Abstract
BACKGROUND
Blood transfusions are common during hematopoietic stem cell transplantation (HSCT) and may contribute to lung injury.
STUDY DESIGN AND METHODS
This study examined the associations between red blood cell (RBC) and platelet (PLT) transfusions and idiopathic pneumonia syndrome (IPS) among 914 individuals who underwent myeloablative allogeneic HSCT between 1997 and 2001. Patients received allogeneic blood transfusions at their physicians' discretion. RBCs, PLTs, and a composite of “other” transfusions were quantified as the sum of units received each 7-day period from 6 days before transplant until IPS onset, death, or Posttransplant Day 120. RBC and PLT transfusions were modeled as separate time-varying exposures in proportional hazards models adjusted for IPS risk factors (age, baseline disease, irradiation dose) and other transfusions. Timing of PLT transfusion relative to myeloid engraftment and PLT ABO blood group (match vs. mismatch) were included as potential interaction terms.
RESULTS
Patients received a median of 9 PLT and 10 RBC units. There were 77 IPS cases (8.4%). Each additional PLT unit transfused in the prior week was associated with 16% higher IPS risk (hazard ratio, 1.16; 95% confidence interval, 1.09–1.23; p < 0.001). Recent RBC and PLT transfusions were each significantly associated with greater risk of IPS when examined without the other; only PLT transfusions retained significance when both exposures were included in the model. The PLT association was not modified by engraftment or ABO mismatch.
CONCLUSION
PLT transfusions are associated with greater risk of IPS after myeloablative HSCT. RBCs may also contribute; however, these findings need confirmation.
Red blood cell (RBC) and platelet (PLT) transfusions are among the most common therapies administered to patients undergoing hematopoietic stem cell transplantation (HSCT).1,2 While lifesaving in some settings, transfusions are also associated with adverse outcomes in critically ill patients including death,3–6 nosocomial infections,3,7 and acute lung injury (ALI).8,9 After HSCT, the development of ALI of noninfectious origin is termed idiopathic pneumonia syndrome (IPS). IPS is a syndrome, defined as widespread noninfectious alveolar injury leading to abnormal pulmonary physiology that is not due to cardiac or renal dysfunction.10 IPS typically occurs within 120 days of HSCT and is associated with 60% to 80% mortality.11–14 Recognized clinical risk factors for IPS include myeloablative conditioning, age over 40 years at time of transplant, severe (Grade III–IV) acute graft-versus-host-disease (GVHD), high-dose total body irradiation (TBI; greater than 12 Gy), and acute leukemia or myelodysplastic syndrome as indication for transplant.10–18 Although blood component transfusion is an established risk factor for ALI in critically ill and injured patients,5,6,9,19–21 the association between transfusions and development of IPS after HSCT has not previously been examined.
The biologic mechanisms linking transfusion to lung injury are unknown; however, research focusing on transfusion-related acute lung injury (TRALI) has led to the emergence of several hypotheses.22–24 An immune-mediated theory involves the interaction between passively transferred donor antibodies against the recipient's human leukocyte antigen (HLA I and II) or human neutrophil antigen (HNA) and recipient white blood cells (WBCs) resulting in immune activation and subsequent lung injury.24–33 While passive transfusion of cognate WBC antibody alone can cause TRALI,34 it is not always sufficient.35–37 The proposed “two-hit” hypothesis suggests that WBC antibodies38 or biologically active substances that accumulate in stored blood components, such as lysophosphatidylcholine species of bioactive lipids,39–41 prime neutrophils and produce lung injury in the setting of an underlying inflammatory insult.42–44 Under the latter hypothesis, PLTs potentially mediate lung injury by interacting with and activating neutrophils and pulmonary vascular endothelium.45,46 In animal models, experimentally reducing host PLT function decreases the risk of TRALI46 and prolongs survival.47 Conceivably, patients treated with HSCT may enter an “inflammatory” state due to the toxic effects of conditioning regimens, engraftment, and/or other immune factors that render them susceptible to similar mechanisms of transfusion-mediated lung injury. In this study, we examined the associations between RBC and PLT transfusions and subsequent development of IPS after HSCT. Our a priori hypothesis was that IPS risk would increase within a week of receiving either RBC or PLT transfusions. We also investigated whether the associations between transfusions and IPS were modified by the timing of transfusion relative to neutrophil engraftment or by receipt of ABO blood group major or minor mismatched PLT units.
MATERIALS AND METHODS
Cohort description, eligibility, and definitions
We performed a retrospective study of patients who underwent myeloablative allogeneic HSCT at the Fred Hutchinson Cancer Research Center (FHCRC) between December 1997 and 2001. The cohort of 1100 myeloablative and nonmyeloablative HSCT recipients was previously studied to identify risk factors for IPS;13 however, blood component transfusions were not specifically examined as potential IPS risk factors. The present analysis was restricted to the subgroup of 917 individuals receiving myeloablative conditioning regimens from which 95% of IPS cases were identified to compare patients with more homogeneous baseline IPS risk factors and transfusion needs. Patients were observed until Posttransplant Day 120, during which time it was expected that the majority of IPS cases would be ascertained.12,15 Three patients were excluded due to withdrawal of consent or imprisonment, leaving 914 available for analysis. All required data elements for this study were present within a rigorously validated research database, including the type, timing, and ABO status of all blood component transfusions.13 This study was approved by the FHCRC Institutional Review Board.
We defined ABO blood group mismatch according to the Puget Sound Blood Center protocol (Seattle, WA), considering a transfused unit to be a match only if identical to both HSCT recipient and stem cell donor blood type.48–50 A minor mismatch was defined as possible isoagglutinin (anti-ABO) antibody in the transfused product against the HSCT donor or recipient cells. A major mismatch represents possible isoagglutinin antibody in the transfusion recipient against the transfused cells. Actual isoagglutinin titers were not available. We defined the time of myeloid engraftment as the first of 3 consecutive days on which the patient had an absolute neutrophil count greater than 500 × 106/L.51
Exposures
Patients were transfused blood at their medical providers' discretion. All blood products transfused to cohort members were prepared at Puget Sound Blood Center according to Food and Drug Administration regulations. RBC components were prepared using US standard centrifugation while PLT units could be produced via single-donor apheresis or by pooling four to six donors' whole blood–derived concentrates. All patients were transfused leukoreduced blood components, which were accepted as “cytomegalovirus (CMV) safe,” up until the day of transplant. After infusion of stem cells or marrow, patients who were CMV seronegative before transplant continued to receive blood components considered CMV safe by leukofiltration or CMV screening. Pooled PLT components underwent filtration for leukoreduction at the time of issue. Apheresis PLTs not meeting leukoreduction standards at the time of collection were filtered at the time of issue. RBC components could have been filtered anytime before issue. Patients who were CMV seropositive could receive CMV-unscreened, nonleukoreduced units after transplant, although may have times received CMV safe components due to inventory. When inventory management practices required the administration of PLTs with plasma ABO incompatible with either donor or recipient, centrifugation to remove all but 100 mL of plasma was performed before issue.
For all analyses, blood transfusions were grouped as RBCs, PLTs, and “other” which included cryoprecipitate, granulocytes, plasma, and whole blood. As transfusion requirements varied throughout the posttransplant period, we modeled blood component transfusions as time-varying exposure variables for all analyses, further described below. We considered only RBC and PLT units administered before the first day of IPS development as units of exposure because the temporal relationship of transfusion relative to IPS onset was unknown for units administered on the day a patient developed IPS.
Outcome
As previously described by Fukuda and colleagues13 each patient's IPS status was determined via abstraction of clinical, radiographic, microbiologic, and histopathologic reports. Two physicians, blinded to the number and timing of blood component transfusions, assigned IPS determinations using standard definitions. IPS was defined using the 1993 National Heart, Lung, and Blood Institute workshop criteria which include multilobar infiltrates on chest imaging, signs or symptoms of pneumonia, abnormal pulmonary physiology characterized by increased alveolar-arterial oxygen difference or new or increased restrictive ventilatory abnormality, and absence of active lower respiratory tract infection by bronchoscopic lavage, lung biopsy, or autopsy.52 IPS was excluded if lavage cultures grew 104 or more colony-forming units of bacteria per milliliter, grew a lung pathogen in the setting of compatible radiographic findings, or met European Organization for Research and Treatment of Cancer and Mycoses Study Group criteria for fungal pneumonia.53,54 Patients with nondiagnostic lavage who responded to antimicrobial therapy and those with suspected fluid overload responsive to diuretics were not considered to have IPS. The date of IPS onset is the first day on which both abnormal chest imaging and pulmonary physiology were noted.
Statistical analysis
We determined cumulative incidences of IPS according to the number of RBC or PLT transfusions received in the 7 days before IPS onset. We used Cox proportional hazards regression models to estimate the independent associations between RBC and PLT transfusion and IPS development.55 RBC, PLT, and other blood component transfusions were individually modeled as time-varying exposure variables defined by running weekly totals.56 The weekly transfusion total was the sum of units received during the 7 days before the analysis day, updated daily for each patient until IPS development, death, or Posttransplant Day 120. Time 0 for the time-to-IPS analysis was the day of transplant, but transfusion values from up to 6 days before transplant were included in the weekly sums when available. We assessed departure from the proportional hazards assumption two ways: first, an interaction term of the exposure with the natural logarithm of analysis day was included in each model; second, we qualitatively compared adjusted hazard ratios (HRs) over four time frames defined by quartiles of IPS onset. Three primary models were examined: one including only the number of RBC transfusions administered as the main exposure, a second examining only the number of PLT transfusions administered as the main exposure, and a third model including both RBCs and PLTs as exposures.
Based on prior studies, the following factors were considered as potential confounders: age at transplant (40 years and under vs. over 40 years), TBI dose (0, 12, or >12 Gy), original disease (chronic myelogenous leukemia or nonmalignant disease, acute leukemia or myelodysplastic syndrome, or other malignancy), stem cell source (marrow or peripheral blood), presence of Grade III to IV acute GVHD, and the weekly transfusion total of any non-RBC and non-PLT blood component.10–18,44,57,58 Acute GVHD was modeled as a time-dependent covariate. To maximize the precision of the estimated HR for the primary outcome, only factors that altered the HR for transfusion's effect on IPS by 10% or more were included in the model.59
Two factors were considered as potential interaction terms (multiplicative scale). First, it is hypothesized that transplanted stem cell engraftment stimulates endothelial inflammation.60,61 Engraftment may prime neutrophils to induce lung injury once exposed to blood component transfusion. As a result, we examined whether the associations between PLT and RBC transfusions and IPS varied by myeloid engraftment status. Forty patients were missing an engraftment day and thus we assumed did not engraft before death. Second, limited inventories commonly necessitate transfusing HSCT recipients with minor, major, or bidirectionally ABO-mismatched PLTs. As prior studies demonstrate decreased survival and more frequent acute GVHD in individuals receiving ABO-mismatched HSCT,62,63 we also examined whether the association between PLT transfusion and IPS varied by receipt of major- or minor-mismatched PLT transfusions. A patient's weekly transfusion total was classified as mismatched if at least one of the units received was mismatched. Fifty-five patients lacked ABO blood group records, resulting in 859 patients available for the interaction term analysis.
Finally, a nested case–control study was performed as a confirmatory analysis of the same associations hypothesized a priori and to increase the ease of interpreting the primary analysis. Each IPS case was matched to two controls on days-from-transplant sampled from the set of patients under study at the time of IPS occurrence. To describe levels of exposure among cases and controls, we categorized RBC, PLT, and other transfusions as zero, 1 to 2, or more than 2 units transfused during the 7 days before the matched day. RBC and PLT sums for the week of interest were also modeled as continuous exposures in a conditional logistic regression model adjusted for days-from-transplant and sums of other components transfused that week. While the primary analysis incorporates each patient's complete transfusion records over time, this analysis examines only the transfusions given in the 7 days before IPS onset among cases, and 7 days before the same posttransplant day among controls still at risk for IPS. To produce estimates comparable to those from the primary analysis, three separate multivariable models were fit including the confounders identified in the proportional hazards analyses (described previously).
Reported p values are two-sided and based on the Wald statistic. Statistical analyses were performed with statistical software (SAS 9, SAS Institute, Inc., Cary, NC; or Stata 12, StataCorp LP, College Station, TX).
RESULTS
Baseline characteristics
Patients in the full cohort were predominantly male and self-reported as Caucasian (Table 1). Fifty-six percent were transplanted for acute leukemia or myelodysplastic syndrome, 39% for chronic leukemia or nonmalignant indication, and 5% for other malignancies such as multiple myeloma. Approximately two-thirds of HSCT recipients were given grafts from marrow source, and the remainder from peripheral blood stem cells. Conditioning therapy included TBI for 58% of patients. One-quarter developed severe acute GVHD. Of the 914 patients at risk, 77 (8%) developed IPS (Fig. 1). IPS onset occurred between Days 4 and 106, with half of all cases occurring by Day 22 and 70% of cases in the first 30 days after transplant.
TABLE 1.
Characteristics of HSCT recipients and grafts
| Patient characteristics | |
| Age (years) | 41 (31–49) |
| Less than 40 | 434 (47) |
| 40 or older | 480 (53) |
| Male | 502 (55) |
| Race, in order of frequency | |
| White | 743 (82) |
| Asian/Pacific Islander | 51 (6) |
| Hispanic | 50 (6) |
| Black | 13 (1) |
| Native American | 13 (1) |
| Other | 33 (4) |
| Transplant characteristics | |
| Stem cell sources | |
| Bone marrow | 598 (65) |
| Peripheral blood | 311 (34) |
| Both of the above | 5 (1) |
| Second transplant | 21 (2) |
| Stem cell donor | |
| Related, HLA matched | 428 (47) |
| Related, HLA mismatched | 55 (6) |
| Unrelated | 431 (47) |
| CMV discordant, donor positive | 134 (15) |
| Dose of TBI | |
| No irradiation | 385 (42) |
| 12 Gy | 256 (28) |
| More than 12 Gy | 273 (30) |
| Methotrexate used | 874 (96) |
| Acute GVHD | |
| None | 145 (16) |
| Grade I–II | 537 (59) |
| Grade III–IV | 229 (25) |
| Days to engraftment (n = 879)† | 19 (16–23) |
Data are reported as median (IQR) or number (%).
Data were missing for 1% or less of records unless otherwise noted.
Fig. 1.
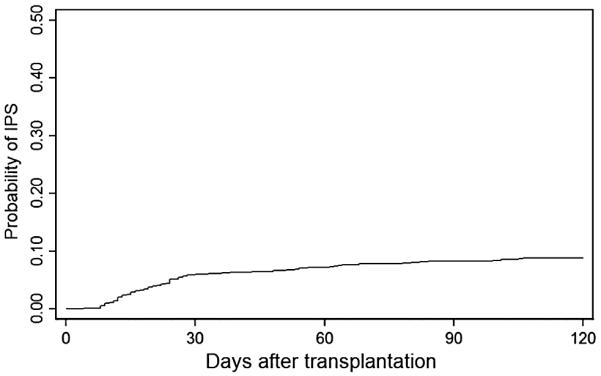
Cumulative incidence of IPS. IPS occurred within 120 days of transplant in 8.4% of 914 patients who underwent myeloablative allogeneic HSCT, 1997 to 2001
Distribution of RBC, PLT, and other transfusions
Approximately one-third of PLTs transfused to the whole cohort were obtained via apheresis and two-thirds from whole blood with multidonor pooling. The median number of transfusions each individual received was 9 PLT units (interquartile range [IQR], 4–19) and 10 RBC units (IQR 5–18). The probability of having received at least 1 RBC unit by Day 30 was 88%, and at least 1 PLT unit was 99%. Both RBC and PLT transfusions were more common early after transplant (Fig. 2). Fifty percent of all RBC transfusions were administered by Posttransplant Day 24 and 75% by Day 67. On average, patients were transfused RBC on 5 separate days (range, 0–34 days). The median weekly sum of RBC transfusions was 2 units between Days 9 and Day 20 posttransplant and was zero during other times. In comparison, 50% of all PLT transfusions were administered by Day 19 post-HSCT and 75% by Day 56. On average, each patient received PLT transfusions on 8 days (range, 0–85 days). The median weekly sum of PLT transfusions ranged from 1 to 3 units between Day 5 and Day 24 after transplant and was zero at other times.
Fig. 2.
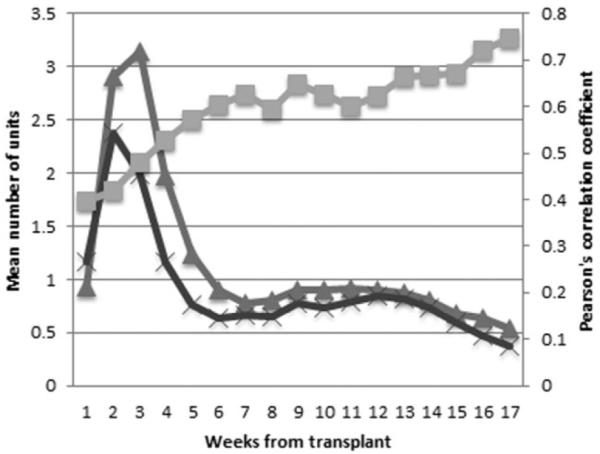
Mean weekly transfusion sums. Mean weekly sums of RBC and PLT transfusions were greatest early after HSCT with increasing correlation throughout observation (range, 0.40–0.75). ( ) Weekly PLT sum; (
) Weekly PLT sum; ( ) weekly RBC sum; (
) weekly RBC sum; ( ) RBC-PLT correlation.
) RBC-PLT correlation.
Throughout the observation period, 17 patients received granulocyte infusions and six received whole blood, representing 0.48% and 0.07% of all transfusions, respectively. Each of these patients also received RBC and PLT transfusions and none subsequently developed IPS. Plasma components were transfused to 178 total recipients, five of whom subsequently developed IPS. Only one of these five received no RBC or PLT in the week before IPS onset. In total, 4 units of cryoprecipitate were transfused to three patients, two of whom developed IPS after also receiving RBC and PLT transfusions in the prior 7 days. Some blood donor ABO records were missing. Among the 859 patients for whom ABO blood group data were complete, 403 (47%) received at least one ABO minor-mismatched unit and 480 (56%) received at least one ABO major-mismatched unit while at risk for IPS.
Association of RBC and PLT transfusions and development of IPS
Among the 914 transplant recipients, age at transplant, disease indicating transplant, and TBI dose were identified as confounders of the relationship between transfusion and IPS risk. In multivariable analysis adjusting for confounders and non-RBC transfusions, we observed a significant association between units of PLTs transfused in the previous week and the development of IPS (HR, 1.18; 95% confidence interval [CI], 1.13–1.24; p = 0.001; Table 2, separate model). The association between units of RBCs transfused and the development of IPS, adjusted for non-PLT blood transfusions and identified confounders, was also significant (HR, 1.17; 95% CI, 1.11–1.23; p < 0.001). However, when the model included the running weekly sum of both PLT and RBC transfusions, PLT transfusions were still associated with IPS (HR, 1.16; 95% CI, 1.09–1.23; p < 0.001) but the association for RBC transfusions was reduced (HR, 1.06; 95% CI, 0.98–1.14; p = 0.16; Table 2, combined models). There was no evidence of departure from the proportional hazards assumption.
TABLE 2.
Multiple Cox regression estimates of IPS risk according to RBC and/or PLT transfusions received in the prior 7 days
| Separate model* |
Combined model† |
|||
|---|---|---|---|---|
| Predictor | HR (95% CI) | p value | HR (95% CI) | p value |
| RBC weekly sum | 1.17 (1.11–1.23) | <0.001 | 1.06 (0.98–1.14) | 0.16 |
| PLT weekly sum | 1.18 (1.13–1.24) | <0.001 | 1.16 (1.09–1.23) | <0.001 |
Model adjusted for age at transplant, irradiation dose, disease indicating transplant, and the sum of non-RBC, non-PLT transfusions from the prior 7 days.
Model included both RBC and PLT transfusion from the prior 7 days in addition to adjusting for confounders.
The timing of transfusion relative to neutrophil engraftment was not observed to modify the association between RBC or PLT transfusion and IPS development (p = 0.99 and p = 0.30 for interaction terms, respectively; Table 3). Likewise, receipt of out-of-group PLT transfusions, represented as both minor and major ABO mismatch, did not modify the association between PLT transfusion and IPS (p = 0.53 and p = 0.49 for interactions, respectively; Table 3).
TABLE 3.
Multiple Cox regression estimates of IPS risk according to RBC and/or PLT transfusions received in the prior 7 days, engraftment status, and PLT ABO mismatch
| Transfusion type | Weekly sum of units | HR (95% CI) | p value |
|---|---|---|---|
| RBC* | Preengraftment | 1.06 (0.94–1.19) | 0.34† |
| Postengraftment | 1.06 (0.97–1.14) | 0.19 | |
| PLT‡ | Preengraftment | 1.15 (1.06–1.24) | <0.001† |
| Postengraftment | 1.18 (1.10–1.26) | <0.001 | |
| All plasma matched | 1.17 (1.10–1.25) | <0.001 | |
| One or more minor mismatch | 1.15 (1.07–1.24) | <0.001 | |
| All cell matched | 1.17 (1.09–1.25) | <0.001 | |
| One or more major mismatch | 1.14 (1.06–1.22) | <0.001 |
Model adjusted for the sums of PLT transfusions and non-PLT, non-RBC transfusions from the prior 7 days, as well as age, irradiation dose, and disease indicating transplant.
Wald statistic for interaction term of transfusion type by engraftment status gave p > 0.05.
Model adjusted for the sums of RBC transfusions and non-PLT, non-RBC transfusions from the prior 7 days and other confounders.
Results from the case–control study
The 77 patients who developed IPS received a median of 2 RBC units, 4 PLT units, and zero other units in the week before IPS onset (Table 4). By comparison, patients selected as controls received a median of 0 RBC units, 1 PLT unit, and 0 other units during the corresponding week. The percentages of cases who received RBC or PLT during the week were 77 and 78%, respectively (Table 5), in contrast to 46 and 59%, respectively, of controls. The multivariable conditional logistic regression analysis adjusted for confounders and weekly sum of non-PLT transfusions estimated that odds of IPS were 1.33 times greater per additional PLT unit transfused in the prior 7 days (odds ratio [OR], 1.33; 95% CI, 1.44–1.55; p = 0.02; Table 6, combined model). The association between units of RBCs transfused and the development of IPS adjusted for non-PLT blood transfusions and identified confounders (OR, 1.22; 95% CI, 1.06–1.40; p = 0.006) decreased when also adjusted for weekly sum of PLT transfusions (OR, 1.04; 95% CI, 0.88–1.22; p = 0.67).
TABLE 4.
Sum of RBC, PLT, and other blood component transfusions in the seven days before IPS onset among 77 cases and 154 controls matched on days-from-transplant
| RBCs |
PLTs |
Other components |
||||
|---|---|---|---|---|---|---|
| Measure | Case | Control | Case | Control | Case | Control |
| Mean (SD) | 2.7 (2.6) | 1.6 (2.3) | 4.5 (4.4) | 2.1 (2.8) | 1.2 (5.2) | 0.1 (0.9) |
| Median (IQR) | 2 (3) | 0 (2) | 4 (6) | 1 (3) | 0 (0) | 0 (0) |
| Range | 0–12 | 0–12 | 0–20 | 0–21 | 0–40 | 0–9 |
TABLE 5.
Percentage of IPS cases and controls exposed to RBC or PLT transfusions in the prior 7 days
| Exposure | Number of units | Cases (n = 77) | Controls (n = 154) |
|---|---|---|---|
| RBCs | 0 | 23.4 | 53.2 |
| 1–2 | 41.6 | 27.3 | |
| >2 | 35.1 | 19.5 | |
| PLTs | 0 | 22.1 | 40.9 |
| 1–2 | 14.3 | 23.4 | |
| >2 | 63.6 | 35.7 |
TABLE 6.
Multiple conditional logistic regression estimates of IPS risk according to RBC and/or PLT transfusions received in the prior 7 days
| Separate model* |
Combined model† |
|||
|---|---|---|---|---|
| Predictor | OR (95% CI) | p value | OR (95% CI) | p value |
| RBC sum | 1.22 (1.06–1.40) | 0.006 | 1.04 (0.88–1.22) | 0.669 |
| PLT sum | 1.35 (1.18–1.54) | <0.001 | 1.33 (1.44–1.55) | <0.001 |
Models adjusted for confounders and the sum of non-PLT, non-RBC transfusions from the prior 7 days.
Models included both RBC and PLT transfusions from the prior 7 days in addition to other confounders and sum of non-PLT, non-RBC transfusions from the prior 7 days.
DISCUSSION
The purpose of our study was to determine the degree to which RBC and PLT transfusions predispose patients receiving an allogeneic HSCT after myeloablative conditioning to IPS. We found both RBC and PLT transfusion to be extremely common, with 96 and 99% of patients transfused with these respective blood components within 120 days after HSCT. After adjusting for confounders, receipt of RBC and PLT components was associated with an estimated 17 and 18% higher IPS risk, respectively, per additional unit transfused in the prior week. The collinear relationship between RBC and PLT transfusions made it impossible to examine their independent effects on IPS development while controlling for one another, but there was a suggestion that PLT transfusions may be more determinative of IPS risk. The nested case–control model validated these results, with a similar pattern of associations identified.
The relationship between receipt of ABO-mismatched blood component transfusion and IPS has not been previously investigated. Approximately one-third of HSCT patients receive a minor, major, or bidirectionally ABO-mismatched transplant,64 which has been associated with shorter survival in some studies.62,63 We hypothesized a priori that IPS would be more likely among transfused patients receiving ABO-mismatched PLT. Out-of-group PLT transfusions were frequently administered to our population of HSCT recipients; however, the association between PLT transfusion and IPS development did not significantly differ according to PLT major or minor ABO mismatch status. At FHCRC, ABO-mismatched PLT transfusions are routinely plasma volume reduced before transfusion. It is possible either that ABO-mismatched PLT transfusions do not contribute importantly to IPS risk or that the related risk is abrogated by reducing plasma-suspended inflammatory mediators. To optimize allocation of limited blood supplies, it will be important to validate our finding of the apparent similar risk of ABO-mismatched PLT transfusion while considering anti-ABO titers and component plasma volume, which were not available for this analysis.
Engraftment syndrome encompasses tissue injury and endothelial inflammation occurring as transplanted cells engraft and may, like IPS, be mediated by systemic inflammation resulting in endothelial cell activation and dysfunction.60,61 In our cohort, the association between RBC or PLT transfusion and IPS did not differ by engraftment status of the transfusion recipient, reflecting that engraftment status was not associated with either the number of PLT transfusions received or the development of IPS.
Given the high frequency of blood product transfusion during HSCT, PLTs and RBCs may contribute in an important and potentially modifiable way to the incidence of IPS. There are no randomized human studies investigating the relationship between blood transfusion and lung injury in the HSCT population. Our study uniquely adds to the existing literature by specifically describing IPS in an allogeneic HSCT population and by examining blood transfusions as independent time-variable IPS risk factors.
Our study has noteworthy limitations. First, given the observational nature of the data, the results could be biased by residual confounding. Importantly, our findings could be consistent with the conclusion that transfusions contribute directly to IPS pathogenesis or alternatively that transfusions act as a marker of severe illness and are indirectly correlated with IPS. Adjusting for the known IPS clinical risk factors accounts for some portion of this indication bias, but possibly not all of it. Second, the cohort was transplanted 10 to 15 years ago at a single institution. It is possible that conditioning and treatment strategies during HSCT have changed since 2001 in a way that blood component exposure is now more or less important. For example, in 2006 the AABB recommended instituting “mitigation” strategies to reduce the occurrence of TRALI.65 These techniques could include precluding women with positive anti-HLA or -HNA screens from donating high-plasma-volume components (i.e., plasma, PLTs). Unfortunately, our data did not allow us to determine the timing of blood component transfusion relative to IPS onset when both occurred the same day. Consequently, we could not determine the percentage of IPS cases meeting the current consensus definition of TRALI.66 TRALI complicating HSCT may be underrecognized and is seldom reported;23,67–70 therefore, it is not known how often IPS represents TRALI and unclear how our results would be impacted by current mitigation techniques.
Another potential limitation relates to research involving clinical syndromes like IPS and ALI. By definition, IPS encompasses a heterogeneous mix of clinical diagnoses (i.e., drug-induced pneumonitis, engraftment syndrome, diffuse alveolar hemorrhage) and histopathologic correlates (i.e., interstitial pneumonitis, diffuse alveolar damage).10 Blood component transfusions might differentially modify mechanistic pathways specific to each lung injury phenotype. Our analyses were insensitive to phenotype-specific associations, which may diminish our ability to detect interactions and limit the generalizability of our findings to all clinical IPS phenotypes.
In conclusion, PLT transfusions were associated with greater risk of IPS in the first 120 days after myeloablative allogeneic HSCT. RBCs may also contribute, but collinearity between these frequent exposures is a challenge to analyzing their independent associations. Future work should seek to confirm these results using updated IPS criteria in the current era of transplant and transfusion practices as well as identify potentially modifiable blood donor and blood component processing characteristics (e.g., donor sex, component storage time) that contribute to IPS risk. Recent studies suggest that transfusing fewer PLTs to patients after chemotherapy or HSCT does not increase incidence of clinically severe bleeding.71–73 Prospective, randomized trials are needed to investigate whether more restrictive blood transfusion practices reduce incidence of or mortality from IPS.
ACKNOWLEDGMENTS
The authors recognize the data management contributions of Gary Schoch, Fred Hutchinson Cancer Research Center, Clinical Research Division, Seattle, Washington, and the scientific content and editorial contributions of Noel Weiss, MD, DrPH, University of Washington School of Public Health.
This research was supported by a grant from National Institute of General Medical Sciences NIGMS K23GM086729 (PI Timothy Watkins) and by research funds from the Puget Sound Blood Center, Seattle, WA.
ABBREVIATIONS
- ALI
acute lung injury
- FHCRC
Fred Hutchinson Cancer Research Center
- HSCT
hematopoietic stem cell transplantation
- IPS
idiopathic pneumonia syndrome
- TBI
total body irradiation
Footnotes
CONFLICT OF INTEREST DM is a paid consultant for Cerus Corporation, Concord, CA. The other authors report no conflicts of interest.
REFERENCES
- 1.Bernstein SH, Nadamanee AP, Vose JM, Tricot G, Fay JW, Negrin RS, DiPersio J, Rondon G, Champlin R, Barnett MJ, Cornetta K, Herzig GP, Giles G, Jr, Keating A, Messner H, Wolff SN, Miller KB, Linker C, Cairo M, Hellmann S, Ashby M, Stryker S, Nash RA. A multicenter study of platelet recovery and utilization in patients after myeloablative therapy and hematopoietic stem cell transplantation. Blood. 1998;91:3509–17. [PubMed] [Google Scholar]
- 2.Radia R, Pamphilon D. Transfusion strategies in patients undergoing stem-cell transplantation. Expert Rev Hematol. 2011;4:213–20. doi: 10.1586/ehm.11.14. [DOI] [PubMed] [Google Scholar]
- 3.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–774. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 4.Hebert PC, Wells G, Blajchmann MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 5.Netzer G, Shah CV, Iwashyna TJ, Lanken PN, Finkel B, Fuchs B, Guo W, Christ JD. Association of RBC transfusion with mortality in patients with acute lung injury. Chest. 2007;132:1116–23. doi: 10.1378/chest.07-0145. [DOI] [PubMed] [Google Scholar]
- 6.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33:1191–8. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 7.Shorr AF, Jackson WL, Kelly KM, Fu M, Kollef MH. Transfusion practice and blood stream infections in critically ill patients. Chest. 2005;127:1722–8. doi: 10.1378/chest.127.5.1722. [DOI] [PubMed] [Google Scholar]
- 8.Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O'Byrne MM, Evenson LK, Malinchoc M, DeGoey SR, Afessa B, Hubmayr RD, Moore SB. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176:886–91. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, Gajic O. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36:1518–22. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 10.Panoskaltsis-Mortari A, Griese M, Madtes DK, Belperio JA, Haddad IY, Folz RJ, Cooke KR. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183:1262–79. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawford SW, Hackman RC. Clinical course of idiopathic pneumonia after bone marrow transplantation. Am Rev Respir Dis. 1993;147:1393–400. doi: 10.1164/ajrccm/147.6_Pt_1.1393. [DOI] [PubMed] [Google Scholar]
- 12.Wingard JR, Mellits ED, Sostrin MD, Chen DY, Burns WH, Santos GW, Vriesendorp HM, Beschomer WE, Saral R. Interstitial pneumonitis after allogeneic bone marrow transplantation: nine-year experience at a single institution. Medicine (Baltimore) 1988;67:175–86. doi: 10.1097/00005792-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda T, Hackman RC, Guthrie KA, Sandmaier BM, Boeckh M, Maris MB, Maloney DG, Deeg HJ, Martin PJ, Storb RF, Madtes DK. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood. 2003;102:2777–85. doi: 10.1182/blood-2003-05-1597. [DOI] [PubMed] [Google Scholar]
- 14.Kantrow SP, Hackman RC, Boeckh M, Myerson D, Crawford SW. Idiopathic pneumonia syndrome: changing spectrum of lung injury after marrow transplantation. Transplantation. 1997;63:1079–86. doi: 10.1097/00007890-199704270-00006. [DOI] [PubMed] [Google Scholar]
- 15.Meyers JD, Gluornoy N, Thomas ED. Nonbacterial pneumonia after allogeneic marrow transplantation: a review of ten years' experience. Rev Infect Dis. 1982;4:1119–32. doi: 10.1093/clinids/4.6.1119. [DOI] [PubMed] [Google Scholar]
- 16.Weiner RS, Bortin MM, Gale RP, Gluckman E, Kay HE, Kolb HJ, Hartz AJ, Rimm AA. Interstitial pneumonitis after bone marrow transplantation: assessment of risk factors. Ann Intern Med. 1986;104:168–75. doi: 10.7326/0003-4819-104-2-168. [DOI] [PubMed] [Google Scholar]
- 17.Sampath S, Schultheiss TE, Wong J. Dose response and factors related to interstitial pneumonitis after bone marrow transplant. Int J Radiat Oncol Biol Phys. 2005;63:876–84. doi: 10.1016/j.ijrobp.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Zhu KE, Hu JY, Zhang T, Chen J, Zhong J, Lu YH. Incidence, risks, and outcome of idiopathic pneumonia syndrome early after allogeneic hematopoietic stem cell transplantation. Eur J Haematol. 2008;81:461–6. doi: 10.1111/j.1600-0609.2008.01149.x. [DOI] [PubMed] [Google Scholar]
- 19.Silverboard H, Aisiku I, Martin GS, Adams M, Rozycki G, Moss M. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. J Trauma. 2005;59:717–23. [PubMed] [Google Scholar]
- 20.Pereboom IT, de Boer MT, Haagsma EB, Hendriks HG, Lisman T, Porte RJ. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analg. 2009;108:1083–91. doi: 10.1213/ane.0b013e3181948a59. [DOI] [PubMed] [Google Scholar]
- 21.Rubenfeld GD, Herridge M. Epidemiology and outcomes of acute lung injury. Chest. 2007;131:554–62. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 22.Toy P, Popovsky MA, Abraham E, Ambruso DR, Holness LG, Kopko PM, McFarland JG, Nathens AB, Silliman CC, Stroncek D. Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33:721–6. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 23.Ganguly S, Carrum G, Nizzi F, Heslop HE, Popat U. Transfusion-related acute lung injury (TRALI) following allogeneic stem cell transplant for acute myeloid leukemia. Am J Hematol. 2004;75:48–51. doi: 10.1002/ajh.10452. [DOI] [PubMed] [Google Scholar]
- 24.Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI) Br J Haematol. 2007;136:788–99. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 25.Davoren A, Curtis BR, Shulman IA, Mohrbacher AF, Bux J, Kwiatkowska BJ, McFarland JG, Aster RH. TRALI due to granulocyte-agglutinating human neutrophil antigen-3a (5b) alloantibodies in donor plasma: a report of 2 fatalities. Transfusion. 2003;43:641–5. doi: 10.1046/j.1537-2995.2003.00374.x. [DOI] [PubMed] [Google Scholar]
- 26.Chapman CE, Stainsby D, Jones H, Love E, Massey E, Win N, Navarerete C, Lucas G, Soni N, Morgan C, Choo L, Cohen H, Williamson LM. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440–52. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 27.Reil A, Keller-Stanislawski B, Guenay S, Bux J. Specificities of leukocyte alloantibodies in transfusion-related acute lung injury and results of leukocyte antibody screening of blood donors. Vox Sang. 2008;95:313–17. doi: 10.1111/j.1423-0410.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- 28.Sachs UJ, Wasel W, Bayat B, Bohle RM, Hattar K, Berghofer H, Reil A, Bux J, Bein G, Santoso S, Weissmann N. Mechanism of transfusion-related acute lung injury induced by HLA class II antibodies. Blood Rev. 2011;2011:669–77. doi: 10.1182/blood-2010-05-286146. [DOI] [PubMed] [Google Scholar]
- 29.Kopko PM, Popovsky MA, MacKenzie MR, Paglierone TG, Muto KN, Holland PV. HLA class II antibodies in transfusion-related acute lung injury. Transfusion. 2001;41:1244–8. doi: 10.1046/j.1537-2995.2001.41101244.x. [DOI] [PubMed] [Google Scholar]
- 30.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–7. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 31.Bux J, Becker F, Seeger W, Kilpatrick D, Chapman J, Waters A. Transfusion-related acute lung injury due to HLA-A2-specific antibodies in recipient and NB1-specific antibodies in donor blood. Br J Haematol. 1996;93:707–13. doi: 10.1046/j.1365-2141.1996.d01-1703.x. [DOI] [PubMed] [Google Scholar]
- 32.Sachs UJ, Hattar K, Weissmann N, Bohle R, Weiss T, Sibelius U, Bux J. Antibody-induced neutrophil activation as a trigger for transfusion-related acute lung injury in an ex vivo rat lung model. Blood. 2006;107:1217–19. doi: 10.1182/blood-2005-04-1744. [DOI] [PubMed] [Google Scholar]
- 33.Saw CL, Hannach B, Petrazsko T, Nickerson P. Blood donors implicated in transfusion-related acute lung injury with patient-specific HLA antibodies are more broadly sensitized to HLA antigens compared to other blood donors. Transfusion. 2012;52:1–8. doi: 10.1111/j.1537-2995.2012.03766.x. [DOI] [PubMed] [Google Scholar]
- 34.Doreen MC, Ouwehand WH, Verhoeven AJ, von dem Borne AE, Kuijpers RW. Adult respiratory distress syndrome after experimental intravenous gamma-globulin concentrate and monocyte-reactive IgG antibodies. Lancet. 1998;352:1601–2. doi: 10.1016/s0140-6736(05)61049-5. [DOI] [PubMed] [Google Scholar]
- 35.Toy P, Hollis-Perry KM, Jun J, Nakagawa M. Recipients of blood from a donor with multiple HLA antibodies: a lookback study of transfusion-related acute lung injury. Transfusion. 2004;44:1683–8. doi: 10.1111/j.0041-1132.2004.04193.x. [DOI] [PubMed] [Google Scholar]
- 36.Kopko PM, Marshall CS, MacKenzie MR, Holland PV. Transfusion-related acute lung injury: report of a clinical look-back investigation. JAMA. 2002;287:1968–71. doi: 10.1001/jama.287.15.1968. [DOI] [PubMed] [Google Scholar]
- 37.Maslanka K, Michur H, Zupanska B, Uhrynowska M, Nowak J. Leucocyte antibodies in blood donors and a look back on recipients of their blood components. Vox Sang. 2007;92:247–9. doi: 10.1111/j.1423-0410.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 38.Van Buren NL, Stroncek DF, Clay ME, McCullough J, Dalmasso AP. Transfusion-related acute lung injury caused by an NB2 granulocyte-specific antibody in a patient with thrombotic thrombocytopenic purpura. Transfusion. 1990;30:42–5. doi: 10.1046/j.1537-2995.1990.30190117629.x. [DOI] [PubMed] [Google Scholar]
- 39.Silliman CC, Clay KL, Thurman GW, Johnson CA, Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–94. [PMC free article] [PubMed] [Google Scholar]
- 40.Maslanka K, Smolensa-Sym G, Michur H, Wrobel A, Lachert E, Brojer E. Lysophosphatidylcholines: bioactive lipids generated during storage of blood components. Arch Immunol Ther Exp (Warsz) 2012;60:55–60. doi: 10.1007/s00005-011-0154-x. [DOI] [PubMed] [Google Scholar]
- 41.Silliman CC, Moore EE, Kelher MR, Khan SY, Gellar L, Elzi DJ. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–54. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silliman CC, Bjornsen AJ, Wyman TH, Kelher MR, Allard J, Bieber S, Voelkel NF. Plasma and lipids from stored platelets cause acute lung injury in an animal model. Transfusion. 2003;43:633–40. doi: 10.1046/j.1537-2995.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 43.Silliman CC, Paterson AJ, Dickey WO, Stroneck DF, Popovsky MA, Caldwell SA, Ambruso DR. The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion. 1997;37:719–26. doi: 10.1046/j.1537-2995.1997.37797369448.x. [DOI] [PubMed] [Google Scholar]
- 44.Silliman CC, Boshkov LK, Mehdizadehkaahi Z, Elzi DJ, Dickey WO, Podlosky L, Clarke G, Ambruso DR. Transfusion-related acute lung injury: epidemiology and prospective analysis of etiologic factors. Blood. 2003;101:454–62. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 45.Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. J Clin Invest. 2006;116:1615–23. doi: 10.1172/JCI27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119:3450–61. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol. 2008;172:1–7. doi: 10.2353/ajpath.2008.070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fung MK, Downes KA, Shulman IA. Transfusion of platelets containing ABO-incompatible plasma. Arch Pathol Lab Med. 2007;121:909–16. doi: 10.5858/2007-131-909-TOPCAP. [DOI] [PubMed] [Google Scholar]
- 49.Lasky LC, Warkentin PI, Kersey HJ, Ramsay NK, McGlave PB, McCullough J. Hemotherapy in patients undergoing blood group incompatible bone marrow transplantation. Transfusion. 1983;23:277–85. doi: 10.1046/j.1537-2995.1983.23483276858.x. [DOI] [PubMed] [Google Scholar]
- 50.O'Donghaile D, Kelley W, Klein HG, Flegel WA. Recommendations for transfusion in ABO-incompatible hematopoietic stem cell transplantation. Transfusion. 2012;52:456–8. doi: 10.1111/j.1537-2995.2011.03465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rihn C, Cilley J, Naik P, Pedicano AV, Mehta J. Definition of myeloid engraftment after allogeneic stem cell transplantation. Haematologica. 2004;89:763–4. [PubMed] [Google Scholar]
- 52.Clark JG, Hansen JA, Hertz MI, Parkman R, Jensen L, Peavy HH. NHLBI workshop summary. Idiopathic Pneumonia Syndrome after bone marrow transplantation. Am Rev Respir Dis. 1993;147:1601–6. doi: 10.1164/ajrccm/147.6_Pt_1.1601. [DOI] [PubMed] [Google Scholar]
- 53.de Jaeger A, Litalien C, Lacroix J, Guertin MC, Infante RC. Protected specimen brush or bronchoalveolar lavage to diagnose bacterial nosocomial pneumonia in ventilated adults: a meta-analysis. Crit Care Med. 1999;27:2548–60. doi: 10.1097/00003246-199911000-00037. [DOI] [PubMed] [Google Scholar]
- 54.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Corokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PM, Stevens A, Walsh TJ. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 55.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society. Series B (Methodological) 1972;34:187–220. [Google Scholar]
- 56.Fisher LD, Lin DY. Time-dependent covariates in the cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–57. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 57.Ho VT, Weller E, Lee SJ, Alyea EP, Antin JH, Soiffer RJ. Prognostic factors for early severe pulmonary complications after HSCT. Biol Blood Marrow Transplant. 2001;7:233–9. doi: 10.1053/bbmt.2001.v7.pm11349809. [DOI] [PubMed] [Google Scholar]
- 58.Sachs UJ, Bux J. TRALI after the transfusion of cross-match-positive granulocytes. Transfusion. 2003;43:1683–6. doi: 10.1111/j.0041-1132.2003.00568.x. [DOI] [PubMed] [Google Scholar]
- 59.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis II: accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–10. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 60.Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011;46:1495–502. doi: 10.1038/bmt.2011.65. [DOI] [PubMed] [Google Scholar]
- 61.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–8. doi: 10.1038/sj.bmt.1703015. [DOI] [PubMed] [Google Scholar]
- 62.Kanda J, Ichinohe T, Matsuo K, Benjamin RJ, Klumpp TR, Rozman P, Blumberg N, Mehta J, Sohn SK, Uchiyama T. Impact of ABO mismatching on the outcomes of allogeneic related and unrelated blood and marrow stem cell transplantations for hematologic malignancies: IPD-based meta-analysis of cohort studies. Transfusion. 2009;49:624–35. doi: 10.1111/j.1537-2995.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- 63.Ozkurt ZN, Yegin ZA, Yenicesu I, Aki SZ, Yagci M, Sucak GT. Impact of ABO-incompatible donor on early and late outcome of hematopoietic stem cell transplantation. Transplant Proc. 2009;41:3851–8. doi: 10.1016/j.transproceed.2009.06.189. [DOI] [PubMed] [Google Scholar]
- 64.Seebach JD, Stussi G, Passweg JR, Loberiza FR, Jr, Gajewski JL, Keating A, Goerner M, Rowlings PA, Tiberghien P, Elfenbein GJ, Gale RP, van Rood JJ, Reddy V, Gluckman E, Bolwell BJ, Klumpp TR, Horowitz MM, Rignden O, Barrett AJ. ABO blood group barrier in allogeneic bone marrow transplantation revisited. Biol Blood Marrow Transplant. 2005;11:1006–13. doi: 10.1016/j.bbmt.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 65.Strong DM, Lipton S. American Association of Blood Banks Association bulletin #06–07 [Internet] 2006 [cited 09 July 2013]. Available from: URL: http://www.aabb.org/resources/publications/bulletins/Pages/ab06-07.aspx.
- 66.Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, Meade M, Morrison D, Pinsent T, Robillard P, Slinger P. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–89. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 67.Leach M, Vora AJ, Jones DA, Lucas G. Transfusion-related acute lung injury (TRALI) following autologous stem cell transplant for relapsed acute myeloid leukaemia: a case report and review of the literature. Transfus Med. 1998;8:333–7. doi: 10.1046/j.1365-3148.1998.00165.x. [DOI] [PubMed] [Google Scholar]
- 68.Citak EC, Kesik V, Atay AA, Sari E, Kismet E, Koseoglu V. Transfusion-related acute lung injury in a child with neuroblastoma during a late engraftment period of autologous stem cell transplantation. Pediatr Transplant. 2008;12:235–7. doi: 10.1111/j.1399-3046.2007.00856.x. [DOI] [PubMed] [Google Scholar]
- 69.Noji H, Schishima T, Ogawa K, Shikama Y, Ohto H, Maruyama Y. Transfusion-related acute lung injury following allogeneic bone marrow transplantation in a patient with acute lymphoblastic leukemia. Intern Med. 2004;43:1068–72. doi: 10.2169/internalmedicine.43.1068. [DOI] [PubMed] [Google Scholar]
- 70.Yui Y, Umeda K, Kaku H, Arai M, Hiramatsu H, Watanabe K, Saji H, Adachi S, Nakahata T. A pediatric case of transfusion-related acute lung injury following bone marrow infusion. Pediatr Transplant. 2007;11:543–6. doi: 10.1111/j.1399-3046.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 71.Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, Gernsheimer TB, Ness PM, Brecher ME, Josephson CD, Konkle BA, Woodson RD, Ortel TL, Hillyer CD, Skerrett DL, McCrae KR, Sloan SR, Uhl L, George JN, Aquino VM, Manno CS, McFarland JG, Hess JR, Leissinger C, Granger S. Dose of prophylactic platelet transfusion and prevention of hemorrhage. N Engl J Med. 2010;362:600–13. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blajchman MA, Slichter S, Heddle NM, Murphy MF. New strategies for the optimal use of platelet transfusions. Hematology Am Soc Hematol Educ Program. 2008;1:198–204. doi: 10.1182/asheducation-2008.1.198. [DOI] [PubMed] [Google Scholar]
- 73.Estcourt L, Stanworth S, Doree C, Hopewell S, Murphy MF, Tinmouth A, Heddle N. Prophylactic platelet transfusion for prevention of bleeding in patients with haematological disorders after chemotherapy and stem cell transplantation. Cochrane Database Syst Rev. 2012;(16):CD004269. doi: 10.1002/14651858.CD004269.pub3. [DOI] [PubMed] [Google Scholar]


