Abstract
In retinal transplantation experiments it is hypothesized that remaining diseased photoreceptor cells in the host retina and inner retinal cells in transplants physically obstruct the development of graft-host neuronal contacts which are required for vision. Recently, we developed methods for the isolation of donor photoreceptor layers in vitro, and the selective removal of host photoreceptors in vivo using biodegradable elastomeric membranes composed of poly(glycerol-co-sebacic acid) (PGS). We also coated PGS membranes with electrospun nanofibers, composed of laminin and poly(epsilon-caprolactone) (PCL), to promote attachment of embryonic retinal explants, allowing the resulting composites to be handled surgically as a single entity. Here, we report subretinal transplantation of these composites into adult porcine eyes. In hematoxylin and eosin stained sections of composite explants after 5–7 days in vitro, excellent fusion of retinas and biomaterial membranes was noted, with the immature retinal components showing laminated as well as folded and rosetted areas. The composite grafts could be transplanted in all cases and, 3 months after surgery, eyes displayed clear media, attached retinas and the grafts located subretinally. Histological examination revealed that the biomaterial membrane had degraded without any signs of inflammation. Transplanted retinas displayed areas of rosettes as well as normal lamination. In most cases inner retinal layers were present in the grafts. Laminated areas displayed well-developed photoreceptors adjacent to an intact host retinal pigment epithelium and degeneration of the host outer nuclear layer (ONL) was often observed together with occasional fusion of graft and host inner layers.
Keywords: Cell adhesion, Elastomer, Nanotopography, Polycaprolactone, Retina, Transplantation
1. Introduction
Retinal transplantation experiments have the common goal of alleviating symptoms in patients suffering from retinal degeneration by replacing diseased photoreceptors with healthy cells. In the past two decades, a multitude of experimental approaches involving many different animal models have been explored but, to date, retinal transplantation is not available as a clinical treatment.
To transfer the retinal transplantation paradigm from the laboratory to the clinic, several key requirements must first be demonstrated. First, photoreceptors must survive the transplantation procedure and avoid rejection in the foreign host. Once in place, grafted cells have to organize properly and display structural properties as well as biochemical machinery compatible with phototransduction. Finally, the transduced signal must be transferred from the grafted cells to host neurons in a manner which allows visual perception.
Early protocols of retinal transplantation involved enzymatically and mechanically disrupted donor tissue in soluble form which, with comparatively little surgical effort, could be transferred to the confined subretinal space of the host eye. These experiments, as well as more recent ones built on the concept of cell-suspensions of retinal, progenitor or stem cells, have shown that disruption of the donor tissue may not be beneficial for graft survival, development, organization and integration [1–4].
To improve graft survival and organization, transplantation of non-disrupted tissue in the form of full-thickness retina has been explored by a few groups [5–8]. The rationale of the full-thickness paradigm is to limit antigen exposure to the host immune system, and retain the laminated organization of the donor retina with its delicate intrinsic microcircuitry. From experiments in large-eye animal models, the surgical procedure for full-thickness transplantation has been shown to be safe and relatively atraumatic to the eye in both normal and diseased eyes. Further, full-thickness grafts develop and retain morphologically normal photoreceptors for extended time periods in the foreign environment. In accordance with the initial hypothesis, these grafts elicit almost no immune response, as long as the graft is kept intact in both allogeneic as well as xenogeneic donor tissue paradigms [9–11].
The remaining issue that needs to be resolved pending clinical trials is the formation of functional connective neuronal networks so that transduced signals from grafted photoreceptors can be transferred to the host CNS. Graft-host neuronal connections have been demonstrated to some extent in the rabbit model [12,13] but not in the more relevant porcine one, in which integration of the two entities is hampered by the presence of remaining inner retinal cells in the transplant and remaining photoreceptors in the host [14]. To enhance the chance of useful integration of host and graft retina, these physical barriers should ideally be removed.
We have previously described the creation of a graft retina composed of isolated photoreceptors supported by Müller cells in an in vitro rodent model [15]. This was accomplished by separating immature full-thickness retinas from their inherent retinal circulation, which, in combination with optic nerve transection, caused regression of inner retinal layer development. Next, we were able to remove photoreceptor cells from a defined area in adult rabbit eyes without damaging remaining inner retinal cells [16]. The photoreceptor cells are dependent on nutritive support from the choroid and, by implanting a 50 μm thick biodegradable PGS elastomer in the subretinal space of normal rabbits, selective outer retinal ischemia was accomplished. Implantation of the PGS membrane, however, did inflict damage on the retinal pigment epithelium. To limit the risk of this and other complications, transplantation of the neuroretinal tissue and implantation of the PGS membrane in the same session would be preferable. To this end, we constructed a composite graft consisting of an immature porcine full-thickness retina fused with a degradable PGS membrane [17]. We found that the PGS membranes coated with electrospun nanofibers adhered very well to the retina after 7 days of co-culture in vitro, and did not separate after placement and ejection from the transplantation instrument. The addition of the PGS membrane had no observed adverse effects on the developing donor tissue.
In the present study, we explore transplantation of the PGS-retina composite graft. We hypothesize that a graft composed of well-organized photoreceptors within a Müller glia cell scaffold fused with a biodegradable membrane will have an increased likelihood of recreating the complete retina with outer layers derived from the graft and inner retinal layers from the host (Fig. 1).
Fig. 1.
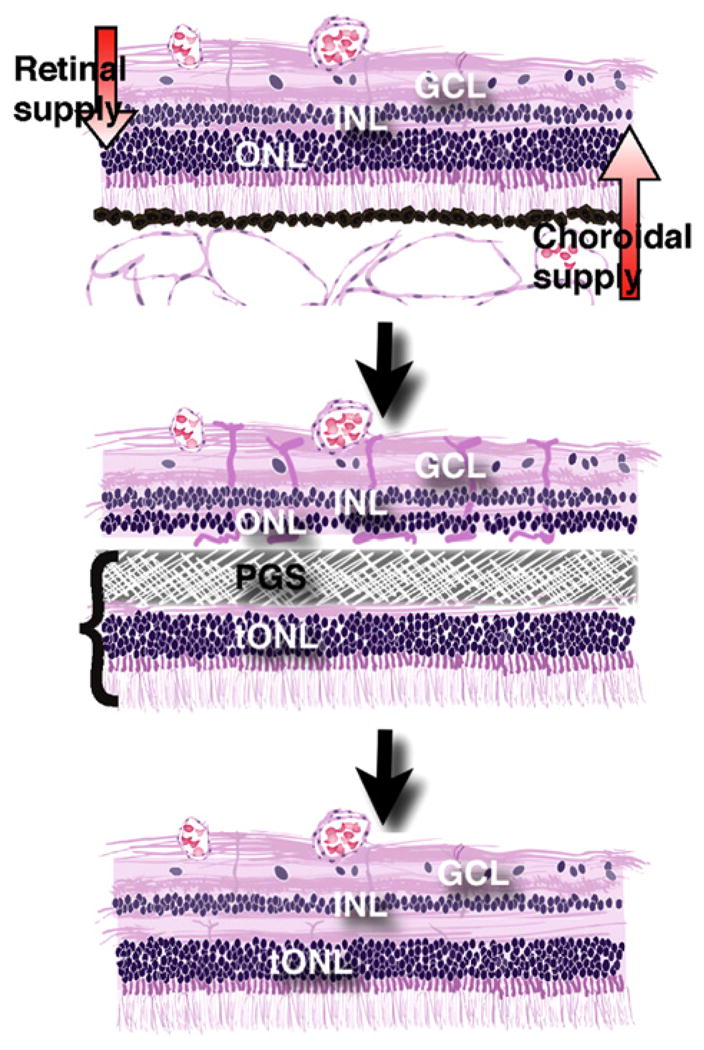
Illustration of the composite retinal graft model. Top: The in vivo host retina is dependent on a dual blood supply. The choroid supplies the photoreceptors in the outer nuclear layer (ONL) while the retinal vessels supply the inner retinal cells in the inner nuclear and ganglion cell layer (INL and GCL). Middle: When the composite graft, consisting of photoreceptors in a transplant outer nuclear layer (tONL) fused with a PGS membrane, is placed in the subretinal space, the membrane blocks the nutritive support to the host ONL which induces ischemia and removes host photoreceptors. Bottom: Following PGS membrane degradation, the remaining inner retina of the host integrates with the transplanted photoreceptors (tONL) creating a new retina with all normal layers.
2. Materials and methods
All compounds and solvents were obtained from Sigma–Aldrich, St. Louis, MO, USA, unless otherwise stated.
2.1. Preparation of poly(glycerol-co-sebacic acid) membranes
Poly(glycerol-co-sebacic acid) (PGS) branched polymer was prepared by poly-condensation as previously described [18]. Briefly, equimolar amounts (0.989 mol) of re-crystallized sebacic acid and anhydrous glycerol were charged to an oven-dried round bottom flask and reacted with stirring at 120 °C under argon. After 21 h, vacuum was applied and the mixture was reacted for another 64 h. The resulting material formed a white wax at room temperature.
Cross-linking of the PGS to an elastomer was carried out in a class 10,000 clean room [16,19]. A 10:1 prepolymer:hardener mixture of poly(dimethylsiloxane) (PDMS) (Sylgard 184, Dow Corning, Midland, MI) was degassed under vacuum and cured in a 10 cm Petri dish for 1 h at 60 °C. The resulting 10 cm diameter PDMS slab was plasma oxidized for 1 min to create a hydrophilic surface. The top surface was spin coated at 3000 rpm for 30 s with a 61.5 wt. % aqueous sucrose solution (saturated at room temperature and 0.45 μm syringe filtered). The sucrose-coated PDMS was subsequently baked at 130 °C for 10 min and transferred to a hotplate at 120 °C. Molten PGS wax (120 °C) was poured onto the sucrose-coated PDMS slab, covering the entire surface, and subsequently cured at 120 °C under a 15 millitorr vacuum until the PGS lost any adhesiveness to a stainless steel spatula.
Subsequently, the mold was submerged in 18 MOhm water overnight and the PGS was peeled from the PDMS underwater. The resultant PGS disk was approximately 3 mm thick.
The PGS disk was cut into 5 × 5 × 3 mm blocks, embedded in Tissue-Tek OCT compound (Sakura, Torrance, CA) and rapidly frozen by immersion in ethanol cooled with dry ice [17]. Frozen PGS was cryo-sectioned into 30 μm membranes at −30 °C with a Shur/Sharp Heavy Duty razor blade (TBS, Durham, NC). Thicker blades (0.5 mm or greater) and slow sectioning speeds were necessary to reduce vibration and warming of the blade from friction. Membrane thickness and uniformity was confirmed by scanning electron microscopy.
2.2. Fabrication of electrospun nanofibers
Nanofibers consisting of poly(epsilon-caprolactone) (PCL) laminin and a blend of 10 wt. % laminin in PCL of approximately 100 nm were electrospun onto 30 micron PGS sheets to create a bi-layer biomaterial membrane. Neuroretinal explants were subsequently placed on a culture membrane with the outer layers facing downward and the PGS-nanofiber membranes on top, with the nanofiber side of the PGS sheet placed onto the inner layers of the explant. Type 1 Laminin was isolated from murine Englebreth-Swarm-Holme tumor as previously described [20]. The laminin was provided as a gift by Stemgent, Inc. (Cambridge, MA). Electrospinning followed previously described protocols [17,21]. For laminin nanofibers, lyophilized laminin was dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFP) at 3 w/v % at 4 °C overnight. The solution was then mounted into an Aladdin syringe pump (World Precision Instruments, Sarasota, FL) using a disposable syringe and an 18 gauge needle. PGS sheets mounted on glass cover slips were placed on the collector plate, and the laminin solution was dispensed at a flow rate of 1 mL/h and driving voltage of 15 kV across a 15 cm vertical gap onto the grounded copper collector, and hence onto the PGS sheets. Samples were dried completely and stored in a dessicant at −20 °C. Laminin-PCL blend nanofibers were electrospun in a similar manner. A solution consisting of 10 wt. % laminin and 90 wt. % PCL with 8 w/v % total polymer dissolved in HFP was used as the initial solution. The same procedure as for laminin was used to electrospin the blend onto PGS sheets, though the driving voltage was increased to 20 kV to compensate for the higher viscosity of this solution. 18 wt. % PCL was dissolved in 1:1 THF:DMF and electrospun at 20 kV in the same manner to fabricate PCL nanofibers.
2.3. Porcine tissue isolation
Eyes from Yorkshire/Hampshire pigs (gestational age of 114 days) were obtained from a local breeder (Lund, Sweden) and used for all experiments. Retinal tissue from E40 (40 days after conception) was used for culturing experiments. The pregnant sow was euthanized by means of captive bolt and incision of the carotid arteries. The fetuses were collected by caesarean section and euthanized by decapitation. Both eyes were enucleated and immediately immersed in ice-cold CO2-independent medium. The neuroretinas were carefully dissected free from the retinal pigment epithelium (RPE) and hyaloid vascular system with fine forceps. The optic nerve was thereafter cut with micro-scissors and neuroretinal pieces measuring approximately 3 × 4 mm were explanted on Millicell®-HA 0.45 μm culture plate inserts with the photoreceptor layer toward the membrane. All neuroretinas were put in culture within 240 min of euthanasia.
Explants were cultured in 1.2 mL Gibco D-MEM F12 medium L-Glutamine (Gibco, Paisley, UK) supplemented with 10 vol. % fetal calf serum. A cocktail containing 2 mm L-glutamine, 100 U/mL penicillin and 100 ng/mL streptomycin was added and the retinas were maintained at 37 °C with 95% humidity and 5% CO2.
All proceedings and treatment of animals were in accordance with the guidelines and requirements of the Government Committee on Animal Experimentation at Lund University and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.4. Co-culture of retinal layers with biomaterial membranes
After two days in vitro, PCL-PGS and Laminin-PCL-PGS membranes measuring 3 × 4 mm were added to the explanted retinal specimens, while remaining pieces were kept in culture without the addition of a membrane as controls. At this time, half of the culture medium was exchanged, and this procedure was then repeated every second day. The composite explants were kept for 5–7 days in vitro. At the end of the culture period, the PGS membrane had attached firmly to the neuroretina.
To establish the fate of the composite explants prior to transplantation, neuroretinal explants with and without PGS membranes were fixed and prepared for histology at the end of the culture period. For these experiments, culture plate inserts were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.2, for 4 h at 4 °C. Several rinses in PB followed, and then the specimens were infiltrated with 0.1 M PB containing sucrose in increasing concentrations, and finally embedded for cryosectioning. After serial sectioning on a cryostat, every 10th slide was stained with hematoxylin and eosin. Photographs were obtained with a digital camera system (Olympus, Tokyo, Japan) and were adjusted for brightness and contrast digitally.
2.5. Surgery
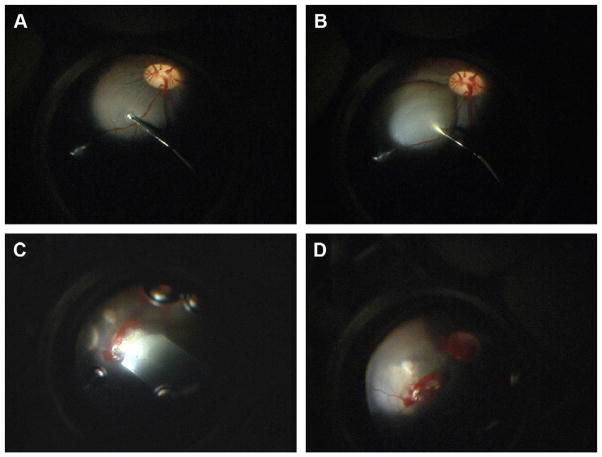
Twelve normal Yorkshire/Hampshire pigs, aged 3 months were used as hosts and received one PGS-retina composite graft in the right eye (PCL-PGS n = 5, and Laminin-PCL-PGS n = 7). The surgical procedure for full-thickness transplantation has been described in detail in previous articles [14]. In summary, a two-port central vitrectomy, including a posterior vitreous detachment, was performed, after which a local retinal bleb was created in a vessel-free area 2–3 mm superior and nasal to the optic nerve by infusing Ames’ medium subretinally (Fig. 2). A second retinotomy was then made to provide a valve. The compound graft, consisting of PGS membrane and neuroretina was transferred from the culture system to an embryonic cryodish filled with Ames’ solution. The Ames’ solution was exchanged several times, to ensure that no culture medium remained with the explants. The graft was drawn into a polyethylene tube (Becton Dickinson and company, MD, USA) attached to a syringe filled with Ames’ solution. The polyethylene tube had an original inner diameter of 1.4 mm and an outer diameter of 1.9 mm, and was flattened mechanically to accommodate the flat PGS-retina graft. The tube was introduced into the eye, and advanced until its tip was adjacent to the first retinotomy. The graft was then eased out, into the subretinal space. Eyes from the first and second day of surgery received a 3 × 4 mm graft, and in these eyes, some bleeding from the retinotomy was evident. On the second day of surgery, smaller 2 × 3 mm grafts were used which reduced the incidence of retinal hemorrhage.
Fig. 2.
Intra-operative photographs, demonstrating the formation of a subretinal bleb (A and B) and introduction of the graft into the subretinal space (C and D).
2.6. Postoperative management and follow-up
No postoperative treatment was given. Eyes were examined externally daily for the first week. An ophthalmoscopic examination was performed on one occasion, 4–6 days postoperatively. At the end of the follow-up period, animals were euthanized by means of captive bolt and incision of the carotid arteries. The eyes were enucleated, a cut made at the pars plana, and the eyes were fixed in 4% paraformaldehyde in 0.1 M PB, pH 7.2, for 30 min. The anterior segment was then removed and the posterior eyecup was fixed in the same solution for 4 h. After fixation, the specimens were handled in the same way as the explants fixed directly after culture (see above).
All procedures and animal treatment were in accordance with the guidelines and requirements of the Government Committee on Animal Experimentation at Lund University and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
3. Results
3.1. Macroscopic findings
At ophthalmoscopic examination, most eyes displayed clear media with the graft placed flat in the subretinal space. One pig showed signs of infection (blepharitis and slight opacification of the cornea) and was terminated 5 days postoperatively. At dissection, the interior of this eye was clear, and the graft was found in place in the subretinal space. Remaining eyes were followed for 3 months. At dissection, these eyes displayed clear media and an attached retina with no signs of inflammation. In most cases, the graft was visible in the subretinal space as a rectangular gray tissue (Fig. 3).
Fig. 3.
Dissected eyecup 3 months after transplantation of a PGS-Retina explant. The graft is present as a gray rectangular shape under the folded host retina.
3.2. Histology
3.2.1. PGS-retina explants
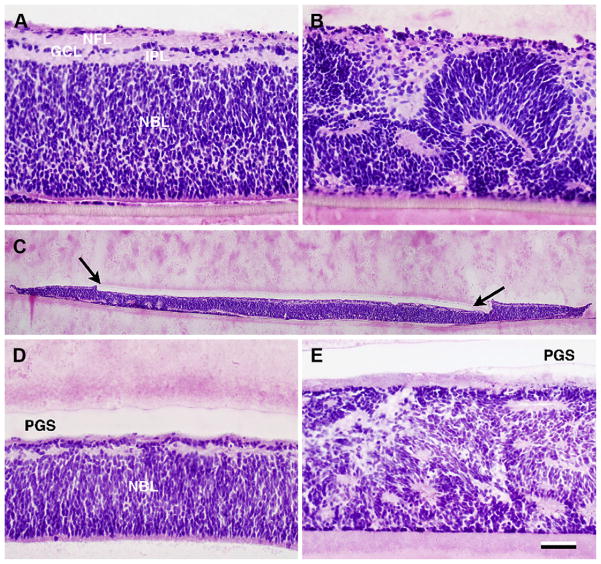
In hematoxylin and eosin stained sections, explants cultured for a total of 7–9 days displayed areas of laminated retina as well as areas with folded retina and rosettes (Fig. 4). Retinal explants without addition of PGS membrane (n = 7) displayed minimal folding/rosettes in one case, mixed rosettes and lamination in 4 cases, and in 2 cases mostly rosettes. Laminin-PCL-PGS explants (n = 5) displayed minimal folding/rosettes in 2 cases, mixed rosettes and lamination in one case, and in 2 cases mostly rosettes. PCL-PGS explants (n = 6) had minimal folding/rosettes in 3 cases, mixed rosettes and lamination in 2 cases, and in one case mostly rosettes. Laminated areas of explants without PGS membrane displayed a neuroblastic cell layer (NBL) consisting of multiple rows of undifferentiated cells, a thin inner plexiform layer (IPL), a ganglion cell layer (GCL) with one row of cells and a nerve fiber layer (NFL) in the innermost part (Fig. 4A). In composite explants, the PGS membrane could be seen well-attached to the inner surface of the immature neuroretina (Fig. 4C–E). Laminated areas were thinner compared to specimens without PGS membrane and consisted of an NBL, a thin IPL and GCL and no discernible NFL (Fig. 4D).
Fig. 4.
Donor tissue after 7–9 days in vitro. Hematoxylin and eosin staining. A–B: E40 neuroretinal explants without addition of PGS membrane. In A, a well laminated explant is seen with a neuroblastic cell layer (NBL) consisting of multiple rows of undifferentiated cells, a thin inner plexiform layer (IPL), a ganglion cell layer (GCL) with 1 row of cells and a nerve fiber layer NFL in the innermost part. In B severe folding of the retinal architecture is seen in another explant. C–E: PGS-Retina explants: In C, the full extent of the composite explant with the Laminin-PCL-PGS membrane (between arrows) attached to the inner retina can be seen. D: Detail. The explanted retina displays an NBL and rudimentary inner layers. E: Rosetted explant without any apparent lamination. Scale bar = 50 μm (A–B and D–E) and 500 μm (C).
3.2.2. Transplanted eyes
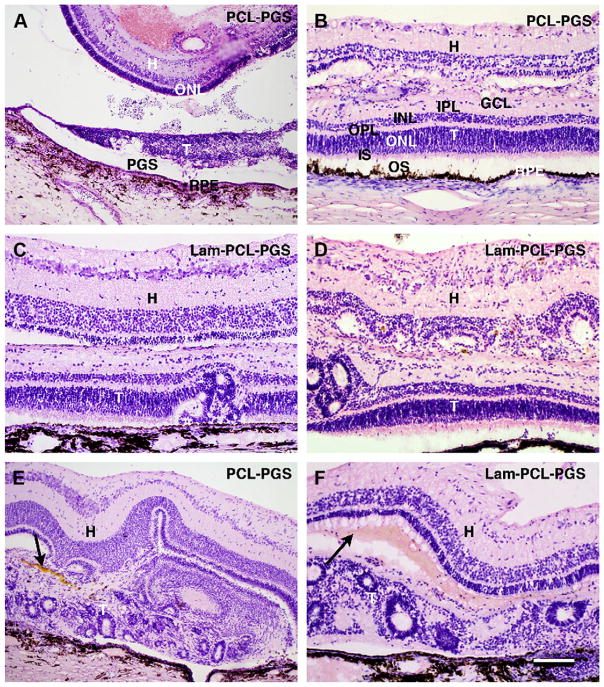
In hematoxylin and eosin stained sections, the one eye with a PCL-PGS composite graft terminated on postoperative day 5 displayed the graft upside down in the subretinal space, with the membrane adjacent to the host retinal pigment epithelium (RPE), and the grafted neuroretina severely degenerated (Fig. 5A). The host retina had an epiretinal hemorrhage in the transplantation area and the outer nuclear layer (ONL) was thinner than normal.
Fig. 5.
PGS-Retina transplants. Hematoxylin and eosin staining. A: PCL-PGS composite graft 5 days postoperatively. The transplant (T) is upside down in the subretinal space with the membrane (PGS) adjacent to the host Retinal Pigment Epithelium (RPE). The grafted neuroretina in severely degenerated. The host retina (H) has an epiretinal hemorrhage and the outer nuclear (ONL) is thinner than normal. B–D: 3-month transplants (T). B–D shows laminated areas where the graft consists of outer segments (OS) apposed to the host Retinal Pigment Epithelium (RPE), inner segments (IS), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), and cells with small perikarya in the ganglion cell layer (GCL). In the host retina (H), the ONL is absent in B and D and severely reduced in C. Fusion of the graft with the inner nuclear layer is evident in B and D while the two entities remain separate in C. E and F show rosetted transplants areas with yellowish and reddish material between the host and graft (arrows). Scale bar = 200 μm (A), and 100 μm (B–F).
In transplanted eyes 3 months postoperatively, grafts were found in all 11 eyes which had received a PGS-retina composite graft (Fig. 5B–E). Eyes with Laminin-PCL-PGS composite grafts (n = 7) displayed a laminated retina with minimal folding/rosettes in one case, mixed rosettes and lamination in 2 cases and mostly rosettes in 4 cases. Eyes with PCL-PGS composite grafts (n = 4) displayed a laminated retina with minimal folding/rosettes in one case, with the remaining 3 consisting mostly of rosettes. Laminated areas of 3-month composite grafts displayed outer segments apposed to the host RPE, inner segments, a well-developed ONL, an outer plexiform layer (OPL), INL, thin IPL and cells with small perikarya in the GCL. The host RPE was found to be continuous in all areas where a laminated graft was found. In rosetted areas, the RPE was occasionally discontinuous, and in one case, an invasion of cells from the choroid was evident. No other signs of inflammation were seen. The host retina appeared normal in all eyes, except for the part covering the transplant. Here, the photoreceptors in the ONL had degenerated to varying degrees. In some sections no ONL was found, while in others, 3–4 rows of cells without outer segments remained. The inner retinal layers were preserved in most cases but occasionally disruption of the INL was evident. The PGS membrane could not be identified in any 3-month specimen. However, between graft and host, especially in areas of rosette formation, yellowish deposits were found in 5 specimens (4 Lam-PCL-PGS and 1 PCL-PGS), and reddish material in one case, suggestive of remnants of foreign material possibly derived from the degraded biomaterial membrane (Fig. 5D–E). Occasionally fusion of graft and host retina was seen and the innermost layers of the graft had integrated with the host INL.
4. Discussion
4.1. Surgical considerations
In the present paper we have explored the concept of retinal transplantation in combination with a degradable membrane for the purpose of enhancing graft-host neuronal integration. Eyes transplanted with the PGS-retina graft fared well postoperatively, displaying clear media, attached retina, and the graft securely in place in the subretinal space, suggesting that the surgical procedure for composite graft transplantation works efficiently.
Transplantation of full-thickness retina has been reported in several animal models previously [6–8,14]. In earlier work in pig and rabbit eyes, we used a siliconized glass cannula for transplantation in which the elastic retinal graft adopted a rolled-up shape. This allowed us to use a relatively small sclerotomy and retinotomy. In the present study, the added PGS membrane made the composite graft less flexible, requiring a different approach using a wider device in which the graft could be kept flat. This, in turn, necessitated a wider sclerotomy and retinotomy, which was associated with some degree of retinal hemorrhage. We are currently developing a more flexible biomaterial membrane, with a lower elastic modulus, to reduce the risk of complications. This may be accomplished by changing the curing time of PGS to reduce the degree of cross-linking, or the selection of different monomer precursors [22,23].
4.2. Transplanted retina
Surviving grafts were found in all transplanted eyes confirming the low degree of antigenicity of full-thickness retina. This also suggests that the addition of PGS membrane does not provoke immune rejection. Laminated areas of the grafts displayed well-developed photoreceptors organized in an outer nuclear layer comparable in size to the one found in the normal adult porcine retina. However, somewhat surprisingly, inner layers also developed and were retained, which hampered neuronal contacts between grafted photoreceptors and the host inner retina. In a previously published paper where E45–E50 porcine retina cultures were explored, we found that addition of PGS membrane drastically suppressed inner retinal cell development while photoreceptors were unaffected [17]. Except for the use of E40 instead of E45–50 porcine retina, the same culturing protocol as in our previous work was used in the present work. In spite of several steps which should theoretically ensure photoreceptor isolation (optic nerve transection, separation from the developing retinal circulation, and placement of PGS membrane on the inner part of the explants), inner layers were observed consistently in transplanted eyes. Cell type differentiation in the porcine retina commences at approximately E39–40 with a large population of cells still retained in an undifferentiated state [24]. The relative immaturity of the E40 specimens may thus account for a seemingly normal inner retinal development in spite of the culturing process. The results suggest that, to obtain photoreceptor isolation in the graft, more mature donor tissue should be used.
The PGS-retina composite grafts all displayed areas of rosette formation. Rosettes are an abnormal form of retinal development where the retina forms folds and then rolls of retinal tissue. The phenomenon has been attributed to abnormal development, and is not seen in the adult retina, even after trauma [25]. The exact origin of rosettes has not been established but they were first described in tumors (retinoblastoma) and later in retinal cultures, as well as in fragmented retinal transplants. Rosettes have been described in full-thickness retinal transplants but, in our previous work, laminated areas have invariably dominated the more atypical rosetted architecture [14]. Photoreceptors in rosettes display short or absent outer segments due to lack of proper contact with the RPE. In addition, visual processing in the retina is completely dependent on a precise organization of the retinal subtypes for transduction, as well as visual processing, and it is unlikely for rosetted grafts to result in functional restoration of vision. A strong association between Müller cell trauma and rosette formation in the developing retina has been postulated [25]. In the present work, the E40 retinal explants with or without added PGS membrane displayed a considerable degree of folding and rosette formation, indicating that the culturing procedure prior to transplantation induces abnormal retinal development. In our previous paper on E45–E50 porcine retina cultures, we found only minimal folding [17]. Further, the addition of PGS membrane attenuated this phenomenon. As mentioned above, E39–40 is a critical time-point in porcine retinal development and it is possible that disturbance of early cell type differentiation disrupts retinal lamination [24]. Again, this indicates that the use of more mature donor tissue may be favorable.
4.3. Host retina
One of the main aims of the present work was to eliminate host photoreceptors without disturbing the inner retinal architecture. In all specimens, the ONL was reduced in the part straddling the transplant and, in some areas, it was completely missing while inner retinas were generally well-preserved. The reduction of ONL was more prominent compared with our earlier work involving transplantation of full-thickness retinas into porcine eyes. This suggests that the PGS membrane contributed to host photoreceptor death [14]. However, the host ONL was still present in some areas, indicating that a membrane with a longer degradation time may be even more effective. This may be accomplished by the selection of different monomer precursors with altered hydrophobicity in order to reduce the rate of hydrolysis of ester bonds in the elastomer [22,23].
5. Conclusion
We show that a composite graft composed of retinal tissue fused with a PGS membrane can be transplanted into an adult porcine eye with minimal complications. The PGS component of the graft degrades without signs of inflammation after inducing selective elimination of host photoreceptors. The remaining grafted retina survives well but often displays an atypical retinal architecture in the form of rosettes which can already be observed prior to transplantation in vitro. In spite of the culturing procedure, inner retinal layers develop and persist in laminated areas of the graft which hampers proper integration of graft photoreceptors with the remaining inner retina of the host. Future work will be directed toward optimization of graft retinal architecture prior to transplantation.
Acknowledgments
We thank Kurt Broderick for assistance with clean room operation (MTL, MIT, Cambridge, MA), Nikki Watson assistance with cryosectioning (Whitehead Institute, Cambridge, MA), Rebekah Neal for electrospinning (University of Virginia, Charlottesville, VA), and Kerry Mahon for providing lab space and materials for electrospinning (Stemgent, Inc., Cambridge, MA). We thank Elizabeth Pritchard for writing assistance (Starnberg, Germany). This work was supported by The Faculty of Medicine, University of Lund, The Swedish Research Council, The Princess Margaretas Foundation for Blind Children, the Torsten and Ragnar Söderberg Foundation, the National Institutes of Health (Grants DE013023 and HL060435), and the Richard and Gail Siegal Gift Fund. C.D.P. was supported by a MIT/CIMIT Medical Engineering Fellowship and a gift to MIT by InVivo Therapeutics Corporation.
Appendix
Figures with essential color discrimination. Figs. 1–5 in this article are difficult to interpret in black and white. The full color images can be found in the on-line version, at doi:10.1016/j. biomaterials.2010.07.026.
References
- 1.Turner JE, Blair JR. Newborn rat retinal cells transplanted into a retinal lesion site in adult host eyes. Brain Res. 1986;391:91–104. doi: 10.1016/0165-3806(86)90011-8. [DOI] [PubMed] [Google Scholar]
- 2.Juliusson B, Bergström A, van Veen T, Ehinger B. Cellular organization in retinal transplants using cell suspensions or fragments of embryonic retinal tissue. Cell Transpl. 1993;2:411–8. doi: 10.1177/096368979300200509. [DOI] [PubMed] [Google Scholar]
- 3.MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–7. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 4.Canola K, Angénieux B, Tekaya M, Quiambao A, Naash MI, Munier FL, et al. Retinal stem cells transplanted into models of late stages of retinitis pigmentosa preferentially adopt a glial or a retinal ganglion cell fate. Invest Ophthalmol Vis Sci. 2007;48(1):446–54. doi: 10.1167/iovs.06-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuschereba ST, Silverman MS. Retinal cell and photoreceptor transplantation between adult New Zealand red rabbit retinas. Exp Neurol. 1992;115(1):95–9. doi: 10.1016/0014-4886(92)90228-i. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh F, Arnér K, Ehinger B. Transplant of full-thickness embryonic rabbit retina using pars plana vitrectomy. Retina. 1998;18:136–42. doi: 10.1097/00006982-199818020-00007. [DOI] [PubMed] [Google Scholar]
- 7.Seiler MJ, Aramant RB. Intact sheets of fetal retina transplanted to restore damaged rat retinas. Invest Ophthalmol Vis Sci. 1998;39(11):2121–31. [PubMed] [Google Scholar]
- 8.Seiler MJ, Aramant RB, Seeliger MW, Bragadottir R, Mahoney M, Narfström K. Functional and structural assessment of retinal sheet allograft transplantation in feline hereditary retinal degeneration. Vet Ophthalmol. 2009;12:158–69. doi: 10.1111/j.1463-5224.2009.00693.x. [DOI] [PubMed] [Google Scholar]
- 9.Wassélius J, Ghosh F. Adult rabbit retinal transplants. Invest Ophthalmol Vis Sci. 2001;42:2632–8. [PubMed] [Google Scholar]
- 10.Engelsberg K, Ghosh F. Transplantation of cultured adult porcine full-thickness retina. Cell Transpl. 2007;16(1):31–9. doi: 10.3727/000000007783464506. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh F, Rauer O, Arnér K. Neuroretinal xenotransplantation to immunocompetent hosts in a discordant species combination. Neuroscience. 2008;152:526–33. doi: 10.1016/j.neuroscience.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh F, Bruun A, Ehinger B. Graft-host connections in long-term full thickness embryonic rabbit retinal transplants. Invest Ophthalmol Vis Sci. 1999;40:126–32. [PubMed] [Google Scholar]
- 13.Ghosh F, Johansson K, Ehinger B. Long-term full-thickness embryonic rabbit retinal transplants. Invest Ophthalmol Vis Sci. 1999;40:133–40. [PubMed] [Google Scholar]
- 14.Ghosh F, Arnér K. Transplantation of full-thickness retina in the normal porcine eye: surgical and morphologic aspects. Retina. 2002;22:478–86. doi: 10.1097/00006982-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh F, Arnér K, Engelsberg K. Isolation of photoreceptors in the cultured full-thickness fetal rat retina. Invest Ophthalmol Vis Sci. 2009;50:826–35. doi: 10.1167/iovs.08-2389. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh F, Neeley WL, Arnér K, Langer R. Selective removal of photoreceptor cells in vivo using the biodegradable elastomer poly(Glycerol sebacate) Tissue Eng Part A. doi: 10.1089/ten.tea.2008.0450. Not available-, ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pritchard CD, Arnér KM, Neal RA, Neeley WL, Bojo P, Bachelder E, et al. The use of surface modified poly(glycerol-co-sebacic acid) in retinal transplantation. Biomaterials. 2010;31(8):2153–62. doi: 10.1016/j.biomaterials.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Ameer GA, Sheppard BJ, Langer RS. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–6. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 19.Neeley WL, Redenti S, Klassen H, Tao S, Desai T, Young MJ, et al. A micro-fabricated scaffold for retinal progenitor cell grafting. Biomaterials. 2008;29 (4):418–26. doi: 10.1016/j.biomaterials.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin and heparin sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–93. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 21.Neal RA, McClugage SG, Link MC, Sefcik LS, Ogle RC, Botchwey EA. Laminin nanofiber meshes that mimic morphological properties and bioactivity of basement membranes. Tissue Eng Part C Methods. 2009;15:11–21. doi: 10.1089/ten.tec.2007.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettinger CJ, Bruggeman JP, Borenstein JT, Langer RS. Amino alcohol-based degradable poly(ester amide) elastomers. Biomaterials. 2008;29(15):2315–25. doi: 10.1016/j.biomaterials.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruggeman JP, de Bruin BJ, Bettinger CJ, Langer R. Biodegradable poly(polyol sebacate) polymers. Biomaterials. 2008;29(36):4726–35. doi: 10.1016/j.biomaterials.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh F, Arnér K. Cell type differentiation dynamics in the developing porcine retina. Dev Neurosci. 2010;32:47–58. doi: 10.1159/000261704. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh F, Juliusson B, Arnér K, Ehinger B. Partial and full-thickness neuroretinal transplants. Exp Eye Res. 1999;68:67–74. doi: 10.1006/exer.1998.0582. [DOI] [PubMed] [Google Scholar]