Abstract
OBJECTIVE
To test whether d-cycloserine, a partial agonist at the glutamatergic N-methyl-D-aspartate receptor, augments and accelerates a full course of comprehensive cognitive behavioral therapy (CBT) in medication-free adults with generalized social anxiety disorder.
METHOD
A randomized placebo-controlled efficacy-study conducted at Boston University, Massachusetts General Hospital, and Southern Methodist University between 9/2007 and 12/2011 of 169 medication-free adults with generalized social anxiety disorder; 144 completed treatment, and 131 completed the follow-up assessments. Patients were randomized to receive 50 mg of d-cycloserine or placebo 1 hour before each of 5 exposure sessions that were part of a 12-session cognitive behavioral group treatment. Response and remission status was determined at baseline, throughout treatment, post-treatment, and at 1, 3, and 6-month follow-up assessments rated by assessors who were blind to treatment condition.
RESULTS
D-cycloserine-augmented and placebo-augmented CBT were associated with similar completion rates (87% and 82%), response rates (79.3% and 73.3%), and remission rates (34.5% and 24.4%) at post-treatment that were largely maintained at follow-up. Although d-cycloserine was associated with a 24–33% faster rate of improvement in symptom severity and remission rates relative to placebo during the 12-week treatment phase, the groups did not differ in response and remission rates.
CONCLUSIONS
D-cycloserine did not augment a full course of comprehensive CBT for social anxiety disorder.
TRIAL REGISTRATION
http://www.ClinicalTrials.gov, ID# NCT00633984, http://www.clinicaltrials.gov/ct2/show/NCT00633984
Keywords: Cognitive behavioral therapy, d-cycloserine, N-methyl-D-aspartate receptor agonist, CBT, social anxiety disorder, social phobia, randomized controlled trial
INTRODUCTION
Social anxiety disorder is one of the most common forms of anxiety disorders (1). Clinical trials have demonstrated only moderate efficacy for anxiolytic pharmacotherapy (e.g., paroxetine, fluoxetine, and phenelzine) and cognitive-behavioral therapy (CBT) (2–5). Attempts to boost treatment response by combining CBT and anxiolytic pharmacotherapy for social anxiety disorder have met with disappointing results (6, 7), with one exception (8).
Recently, a novel strategy has emerged for the combination of pharmacotherapy and CBT to improve outcomes. This strategy is the result of research studies that have mapped some of the core pathways involved in fear extinction (9). Fear learning and extinction are both blocked by antagonists at the glutametergic N-methyl-D-aspartate (NMDA) receptor. D-cycloserine, an analogue of D-alanine and a partial agonist at the NMDA receptor, augments learning in animals and in some human trials (10). Moreover, the process of extinction of conditioned fear is facilitated by d-cycloserine given in individual doses prior to or soon after extinction trials in animals (11–13). Use of acute dosing as opposed to chronic dosing of d-cycloserine may be critical to its intended effect on NMDA receptor activity (14–16).
The efficacy of d-cycloserine in animal models led to the application of d-cycloserine for humans, because exposure-based treatments in humans, such as CBT, rely on extinction to treat the core fears underlying anxiety disorders (17). Some preliminary studies report that d-cycloserine augmentation effects of CBT, as compared to placebo, might be effective for a variety of anxiety disorders (18), including social anxiety disorder (19, 20). However, other studies report weaker or no effects of d-cycloserine as a CBT augmentation strategy for treating post-traumatic stress disorder (21, 22) and obsessive-compulsive disorder (16, 23, 24). Two recent studies examining patients with obsessive-compulsive disorder (23, 25) and one trial with post-traumatic stress disorder (21) suggest that d-cycloserine may speed response to treatment rather than fundamentally change the outcome of treatment (14, 26, 27).
The interpretation of this literature is complicated by the small sample sizes of these studies and by inconsistencies in study design, target disorder, and drug administration schedules of d-cycloserine. The most promising results, thus far, have come from preliminary studies that administered 50 mg of d-cycloserine acutely 1 hour before an abbreviated (4–5 session) form of CBT delivered to small samples of patients with social anxiety disorder (19, 20). Therefore, we adopted the same drug dosing and dosing schedule that were used in these earlier trials. However, the results of these proof-of-concept studies are of limited practical relevance and many patients remain symptomatic after such ultra-brief treatments. In order to examine whether d-cycloserine improves speed or quality of response to a full course of CBT for social anxiety disorder, this study was designed to examine the short-term and long-term effects of 50 mg of d-cycloserine administered acutely during the course of comprehensive CBT for social anxiety disorder in an adequately powered, larger scale, double-blind, randomized and placebo-controlled trial. We hypothesized that d-cycloserine would facilitate CBT for social anxiety disorder, resulting in faster treatment gains and greater treatment response and remission rates at post-treatment and at the follow-up periods as compared to placebo-augmented CBT.
METHODS
Participants
Prospective participants were recruited between September 2007 and June 2011 through referrals to the three study sites (Boston University [BU], Massachusetts General Hospital [MGH], and Southern Methodist University [SMU]), from other area clinical facilities and programs, and from advertisements. Inclusion criteria were: 1) 18 years of age or older; and 2) current diagnosis of generalized social anxiety disorder that is designated by the patient as the most important source of current distress or interference. The diagnosis was defined based on the Diagnostic and Statistical Manual for Mental Disorders (Fourth Edition), DSM-IV criteria; 3) a total score on the Liebowitz Social Anxiety Scale (28) of 60 or greater; 4) no clinically significant abnormalities based on a physical examination, electrocardiogram, and laboratory findings; and 5) willingness and ability to comply with the requirements of the study protocol. Exclusion criteria included: 1) a lifetime history of bipolar disorder, schizophrenia, psychosis, delusional disorders or obsessive-compulsive disorder; an eating disorder in the past 6 months; organic brain syndrome, mental retardation or other cognitive dysfunction that could interfere with the capacity to engage in therapy; a history of substance or alcohol abuse or dependence (other than nicotine or caffeine) in the last 6 months or otherwise unable to commit to refraining from alcohol use during the treatment period of the study; 2) posttraumatic stress disorder within the past 6 months (entry of patients with other mood or anxiety disorders were permitted if the social anxiety disorder was judged to be the predominant disorder, in order to increase accrual of a clinically relevant sample); 3) significant suicidal ideation as suggested by a score of 3 or greater on item # 10 of the Montgomery Asberg Depression Rating Scale (29) (or presence of suicidal behaviors within 6 months prior to intake; 4) concurrent psychotropic medication (e.g., antidepressants, anxiolytics, beta blockers) for at least 2 weeks prior to entering the study; 5) significant personality dysfunction likely to interfere with study participation; 6) serious medical illness or instability for which hospitalization may be likely the following year; 7) a current or past history of seizures; 8) pregnant or lactating women, and women of childbearing potential who are not using medically accepted forms of contraception; 9) any concurrent psychotherapy initiated within 3 months of baseline, or ongoing psychotherapy of any duration directed specifically toward treatment of social anxiety disorder. Prohibited psychotherapy included CBT or psychodynamic therapy focusing on exploring specific, dynamic causes of the phobic symptomatology and providing management skills. General supportive therapy initiated more than 3 months prior was acceptable; 10) prior non-response to adequately-delivered exposure (i.e., as defined by the patient’s report of receiving specific and regular exposure assignments as part of a previous treatment; 11) a history of head trauma causing loss of consciousness, seizure or ongoing cognitive impairment; 12) patients receiving isoniazid; and 13) inability to understand study procedures and participate in the informed consent process.
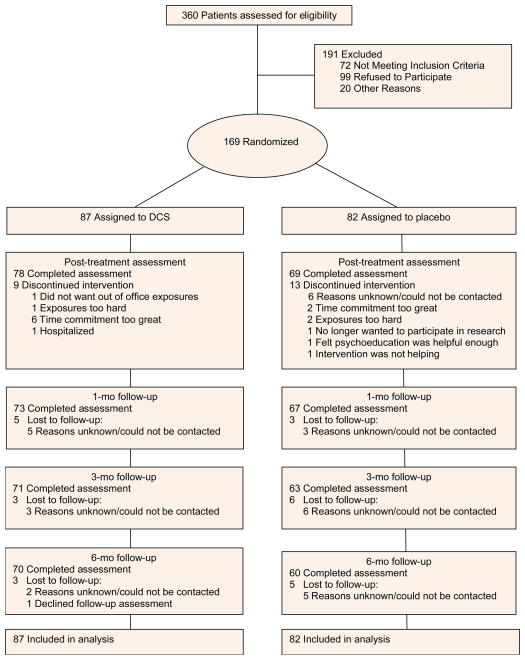
Study participants underwent a two-step screening evaluation, consisting of 1) telephone screening, and 2) psychiatric assessment and medical history taking with physical examination. A CONSORT diagram of participant flow is depicted in Figure 1. Patients in the d-cycloserine-augmented CBT group did not differ from the placebo-augmented CBT group in their mean age (34.6 vs. 30.5). Table 1 summarizes other demographic information for those patients who were randomized. The two treatment conditions did not differ on any of the demographic data at baseline, except that there were more males (64%) receiving d-cycloserine-augmented CBT than pill placebo-augmented CBT [49%; 2-tailed Fisher’s Exact Test; p = .045].
Figure 1.
Flow of Patients From Randomization Through Analysis.
TABLE 1.
Baseline Demographics
| Variable | D-cycloserine-augmented CBT (N=87) | Placebo-augmented CBT (N=82) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Male | 56 | 64.4 | 40 | 48.8 |
| Female | 31 | 35.6 | 42 | 51.2 |
| Race | ||||
| White | 61 | 70.1 | 61 | 74.4 |
| African American | 8 | 9.2 | 8 | 9.8 |
| Asian | 13 | 15.0 | 7 | 8.5 |
| Native Hawaiian | 0 | 0.0 | 1 | 1.2 |
| Native American/Alaska Native | 1 | 1.1 | 1 | 1.2 |
| Other | 4 | 4.6 | 4 | 4.9 |
| Ethnicity | ||||
| Not Hispanic or Latino | 78 | 89.7 | 73 | 89.0 |
| Hispanic or Latino | 9 | 10.3 | 9 | 11.0 |
| Marital Status | ||||
| Single | 47 | 54.0 | 57 | 69.5 |
| Living with partner | 10 | 11.5 | 5 | 6.1 |
| Married | 25 | 28.7 | 16 | 19.5 |
| Divorced | 5 | 5.7 | 2 | 2.4 |
| Widowed | 0 | 0.0 | 1 | 1.2 |
| Separated | 0 | 0.0 | 1 | 1.2 |
| Highest Education | ||||
| Graduate school | 25 | 28.7 | 20 | 24.4 |
| College graduate | 32 | 36.8 | 37 | 45.1 |
| Partial college | 25 | 28.7 | 19 | 23.2 |
| High school graduate | 4 | 4.6 | 5 | 6.1 |
| Partial high school | 1 | 1.1 | 1 | 1.2 |
| Highest Occupation | ||||
| Executive | 7 | 8.0 | 3 | 3.7 |
| Manager/professional | 34 | 39.1 | 31 | 37.8 |
| Administrative | 12 | 13.8 | 13 | 15.9 |
| Clerical | 6 | 6.9 | 9 | 11.0 |
| Skilled | 15 | 17.2 | 6 | 7.3 |
| Semi-skilled | 7 | 8.0 | 5 | 6.1 |
| Unskilled | 6 | 6.9 | 10 | 12.2 |
| Never worked | 0 | 0.0 | 5 | 6.1 |
| Living Situation | ||||
| Urban | 46 | 52.9 | 41 | 50.0 |
| Suburban | 35 | 40.2 | 37 | 45.1 |
| Rural | 6 | 6.9 | 4 | 4.9 |
| Income | ||||
| 0 | 7 | 8.0 | 6 | 7.3 |
| Not given | 16 | 18.4 | 15 | 18.3 |
| $0–$4,999 | 4 | 4.6 | 5 | 6.1 |
| $5,000–$9,999 | 1 | 1.1 | 4 | 4.9 |
| $10,000–$24,999 | 6 | 6.9 | 9 | 11.0 |
| $25,000–$34,999 | 7 | 8.0 | 5 | 6.1 |
| $35,000–$49,999 | 10 | 11.5 | 11 | 13.4 |
| $50,000–$74,999 | 12 | 13.8 | 8 | 9.8 |
| Greater than $75,000 | 24 | 27.6 | 18 | 22.0 |
| Missing | 0 | 0.0 | 1 | 1.2 |
| Occupational Status | ||||
| Not applicable | 9 | 10.3 | 11 | 13.4 |
| Full-time employment | 45 | 51.7 | 39 | 47.6 |
| Part-time employment | 10 | 11.5 | 12 | 14.6 |
| Dependent on spouse or is a student | 17 | 19.5 | 17 | 20.7 |
| Missing | 0 | 0.0 | 1 | 1.2 |
Study Design
Eligible participants were enrolled into a 12-session CBT protocol across three study sites (BU, MGH, and SMU), all using identical study protocols. One of the sites (MGH) is well known for conducting pharmacological studies, whereas SMU and BU are specialized in providing psychological interventions. At session 3, patients were randomly assigned to receive oral administration of either 50 mg of d-cycloserine (N=87) or pill placebo (N=82) 1-hour prior to sessions 3–7. Assignment to treatment condition was determined by a computer-generated allocation schedule, involving stratification by baseline social anxiety disorder severity (i.e., Liebowitz Social Anxiety Scale score ≤ 70 or ≥ 70) (28). Assessment of primary outcome variables was conducted during the treatment phase at baseline, sessions 2 through 8, 10 and 12, post-treatment (week 13), and during a follow-up phase at 1-month (week 17), 3-month (week 25), and 6-month (week 37) follow-up. The trial was sufficiently powered to detect a medium-sized effect (d = .5) at 85% power and P < .05 at post treatment. Furthermore, the study was sufficiently powered to detect a medium-sized group difference (d = .5) at 80% power and P < .05 at the 6-month follow-up, which was the assessment with the smallest number of subjects available for the analyses. The protocol was approved by the Institutional Review Boards of BU, MGH and SMU, respectively. After complete description of the study to the subjects, written informed consent was obtained.
Assessment Measures and Procedures
Initial diagnoses were performed by trained study therapists using the Structured Clinical Interview for DSM-IV (30) at the MGH and SMU site and the Anxiety Disorders Interview Schedule for DSM-IV (31) at the BU site. In addition, the Liebowitz Social Anxiety Scale was administered to assess for the generalized social anxiety disorder diagnostic subtype specifier. The blinded assessments were conducted by a masters or doctoral level clinician trained in these assessments. The integrity and reliability of the diagnostic and efficacy evaluations was established through training by the MGH site and maintained by providing the clinicians with weekly supervision and feedback based on approximately 20% of the audio recorded assessment interviews.
The primary outcomes were clinical global impression ratings tailored to social anxiety as assessed by the Liebowitz Social Anxiety Scale rating scale (28) and the Social Phobic Disorders Severity and Change Form (32) which yielded two continuous measures, a Social Phobic Disorders Severity score and a Social Phobic Disorders Change score. These scores are the Clinical Global Impression scores (severity and improvement, respectively) with wordings that have been modified to specifically measure symptoms of social anxiety disorder (32). These clinical interviews were conducted by an interviewer blind to treatment assignment.
Definition of Response and Remission
Response was defined as an improvement score of 1 (“very much improved”) or 2 (“much improved”) on the Social Phobic Disorders Change score; remission was defined as an improvement score of 1 or 2 on the Social Phobic Disorders Change score and a Liebowitz Social Anxiety Scale total score < 30.
Study Interventions
CBT
All therapists followed the same protocol, adhering to standard treatment protocols consisting of 12 weekly, 2.5-hour sessions conducted in a group format with 4–6 patients and 2 therapists per group (33). The content of these first 2 sessions followed the treatment protocol developed by Heimberg and colleagues (34) with remaining sessions adhering to the exposure targets and strategies described by Hofmann and Otto (33). Sessions 3–7 emphasized exposure to feared cues (e.g., public speaking, calling a stranger, returning goods to a store) with the aim to achieve fear extinction. Session 8–12 involved a combination of cognitive restructuring and individually-tailored in vivo exposure practices designed to challenge the patient’s maladaptive beliefs about the negative consequences of social mishaps. In addition, patients received homework assignments between sessions to practice the techniques they learned during the sessions. All therapists were trained and supervised by senior clinicians (SGH, JAJS, LM) and participated in weekly cross-site supervision led by Drs Hofmann and Smits.
Medication
Study capsules were prepared containing: (a) 50 mg d-cycloserine or (b) pill placebo and were administered by a nurse or psychiatrist 1-hour prior to sessions 3–7. All capsules were identical in appearance to maintain the blind. The capsules of the study pills (size #0) were compounded by Abrams Royal Pharmacy in Dallas, TX (for SMU and BU sites) or the MGH pharmacy (for MGH site). The active drug capsules contained d-cycloserine 50mg (derived from Seromycin 250mg capsules) and polyethylene glycol 3350 powder, while the matching placebo capsules contained only the polyethylene glycol 3350 powder. All subjects were observed when they ingested the pill.
Data Analysis Plan
The primary analyses were conducted using mixed effects regression models for continuous outcomes (Social Phobic Disorders Severity and Liebowitz Social Anxiety Scale scores), and generalized linear mixed models (mixed effects regression models using a logistic linking function) for dichotomous outcomes (Remission and Response). These analytical approaches allow the inclusion of all subjects, regardless of missing data, thereby improving power and the generalizability of the results and are the recommended approaches to analyzing longitudinal psychiatric data (35). Because the growth curve of the outcome measures changed markedly from treatment through follow-up, the growth curve was modeled as “discontinuous” (36), allowing all growth curve parameters to change from the treatment phase to the follow-up phase. The predictors in the models were Time (t), t2, treatment condition (T), T x t and T x t2. In addition, because of the disproportionally higher rate of males in the d-cycloserine-augmented CBT condition, sex along with the interaction of sex and Time were added as additional covariates in the model. Because initial severity is likely to be related to rate of improvement in outcomes, we also added initial severity, and the interaction of initial severity with Time, as additional predictors of outcome to reduce variance in outcome. The growth curves during the treatment phase and the follow-up periods were linear for both of the continuous outcome measures (Social Phobic Disorders Severity and Liebowitz Social Anxiety Scale scores), but were curvilinear for the treatment phase in the analysis of the dichotomous outcomes (Remission and Response). Thus, the quadratic terms for the growth curve were omitted from the mixed effects regression model analyses of Social Phobic Disorders Severity and Liebowitz Social Anxiety Scale scores, and retained in the generalized linear mixed model analyses for the treatment phase of the analysis of response and remission. Neither sex nor the interaction of sex and Time were significant predictors of any of the outcomes.
RESULTS
Treatment Attrition and Integrity
The attrition rates during the 12-week treatment phase were low and did not differ significantly across conditions (10.3% for the d-cycloserine-augmented CBT condition vs. 15.9% for the placebo-augmented CBT condition, Fisher Exact Test, P>.3). The reasons for attrition are listed in Figure 1. Similarly, attrition was low during the follow-up phase (11.5% and 11.0% for the d-cycloserine-augmented CBT and placebo-augmented CBT conditions, respectively; Fisher Exact Test, P>.5). The mean number of CBT sessions attended was 10.8 (SD=2.19) in the d-cycloserine-augmented condition and 10.6 (SD=2.14) in the placebo-augmented condition (t167=.45, P>.6). Similarly, compliance with pill administration was high, with 78.1% of patients receiving all pill administrations (d-cycloserine: 75.9%; placebo: 80.5%; Fisher Exact Test, P>.2).
At the beginning of sessions 3–7 when the study pills were administered, patients were asked to indicate whether they believe the pill consisted of (1) d-cycloserine or (2) placebo or (3) whether they were unable to guess. Approximately one-third to one-half of all patients (30.9%–46.2%, depending on group and session) in both conditions reported that they were unable to guess their treatment condition (all chi-square tests showed Ps > .08). Among patients who guessed either of the two drug conditions, those who received d-cycloserine did not differ from those who received placebo in their guess that they received d-cycloserine at session 3 (d-cycloserine group: 50.0% vs. placebo: 75.0%), 4 (d-cycloserine group: 40.0% vs. placebo: 47.6%), 5 (d-cycloserine group: 35.7% vs. placebo: 47.4%), 6 (d-cycloserine group: 33.3% vs. placebo: 45.7%), and 7 (d-cycloserine group: 38.6% vs. placebo: 37.8%, all Fisher Exact Tests showed Ps >.2).
Baseline Severity
There were no differences between-groups at baseline on the Liebowitz Social Anxiety Scale [81.34 (15.06) vs. 81.98 (17.15)], Social Phobic Disorders Severity Scale [5.33 (0.83) vs. 5.26 (0.86)] or depressive symptom severity (all P’s>.40). The median number of comorbid diagnoses in both groups was 1.
Primary Analyses: Response and Remission Rates
An intent-to-treat analysis using the last observation carried forward approach revealed response rates of 79.3% in the d-cycloserine-augmented CBT group and 73.2% in the placebo-augmented treatment group (P >.3). The remission rates of these intent-to-treat analyses were 34.5% and 24.4% for the d-cycloserine and placebo-augmented CBT groups, respectively (P >.15).
The follow-up analyses using traditional intent-to-treat analyses also revealed no significant differences (all Ps>.5) between the d-cycloserine and placebo-augmented CBT group in the response rates at 1-month follow-up (74.7% vs.75.6%, respectively), 3-month follow-up (73.6% vs. 70.7%, respectively), or at 6-month follow-up (70.1% vs. 74.4%, respectively). Similarly, these analyses revealed no between-group differences for remission rates (all Ps >.4) at 1-month follow-up (33.3% vs. 28.0%, respectively), 3-month follow-up (29.9% vs. 28.0% respectively), or at 6-month follow-up (29.9% vs. 28.0%, respectively).
The mixed effects regression models revealed that at post-treatment, patients who received d-cycloserine showed lower global illness severity scores, as measured with the Social Phobic Disorders Severity scores, than patients who received placebo (estimated parameter for treatment effect: .43; t149=2.05, P=.04). We also observed a statistical trend towards lower scores in the Liebowitz Social Anxiety Scale at the post-treatment assessment with d-cycloserine compared to placebo (estimated parameter for treatment effect: 5.22; t146=1.75; P=.08). Table 2 presents the unadjusted means and 95% confidence intervals (CI).
TABLE 2.
Unadjusted Outcomes at Posttreatment and Follow-up Assessments
| Variable | D-cycloserine-augmented CBT (N=87) | Placebo-augmented CBT (N=82) | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
|
| ||||
| Global Illness Severity (SPD-S) | ||||
| Posttreatment | 2.68 | 2.38–2.98 | 2.95 | 2.64–3.27 |
| 1-mo Follow-up | 2.77 | 2.47–3.07 | 2.79 | 2.47–3.10 |
| 3-mo Follow-up | 2.90 | 2.58–3.22 | 2.86 | 2.52–3.19 |
| 6-mo Follow-up | 2.91 | 2.59–3.23 | 2.75 | 2.41–3.09 |
| Social Anxiety Severity (LSAS) | ||||
| Posttreatment | 39.19 | 34.79–43.59 | 42.44 | 37.94–47.03 |
| 1-mo Follow-up | 39.59 | 35.15–44.03 | 40.42 | 35.79–45.05 |
| 3-mo Follow-up | 40.71 | 36.01–45.40 | 40.91 | 36.01–45.82 |
| 6-mo Follow-up | 42.63 | 37.63–47.63 | 40.58 | 35.34–45.82 |
|
| ||||
| N | % | N | % | |
|
| ||||
| Response | ||||
| Posttreatment | 80 | 79.3 | 67 | 73.2 |
| 1-mo Follow-up | 72 | 74.7 | 67 | 75.6 |
| 3-mo Follow-up | 70 | 73.6 | 63 | 70.7 |
| 6-mo Follow-up | 69 | 70.1 | 59 | 74.4 |
| Remission | ||||
| Posttreatment | 80 | 34.5 | 67 | 24.4 |
| 1-mo Follow-up | 72 | 33.3 | 67 | 28.0 |
| 3-mo Follow-up | 70 | 29.9 | 63 | 28.0 |
| 6-mo Follow-up | 69 | 29.9 | 59 | 28.0 |
Secondary Analyses: Slope Analyses and Speed of Response
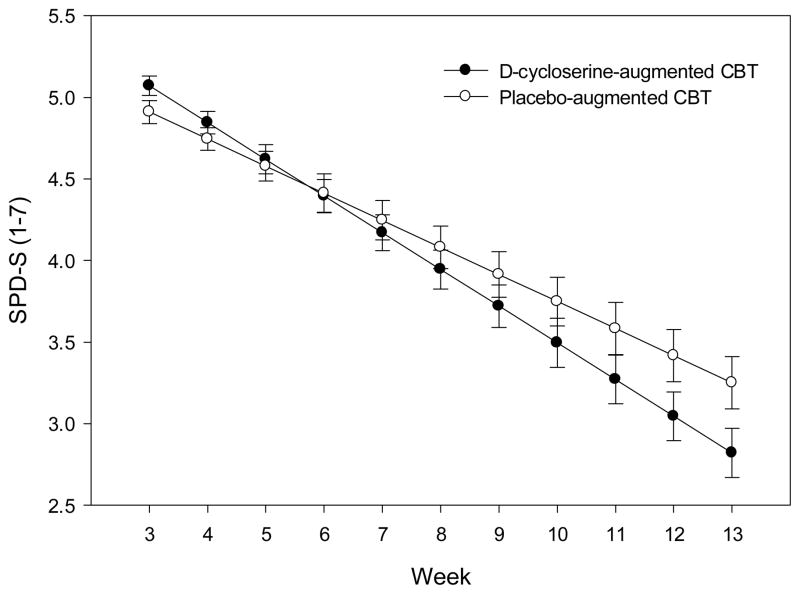
During the treatment phase, patients who received d-cycloserine-augmented CBT evidenced a faster slope of improvement on global illness severity (Social Phobic Disorders Severity scores; estimated parameter for treatment effect: .06; t129=2.76, P=.006; Figure 2), on social anxiety symptoms severity scores (Liebowitz Social Anxiety Scale; estimated parameter for treatment effect: .65; t147=2.18, P=.03), and on remission rates (estimated parameter for treatment effect: −.10; t1661=2.59, P=.01) as compared to those assigned placebo-augmented CBT. Compared to patients who received placebo-augmented CBT, patients who received d-cycloserine showed a 33% faster rate of improvement in Social Phobic Disorders Severity scores, a 24% faster reduction in Liebowitz Social Anxiety Scale scores and a 30% faster increase in remission rates during treatment. There was no between-group difference on slopes of change in response rates (P>.6).
Figure 2. Mean Growth Curves of Social Phobic Disorders Severity Scale (SPD-S) Scores.
Changes in Social Phobic Disorders Severity Scale scores during d-cycloserine-augmented cognitive behavioral therapy and placebo-augmented cognitive behavioral therapy for social anxiety disorder. Randomization to the two study groups occurred at week 3. The study pills were administered weekly 1 hour prior to each of 5 sessions between week 3 and 7. The post-treatment assessment point was at week 13. Error bars are standard deviations.
During the follow-up period, the mixed effects regression model and generalized linear mixed model analyses showed that none of the slopes of change for any of the outcomes (Social Phobic Disorders Change scores, Liebowitz Social Anxiety Scale, response, and remission) varied as a function of condition (all Ps>.08). Patients receiving d-cycloserine did not evidence significantly better outcomes on any of the measures at the post-treatment or follow-up assessments (all Ps >.3). Unadjusted means and 95% confidence intervals (CI) for these outcomes appear in Table 2.
DISCUSSION
This is the first large-scale, randomized, placebo-controlled clinical trial to evaluate d-cycloserine as an augmentation strategy for a full course and comprehensive CBT intervention for social anxiety disorder. In contrast, to our prediction, d-cycloserine-augmented CBT was not superior to placebo-augmented CBT in completion rates, response rates, and remission rates at post-treatment or at follow-up. These findings are at odds with our earlier pilot study (19), an independent replication (19), and other small-scale proof-of-concept trials with other anxiety disorders, including panic disorder (37) and height phobia (38). Instead, our findings are in line with other studies (21, 23, 24) suggesting that d-cycloserine does not amplify the effects of CBT at major endpoints, but may temporarily accelerate therapy gains.
One important difference between these previous studies and the current trial is the length of treatment. Specifically, in our earlier study (19) and the subsequent replication (20), the sessions consisted of only four 1-hour public speaking exposures. The greater number and more tailored exposure sessions in the current study might have provided less room for a memory enhancement effect of d-cycloserine, as suggested by the response rates of greater than 70%, which is higher than what has typically been reported in studies examining traditional CBT protocols for social anxiety disorder. For example, a previous large-scale study that employed similar study criteria reported an intent-to-treat response rate of 50.8% after 12 weekly sessions of traditional CBT plus pill placebo (39). Another more recent trial reported only 47.1% response after monotherapy of traditional CBT (40). The treatment protocol utilized in the present study differed from these traditional CBT approaches primarily in the nature of the exposure practices. The current protocol included in-vivo social mishap exposures, which were also encouraged as homework practices in between the sessions. This might have contributed to the relatively high response rates in both groups. Both treatments were further associated with relatively low dropout rates, which did not differ between the two treatments.
Our study provides evidence for limitations of d-cycloserine as a combination strategy with a full course of comprehensive CBT. Although patients who received d-cycloserine-augmented CBT showed a 24–33% faster rate of improvement in symptom severity and remission rates relative to those who received placebo-augmented CBT, no between-group differences were evident in response or remission rates at post-treatment or any of the follow-up assessments. However, it is possible that a difference in dosing or dose timing of d-cycloserine might have led to different results. It is further possible that d-cycloserine is more effective for some subgroups of patients than for others, including those with a specific genotype and subjects who demonstrated greater within-session progress. Another limitation is the relatively stringent, although not untypical, study criteria. However, only 20% of patients (71 out of 360) were excluded because of the study criteria.
In sum, the results of this first large-scale placebo-controlled randomized trial showed that d-cycloserine did not augment the efficacy of CBT for social anxiety disorder. However, in line with other studies (14, 27), d-cycloserine might temporarily accelerate the speed of treatment response. This acceleration may provide early treatment benefit, affording a potential role for d-cycloserine in reducing patient distress during exposure treatments. This might enhance the acceptability of treatment and limit avoidance tendencies of patients with anxiety disorders that can hinder the success of CBT. However, this study tests the use of d-cycloserine as a routine augmentation strategy for a full course of CBT for social anxiety disorder. We recommend that future studies might examine whether any conditions and/or patient characteristics exist for which d-cycloserine augmentation might be indicated.
Acknowledgments
Funding/Support: This study was funded by NIH grants R01MH078308 (Hofmann) and R01MH075889 (Pollack) from the National Institute of Mental Health.
Role of the sponsor: The sponsor (NIH) had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosures: Dr. Hofmann reported receiving royalties from multiple publishers, including Routledge, the publisher of the CBT manual used in this study. Dr. Smits reported receiving royalties from various book publishers unrelated to this study. Dr. Otto noted serving as a consultant for MicroTransponder Inc. and reported receiving royalties from multiple publishers, including Routledge, the publisher of the CBT manual used in this study. Dr. Pollack noted the following disclosures for the preceding 12 months: Advisory Boards and Consultation: Eli Lilly, Medavante, Otsuka., Targia Pharmaceuticals; Research Grants: Bristol Myers Squibb, Euthymics, Forest Laboratories, Glaxo SmithKline, NCCAM, NIDA, NIMH, Equity: Medavante, Mensante Corporation, Mindsite, Targia Pharmaceuticals. Royalty/patent: SIGH-A, SAFER interviews. Dr. Meuret reported serving as a consultant for Palo Alto Health Sciences Inc.
Additional Contributions: The authors would like to thank Stephen Wisniewski, Ph.D. for providing free statistical consultation during the design development and Richard G. Heimberg, Ph.D. for providing a 1-day training and consultation workshop in cognitive behavioral group therapy prior to participant enrollment. He received compensation for this contribution. We would also like to thank Ashley Witt, B.A., and Shelley Capozzoli, B.A. at Boston University for their help with the data management and data entry. We also thank the following individuals for serving as therapists or clinical assessors in the study: Allison Applebaum, Ph.D., Anu Asnaani, M.A. Jacqueline Bullis, M.A., Cassidy A. Gutner, M.A., John A. Richey, Ph.D., Alice T. Sawyer, M.A., Maria Steenkamp, Ph.D. (at Boston University); Lindsey DeBoer, M.A., Deborah Corbitt-Shindler, M.A., Katherine Croft, M.A., Catherine Dodson, M.A., Pamela Handelsman, B.A., Grant Holland, M.A., Kristin Julian, M.A., Erica Simon, M.A., Anne Miller, M.A., Matthew Leahy, Ph.D. (at Southern Methodist University); and Ryan Jacoby, B.A., Meghan Keogh, Ph.D., Libby Marks, B.A., Laura Morris, B.A., Don Robinaugh, M.A., Sharon Sung, Ph.D., (at Massachusetts General Hospital). These individuals received financial contribution for their institutional efforts.
Previous Presentations: Initial results have been presented at the annual meetings of the Anxiety Disorders Association of American, April, 2012, Washington, DC, and the Society for Biological Psychiatry, May 2012, Philadelphia, PA.
Author Contributions: Drs Hofmann and Pollack had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hofmann, Pollack, Smits, Simon, Otto, Meuret.
Acquisition of data: Hofmann, Pollack, Smits, Marques, Meuret, Tart, Fang.
Analysis and interpretation of data: Rosenfield, Hofmann, Pollack, Smits, Simon, Fang.
Drafting of the manuscript: Hofmann, Rosenfield, Smits.
Critical revision of the manuscript for important intellectual content: Pollack, Simon, Otto, Meuret.
Statistical analysis: Rosenfield, Hofmann, Smits.
Obtained funding: Hofmann, Pollack.
Administrative, technical, or material support: Hofmann, Pollack, Smits, Otto, Tart.
Study supervision: Hofmann, Pollack, Smits, Simon, Meuret.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walter EE. Lifetime prevalence and age-of-onset distribution of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Davidson JR, Foa EB, Huppert JD, Keefe FJ, Franklin ME, Compton JS, Zhao N, Connor KM, Lynch TR, Gaddle KM. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61:1005–1013. doi: 10.1001/archpsyc.61.10.1005. [DOI] [PubMed] [Google Scholar]
- 3.Clark DM, Ehlers A, McManus F, Hackmann A, Fennell M, Campbell H, Flower T, Davenport C, Louis B. Cognitive therapy versus fluoxetine in generalized social phobia: a randomized placebo-controlled trial. J Consult Clin Psychol. 2003;71:1058–1067. doi: 10.1037/0022-006X.71.6.1058. [DOI] [PubMed] [Google Scholar]
- 4.Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, Holt CS, Welkowitz LA, Juster HR, Campeas R, Bruch MA, Cloitre M, Fallon B, Klein DF. Cognitive behavioral group therapy vs. phenelzine therapy for social phobia. Arch Gen Psychiatry. 1998;55:1133–1141. doi: 10.1001/archpsyc.55.12.1133. [DOI] [PubMed] [Google Scholar]
- 5.Stein MB, Liebowitz MR, Lydiard RB, Pitts CD, Bushnell W, Gergel I. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280:708–713. doi: 10.1001/jama.280.8.708. [DOI] [PubMed] [Google Scholar]
- 6.Blomhoff S, Haug TT, Hellstroem K, Holme I, Humble M, Madsbu HP, Wold JE. Randomised controlled general practice trial of sertraline, exposure therapy and combined treatment in generalised social phobia. Br J Psychiatry. 2001;179:23–30. doi: 10.1192/bjp.179.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clin Psychol Sc Pract. 2005;12:72–86. [Google Scholar]
- 8.Blanco C, Heimberg RG, Schneier FG, Fresco DM, Chen H, Turk CL, Vermes D, Erwin BA, Schmidt AB, Juster HR, Campeas R, Liebowitz MR. A placebo-controlled trial of phenelzine, cognitive behavioral group therapy, and their combination for social anxiety disorder. Arch Gen Psychiatry. 2010;67:286–296. doi: 10.1001/archgenpsychiatry.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falls WA, Miserendino MJD, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers KM, Davis M. Behavioral and neural analysis of extinction: a review. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 11.Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of conditioned fear as assessed by freezing in rats. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- 12.Davis M, Walker DL, Meyers KM. Role of the amygdale in fear extinction measured with potentiated startle. Ann N Y Acad Sci. 2003;985:218–232. doi: 10.1111/j.1749-6632.2003.tb07084.x. [DOI] [PubMed] [Google Scholar]
- 13.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegmund A, Golfels F, Finck C, Halisch A, Räth D, Plag J, Ströhle A. D-cycloserine does not improve but might slightly speed up the outcome of in-vivo exposure therapy in patients with severe agoraphobia and panic disorder in a randomized double blind clinical trial. J Psychiatr Res. 2011;45:1042–1047. doi: 10.1016/j.jpsychires.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Guastella AJ, Dadds M, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of D-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Storch EA, Merlo LJ, Bengtson M, Murphy TK, Lewis MH, Yang MC, Jacob ML, Larson M, Hirsh A, Fernandez M, Geffken GR, Goodman WK. D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2007;22:230–237. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- 17.Berry AC, Rosenfield D, Smits JAJ. Extinction retention predicts improvement in social anxiety symptoms following exposure therapy. Depress Anxiety. 2009;26:22–27. doi: 10.1002/da.20511. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann SG, Sawyer AT, Asnaani A. D-cycloserine as an augmentation strategy for cognitive behavioral therapy for anxiety disorders: an update. Curr Pharm Des[0] doi: 10.2174/138161212803530916. (Epub ahead of print, May 24, 2012) [DOI] [PubMed] [Google Scholar]
- 19.Hofmann SG, Meuret AE, Smits JAJ, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. Augmentation of exposure therapy for social anxiety disorder with d-cycloserine. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 20.Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of d-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 21.de Kleine RA, Hendriks GJ, Kusters WJ, Broekman TG, van Minnen A. A randomized placebo-controlled trial of d-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Litz BT, Salters-Pedneault K, Steenkamp M, Hermos JA, Bryant RA, Otto MW, Hofmann SG. A randomized placebo-controlled trial of d-cycloserine and exposure therapy for post-traumatic stress disorder. J Psychiatr Res. 2012;46:1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL. Augmentation of behavior therapy with d-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- 24.Storch EA, Murphy TK, Goodman WK, Geffken GR, Lewin AB, Henin A, Micco JA, Sprich S, Wilhelm S, Bengston M, Geller DA. A preliminary study of d-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2010;68:1073–1076. doi: 10.1016/j.biopsych.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Chasson GS, Buhlmann U, Tolin DF. Need for speed: evaluating slopes of OCD recovery in behavior therapy enhanced with d-cycloserine. Behav Res Ther. 2010;48:675–679. doi: 10.1016/j.brat.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann SG, Smits JAJ, Asnaani A, Gutner CA, Otto MW. Cognitive enhancers for anxiety disorders. Pharmacol Biochem Behav. 2011;99:275–284. doi: 10.1016/j.pbb.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatr. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- 29.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 30.First M, Spitzer RL, Gibbon M, Williams J. Structured clinical interview for DSM-IV Axis I disorders, Patient edition. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 31.DiNardo PA, Brown TA, Barlow DH. Anxiety disorders interview schedule for DSM-IV: lifetime version (ADIS-IV-L) Graywind Publications Incorporated; 1994. [Google Scholar]
- 32.Liebowitz MR, Schneier FR, Campeas R, Hollander E, Hatterer J, Fyer A, Gorman J, Papp L, Davies S, Gully R, Klein DF. Phenelzine vs. atenolol in social phobia: a placebo-controlled comparison. Arch Gen Psychiatry. 1992;49:290–300. doi: 10.1001/archpsyc.49.4.290. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann SG, Otto MW. Cognitive-behavior therapy for social anxiety disorder: evidence-based and disorder-specific treatment techniques. New York, NY: Routledge; 2008. [Google Scholar]
- 34.Hope DA, Heimberg RG, Turk CL. Managing social anxiety: a cognitive behavioral therapy approach: therapist guide. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 35.Hammer RM, Simpson PM. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. Am J Psychiatry. 2009;166:639–641. doi: 10.1176/appi.ajp.2009.09040458. [DOI] [PubMed] [Google Scholar]
- 36.Singer JD, Willett JB. Applied longitudinal data analysis. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- 37.Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, Hofmann SG, Eisenmenger K, Krystal JH, Pollack MH. The efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2009;67:365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 38.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of d-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 39.Davidson JR, Foa EB, Huppert JD, Keefe FJ, Franklin ME, Compton JS, Zhao N, Connor KM, Lynch TR, Gadde KM. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61:1005–1013. doi: 10.1001/archpsyc.61.10.1005. [DOI] [PubMed] [Google Scholar]
- 40.Blanco C, Heimberg RG, Schneier FR, Fresco DM, Chen H, Turk CL, Vermes D, Erwin BA, Schmidt AB, Juster HR, Campeas R, Liebowitz MR. A placebo-controlled trial of phenelzine, cognitive behavioral group therapy, and their combination for social anxiety disorder. Arch Gen Psychiatry. 2010;67:286–295. doi: 10.1001/archgenpsychiatry.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]