SUMMARY
In the developing vertebrate nervous system, both neural crest and sensory neurons form at the boundary between non-neural ectoderm and the neural plate. From an in situ hybridization based expression analysis screen, we have identified a novel zebrafish mutation, narrowminded (nrd), which reduces the number of early neural crest cells and eliminates Rohon-Beard (RB) sensory neurons. Mosaic analysis has shown that the mutation acts cell autonomously suggesting that nrd is involved in either the reception or interpretation of signals at the lateral neural plate boundary. Characterization of the mutant phenotype indicates that nrd is required for a primary wave of neural crest cell formation during which progenitors generate both RB sensory neurons and neural crest cells. Moreover, the early deficit in neural crest cells in nrd homozygotes is compensated later in development. Thus, we propose that a later wave can compensate for the loss of early neural crest cells but, interestingly, not the RB sensory neurons. We discuss the implications of these findings for the possibility that RB sensory neurons and neural crest cells share a common evolutionary origin.
Keywords: narrowminded, Neural crest, Sensory neurons, Cell signalling, Zebrafish
INTRODUCTION
There are two cell types at the lateral edge of the neural plate which can be distinguished during early neural development in zebrafish: the neural crest and RB sensory neurons. Progenitors for both the neural crest and RB sensory neurons constitute the most lateral (future dorsal) of the three longitudinal stripes of primary neurons within the neural plate. The other two stripes of primary neurons include a medial (future ventral) domain, which gives rise to primary motor neurons, and an intermediate domain, which generates interneurons, including commissural neurons. RB neurons are characteristically large neurons which have central and peripheral projections that innervate the skin. In Xenopus and zebrafish, RB neurons are born at the end of gastrulation and upon differentiation transduce mechanosenory stimuli (response to touch) via the primary nervous system in the larvae (Lamborghini, 1980; Moody, 1989). RB neurons are subsequently lost in the adult and are thought to undergo programmed cell death (Lamborghini, 1987). Consistent with the notion that neural crest cells and RB neurons both arise from the lateral neural plate (Le Dourain, 1982; Selleck and Bronner-Fraser, 1995; Raible and Eisen, 1992), recent lineage analysis indicates that neural crest cells and RB neurons are intermingled (R. Cornell and J. Eisen, personal communication). Similarly, ablation of the neural crest in amphibian embryos also deletes the RB neurons from a given segment (Chibon, 1965). Thus, RB neurons and the neural crest may share a common precursor and/or require the same set of inductive cues.
During development of the zebrafish, Danio rerio, following the induction of neural tissue, the presumptive neural plate undergoes neurulation to form a neural keel prior to cavitation and subsequent formation of the neural tube (Schmitz et al., 1993; Papan and Campos-Ortega, 1994). Neural crest cells emerge from the dorsal surface of the neural keel and form a ‘crest’. They then migrate to give rise to a wide variety of derivatives, including sensory and sympathetic ganglia of the peripheral nervous system, pigment cells, and cartilage of the face (Le Dourain, 1982; Raible and Eisen, 1992). It has been well documented for zebrafish (Raible and Eisen, 1994) as well as other vertebrates (Weston and Butler, 1966; Serbedzija et al., 1989), that neural crest formation takes place in successive waves during which migration is spatially and temporally ordered. In the trunk, the earliest cells to migrate travel along the ventromedial pathway and give rise to sympathetic and sensory ganglia, while later cells that migrate along the dorsolateral pathway generate mainly pigment cells. In the chick embryo, experiments in which late migrating cells were placed into the early migratory pathway, revealed that these late migrating cells are restricted in their developmental potential (Artinger and Bronner-Fraser, 1992). However, if the early cells are ablated and the late migrating cells are transplanted and/or allowed to migrate, they are able to form all the derivatives of the early migrating cells, including sympathetic ganglia and cranial derivatives (Raible and Eisen, 1994, 1996; Baker et al., 1997). Thus, at least in the chick trunk, it appears that the late migrating cells can compensate for the absence of the early migrating cells.
Neural crest cells appear to be induced by interactions between the neural ectoderm and non-neural ectoderm. In amphibians, transplantation of presumptive neural tissue into belly ectoderm produces pigment at the boundaries (Moury and Jacobson, 1989, 1990). Furthermore, experiments in the chick have shown that Slug-expressing neural crest cells arise from the border of the neural plate and epidermis (Nieto and Wilkinson, 1994; Selleck and Bronner-Fraser, 1995; Dickinson et al., 1995). BMP-4/7, Wnts and FGF have all been implicated as diffusible factors involved in neural crest induction (Liem et al., 1995; Mayor et al., 1997; Neave et al., 1997). For example, when chick intermediate neural plates (stage 8–10) were co-cultured in the presence of BMP-4/7-expressing COS cells, multiple neural crest derivatives were produced. When BMP-4 is ectopically expressed in zebrafish the number of RB cells is increased (Neave et al., 1997). Dorsalized zebrafish mutants that lack functional BMP signaling show defects in neural crest cell patterning (Nguyen et al., 1998). These mutants provide a new perspective on the role of BMPs in neural crest determination, suggesting that the level of BMP activation is important. In contrast, very little is known about the molecules which act cell autonomously to allow cells to differentiate in response to these inducing factors. Our aim was to use the zebrafish system to identify genetic components of the regulatory cascades involved in neural crest induction and primary neurogenesis.
Zebrafish represents a powerful model system to determine the genetic mechanisms involved in pattern formation. Several systematic large scale genetic screens for patterning mutations have recently been completed (Driever et al., 1996; Haffter et al., 1996; and Development 123, 1–481). Although these screens have shed light on many processes during embryogenesis, only a small number of mutations were found to affect the development of the primary nervous system and early specification of the neural crest (but see Jiang et al., 1996; Kelsh et al., 1996). We designed an in situ hybridization screen, using DeltaB or HuC to detect primary neurons, to isolate mutations with defects in the formation of the primary neurons within the neural plate or in the neural crest (A. B. C., K. B. A. and W. D., unpublished). Similar screens have recently been used successfully to recover subtle but specific mutant phenotypes (for instance, Henion et al., 1996; Moens et al., 1996). From our screen, we isolated a number of mutants including narrowminded (nrd). Mutant nrd embryos lack RB neurons and have abnormal neural crest development. While mutant embryos never regenerate RB neurons, it is remarkable that all types of neural crest cell derivatives are present later in development, although they appear reduced in cell number. Consistent with the analysis of expression patterns by in situ hybridization, the phenotype is characterized by embryos having little if any response to touch, a reduction in the size of the trigeminal ganglion, reduced pigmentation, and fin degeneration. We conclude that the nrd mutation identifies a common genetic requirement for production of early neural crest and RB neurons. We further demonstrate that nrd functions cell autonomously and is required for the expression of genes (otx2 and dlx3) that normally mark the neural plate border. Thus, nrd appears to a gene involved in the response to signals responsible for inducing neural crest and RB neurons, and its identification may indicate a common origin of primary sensory neurons and neural crest.
MATERIALS AND METHODS
Isolation of mutation
Fish embryos were maintained according to Solnica-Krezel et al., (1994) and Westerfield (1994). nrd was isolated as part of a screen based on in situ hybridization that will be described in detail elsewhere (A. B. C., K. B. A. and W. D., unpublished). Briefly, AB strain males were mutagenized with ethyl nitrosourea (ENU), and following mutagenesis, they were given 4 weeks for mutagenized spermatogonial cells to mature and populate the sperm compartment. The G0 males were then bread to EK strain females (EK strain derived from fish obtained from Ekk Will Aquafarms, Gibsonton, FL; a strain similar to AB that was bread for several generations and screened for the absence of lethal mutations) and the F1 progeny was allowed to mature. Production of gynogenetic progeny was used to reveal mutant phenotypes directly in progeny of F1 females (Streisinger et al., 1981). In total, progeny from 1250 mutagenized females was analyzed. Embryos were maintained at 28.5°C until they had reached the 2–3 somite stage (12 hours) and were screened with a cocktail of in situ hybridization probes that detect specific neuronal populations within the neural plate: deltaB (Haddon et al., 1998) and ElavC/HuC (Good, 1995; Kim et al., 1997) were used to identify primary neurons and the area of premigratory neural crest formation; Krox20, for rhombomere 3 and 5, and her5 for the midbrain/hindbrain boundary (MHB). One mutation that specifically affected neuronal development was named narrowminded. The allele designation of the mutant allele investigated in this manuscript is nrdm805.
In situ hybridization
In situ hybridization was performed as described by Oxtoby and Jowett (1993); Schier et al. (1996). Briefly, embryos were hybridized with DIG-labeled probes in 50% formamide at 70°C overnight. In the case of snail1 and snail2, 65% formamide was used, as described by Thisse (1995). Antibodies to DIG conjugated with alkaline phosphatase (AP) were used and visualized with NBT/BCIP.
Antibody staining
Antibody staining was done as described by Solnica-Krezel and Driever (1994). Briefly, embryos were fixed for 1–2 hours in 4% paraformaldehyde at room temperature. After a rinse in PBT, embryos older than 24 hpf (hours post-fertilization) were treated in 10 mg/ml Proteinase K for 5 minutes, followed by rinsing in PBT. Blocking was done for 1–4 hours with 10% goat serum in PBT. Embryos were incubated in primary antibody (anti-α-tubulin and HNK-1; Sigma) overnight at 4°C. After primary incubation, the embryos were extensively rinsed for 6 hours to overnight in PBT. Embryos were incubated with secondary antibody overnight at 4°C. Fluoresceinconjugated secondary antibodies were rinsed and postfixed in 4% paraformaldehyde. HRP-conjugated antibodies were rinsed and visualized with DAB using an ABC-peroxidase kit (Vector Laboratories).
Mosaic analysis
Genetic mosaics were generated as described by Schier et al. (1997). Donor embryos from heterozygote nrd fish were injected with 5% lysinated rhodomine dextran (LRD; Mr 10×103, Molecular Probes, Eugene, OR) in 200 mM KCl at the 1–8 cell stage. Embryos were allowed to develop for 4–5 hours until mid-blastula stage. At this time, 20–40 donor cells were removed from the donor and placed into an unlabelled host of similar age. Embryos were then allowed to develop until 24 hpf, fixed in 4% paraformaldehyde and screened with specific markers and by morphology for the presence of RB neurons. RB neurons are identified as large dorsally located neurons which distinctly express the HNK-1 eptiope (anti-HNK-1 from Sigma, C-0678).
RESULTS
nrd mutants lack lateral primary neurons
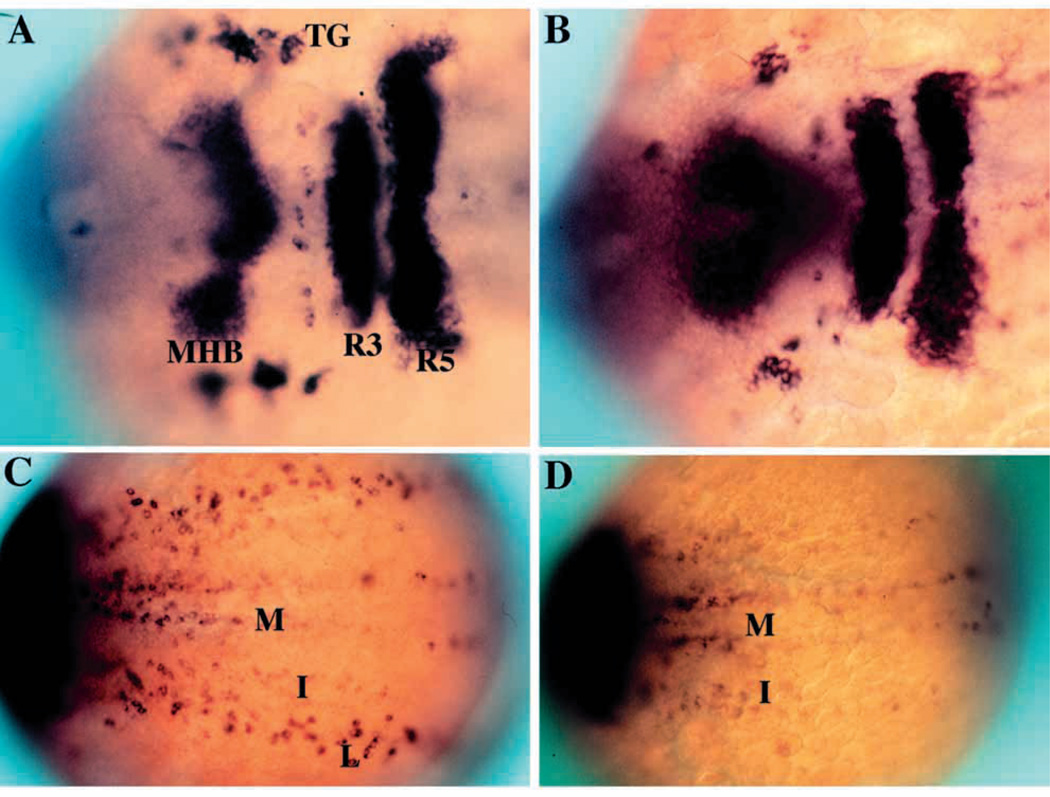
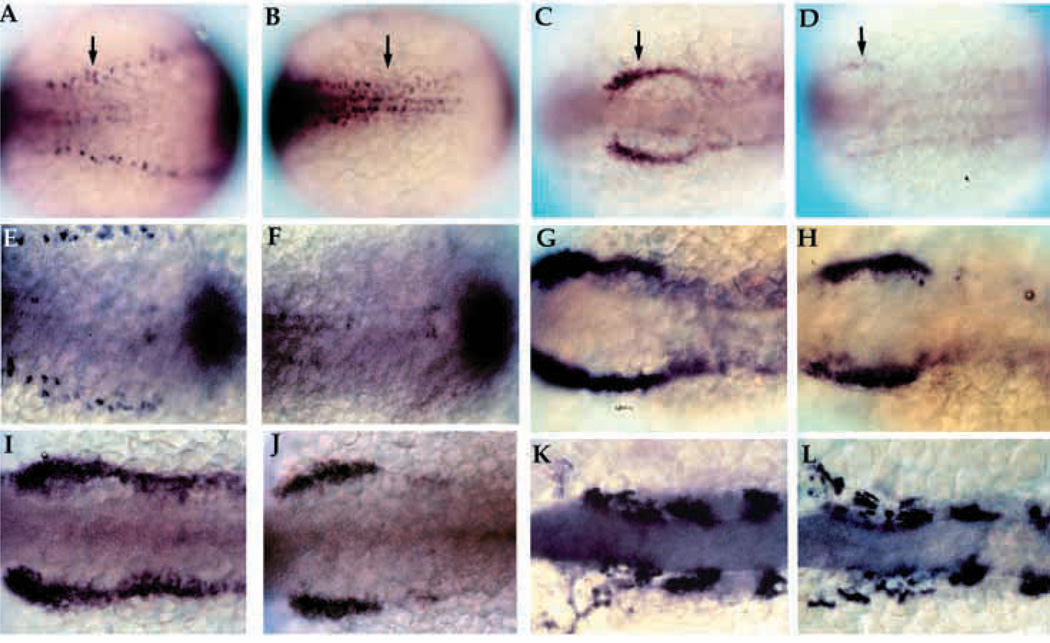
nrd was isolated during a mutagenesis screen in which detection of mutant phenotypes was performed using in situ hybridization to visualize changes in gene expression patterns. We used the expression patterns of HuC, Krox20, and her-5 as markers to detect changes in the neurogenesis and neural crest pattern (Fig. 1). Early in development (2–3 somite stage), HuC demarcates the three longitudinal stripes of primary neurons within the neural plate as well as neurons within the trigeminal ganglion (Fig. 1A,C). Probes for Krox20 identify rhombomeres 3 and 5 while probes for her5 delineate the midbrain-hindbrain boundary (Fig. 1A,C). A cocktail of these three probes identifies alterations in the pattern of neurogenesis as well as in anteroposterior patterning. nrd was identified from the screen since affected embryos lack the lateral stripe of HuC expressing primary neurons (Fig. 1B,D). The lateral stripe of neurons corresponds to the lateral edge of the neural plate where both RB neurons and neural crest form, and is consistent with a close spatial relationship between them. As judged from the late morphological phenotype (Fig. 3), in which the RB neurons are completely absent, all types of neural crest derivatives are present, but reduced in cell number. The intermediate and medial stripes of primary neurons, as well as Krox20 and her5 expression, appear normal in nrd mutant embryos, indicating that nrd specifically affects the lateral domain in the neural plate and does not affect the formation of the midbrain-hindbrain boundary or rhombomere 3 and 5.
Fig. 1.
Phenotypic basis for isolation of nrd during the genetic screen. Dorsal views of 2–3 somite stage haploid embryos (anterior to the right) upon analysis by in situ hybridization utilizing a combination of RNA probes: her5 (to visualize the midbrain-hindbrain boundary), Krox20 (to visualize rhombomeres 3 and 5) and HuC (to visualize trigeminal ganglia (TG) and medial (M), intermediate (I) and lateral (L) domains of primary neurons). Wild type (A,C) and nrd (B,D) embryos with view of anterior (A,B) and trunk neural plate (C,D) region. nrd has normal anterior-posterior patterning but lacks the most lateral stripe of primary neurons.
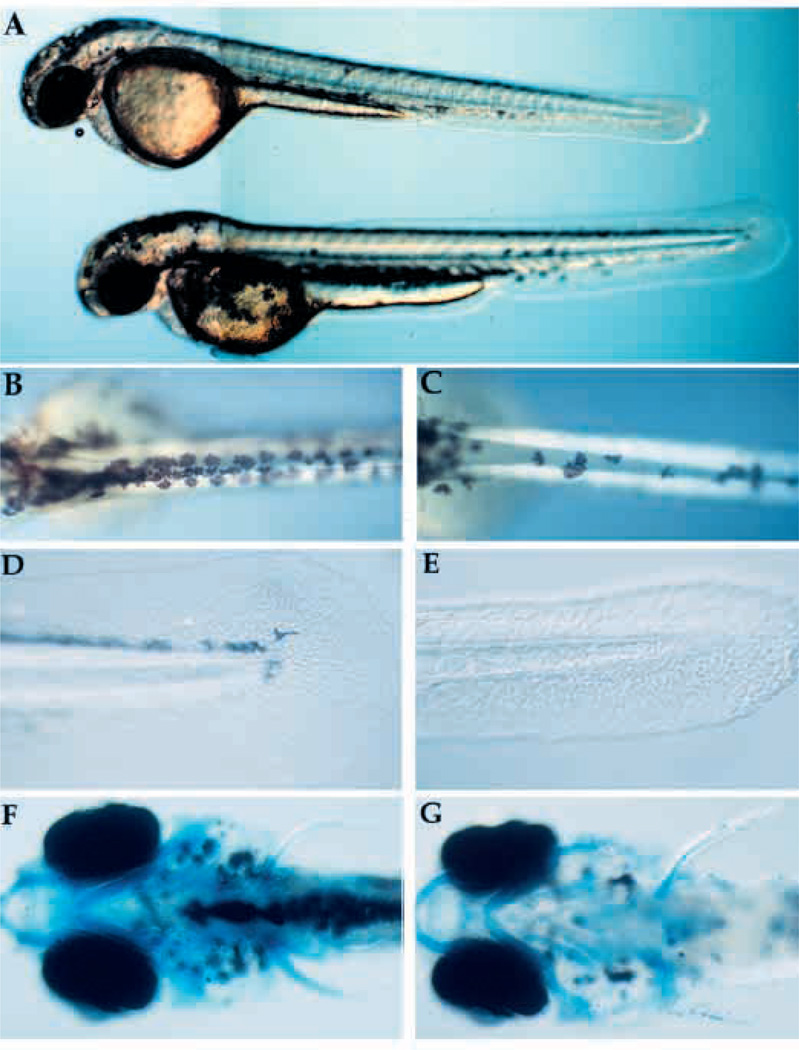
Fig. 3.
Visible phenotype of nrd and defects in formation of neural crest derivatives. (A) Whole live nrd (top) and wild-type sibling (bottom) at 48 hpf (lateral view). Wild-type (B,D,F) and nrd (C,E,G) siblings (10×). nrd mutant larvae have a smaller number of pigment cells (B,C: dorsal view), reduced fin mesenchyme (D,E: lateral view) and a normal pattern of cartilage (F,G: ventral view, visualized in whole mount by alcian blue stain. The slight reduction of density of the stain is not observed in serial sections).
Loss of HuC expression in the lateral domain corresponds to loss of Rohon-Beard neurons
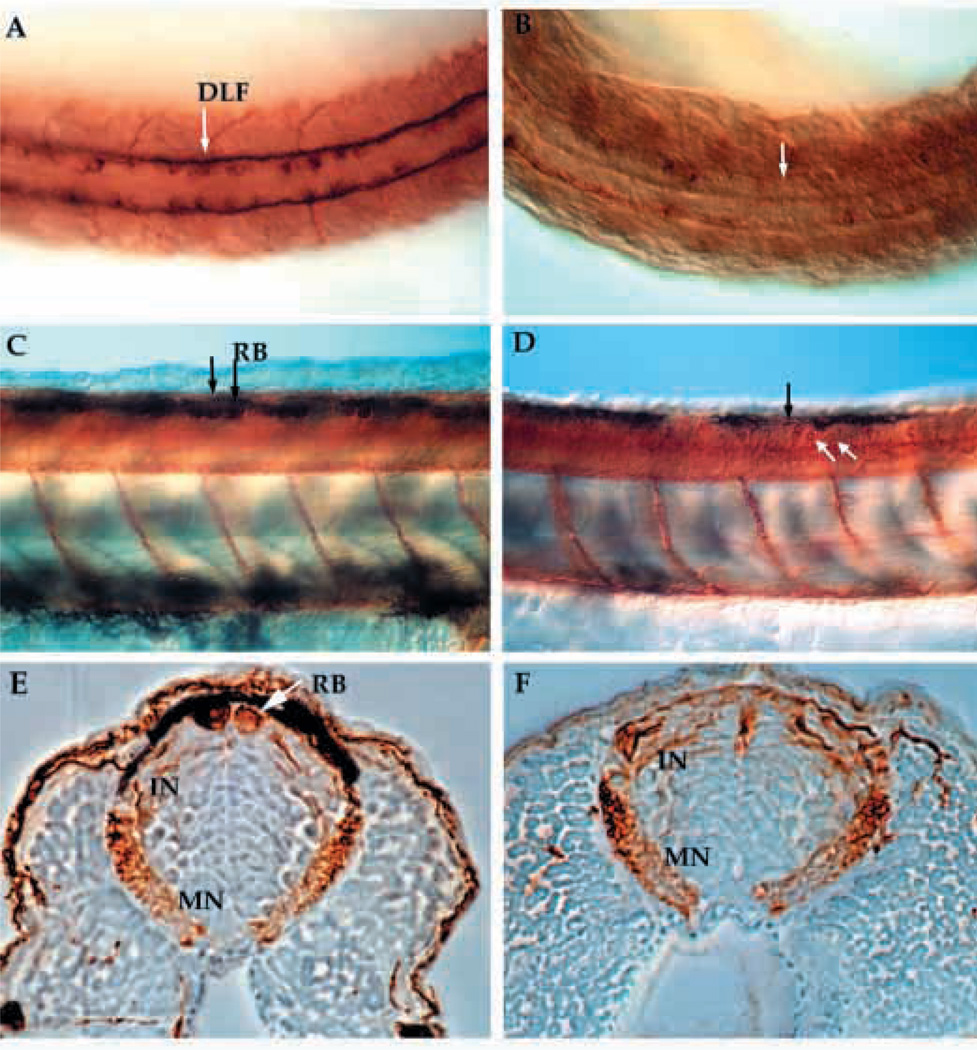
RB neurons are large sensory neurons that form in groups of 3 to 5 per segment as part of the zebrafish primary nervous system (Metcalfe et al., 1990). In nrd, these large neurons are almost completely absent whereas commissural neurons, an interneuron which sends projections ventrally across the floor plate, as shown by anti-acetylated α -tubulin staining at 24 hpf, appear to be present (Fig. 2A,B). RB neurons, along with some specific commissural neurons, send their axons along the dorsal lateral fasciculas (DLF), which is also severely reduced in size (Fig. 2A,B). Since there are less neurons and neural crest cells within the lateral domain, we examined whether this was accompanied by an expansion of other types of neurons within the neural plate, in particular the adjacent commissural neurons. Commissural neuron cell bodies and axons appear to be in the correct location (Table 1), but are reduced slightly in number when compared to wild type. At 48 hpf, RB neurons are clearly absent, whereas commissural neurons are present (Fig. 2C,D; white arrow in D points to commissural neurons; transverse section 2E,F). Occasionally, we observe a large RB-like neuron in the dorsal spinal cord, although this is a rare event. The lack of RB neurons likely explains the absence of a touch response when embryos are prodded on the flank. The head touch response appears normal, perhaps due to other sensory input via the trigeminal ganglia. The small number of interneurons that project along the DLF, which are known as the DL1 (Kuwada, 1986), are still present in nrd.
Fig. 2.
Anti-acetylated α-tubulin immunostained whole-mount embryos at 24 hpf (A,B) and 48 hpf (C–F) reveal the neuronal pattern in nrd. Dorsal view of the neuronal pattern of a 24 hpf wild-type embryo (A) shows large RB neurons (arrow; large brown cells) as well as a distinct DLF (two parallel tracts of axons). In nrd (B), the embryos have very little dorsal expression of α-tubulin, except in a few scattered commissural neurons (dark brown stained cells). At 48 hpf, the differentiation of many neuronal cell types including large RB neurons can be seen in wild-type embryos (C, arrows and lateral view). In nrd (D), other neurons such as commissural neurons appear to develop normally (white arrows), but RB neurons do not form (black arrow point to area where RBs should form). (E,F) Transverse sections of embryos in C and D (embryos were embedded in plastic and sectioned at a thickness of 3 µm). In wild-type embryos, RBs are large neurons at the dorsal most aspect of the neural tube (E), while nrd embryos lack RBs. RB, Rohon Beard cell; DLF, dorsal longitudinal fascicle; In, interneuron; MN, motorneuron.
Table 1.
Presence of commissural neurons and dorsal root ganglion neurons in wild-type and narrowminded zebrafish
| Number of commissural neurons* |
Number of DRG neurons‡ |
|
|---|---|---|
| Wild type | average=13.25 | average=2.04 |
| range=±1 n=4 | range=±0–1 n=10 | |
| narrowminded | average=10.25 | average=1.22 |
| range=±1–2 n=4 | range=±0–1 n=8 |
Commissural neurons were counted over 25 somite lengths at 24 hpf.
DRGs were counted over 18 somite lengths at 72 hpf.
The (n) refers to the number of fish with 10–18 ganglia per fish counted. The ganglia generally contained 2–3 cell in the wild type and 1–2 in the mutant, revealing a loss of 1 neuron per ganglia.
Reduction of neural crest cell derivatives
Both RB neurons and neural crest cells form in the lateral stripe of primary neurogenesis at the edge of neural plate. Neural crest cells segregate from the boundary of the neural plate and epidermis early in development and migrate extensively from the dorsal aspect of the neural keel to form a wide variety of derivatives. In nrd mutant embryos, at 48 hours of development all the types of neural crest derivatives are present, but most are reduced. Some neural crest derivatives like the pigment cells and fin mesenchyme can be identified in live embryos. A photomicrograph of wild-type and nrd embryos at 48 hpf shows that pigment cells are reduced in number and tend not to spread over the yolk, as they do in wild-type embryos (Fig. 3A–C). The fin mesenchyme was also affected in homozygous mutant embryos. This was quite striking when looking at the embryo at 48 hours of development, especially in the tail fin (Fig. 3D,E). We ascribe the visible defect in the fin to a loss of mesenchyme derived from the neural crest. No morphological abnormalities were observed, however, in the cartilage of the jaw and branchial region, which were also derived from the neural crest (Fig. 3F,G).
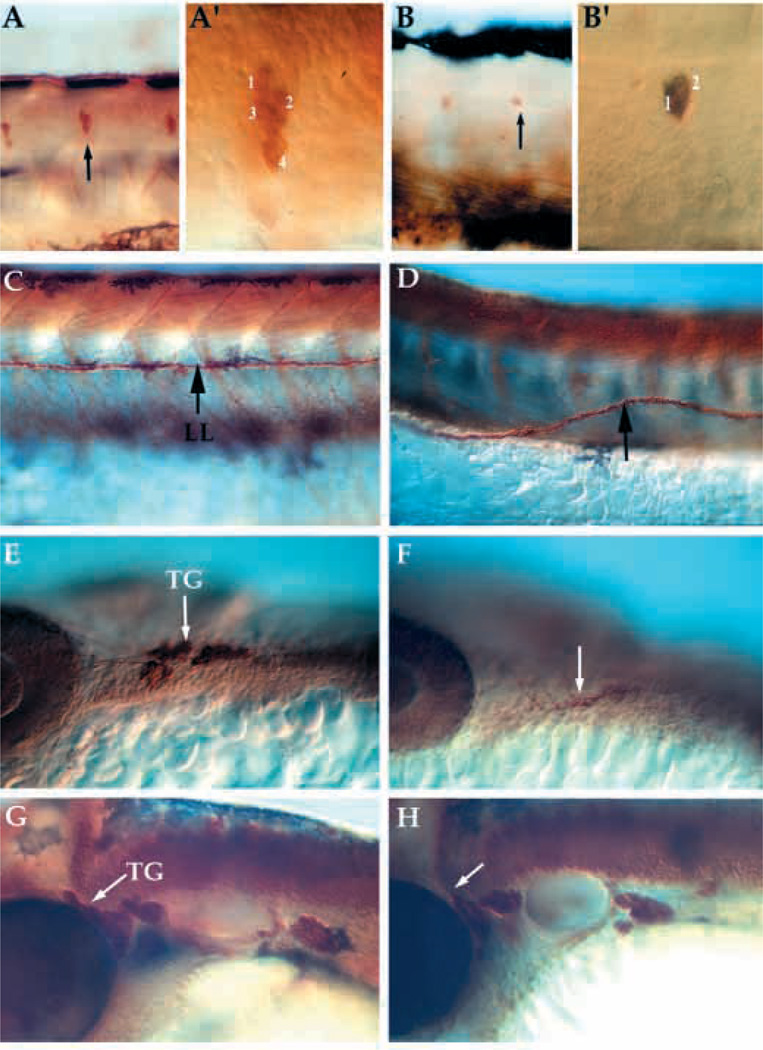
Dorsal root ganglia (DRGs) are neural crest-derived sensory neurons that differentiate later than the RB neurons and are part of the peripheral nervous system. Their distribution was compared using anti-HuC antibodies in wild-type and nrd mutant embryos. DRGs are present in nrd mutant embryos, but fewer cells per ganglia were detected (Fig. 4A,B; higher magnification in A′ and B′). The number of cells in each ganglion was reduced by about 1 cell per ganglion at 48 hpf (Table 1; number of cells/ganglion). The ganglia seemed to form in the correct location, but were slightly misaligned relative to the horizontal myoseptum (see Fig. 4B). The neuromasts were similarly misaligned (not shown) and the lateral line was often not straight, curving dorsally and ventrally (Fig. 4C,D). Neural crest contribute to the dorsal lobe of the trigeminal ganglia, while the ventral-most lobe is derived from ectodermal placodes, as described in chick embryos by Noden (1991). The trigeminal ganglion was reduced in size at 24 hours (Fig. 4E,F), but by 48 hours appeared to be almost the same size as in wild type (Fig. 4G,H). This suggests some compensation from either the placode-derived portion of the ganglion or by contributions from the later migrating neural crest.
Fig. 4.
Lateral views of whole mount embryos immunostained for expression of acetylated α-tubulin and anti-HuC in neural crest derivatives at 24–72 hpf. Pattern of DRG, lateral line pathfinding, trigeminal ganglia formation in wild-type (A,C, trunk; E,G, head) and nrd mutant embryos (B,D, trunk; F,K, head). nrd embryos have a reduction in the number of neurons per ganglion in the DRG at 72 hpf (B; see also Table 1) and the neuromasts (not shown) (A′,B′) High magnification images of the ganglion (arrows in A and B) to show the reduction in the number of cells per ganglion. (C,D) The lateral line (LL) pathfinding is misaligned (arrow) at 72 hpf. Within the trigeminal ganglia (TG), cells derive from the neural crest as well as ectodermal placode. At 24 hpf (E,F), mutant embryos (F) show a reduction in the dorsal ganglia corresponding to the neural crest derived portion (arrows). But by 48 hpf (G,H), the trigeminal ganglia in the mutant (H) is close to the normal size, suggesting that the placodal or late migrating neural crest population may be compensating for the loss of the neural crest. DRG, dorsal root ganglia.
Early pattern of primary neurons and neural crest
To begin to understand the effect of nrd on early development, we examined the expression of genes that demarcate the nascent neural plate-epidermal boundary. At the 2-somite stage, there was a marked loss of expression of markers for the lateral longitudinal stripes of primary neurons such as HuC and deltaB (Fig. 5A,B). Expression of markers specific for neural crest, such as snail2 (Fig. 5C,D; Thisse et al., 1995) and Fkd6 (Fig. 5I,J; Odenthal et al., 1998), were reduced but not completely absent. In contrast, the expression of BMP-4 did not differ markedly from that in wild-type embryos at the 2–3 somite stage, as shown in Fig. 5E and F by double expression staining for HuC (to identify genotype) and BMP-4. snail2 expression at the 2 somite stage was severely reduced, suggesting a reduction in differentiating neural crest cells at this stage. Observations made at slightly later stages (between 4–10 somites) with the neural crest-specific markers, snail2 and dlx2, showed that the neural crest population seems to recover. At the 4–5 somite stage, snail2 appeared to be re-expressed (Fig. 5G,H) and by the 10 somite stage, the expression of dlx2 was only slightly reduced (Fig. 5K,L). Although nrd appeared to affect neural crest cells and RB progenitors at the border of the neural plate, the expression of the dorsal neural tube markers pax3 and pax7 appeared normal (data not shown). Thus, we conclude that nrd does not perturb the overall dorsoventral pattern of the neural tube.
Fig. 5.
Early development of primary neurons and neural crest in nrd. Whole-mount in situ hybridization of 2–3 somite stage zebrafish embryos with HuC (A,B; 20×), snail2 at the 2 somite stage (C,D; 20×), BMP-4 (E,F; 32×), snail2 at the 4–5 somite stage (G,H; 32×), fkd6 at the 2–3 somite stage (I,J; 32×), and dlx2 at the 10 somite stage (K,L; 32×). All images show dorsal views of embryos orientated anterior to the left. Wildtype (A,C,E,G,I,K) and nrd mutant (B,D,F,H,J,L) embryos. nrd embryos shows a lack of HuC expression in the lateral most stripe of primary neurons, corresponding to the lateral edge of the neural plate (arrows in A,B). snail2 expression indicates a severe reduction in the neural crest population in the mutant embryos (arrows in C,D). This is also consistant with effects on the neural crest expression domain of fkd6, which appears reduced in nrd although less severe than snail2 (I,J). BMP-4 expression appears normal in nrd (compare E and F; double stain with HuC to identify mutant embryos). Later in development, at the 4–5 somite stage, snail2 expression appears to be upregulated again (compare nrd, in H, to wild type, in G) and is almost indistinguishable from wild type at the 10 somite stage, shown here with dlx2 expression (K,L).
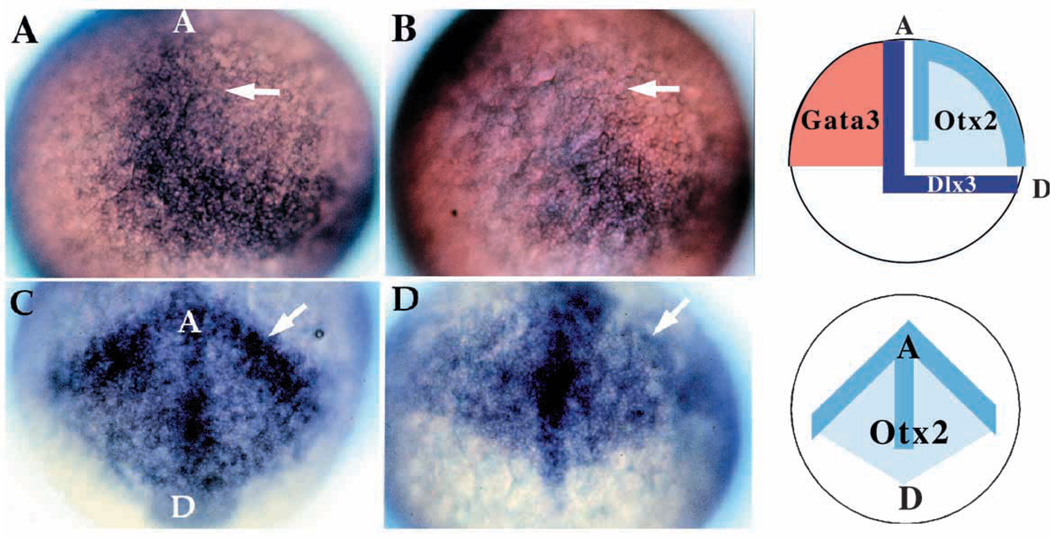
The above results imply that nrd is important in the establishment of the lateral boundary of the neural plate and/or the induction of cell specification in this region. Thus, we examined early markers of boundary formation. Interestingly, we observed a reduction in dlx3 (Akimenko et al., 1994) and otx2 (Li et al., 1994; Fig. 6), suggesting that nrd acts as early as gastrulation. At 90% epiboly, dlx3 expression, which normally marks the border between the neural plate and nonneural ectoderm, probably including the premigratory neural crest and placodal populations, was restricted to the posteriordorsal most region in nrd mutants, while the staining within the border of the neural plate and non-neural ectoderm was absent (Fig. 6A,B). otx2 is normally expressed in the presumptive forebrain, with stronger expression along the boundaries of the neural plate at this stage. otx2 remained expressed in the neural plate of nrd, but the stronger border expression appeared missing (Fig. 6C,D). Non-neural ectoderm was unaffected, as assayed for by GATA3 expression (data not shown). Therefore, we conclude that nrd is necessary to establish the neural plate-epidermal boundary.
Fig. 6.
Patterning abnormalities in the early neural plate. Expression of genes involved in patterning the early neural plate visualized by whole-mount in situ hybridization for dlx3 (A,B; 20× ) and otx2 (C,D; 20× ) at late gastrulation stages (90% epiboly). Wild-type (A) and mutant (B) embryos indicate the lack of the anterior-medial domain of dlx3 (arrow) in nrd. In these embryos, anterior is to the top, dorsal to the right. (C,D) Anterior view of otx2 expression in wild type (C) and nrd (D): at the border region of the neural plate otx2 expression level is reduced (arrow) but appears to extend in a pattern of normal shape in mutant embryos. In C and D, the anterior edge of the neural plate is to the top, with a dorsal and posterior neural plate toward the bottom. Schematic diagrams illustrate the normal expression domains of dlx3 in the neural crest and placodal domain, between the neural and non-neural ectoderm, otx2 in the neural plate, and GATA3 in the non-neural ectoderm in wild-type embryos. GATA3 expression is not affected in nrd embryos (not shown).
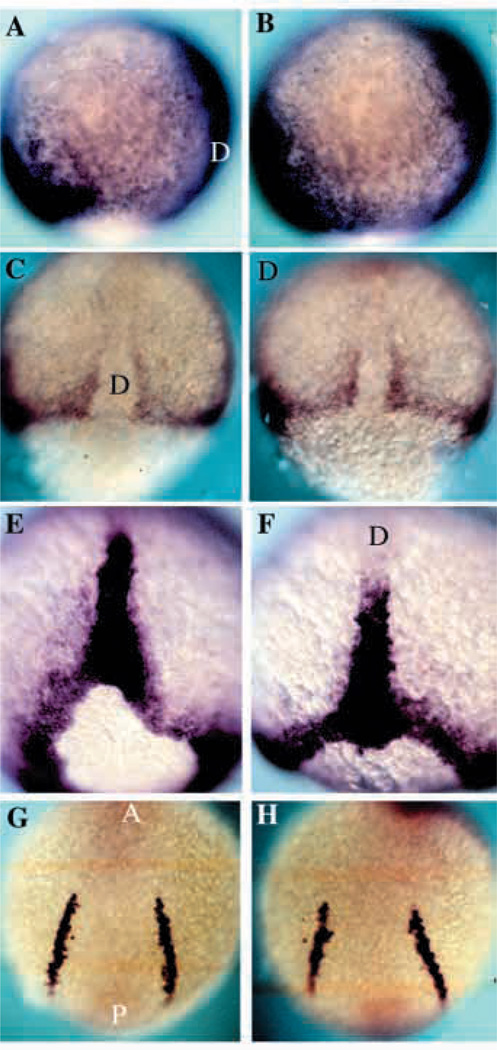
Dorsoventral patterning in nrd
The overall dorsoventral and anteroposterior patterning of the embryo was overtly normal by external criteria. In order to confirm a lack of an effect on early pattern, we examined several mesodermal markers in the later stages of gastrulation (90% epiboly) to the 5 somite stage. We examined the expression genes which are expressed ventrally, intermediately, and dorsally in the zebrafish gastrula. Zebrafish BMP-4 (Nikaido et al., 1997) in the ventral mesoderm, snail1 (Thisse et al., 1993) in the paraxial mesoderm and Brachyury (Schulte-Merker et al., 1994) in the dorsalmost mesoderm and the notochord, all showed normal patterns of expression in nrd (Fig. 7B,D,F) as compared to wild type (Fig. 7A,C,E). Further, we observed a normal pattern of expression of genes expressed in the derivatives of the mesoderm, including SCL (Fig. 7G,H; Gering et al., 1998) in the blood, paxb in blood and pronepheric region (not shown), and myoD in the somitic mesoderm (not shown). The expression of SCL is directly below, in the mesoderm layer, to the neural crest forming region. This suggests that nrd does not perturb dorsoventral pattern and that defects in nrd are not secondary to alterations in dorsovental pattern.
Fig. 7.
DV patterning is normal in nrd. Expression analysis of the mesodermal genes BMP-4, Snail1, Brachyury and SCL in wild-type (A,C,E,G) and nrd (B,D,F,H) embryos at 80–90% epiboly to 5 somite stage. (A,B) BMP-4 is expressed on the ventral side of the gastrula and the leading edge of the invagingating tissue on the dorsal side in both wild-type and nrd embryos. Anterior top and dorsal to the right. (C,D) Dorsal view of the expression of snail1 as seen by in situ hybridization shows the normal paraxial mesoderm in nrd (anterior to the top). (E,F) Zebrafish Brachyury is expressed in a ring around the blastopore and in the forming notochord at the end of gastrulation. nrd embryos show a normal pattern of expression of Brachyury (F) as compared to wild type (E). Dorsal view, anterior to the top in both E and F. (G,H) Expression of SCl in the blood precursors at the 5 somite stage shows normal development of a mesoderm derivative, blood, at a slightly later stage in an nrd (H) embryo compared to a wild type (G). This region is just ventral to the neural crest forming region. (Note: nrd embryos presented in this figure were from nrd mutant clutches in which homozygous were not distinguishable from heterozygous siblings) D, dorsal; A, anterior; P, posterior.
nrd is required cell autonomously to make RB neurons
Creating genetic mosaics by combining mutant with nonmutant tissue allows predictions to be made about how a mutant zebrafish gene acts in a signaling pathway. If, for instance, a mutated locus were to encode a diffusible signal, the phenotype might be rescued when mutant cells were transplanted into a wild-type environment. Failure to rescue would indicate that the gene acts cell autonomously. A cell autonomous mutation, however, would not prevent wild-type cells from differentiating appropriately in a mutant environment. Mosaic analysis was performed within mutant clutches of nrd (Table 2). Lysinated rhodamine dextran (LRD)-labeled cells from wild-type embryos were transplanted into nrd a mutant embryos and RB differentiation was observed. RB neurons were recognized by their characteristic projection pattern and dorsal position in the neural tube, as visualized with the neuronal marker, anti-HNK-1 antibody. An example is shown in Fig. 8A,B, where two wild-type cells (red) placed within a mutant environment formed RB neurons and expressed HNK-1 (green; cells appear in yellow due to the overlap of both LRD and anti HNK-1 immunofluorescence). In contrast, mutant nrd cells placed into a wild-type environment failed to differentiate into RB neurons. Although mutant cells were seen in dorsal locations characteristic of RB neurons, the cells never expressed HNK-1 (Fig. 8D–F). Thus, nrd is required cell autonomously for RB neuron differentiation. We next asked if the mutant cells were able to form other types of neurons in the mutant environment. Mutant cells transplanted into a mutant environment formed commissural neurons (Fig. 8G,H) with proper axonal pathfinding (see axon projecting ventrally across the floor plate [out of the plane of focus] to the other side of neural tube). This is consistent with the presence of commissural neurons within the neural tube in the absence of RB neurons in nrd embryos.
Table 2.
Cell-autonomous action of nrd. Cell transplantation experiments providing donor clones in the dorsal spinal cord
| Transplantation donor into recipient |
Number of recipient embryos with donor derived RBs |
Total number of recipients with donor derived cells in the dorsal spinal cord* |
|---|---|---|
| Mutant into wildtype | 0 | 6 |
| Wildtype into mutant | 3 | 3 |
| Mutant into mutant | 0 | 1 |
Note: the total number of surviving transplants analyzed to obtain these numbers was 286. It is a rare event to find cells within the dorsal spinal cord.
Fig. 8.
Cell-autonomous action of nrd as revealed by mosaic analysis. Results of cell transplantation experiments between wild-type and nrd embryos viewed by confocal microscopy (A,B,D-H; all images are anterior to the left, dorsal up. Some images were flipped horizontal to maintain consistant antero-posterior orientation.). Low (A) and high (B) magnification of wild-type cells (LRD-labeled red) transplanted into a mutant environment that are able to differentiate into RB neurons (as revealed by HNK-1 antibody staining, green; where HNK-1 epitope expression is present in the same cell that contains the lineage label, the cells appear yellow). (C) Nomarski image of a similar live embryo to illustrate the location of the labelled cells in the dorsal neural tube (square indicates approximate location of confocal optical section for all embryos shown). Mutant cells (D–F, red) transplanted into a wild-type environment have never been observed to be able to form RB neurons although they can form other neuronal cell types in or near the RB forming domain of the neural cord. (E,F) Higher magnification views of two different optical planes showing that mutant cells (red) located within the wild-type domain of HNK-1-expressing cells are able to form commissural neurons (see axonal processes in F). Low and high magnification view of a mutant cell (G,H; red) transplanted into a mutant environment that forms commissural neurons with correct pathfinding ability. This axon can be seen crossing the floorplate on the contralateral side and projecting along the DLF D, dorsal spinal cord; FP, floor plate; SC, spinal cord; N, notochord.
DISCUSSION
Primary neurons in the neural plate of the zebrafish embryo form in three longitudinal domains: medial, intermediate and lateral. The narrowmindedm805 (nrd) mutation was isolated in a genetic screen aimed specifically at isolating zebrafish mutations affecting the pattern of primary neurons based on the absence of neuronal marker gene expression in the lateral domain of the neural plate. Homozygous nrd mutant embryos have defects in two cell types previously thought to be under separate control. First, nrd mutants lack primary neurons in the lateral domain and this later corresponds to the absence of RB sensory neurons. Second, there is a severe reduction in the expression of early neural crest marker genes such as snail2 which correlates with later defects in all derivatives of the neural crest. Neural crest derivatives such as the dorsal root ganglia (DRG), trigeminal ganglia (TG), pigment cells, and fin mesenchyme are all present but cell numbers are typically reduced. The mutation has been shown to act early in development, with a loss of expression of the neural crest marker dlx3 at the neural plate border as early as 90% epiboly. Comparison of snail2 expression in nrd mutants at two stages of development shows that the dramatic reduction of snail2 expression seen at the 2 somite stage is no longer apparent at the 10 somite stage, which suggests that the embryo is capable of compensating for the loss of some early neural crest cells. The absence of RB neurons is permanent, since, unlike the neural crest cells, RB neurons do not regenerate later in development.
Defects in nrd mutants are not secondary to defects in early dorsoventral patterning
BMP signaling plays an important role in determining both neural crest cell and RB neuron fate. It is possible that defects in the BMP pathway are responsible for defects in these tissues. A gradient of BMP activity in the ectoderm likely contributes to the formation of the boundary between the neural plate and epidermis; regions of the ectoderm associated with a high level of BMP signaling adopt an epidermal fate while regions with a low level of BMP activity adopt a neural fate. The neural crest and the RB neurons are generated adjacent to this boundary in a domain that reflects an intermediate level of BMP activity (Nguyen et al., 1998) and/or because of local interactions at the boundary, possibly mediated by BMPs, FGFs, and/or Wnts (Mayor et al., 1995, 1997; LaBonne and Bronner-Fraser, 1998) or other signals. somitabun and snailhouse mutant embryos, which have reduced BMP signaling, are characterized by a moderate and mild dorsalized phenotype respectively, and have expanded domains of neural crest differentiation corresponding to expanded domains of intermediate BMP signaling levels (Nguyen et al., 1997). swirl mutants, which have a loss of BMP2b signaling, develop a severely dorsalized phenotype and have no neural crest cells (Nguyen et al., 1997). This may be due to the absence of the domain of intermediate BMP signaling and/or because BMP2b signals are specifically required for differentiation of these cells at the neural plate boundary. The nrd phenotype, however, does not include any obvious effects on dorsoventral patterning, and the dorsoventral position of genes expressed in the mesoderm underlying the neural plate boundary is not altered (Fig. 7). These results indicate that the loss of RB neurons and neural crest is not caused by a change in the size of the neural plate. However, the expression level of several genes expressed at the neural plate boundary, as judged by stain intensity, is significantly reduced, which is consistent with nrd having an effect on the specification of cells at the boundary of the neural plate. Moreover, the loss of expression of dlx3 and otx2 at the neural plate border suggests that nrd is required as early as during gastrulation to pattern neural crest and Rohon-Beard progenitors.
nrd is required for reception or interpretation of signals in the lateral neural plate
To examine whether the phenotype in nrd mutants arises from a defect in producing, receiving or interpreting a signal required for generation of both RB neurons and neural crest cells, cells from wild-type embryos were transplanted into mutant embryos. Wildtype cells were able to differentiate into RB neurons in the mutant dorsal spinal cord. This suggests that signals for RB differentiation are present in the mutant embryos, but that they can be received or interpreted only by wild type cells. We cannot yet rule out the possibility that nrd is required non-cell autonomously but acts prior to the time of transplantation (for instance, early blastula). An example for such behavior has recently been demonstrated for the oep mutation (Schier et al., 1997; Zhang et al., 1998).
What signal might the nrd mutants be incapable of receiving or interpreting? Previous studies have identified a number of signaling pathways involved in RB and neural crest cell differentiation. These include the BMPs mentioned above as well the members of the Wnt and FGF families. Overexpression of a truncated FGF receptor (XFD) or dominant negative Xwnt-8 in Xenopus, inhibited expression of Xslug, which marks the neural crest (Mayor et al., 1997; LaBonne and Bronner-Fraser, 1998). Thus, FGFs and Wnts might play a role in setting the competence of the neural ectoderm to respond to BMP signals from the epidermis to induce neural crest, or act directly as an inducer of this fate.
Further, ectopic expression of BMP-4 did not produce RB neurons in nrd (data not shown) as it did in wild-type embryos (Neave et al., 1997). This is consistent with, but does not prove, that nrd cells are defective in receiving or interpreting a BMP signal. The ability of FGF or Wnt signaling pathways to induce RB neurons in nrd mutants has not been investigated, since earlier defects based on overexpression of these factors might obscure the effects on RB neuron specification. Thus, the nrd mutation could also cause a defect in reception or interpretation of these signals.
nrd is required for RB neurons and neural crest cells
One of the most striking observations we have made is that nrd is required specifically for generation of both RB and neural crest cells. While the swirl/bmp2b mutation affects neural crest cell patterning, its defect is a consequence of expansion of the dorsal and dorsolateral portion of the neural plate at the expense of more lateral tissue, which includes the RB neurons, neural crest, placodal derivatives and epidermis. nrd is unique in its selective effect on RB neurons and early neural crest determination, and provides important insight into genetic requirements for the formation of both of these cell types.
While the relationship between the neural crest cells and RB neurons is poorly understood, lineage analysis studies provide evidence for a potential link between these tissues (R. Cornell and J. Eisen, personal communication). Both cell types can arise from an intermingled population in the lateral neural plate. In addition to the overlap in lineage, the expression of many genes, such as several Deltas, HuC, msxb, snail2 and fkd6, overlap in the RB neuron and neural crest producing region. Furthermore, the activity of neurogenic genes influences whether cells become RB neurons or neural crest cells (as suggested by Jiang et al., 1996, Turner and Weintraub, 1994; R. Cornell and J. Eisen, personal communication). Inhibiting RB neuron differentiation by disrupting lateral inhibition forces these progenitors to adopt a neural crest fate. Although we cannot yet rule out the possibility that nrd is required independently in both RB neuron and neural crest lineages, the loss of both cell types in nrd mutants supports the simpler and intriguing possibility that the gene is required for specification of a common progenitor.
nrd is required for early, but not late, emigrating neural crest cells
It is particularly interesting that while there was an early decrease in expression of neural crest markers like snail2, opl, fkd6 and dlx2 in 2-somite stage embryos, levels of these genes appeared nearly normal by the 10 somite stage. One interpretation of this result is that nrd is specifically required for an early wave of neurogenesis and neural crest formation, when cells in the lateral neural plate generate both of these cell types. Loss of early progenitors for neural crest cell and RB neurons would account for the early nrd phenotype. Since neural crest formation is a continuous process, progenitors required for subsequent waves of neural crest formation might not be affected in nrd mutants and late progenitors could continue to produce neural crest cells from the dorsal neural keel/spinal cord. This model would account for the recovery of neural crest marker expression levels in older embryos. The late progenitors may participate in developmental regulation that leads to compensation of some deficits produced by loss of early progenitors in nrd mutants. These late progenitors may, however, appear incapable of compensating for the loss of RB neurons.
Early crest progenitors produce large sensory RB neurons and other neural crest derivatives in zebrafish
The idea that early lateral plate progenitors give rise to both large sensory neurons like RB neurons and other neural crest derivatives is reminiscent of the situation in mouse and rat where single neural crest progenitors also give rise to large sensory neurons. Consistent with this idea, specific proneural genes, neurogenin2 and neurogenin1, have been shown to be responsible for determination of sensory ganglia derived from early and late neural crest progenitors (Ma et al., 1996, 1998). A knock-out of neurogenin2 results in a reduction in early but not late neural crest derivatives generated by early progenitors that specifically require neurogenin2 (Fode et al., 1998; Ma et al., 1999). Furthermore, neurogenin1 knock-out mice have a defect in later neural crest derivatives suggesting that these genes work in combination to generate the complete array of neural crest derivatives in the mouse.
Evolutionary implications of the nrd phenotype
Parallels between phenotypes in the neurogenin2 knockout mouse and nrd mutant zebrafish are striking. They support the idea that the fate of neuronal cells at the lateral edge of the neural plate shows similarities to that of the neural crest cells derived from early neural crest progenitors. This suggests the hypothesis that nrd mutants might specifically lack an early progenitor population whose determination requires the function of the neurogenin2 orthologue. Previous studies in Xenopus and zebrafish defined the role of the proneural genes like neurogenin and of neurogenic genes like Notch and Delta in the determination of primary neurons in three longitudinal domains in the neural plate, a process that is similar to selection of neural fate in the Drosophila neuroectoderm (Coffman et al., 1993; Chitnis et al., 1995; Turner and Weintraub, 1994; Jiang et al., 1996; Haddon et al., 1998, Appel and Eisen, 1998). Our results suggest parallels between cell fate determination in the lateral domain of primary neurons in zebrafish and cells derived from the early neural crest progenitors in mice. These studies together indicate important potential evolutionary links during the process of neurogenesis in widely different species.
nrd also provides further evidence that there may be an early evolutionary relationship between cell types within the lateral neural plate. This is consistent with the expression of homologues of neural crest regulatory genes within a region that also gives rise to neurons in other vertebrates and chordates. Recent isolation of snail and slug from the chordate, Ciona intestinalis, as well as distalless from Amphioxus, reveal expression at the border of neuroectoderm and ectoderm, reminiscent of their expression in vertebrates (Corbo et al., 1997; Holland et al., 1996; Baker and Bronner-Fraser, 1997), indicating ancient mechanisms of cell fate control in this region. It appears plausible that the first step in neural crest evolution might have been the emigration of a RB-like sensory neuron from within the dorsal neural tube (Fritzsch and Northcutt, 1993). Such an evolutionary scenario, indicating a common origin of both cell types, is further supported by the lack of RB neurons and neural crest cells in nrd.
In summary, we have identified a gene that is specifically required for receiving or interpreting signals in the lateral neural plate that are responsible for the determination of RB neurons and early neural crest cells. This gene may be required for determination of a specific early progenitor that generates both RB and neural crest cells, or for an initial differentiation decision common to both lineages. The existence of such a progenitor would suggest an important new parallel between cells in the lateral proneural domain and cells derived from the earliest neural crest progenitors in early chordates, fish and mice.
Acknowledgments
K. B. A. would like to thank M. M. for continuing to support this project in his laboratory. We would like to thank Drs M. Artinger, M. Bronner-Fraser, R. Cornell, A. Manjubar, E. Raz and M. Selleck for critical reading of the manuscript, numerous investigators for sharing data prior to publication, and Dr Su Guo, Lauren Cosgrove, Gerlinda Wussler, Donald Saugaro and Elizabeth Laver for assistance during the screen; members of the Boston Driever lab for useful discussions; Michael Raffin for technical assistance and members of the Mercola lab. Also Dr Len Zon and Steve Pratt for allowing use of their fish facility, and the following investigators for sharing constructs: J. Rutenberg for XBMP-4, N. Ueno for BMP-4, J. Lewis for DeltaB, J. Grinblat and H. Sive for Opl, H.-C. Seo and A. Fjose for pax3 and pax7, E. Liao for SCL, C. Thisse for Snail1 and Snail2, This work is supported by a NICHD postdoctoral fellowship HD08209 to K. B. A., Council for Tobacco Research 47571 and American Cancer Society RPG-97-121-01-DDC to M. M. and NIMH MH56552 and NICHD HD29761 to W. D.
REFERENCES
- Akimenko MA, Ekker M, Wegner J, Lin W, Westerfield M. Combinatorial expression of three zebrafish genes related to distal-less: part of a homeobox gene code for the head. J. Neurosci. 1994;6:3475–3486. doi: 10.1523/JNEUROSCI.14-06-03475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel B, Eisen JS. Regulation of neuronal specification in the zebrafish spinal cord by Delta function. Development. 1998;135:371–380. doi: 10.1242/dev.125.3.371. [DOI] [PubMed] [Google Scholar]
- Artinger KB, Bronner-Fraser M. Partial restriction in the developmental potential of late emigrating neural crest cells. Dev. Biol. 1992;149:149–157. doi: 10.1016/0012-1606(92)90271-h. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. The origins of the neural crest. Part I: embryonic induction. Mech. Dev. 1997;69:3–11. doi: 10.1016/s0925-4773(97)00132-9. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M, Le Douarin NM, Teillet MA. Early- and late-migrating cranial neural crest cell populations have equivalent developmental potential in vivo. Development. 1997;124:3077–3087. doi: 10.1242/dev.124.16.3077. [DOI] [PubMed] [Google Scholar]
- Chibon P. Analyse experimentale de la regionalisaton et des capacites morphogenetiquers de la crete neurale chez l Amphibien Urodele Pleurodeles waltiii Michah. Mem. Soc. Fr. Zool. 1965;36:1–107. [Google Scholar]
- Chitnis A, Henrique D, Lewis J, Ish-Horowicz D, Kintner C. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature. 1995;375:761–766. doi: 10.1038/375761a0. [DOI] [PubMed] [Google Scholar]
- Coffman CR, Skoglund P, Harris WA, Kintner CR. Expression of an extracellular deletion of Xotch diverts cell fate in Xenopus embryos. Cell. 1993;73:659–671. doi: 10.1016/0092-8674(93)90247-n. [DOI] [PubMed] [Google Scholar]
- Corbo JC, Erives A, Di Gregorio A, Chang A, Levine M. Dorsoventral patterning of the vertebrate neural tube is conserved in a protochordate. Development. 1997;124:2335–2344. doi: 10.1242/dev.124.12.2335. [DOI] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Nalicki J, Stemple DL, Stainier DYR, Zwartkruis F, Abdelilah S, Rangini Z, Belak J, Boggs C. A genetic screen for mutations affecting embrogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- Dickinson M, Selleck M, McMahon A, Bronner-Fraser M. Dorsalization of the neural tube by the non-neural ectoderm. Development. 1995;121:2099–2106. doi: 10.1242/dev.121.7.2099. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Northcutt RG. Cranial and spinal nerve organizations in amphioxus and lamreys: evidence for an ancestral craniate pattern. Acta Anat. 1993;148:96–109. doi: 10.1159/000147529. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–94. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Gering M, Rodaway ARB, Patient RK, Green AR. The SCL gene specifies haemangioblast development from early mesoderm. EMBO J. 1998;17:4029–4045. doi: 10.1093/emboj/17.14.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good PJ. A conserved family of elav-like genes in vertebrates. Proc. Natn. Acad. Sci. USA. 1995;92:4557–4561. doi: 10.1073/pnas.92.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon C, Smithers L, Schneider-Maunoury S, Coche T, Henrique D, Lewis J. Multiple delta genes and lateral inhibition in zebrafish primary neurogenesis. Development. 1998;125:359–370. doi: 10.1242/dev.125.3.359. [DOI] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van-Eeden FJM, Jiang Y-J, Heisenberg C-P, Kelsh RN, Furutani-Seiki M, Warga RM, Vogelsang E, Beuchle D, Schach U, Fabian C, Nüsslein-Vollard C. The identifcation of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- Henion PD, Raible DW, Beattie CE, Stoesser KL, Weston JA, Eisen JS. Screen for mutations affecting development of Zebrafish neural crest. Dev. Genet. 1996;18:11–17. doi: 10.1002/(SICI)1520-6408(1996)18:1<11::AID-DVG2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Holland ND, Panganiban G, Henyey EL, Holland LZ. Sequence and developmental expression of AmphiDll, an amphioxus Distal-less gene transcribed in the ectoderm, epidermis and nervous system: insights into evolution of craniate forebrain and neural crest. Development. 1996;122:2911–2920. doi: 10.1242/dev.122.9.2911. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Brand M, Heisenberg CP, Beuchle D, Furutani-Seiki M, Kelsh RN, Warga RM, Granato M, Haffter P, Hammerschmidt M, Kane DA, Mullins MC, Odenthal J, van Eeden FJ, Nüsslein-Volhard C. Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio . Development. 1996;123:205–216. doi: 10.1242/dev.123.1.205. [DOI] [PubMed] [Google Scholar]
- Kelsh RN, Brand M, Jiang Y-J, Heisenberg C-P, Lin S, Haffter P, Odenthal J, Mullins MC, van-Eeden FJM, Furutani-Seiki M, Granto M, Hammerschmidt M, Kane DA, Warga RM, Beuchle D, Vogelsang E, Nusslein-Vollard C. Analysis of neural crest develoment using zebrafish pigmentation mutations. Development. 1996;123:369–389. doi: 10.1242/dev.123.1.369. [DOI] [PubMed] [Google Scholar]
- Kim CH, Bae YK, Yamanaka Y, Yamashita S, Shimizu T, Fujii R, Park HC, Yeo SY, Huh TL, Hibi M, Hirano T. Overexpression of neurogenin induces ectopic expression of HuC in zebrafish. Neurosci. Lett. 1997;239:113–116. doi: 10.1016/s0304-3940(97)00908-7. [DOI] [PubMed] [Google Scholar]
- Kuwada JY. Cell recognition by neuronal growth cones in a simple vertebrate embryo. Science. 1986;233:740–746. doi: 10.1126/science.3738507. [DOI] [PubMed] [Google Scholar]
- Lamborghini JE. Rohon-Beard cells and other large neurons in Xenopus embryos originate during gastrulation. J. Comp. Neurol. 1980;189:323–333. doi: 10.1002/cne.901890208. [DOI] [PubMed] [Google Scholar]
- Lamborghini JE. Disappearance of Rohon-Beard neurons from the spinal cord of larval Xenopus laevis. J. Comp. Neurol. 1987;264:47–55. doi: 10.1002/cne.902640105. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998;13:2403–14. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. The Neural Crest. New York: Cambridge Univ. Press; 1982. [Google Scholar]
- Li Y, Allende ML, Finkelstein R, Weinberg ES. Expression of two zebrafish orthodenticle-related genes in the embryonic brain. Mech. Dev. 1994;3:229–244. doi: 10.1016/0925-4773(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and Neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13:1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Mayor R, Guerrero N, Martinez C. Role of FGF and noggin in neural crest induction. Dev. Biol. 1997;189:1–12. doi: 10.1006/dbio.1997.8634. [DOI] [PubMed] [Google Scholar]
- Mayor R, Morgan R, Sargent MG. Induction of the prospective neural crest of Xenopus. Development. 1995;121:767–777. doi: 10.1242/dev.121.3.767. [DOI] [PubMed] [Google Scholar]
- Metcalfe WK, Myers PZ, Trevarrow B, Bass MB, Kimmel CB. Primary neurons that express the L2/HNK-1 carbohydrate during early development in the zebrafish. Development. 1990;100:491–504. doi: 10.1242/dev.110.2.491. [DOI] [PubMed] [Google Scholar]
- Moens CB, Yan YL, Appel B, Force AG, Kimmel CB. valentino: a zebrafish gene required for normal hindbrain segmentation. Development. 1996;122:3981–3990. doi: 10.1242/dev.122.12.3981. [DOI] [PubMed] [Google Scholar]
- Moody SA. Quantitative lineage analysis of the origin of frog primary motor and sensory neurons from cleavage stage blastomeres. J. Neurosci. 1989;9:2919–2930. doi: 10.1523/JNEUROSCI.09-08-02919.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moury JD, Jacobson AG. Neural fold formation at newly created boundaries between neural plate and epidermis in the axolotl. Dev. Biol. 1989;133:44–57. doi: 10.1016/0012-1606(89)90295-9. [DOI] [PubMed] [Google Scholar]
- Moury JD, Jacobson AG. The origns of neural crest cells in the axolotl. Dev. Biol. 1990;141:243–253. doi: 10.1016/0012-1606(90)90380-2. [DOI] [PubMed] [Google Scholar]
- Neave B, Holder N, Patient R. A graded response to BMP-4 spatially coordinates patterning of the mesoderm and ectoderm in the zebrafish. Mech. Dev. 1997;62:183–195. doi: 10.1016/s0925-4773(97)00659-x. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev. Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Wilkinson DG. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Tada M, Takashi S, Ueno N. Conservation of BMP signaling in zebrafish mesoderm patterning. Mech. Dev. 1997;61:45–88. doi: 10.1016/s0925-4773(96)00625-9. [DOI] [PubMed] [Google Scholar]
- Noden DM. Vertebrate craniofacial development: the relation between ontogenetic process and morphological outcome. Brain, Behavior and Evolution. 1991;38:190–225. doi: 10.1159/000114388. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Nusslein-Volhard C. Fork head domain genes in zebrafish. Dev. Genes Evol. 1998;208:245–258. doi: 10.1007/s004270050179. [DOI] [PubMed] [Google Scholar]
- Oxtoby E, Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993;21:1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papan C, Campos-Ortega JA. On the formation of the neural keel and neural tube in zebrafish Danio (Brachydanio) rerio . Wilhelm Roux’s Arch. Dev. Biol. 1994;203:178–186. doi: 10.1007/BF00636333. [DOI] [PubMed] [Google Scholar]
- Raible DW, Eisen JS. Segregation and early dispersal of neural crest cells in the embryonic zebrafish. Dev. Dyn. 1992;195:29–42. doi: 10.1002/aja.1001950104. [DOI] [PubMed] [Google Scholar]
- Raible DW, Eisen JS. Restriction of neural crest cell fate in the trunk of the embryonic zebrafish. Development. 1994;120:495–503. doi: 10.1242/dev.120.3.495. [DOI] [PubMed] [Google Scholar]
- Raible DW, Eisen JS. Regulative interactions in zebrafish neural crest. Development. 1996;122:501–507. doi: 10.1242/dev.122.2.501. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Harvey M, Malicki J, Solnica-Krezel L, Stainier DY, Zwartkruis F, Abdelilah S, Stemple DL, Rangini Z, Yang H, Driever W. Mutations affecting the development of the embryonic zebrafish brain. Development. 1996;123:165–178. doi: 10.1242/dev.123.1.165. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Helde KA, Talbot WS, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- Schmitz X, Papan C, Campos-Ortega JA. Neurulation in the anterior trunk region of the zebrafish Brachydanio rerio. Wilhelm Roux’s Arch. Biol. 1993;203:250–259. doi: 10.1007/BF00363214. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Hammerschmidt M, Beuchle D, Cho KW, De Robertis EM, Nusslein-Volhard C. Expression of zebrafish goosecoid and no tail gene products in wild-type and mutant no tail embryos. Development. 1994;120:843–852. doi: 10.1242/dev.120.4.843. [DOI] [PubMed] [Google Scholar]
- Selleck MAJ, Bronner-Fraser M. Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development. 1995;121:526–538. doi: 10.1242/dev.121.2.525. [DOI] [PubMed] [Google Scholar]
- Serbedzija G, Bronner-Fraser M, Fraser S. Vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development. 1989;106:806–816. doi: 10.1242/dev.106.4.809. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L, Driever W. Microtubule arrays of the zebrafish yolk cell: organization and function during epiboly. Development. 1994;120:2443–2455. doi: 10.1242/dev.120.9.2443. [DOI] [PubMed] [Google Scholar]
- Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish Brachydanio rerio. Nature. 1981;291:293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Postlethwait JH. Expression of snail2, a second member of the zebrafish family, in cephalic mesendoderm and presumptive neural crest of wild type and spadetail mutant embryos. Dev. Biol. 1995;172:86–99. doi: 10.1006/dbio.1995.0007. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene, Orgeon: University of Orgegon Press; 1994. [Google Scholar]
- Weston JA, Butler SL. Temporal factors affecting the localization of neural crest cells in chick embryos. Dev. Biol. 1966;14:246–266. doi: 10.1016/0012-1606(66)90015-7. [DOI] [PubMed] [Google Scholar]
- Zhang J, Talbot WS, Schier AF. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell. 1998;92:241–251. doi: 10.1016/s0092-8674(00)80918-6. [DOI] [PubMed] [Google Scholar]