Abstract
Objective
Endothelial dysfunction is a possible mechanism to explain the association between atherosclerosis and kidney disease. This study evaluated circulating soluble endothelial cell–selective adhesion molecule (sESAM), a marker of endothelial dysfunction, as a risk factor for kidney function decline and albuminuria.
Approach and Results
In the Heart and Soul Study, we measured sESAM from baseline serum samples and defined elevated levels of sESAM by the highest quartile (quartile 4 [Q4]: >65.4 ng/mL). We evaluated the associations of high sESAM with baseline estimated glomerular filtration rate (eGFR) and ratio of urine albumin to creatinine (ACR), and with longitudinal changes in eGFR and ACR. Among 990 participants with sESAM measurements, median sESAM was 54.5 ng/mL (interquartile range, 45.3–65.8). After multivariable adjustment, elevated levels of sESAM were strongly and independently associated with baseline reduced eGFR <60 mL/min per 1.73 m2 (odds ratio [OR], 11.44; P<0.0001) and ACR ≥30 mg/g (OR, 5.23; P<0.0001). Associations of sESAM (Q4 versus quartile 1 [Q1]) with change in ACR (β=54.47; P<0.0001) were also significant after full adjustment. The association with change in eGFR (1.56%; P=0.0049) was not statistically significant after application of the Bonferroni correction for multiple markers. In unadjusted models, sESAM was associated with rapid kidney function loss, defined as 3% annual eGFR decline (OR, 2.28; P=0.0003), although this was attenuated by adjustment (OR, 2.11; P=0.0095).
Conclusions
sESAM is associated with albuminuria and reduced kidney function in both cross-sectional and longitudinal analyses. These findings implicate endothelial dysfunction as a potential contributor to the elevated kidney disease risk in persons with cardiovascular disease.
Keywords: albuminuria, atherosclerosis, kidney diseases
Clinical cardiovascular disease (CVD) is independently associated with kidney function decline and development of chronic kidney disease (CKD), defined by estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2.1 Mechanisms explaining the higher risk of CKD in this population are incompletely understood.2 Albuminuria occurs in many individuals with CVD3 and may be the result of a defect in the endothelial surface monolayer.4 Endothelial dysfunction is observed in early stages of atherosclerosis and contributes to its progression to more advanced disease.5,6 Because endothelial dysfunction in atherosclerosis is a systemic process,7 its effects on renal vasculature may contribute to the development and progression of CKD among patients with atherosclerotic CVD.7
Endothelial dysfunction is associated with impaired endothelium-dependent vasodilation and has been observed in persons with proinflammatory or prothrombotic states, including dyslipidemia, coronary artery disease, congestive heart failure, and peripheral artery disease.5,8 Abnormal endothelial function has also been described in early kidney disease9,10 and may be responsible for albuminuria,4 although the role of glomerular endothelial cells in the onset of albuminuria remains controversial.11 Endothelial dysfunction can be assessed by brachial artery flow–mediated dilation,12 but this measurement is methodologically challenging.8 Identification of specific serum markers of endothelial function is an active area of research13; one example is endothelial cell-selective adhesion molecule (ESAM), which is expressed in vascular endothelial cells.14 Increased expression of ESAM is associated with susceptibility to atherosclerosis.15 In the Dallas Heart Study, a population-based cohort, circulating soluble ESAM (sESAM) was associated with subclinical CVD assessed by coronary artery calcium, abdominal aortic wall thickness, aortic plaque burden, and aortic compliance.15 sESAM is also expressed at the glomerulus.16 The association of this selective endothelial function marker with kidney disease in patients with preexisting atherosclerotic heart disease is unknown.
We investigated the associations between sESAM and kidney outcomes among ambulatory individuals with stable coronary heart disease in the Heart and Soul Study cohort. We evaluated a comprehensive array of kidney outcomes, including baseline reduced eGFR and ratio of albumin to creatinine (ACR), change in eGFR, change in ACR, incident reduced eGFR <60 mL/min per 1.73 m2, and rapid kidney function decline. Understanding these associations may help to delineate the role of endothelial dysfunction in kidney disease onset, particularly among patients with established CVD.
Materials and Methods
The Materials and Methods section is available in the online-only Supplement.
Results
Participant Characteristics
Among 990 individuals with ischemic heart disease and serum measurements of sESAM at baseline, mean age was 66.7 (±11) years, 81% were men, 60% were white, 70% had hypertension, and 26% had diabetes mellitus. Mean eGFRcreatinine-cystatin C (cr-cys) at baseline was 70.6 (±22.2) mL/min per 1.73 m2, and the prevalence of eGFRcr-cys <60 mL/min per 1.73 m2 at baseline was 31%. Individuals in the highest quartile of sESAM were more likely to be older, men, and white, and to have much lower baseline eGFR and higher baseline ACR and modestly lower high-density lipoprotein and low-density lipoprotein levels (Table 1).
Table 1.
Baseline Characteristics by Quartile of Soluble Endothelial Cell–Selective Adhesion Molecule
| Q1 (0.4–45.1 ng/mL) | Q2 (45.1–53.8 ng/mL) | Q3 (53.8–65.3 ng/mL) | Q4 (65.4–130 ng/mL) | P Value | |
|---|---|---|---|---|---|
| Age, y* | 62 (10) | 66 (10) | 67 (11) | 72 (10) | 0.0001 |
| Men* | 73% | 80% | 86% | 86% | 0.0001 |
| Race | |||||
| White | 44% | 60% | 67% | 69% | 0.0001 |
| Latino | 9% | 11% | 8% | 7% | |
| Asian | 14% | 12% | 11% | 9% | |
| Black | 28% | 15% | 12% | 11% | |
| Other | 4% | 3% | 2% | 4% | |
| Tobacco, pack-years* | 22 (22) | 21.1 (22.5) | 20.9 (21.7) | 21.3 (23) | 0.8596 |
| HDL, mg/dL* | 46 (14) | 46 (13) | 44 (15) | 46 (14) | 0.0374 |
| LDL, mg/dL* | 110 (35) | 105 (31) | 102 (35) | 101 (34) | 0.0012 |
| C-reactive protein, mg/L | 4.4 (6.8) | 4.5 (10.3) | 3.9 (5.3) | 5.5 (9.5) | 0.2219 |
| Hypertension | 70.5% | 64.8% | 70.2% | 75.6% | 0.075 |
| Type 2 diabetes mellitus | 23% | 24% | 29% | 29% | 0.276 |
| ACEI/ARB | 45.5% | 50% | 56.6% | 52.8% | 0.092 |
| Statins | 59.5% | 70% | 65% | 63% | 0.133 |
| eGFR, mL/min per 1.73 m2 | |||||
| cr* | 76 (19) | 68 (15) | 65 (18) | 52 (20) | 0.0001 |
| cys* | 85 (21) | 77 (17) | 70 (18) | 54 (23) | 0.0001 |
| cr-cys* | 86 (19) | 77 (17) | 70 (18) | 52 (19) | 0.0001 |
| cr-cys <60 | 14.8% | 16.0% | 27.1% | 58.1% | 0.0001 |
| ACR, mg/g† | 8.0 (4.4–13.8) | 7.8 (4.1–13.9) | 8.3 (4.9–17.1) | 11.8 (6.9–54) | 0.0001 |
| ACR ≥30 mg/g | 10.4% | 9.5% | 12.3% | 31.6% | 0.0001 |
Values reported as *mean (SD) or †median (IQR). ACEI indicates angiotensin-converting enzyme inhibitor; ACR, albumin-to-creatinine ratio; ARB, angiotensin receptor blocker; cr, creatinine; cys, cystatin C; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; Q, quartile; and SD, standard deviation.
Associations of sESAM With Baseline Reduced eGFR and Albuminuria
sESAM was strongly associated with eGFR <60 mL/min per 1.73 m2 at baseline. After full adjustment, the highest quartile had 11-fold increased odds of eGFR <60 mL/min per 1.73 m2 (Table 2). Similarly, the highest quartile had 5-fold increased odds of baseline urine ACR ≥30 mg/g (Table 2). These associations remained strong after additional adjustment for ACR and were attenuated by adjustment for eGFR. These findings were especially strong among black individuals (adjusted odds ratio [OR] for eGFR <60: 70; P<0.0001; adjusted OR for ACR ≥30: 16; P=0.0001).
Table 2.
Associations of Quartiles of Soluble Endothelial Cell–Selective Adhesion Molecule With Baseline CKD Assessed by eGFR and ACR
| Q1 | Q2 | Q3 | Q4 | P Overall | |
|---|---|---|---|---|---|
| eGFR <60 mL/min per 1.73 m2 | |||||
| Unadjusted OR | Ref | 1.41 | 3.22 | 16.0 | <0.0001 |
| 99.83% CI | 0.59–3.36 | 1.46–7.11 | 7.43–34.41 | ||
| P value | 0.2212 | <0.0001 | <0.0001 | ||
| Adjusted* OR | Ref | 1.12 | 2.56 | 11.44 | <0.0001 |
| 99.83% CI | 0.43–2.89 | 1.07–6.12 | 4.89–26.76 | ||
| P value | 0.7454 | 0.0016 | <0.0001 | ||
| Adjusted* plus ACR | Ref | 1.12 | 2.53 | 9.58 | <0.0001 |
| 99.83% CI | 0.42–3.03 | 1.02–6.3 | 3.87–23.73 | ||
| P value | 0.7177 | 0.0014 | <0.0001 | ||
| ACR ≥30 mg/g | |||||
| Unadjusted OR | Ref | 0.75 | 1.12 | 3.52 | <0.0001 |
| 99.83% CI | 0.28–2.02 | 0.45–2.75 | 1.61–7.69 | ||
| P value | 0.3557 | 0.7044 | <0.0001 | ||
| Adjusted* OR | Ref | 0.95 | 1.36 | 5.23 | <0.0001 |
| 99.83% CI | 0.31–2.89 | 0.47–3.93 | 1.96–13.91 | ||
| P value | 0.8788 | 0.3598 | <0.0001 | ||
| Adjusted* plus eGFR | Ref | 0.82 | 1.05 | 2.83 | 0.0006 |
| 99.83% CI | 0.27–2.52 | 0.35–3.11 | 0.93–8.6 | ||
| P value | 0.5888 | 0.8965 | 0.00334 | ||
Threshold P value for significance=0.0017. P overall gives results of test of heterogeneity. ACR indicates albumin-to-creatinine ratio; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; OR, odds ratio; Q, quartile; and Ref, reference.
Adjusted for age, race, sex, hypertension, pack-years, type 2 diabetes mellitus, high-density lipoprotein, low-density lipoprotein, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, statins, and high-sensitivity C-reactive protein.
Associations With Change in Urine ACR
Compared with the lowest quartile, adjusted mean increase in ACR from baseline to year 5 was 54 mg/g higher among patients in the highest sESAM quartile (Table 3). The adjusted mean increase in ACR was similar among individuals with preserved and reduced eGFR. Our sensitivity analysis using proportional odds and ordinal categories of ACR change gave similar results (OR, 2.55; P=0.0171).
Table 3.
Adjusted* 5-Year Change in Albumin-to-Creatinine Ratio (mg/g) by Quartile of Soluble Endothelial Cell–Selective Adhesion Molecule
| Entire Cohort (N=990) | eGFR ≥60 (N=678) | eGFR <60 (N=303) | |
|---|---|---|---|
| Q1 | Ref | Ref | Ref |
| Q2 | 7.92 (P=0.4298) | 2.26 (P=0.8255) | 21.68 (P=0.6827) |
| Q3 | 11.44 (P=0.3424) | −0.91 (P=0.9358) | 14.06 (P=0.7608) |
| Q4 | 54.47 (P<0.0001) | 40.35 (P=0.0041) | 63.21 (P=0.1458) |
| P overall | 0.0001 | 0.0143 | 0.2148 |
Threshold P value for significance=0.0017. P overall gives results of test of heterogeneity. ACR indicates albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; Q, quartile; and Ref, reference.
Adjusted for age, race, sex, hypertension, pack-years, type 2 diabetes mellitus, high-density lipoprotein, low-density lipoprotein, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, statins, high-sensitivity C-reactive protein, and baseline ACR.
Associations With Changes in Kidney Function
The highest quartile of sESAM compared with the lowest was associated with a loss in eGFRcr-cys of 1.56% per year after full adjustment (P=0.0049; Figure). This association was minimally affected by additional adjustment for ACR (1.36%; P=0.0166). These results were not statistically significant at the experiment-wide P=0.0017.
Figure.
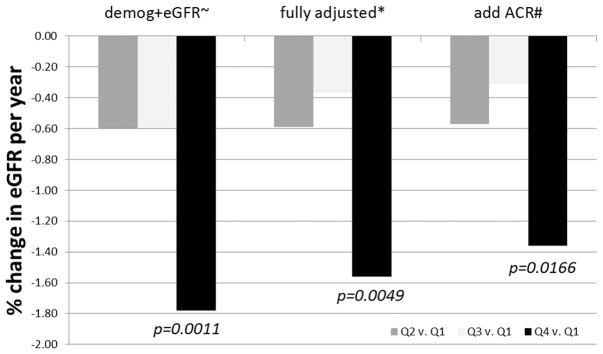
Quartiles of soluble endothelial cell–selective adhesion molecule after sequential adjustment for demographics, covariates, and albumin-to-creatinine ratio and associations with annual percent change in estimated glomerular filtration rate. ~Adjusted for demographics and baseline eGFR only; *Adjusted for age, race, sex, htn, BMI, smoking, T2DM, HDL, LDL, ACEI/ ARB, statins, CRP, baseline eGFR; #Above covariates plus ACR. Threshold P-value for significance=0.001724.
In unadjusted models, when compared with the lower 3 quartiles, the highest quartile of sESAM was associated with rapid loss in kidney function, defined as a loss of eGFRcr-cys of >3% per year (Table 4). Adjusted models were not significant after applying the 29-marker Bonferroni correction to the P value. sESAM was not associated with incident reduced eGFR <60 mL/min per 1.73 m2 (adjusted OR, 1.32; P=0.56).
Table 4.
Associations of Quartiles of Soluble Endothelial Cell–Selective Adhesion Molecule With Rapid Loss (>3% Per Year) in Kidney Function
| Unadjusted OR; 99.83% CI; P Value | Adjusted* OR; 99.83% CI; P Value | Adjusted* Plus ACR; 99.83% CI; P Value | |
|---|---|---|---|
| Q1 | Ref | Ref | Ref |
| Q2 | 1.18; 0.44–3.12; 0.6018 | 1.09; 0.38–3.13; 0.8056 | 1.12; 0.37–3.36; 0.7489 |
| Q3 | 1.16; 0.43–3.13; 0.6359 | 0.94; 0.3–2.93; 0.8702 | 0.99; 0.31–3.19; 0.9869 |
| Q4 | 2.52; 0.99–6.41; 0.0019 | 2.13; 0.65–7.01; 0.0465 | 2.01; 0.58–6.93; 0.0783 |
| P overall | 0.0043 | 0.0659 | 0.0157 |
| Q4 vs Q1–Q3 | 2.28; 1.11–4.65; 0.0003 | 2.11; 0.86–5.22; 0.0095 | 1.94; 0.76–4.93; 0.0265 |
Threshold P value for significance=0.0017. P overall gives results of test of heterogeneity. ACR indicates albumin-to-creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; OR, odds ratio; Q, quartile; and Ref, reference.
Adjusted for age, race, sex, hypertension, pack-years, type 2 diabetes mellitus, high-density lipoprotein, low-density lipoprotein, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, statins, high-sensitivity C-reactive protein, and baseline eGFR.
Discussion
In a cohort of individuals with known ischemic heart disease, we found higher levels of sESAM to be strongly associated with baseline reduced eGFR and higher ACR. sESAM was significantly associated with worsening ACR after full multivariable adjustment and with rapid kidney function loss in unadjusted models only. sESAM was not significantly associated with annual eGFR loss after application of Bonferroni correction for multiple markers tested on the same platform on which sESAM was measured. Higher sESAM may indicate early endothelial dysfunction that precedes kidney function decline among individuals with established CVD.
This is the first study to our knowledge to examine the relationship between sESAM and changes in kidney function in individuals with established atherosclerosis. CKD is a frequent complication of CVD17–19 and accelerates risk of cardiovascular mortality in this population.20 Previous work by Gold et al21 has shown that sESAM is associated cross-sectionally with CKD. It is possible that sESAM is associated with CKD and kidney function decline in our study only as a result of reduced kidney filtration of this molecule. However, we adjusted for baseline kidney function and for known confounders, and our associations remained significant. Furthermore, the strong association of sESAM with ACR and change in ACR was independent of kidney filtration function assessed by eGFR, which implicates endothelial dysfunction in glomerular capillary disruption and consequent increases in ACR.4
sESAM regulates vascular permeability, and sESAM may regulate albumin extravasation at the level of the glomeruli in hyperglycemia.11 In a longitudinal study of 88 individuals with type 2 diabetes mellitus, Kacso et al22 found an increase in albuminuria over 12 to 24 months in individuals with lower sESAM levels. This finding is in contrast to our study. However, our study was larger and included only 26% diabetic subjects, and these relationships may differ in the absence of overt hyperglycemia.11 Our study detected a much larger magnitude of change in ACR and had a longer follow-up time. Furthermore, the Kacso study22 did not observe associations of sESAM with a change in eGFR. Decreased sESAM expression may play a role in early diabetic nephropathy,11 and our study could not address different stages of diabetes mellitus. Our study adjusted for the presence of diabetes mellitus, and our associations remained strong.
Our findings are of interest for several reasons. Although this study focused on the association of CVD with development and progression of CKD, the increased cardiovascular risk in individuals with preexisting CKD is substantial, and the mediators remain incompletely characterized.23 Candidate mechanisms for CKD to CVD risk association include inflammation, abnormal bone mineral calcification, and other conditions associated with CKD.24,25 Endothelial dysfunction is considered a prodromal phase of atherosclerosis in CKD. However, atherosclerosis often precedes CKD diagnosis,26 and efforts to identify early parallel processes of vascular dysregulation may be a useful framework for identifying common mechanisms for both diseases. As a marker of endothelial function, sESAM may help to explain the early pathogenesis of kidney abnormalities in the setting of known CVD.
Our study has several strengths. The Heart and Soul Study cohort is a unique cohort in which to examine the relationship between CVD and CKD. This cohort has robust measurements of sESAM and repeated measures of eGFR and ACR in 990 individuals. The coefficients of variation of biomarker measurement were not perfect but were comparable to those of other studies and are low for a new biomarker.27 Also, our samples were all measured in concert, minimizing risks of drift among biomarker samples.
Our study has several limitations. First, the endothelial function biomarkers are only available on baseline serum samples. Although we have robust measurements of kidney function at baseline and at 5 years of follow-up, this cohort has a relatively low prevalence and incidence of kidney disease. We had inadequate power to stratify by race, and there were relatively few women in this cohort. Exploratory analyses suggest that further investigations into racial differences may be revealing, especially in light of higher prevalence of apolipoprotein L1 risk variants among the black population.28 Finally, we were underpowered to detect associations with more severe changes in kidney function, such as rapid kidney function loss, decline in eGFR >50%, incident end-stage renal disease, or increase in ACR >50%. Application of the Bonferroni correction suggests that our results for changes in eGFR may be attributable to chance.
In conclusion, elevated sESAM may indicate increased risk of progressive albuminuria and rapid kidney function loss in individuals with known atherosclerotic disease. The accelerated kidney function loss is independent of baseline reduced eGFR, albuminuria, hypertension, and diabetes mellitus. This study indicates a possible role for endothelial function in the development and progression of CKD among individuals with preexisting CVD. Future investigations should focus on the pathophysiology of atherosclerosis and endothelial dysfunction as determinants of kidney function decline.
Significance.
The risk of kidney disease in individuals with established cardiovascular disease is high. Mechanisms of onset of kidney disease are unclear and do not seem to be explained exclusively by traditional risk factors such as diabetes mellitus and hypertension. Endothelial dysfunction may be a pathogenic mechanism in the onset of kidney disease in systemic atherosclerosis. In the Heart and Soul Study, a cohort of individuals with established ischemic heart disease, we evaluated associations between soluble endothelial cell–selective adhesion molecule, a marker of endothelial dysfunction, and kidney function assessed by estimated glomerular filtration rate and albuminuria. Our findings suggest a role for endothelial dysfunction in the onset of kidney disease in this population.
Acknowledgments
Sources of Funding
This work was supported by Meyeon Park’s NIH/NIDDK F32DK093231 and American Heart Association 11POST7230046 (to M. Park). M.S. is supported by R01 AG034853-04, 5R01AG027002-06, and 5R01DK087961-02. C.P. is funded by NIH/NIDDK 1K23DK082793. P.G. is funded by NIH R01 DK080662 and NIH 1 P50 DA036109. The Heart and Soul Study was funded by the Department of Veteran Affairs (Epidemiology Merit Review Program), Washington, DC; grant R01 HL-079235 from the National Heart, Lung, and Blood Institute, Bethesda, MD; the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), Princeton, NJ; the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), New York, NY; and the Ischemia Research and Education Foundation, South San Francisco, CA.
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.113.301806/-/DC1.
Disclosures
None.
References
- 1.Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D, Sarnak MJ, Weiner DE. Cardiovascular disease and subsequent kidney disease. Arch Intern Med. 2007;167:1130–1136. doi: 10.1001/archinte.167.11.1130. [DOI] [PubMed] [Google Scholar]
- 2.Moody WE, Edwards NC, Madhani M, Chue CD, Steeds RP, Ferro CJ, Townend JN. Endothelial dysfunction and cardiovascular disease in early-stage chronic kidney disease: cause or association? Atherosclerosis. 2012;223:86–94. doi: 10.1016/j.atherosclerosis.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 3.Mann JFE, Yi Q-L, Gerstein HC. Albuminuria as a predictor of cardiovascular and renal outcomes in people with known atherosclerotic cardiovascular disease. Kidney Int. 2004;66:S59–S62. doi: 10.1111/j.1523-1755.2004.09215.x. [DOI] [PubMed] [Google Scholar]
- 4.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, Bates DO, Peti-Peterdi J. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23:1339–1350. doi: 10.1681/ASN.2012010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juonala M, Viikari JS, Laitinen T, Marniemi J, Helenius H, Rönnemaa T, Raitakari OT. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation. 2004;110:2918–2923. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 7.Anderson TJ, Gerhard MD, Meredith IT, Charbonneau F, Delagrange D, Creager MA, Selwyn AP, Ganz P. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75:71B–74B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- 8.Ganz P, Vita JA. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation. 2003;108:2049–2053. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- 9.Stam F, van Guldener C, Becker A, Dekker JM, Heine RJ, Bouter LM, Stehouwer CDA. Endothelial dysfunction contributes to renal function–associated cardiovascular mortality in a population with mild renal insufficiency: the Hoorn study. J Am Soc Nephrol. 2006;17:537–545. doi: 10.1681/ASN.2005080834. [DOI] [PubMed] [Google Scholar]
- 10.Yilmaz MI, Stenvinkel P, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Çakar M, Altun B, Yenicesu M, Carrero JJ. Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol Dial Transplant. 2011;26:3537–3543. doi: 10.1093/ndt/gfr081. [DOI] [PubMed] [Google Scholar]
- 11.Hara T, Ishida T, Cangara HM, Hirata K. Endothelial cell-selective adhesion molecule regulates albuminuria in diabetic nephropathy. Microvasc Res. 2009;77:348–355. doi: 10.1016/j.mvr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 13.Dubin R, Li Y, Ix JH, Shlipak MG, Whooley M, Peralta CA. Associations of pentraxin-3 with cardiovascular events, incident heart failure, and mortality among persons with coronary heart disease: data from the Heart and Soul Study. Am Heart J. 2012;163:274–279. doi: 10.1016/j.ahj.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue M, Ishida T, Yasuda T, Toh R, Hara T, Cangara HM, Rikitake Y, Taira K, Sun L, Kundu RK, Quertermous T, Hirata K. Endothelial cell-selective adhesion molecule modulates atherosclerosis through plaque angiogenesis and monocyte-endothelial interaction. Microvasc Res. 2010;80:179–187. doi: 10.1016/j.mvr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Rohatgi A, Owens AW, Khera A, Ayers CR, Banks K, Das SR, Berry JD, McGuire DK, de Lemos JA. Differential associations between soluble cellular adhesion molecules and atherosclerosis in the Dallas Heart Study: a distinct role for soluble endothelial cell- selective adhesion molecule. Arterioscler Thromb Vasc Biol. 2009;29:1684–1690. doi: 10.1161/ATVBAHA.109.190553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasdala I, Wolburg-Buchholz K, Wolburg H, Kuhn A, Ebnet K, Brachtendorf G, Samulowitz U, Kuster B, Engelhardt B, Vestweber D, Butz S. A transmembrane tight junction protein selectively expressed on endothelial cells and platelets. J Biol Chem. 2002;277:16294–16303. doi: 10.1074/jbc.M111999200. [DOI] [PubMed] [Google Scholar]
- 17.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 18.Nakano T, Ninomiya T, Sumiyoshi S, Fujii H, Doi Y, Hirakata H, Tsuruya K, Iida M, Kiyohara Y, Sueishi K. Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: the Hisayama study. Am J Kidney Dis. 2010;55:21–30. doi: 10.1053/j.ajkd.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 19.McClellan WM, Langston RD, Presley R. Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J Am Soc Nephrol. 2004;15:1912–1919. doi: 10.1097/01.asn.0000129982.10611.4c. [DOI] [PubMed] [Google Scholar]
- 20.Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 21.Gold L, Ayers D, Bertino J, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One. 2010;5:e15004. doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kacso I, Bondor C, Kacso G. Low serum endothelial cell-selective adhesion molecule predicts increase in albuminuria in type 2 diabetes patients. Int Urol Nephrol. 2013:1–8. doi: 10.1007/s11255-012-0365-z. [DOI] [PubMed] [Google Scholar]
- 23.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 24.Recio-Mayoral A, Banerjee D, Streather C, Kaski JC. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease–a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis. 2011;216:446–451. doi: 10.1016/j.atherosclerosis.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 26.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Siscovick D, Sarnak MJ. Clinical and subclinical cardiovascular disease and kidney function decline in the elderly. Atherosclerosis. 2009;204:298–303. doi: 10.1016/j.atherosclerosis.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yilmaz MI, Sonmez A, Ortiz A, Saglam M, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Çakar M, Egido J, Altun B, Yenicesu M, Blanco-Colio LM, Carrero JJ. Soluble TWEAK and PTX3 in nondialysis CKD patients: impact on endothelial dysfunction and cardiovascular outcomes. Clin J Am Soc Nephrol. 2011;6:785–792. doi: 10.2215/CJN.09231010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol. 2011;22:2098–2105. doi: 10.1681/ASN.2011050519. [DOI] [PMC free article] [PubMed] [Google Scholar]


