Abstract
An established neural biomarker of autism spectrum disorder (ASD) has the potential to provide novel biological and pharmacological targets for treatment. Lower level of inhibition in brain circuits is a leading biomarker candidate. A physiological investigation of the functional levels of inhibition in the cortex of individuals with autism can provide a strong test of the hypothesis. The amplitude of cortical response to the stimulation of adjacent fingers is controlled by the level of cortical inhibition and provides just such a test. Using magnetoencephalography, we recorded the response of the somatosensory cortex to the passive tactile stimulation of the thumb (D1), and index finger (D2), and to the simultaneous stimulation of both fingers combined (D1, D2) of the dominant (right) hand of young subjects with and without autism. For each participant, we measured the response to the stimulation of both fingers combined (D1, D2) relative to the post hoc sum of the responses to the stimulation of each finger alone (D1+D2) in multiple different ways and linearly regressed the ASD and neurotypical (NT) groups’ responses. The resulting slopes were then compared: Smaller slope values imply attenuated response to paired finger stimulation, and enhanced levels of inhibition. The short-latency M40 and mid-latency M80 response slopes of the group with autism obtained in different ways were either significantly smaller, or statistically indistinguishable from NT. The result does not support reduced inhibition in the somatosensory cortex of individuals with autism, contrary to the seminal hypothesis of reduced inhibition. Implications are discussed including refinements of current theory.
Keywords: evoked potentials, homeostasis, somatosensory cortex, cortical interaction, finger representation, source modeling, tactile
Introduction
Autism spectrum disorder (ASD) is a developmental disorder for which an objective medical test is not yet available, but rather trained clinicians conduct extensive interviews with the individual and their caregiver and perform rigorous behavioral evaluations of the individual in accordance with a manual. New diagnostic guidelines will come into effect as early as next year. New altered criteria by which individuals will be categorized as being included within the spectrum of autism disorders is likely to make the diagnostic process more difficult. The impending change and rising uncertainty in the diagnostic community has enhanced the imperative to find a biomarker that will eventually lead to the creation of an objective medical test that will complement behavioral evaluations and aid the clinician in making a correct and timely diagnosis. Moreover, an objective neural biomarker of autism has the potential to provide additional biological targets for intervention and treatment.
One leading candidate biomarker is reduced levels of inhibition and imbalance in the inhibition/excitation ratio in the brains of individuals with autism [Rubenstein & Merzenich, 2003]. Evidence for reduced GABAergic inhibition and abnormal glutamatergic transmission in autism stems from genetic [DiCicco-Bloom et al., 2006; Polleux & Lauder, 2004], and anatomical studies [Courchesne et al., 2001; Herbert et al., 2003]. A functional or physiological test of differences in inhibition in the brains of individuals with ASD would provide, arguably, a direct and rigorous test of the reduced inhibition hypothesis.
Electrophysiological recordings of the cortex have shown that the simultaneous mechanical or electrical stimulation of adjacent fingers of the hand (e.g. thumb and index finger) suppresses the response of somatosensory cortex: The magnitude of the cerebral evoked potential in response to the simultaneous moderate or strong stimulation of both fingers is less than that predicted from the simple addition of the potentials generated by the individual stimulation of each finger. The amount of attenuation in the cortical response to the combined simultaneous stimulation of neighboring fingers relative to the arithmetic sum of the responses to the individual stimulation of each finger is proportional to the level of cortical inhibition [Friedman, Chen, & Roe, 2008; Gandevia, Burke, & McKeon, 1983; Greek, Chowdhury, & Rasmusson, 2003; Hsieh, Shima, Tobimatsu, Sun, & Kato, 1995]. That is to say, the more sublinear the combined response relative to the arithmetic sum of the responses, the greater is the level of cortical inhibition. Studies of paired finger stimulation can thus provide a physiological window into the level of inhibition [Gandevia et al., 1983; Greek et al., 2003; Hsieh et al., 1995].
In order to assay inhibition from a physiological perspective in autism, we investigated and compared the cortical response to paired finger stimulation in the brains of individuals with ASD using magnetoencephalography (MEG). The response to the stimulation of adjacent fingers of the dominant hand is plotted with respect to the response to the post hoc sum of the responses to the individual stimulation of the same fingers. The plotted data are linearly regressed for each group separately, and the slopes thus provide a summary statistic of the degree of sublinearity of the combined response of the neurotypical (NT) and ASD groups. There are three possible outcomes. First, the slopes of both groups is less than unity (= 1) indicating sublinearity of response to paired stimulation (and cortical inhibition as well), but the slope for the ASD group is significantly greater than that for the NT group. This would provide experimental support for the idea that inhibition is reduced in the brains of individuals with ASD [Hussman, 2001]. Alternatively, the slopes of the two groups, while being significantly less than unity, do not differ statistically from one another’s, which would not bolster the reduced inhibition hypothesis. At the other extreme, significantly lower slope for the ASD group is a possibility as well. Such a finding would argue against the idea that inhibition levels are lower throughout the brain in autism, but is rather consistent with the idea that there are local brain areas of increase and of decrease in inhibition level that would point to spatial imbalance in inhibition levels in the brains of individuals with autism. The last outcome could have different but equally important implications in the quest for a biomarker and possible treatment.
Method
Participants
MEG signals from 13 individuals with a diagnosis of ASD (18.7 ± 1.0 years old; four female) and 17 typically developing, or NT, individuals (19.2 ± 1.2 years old; four female) were recorded. The groups were matched for age (P = 0.83, two-tailed t-test) and gender (P = 0.69, Fisher’s exact test). All individuals in the autism group met our research criteria for an ASD, as determined by using the Autism Diagnostic Observation Schedule [Lord, Rutter, DiLavore, & Risi, 1999] and the Autism Diagnostic Interview, Revised [Rutter, Le Couteur, & Lord, 2003] administered by trained clinicians. Five individuals in the autism group had been clinically classified as pervasive developmental disorder—not otherwise specified, one as Asperger syndrome and the remaining seven as autistic disorder. Potential participants were excluded when there was evidence of brain injury, seizure disorder, or neurotropic infection or disease, or if they had a history of identified severe psychopathology such as bipolar disorder, schizophrenia, or behavior problems severe enough to make accurate and reliable testing difficult. All participants were right handed as determined by the Edinburgh Handedness Inventory [Oldfield, 1971]. All individuals with autism had strong verbal skills, and were without intellectual disability: full-scale intelligence quotients (IQs) and verbal IQs derived from the Wechsler Abbreviated Scale of Intelligence [Wechsler, 1999] were greater than 85 (full-scale IQ: 103.7 ± 4.5; verbal IQ: 101.9 ± 4.9; performance IQ: 103.1 ± 4.5). NT participants were volunteers without a history of ASD or other major developmental or psychiatric illness. Their IQs were above average (full-scale IQ: 118 ± 3.0; verbal IQ: 113.0 ± 4.0; performance IQ: 118.0 ± 2.0) and were significantly higher than those of the ASD group (full-scale IQ: P = 0.02; verbal IQ: P = 0.017; performance IQ: P = 0.012).
Participants did not have to perform any cognitive task; therefore, any differences in signal between the groups is unlikely to be based on differences in IQ (see Supplementary Materials for correlations between the extracted MEG signals and IQ measures). After complete description of the study to the study participants, written informed consent was obtained under a protocol approved by the University of Texas Health Science Center–Houston and the University of Houston.
Stimuli
Pneumatically driven mechanical taps (25 pounds per square inch, or 25 psi) of 40-ms duration (20-ms rise time) were delivered individually to the distal tips of the thumb (D1), index finger (D2), and a combination of both (D1, D2) of the dominant hand of participants in separate blocks of epochs. This is a benign tactile stimulus that elicits a mild sensation on the skin; none of the participants indicated any discomfort with this procedure, but the stimulus amplitude (25 psi) is nonetheless clearly above the sensory detection threshold of 17 psi [Zhu et al., 2009]. Each finger had its own dedicated pressure transducer. Participants were told that a pressure pulse will be delivered during which they were supposed to close their eyes, relax, and stay still. As mentioned above, there was no task to perform and therefore, no demand on participants’ cognition. A training block containing five epochs before the experimental recordings helped familiarize participants with the stimuli.
Procedure
Participants lay supine on a comfortable bed and kept their eyes closed. Fiducial markers were placed on their forehead and in the ears. The locations of the fiducial markers were recorded into the computer by means of a digitizer (stylus pen). The digitizer was slowly rolled over the participant’s scalp, and the shape of his or her head was thus recorded. Using the digitization points and the fiducial marker locations, a single sphere head model was created that best fit each participant’s head (Fieldtrip toolbox, MATLAB; The Mathworks, Inc., Natick, MA).
MEG Recordings
All MEG recordings used a whole-head neuromagnetometer containing an array of 248 gradiometers (Magnes WH3600, 4D Neuroimaging Inc., San Diego, CA, USA). The instruments were placed in a magnetically shielded and sound attenuated room (Vacuumschmelze Gmbh & Co., KG, Hanau, Germany). In separate blocks, we ran 2000 epochs of stimulation of the index finger (D2), 700 epochs of stimulation of the thumb (D1), and 700 epochs of stimulation of the both fingers combined (D1,D2). The additional epochs of D2 stimulation were for investigating the effects of continual stimulation on neural response and, as such, are for an altogether different study. It is important to note though at this juncture that 700 epochs of stimulation produced a robust signal as indicated by the goodness of fits and correlations of the resulting equivalent current dipole sources (see Supplementary Materials). A single epoch lasted 575 ms and included a 120 ms prestimulus baseline. Data were acquired with a 1.0-Hz high-pass cutoff at a sampling rate of 290 Hz. Portions of the signal that were correlated to sensors placed far away from the head were likely to be noise and were subtracted out. Epochs remaining were used for analysis.
Analysis
Prior to analysis, epochs containing exaggerated moments such as eye blinks (peak-to-peak deflections >2pT) were discarded. The criteria caused us to discard from the NT and ASD groups respectively 8.2% ± 1.4% and 6.2% ± 1.7% of D1 stimulation epochs, 7.2% ± 1.8% and 9.1% ± 1.7% of D2 stimulation epochs, and 9.2% ± 0.6% and 13.1% ± 4.3% of D1,D2 stimulation epochs. Statistical tests on arcsine transformed percent values confirmed that the between-group percentage of epochs discarded was indistinguishable (D1: P = 0.376; D2: P = 0.513; D1,D2: P = 0.407). Remaining epochs were used for analysis.
Source modeling
MEG data from remaining epochs (e.g. D2data248×122) and the participant’s single sphere head model were combined to obtain a best fitting dipole model, utilizing the Fieldtrip toolbox in MATLAB. The best fitting dipole is the one that has the least squared error between modeled and actual data and is chosen from candidates between 30 and 100 ms following stimulus onset—this corresponds to 22 time points at a sampling rate of 290 Hz—for each condition (D1, D2, and D1,D2 stimulation) separately. Typically, the best fitting dipole is obtained from the signal in the time interval around the M80 component of the response. Dipole coordinates and orientations were computed for the best fitting dipole thus obtained. Next, for the dipole, a forward solution, termed a lead field (e.g. D2lf1×248), was computed which contains the evoked field distributions of all 248 MEG channels. The lead field was used to compute the time series of the response of the dipole source to the tactile stimulus. Relatively high goodness of fit values and moderately high correlations between actual data and dipole-modeled data were obtained (see Supplementary Materials) indicating that the acquired MEG signals and source localization were of reasonably high quality.
Computation of M40, M80
The M40 and M80 responses of the best fitting dipole obtained for each stimulus condition described above (see Source modeling) were obtained using the procedure described below. For the two somatosensory evoked field (SSEF) components, namely the M40 and the M80, the time point following stimulus onset at which the signal deviated from prestimulus baseline was obtained. For a given component, its half maximum value, defined as the signal amplitude halfway between those at the starting time point of the component and the component’s peak, was obtained. The time series was linearly interpolated by a factor of 1000 in order to obtain a more precise estimate of the location of the half maximum in the time series. The amplitude of the given component was defined as the area of the signal (in femtoTesla × milliseconds) under the waveform that lay between the locations of the half maxima on either side of the component peak (see Fig. S1 for a visual depiction of the application of the area measure). The area measure has been used extensively in electroencephalography studies [Hillyard, Squires, Bauer, & Lindsay, 1971; Picton & Hillyard, 1988; Viswanathan & Jansen, 2010] and is generally chosen to reduce the variability inherent in determining a single peak in a given component. Moreover, the area measure naturally utilizes more of the signal, i.e. averages over a wider range of time durations, than an amplitude peak measure, thereby providing a higher signal to noise ratio or SNR.
For each individual subject, the above set of calculations was repeated to obtain responses to the stimulation of D1 alone, D2 alone and D1, D2 combined. For each group (ASD, NT), the response to the combined simultaneous stimulation of fingers D1 and D2 (D1, D2) was plotted (ordinate) with respect to the sum of the responses to the individual stimulations of D1 and D2 (D1+D2). The paired finger/single finger response ratios for a given group (NT or ASD) were fitted with a straight line using a least squares criterion.
Statistics
SPSS was used for statistical analyses (SPSS Inc., Chicago, IL, USA). Student’s t-test (two-tailed) examined the validity of the following null hypotheses: (a) D1, D2 vs. D1+D2 slope for each group (ASD, NT) does not differ significantly from 1.0 (when MEG response to the combined stimulation of both fingers is equal to the arithmetic sum of the individual MEG responses); (b) slopes of the optimal least squares linear regressors of the ASD and NT groups do not differ; and (c) D1,D2/D1+D2 response ratios of the two groups (ASD, NT) do not significantly differ from one another.
Results
M40
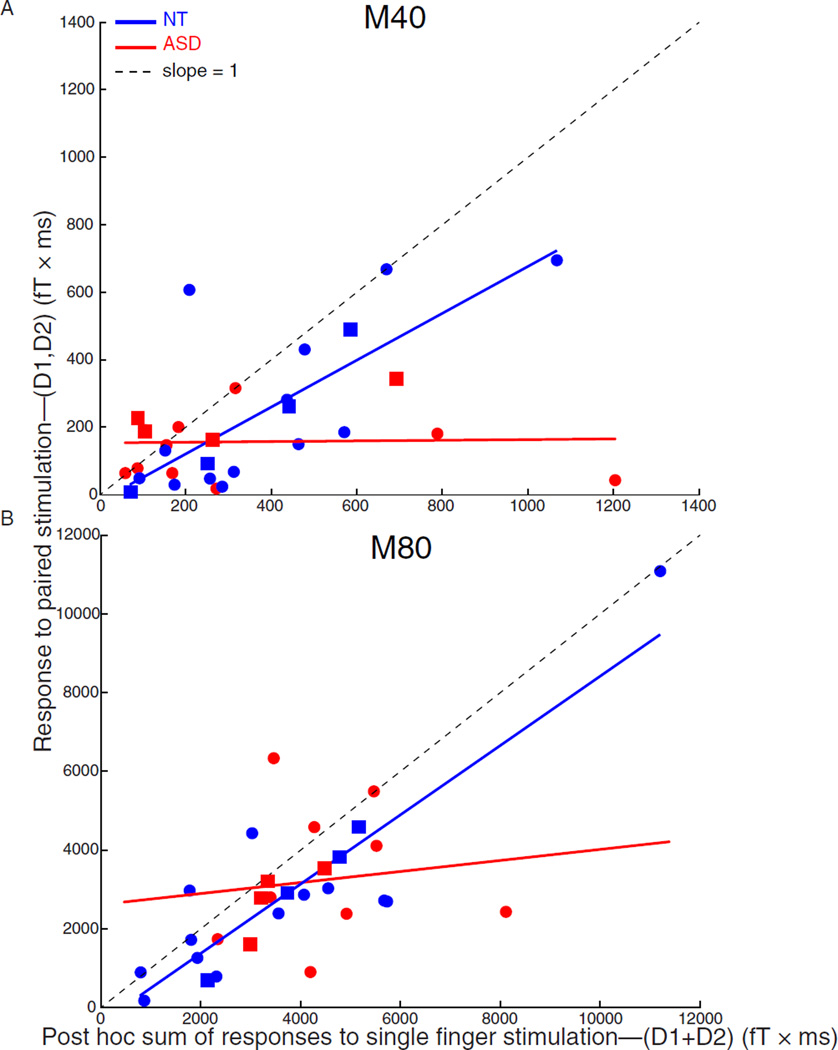
Figure 1A shows data for the short-latency M40 component of the MEG response analyzed using the source modeling approach—the responses to each finger and their combination are modeled separately—and plots the post hoc sum of the M40 responses to the individual stimulation of fingers D1 and D2 (D1+D2) vs. the M40 response to the combined simultaneous stimulation of both fingers (D1, D2), and the corresponding least squares straight line fits. The slope of the D1, D2 vs. D1+D2 responses of the ASD group was significantly less than 1.0 (slope = 0.01 ± 0.09 (standard deviation), t(11) = 11.12, P < 0.0001), indicating a sublinear response to paired tactile stimulation. In the NT group on the other hand, the response to the combined stimulation of both fingers was comparable to the sum of the responses to the tactile stimulation of each finger separately (slope = 0.69 ± 0.17, t(15) = 1.80, P = 0.09). The difference in slopes of the two groups was significant (t(26) = 3.58, P = 0.001). Thus, the short-latency M40 response to the paired stimulation of fingers D1 and D2 in the brains of individuals with ASD was significantly weaker than that in the brains of NT individuals. Results obtained using singular value decomposition (Fig. S2A) were in the same general direction: The slopes fitting the NT group M40 data were greater than the respective slopes fitting the ASD group data, and furthermore, reached significance (see Supplementary Materials).
Figure 1.
The response of the autism spectrum disorder (ASD) and typically developing (NT) groups to paired versus single finger stimulation and linear fits. The SSEF response to the combined, simultaneous stimulation of the thumb and index finger (D1, D2; ordinate) is plotted with respect to the post-hoc sum of the responses to the stimulation of each finger alone (D1+D2). Each point represents a single participant (ASD: red; NT: blue; males: circles; females: squares). The paired/individual response ratios were linearly fitted and the resulting slopes for the ASD and NT groups were compared to a slope of unity (= 1; dotted line) and to each other. (A) The short-latency M40 responses of the ASD and NT groups and linear fits are shown. (B) The mid-latency cortical M80 response of the ASD and NT groups and linear fits are shown. In both cases, the slopes of the two groups significantly differ from one another.
A different approach we employed was to compute the ratios (D1,D2/D1+D2) for each individual and then compare the average ratios for the ASD and NT groups. Using this approach, we found that the ratios for the ASD (0.87 ± 0.20) and NT (0.63 ± 0.16) groups were statistically indistinguishable (t(28) = 0.56, P = 0.582; t-test conducted on log transformed values—see [Fleming & Wallace, 1986]).
It is notable that the response to combined stimulation relative to the responses to the individual stimuli was studied, as this normalizes for differences in responsiveness to tactile stimulation, even if they exist. Further analysis demonstrated that no between-group differences in neural excitability to tactile stimulation do not exist anyway: M40 response magnitudes of the ASD and NT groups to stimulating D1 alone (t(28) = 1.26, P = 0.22) or D2 alone (t(28) = 0.86, P = 0.40) did not find significant differences. That is to say, the brains of individuals with ASD are not less responsive to tactile stimulation.
In summary, even though there are small differences in the details of the results obtained using different analytical approaches and measures, there is one overriding commonality that cuts across all: the short-latency M40 cortical response to paired tactile stimulation was not significantly stronger, and often, significantly weaker, in the autism group as compared with their control counterparts.
M80
Figure 1B shows data for the mid-latency M80 component of the MEG response analyzed using the source modeling approach. The regression slope of the D1, D2 vs. D1+D2 response ratio of the ASD group was significantly less than 1.0 (slope = 0.14 ± 0.31, t(11) = 2.75, P = 0.019), whereas the corresponding value for the NT group was indistinguishable from 1.0 (slope = 0.88 ± 0.12, t(15) = 1.02, P = 0.32). The difference in slope between the two groups was significant (t(26) = 2.22, P = 0.033; Fig. 1B). Thus, the normalized mid-latency M80 response to the combined stimulation of fingers D1 and D2 in the ASD group was smaller, not greater, than that in the NT group [as in the case of the M40, there was no difference observed between the two groups in the magnitude of the M80 to D1 (t(28) = 0.57, P = 0.57) or D2 (t(28) = 0.57, P = 0.57)] stimulation. Results obtained using other analytical approaches, i.e. the vector interaction ratio (Fig. S3) and singular value decomposition (Fig. S2B), were remarkably consistent: the cortical response to paired tactile stimulation was never stronger, and often, significantly weaker, in the brains of individuals with ASD than that in the brains of NT individuals. We also compared the response ratios (D1,D2/D1+D2) of the ASD and NT groups and again found no difference between the ASD (0.80 ± 0.11) and NT (0.77 ± 0.09) groups (t(28) = 0.14, P = 0.886).
Discussion
The present study was designed to provide a physiological test—a window into the brain in action as such—of the seminal hypothesis of reduced inhibition in the brains of individuals with autism. Using high-resolution, whole-head MEG, we compared the cortical response to the simultaneous tactile stimulation of the thumb and index finger in individuals with ASD vs. NT individuals. Because there is no tried and tested measure, we employed a variety of analysis methods. The different methods yielded findings that differed in details, but otherwise converged to the same basic result: The somatosensory cortex of the autism group did not respond more strongly to paired tactile stimulation than control.
Before discussing possible implications of our finding, a brief discussion of the relative merits of the various techniques used here bear mention. In particular, calculating the ratio of the responses to paired over single finger mechanical stimulation and comparing the average values between the two groups appears straightforward. However, it runs into one problem: the measure is particularly sensitive to outliers, as even a single small (or large) ratio will drag down (or up) the mean and affect the statistic with it. By comparison, the linear regression approach is somewhat more robust to outliers. In fact, the response to the combined stimulation of two adjacent fingers ought to be less, ipso facto, than the post hoc summed response to the stimulation of each finger alone—this would be reflected in a ratio less than one—but there are instances of where this reasonable assumption is violated for individual ASD and NT participants (see Fig. 1), and, in the case of the values obtained using the vector interaction method, the mean D1,D2/D1+D2 ratio of NT participants is greater than one (see Supplementary Materials). On the other hand, the slopes of the linear regressors obtained from all three analysis methods (dipole modeling, vector interaction, and singular value decomposition (SVD)) are all less than one, which is in line with expectation and with the premise of our study. Thus, while we report here the results of both measures—slopes of linear regressors and raw ratios—we believe that the former is a more robust and reliable method to addressing the main question driving the present study, and has been successfully used before in a previous study [Coskun, Loveland, Pearson, Papanicolaou, & Sheth, 2013]. Nonetheless, because single-cell physiology is the only way of incontrovertibly settling the methodological question but is not practical or ethical (in fairness, the regression method assumes a linear relationship, which is by no means proven either), our conclusions have to be tempered by the lack of a significant finding from the computation of ratios. Albeit, there are implications arising from these conclusions and they are discussed below.
Implications: Reduced Inhibition Hypothesis
As explained in the Introduction, a weaker physiological response to paired finger stimulation in the ASD group implies higher, not lower, level of inhibition in their somatosensory cortex. Our findings thus fail to support the claim of reduced inhibition in the brains of individuals with autism, and appears to go against the grain of past theoretical claims, anatomical and genetic studies, and behavioral findings [Casanova, Buxhoeveden, & Gomez, 2003; Fatemi et al., 2002; Hussman, 2001; Keita, Mottron, & Bertone, 2010; Rubenstein & Merzenich, 2003; Tannan, Holden, Zhang, Baranek, & Tommerdahl, 2008; Tommerdahl, Tannan, Cascio, Baranek, & Whitsel, 2007; Tommerdahl, Tannan, Holden, & Baranek, 2008].
Functional studies of inhibition in humans have been conducted in recent years using sensory and sensorimotor gating. Sensory gating, which is the filtering out of irrelevant or repeated stimuli by the brain, is believed to be a physiological measure of inhibition in the brain. The paired click paradigm and the amplitude of the P50 component of the auditory evoked potential to the second click is a noninvasive means of measuring sensory gating in the auditory cortex. Using this paradigm, Kemner, Oranje, Verbaten, and van Engeland (2002) found normal P50 gating in “high-functioning” children with autism, indicating no difference in the putative early, inhibitory processes related to P50 gating. An audiovisual gating paradigm on adult males with ASD similarly revealed no differences in suppression of the P50 component compared with controls (Magnee, Oranje, van Engeland, Kahn, & Kemner, 2009). A different group of investigators replicated the negative finding in high-functioning children with autism, but also found a small but significant reduction in P50 amplitude in children with autism having low IQs (Orekhova et al., 2008). Sensorimotor gating studies of autism, which examine motor response and engage corticostriatal circuits of the brain, showed a significant deficit in adult males with Asperger syndrome and autism (McAlonan et al., 2002; Perry, Minassian, Lopez, Maron, & Lincoln, 2007). Taken together, the studies on gating are a mixed bag in terms of what they inform us about inhibition levels in ASD: auditory and audiovisual gating show little difference in the level of inhibition in the auditory cortex of individuals with ASD, whereas sensorimotor gating studies imply reduced inhibition levels in corticostriatal brain circuits. Finally, the present study suggests, if anything, enhanced inhibition localized to the somatosensory cortex in the brains of individuals with autism.
The lack of a clear and consistent finding regarding inhibition levels across the brain leads us to speculate the existence of interspersed regions of increased and decreased inhibition throughout the brains of individuals with ASD. These islands of excitation and inhibition may even characterize the brains of individuals with autism. It has been noted before that an imbalance of excitation and inhibition in either direction is likely to lead to profound differences in network dynamics, neural synchrony, and even behavior (Gibson, Bartley, Hays, & Huber, 2008). It may be that global increase or decrease in inhibition across the entire brain can be offset by homeostatic mechanisms (e.g. a long-term decrease in neuronal excitability can counteract an overall decrease in inhibition), but a localized patchwork of increases and decreases of inhibition across the brain is more difficult to naturally offset. It remains to be seen if the putative patchy imbalance in inhibition is correlated with significant, uncompensated alterations in brain functioning and behavior observed in the autism syndrome.
Limitations and Future Directions
Here, we investigated cortical response to the stimulation of a pair of adjacent fingers. Investigations of brain responses to pairs of nonadjacent fingers (D1,D3/D1,D4/D1,D5) is likely to yield insight into the upper and lower limits respectively of inhibition in the somatosensory pathway of individuals with (and without) autism.
Increasing sample size will improve the generalizability of our findings. The exclusion of female participants, the Asperger’s syndrome participant, or the individuals with pervasive developmental disorder–not otherwise specified (PDD-NOS) did not qualitatively affect the basic findings (Supplementary Materials). It bears mention that distinctions between subdiagnoses within the spectrum, e.g. Asperger’s, will no longer hold under new diagnostic criteria that will be adopted next year.
It is also desirable that, when a measure of somatosensory activity is used, its relationship to tactile capabilities of the subjects is measured in tandem. Unfortunately, our study did not measure the sensory capabilities of the two groups. A correlational study measuring tactile discrimination in individuals with ASD and physiological assays of inhibition in somatosensory cortex is a logical next step.
Finally, the task-free, preattentive nature of our experiment holds promise for studying the brains of young children with autism as well as of individuals with autism with intellectual disability or impaired verbal skills—two populations that are not that commonly studied using these methods. Furthermore, the idea of probing the brain response to multiple stimuli is a simple one that is readily extendable to other sensory modalities and to stimuli with clear emotional and/or social content— domains that are at the core of an autism diagnosis.
Conclusions
Unlike studies to date that focus on structural, anatomical, or chemical assessments of brain circuitry and inhibition in autism, the present study performed a functional, physiological probe of inhibition levels in the brains of individuals with autism. We found that the level of inhibition in the somatosensory cortex of individuals with autism is either comparable to or greater than control levels. Proposed pharmacological treatments that globally enhance inhibition level in order to alleviate symptoms of the autism syndrome could enhance already high levels of inhibition and dramatically alter the processing of touch in individuals with autism, perhaps in negative ways. Analogous investigations of cortical response to the simultaneous stimulation of neighboring sites in the periphery of other sensory modalities can provide a powerful and noninvasive means of probing inhibition levels in different areas of the brain, and thereby help refine current theory and search for a neural biomarker of autism.
Supplementary Material
Acknowledgments
The research was supported by a grant from the National Alliance for Autism Research—Autism Speaks (BRS). MAC was supported in part by a Presidential fellowship from the University of Houston. KAL and DAP were supported by the NIH: P01 HD035471 (KAL) and R01 MH072263 (DAP).
Footnotes
Financial Disclosures
The authors report no competing interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Figure S1 A representative M80 area calculation is shown. A is the amplitude of the M80 component of the response from baseline (more specifically the first point of the post-stimulus response that is significantly different from baseline) to the peak of the response. A/2 represents half the value. The hatched area represents the full width at half maximum (FWHM) and the integral of the hatched area is the result of the calculation. The area of the M40 component is computed in a similar manner.
Figure S2 The response of the autism spectrum disorder (ASD) and neurotypical (NT) groups to paired versus single finger stimulation and linear fits. SSEF response ratios were computed using the virtual sensor approach (SVD). (A) The short-latency M40 responses of the ASD and NT groups and linear fits are shown. (B) The mid-latency cortical M80 response of the ASD and NT groups and linear fits are shown.
Figure S3 The response of the autism spectrum disorder (ASD) and neurotypical (NT) groups to paired versus single finger stimulation and linear fits. SSEF response ratios were computed using vector interaction. (A) The short-latency M40 responses of the ASD and NT groups and linear fits are shown. (B) The mid-latency cortical M80 response of the ASD and NT groups and linear fits are shown.
References
- Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: Implications for autisim. Neuroscientist. 2003;9:496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- Coskun MA, Loveland KA, Pearson DA, Papanicolaou AC, Sheth BR. Functional assays of local connectivity in the somatosensory cortex of individuals with autism. Autism Research. 2013;6:190–200. doi: 10.1002/aur.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, et al. The developmental neurobiology of autism spectrum disorder. Journal of Neuroscience. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biological Psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fleming PJ, Wallace JJ. How not to lie with statistics: The correct way to summarize benchmark results. Communications of the ACM. 1986;29:218–221. [Google Scholar]
- Friedman RM, Chen LM, Roe AW. Responses of areas 3b and 1 in anesthetized squirrel monkeys to single-and dual-site stimulation of the digits. Journal of Neurophysiology. 2008;100:3185–3196. doi: 10.1152/jn.90278.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Burke D, McKeon BB. Convergence in the somatosensory pathway between cutaneous afferents from the index and middle fingers in man. Experimental Brain Research. 1983;50:415–425. doi: 10.1007/BF00239208. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. Journal of Neurophysiology. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greek KA, Chowdhury SA, Rasmusson DD. Interactions between inputs from adjacent digits in somatosensory thalamus and cortex of the raccoon. Experimental Brain Research. 2003;151:364–371. doi: 10.1007/s00221-003-1493-6. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126(Pt 5):1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Squires KC, Bauer JW, Lindsay PH. Evoked potential correlates of auditory signal detection. Science. 1971;172:1357–1360. doi: 10.1126/science.172.3990.1357. [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Shima F, Tobimatsu S, Sun SJ, Kato M. The interaction of the somatosensory evoked potentials to simultaneous finger stimuli in the human central nervous system. A study using direct recordings. Electroencephalography and Clinical Neurophysiology. 1995;96:135–142. doi: 10.1016/0168-5597(94)00251-9. [DOI] [PubMed] [Google Scholar]
- Hussman JP. Suppressed GABAergic inhibition as a common factor in suspected etiologies of autism. Journal of Autism and Developmental Disorders. 2001;31:247–248. doi: 10.1023/a:1010715619091. [DOI] [PubMed] [Google Scholar]
- Keita L, Mottron L, Bertone A. Far visual acuity is unremarkable in autism: Do we need to focus on crowding. Autism Research. 2010;3:333–341. doi: 10.1002/aur.164. [DOI] [PubMed] [Google Scholar]
- Kemner C, Oranje B, Verbaten MN, van Engeland H. Normal P50 gating in children with autism. The Journal of Clinical Psychiatry. 2002;63:214–217. doi: 10.4088/jcp.v63n0307. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule (ados) manual. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Magnee MJ, Oranje B, van Engeland H, Kahn RS, Kemner C. Cross-sensory gating in schizophrenia and autism spectrum disorder: EEG evidence for impaired brain connectivity. Neuropsychologia. 2009;47:1728–1732. doi: 10.1016/j.neuropsychologia.2009.02.012. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, et al. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125(Pt 7):1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsycholologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofyev AO, Nygren G, Gillberg C, Elam M. Sensory gating in young children with autism: Relation to age IQ EEG gamma oscillations. Neuroscience Letters. 2008;434:218–223. doi: 10.1016/j.neulet.2008.01.066. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biological Psychiatry. 2007;61:482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Picton TW, Hillyard SA. Endogenous event-related potentials. In: Picton TW, editor. human event-related potentials eeg handbook (revised series) Vol. 3. Amsterdam: Elsevier; 1988. pp. 361–426. [Google Scholar]
- Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes, Brain and Behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism diagnostic interview—revised manual. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Tannan V, Holden JK, Zhang Z, Baranek GT, Tommerdahl MA. Perceptual metrics of individuals with autism provide evidence for disinhibition. Autism Research. 2008;1:223–230. doi: 10.1002/aur.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerdahl M, Tannan V, Cascio CJ, Baranek GT, Whitsel BL. Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Research. 2007;1154:116–123. doi: 10.1016/j.brainres.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerdahl M, Tannan V, Holden JK, Baranek GT. Absence of stimulus-driven synchronization effects on sensory perception in autism: Evidence for local under-connectivity. Behavioral and Brain Functions. 2008;4:19–27. doi: 10.1186/1744-9081-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan D, Jansen BH. The effect of stimulus expectancy on dishabituation of auditory evoked potentials. International Journal of Psychophysiology. 2010;78:251–256. doi: 10.1016/j.ijpsycho.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence Manual. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Zhu Z, Zumer JM, Lowenthal ME, Padberg J, Recanzone GH, et al. The relationship between magnetic and electrophysiological responses to complex tactile stimuli. BMC Neuroscience. 2009;10:4. doi: 10.1186/1471-2202-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.