokines
ELISA-based assay kits were purchased from R&D System (Minneapolis, MN) and used to quantitate Spp1 (osteopontin) and Ccl2 (Mcp1) in BAL fluid according to manufacturer’s instruction. Fifty µl of BAL fluid from 4 day, and 7, 14 and 28 week post exposure were used for the Spp1 assay. For Ccl2, BAL fluid was diluted 1:1 with assay diluents to increase the test’s dynamic range.
Multiplex Immunoassay
The LINCOplex Rat Cytokine/Chemokine Multiplex Immunoassay (LINCO Research Inc., St. Charles, MO) was performed per manufacturer’s instructions on BAL fluid (4 day and 28 week post exposure) to determine protein expression of 24 cytokines and chemokines on the Bio-Rad Bio-plex System (Bio-Rad, Hercules, CA).
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis and Immunoblotting
Western blot analysis was performed as described (Langley et al. 2010). Snap-frozen whole lung lobes were washed with PBS and homogenized/lysed in ice-cold RIPA buffer, centrifuged (14,500 × g for 20 min) and supernatants were collected; protein concentration was determined using the BCA Protein Assay kit (Bio-Rad). Protein samples were resolved on 10, 12.5, or 15% SDS Criterion Gels (Bio-Rad) in a criterion-gel apparatus (Bio-Rad). Resolved proteins were transferred onto a nitrocellulose membrane using a wet transfer unit. Membranes were probed for goat antiserum Sod2, Arg1, Mmp12, and actin antibodies (Santa Cruz Biotechnologies, Santa Cruz, CA). The membranes were extensively washed with TBST (Tris-buffered saline, pH 8, containing 0.05% Tween 20), incubated 1 hr at room temp with peroxidase-linked anti-goat IgG (Santa Cruz Biotechnologies). Blots were placed in enhanced chemiluminescence reagents (Amersham, Arlington Heights, IL) for 1 min followed by exposure to X-ray film (Amersham) for 1–3 min. The films were developed by an automated film processor (Kodak, New Haven, CT). The membrane bands were scanned and quantified with a Bio-Rad GS-800 scanner and Quantity One software. Densitometry is presented as fold change over control. Statistical analysis was determined using 5 animals per group.
Matrix Metalloprotease Zymography
Mmp2 and Mmp9 were measured in BAL fluid as described (Seagrave et al. 2004). Briefly, 150 µl of BAL fluid was combined with 50 µl of 4× Laemmli buffer without reducing agents. Twenty µl of the mixture was subject to electrophoresis on 7.5% SDS gels with 0.15% gelatin using a Bio-Rad mini-PROTEAN apparatus at 150 mV for 1 hr. The gels were washed thrice (2.5% Triton X-100, PBS), and then incubated for 24 hr at 37°C in 100 mM Tris (pH 7.4, containing 5 mM CaCl2 and 10 µM ZnCl2). The gels were fixed for 30 min in 40% methanol and 7% acetic acid, and then stained with 0.2% Coomassie Blue G250 in fixing solution. The bands were analyzed using a Bio-Rad GS-800 scanner and Quantity One software (Bio-Rad).
Statistics
The results were analyzed for statistical significance by GraphPad Prism Software 3.0 (GraphPad Inc., San Diego, CA); p values of ≤ 0.05 were considered significant. Error bars in all figures represent the standard error of mean. Gene Ontology pathway analysis of the differentially affected genes was performed with the GeneGo program (Thompson-Reuters, St. Joseph, MI). Sixty-five genes mapped to assigned gene ontology entries and were processed with the GO Cellular Process enrichment analysis. The probability of a random intersection between a set of ID with ontology entries is estimated as a p-value of a hypergeometric intersection.
RESULTS
MICROARRAY ANALYSIS
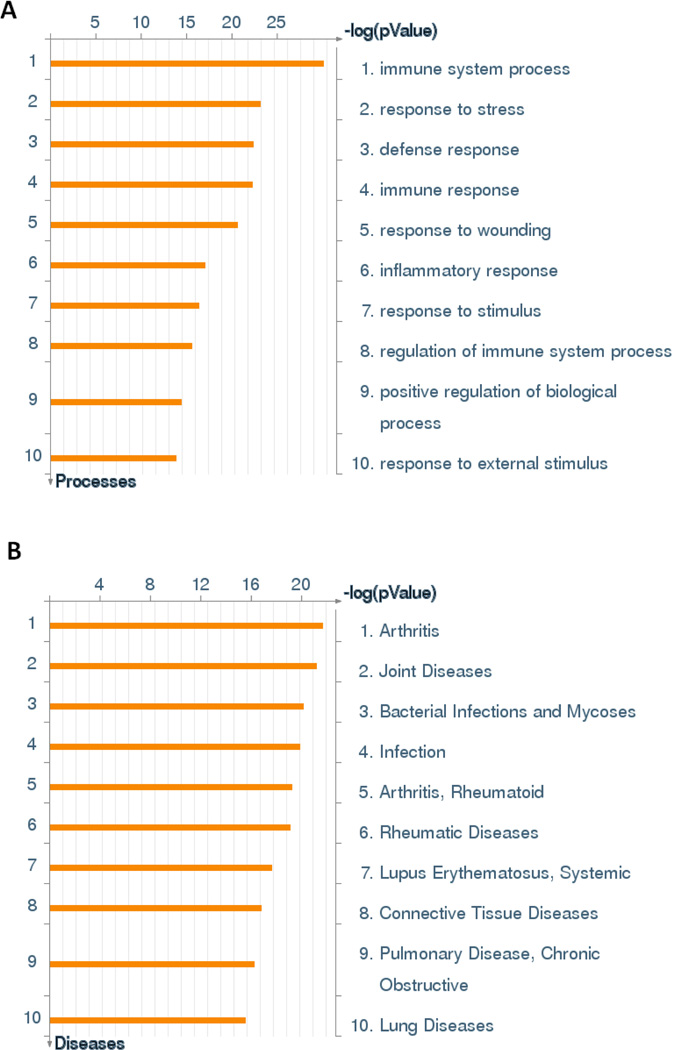
To determine the transcriptional changes in the sub-chronic silicosis model, RNA was isolated from the lungs of control rats and rats at 28 week after sub-chronic silica inhalation exposure. The RNAs were subjected to Affymetrix analysis that showed a significant upregulation of 144 genes and downregulation of 7 genes (Table 2). The GeneGo pathway analysis suggested that sub-chronic silica exposures produce a significant enrichment of genes that participate in immunity, wound healing and stress response (Figure 1a). Interestingly, the GeneGo analysis also indicated links to known silicosis related diseases including rheumatoid arthritis and joint disease (Otsuki et al. 2007), lupus (Otsuki et al. 2007), and chronic obstructive pulmonary disease (Rushton 2007) (Figure 1b). The most significantly affected gene was lactoperoxidase (Lpo) with a marked 101 fold increase. Relatively little is known of the function of Lpo; however, it has been implicated in bactericidal effects and reduction of H2O2 (Davies et al. 2008). Among the genes upregulated by sub-chronic silica inhalation were those that affect lung fibrosis: Spp1 (2.8 fold), Ccl7 (9.1 fold), Ccl2 (8.5 fold); oxidative stress: Sod2 (2.1-fold induction), Arg1 (3.1-fold induction), and tissue remodeling: Mmp12 (11.3 fold). Increases in acute phase response and complement cascade genes (e.g. Orm1, Lcn2, Saa3, C1qa, C2, C3, C4ba) suggested a proinflammatory response in the chronic silicosis model. Although chronically silica-exposed lungs at around 28 week post-silica exposure exhibited a significant increase in cellular infiltration (Langley et al. 2010), microarray analysis did not indicate a significant rise in the expression of prototypic proinflammatory cytokines (e.g. Tnfα, Il11β, Il66, and Ifnγ; data not shown). These results suggest that sub-chronic silica inhalation upregulates the expression of profibrotic and redox genes, but surprisingly without significantly affecting the expression of typical proinflammatory genes.
TABLE 2.
GENES DISPLAYING 2-FOLD OR GREATER CHANGES IN THE RAT LUNGS OF 28 WEEK POST SILICA INHALATION EXPOSURE
| GenBank | Gene | Gene Name | Fold Change | q- score |
|---|---|---|---|---|
| BI292231 | Lpo | Lactoperoxidase | 101.40 | 6.00 |
| NM_053288 | Orm1 | orosomucoid 1 | 19.40 | 8.37 |
| BF282318 | Saa3 | Serum A A3 | 14.70 | 7.85 |
| NM_053963 | Mmp12 | matrix metalloproteinase 12 | 11.40 | 4.92 |
| BF419899 | Ccl7 | C-C motif chemokine 7 precursor | 9.09 | 4.92 |
| NM_031530 | Ccl2 | chemokine (C-C motif) ligand 2 | 8.55 | 3.96 |
| NM_019214 | Slc26a4 | solute carrier family 26, member 4 | 7.63 | 2.60 |
| AW435415 | AW435415 | 6.71 | 5.90 | |
| AA875647 | AA875647 | 5.99 | 4.92 | |
| AA942745 | EST198244 | 5.32 | 4.92 | |
| NM_053333 | Retnla | resistin like alpha | 4.98 | 4.92 |
| NM_031598 | Pla2g2a | Phospholipase A2, group IIA | 4.76 | 9.31 |
| BF404154 | BF404154 | 4.42 | 2.60 | |
| AI407487 | EST235776 | 4.13 | 5.57 | |
| AA957923 | Mcpt2 | mast cell protease 2 | 4.08 | 7.70 |
| AI176732 | EST220325 | 3.86 | 8.37 | |
| NM_130741 | Lcn2 | lipocalin 2 | 3.75 | 4.92 |
| BG372891 | BG372891 | 3.70 | 7.70 | |
| AI639227 | Chia | chitinase, acidic | 3.58 | 4.92 |
| BG379338 | BG379338 | 3.42 | 4.92 | |
| AI714002 | LOC291234 | LOC291234 | 3.37 | 4.46 |
| AA892854 | EST196657 | 3.32 | 6.34 | |
| AI169984 | EST215900 | 3.30 | 2.62 | |
| X73371 | Fcgr2b | Fc receptor, IgG, low affinity IIb | 3.30 | 6.61 |
| AF268593 | Itgam | integrin alpha M (aka CD11b) | 3.25 | 5.90 |
| AI548358 | AI548358 | 3.19 | 4.92 | |
| NM_017134 | Arg1 | arginase 1 | 3.14 | 4.92 |
| BI295964 | LOC289235 | LOC289235 | 3.07 | 2.60 |
| BE113362 | LOC289993 | 3.04 | 4.62 | |
| AA945643 | Chi3l1 | chitinase 3-like 1 | 2.98 | 4.92 |
| NM_012582 | Hp | haptoglobin | 2.93 | 7.85 |
| BF282961 | LOC292594 | LOC292594 | 2.92 | 6.00 |
| NM_012830 | Cd2 | CD2 antigen | 2.90 | 7.70 |
| AF129400 | Fxyd2 | FXYD ion transport regulator 2 | 2.90 | 3.96 |
| NM_017335 | RNU28927 | GABA transporter | 2.89 | 4.46 |
| BI296811 | Pumag | Puma-g | 2.87 | 6.00 |
| X68657 | Mcpt10 | mast cell protease 10 | 2.87 | 8.37 |
| U22414 | Ccl3 | chemokine (C-C motif) ligand 3 | 2.87 | 2.60 |
| AI716211 | LOC302884 | LOC302884 | 2.85 | 9.15 |
| NM_022391 | Pttg1 | pituitary tumor-transforming 1 | 2.84 | 6.34 |
| NM_031560 | Ctsk | cathepsin K | 2.83 | 4.92 |
| BF418957 | LOC298566 | LOC298566 | 2.82 | 2.60 |
| AB001382 | Spp1 | secreted phosphoprotein 1 | 2.80 | 3.96 |
| AI172302 | EST218303 | 2.80 | 3.60 | |
| AA964152 | LOC311336 | LOC311336 | 2.76 | 5.58 |
| AI578135 | AI578135 | 2.75 | 4.92 | |
| NM_012516 | C4bpa | complement 4 binding protein alpha | 2.75 | 2.60 |
| AF411318 | Mt1a | Metallothionein | 2.74 | 7.85 |
| AI716456 | AI716456 | 2.71 | 2.62 | |
| U05341 | Cdc20 | cell cycle protein p55CDC | 2.69 | 4.92 |
| BF417541 | BF417541 | 2.68 | 4.92 | |
| NM_024157 | Cfi | complement factor I | 2.67 | 2.60 |
| AI411057 | LOC361422 | 2.67 | 4.62 | |
| AI237698 | EST234260 | 2.64 | 2.60 | |
| BE096731 | LOC302976 | LOC302976 | 2.63 | 5.90 |
| BF281153 | Ns5atp9 | Ns5atp9 protein | 2.59 | 5.58 |
| AI044644 | AI044644 | 2.56 | 7.70 | |
| U06434 | Scya4 | small inducible cytokine A4 | 2.56 | 4.92 |
| AI227769 | EST224464 | 2.55 | 2.62 | |
| AI137672 | AI137672 | 2.54 | 8.62 | |
| AW434057 | C1qb | complement 1,q beta | 2.54 | 6.61 |
| AI502757 | AI502757 | 2.53 | 8.62 | |
| AA944180 | EST199679 | 2.52 | 7.70 | |
| NM_013169 | Cd3d | CD3 antigen delta polypeptide | 2.52 | 4.92 |
| AA819819 | LOC299206 | LOC299206 | 2.52 | 7.70 |
| NM_016994 | C3 | complement component 3 | 2.51 | 5.16 |
| BM386789 | Rgs1 | regulator of G-protein signaling 1 | 2.47 | 9.15 |
| BF420720 | LOC303505 | LOC303505 | 2.44 | 7.85 |
| BI286015 | LOC362423 | LOC362423 | 2.44 | 6.61 |
| AW915948 | LOC315609 | LOC315609 | 2.42 | 5.58 |
| AA818264 | LOC288589 | LOC288589 | 2.37 | 7.85 |
| NM_012646 | RT1-N1 | RT1 class Ib gene, H2-TL-like, grc | 2.37 | 4.92 |
| AW534046 | AW534046 | 2.36 | 5.43 | |
| AW251927 | LOC300024 | LOC300024 | 2.35 | 5.58 |
| BI290159 | LOC300732 | LOC300732 | 2.35 | 3.96 |
| AA849031 | Lcptp | protein-tyrosine phosphatase, type 7 | 2.34 | 3.60 |
| BI295614 | BI295614 | 2.34 | 9.31 | |
| L05175 | Gzmm | lymphoctye Met-ase 1 | 2.33 | 4.92 |
| NM_012580 | Hmox1 | Heme oxygenase 1 | 2.33 | 4.46 |
| AF200684 | Slc7a7 | solute carrier family 7, member 7 | 2.33 | 4.46 |
| NM_133540 | Nkg7 | natural killer cell group 7 | 2.33 | 5.58 |
| BG057530 | BG057530 | 2.31 | 4.92 | |
| AI409259 | LOC315298 | LOC315298 | 2.31 | 4.92 |
| BM385445 | Top2a | topoisomerase (DNA) 2 alpha | 2.31 | 4.62 |
| AA945909 | LOC315609 | LOC315609 | 2.30 | 6.85 |
| AI411699 | EST239993 | 2.29 | 5.90 | |
| NM_053372 | Slpi | secretory leukocyte protease inhibitor | 2.29 | 6.34 |
| BG375029 | BG375029 | 2.29 | 4.62 | |
| AI716125 | C2 | complement component 2 | 2.28 | 2.60 |
| AI179539 | LOC287961 | LOC287961 | 2.27 | 5.90 |
| NM_053415 | Gpr9 | G protein-coupled receptor 9 | 2.26 | 7.70 |
| AI639401 | LOC310588 | LOC310588 | 2.26 | 5.58 |
| BM384693 | LOC297339 | LOC297339 | 2.25 | 2.60 |
| AA859652 | Slc16a6 | solute carrier family 16, member 6 | 2.22 | 3.96 |
| NM_019323 | Mcpt9 | mast cell protease 9 | 2.20 | 4.92 |
| BI278479 | LOC299340 | LOC299340 | 2.19 | 5.43 |
| BM391096 | BM391096 | 2.19 | 5.43 | |
| M17153 | Fcer1a | Fc receptor, IgE, HA I, alpha | 2.19 | 3.60 |
| NM_019296 | Cdc2a | cell division cycle 2 homolog A | 2.17 | 5.58 |
| BM387946 | Zap70 | zeta-chain (TCR) 70kDa | 2.17 | 2.60 |
| NM_001108978 | Pik3cd | phosphoinositide-3-kinase catalytic d | 2.17 | 8.37 |
| AI411618 | LOC362634 | LOC362634 | 2.16 | 4.62 |
| BE099038 | BE099038 | 2.16 | 4.92 | |
| BE116855 | BE116855 | 2.16 | 6.85 | |
| AI144884 | AI144884 | 2.16 | 2.60 | |
| AA799569 | LOC288774 | LOC288774 | 2.15 | 4.92 |
| BF407234 | BF407234 | 2.15 | 2.60 | |
| X03369 | Tubb2b | beta-tubulin T beta15 | 2.14 | 4.92 |
| BI284779 | LOC303664 | LOC303664 | 2.14 | 6.61 |
| AA892254 | Sod2 | superoxide dismutase 2 | 2.14 | 8.37 |
| AA849399 | EST192166 | 2.14 | 5.16 | |
| NM_030853 | Lat | linker for activation of T cells | 2.12 | 4.46 |
| BG374683 | BG374683 | 2.12 | 9.31 | |
| NM_030834 | Mct3 | monocarboxylate transporter | 2.12 | 8.37 |
| BE097195 | BE097195 | 2.12 | 7.70 | |
| BF400937 | BF400937 | 2.11 | 4.92 | |
| NM_022849 | Dmbt1 | deleted in malignant brain tumors 1 | 2.11 | 5.90 |
| BF283798 | LOC313211 | LOC313211 | 2.10 | 5.16 |
| BI289378 | LOC294430 | LOC294430 | 2.10 | 2.62 |
| AI233740 | LOC286921 | aldose reductase-like protein | 2.10 | 2.62 |
| NM_012645 | RT1-Aw2 | RT1 class Ib, locus Aw2 | 2.09 | 5.58 |
| BE117044 | BE117044 | 2.09 | 6.85 | |
| AI232788 | Cyba | cytochrome b558 alpha-subunit | 2.07 | 4.92 |
| NM_133298 | Gpnmb | glycoprotein nmb | 2.07 | 7.85 |
| NM_133416 | Bcl2a1 | BCL2-related protein A1 | 2.07 | 4.92 |
| BF419129 | BF419129 | 2.07 | 6.61 | |
| L23128 | RT1-N1 | RT1 class Ib, H2-TL-like, grc (N1) | 2.07 | 7.70 |
| BE112927 | LOC303073 | LOC303073 | 2.06 | 7.70 |
| AI045321 | C2ta | MHC class II transactivator | 2.06 | 5.58 |
| NM_022634 | Lst1 | leucocyte specific transcript 1 | 2.06 | 6.85 |
| NM_030845 | Gro1 | gro | 2.06 | 6.85 |
| NM_053348 | Fetub | fetuin beta | 2.05 | 2.62 |
| AI535104 | AI535104 | 2.04 | 5.16 | |
| AW532179 | AW532179 | 2.04 | 9.31 | |
| AJ302054 | Dlm1 | activated macrophage protein DLM-1 | 2.04 | 5.90 |
| AA859768 | AA859768 | 2.03 | 8.37 | |
| BF396319 | LOC289397 | LOC289397 | 2.03 | 6.34 |
| BM389005 | BM389005 | 2.03 | 3.60 | |
| BE106297 | LOC293783 | LOC293783 | 2.03 | 2.62 |
| AW919577 | Tcrb | T-cell receptor beta chain | 2.02 | 3.60 |
| NM_017196 | Aif1 | allograft inflammatory factor 1 | 2.02 | 5.58 |
| M24026 | RT1-Aw2 | RT1 class Ib, locus Aw2 | 2.02 | 5.90 |
| BI275818 | Serpine2 | serine proteinase inhibitor, E, 2 | 2.01 | 4.62 |
| NM_133303 | Bhlhb3 | basic helix-loop-helix, class B3 | 2.00 | 2.62 |
| BG664080 | BG664080 | 0.47 | 9.31 | |
| BF399855 | Serpinb10 | serine proteinase inhibitor, B, 10 | 0.47 | 5.16 |
| AW921479 | AW921479 | 0.45 | 8.37 | |
| AW525048 | AW525048 | 0.45 | 5.43 | |
| AA926082 | AA926082 | 0.45 | 9.31 | |
| AI043817 | AI043817 | 0.42 | 9.31 | |
| AW532942 | AW532942 | 0.36 | 9.31 |
Figure 1. Inflammation and Stress Responses are Upregulated in Sub-Chronic Silica Exposure.
Analysis performed as described in materials and methods. (A) Significantly enriched GO cellular processes. (B). GO Disease biomarker analysis.
Validation of Affymetrix Data
The microarray data analysis was carried out at 28 week post-silica exposure; however, it is possible that a proinflammatory response existed prior to granuloma formation. Therefore, to validate Affymetrix data and extend them to various times during granuloma formation, the lung expression of selected genes by qPCR, ELISA, Luminex bead-based assays, and/or Western blot/zymography analysis was examined at various times between 4 day and 28 week post-silica exposure. To compare the expression with acute silicosis, lung samples from an acute silicosis model were also included at 14 days after high-dose silica instillation. These analyses produced 4 major results:
Proinflammatory cytokines are not upregulated in sub-chronic silicosis
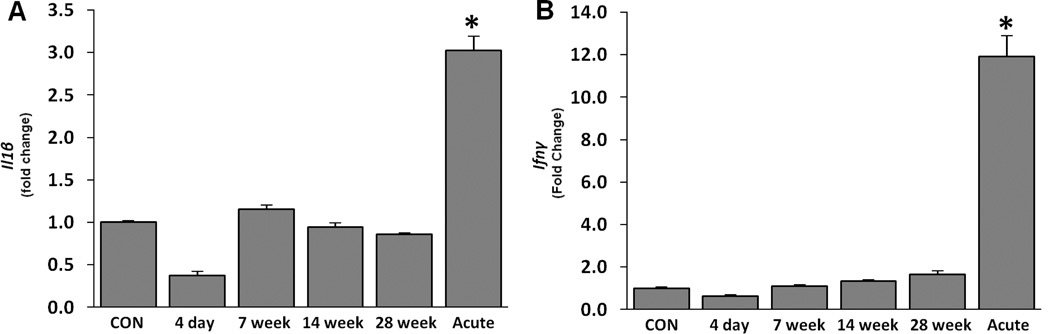
The microarray analysis of sub-chronic silica exposed lungs did not show any significant changes in the expression of proinflammatory cytokines at 28 week post-silica inhalation. To confirm these results and evaluate the expression prior to granuloma formation, the lung mRNA expression of Il1β (Figure 2A) and IFN-γ (Figure 2B) was analyzed by qPCR at 4 day, 7 week, 14 week, and 28 week after sub-chronic silica inhalation, and compared the results with acute high-dose silica exposed lungs (14 day post silica exposure). Sub-chronic silica exposure did not lead to any significant change in the transcriptional expression of these proinflammatory cytokines. Similarly, BAL content of 24 common proinflammatory cytokines and chemokines was analyzed by a multiplex bead-based immunoassay. The results indicated an absence of any significant change in proinflammatory cytokines/chemokines at 4 day and 28 week after sub-chronic silica exposure (Table 3). On the other hand, BAL fluid from acute silicotic lungs contained high levels of Il11β, Il6, Il9, Il10, Il17, Il18, Ifnγ, Ccl2, Gro-Kc, and Leptin. Eotaxin, Gcsf, Il1α, Il2, Il5, and Ip10 remained unchanged in both the sub-chronic and acute silicosis models (Table 4). It should be noted that while the Luminex assay did not detect significant changes in Ccl2 in the sub-chronic silicosis model, the ELISA assay did show significant changes in this chemokine (see below). This suggests that the dynamic range of the Luminex assay is not sensitive enough to detect low level changes of some cytokines/chemokines. Despite this limitation, the results indicate that it is unlikely the prototypic proinflammatory cytokines are critical in the development of chronic silicosis.
Figure 2. Il1β and Ifnγ are upregulated in acute but unchanged in sub-chronic silicosis.
Changes seen in whole lung mRNA (A) Il1β and (B) Ifnγ as determined by qPCR in sub-chronic silicosis (4 day, 7 week, 14 week, 28 week; low dose post-silica inhalation exposure) and acute silicosis (Acute; 14 day high dose post-silica intratracheal exposure) as described in the materials and methods. Samples were normalized against β-Actin expression and were presented as fold change compared to control (CON). No significant changes in controls between the various time points were noted (data not shown). Analysis was determined in 5 rats per group against appropriate controls. * p ≤ 0.05
TABLE 3.
CYTOKINES AND CHEMOKINES WERE UPREGULATED IN ACUTE, BUT NOT CHRONIC SILICA EXPOSURE
| Cytokine/ Chemokine |
Control (pg/ml) |
4-day exposure (pg/ml) |
28-week exposure (pg/ml) |
Acute exposure (pg/ml) |
|---|---|---|---|---|
| Gmcsf | 14.6 ± 3.9 | 6.1 ± 1.3 | 6.6 ± 1.0 | 31.4 ± 4.4 * |
| Ccl2 | 5.9 ± 1.3 | 5.530 ± 1.1 | 13 ± 3.4 | 226 ± 25 * |
| Leptin | 2.1 ± 0.6 | 1.4 ± 0.4 | 1.0 ± 0.1 | 63 ± 32 * |
| Mip1α | BD | BD | BD | 6.9 ± 2.4 † |
| Il1β | 1.5 ± 0.3 | BD | BD | 12.8 ± 4.1 * |
| Il6 | 27.5 ± 5.3 | 27.3 ± 15 | 28 ± 5 | 141.2 ± 49 * |
| Il9 | 52 ± 34.4 | 5.4 ± 2.7 | 9.3 ± 4.3 | 1960 ± 924 * |
| Il13 | 2.6 ± 1.0 | BD | 2.6 ± 2.3 | 10.6 ± 8.6 |
| Il10 | 2.8 ± 0.6 | 2.2 ± 0.7 | 19.4 ± 17 | 2697 ± 2568 |
| Il12(p70) | 2.2 ± 0.5 | 1.7 ± 0.9 | 1.6 ± 0.51 | 4.9 ± 0.7 * |
| Ifnγ | 1.8 ± 0.5 | BD | BD | 314 ± 144 * |
| Il17 | 1.9 ± 0.3 | BD | 2.2 ± 0.5 | 50.4 ± 24 * |
| Il18 | 37.4 ± 15 | 5.2 ± 2.3 | 85 ± 58.4 | 4348 ± 1743 * |
| Gro-Kc | 18.4 ± 5.3 | 15.0 ± 2.3 | 15 ± 3.4 | 204.1 ± 4.2 * |
| Rantes | 148.0 ± 18.1 | 130.4 ± 18.1 | 149.2 ± 20.2 | 270.5 ± 10 * |
| Tnfα | BD | BD | BD | 2.8 ± 0.5 † |
BD: Below detection limits
: Unable to perform reliable statistics.
Tested, but no significant changes seen or below detection limits: Eotaxin, GcsfF, Il1α, Il2, IL-5, Ip10
Changes in Redox Enzymes in chronic silicosis
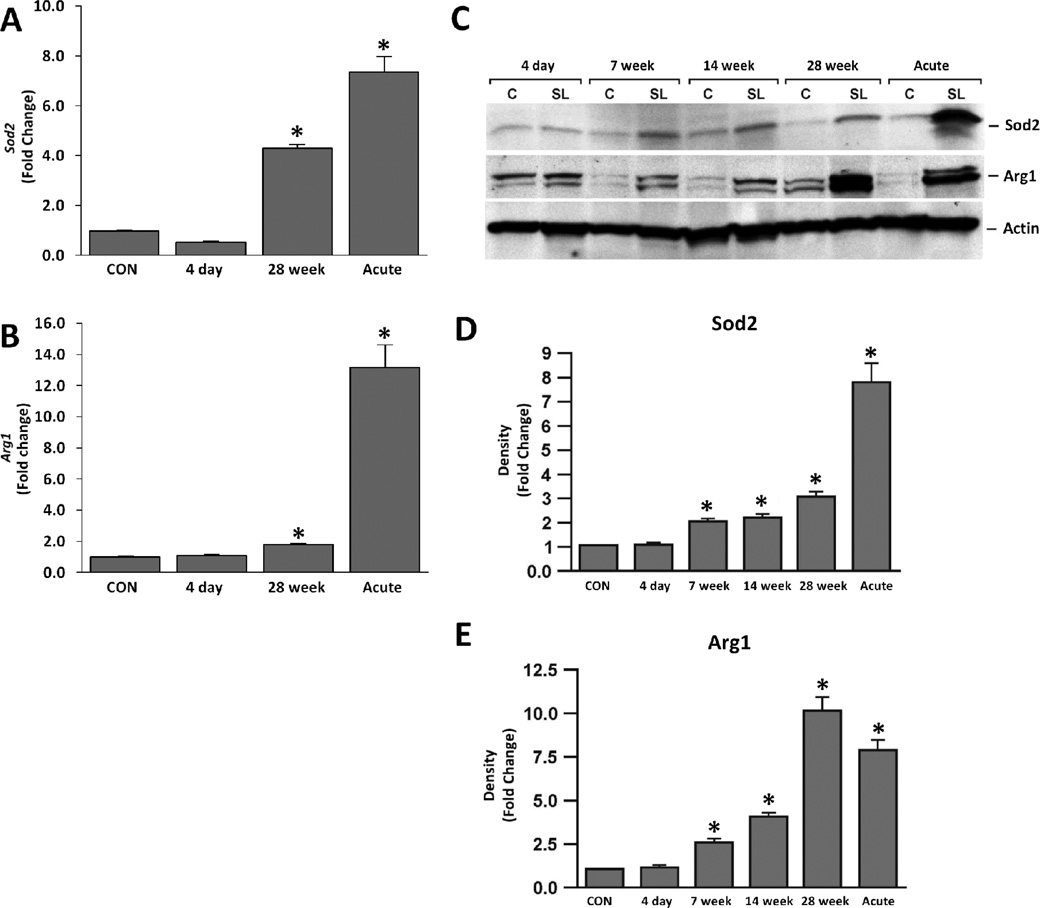
Microarray analysis indicated a significant rise in the redox enzymes Sod2 and Arg1 in the sub-chronic silicosis model (Table 2). To validate the results, qPCR was performed to determine the transcriptional expression of these enzymes in silica-exposed lung samples at 4 day and 28 week after silica inhalation, and compared it with lung samples from acute silicosis at 14 day after silica instillation. Results suggest that sub-chronic (28 week) silica exposed lungs had significantly upregulated mRNA expression of Sod2 and Arg1 (Figure 3A and B). However, samples obtained at 4 day after silica exposure did not show significant changes in the expression of these genes. The Western blot analysis of whole lung lysates from silica-exposed rats indicated that expression of these enzymes rose between 4 day and 7 week after silica exposure (Figure 3C–E). Levels of Sod2 and Arg1 proteins were also increased in acute silicosis. Thus, expression of redox enzymes is elevated in both acute and sub-chronic silicosis models; however, in the sub-chronic silicosis model, these redox enzymes had not changed at 4 days after the end of silica exposure, but were clearly changed at 7 week after silica treatment when granulomatous changes began (Langley et al. 2004). It is possible that redox enzymes started to rise sometime between 4 days and 7 week after silica exposures. Progressive time dependent increase in redox-related enzymes was also observed by Porter et al. (2006).
Figure 3. Silica exposure leads to moderate increases in oxidative markers, while acute silica exposure leads to robust changes in oxidative markers.
Changes found in oxidative stress markers (A) Sod2 and (B) Arg1 in sub-chronic silica (4 day and 28 week post-exposure) and acute silica exposures in the whole lung mRNA (qPCR). No significant changes in controls between the various time points were noted (data not shown). Normalization and analysis as described in Figure 1. C). Whole lung lysate Western Blot analysis of oxidative stress markers (Sod2, Arg1) over the time course of sub-chronic and acute silica models for controls (C) and silica (SL). Data are representative of 5 rats per group; β-Actin was used to confirm equal loading. Densitometry analysis of Western Blots for (D) Sod2 and (e) Arg1. Lanes were normalized against the density readings of β-Actin and are presented as fold change as compared to controls. Analysis was determined in 5 rats per group against appropriate time-sensitive controls. * p ≤ 0.05.
Changes in fibrotic chemokines in chronic silicosis
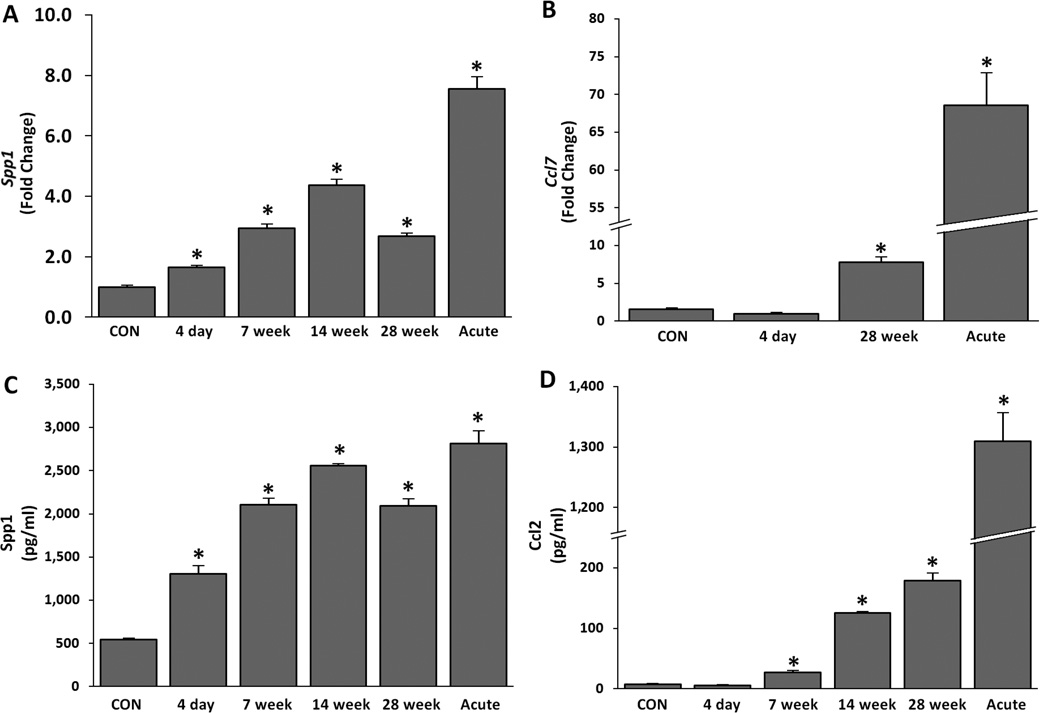
Microarray analysis indicated that transcription of profibrotic chemokines Spp1, Ccl2, Ccl3, and Ccl7 was strongly upregulated in the sub-chronic silicosis model (Table 2). To verify these results, RNA samples were analyzed by qPCR for the expression of Spp1 (Figure 4A) and Ccl7 (Figure 4B). Both were significantly increased at 28 week. The expression of both chemokines was markedly upregulated in the acute silicosis model. The protein content of Spp1 (Figure 4C) and Ccl2 (Figure 4D) in BAL fluids was also significantly elevated in sub-chronic and 14 day acute silicotic lungs. Spp1 mRNA and protein was increased as early as 4 days after the sub-chronic silica exposures, while Ccl2 protein content rose by 7 week post-exposure. Thus, profibrotic expression is presented in acute and initiated early in the development of sub-chronic silicosis.
Figure 4. Silica exposure increases pro-fibrotic chemokines in sub-chronic and acute silica exposure during granuloma formation.
Whole lung mRNA changes of chemokines (A) Spp1 and (B) Ccl7 as determined by qPCR. (C) Spp1 and (D) Ccl2 ELISA of BAL fluid (5 rats per group) over the time course of sub-chronic and acute silica exposures. Spp1 acute ELISA values exceeded the dynamic test range. No significant changes in controls between the various time points were noted (data not shown). Normalization and analysis performed as described in Figure 1. * p ≤ 0.05.
Silicosis increases the activity of airway remodeling enzymes
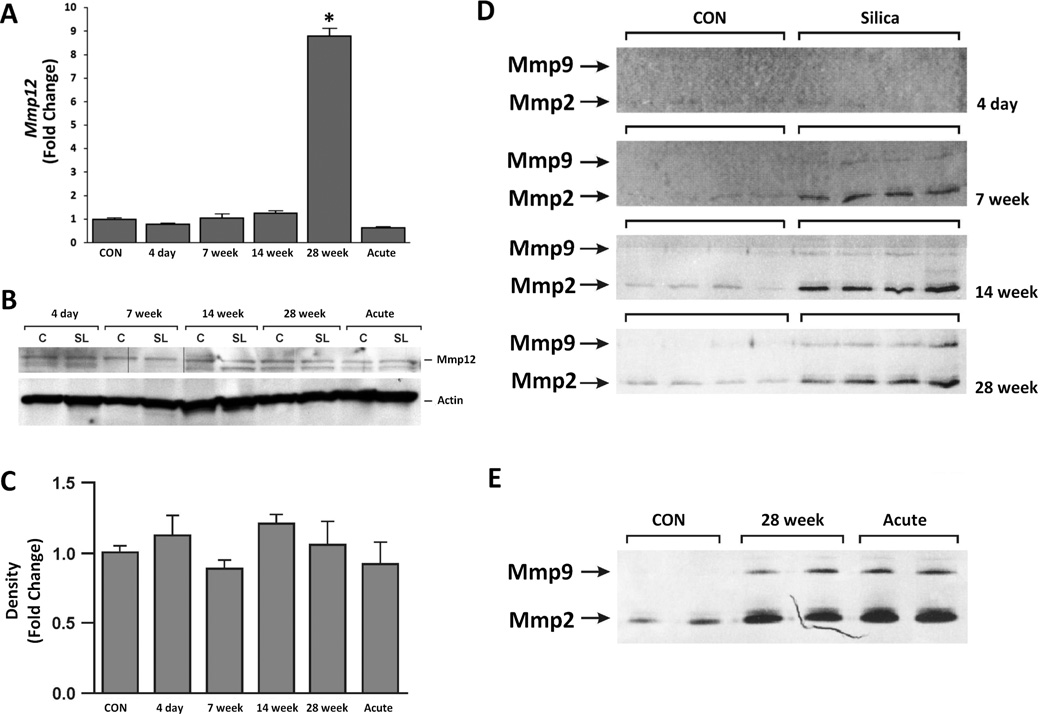
Metalloproteinases (gelatinases and elastases) degrade extracellular matrix and have been implicated in airway remodeling and granuloma formation (Scabilloni et al. 2005; Atkinson and Senior 2003; Corbel et al. 2002). Microarray analysis suggested that the expression of the elastase Mmp12 was significantly upregulated in the sub-chronic silicosis model (Table 2). While qPCR analysis confirmed a significant rise in Mmp12 gene expression in lung tissues at 28 week post-exposure (Figure 5A), Western blot analysis of whole lungs did not show any marked change in the protein content in acute or sub-chronic silicosis models (Figure 5B and C). Although the microarray analysis did not show any significant changes in the mRNA expression of Mmp2 and Mmp9, zymography of BAL fluids demonstrated increased activities in the BAL fluids at 7–28 week (Figure 5D), and a similar rise in the activity of these enzymes in BALF from acute silicotic lungs (Figure 5E).
Figure 5. While Mmp12 protein level is unchanged, Mmp2 and Mmp9 enzymatic activity are increased in chronic silica exposure during granuloma formation and markedly upregulated after acute silica exposure.
A). qPCR of Mmp12 in whole lungs from sub-chronic silicosis across the time course, and 14 day post acute silica exposure. B) Western blot analysis of Mmp12 in whole lungs. C) Densitometry of Western Blots for Mmp12. D) Zymography of BAL fluids were performed as described in the Materials and Methods to determine Mmp2 and Mmp9 enzymatic activity; the contrast was inverted. Each lane represents a unique animal in CON and sub-chronic silicosis (4 day, 7 week, 14 week and 28 week post exposure). E). Zymography comparison of BAL fluids from CON, 28 week post sub-chronic silica exposure and 14 day post acute silica exposure. Each lane represents a unique animal. * p ≤ 0.05.
DISCUSSION
The molecular understanding of silicosis, which has been largely generated from animal models of acute silicosis using inhalation and IT instillation of silica, has led to a general belief that lung inflammation and apoptosis are hallmarks of silicosis (Borges et al. 2002; 2001), and preventing apoptosis/inflammation might attenuate the disease (Rimal et al. 2005). However, acute silicosis is exceedingly rare in humans and the most prevalent form of silicosis – occupational chronic silicosis – is asymptomatic for decades after silica exposures (Chong et al. 2006). It is unlikely that significant lung inflammation and/or tissue destruction might go unnoticed for many years during the development of chronic silicosis. Moreover, in a rat model of sub-chronic silicosis, where animals were exposed by inhalation to occupationally relevant doses of silica, granulomas developed many months after silica inhalation was stopped, and the granulomas resembled human silicotic granulomas in their structure and histopathology. Furthermore, the granuloma formation was not associated with significant inflammation or cell death in early stages and only at late stages (Langley et al. 2004; 2010). Similarly, cell death or tissue destruction was not seen when occupationally relevant doses of silica were instilled intratracheal in rat lungs (Porter et al. 2002a).
A striking observation was that the Affymetrix analysis did not show any change in the expression of the prototypic proinflammatory cytokines Tnfα, Il1β, Il66, and Ifnγ. Although at late stages of the sub-chronic silicosis model (28 week) lungs exhibited significant increases in lymphocytes and neutrophils in the lung (Langley et al. 2004; 2010); it was possible that changes in proinflammatory cytokine gene expression were too low to detect by Affymetrix analysis and/or the putative increase involved posttranscriptional rather than transcriptional changes. Therefore, several assays were used to validate the microarray data and compared the results with lung samples from lungs of the acute IT instillation silicosis model, where inflammatory responses were robust. While the inhalation model developed by Porter et al. (2002b; 2001) is more relevant in exposure delivery, deposition and timeframe, the silica instillation model was selected for comparison because of its immediate development of acute silicosis and similarity to the Borges et al. (2001) mouse model. Indeed, qPCR analysis of acute silicotic lung samples showed significant increases in the mRNA expression of Tnfα, Il1β, Il6, and Ifnγ, and the Luminex multiplex assay indicated elevated presence of these cytokines in the BAL fluid from acute silicotic lungs. These results confirmed the published data in animal models, where BAL fluid from acute silicotic lungs displayed the presence of high concentrations of Tnfα, Il1β, Il6, and Ifnγ (Rimal et al. 2005). On the other hand, qPCR and the multiplex assay did not indicate any significant change in the expression of these cytokines in the sub-chronic silicosis model. A possible explanation is that these cytokines are primarily made by leukocytes, and at best leukocytic infiltration in sub-chronic silica exposed lungs is limited and occurs when the granulomas are well established (Langley et al. 2004; 2010). Moreover, recent evidence clearly suggests that fibrotic changes in silicosis may develop in the absence of proinflammatory cytokines (Giordano et al. 2010; Lo Re et al. 2010). However, it should be emphasized that the expression of these cytokines was examined in the sub-chronic silicosis model at specified times (i.e., 4 day, and 7, 14 and 28 week); it is possible that a spurt of inflammatory cytokines was produced between 4 day and 7 week. This is a highly unlikely scenario because the insulting agent (silica) and, as discussed below, the oxidative stress remained in the lungs throughout granuloma formation. Further, other than a decrease in the number of foamy (i.e., silica containing) macrophages, no significant changes in leukocytic/monocytic infiltration, LDH or total protein was observed in the BAL fluid from between 4 to 28 day post sub-chronic silica exposure (Langley et al. 2004). It is possible that proinflammatory cytokines were upregulated within the microdomain of granulomas and/or specific cell types (i.e., alveolar/epithelioid macrophages) at a concentration below the detection range of our assays. It should also be noted that the inflammatory response was not determined at any time point during the 6 week silica exposure period and an inflammatory response might have occurred during this time frame and receded before the first sacrifice at 4 day post silica inhalation. On the other hand, it is more likely that the granulomatous changes in lung induced by chronic occupationally relevant doses of silica inhalation are not necessarily dependent on the significant presence of prototypic proinflammatory cytokines. Future experiments using knockout mice such as Tnfα−/−, Il1β−/− may provide a more definitive answer about the role of proinflammatory cytokines in acute and chronic silicosis. Despite the lack of prototypic proinflammatory cytokine response, the microarray analysis indicated an increased expression of some complement genes, including C3, C1qb, Cf1 and C4bpa. In some situations, complement proteins C3a and C5a induced proinflammatory cytokines such as TNF-α and IL-1 (Peng et al. 2009; Takabayashi et al. 2004). However, C3a and C5a require C3aR and C5aR, respectively to induce proinflammatory cytokines. A significant increase in the expression of C5, C3ar, or C5ar was not found. Further, the generation of proinflammatory response does not necessarily require complement cascade and C3−/− mice showed elevated Tnf-α expression after ethanol consumption (Pritchard et al. 2007). In addition, the increased expression of proinflammatory cytokines in response to C3a and C5a may depend upon the cell type; thus C3a and/or C5a proinflammatory cytokines rose in microglia but the cytokine response in astrocytes fell (Ingersoll et al. 2010). Thus at this time, the significance of enhanced expression of some complement genes to the development of silicosis is not clear.
There is ample evidence that silica induces oxidative stress such as production of ROS in cell cultures and in vivo (Shi et al. 1998; Shen et al. 2001; Zeidler et al. 2003). Affymetrix analysis indicated that at 28 week after silica inhalation, several redox-related genes that protect against oxidative stress, such as Sod2 and Arg1 were upregulated in the sub-chronic silicosis model. Sod2 is the primary defense against toxicity of superoxide anions generated by oxidative stress (Martin et al. 2006), and Arg1 metabolizes arginine and reduces its availability for nitric oxide synthase, leading to reduced production of nitric oxide. Increased Arg1 immunoreactivity was also observed in an animal model of acute silicosis (Poljakovic et al. 2007). Our qPCR analysis confirmed the enhanced expression of Arg1 and Sod2, and Western blot analysis indicated continued progressive increase in Arg1 and Sod2 during the development of sub-chronic silicosis, reaching the highest level at 28 week post-silica inhalation. These redox enzymes are part of the defense mechanism against oxidative stress; so it is likely that in the sub-chronic silicosis model, the local milieu in the lung continues to trigger this response. Interestingly, at 28 week after silica inhalation, most silica was cleared from the lungs, and the only remaining silica particles within the lung were confined to the epithelioid macrophages (Langley et al. 2004). Data suggest that silica within the epithelioid macrophages is the major contributor to the redox response in sub-chronic silicosis, and support the observation that a tunnel worker, in spite of retiring and exhibiting good lung function and excellent silica clearance, developed CT-scan detectable silicosis decades after retirement (Arakawa et al. 2005).
Silicosis is a fibrotic disease, and microarray as well as qPCR analysis indicated that the gene expression of the profibrotic chemokines Spp1 (osteopontin), Ccl2 (MCP-1), and Ccl7 (MCP-3) were significantly upregulated. Similarly, Spp1 and Ccl7 mRNAs were expressed markedly in acute silicosis. ELISA also detected the increased presence of Spp1 and Ccl2 proteins in acute silicosis and throughout the development of sub-chronic silicosis. The profibrotic chemokines Spp1, Ccl2, and Ccl7, particularly Spp1, have been implicated in pulmonary fibrosis (Chensue et al. 1997; Shang et al. 2002; Mangum et al. 2004; Li et al. 2004; Pardo et al. 2005; Helming and Gordon 2009). Spp1−/− mice exhibit decreased collagen type I expression (Berman et al. 2004) and significantly reduced numbers of macrophage-derived epithelioid cells, MGC, and pulmonary granulomas induced by Schistosoma mansoni eggs (O'Regan et al. 2001). Macrophages, epithelial cells, and endothelial cells activated in acute and sub-chronic silicosis, are the major source of Spp1, Ccl2, and Ccl7 (Shang et al. 2002; Pardo et al. 2005; Serlin et al. 2006; Meyer-Hoffert et al. 2003). These chemokines are also upregulated in human fibrotic diseases such as idiopathic pulmonary fibrosis (Pardo et al. 2005) and tuberculosis (Nau et al. 1997). Data showed that sub-chronic silicosis model is associated with anti-apoptotic responses (Langley et al. 2010), and interestingly, Spp1 treatment suppressed apoptosis in tubulointerstitial cells (Li et al. 2004). While genomic and/or pharmaceutical interventions studies are needed to validate these findings, Ccl2, Ccl7, and Spp1 are likely to be critical in the fibrotic response in both acute and sub-chronic silicosis models, and the marked increases in these fibrotic mediators in acute silicosis may contribute to the rapid onset of fibrosis in this disease.
The discrete and well-organized development of granulomas in human silicosis (Hnizdo and Vallyathan 2003; Chong et al. 2006) and animal models of sub-chronic silicosis (Langley et al. 2010) suggests that fibrosis and remodeling are critical components of the disease and might be initiated by epithelioid macrophages and MGC. These cells are CD11b+, multinucleated and formed by fusion of macrophages. Cathepsin K, CCL2 and MMP9 have been implicated in macrophage fusion (Helming and Gordon 2009), and as seen by microarray analysis, immunoreactivity, and/or zymography, the expression of these proteins/enzymes is upregulated in the sub-chronic silicosis model. Mmp2 and Mmp9 are enzymatically activated as early as 7 week post-silica inhalation; this activity is also increased in acute silicosis. These enzymes might lead to breakdown of extracellular matrix in silicosis. While microarray analysis indicated a marked rise in Mmp12 gene expression in the sub-chronic silica exposed lungs, surprisingly, Western blot analysis did not confirm the Affymetrix finding. The lack of Mmp12 proteomic validation may be due to post-transcriptional mechanism(s) and/or poor reactivity of anti-Mmp12 antibody. However, because Mmp12 was not increased in acute silicosis, it is likely that this metalloproteinase does not play a significant role in silicosis.
Histopathologically acute and chronic silicotic granulomas are distinct: acute silicotic granulomas are mostly loosely aggregated histiocytes, while the granulomas arising in chronic silica exposed lungs are well defined structures composed of epithelioid macrophages and MGC. Overall, the results presented herein show that sub-chronic silicosis model is associated with increased expression of profibrotic chemokines and extracellular matrix-degrading enzymes. Although at present the mechanism is unclear, oxidative stress may play an important role in the granuloma formation. Nonetheless, the prototypic proinflammatory cytokines Tnfα, Il1β, Il6, and Ifnγ do not appear to be significantly involved in the granuloma formation in sub-chronic silicosis, thus supporting recent findings that proinflammatory cytokines may not be indispensable for fibrosis (Giordano et al. 2010; Lo Re et al. 2010). The observation that corticosteroids are ineffective in preventing the development of silicotic granulomas in humans (Chong et al. 2006) further supports the expendable role of lung inflammation in the granulomatous process in silicosis; however, Spp1 may be a potential target for therapeutic intervention in silicosis.
Acknowledgements
These studies were supported in part by grants from the NIH (R01 DA017003 and R01 DA04208-17), from the U.S. Army (W81XWH-04-C-0071), and LRRI internal funds. We thank Dr. Gavin Pickett and Mrs. Marilee Morgan of the Keck-UNM Genomics Resource (University of New Mexico Cancer Research Center, Albuquerque, NM) for their help with Affymetrix analysis.
References
- Arakawa H, Honma K, Saito Y, Morikubo H, Shida H. Delayed development of silicoproteinosis with diffuse interstitial fibrosis: 16-year follow-up with autopsy correlation. Eur Radiol. 2005;15:2210–2211. doi: 10.1007/s00330-005-2761-6. [DOI] [PubMed] [Google Scholar]
- Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- Barbarin V, Xing Z, Delos M, Lison D, Huaux F. Pulmonary overexpression of IL-10 augments lung fibrosis and Th2 responses induced by silica particles. Am J Physiol Lung Cell Mol Physiol. 2005;288:L841–L848. doi: 10.1152/ajplung.00329.2004. [DOI] [PubMed] [Google Scholar]
- Barbaro M, Cutroneo G, Costa C, Sciorio S, Trimarchi F, Favaloro A, Fenga C, Barbaro Martino L, Spatari G, Abbate C, Bramanti P. Early events of experimental exposure to amorphous and crystalline silica in the rat: time course of surfactant protein D. Ital J Anat Embryol. 2002;107:243–256. [PubMed] [Google Scholar]
- Berman JS, Serlin D, Li X, Whitley G, Hayes J, Rishikof DC, Ricupero DA, Liaw L, Goetschkes M, O'Regan AW. Altered bleomycin-induced lung fibrosis in osteopontin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1311–L1318. doi: 10.1152/ajplung.00394.2003. [DOI] [PubMed] [Google Scholar]
- Borges VM, Falcao H, Leite-Junior JH, Alvim L, Teixeira GP, Russo M, Nobrega AF, Lopes MF, Rocco PM, Davidson WF, Linden R, Yagita H, Zin WA, DosReis GA. Fas ligand triggers pulmonary silicosis. J Exp Med. 2001;194:155–164. doi: 10.1084/jem.194.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges VM, Lopes MF, Falcao H, Leite-Junior JH, Rocco PR, Davidson WF, Linden R, Zin WA, DosReis GA. Apoptosis underlies immunopathogenic mechanisms in acute silicosis. Am J Respir Cell Mol Biol. 2002;27:78–84. doi: 10.1165/ajrcmb.27.1.4717. [DOI] [PubMed] [Google Scholar]
- Castranova V. Signaling pathways controlling the production of inflammatory mediators in response to crystalline silica exposure: role of reactive oxygen/nitrogen species. Free Radic Biol Med. 2004;37:916–925. doi: 10.1016/j.freeradbiomed.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Castranova V, Porter D, Millecchia L, Ma JY, Hubbs AF, Teass A. Effect of inhaled crystalline silica in a rat model: time course of pulmonary reactions. Mol Cell Biochem. 2002;234–235:177–184. [PubMed] [Google Scholar]
- Chensue SW, Warmington K, Ruth JH, Lukacs N, Kunkel SL. Mycobacterial and schistosomal antigen-elicited granuloma formation in IFN-gamma and IL-4 knockout mice: analysis of local and regional cytokine and chemokine networks. J Immunol. 1997;159:3565–3573. [PubMed] [Google Scholar]
- Chong S, Lee KS, Chung MJ, Han J, Kwon OJ, Kim TS. Pneumoconiosis: comparison of imaging and pathologic findings. Radiographics. 2006;26:59–77. doi: 10.1148/rg.261055070. [DOI] [PubMed] [Google Scholar]
- Corbel M, Belleguic C, Boichot E, Lagente V. Involvement of gelatinases (MMP-2 and MMP-9) in the development of airway inflammation and pulmonary fibrosis. Cell Biol Toxicol. 2002;18:51–61. doi: 10.1023/a:1014471213371. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1199–1234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubini B, Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med. 2003;34:1507–1516. doi: 10.1016/s0891-5849(03)00149-7. [DOI] [PubMed] [Google Scholar]
- Giordano G, van den Brule S, Lo Re S, Triqueneaux P, Uwambayinema F, Yakoub Y, Couillin I, Ryffel B, Michiels T, Renauld JC, Lison D, Huaux F. Type I interferon signaling contributes to chronic inflammation in a murine model of silicosis. Toxicol Sci. 2010;116:682–692. doi: 10.1093/toxsci/kfq158. [DOI] [PubMed] [Google Scholar]
- Helming L, Gordon S. Molecular mediators of macrophage fusion. Trends Cell Biol. 2009;19:514–522. doi: 10.1016/j.tcb.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Hnizdo E, Sluis-Cremer GK. Risk of silicosis in a cohort of white South African gold miners. Am J Ind Med. 1993;24:447–457. doi: 10.1002/ajim.4700240409. [DOI] [PubMed] [Google Scholar]
- Hnizdo E, Vallyathan V. Chronic obstructive pulmonary disease due to occupational exposure to silica dust: a review of epidemiological and pathological evidence. Occup Environ Med. 2003;60:237–243. doi: 10.1136/oem.60.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll SA, Martin CB, Barnum SR, Martin BK. CNS-specific expression of C3a and C5a exacerbate demyelination severity in the cuprizone model. Mol Immunol. 2010;48:219–230. doi: 10.1016/j.molimm.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley RJ, Kalra R, Mishra NC, Hahn FF, Razani-Boroujerdi S, Singh SP, Benson JM, Pena-Philippides JC, Barr EB, Sopori ML. A biphasic response to silica: I. Immunostimulation is restricted to the early stage of silicosis in lewis rats. Am J Respir Cell Mol Biol. 2004;30:823–829. doi: 10.1165/rcmb.2003-0284OC. [DOI] [PubMed] [Google Scholar]
- Langley RJ, Mishra NC, Pena-Philippides JC, Hutt JA, Sopori ML. Granuloma formation induced by low-dose chronic silica inhalation is associated with an anti-apoptotic response in Lewis rats. J Toxicol Environ Health A. 2010;73:669–683. doi: 10.1080/15287390903578521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Yang CW, Park JH, Lim SW, Sun BK, Jung JY, Kim SB, Kim YS, Kim J, Bang BK. Pravastatin treatment attenuates interstitial inflammation and fibrosis in a rat model of chronic cyclosporine-induced nephropathy. Am J Physiol Renal Physiol. 2004;286:F46–F57. doi: 10.1152/ajprenal.00428.2002. [DOI] [PubMed] [Google Scholar]
- Lo Re S, Dumoutier L, Couillin I, Van Vyve C, Yakoub Y, Uwambayinema F, Marien B, van den Brule S, Van Snick J, Uyttenhove C, Ryffel B, Renauld JC, Lison D, Huaux F. IL-17A-producing gammadelta T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis. J Immunol. 2010;184:6367–6377. doi: 10.4049/jimmunol.0900459. [DOI] [PubMed] [Google Scholar]
- Mangum J, Bermudez E, Sar M, Everitt J. Osteopontin expression in particle-induced lung disease. Exp Lung Res. 2004;30:585–598. doi: 10.1080/01902140490476346. [DOI] [PubMed] [Google Scholar]
- Martin FM, Bydlon G, Friedman JS. SOD2-deficiency sideroblastic anemia and red blood cell oxidative stress. Antioxid Redox Signal. 2006;8:1217–1225. doi: 10.1089/ars.2006.8.1217. [DOI] [PubMed] [Google Scholar]
- Meyer-Hoffert U, Lezcano-Meza D, Bartels J, Montes-Vizuet AR, Schroder JM, Teran LM. Th2- and to a lesser extent Th1-type cytokines upregulate the production of both CXC (IL-8 and gro-alpha) and CC (RANTES, eotaxin, eotaxin-2, MCP-3 and MCP-4) chemokines in human airway epithelial cells. Int Arch Allergy Immunol. 2003;131:264–271. doi: 10.1159/000072138. [DOI] [PubMed] [Google Scholar]
- Nau GJ, Guilfoile P, Chupp GL, Berman JS, Kim SJ, Kornfeld H, Young RA. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc Natl Acad Sci U S A. 1997;94:6414–6419. doi: 10.1073/pnas.94.12.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan AW, Hayden JM, Body S, Liaw L, Mulligan N, Goetschkes M, Berman JS. Abnormal pulmonary granuloma formation in osteopontin-deficient mice. Am J Respir Crit Care Med. 2001;164:2243–2247. doi: 10.1164/ajrccm.164.12.2104139. [DOI] [PubMed] [Google Scholar]
- Otsuki T, Maeda M, Murakami S, Hayashi H, Miura Y, Kusaka M, Nakano T, Fukuoka K, Kishimoto T, Hyodoh F, Ueki A, Nishimura Y. Immunological effects of silica and asbestos. Cell Mol Immunol. 2007;4:261–268. [PubMed] [Google Scholar]
- Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, Kaminski N. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2:e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Li K, Sacks SH, Zhou W. The role of anaphylatoxins C3a and C5a in regulating innate and adaptive immune responses. Inflamm Allergy Drug Targets. 2009;8:236–246. doi: 10.2174/187152809788681038. [DOI] [PubMed] [Google Scholar]
- Poljakovic M, Porter DW, Millecchia L, Kepka-Lenhart D, Beighley C, Wolfarth MG, Castranova V, Morris SM., Jr Cell- and isoform-specific increases in arginase expression in acute silica-induced pulmonary inflammation. J Toxicol Environ Health A. 2007;70:118–127. doi: 10.1080/15287390600755075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DW, Barger M, Robinson VA, Leonard SS, Landsittel D, Castranova V. Comparison of low doses of aged and freshly fractured silica on pulmonary inflammation and damage in the rat. Toxicology. 2002a;175:63–71. doi: 10.1016/s0300-483x(02)00061-6. [DOI] [PubMed] [Google Scholar]
- Porter DW, Millecchia LL, Willard P, Robinson VA, Ramsey D, McLaurin J, Khan A, Brumbaugh K, Beighley CM, Teass A, Castranova V. Nitric oxide and reactive oxygen species production causes progressive damage in rats after cessation of silica inhalation. Toxicol Sci. 2006;90:188–197. doi: 10.1093/toxsci/kfj075. [DOI] [PubMed] [Google Scholar]
- Porter DW, Ramsey D, Hubbs AF, Battelli L, Ma J, Barger M, Landsittel D, Robinson VA, McLaurin J, Khan A, Jones W, Teass A, Castranova V. Time course of pulmonary response of rats to inhalation of crystalline silica: histological results and biochemical indices of damage, lipidosis, and fibrosis. J Environ Pathol Toxicol Oncol. 2001;20(Suppl 1):1–14. [PubMed] [Google Scholar]
- Porter DW, Ye J, Ma J, Barger M, Robinson VA, Ramsey D, McLaurin J, Khan A, Landsittel D, Teass A, Castranova V. Time course of pulmonary response of rats to inhalation of crystalline silica: NF-kappa B activation, inflammation, cytokine production, and damage. Inhal Toxicol. 2002b;14:349–367. doi: 10.1080/08958370252870998. [DOI] [PubMed] [Google Scholar]
- Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, Medof ME, Nagy LE. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology. 2007;132:1117–1126. doi: 10.1053/j.gastro.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani-Boroujerdi S, Sopori ML. Early manifestations of NNK-induced lung cancer: role of lung immunity in tumor susceptibility. Am J Respir Cell Mol Biol. 2007;36:13–19. doi: 10.1165/rcmb.2005-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimal B, Greenberg AK, Rom WN. Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulm Med. 2005;11:169–173. doi: 10.1097/01.mcp.0000152998.11335.24. [DOI] [PubMed] [Google Scholar]
- Rushton L. Chronic obstructive pulmonary disease and occupational exposure to silica. Rev Environ Health. 2007;22:255–272. doi: 10.1515/reveh.2007.22.4.255. [DOI] [PubMed] [Google Scholar]
- Scabilloni JF, Wang L, Antonini JM, Roberts JR, Castranova V, Mercer RR. Matrix metalloproteinase induction in fibrosis and fibrotic nodule formation due to silica inhalation. Am J Physiol Lung Cell Mol Physiol. 2005;288:L709–L717. doi: 10.1152/ajplung.00034.2004. [DOI] [PubMed] [Google Scholar]
- Seagrave J, Barr EB, March TH, Nikula KJ. Effects of cigarette smoke exposure and cessation on inflammatory cells and matrix metalloproteinase activity in mice. Exp Lung Res. 2004;30:1–15. doi: 10.1080/01902140490252858. [DOI] [PubMed] [Google Scholar]
- Serlin DM, Kuang PP, Subramanian M, O'Regan A, Li X, Berman JS, Goldstein RH. Interleukin-1beta induces osteopontin expression in pulmonary fibroblasts. J Cell Biochem. 2006;97:519–529. doi: 10.1002/jcb.20661. [DOI] [PubMed] [Google Scholar]
- Shang XZ, Chiu BC, Stolberg V, Lukacs NW, Kunkel SL, Murphy HS, Chensue SW. Eosinophil recruitment in type-2 hypersensitivity pulmonary granulomas: source and contribution of monocyte chemotactic protein-3 (CCL7) Am J Pathol. 2002;161:257–266. doi: 10.1016/S0002-9440(10)64177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HM, Zhang Z, Zhang QF, Ong CN. Reactive oxygen species and caspase activation mediate silica-induced apoptosis in alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2001;280:L10–L17. doi: 10.1152/ajplung.2001.280.1.L10. [DOI] [PubMed] [Google Scholar]
- Shi X, Castranova V, Halliwell B, Vallyathan V. Reactive oxygen species and silica-induced carcinogenesis. J Toxicol Environ Health B Crit Rev. 1998;1:181–197. doi: 10.1080/10937409809524551. [DOI] [PubMed] [Google Scholar]
- Takabayashi T, Shimizu S, Clark BD, Beinborn M, Burke JF, Gelfand JA. Interleukin-1 upregulates anaphylatoxin receptors on mononuclear cells. Surgery. 2004;135:544–554. doi: 10.1016/j.surg.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Zeidler PC, Roberts JR, Castranova V, Chen F, Butterworth L, Andrew ME, Robinson VA, Porter DW. Response of alveolar macrophages from inducible nitric oxide synthase knockout or wild-type mice to an in vitro lipopolysaccharide or silica exposure. J Toxicol Environ Health A. 2003;66:995–1013. doi: 10.1080/15287390306395. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Hartsky MA, Warheit DB. Time course of quartz and TiO(2) particle-induced pulmonary inflammation and neutrophil apoptotic responses in rats. Exp Lung Res. 2002;28:641–670. doi: 10.1080/01902140260426742. [DOI] [PubMed] [Google Scholar]