Abstract
Objective
To compare the incidence rates of malignancy among psoriatic arthritis (PsA) and rheumatoid arthritis (RA) patients in the Consortium of Rheumatology Researchers of North America (CORRONA) registry.
Methods
We analyzed 2,970 PsA patients with 7133 patient years (PY) of follow-up, and 19,260 RA patients with 53864 PY of follow-up. Using a standardized adjudication process, we identified 40 confirmed malignancies in PsA and 307 confirmed malignancies in RA. Incidence rates (IRs) were calculated per 100 PY. Incidence rate ratios (IRRs) were estimated, adjusted for age, gender, disease duration, body mass index, disease activity, year of enrollment, and medication use.
Results
The overall malignancy incidence per 100 PY was similar between PsA and RA patients, 0.56 (95% CI 0.40, 0.76) for PsA and 0.56 (95% CI 0.50, 0.63) for RA. Non-melanoma skin cancer was the most common type of cancer in the overall cohort, with an IR of 0.21 (95%CI 0.12, 0.35) in PsA, and 0.20 (95%CI 0.17, 0.24) in RA, with a calculated IRR of 1.05 (95%CI 0.61, 1.80), p=0.85. Lymphoma rates were similar in PsA vs. RA, 0.04 (95% CI 0.01, 0.12) vs. 0.04 (95% CI 0.02, 0.06), IRR 1.00 (0.17, 3.11), p=0.67. The adjusted IRR of malignancy in PsA vs. RA was 1.18 (0.82, 1.69), p=0.37).
Conclusion
The incidence rate across malignancy subtypes were similar in PsA and RA cohorts from a United States registry.
Psoriatic arthritis (PsA) is a multisystem inflammatory disease characterized by inflammation of both skin and joints. Therefore, PsA shares some clinical features of both rheumatoid arthritis (RA) and skin psoriasis (PsO), where malignancy risk has been more extensively studied. Several large cohort studies have found an increased overall risk of malignancy (1, 2) as well as lymphoma and hematologic cancers (3, 4) in RA compared with the general population. Similarly, a higher incidence of malignancy has been demonstrated in patients with PsO (5–7), including non-melanoma skin cancers (7, 8) and lymphoma (9).
It is unclear whether malignancy risk in PsA can be extrapolated from previous studies of patients with RA and PsO. For example, patients with RA have been found to have higher levels of disease activity such as tender and swollen joint counts and ESR, and more radiographic damage than patients with PsA (10). This may lead to a possible decreased risk of malignancy in PsA patients as compared to their RA counterparts given that chronic inflammation is a risk factor for certain malignancies in patients with inflammatory arthritis (11). In contrast, the additive impact of inflammation from both skin and joint disease may put PsA patients at increased risk of malignancy as compared to those with RA or PsO who have solely skin disease or joint disease alone. To date there has been only one large prospective study of 665 patients from Canada that examined incidence of malignancy in patients with PsA and showed that malignancy rates were not higher in PsA compared with the general population (12). Based on our literature review, there are no studies comparing the incidence of malignancy and factors associated with malignancy in PsA and RA.
Therefore, we compared the incidence of malignancy between PsA and RA patients enrolled in the Consortium of Rheumatology Researchers of North America (CORRONA) registry, a large prospective United States (U.S.) cohort. In addition, we evaluated demographic and disease-related variables associated with malignancy in both PsA and RA. We hypothesized that malignancy rates will be similar between PsA and RA patients in CORRONA.
Methods
CORRONA registry description
CORRONA is a multi-centered, longitudinal registry which includes 85 academic and private clinical sites across the U.S., with 4216 PsA and 26133 RA patients enrolled from August 2003 to October 2010. The details of CORRONA registry have been previously published (13). Briefly, clinical information of enrolled subjects including disease duration, comorbidities, medications, measures of disease activity, and adverse events is collected using comprehensive questionnaires completed by both patients and participating rheumatologists. Prior to 2013, the CORRONA PsA registry did not collect information on axial disease or skin disease in PsA and did not collect identifying data that would enable linking the information from the CORRONA registry to the National Death Index Database or other national databases. Questionnaires were completed at patient enrollment and follow-up encounters requested at three to six month intervals. The CORRONA registry is approved by the institutional review boards of participating academic sites and a central institutional review board for community-based private sites. All patients sign informed consent before participation.
Study Population
We included all PsA and RA patients followed in the CORRONA registries between August 2003 and October 2010 who had at least 2 study visits during this time period. In order to capture the incidence of new malignancy, we excluded patients with a prior history of malignancy and patients with only one CORRONA visit, similar to other studies (14, 15). History of malignancy was documented in the physician form and based on either patient report or medical records. Additional cases of prior malignancy history were found during adjudication process (see below).
Malignancy adjudication
We used a previously validated structured adjudication process described elsewhere (16). Briefly, we examined cases of physician-reported malignancies that are initially reported at follow-up study visits, and subsequently confirmed using an adverse event form completed by the rheumatologist at the reporting site. Supporting medical records such as biopsy reports, oncology specialist office notes, etc. are collected by the patient’s rheumatologist and sent to CORRONA for centralized adjudication. Adverse event forms and source documents were independently reviewed by two investigators, with a third reviewer serving as a “tiebreaker.” Cases of malignancy were classified as “definite,” “probable,” “possible,” and “not a malignancy.” Only cases that had both the date of diagnosis and histology information were deemed “definite” or “probable” and considered confirmed incident malignancies. For patients with more than one malignancy reported during the follow-up period, only the first event was used in the analysis.
Statistical Analysis
For patients with malignancy, variables were compared at the CORRONA follow-up visit where the malignancy was reported. For patients without malignancy, variables were compared at the last recorded follow-up visit. We used the student’s t-test (or its non-parametric alternative, Wilcoxon rank sum test) to evaluate the differences between distributions of continuous variables, and chi-square (or Fisher’s exact test when appropriate) to evaluate the association between categorical variables. Differences were considered statistically significant if p<0.05 (two-tailed). Continuous variables were reported as mean ± standard deviation (SD). Categorical variables were reported as percentages and frequencies.
First, we calculated the incidence rates of total confirmed malignancies and specific malignancies types with 95% confidence intervals (CIs) for both PsA and RA groups. Then, we used multivariate Poisson and Cox regression models to derive the incidence rate ratios (IRRs) and hazard ratios (HRs) of overall malignancy rates among PsA and RA patients in CORRONA and to test the significance of an indicator variable for PsA vs. RA. Our final models were adjusted for age (in 5-year increments), sex, race, year of enrollment into CORRONA, disease duration, disease activity measures, smoking, alcohol use, BMI, and family history of malignancy.
Following 2010 EULAR recommendations for reporting results from biologics registries in rheumatology (17), we conducted several sensitivity analyses and used various definitions of disease activity and medication exposures to study an association between malignancy incidence and disease state (PsA vs. RA). Disease activity measures, CDAI and mHAQ, were examined as continuous variables and categorical variables (18), as well as cumulative scores, defined as a cumulative average over each time interval during follow-up). Smoking was analyzed as past smoker, and current smoker compared to “never smoked.” Age was analyzed as a continuous variable and as a time scale variable for survival analysis and for Poisson regression models. Since we were interested in comparing the incidence of malignancy between PsA and RA, and because we obtained similar results using survival analysis and Poisson regression models, we only presented Poisson regression models in the manuscript. Our final regression models were tested for overdispersion (p>0.99) demonstrating an appropriate model fit (19).
For our main analyses, we also included use of biologics and DMARDs which were analyzed as “ever exposed” given the likely longer term effects of DMARDs on malignancy risk. In an effort to account for the duration of the medication exposure, we also conducted additional sensitivity analyses using the following 5 mutually exclusive medication categories: current methotrexate without current or prior biologic use; current biologic without current or prior methotrexate use; current biologic with current or prior methotrexate use; non-methotrexate DMARD with prior methotrexate use only, and no current or prior biologic use; and non-methotrexate DMARD without current or prior methotrexate or biologic. In this analysis some patients may have contributed to different categories at different time periods.
To account for a possible left-censorship bias, a bias that arises when people who are more ill are lost to follow-up, as people who may be exposed to certain medications may die before entering a registry (17), we conducted a subgroup analysis that included only patients with a new diagnosis of PsA and RA, defined as disease duration of less than one year.
Finally, to account for the demographic differences observed between PsA and RA groups, we 1:1 matched PsA and RA patients by sex, age and race. In this sub-analysis, age was matched as younger than 25 being the first category, with 5 year increments after that. Forty five PsA patients without one of the three matching criteria were not matched. The final matching RA sample included 2925 patients with 32 malignancies.
Results
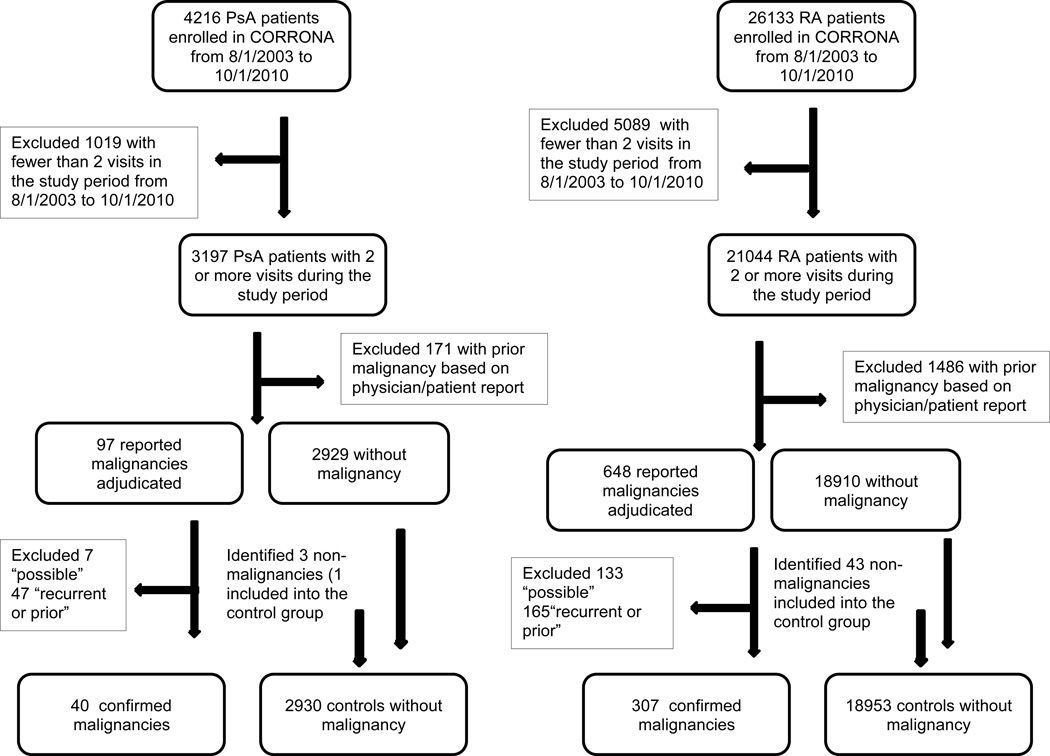
There were 4216 PsA and 26133 RA patients enrolled in the registries between August 2003 and October 2010. We excluded 1019 PsA and 5089 RA patients with fewer than 2 visits during the study period between August 2003 and October 2010 (see Figure 1). We also excluded 171 PsA and 1486 RA with prior malignancy history documented in the physician form or patient self-report. We adjudicated 97 malignancies in PsA (40 confirmed, 47 with recurrent or prior malignancy, 7 “possible”, and 3 non-malignancies) and 648 malignancies in RA (307 confirmed, 133 “possible”, 165 recurrent or prior, 43 non-malignancies). In both RA and PsA CORRONA populations, compared to the excluded patients, patients included in the study were younger, more likely to be Caucasian, had longer disease duration, and were more likely to use methotrexate and anti-TNF agents (Table 1), although the magnitude of these differences was relatively small.
Figure 1.
Inclusion/Exclusion criteria flow chart
Table 1.
Baseline CORRONA visit comparisons of RA and PsA patients that were included in the study with patients not included in the study*
| RA | PsA | |||||
|---|---|---|---|---|---|---|
| Excluded | Analytic Sample |
P- value |
Excluded | Analytic Sample |
P-value | |
| N=6873 | N=19260 | N=1246 | N=2970 | |||
| Age, years | 59.6 (14.3) | 57.6 (13.5) | <0.001 | 52.2 (13.8) | 51.1 (12.5) | 0.03 |
| Female | 5119 (75.3) | 14676 (76.7) | 0.020 | 671 (54.3) | 1524 (51.6) | 0.12 |
| Caucasian | 5520 (81.7) | 15866 (83.5) | <0.001 | 1097 (89.0) | 2698 (91.6) | 0.006 |
| Disease duration, years | 9.0 (9.9) | 9.4 (9.6) | 0.01 | 7.7 (9.1) | 8.4 (8.6) | 0.02 |
| CRP, mg/l | 3.5 (9.5) | 3.0 (8.5) | 0.005 | 3.0 (9.5) | 2.3 (6.9) | 0.13 |
| CDAI (0,76) | 14.4 (13.9) | 13.5 (12.6) | <0.001 | 11.6 (10.9) | 10.3 (10.3) | <0.001 |
| DMARD history | 7072 (86.8) | 17532 (91.0) | <0.001 | 1183 (81.1) | 2629 (88.5) | <0.001 |
| MTX history | 4924 (71.6) | 14754 (76.6) | <0.001 | 1016 (81.5) | 2018 (89.2) | <0.001 |
| anti-TNF history | 2791 (40.6) | 8766 (45.5) | <0.001 | 653 (52.4) | 1696 (57.1) | 0.005 |
Dichotomous variables are expressed as number (%); continuous variables are expressed as mean (SD)
Baseline characteristics of PsA and RA groups
The final analysis included 2,970 PsA patients with 7133 PY of follow-up, and 19,260 RA patients with 53864 PY of follow up. The average follow-up time was 2.79 (SD 2.09) years for PsA and 2.57 (SD 1.87) years for RA patients. The clinical characteristics of the PsA and RA groups are shown in Table 2. As expected, PsA patients were younger, included a higher proportion of men, and a higher proportion of Caucasians, compared to RA patients. PsA patients also had a higher BMI, a shorter disease duration, were more likely to consume alcohol, but were less likely to smoke. The majority of patients in both groups had low-moderate CDAI scores. Current and “ever” anti-TNF use was higher in the PsA group compared with RA group, while current or “ever” methotrexate use was higher in the RA group.
Table 2.
Bivariate comparisons of PsA and RA patients, last visit*
| All PsA |
All RA |
PsA, without malignancy |
PSA, with malignancy |
P- value |
RA, without malignancy |
RA, with malignancy |
P- value |
|
|---|---|---|---|---|---|---|---|---|
| N=2970 | N=19260 | N=2930 | N=40 | N=18953 | N=307 | |||
| Age, years | 53.6 (12.6) | 60.2 (13.6) | 53.5 (12.6) | 62.5 (11.7) | <0.001 | 60.1 (13.7) | 66.1 (11.4) | <0.001 |
| Female | 1525 (51.6) | 14709 (76.7) | 1506 (51.7) | 19 (47.5) | 0.598 | 14490 (76.8) | 219 (71.6) | 0.033 |
| Caucasian | 2700 (91.6) | 15905 (83.5) | 2661 (91.6) | 39 (97.5) | 0.178 | 15632 (83.4) | 273 (89.8) | 0.003 |
| Current smoker | 433 (14.6) | 3136 (16.4) | 429 (14.7) | 4 (10.0) | 0.404 | 3098 (16.4) | 38 (12.4) | 0.058 |
| Past smoker | 866 (29.3) | 6156 (32.1) | 846 (29.0) | 20 (50.0) | 0.004 | 6026 (31.9) | 130 (42.3) | <0.001 |
| Alcohol use | 1672 (58.6) | 8321 (44.9) | 1653 (58.8) | 19 (48.7) | 0.206 | 8196 (45.0) | 125 (43.1) | 0.526 |
| BMI, kg/m2 | 31.2 (7.4) | 29.1 (7.2) | 31.2 (7.5) | 31.4 (6.2) | 0.859 | 29.1 (7.2) | 28.2 (6.6) | 0.022 |
| Disease duration, years | 10.8 (9.0) | 12.1 (10.1) | 10.7 (9.0) | 13.5 (10.9) | 0.053 | 12.0 (10.1) | 14.2 (10.0) | <0.001 |
| CRP, mg/L | 1.6 (4.1) | 2.3 (6.5) | 1.6 (4.1) | 1.4 (2.2) | 0.913 | 2.3 (6.5) | 3.5 (9.5) | 0.050 |
| CDAI, (0–76) | 8.1 (9.2) | 10.3 (10.9) | 8.1 (9.2) | 8.7 (9.0) | 0.674 | 10.3 (10.9) | 9.6 (10.0) | 0.219 |
| DMARD history | 2857 (96.2) | 18982 (98.6) | 2817 (96.1) | 40 (100.0) | 0.206 | 18676 (98.5) | 306 (99.7) | 0.098 |
| MTX history | 2278 (76.7) | 16803 (87.2) | 2242 (76.5) | 36 (90.0) | 0.045 | 16524 (87.2) | 279 (90.9) | 0.054 |
| anti-TNF history | 2088 (70.3) | 11388 (59.1) | 2060 (70.3) | 28 (70.0) | 0.966 | 11209 (59.1) | 179 (58.3) | 0.768 |
| Other biologic history | 70 (2.4) | 2379 (12.4) | 70 (2.4) | 0 (−0.0) | 0.323 | 2349 (12.4) | 30 (9.8) | 0.166 |
| Current DMARD | 2553 (86.0) | 17100 (88.8) | 2522 (86.1) | 31 (77.5) | 0.121 | 16850 (88.9) | 250 (81.4) | <0.001 |
| Current MTX | 1336 (45.0) | 11445 (59.4) | 1320 (45.1) | 16 (40.0) | 0.524 | 11272 (59.5) | 173 (56.4) | 0.269 |
| Current anti-TNF | 1712 (57.6) | 7301 (37.9) | 1695 (57.8) | 17 (42.5) | 0.051 | 7213 (38.1) | 88 (28.7) | <0.001 |
| Current other biologic | 23 (0.8) | 1530 (7.9) | 22 (0.8) | 1 (2.5) | 0.210 | 1516 (8.0) | 14 (4.6) | 0.027 |
| Family history of cancer | 1290 (43.4) | 9019 (46.8) | 1275 (43.5) | 15 (37.5) | 0.446 | 8863 (46.8) | 156 (50.8) | 0.158 |
Dichotomous variables are expressed as number (%); continuous variables are expressed as mean (SD)
Bivariate malignancy/non-malignancy comparisons within each disease category
Table 2 shows demographic and disease-specific variables for registry patients with and without malignancy. In both PsA and RA groups, patients with malignancy were older at the time of the last visit/malignancy diagnosis, had a slightly longer disease duration, and were more likely to be ex-smokers, but not current smokers. Ninety percent of PsA patients with malignancy were ever exposed to methotrexate compared to 77% of patients without malignancy (p=0.045). In the RA group, 91% of patients with malignancy were exposed to methotrexate compared with 87% of RA patients without malignancy (p=0.054). Forty three percent of PsA patients with malignancy were currently on anti-TNF, compared with 58% of PsA patients without malignancy (p=0.051). In the RA group, 29% of patients with malignancy were currently on anti-TNF compared with 38% of patients without malignancy (p<0.001).
Malignancy Incidence in PsA and RA
Table 3 describes the type of cancers identified in the registry, and shows the unadjusted comparison of the malignancy incidence between PsA and RA groups. The overall incidence of malignancy was similar between the two groups (0.56 (95% CI 0.40, 0.76) per 100 PY for PsA vs. 0.56 (95% CI 0.50, 0.63) for RA, incidence rate ratio (IRR) =1.00 (95% CI 0.70, 1.36), p=0.86). NMSC was the most common type of cancer identified in both groups (0.21 (95%CI 0.12, 0.35) per 100 PY’s in PsA and 0.20 (95%CI 0.17, 0.24) per 100 PY’s in RA, IRR 1.05 (95%CI 0.61, 1.80), p=0.85). Lymphoma rates were also similar in PsA vs. RA, 0.04 (0.01, 0.12) vs.0.04 (0.02, 0.06) per 100 PY, IRR1.00 (0.17, 3.11), p=0.67. In addition to the cancers listed in the table, there were several cancer types in the RA group with only a small number of reported cases: uterine (4), head and neck (1), bile duct (1), with only one reported case in the PsA cohort for each of these malignancies.
Table 3.
Incidence Rates of Malignancy in PsA and RA per 100 Patient Years
| PsA | RA | PsA vs. RA | ||||||
|---|---|---|---|---|---|---|---|---|
| Count | Total PY for Event |
IR (95% CI) | Count | Total PY for Event |
IR (95% CI) | IRR | P value | |
| All cancers* | 40 | 7156.1 | 0.56 (0.40, 0.76) | 307 | 54709.7 | 0.56 (0.50, 0.63) | 1.00 (0.70, 1.36) | 0.864 |
| NMSC | 15 | 7099.0 | 0.21 (0.12, 0.35) | 109 | 54136.8 | 0.20 (0.17, 0.24) | 1.05 (0.61, 1.80) | 0.854 |
| non-NMSC** | 25 | 7121.9 | 0.35 (0.23, 0.52) | 198 | 54387.2 | 0.36 (0.32, 0.42) | 0.97 (0.61, 1.42) | 0.725 |
| Solid | 20 | 7112.8 | 0.28 (0.17, 0.43) | 168 | 54294.0 | 0.31 (0.26, 0.36) | 0.90 (0.57, 1.45) | 0.691 |
| Breast | 7 | 3580.0 | 0.20 (0.08, 0.40) | 55 | 41025.4 | 0.13 (0.10, 0.17) | 1.54 (0.67, 3.23) | 0.338 |
| Prostate | 3 | 3477.4 | 0.09 (0.02, 0.25) | 20 | 12747.5 | 0.16 (0.10, 0.24) | 0.56 (0.16, 1.86) | 0.338 |
| Colorectal | 3 | 7073.9 | 0.04 (0.01, 0.12) | 14 | 53847.2 | 0.03 (0.01, 0.04) | 1.33 (0.47, 5.70) | 0.438 |
| Melanoma | 3 | 7068.8 | 0.04 (0.01, 0.12) | 15 | 53850.4 | 0.03 (0.02, 0.05) | 1.33 (0.44, 5.28) | 0.502 |
| Hematologic | 5 | 7074.1 | 0.07 (0.02, 0.16) | 30 | 53907.6 | 0.06 (0.04, 0.08) | 1.17 (0.36, 2.89) | 0.971 |
| Lymphoma | 3 | 7069.7 | 0.04 (0.01, 0.12) | 21 | 53874.8 | 0.04 (0.02, 0.06) | 1.00 (0.17, 3.11) | 0.668 |
| Multiple myeloma | 1 | 7067.1 | 0.01 (0.00, 0.08) | 1 | 53821.2 | 0.00 (0.00, 0.01) | 7.78 (0.48, 122.23) | 0.150 |
| Leukemia | 1 | 7067.1 | 0.01 (0.00, 0.08) | 8 | 53840.2 | 0.01 (0.01, 0.03) | 1.00 (0.12, 7.64) | 0.966 |
All cancers=NMSC + non-NMSC.
Non-NMSC=Solid + Hematologic
Multivariate models comparing malignancy incidence in PsA and RA
Poisson regression models for malignancy incidence between PsA and RA are presented in Table 4. Model 1 includes the entire study population of PsA and RA patients, and defines drug exposure as “ever exposed.” In this model, older age at the time of the last visit, past smoking history (but not current smoking history), and cumulative average CDAI are associated with an increased risk of malignancy, adjusted for sex, disease duration, BMI, alcohol use, family history of malignancy, and year of enrollment into the registry. Of particular note, history of methotrexate use (IRR 1.55, 95% CI 1.08, 2.23, p=0.018), but not history of anti-TNF use, was associated with an increased risk of malignancy in the primary model. We noted an association between year of enrollment and malignancy incidence that was incorporated into the multivariable models. Disease duration was also not associated with an increased risk of malignancy after adjusting for the enrollment period.
Table 4.
Poisson regression models estimating malignancy risk (Incidence rate ratio) in PsA compared with RA*
| Model 1 | Model 2 | |
|---|---|---|
| PsA vs. RA | 1.17 (p=0.370)(0.82, 1.69) | 1.18 (p=0.473)(0.75, 1.85) |
| Age, 5 year increments | 1.21 (p=0.000)(1.16, 1.27) | 1.24 (p=0.000)(1.17, 1.31) |
| Male | 1.17 (p=0.200)(0.92, 1.49) | 1.23 (p=0.167)(0.92, 1.64) |
| Disease Duration, 1 year increments | 1.03 (p=0.212)(0.98, 1.08) | 1.03 (p=0.280)(0.97, 1.10) |
| BMI | 1.00 (p=0.942)(0.98, 1.02) | 1.00 (p=0.963)(0.98, 1.02) |
| Smoking status | ||
| Never smoked | 1.00 | |
| Current smoker | 1.08 (p=0.677)(0.75, 1.55) | 0.85 (p=0.483)(0.53, 1.35) |
| Past smoker | 1.43 (p=0.003)(1.13, 1.81) | 1.19 (p=0.238)(0.89, 1.58) |
| Alcohol use | 1.02 (p=0.893)(0.81, 1.26) | 1.04 (p=0.784)(0.79, 1.37) |
| Family history of cancer | 0.98 (p=0.848)(0.79, 1.21) | 1.12 (p=0.384)(0.86, 1.46) |
| CDAI, cumulative average | 0.93 (p=0.041)(0.88, 1.00) | 0.94 (p=0.130)(0.86, 1.02) |
| Year of enrollment | ||
| before 2004 | 1.00 | 1.00 |
| 2005–2006 | 0.57 (p=0.002)(0.41, 0.81) | 0.63 (p=0.037)(0.40, 0.97) |
| 2007–2008 | 1.56 (p=0.000)(1.22, 1.99) | 1.91 (p=0.000)(1.42, 2.56) |
| 2009–2010 | 1.16 (p=0.532)(0.72, 1.88) | 1.54 (p=0.124)(0.89, 2.67) |
Model 2 includes time-varying drug exposure categories, that includes 2737 PsA patients with 27 malignancies, and 18160 RA patients with 203 malignancies. In this model, patient age was associated with an increased risk of malignancy, adjusting for the same factors adjusted in Model 1. With respect to drug exposure, current methotrexate use (without current or prior biologic use) was associated with an elevated risk estimate of cancer (IRR 1.77, 95% CI 0.92 – 3.40, p=0.087), although the confidence interval crosses unity). Importantly, there was no significant difference in the estimated risk of malignancy in PsA patients compared to RA patients in both models (IRR 1.17 (95% CI 0.82, 1.69), p=0.370 in Model 1, and 1.18 (95% CI 0.75, 1.85), p=0.473 in Model 2). We also compared malignancy incidence in PsA and RA using Cox proportional hazard models with age as the underlying time scale, and obtained similar results after adjusting for the variables used in Model 1 and Model 2 above (data not shown).
To account for a possible left-censorship bias, we performed a sensitivity analysis that includes only patients with a new diagnosis of PsA or RA (318 PsA patients with 3 malignancies, and 1786 RA patients with 18 malignancies). Although the numbers of patients and cancers in this model are small, the risk estimate of malignancy for PsA versus RA patients was similar (data not shown).
Similarly, in another sensitivity analysis with the age-, sex- and race-matched subgroup of PsA and RA patients, there was no difference between malignancy rates in PsA compared with RA (data not shown).
Discussion
Currently, there is a paucity of studies evaluating malignancy risk in PsA patients. Based on our results, the incidence rate of overall malignancy and cancer subtypes, including NMSC and lymphoma, were similar in PsA and RA patients in the CORRONA registry, even after adjusting for multiple potential confounders including age, race, sex, disease activity, disease duration, year of enrollment, and medication use. Our study has several strengths, including the large size, diversity and prospective nature of the CORRONA registry. Information on adverse events was obtained through both patient reports and the treating rheumatologist. In addition, all reported cases went through a structured confirmation process using multiple reviewers. This is one of the major strengths of our study design, as patient or physician reporting alone may not accurate enough for large epidemiologic studies of cancer incidence (16).
Although our study was not specifically designed to look for an association between medication exposures and malignancies, we did not find an association between anti-TNF exposure and cancer in this cohort of patients with inflammatory arthritides. Whether the use of immunomodulatory medications, specifically the TNF inhibitors, affects cancer risk is controversial, as TNF has effects not only on inflammation, but also apoptosis, cell survival, and cancer surveillance.
A 2006 systematic review of randomized control trials found an increased risk of malignancy in patients with RA treated with anti-TNF therapy (20). In contrast, later published metanalyses (21, 22) and both a large Swedish national population based cohort study (23) and Danish national arthritis registry study (DANBIO)(14, 24) have not been able to replicate this increased cancer risk in anti-TNF treated RA patients. In addition, a 2011 meta-analysis of prospective, observational studies that included not only patients with RA, but also patients with PsA and ankylosing spondylitis, did not find an increased risk of solid malignancy or lymphoma, although it did find an increased risk of skin cancers including melanoma (25).
Though there is less available data looking specifically at psoriatic disease, a recent meta-analysis of randomized controlled trials of 6810 patients (5427 with PsO and 1383 patients PsA) did not observe an increased malignancy risk with the use of anti-TNF inhibitors (26). In another study, medication exposure also had no effect on malignancy risk in the Canadian PsA cohort, although only a small percentage of patients (9.7% in the non-malignancy group and 2.9% of patients in the malignancy group) had been exposed to biologic agents (12).
In contrast to the results for TNF inhibitors, we did find that history of methotrexate exposure was associated with an increased risk of malignancy in our primary analyses of this mixed RA/PsA inflammatory arthritis cohort. Since the introduction of methotrexate for the treatment of inflammatory arthritis and psoriasis, there have been questions regarding its oncogenicity. Epstein Barr Virus (EBV)-associated lymphoproliferative disorders have been reported during treatment with low dose MTX treatment in both rheumatoid arthritis (27, 28) and psoriasis (29, 30), although not all lymphoproliferative disorders are associated with EBV. Regression of these tumors after withdrawal of the drug in some patients is well described (31).
Possible mechanisms for its association with increased malignancy risk include its antimetabolic properties and possible decreased cancer surveillance from resulting chronic immunosuppression (28, 32). These effects are likely to be different when the drug is used over a relatively finite period in the treatment of malignancies versus the chronic antimetabolic regimens used to treat RA and PsA.
Conflicting results have been observed from other large cohort studies of RA patients. Three cohort studies have not found a relationship between methotrexate use and malignancy (33–35). In contrast, a large cohort of RA patients from the UK receiving non-biologic DMARDS, of which the large majority (95%) were taking methotrexate alone or in combination with other DMARDs, found a higher incidence of malignancy as compared to the general population (24). A recent pharmacoepidemiologic study from the RA cohort in the CORRONA registry also reported a higher risk of cancer for patients on methotrexate compared to other non-biologic DMARDs (36).
In psoriasis patients, two small cohort studies did not find an increase risk of malignancy with methotrexate exposure (37, 38), while one larger U.S. cohort study of 1,380 PUVA treated patients found an increased risk of lymphoma in those with over 3 years of methotrexate exposure as compared to the general population (39). Thus, there is conflicting evidence on the risk of malignancy associated with methotrexate use. These conflicting results are likely due to a variety of factors, including differences in study design, comparator drugs, background population, exposure definitions and duration of follow-up.
We recognize that studies reporting the association between any DMARD use and malignancy in data registries and retrospective studies could be influenced by a number of factors, including unmeasured residual confounders, underreporting, and selection bias. Therefore, neither our study results, nor the results of other observational studies, are conclusive on this issue. Nevertheless, even with these acknowledged uncertainties, we believe that our findings suggest that further research is needed on malignancy risk associated with methotrexate. Of equal importance, our study adds to the growing literature from registries that TNF inhibitors are not associated with increased malignancy risk.
Since skin cancer is of special concern in PsO and PsA (40), we also compared NMSC rates between CORRONA and several historical control populations to place our findings in a context of previously reported NMSC rates. The highest incidence comes from north-central New Mexico cohort, a high risk geographic area due to a high UV light index, where the overall incidence rates of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) were 0.93 and 0.36 per 100 PY’s respectively for men, and 0.49 and 0.15 for BCC and SCC respectively for women (41). A lower incidence was found in a Minnesota cohort with rates of BCC were 0.03 and 0.02 per 100 PY’s in women and men, respectively. SCC incidence rate was 0.004 per 100 PY’s in both genders (42). Lower rates in this cohort may be explained by a lower median age (33.3 years) in this study and relative low UV light index in this area. The overall rate of NMSC in the CORRONA PsA cohort of 0.21 per 100 PY’s falls in the middle of these two high and low risk populations. The incidence rate is also similar to national incidence estimated by Miller and Weinstock, with a SCC incidence of 0.03 and 0.08 per 100 PY in women and men respectively, and BCC incidence of 0.21 and 0.41 per 100 PY’s in women and men (43).
Importantly, the incidence of NMSC of the CORRONA PsA group may vary from what has been reported in the psoriasis literature, where higher rates of NMSC have been reported. Since skin directed therapy, including both psoralen-ultraviolet A (PUVA) and ultraviolet B (broad band and narrow-band), is indicated for cutaneous but not rheumatologic disease activity, cutaneous-predominant psoriasis patients may be more likely to experience the pro-carcinogenic effects of light therapy than patients with more active joint disease. This concept is supported by a recent review of the clinical trial safety analysis of etanercept in RA, PsA, and PsO, wherein the rate of squamous cell carcinoma in psoriasis etanercept-treated patients, but in neither RA nor PsA, was significantly elevated (44). Whether this is due to variable rates of light therapy exposure in the PsA and PsO groups or related to other pathogenic exposures or biological processes remains unknown.
There are several limitations to this study. The information about skin disease in PsA patients and axial involvement was not available in the CORRONA registry during the study period, and, therefore, we were unable to adjust for these variables in our analyses. More detailed information on severity of skin disease and spinal involvement were added to the PsA data collection forms beginning in early 2013, but were not available for the study period. Furthermore, the overall number of malignancy events was modest for the PsA cohort, which may affect the precision of risk estimates for PsA. Nevertheless, it should be noted that the PsA cohort in the CORRONA registry is one of the largest PsA registries to our knowledge.
Although formal comparisons cannot be made due to the methodological differences between the CORRONA registry and data from other studies, the overall rates of malignancy ascertained in our study were similar to the rates reported in the recent meta-analysis of randomized trials of RA patients treated with biologic therapy (45). On the other hand, the malignancy rates in the CORRONA registry were noted to be approximately 50% lower than the rates reported in the RA population from the DANBIO registry (a Danish database of arthritis patients)(14). Like many registry studies, we recognize that there may be malignancy cases that were not reported during study visits by patients or treating providers, leading to underascertainment. However, systematic underascertainment in both PsA and RA CORRONA registries should not limit the internal validity of our study results on the comparative cancer risk in RA versus PsA participants. A small number of malignancy cases were not confirmed due to lack of supporting source documents, despite multiple attempts by the registry to obtain such information. It is also possible that a higher proportion of these patients did develop incident malignancies but were lost to follow-up, since cancer treatment or mortality may affect ability to comply arthritis care and study visits. Because of patient confidentiality considerations, there is no patient identifying data in the CORRONA registry that would allow us to link our data to the National Death Index database to check for unreported cancer cases. In addition, it is possible that the rates of malignancy could be different in the US where the penetration of biologic drugs is higher than that found in European registries, although this would not affect RA versus PsA patients differentially and should not impact the internal validity of our study results.
Since the main objective of this analysis was to compare incident malignancies in PsA and RA, we excluded individuals with only one registry visit. Therefore, some of the malignancies may have not been captured because of loss to follow up, leading to a possible selection bias. However, this selection bias would not be differentially affected by the disease status (PsA vs. RA), and should not affect the conclusions of our study. Despite of these limitations, this is the first study to investigate malignancy incidence in a large U.S. cohort of patients with PsA, and to compare the incidence of malignancy between patients with PsA and RA. Therefore, these data are particularly relevant when evaluating the comparative incidence of malignancy in a US population.
As the available treatments for inflammatory arthritis expand, more emphasis is being placed on treating associated comorbidities and determining adverse events from therapy. In comparing the two cohorts with inflammatory arthritis, we did not observe an increased cancer risk in patients with PsA as compared to RA across malignancy subtypes.
Table 5.
Poisson regression models estimating malignancy risk (Incidence rate ratio) in PsA compared with RA* (Continued)
| Model 1 | Model 2 | |
|---|---|---|
| History of medication exposure | ||
| non-methotrexate DMARD | 1.00 | |
| methotrexate | 1.55 (p=0.018)(1.08, 2.23) | |
| anti-TNF | 1.05 (p=0.692)(0.84, 1.31) | |
| other Biologic | 1.14 (p=0.513)(0.77, 1.69) | |
| Current medication use | ||
| non-methotrexate DMARD without current or history of methotrexate or biologic | 1.00 | |
| methotrexate, no current or prior biologic use | 1.77 (p=0.087)(0.92, 3.40) | |
| biologic, no current or prior methotrexate use | 1.42 (p=0.427)(0.60, 3.34) | |
| biologic with current or prior methotrexate use | 1.29 (p=0.449)(0.67, 2.48) | |
| non-methotrexate DMARD, prior methotrexate, but no current or history of biologic | 1.60 (p=0.319)(0.64, 4.02) |
Results shown as Incident rate ratios with 95% confidence intervals and p-values
Acknowledgments
The CORRONA registry is currently supported by Abbott, Amgen, Astra Zeneca, Genentech, Janssen, Lilly, Pfizer, and UCB through contracted subscriptions to the database. Funding for this project was provided by the American College of Rheumatology Research and Education Foundation Rheumatology Scientist Development Award and Empire Clinical Research Investigator Program (ECRIP) Award to Dr. Broder. However, the study design, data analysis, and reporting of results of this study were performed independent of all funding sources.
References
- 1.Chen YJ, Chang YT, Wang CB, Wu CY. The risk of cancer in patients with rheumatoid arthritis: a nationwide cohort study in Taiwan. Arthritis Rheum. 2011;63(2):352–358. doi: 10.1002/art.30134. [DOI] [PubMed] [Google Scholar]
- 2.Hakulinen T, Isomaki H, Knekt P. Rheumatoid arthritis and cancer studies based on linking nationwide registries in Finland. Am J Med. 1985;78(1A):29–32. doi: 10.1016/0002-9343(85)90242-6. [DOI] [PubMed] [Google Scholar]
- 3.Mellemkjaer L, Linet MS, Gridley G, Frisch M, Moller H, Olsen JH. Rheumatoid arthritis and cancer risk. Eur J Cancer. 1996;32A(10):1753–1757. doi: 10.1016/0959-8049(96)00210-9. [DOI] [PubMed] [Google Scholar]
- 4.Thomas E, Brewster DH, Black RJ, Macfarlane GJ. Risk of malignancy among patients with rheumatic conditions. Int J Cancer. 2000;88(3):497–502. [PubMed] [Google Scholar]
- 5.Boffetta P, Gridley G, Lindelof B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol. 2001;117(6):1531–1537. doi: 10.1046/j.0022-202x.2001.01520.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen YJ, Wu CY, Chen TJ, Shen JL, Chu SY, Wang CB, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65(1):84–91. doi: 10.1016/j.jaad.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 7.Olsen JH, Moller H, Frentz G. Malignant tumors in patients with psoriasis. J Am Acad Dermatol. 1992;27(5 Pt 1):716–722. doi: 10.1016/0190-9622(92)70244-a. [DOI] [PubMed] [Google Scholar]
- 8.Frentz G, Olsen JH. Malignant tumours and psoriasis: a follow-up study. Br J Dermatol. 1999;140(2):237–242. doi: 10.1046/j.1365-2133.1999.02655.x. [DOI] [PubMed] [Google Scholar]
- 9.Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Invest Dermatol. 2006;126(10):2194–2201. doi: 10.1038/sj.jid.5700410. [DOI] [PubMed] [Google Scholar]
- 10.Reddy SM, Anandarajah AP, Fisher MC, Mease PJ, Greenberg JD, Kremer JM, et al. Comparative analysis of disease activity measures, use of biologic agents, body mass index, radiographic features, and bone density in psoriatic arthritis and rheumatoid arthritis patients followed in a large U.S. disease registry. J Rheumatol. 2010;37(12):2566–2572. doi: 10.3899/jrheum.100483. [DOI] [PubMed] [Google Scholar]
- 11.Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54(3):692–701. doi: 10.1002/art.21675. [DOI] [PubMed] [Google Scholar]
- 12.Rohekar S, Tom BD, Hassa A, Schentag CT, Farewell VT, Gladman DD. Prevalence of malignancy in psoriatic arthritis. Arthritis Rheum. 2008;58(1):82–87. doi: 10.1002/art.23185. [DOI] [PubMed] [Google Scholar]
- 13.Kremer J. The CORRONA database. Ann Rheum Dis. 2005;64(Suppl 4):iv37–iv41. doi: 10.1136/ard.2005.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreyer L, Mellemkjaer L, Andersen AR, Bennett P, Poulsen UE, Juulsgaard Ellingsen T, et al. Incidences of overall and site specific cancers in TNFalpha inhibitor treated patients with rheumatoid arthritis and other arthritides - a follow-up study from the DANBIO Registry. Ann Rheum Dis. 2013;72(1):79–82. doi: 10.1136/annrheumdis-2012-201969. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56(9):2886–2895. doi: 10.1002/art.22864. [DOI] [PubMed] [Google Scholar]
- 16.Fisher MC, Furer V, Hochberg MC, Greenberg JD, Kremer JM, Curtis JR, et al. Malignancy validation in a United States registry of rheumatoid arthritis patients. BMC Musculoskelet Disord. 2012;13:85. doi: 10.1186/1471-2474-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon WG, Carmona L, Finckh A, Hetland ML, Kvien TK, Landewe R, et al. EULAR points to consider when establishing, analysing and reporting safety data of biologics registers in rheumatology. Ann Rheum Dis. 2010;69(9):1596–1602. doi: 10.1136/ard.2009.125526. [DOI] [PubMed] [Google Scholar]
- 18.Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63(3):573–586. doi: 10.1002/art.30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cameron AC, Trivedi PK. Regression analysis of count data. Cambridge, UK; New York, NY, USA: Cambridge University Press; 1998. [Google Scholar]
- 20.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 21.Alonso-Ruiz A, Pijoan JI, Ansuategui E, Urkaregi A, Calabozo M, Quintana A. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metaanalysis of efficacy and safety. BMC Musculoskelet Disord. 2008;9:52. doi: 10.1186/1471-2474-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leombruno JP, Einarson TR, Keystone EC. The safety of anti-tumour necrosis factor treatments in rheumatoid arthritis: meta and exposure-adjusted pooled analyses of serious adverse events. Ann Rheum Dis. 2009;68(7):1136–1145. doi: 10.1136/ard.2008.091025. [DOI] [PubMed] [Google Scholar]
- 23.Askling J, van Vollenhoven RF, Granath F, Raaschou P, Fored CM, Baecklund E, et al. Cancer risk in patients with rheumatoid arthritis treated with anti-tumor necrosis factor alpha therapies: does the risk change with the time since start of treatment? Arthritis Rheum. 2009;60(11):3180–3189. doi: 10.1002/art.24941. [DOI] [PubMed] [Google Scholar]
- 24.Mercer LK, Davies R, Galloway JB, Low A, Lunt M, Dixon WG, et al. Risk of cancer in patients receiving non-biologic disease-modifying therapy for rheumatoid arthritis compared with the UK general population. Rheumatology (Oxford) 2013;52(1):91–98. doi: 10.1093/rheumatology/kes350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariette X, Matucci-Cerinic M, Pavelka K, Taylor P, van Vollenhoven R, Heatley R, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70(11):1895–1904. doi: 10.1136/ard.2010.149419. [DOI] [PubMed] [Google Scholar]
- 26.Dommasch ED, Abuabara K, Shin DB, Nguyen J, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64(6):1035–1050. doi: 10.1016/j.jaad.2010.09.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariette X, Cazals-Hatem D, Warszawki J, Liote F, Balandraud N, Sibilia J, et al. Lymphomas in rheumatoid arthritis patients treated with methotrexate: a 3-year prospective study in France. Blood. 2002;99(11):3909–3915. doi: 10.1182/blood.v99.11.3909. [DOI] [PubMed] [Google Scholar]
- 28.Georgescu L, Quinn GC, Schwartzman S, Paget SA. Lymphoma in patients with rheumatoid arthritis: association with the disease state or methotrexate treatment. Semin Arthritis Rheum. 1997;26(6):794–804. doi: 10.1016/s0049-0172(97)80023-6. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki M, Hirano S, Ito H, Matsubara D, Kubota K, Takeda Y, et al. Pulmonary lymphoma developed during long-term methotrexate therapy for psoriasis. Respirology. 2007;12(5):774–776. doi: 10.1111/j.1440-1843.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 30.Paul C, Le Tourneau A, Cayuela JM, Devidas A, Robert C, Molinie V, et al. Epstein-Barr virus-associated lymphoproliferative disease during methotrexate therapy for psoriasis. Archives of dermatology. 1997;133(7):867–871. [PubMed] [Google Scholar]
- 31.Salloum E, Cooper DL, Howe G, Lacy J, Tallini G, Crouch J, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14(6):1943–1949. doi: 10.1200/JCO.1996.14.6.1943. [DOI] [PubMed] [Google Scholar]
- 32.Kremer JM. Is methotrexate oncogenic in patients with rheumatoid arthritis? Semin Arthritis Rheum. 1997;26(6):785–787. doi: 10.1016/s0049-0172(97)80021-2. [DOI] [PubMed] [Google Scholar]
- 33.Moder KG, Tefferi A, Cohen MD, Menke DM, Luthra HS. Hematologic malignancies and the use of methotrexate in rheumatoid arthritis: a retrospective study. Am J Med. 1995;99(3):276–281. doi: 10.1016/s0002-9343(99)80160-0. [DOI] [PubMed] [Google Scholar]
- 34.Bologna C, Picot MC, Jorgensen C, Viu P, Verdier R, Sany J. Study of eight cases of cancer in 426 rheumatoid arthritis patients treated with methotrexate. Ann Rheum Dis. 1997;56(2):97–102. doi: 10.1136/ard.56.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchbinder R, Barber M, Heuzenroeder L, Wluka AE, Giles G, Hall S, et al. Incidence of melanoma and other malignancies among rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum. 2008;59(6):794–799. doi: 10.1002/art.23716. [DOI] [PubMed] [Google Scholar]
- 36.Solomon DH, Kremer JM, Fisher M, Curtis JR, Furer V, Harrold LR, et al. Comparative cancer risk associated with methotrexate, other non-biologic and biologic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum. 2013 doi: 10.1016/j.semarthrit.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Bailin PL, Tindall JP, Roenigk HH, Jr, Hogan MD. Is methotrexate therapy for psoriasis carcinogenic? A modified retrospective-prospective analysis. JAMA. 1975;232(4):359–362. [PubMed] [Google Scholar]
- 38.Nyfors A, Jensen H. Frequency of malignant neoplasms in 248 long-term methotrexate-treated psoriatics. A preliminary study. Dermatologica. 1983;167(5):260–261. doi: 10.1159/000249793. [DOI] [PubMed] [Google Scholar]
- 39.Stern RS. Lymphoma risk in psoriasis: results of the PUVA follow-up study. Archives of dermatology. 2006;142(9):1132–1135. doi: 10.1001/archderm.142.9.1132. [DOI] [PubMed] [Google Scholar]
- 40.Husni ME, Mease PJ. Managing comorbid disease in patients with psoriatic arthritis. Curr Rheumatol Rep. 2010;12(4):281–287. doi: 10.1007/s11926-010-0112-3. [DOI] [PubMed] [Google Scholar]
- 41.Athas WF, Hunt WC, Key CR. Changes in nonmelanoma skin cancer incidence between 1977–1978 and 1998–1999 in Northcentral New Mexico. Cancer Epidemiol Biomarkers Prev. 2003;12(10):1105–1108. [PubMed] [Google Scholar]
- 42.Christenson LJ, Borrowman TA, Vachon CM, Tollefson MM, Otley CC, Weaver AL, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294(6):681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 43.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30(5 Pt 1):774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 44.Gottlieb AB, Gordon K, Giannini EH, Mease P, Li J, Chon Y, et al. Clinical trial safety and mortality analyses in patients receiving etanercept across approved indications. J Drugs Dermatol. 2011;10(3):289–300. [PubMed] [Google Scholar]
- 45.Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA, Pollono EN, Cueto JP, Gonzales-Crespo MR, et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. JAMA. 2012;308(9):898–908. doi: 10.1001/2012.jama.10857. [DOI] [PubMed] [Google Scholar]