Abstract
Recent studies have suggested that pan inhibitors of dipeptidyl peptidase-4 activity and/or structure homologs (DASH), including ARI-4175, can mediate tumor regression by immune-mediated mechanisms. This study assessed the potential of combining ARI-4175 with cancer vaccines. We evaluated ARI-4175's effect on immunogenic modulation, ability to sensitize tumor cells to antigen-specific CTL killing, effect on immune-cell subsets and function, and antitumor activity in 2 tumor models, both as a monotherapy and in combination with a recombinant viral or dendritic cell (DC)-based tumor-cell vaccine.
ARI-4175's effects on the growth, surface phenotype, and antigen-specific CTL-mediated lysis of murine and human carcinoma cell lines were assessed in vitro. In vivo, C57BL-6 mice were treated orally with ARI-4175, after which splenocytes were assessed by flow cytometry and functional assays. Antitumor studies were performed in murine models of colon carcinoma (MC38-CEA+ in CEA-transgenic C57BL-6 mice) and rhabdomyosarcoma (M3-9-M in C57BL-6 mice). Mice received oral ARI-4175 alone or in combination with a vaccine consisting of recombinant vaccinia/fowlpox CEA-TRICOM (colon model) or a DC-based tumor-cell vaccine (rhabdomyosarcoma model).
Exposure to ARI-4175 had no effect on the proliferation or viability of carcinoma cells in vitro; however, it did alter tumor phenotype, making murine and human tumor cells more sensitive to antigen-specific CTL killing. Assessment of immune-cell subsets and function indicated that ARI-4175 increased levels of natural killer cells and DCs. Detrimental immune effects, including reduced T effector cells and increased immunosuppressive cells (Tregs, MDSCs), were normalized when treatment stopped, suggesting that scheduling is critical when combining this agent with vaccine. As a monotherapy, ARI-4175 had potent antitumor activity in both tumor models, and had even greater effects when combined with a vaccine (either DC-based or poxviral vector based). These findings provide the rationale for the combined use of cancer immunotherapy with DASH enzyme inhibitors such as ARI-4175.
Keywords: Immunogenic modulation, T-cell response, DASH family enzymes, Immunotherapy, Vaccine
1. Introduction
Mammalian post-proline cleaving enzymes form a sub-family of serine proteases called the dipeptidyl peptidase (DPP)-4 and/or structural homologs (DASH). DASH comprise DPP4, DPP2, DPP8, DPP9, fibroblast activation protein (FAP) and prolyl endopeptidase [1, 2]. The DPPs selectively cleave after proline or alanine residues when either is located as the penultimate residue at the N-terminus of peptides. It has been proposed that conserved prolines act as regulatory elements in biologically active peptides, for which post-proline cleaving proteases in the DASH sub-family provide checkpoints [1]. The mature forms of several chemokines frequently possess N-terminally penultimate proline or alanine, suggesting that their biological activity might be regulated by DPP activity [3, 4]. This has been shown to be the case for the chemokine stromal cell-derived factor-1 in vivo [5, 6], and more recently, for GM-CSF, G-CSF, IL-3, and erythropoietin, in the homeostatic regulation of hematopoiesis [7]. It has previously been reported preclinically that pharmacological inhibition of the enzymatic activity of DPP4 and/or DASH with dipeptide boronic acids can mediate tumor regression in an immune-mediated manner [8-10]. In the clinic a DASH inhibitor PT-100 achieved some partial and complete responses [11, 12] but has since been discontinued. A second generation pan-inhibitor of DASH, ARI-4175, has been reported to induce significant immune-dependent anti-tumor activity against established rhabdomyosarcoma preclinically [13].
Immunotherapeutic vaccination is being investigated with renewed interest as a treatment for a wide spectrum of human cancers [14]. In the present preclinical study, we have investigated the ability of ARI-4175 to enhance tumor immunity in response to active vaccination. ARI-4175 was combined with either a recombinant vaccinia/fowlpox vaccine targeting the tumor associated antigen (TAA) CEA in CEA expressing colon carcinoma, or with a DC-based tumor cell vaccine targeting M3-9-M cells in a model of rhabdomyosarcoma. We hypothesized that ARI-4175 might interact directly with tumor cells to sensitize them to cytotoxic T lymphocyte (CTL)-mediated killing. We have investigated: a) the in vitro effect of ARI-4175 on tumor cell killing by CTLs specific for TAAs, b) modulation of immune cell subsets and functions resulting from exposure to ARI-4175 in vivo, and c) antitumor effects of ARI-4715 in two tumor models when administered either as a monotherapy or in combination with a recombinant viral or DC tumor cell vaccine. The results support the future clinical translation of ARI-4175 as an adjuvant to tumor vaccines.
2. Materials and methods
2.1. Tumor cell lines
Murine colon carcinoma (MC38) expressing human CEA (MC38-CEA) were generated and maintained as previously described [15]. Murine rhabodomyosarcoma (M3-9-M) cells have been previously described [16]. Human prostate (LNCaP), breast (MDA-MB-231), and colon (SW480) tumor cells were purchased and maintained as directed (American Type Culture Collection, ATCC, Manassas, VA).
2.2. Drug preparation and treatment
ARI-4175 was provided by Tufts University School of Medicine Biochemistry Department (Boston, MA) and stored at –80°C. Concentrated aliquots of ARI-4175 (100 mM) were prepared by dissolving ARI-4175 in 0.1 N HCl. For in vivo studies, aliquots were diluted with saline immediately prior to administration to a final concentration of 1 mg/mL. ARI-4175 was administered by oral gavage (0.2 cc) at a dose of 200 μg/day; HCl (0.1 N) diluted in saline served as the vehicle control.
2.3. Assessment of phenotypic modulation
Murine and human carcinoma cells were treated for 72 h with 10 μM ARI-4175 and assessed for changes in a variety of immunologically relevant cell-surface molecules [17]. Murine cells were stained using the following antibodies: anti-H2Kb/H2Db-FITC, anti-H2Kd/H2Dd-APC, anti-CD54 (ICAM-1)-PE, anti-CD95 (Fas)-PE-Cy7 (BD Biosciences, San Diego, CA), anti-Col-1 (CEA)-FITC ([18]), and anti-Calreticulin-PE (R&D Systems, Minneapolis, MN). Human cells were assessed with: anti-CD95 (Fas)-PerCP-Cy5.5, anti-CD54 (ICAM-1)-PE, anti-CD227 (MUC1)-FITC, anti-HLA-A2-FITC, (BD Biosciences), anti-CD66 (CEA)-APC (Miltenyi Biotech, Auburn, CA) and anti-Calreticulin-PE (R&D Systems). Stained cells were acquired on an LSR II flow cytometer and analyzed using FloJo software. Isotype staining was < 5% for all samples analyzed. Proteins were scored as up-regulated if either the percent of cells or the mean fluorescence intensity (MFI) was increased by > 10% relative to untreated and vehicle-treated controls.
2.4. CTL lines and cytotoxicity analysis
CD8+ CTLs that kill in an antigen specific and haplotype (HLA) restricted manner were generated and used as described: Murine: CEA-specific (H-2Db-restricted), identifying the peptide epitope CEA526-533 (EAQNTTLY) [19], and p15E-specific (H-2Kb-restricted; designated gp-70), recognizing the peptide epitope p15E604-611 (KSPWFTTL) [20]. Human: HLA-A2-restricted CEA-specific, recognizing the CEA peptide epitope YLSGANLNL (CAP-1) [21]; PSA-specific, identifying the PSA peptide epitope VISNDVCAQV [22]; and MUC1-specific, recognizing the MUC1 peptide epitope ALWGQDVTSV [23]. Cancer cells were treated with 10 μM ARI-4175 for 72 h and used as targets in a standard cytotoxicity assay using 111In, as previously described [17].
2.5. Inoculation and measurement of tumor cells
Single-cell suspensions of MC38-CEA cells (3×105) were injected subcutaneously into the backs of female CEA-transgenic (CEA-tg) C57BL/6 mice, while M3-9-M cells (1×106) were injected intramuscularly into the gastrocnemius muscle of female C57BL/6 mice. Injections were administered in 0.2 mL PBS in nonanaesthetized mice. Tumors were measured in 2 dimensions, length (L) and width (W), 2–3 times/week by digital caliper. Volumes in the subcutaneous model were calculated by the formula L×W2/2. Volumes in the intramuscular model were calculated by measuring the entire leg and using the formula ((L/2)×(W/2)×[(L+W/2)/2]×4/3—π). Mice were euthanized when tumor diameters reached 2 cm, in accordance with animal protocols.
2.6. Effect of ARI-4175 on immune-cell subsets and function
Nontumor-bearing female C57BL/6 mice were given ARI-4175 for either 3 days (followed by 4 days of vehicle), 5 days (followed by 2 days of vehicle), or 7 days; control mice received vehicle alone. On day 7, spleens were removed and pressed through a 70-μm cell strainer (BD Bioscience), red blood cells were lysed and Fc receptors blocked with anti-CD16/CD32 (2.4 G2). Immune subsets were identified by staining 1×106 cells from 5 individual animals with the indicated antibodies: T cells (anti-CD3-V500, anti-CD4-AF700, and anti-CD8-pacific blue), B cells (anti-CD19-PerCP-Cy5.5), NK cells (anti-CD49b-FITC), DCs (anti-CD11c-FITC and anti-MHC-II (I-A/I-E)-APC), MDSCs (anti-CD11b-PerCP-Cy5.5 and anti-GR1-V450), and Tregs (anti-CD4-AF700, anti-CD25-APC, and intracellular anti-FoxP3-PE). All antibodies, except for CD25-APC and FoxP3-PE (purchased from eBioscience) were obtained from BD Bioscience.
The effect of ARI-4175 on antigen-presenting cells (APCs) was determined using an allostimulatory (H-2b vs. H-2d) mixed lymphocyte reaction. The effect of ARI-4175 on T-cell function was analyzed by anti-CD3 proliferation assay. For both functional assays, [3H]thymidine was added during the final 18 h and incorporation was measured.
2.7. Vaccine preparation
The DC vaccine was prepared by harvesting bone marrow from female C57BL/6 mice as described [24]. The M3-9-M DC vaccine or sham vaccine was administered to mice by intraperitoneal injection on day 8 (1×106 cells/0.2 cc). Recombinant modified vaccinia Ankara (rMVA) and recombinant fowlpox (rF) viruses containing transgenes for the murine costimulatory molecules B7.1, ICAM-1, and LFA-3 (designated TRICOM), in combination with the CEA transgene (rMVA/rF-CEA-TRICOM), have been previously described [25]. rMVACEA-TRICOM was administered subcutaneously as a prime, and rF-CEA-TRICOM as weekly boosts at 1×108 pfu/mouse [26, 27].
2.8. Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA). Differences between treatments were evaluated using an unpaired Student's t-test with a 2-tailed distribution or one-way analysis of variance. Subsequent comparisons were made using Newman-Keuls tests. Survival was analyzed by Kaplan-Meier curves. Results are reported as P values, calculated using a confidence interval of 95%.
3. Results
3.1. Effect of ARI-4175 on murine tumor cells in vitro: proliferation, cytotoxicity, immunogenic modulation, and sensitivity to cytotoxic T-cell killing
To determine whether ARI-4175 has a direct proliferative or cytotoxic effect on cancer cells, murine carcinoma cells were exposed in vitro to 0.1–10 μM of ARI-4175 in a dose-response study (Fig. 1A). MC38-CEA cells treated with ARI-4175 showed no change in cell number relative to untreated or vehicle-treated controls. Murine carcinoma cells exposed to ARI-4175 (10 μM for 72 hours) were analyzed for surface expression of Fas, ICAM-1, CEA, MHC I, and calreticulin, each of which has been shown to enhance antitumor T-cell responses through diverse mechanisms [17, 28-33]. Relative to vehicle control, MC38-CEA cultures treated with ARI-4175 increased expression of Fas (1.7-fold), ICAM-1 (1.4-fold) and MHC I (H2Db, 1.5-fold; H2Kb, 1.2 fold), whereas cell surface levels of CEA and calreticulin were unaltered by ARI-4175.
Figure 1. Effect of ARI-4175 on growth and cell-surface phenotype of murine MC38-CEA colon carcinoma cells and their sensitivity to CTL-mediated killing.
A. At varying concentrations, ARI-4175 does not alter the growth of MC38-CEA cells. Vehicle-treated and untreated cells served as controls; medium and compound were replenished daily. The total number of viable cells was determined at various time points by trypan blue exclusion. Data represent mean ± SEM from 2 replicate wells. B. MC38-CEA cells undergo immunogenic modulation following exposure to ARI-4175. Cells were treated daily with 10 μM ARI-4175 for 72 h, harvested, and analyzed by flow cytometry for cell-surface expression of Fas, ICAM-1, CEA, MHC I (H2Kb/H2Db), and calreticulin. Vehicle-treated and untreated cells served as controls. Numbers indicate percentage of positive cells (MFI in parentheses). Bold type indicates marked up-regulation (> 10% increase in percent of cells or MFI) relative to controls. C. Exposure of MC38-CEA cells to ARI-4175 increases sensitivity to antigen-specific CTL killing. Tumor cells treated with 10 μM ARI-4175 for 72 h were used as targets in an 18-h CTL lysis assay. CEA- or gp70-specific CD8+ T cells were used as effector cells at an effector:target ratio of 30:1 and 50:1, respectively. Data represent mean ± SEM for 3 replicate wells. Asterisks denote significance (P < 0.05) relative to untreated wells. Data are representative of one experiment. Experiments were repeated 2–4 times with similar results.
To determine the functional significance of these phenotypic changes induced by ARI-4175, MC38-CEA cells were either untreated or treated with ARI-4175 and subsequently cocultured with CEA- or gp-70-specific CTLs. CEA-specific T cells killed untreated or vehicle-treated MC38-CEA cells at a level of 14% to 20%. Treating targets with ARI-4175 markedly increased lysis 4.5-fold. Similarly, gp-70-specific T cells killed MC38-CEA cells that were untreated or vehicle-treated at a level of 29% to 31%, while treatment of these targets with ARI-4175 increased lysis to 43% (Fig. 1C). These data indicate that exposure of murine cells to ARI-4175 enhances antigen-specific CTL-mediated killing of tumor cells, and that this effect extends to a variety of TAAs.
3.2. In vivo effect of orally administered ARI-4175 on murine tumor models
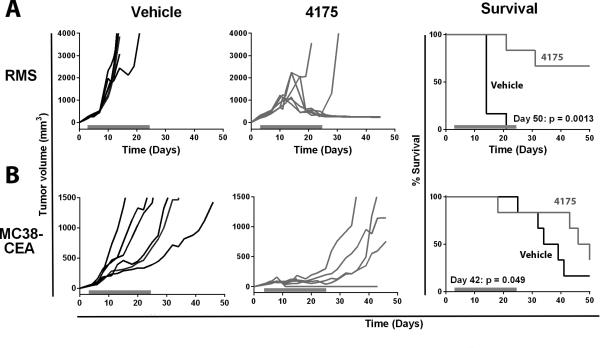
Our first in vivo test of ARI-4175 was in an immunogenic rhabdomyosarcoma model in C57BL/6 mice in which M3-9-M cells were orthotopically transplanted in the gastrocnemius muscle. After tumor inoculation, 200 μg of ARI-4175 was administered daily, beginning when tumors became visible (day 3). After initial progression of M3-9-M tumors during the first week of treatment, tumors started regressing, and on day 21 when 100% of the vehicle-administered mice had to be euthanized because their tumors had reached the ethical limit, 5 of 6 mice (83%) treated with ARI-4175 were still surviving (Figure 2A). When treatment was discontinued (day 24), 4 out of 5 mice remained tumor free (P = 0.0013).
Figure 2. ARI-4175 as monotherapy decreased tumor volume and improved survival in M3-9-M and MC38-CEA tumor models.
A. CEA-tg C57BL/6 mice were implanted on day 0 with 1×106 M3-9-M cells intramuscularly on the flank. B. CEA-tg C57BL/6 mice were implanted on day 0 with 3×105 MC38-CEA cells subcutaneously on the back. In both models, mice were given ARI-4175 (200 μg/mouse/day) or vehicle by gavage beginning on day 4 when tumors became visible. Mice were treated continuously from day 4 to day 25 (indicated by gray bars). Tumor volume was assessed 2–3 times/week. Statistical analysis of survival based on the Wilcoxon rank-sum test was performed on the indicated days.
We also tested ARI-4175 as a monotherapy in a nonimmunogenic model, using CEA-tg mice bearing MC38-CEA tumors. ARI-4175 (200 μg) was administered daily beginning when tumors became palpable (day 4). As shown in Figure 2B, ARI-4175 suppressed tumor growth during the time the drug was administered. By day 24, tumor volumes were significantly reduced in mice given ARI-4175 (mean: 129 mm3) compared to mice given vehicle (1,498 mm3) (P < 0.0043). However, after ARI-4175 treatment was discontinued, tumor growth resumed at a rate comparable to that observed in vehicle-treated mice. In this model, ARI-4175 significantly improved survival compared to mice given vehicle (P = 0.049).
3.3. Effect of orally administered ARI-4175 on murine immune-cell subsets and function
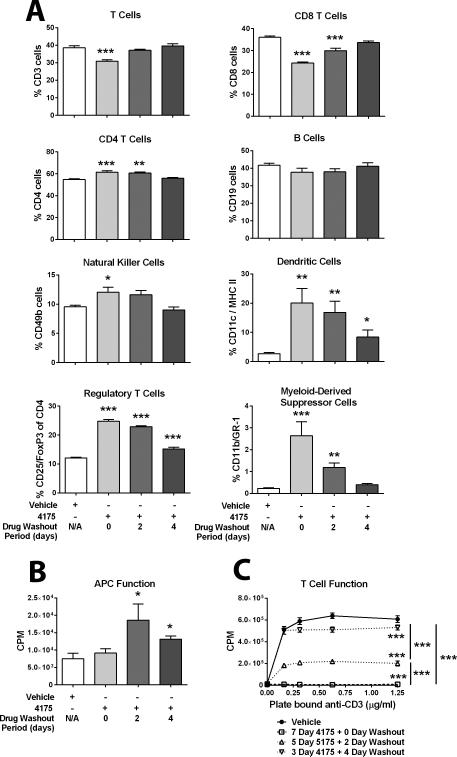
Because the goal of these studies was to determine the optimal timing and schedule for combining ARI-4175 with immunotherapy, we evaluated splenocytes from nontumor-bearing mice treated with ARI-4175 for either 7 days, 5 days (followed by 2 days of vehicle), or 3 days (followed by 4 days of vehicle). As shown in Figure 3A, 7 days of continuous treatment with ARI-4175 had varied effects on immune-cell subsets as assessed by flow cytometry. Specifically, continuous treatment with ARI-4175 decreased the percentage of CD3+ T lymphocytes, increased the percentage of CD4+ T lymphocytes , NK cells, and DCs, but had no notable effects on the percentage of B lymphocytes. CD3+ and CD8+ T lymphocytes returned to normal levels within 2 and 4 days, respectively, after stopping ARI-4175 treatment. Assessment of regulatory cells revealed that ARI-4175 markedly increased Tregs and MDSCs. Tregs remained markedly elevated after ARI-4175 treatment was suspended; however, 4 days after treatment ended, Tregs accounted for only 15% of CD4+ T cells. MDSCs similarly increased with ARI-4175 therapy, but within 4 days of terminating ARI-4175, MDSC levels returned to the baseline levels observed in vehicle-treated mice.
Figure 3. Effect of ARI-4175 on splenic immune-cell subsets and function.
Female C57BL/6 mice were given ARI-4175 (200 μg/mouse/day) by gavage for either 3 days (followed by 4 days of vehicle), 5 days (followed by 2 days of vehicle), or 7 days (followed by 0 days of vehicle). Control mice received vehicle for 7 days. A. ARI-4175 has varied effects on immune-cell subsets. On day 7, the percent of indicated immune-cell populations in individual mice (n = 3–5) was determined by flow cytometric analysis with the indicated markers. Data are representative of one experiment and are presented as mean ± SEM. B. ARI-4175 enhanced allostimulatory (H-2b vs. H-2d) activity. On day 7, splenocytes from ARI-4175-treated female C57BL/6 mice (effector cells) were cocultured with splenocytes from untreated Balb/c mice (target cells) for 4 days at an effector:stimulator ratio of 1:1 to 32:1. [3H]Thymidine was added during the final 18 h and incorporation was measured. Data shown represent an effector:stimulator ratio of 1:1 and mean ± SEM for triplicate wells. C. ARI-4175 reduced T-cell function. On day 7, splenocytes from ARI-4175-treated female C57BL/6 mice were incubated in the presence of increasing concentrations of plate-bound anti-CD3 for 3 days. [3H]Thymidine was added during the final 18 h and incorporation was measured. Data represent mean ± SEM for triplicate wells. Asterisks denote statistical significance (*P < 0.05, **P < 0.01, ***P < 0.001) relative to vehicle-treated mice.
We next evaluated the effect of ARI-4175 on the function of APCs using an allostimulatory (H-2b vs. H-2d) assay, where the APC population came from mice treated continuously with ARI-4175. APC function remained similar to that in control mice after 7 days of continuous treatment with ARI-4175; however, APC function significantly increased (74% to 146%) in mice after shorter periods of ARI-4175 administration followed by 2 or 4 days of drug washout (Figure 3B). Given the marked effects of ARI-4175 on splenic Tregs and MDSCs, we additionally evaluated the ability of splenocytes isolated from ARI-4175-treated mice to proliferate in response to anti-CD3. Continuous administration of ARI-4175 for 7 days abrogated the ability of T cells to proliferate in response to anti-CD3; however, this reduction in proliferation was significantly lessened following a shorter period of ARI-4175 administration followed by a 2- or 4-day drug holiday.
3.4. Effect of ARI-4175 in combination with cancer vaccines in M3-9-M and MC38-CEA tumor models
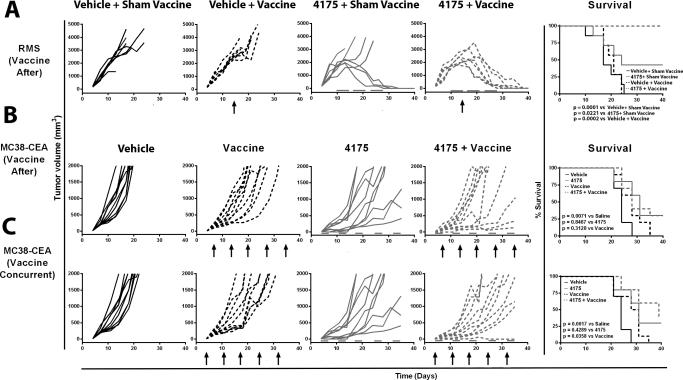
Based on the above findings, in the following studies ARI-4175 was administered for either 5 days/week (M3-9-M model) or 3 days/week (MC38-CEA model). We first analyzed the combination of ARI-4175 and a DC-based M3-9-M tumor-cell vaccine in the M3-9-M model. The M3-9-M DC-based vaccine and sham vaccine were given on day 14 after tumor-cell inoculation, either alone or in combination with a 3-week course of ARI-4175, started on day 10 after tumor-cell inoculation. The 3-week course of ARI-4175 (administered 5 consecutive days/week followed by a 2-day break) mediated tumor regression in 3/7 mice (Fig. 4A), while in mice given vaccine alone, tumor progression was similar to that observed in mice given sham vaccine. In contrast, the combination of vaccine and ARI-4175 resulted in tumor eradication in 7/7 mice. Moreover, the combination of ARI-4175 plus DC-based vaccine significantly improved survival compared to ARI-4175 plus sham vaccine (P = 0.0221), vehicle plus DC-based vaccine (P = 0.0002), and vehicle plus sham vaccine (P = 0.0001).
Figure 4. ARI-4175 given in combination with vaccine decreased tumor volume and improved survival in M3-9-M and MC38-CEA tumor models.
A. For the M3-9-M model, C57BL/6 mice were implanted on day 0 with 1×106 cells intramuscularly on the flank. Mice were given ARI-4175 (200 μg/mouse/day) or vehicle by gavage 5 times/week on days 10–14, 17–21, and 24–28. On day 14, mice received an intraperitoneal injection of DC-based vaccine (pulsed with irradiated tumor) or sham vaccine (non-pulsed DCs). B. For the MC38-CEA model, where vaccine was administered following ARI-4175, CEA-tg C57BL/6 mice were implanted on day 0 with 3×105 MC38-CEA cells subcutaneously. Mice were given either ARI-4175 (200 μg/mouse/day) or vehicle by gavage 3 days/week on days 4–6, 11–13, 18–20, 25–27, 32–34, and 39–41. Vaccinated mice received a prime of MVA-CEA-TRICOM on day 6 and weekly boosts with rF-CEA-TRICOM. C. For the MC38-CEA model, where vaccine was administered concurrently with ARI-4175, CEA-tg C57BL/6 mice were implanted on day 0 with 3×105 MC38-CEA cells subcutaneously. Mice were given either ARI-4175 (200 μg/mouse/day) or vehicle by gavage 3 times/week on days 4–6, 11–13, 18–20, 25–27, 32–34, and 39–41. Vaccinated mice received a prime with MVA-CEA-TRICOM on day 4 and weekly boosts with rF-CEA-TRICOM. Tumor volume was assessed 2–3 times/week. Statistical analysis of survival based on the Wilcoxon rank-sum test was performed on day 40. Arrows indicate vaccination. Gray bars indicate duration of ARI-4175 treatment.
In the MC38-CEA model, we evaluated ARI-4175 in combination with a prime with MVA-CEA-TRICOM, followed by weekly boosts with rF-CEA-TRICOM. In both treatment schedules, ARI-4175 was administered for 3 consecutive days/week followed by a 4-day break, in a 5-week course beginning 4 days after tumor-cell implantation. In the first schedule (Fig. 5B), vaccine was initiated on day 7, after ARI-4175 therapy had already been started. On day 21, mean tumor volume was reduced 57% in mice receiving ARI-4175 compared to mice given vehicle (P = 0.0052). The combination of ARI-4175 followed by vaccine did not significantly improve tumor progression relative to ARI-4175 alone (P = 0.5678, day 21) or vaccine alone (P = 0.0749, day 21). We next evaluated whether the therapeutic effect of ARI-4175 in combination with vaccine could be improved by administering vaccine concurrently (beginning on day 4) with ARI-4175 (Fig. 5C). In this schedule, the combination of ARI-4175 with vaccine significantly reduced tumor growth (77%) compared to vehicle (P < 0.0001, day 21) and vaccine alone (64%, P = 0.0052, day 21).
Figure 5. Effect of ARI-4175 on growth, cell-surface phenotype, and invasive capacity of human carcinoma cells.
A. At varying concentrations, ARI-4175 did not alter the growth of human prostate (LNCaP), colon (SW480), and breast (MDA-MB-231) cancer cells. Vehicle-treated and untreated cells served as controls, and medium and compound were replenished daily. The total number of viable cells was determined at various time points by trypan blue exclusion. Data represent mean ± SEM from 2 replicate wells. B. Human tumor cells undergo immunogenic modulation following exposure to ARI-4175. Cells were treated daily for 72 h with 10 μM ARI-4175, harvested, and analyzed by flow cytometry for cell-surface expression of Fas, ICAM-1, CEA, MUC1, MHC I, and calreticulin. Vehicle-treated and untreated cells served as controls. Numbers indicate percentage of positive cells (MFI in parentheses). Bold type indicates marked up-regulation (> 10% increase in percent of cells or MFI) relative to controls.
3.5. Effect of ARI-4175 on growth, immunogenic modulation of human carcinoma cells
To determine whether ARI-4175 has a direct proliferative or cytotoxic effect on human cancer cells, we performed in vitro dose-response studies using LNCaP, SW480, and MDA-MB-231 cells. Exposing any of these cells to ARI-4175 (0.1–10 μM) had no effect on cell number relative to untreated or vehicle-treated controls (Fig. 5A). We next examined the potential of ARI-4175 to alter the phenotype of human tumor cells, focusing on molecules that have been implicated in enhancing antitumor T-cell responses [17, 28-33]. Human tumor cells were treated with 10 μM ARI-4175 for 72h and analyzed for expression of cell-surface Fas, ICAM-1, the TAAs CEA and MUC1, MHC I (HLA-A2), and calreticulin. Relative to vehicle and untreated controls, Fas and HLA-A2 increased in 1/3 cell lines; ICAM-1, CEA, and calreticulin were elevated in 2/3 cell lines, and MUC1 was up-regulated in all 3 cell lines following treatment with ARI-4175 (Fig. 5B).
3.6. Effect of ARI-4175 on the sensitivity of human carcinoma cells to antigen-specific CTL killing
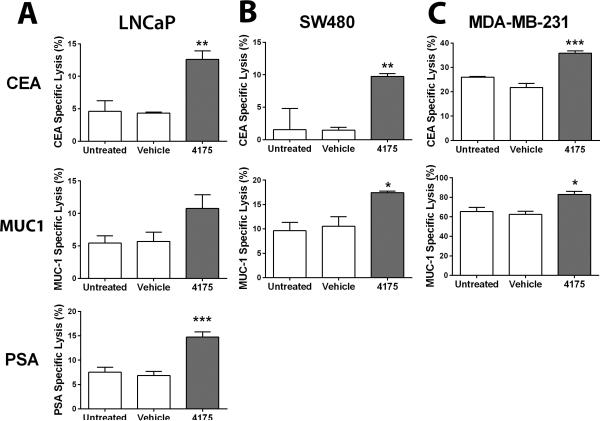
To determine the functional significance of the phenotypic changes induced by ARI-4175 in human LNCaP, SW480, and MDA-MB-231 cells, cultures were either untreated or treated with ARI-4174 or vehicle for 3 days, and subsequently cocultured with CEA-, MUC1-, and/or PSA-specific CTLs. ARI-4175 pretreatment of LNCaP tumor cells markedly increased killing by CEA-, MUC1-, and PSA-specific T cells over the level seen against untreated or vehicle-treated control cells (Fig. 6A). Similarly results were seen with with pretreated SW480 (Fig. 6B) and MDA-MB-231 tumor cells (Fig. 6C) cocultured with CEA- and MUC1-specific CTLs. These data indicate that exposure of a variety of human carcinoma cells to ARI-4175 enhances antigen-specific CTL-mediated killing, and that this effect can be extended to a variety of TAAs.
Figure 6. Effect of ARI-4175 on the sensitivity of human carcinoma cells to antigen-specific CTL killing.
Exposing human carcinoma cells to ARI-4175 increases sensitivity to antigen-specific CTL killing. Human cancer cells of the (A) prostate (LNCaP), (B) colon (SW480), and (C) breast (MDA-MB-231) treated with 10 μM ARI-4175 for 72 h were used as targets in an 18-h CTL lysis assay. CEA-, MUC1-, or PSA-specific CD8+ T cells were used as effector cells at an effector:target ratio of 30:1. Data represent mean ± SEM for 3 replicate wells. Asterisks denote significance (*P < 0.05, **P < 0.01, ***P < 0.001) relative to untreated wells. Data are representative of one experiment. Experiments were repeated 2–4 times with similar results.
4. Discussion
In previous studies, DASH inhibitors PT-100 and ARI-4175 were shown to upregulate cytokine and/or chemokine expression, modulate the trafficking of DCs and immunoregulatory cells, and activate or accelerate T-cell dependent immune responses against solid tumors [9, 10]. Our present studies revealed a new aspect of the antitumor activity of ARI-4175: the direct sensitization of tumor cells to T-cell mediated killing. An analysis of immune cell populations exposed to ARI-4175 revealed time-dependent changes in both effector T-cells and Tregs and enabled us to optimize ARI-4175 dosing schedule for immune stimulation in an investigation of adjuvant activity in combination with recombinant viral CEA-TRICOM and a DC tumor vaccine.
ARI-4175 was not directly cytotoxic for murine and human tumor cells in vitro (Fig. 1A and 5A). However, ARI-4175 produced increases in the levels of Fas, ICAM-1, MHC class I, and TAAs—MUC1 and CEA—expressed on the tumor cell surface (Figs. 1B and 5B). Each of these immunologically relevant molecules have been previously shown to be involved in the cell-to-cell interactions by which CTLs kill tumor cells. For example, binding of Fas to the Fas receptor on CTLs is one of the main mechanism by which CTLs induce apoptosis [17], while ICAM-1 has both co-stimulatory and cell adhesive properties, with increased levels correlating with CTL binding to tumor cell targets and enhanced cytotoxicity [34]. Moreover, simultaneous upregulation of MHC class I with the TAAs CEA and MUC-1 in tumor cells should increase the efficiency of immune surveillance [30, 32]. Consistent with the observed phenotypic modification of the tumor cells by ARI-4175, their sensitivity to killing by TAA-specific CTL in vitro was significantly increased (Fig. 1C and 6). Whether exposure of murine or human cancer cells to ARI-4175 similarly increases their susceptibility to non-antigen and/or non-immune killing remains to be tested.
In the present study we found in the M3-9-M rhabdomyosarcoma and MC38-CEA colon cancer models that daily administration of ARI-4175 on every consecutive day from day 4, when tumors become visible, until day 25 produced strong tumor responses: tumor regression and rejection in the M3-9-M model and greatly reduced tumor growth in the MC38-CEA model (Fig. 2). In the analysis of ARI-4175 effects on immune cell subsets in vivo, we observed that T-cell anergy appeared to be quickly reversed once drug treatment was stopped in vitro (Fig. 3C). This led us to reason that the ARI-4175 treatment schedule for investigation of vaccine adjuvant activity should include drug holidays in order to reduce possible immunosuppressive effects. We sought to improve efficacy of the MC38-CEA tumors to ARI-4175 in vivo (Fig. 2B) by adjusting the ARI-4175 schedule to 3 days on drug and 4 days off in each week of treatment in combination with the recombinant virus CEA-TRICOM vaccine. The vaccine was administered either after ARI-4175 administration had started (Fig. 4B), or concurrently with the drug (Fig. 4C). We found that the tumor response was most improved by the latter regimen.
As an adjuvant to the CEA-TRICOM and DC tumor vaccines, ARI-4175 produced superior tumor responses when added to the DC vaccine. The reason for this difference might lie in design of the two vaccines and tumor models examined. The CEA-TRICOM vaccine is designed to elicit a T-cell response against MHC-binding peptides derived from human CEA; however in CEA-tg mice bearing subcutaneous MC38-CEA expressing tumors, the vaccine must overcome the hurdle of thymic tolerance to human CEA. This is in contrast to the DC vaccine in the rhabdomyosarcoma tumor model, where the DCs have the opportunity to sample all possible M-3-9-M tumor antigens upon ingestion of the apoptotic bodies derived from the M3-9-M tumor cell line. These antigens could include mutated proteins that are unique to the tumor cell, wild-type gene products that are overexpressed by the tumor cell, or differentiation antigens, which due to age- or anatomically-restricted expression might fail to establish thymic tolerance. Thus, the tolerance barrier in M3-9-M DC vaccination is lower than that faced by CEA-TRICOM in the MC38-CEA model. The results of the present study appear to indicate that ARI-4175 is well suited as an adjuvant to DC tumor vaccines that are designed to incorporate a large repertoire of TAAs and thereby provide the immune system with the opportunity to respond against antigens that have escaped thymic tolerance.
The development of cancer vaccines has been hampered by a lack of appropriate adjuvant therapies. Our results provide a rationale for the combination of ARI-4175 with immunotherapeutic vaccines in a clinical setting. Our results indicate that the timing and schedule of ARI-4175 administration in relation to vaccine is critical, and that when used as a vaccine adjuvant, ARI-4175 should include drug holidays in order to reduce the possible immunosuppressive effects and maximize the immune beneficial effects of this agent. Interruption of drug dosing should not affect the sensitization of tumor cells to CTL killing, as a short duration (only 3 days) of exposure to ARI-4175 was sufficient to increase the susceptibility of tumor cells to lysis. To date, ARI-4175 remains at the pre-clinical stages of development and needs to be tested in a phase I trial before it can be combined with a vaccine platform.
Highlights.
ARI-4175 modulated the phenotype of immunologically relevant molecules.
ARI-41745 increased the sensitivity of carcinoma cells to CTL-mediated lysis.
ARI-4175 modulated immune-cell subsets and function in nontumor-bearing mice.
ARI-4175 suppressed the growth of rhabdomyosarcoma and MC38-CEA tumors in vivo.
ARI-4175 used in combination with 2 vaccine platforms enhanced antitumor activity
Acknowledgements
The authors thank Dr. Jeffrey Schlom for his helpful suggestions and Marion Taylor for excellent technical assistance. We also thank Bonnie L. Casey for editorial assistance in the preparation of this manuscript.
Funding
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sedo A, Malik R. Dipeptidyl peptidase IV-like molecules: homologous proteins or homologous activities? Biochim Biophys Acta. 2001;1550(2):107–16. doi: 10.1016/s0167-4838(01)00278-3. [DOI] [PubMed] [Google Scholar]
- 2.Yu DM, et al. The dipeptidyl peptidase IV family in cancer and cell biology. FEBS J. 2010;277(5):1126–44. doi: 10.1111/j.1742-4658.2009.07526.x. [DOI] [PubMed] [Google Scholar]
- 3.Mentlein R. Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept. 1999;85(1):9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 4.Gorrell MD. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin Sci (Lond) 2005;108(4):277–92. doi: 10.1042/CS20040302. [DOI] [PubMed] [Google Scholar]
- 5.Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169(12):7000–8. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 6.Christopherson KW, 2nd, et al. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305(5686):1000–3. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 7.Broxmeyer HE, et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med. 2012;18(12):1786–96. doi: 10.1038/nm.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams S, et al. PT-100, a small molecule dipeptidyl peptidase inhibitor, has potent antitumor effects and augments antibody-mediated cytotoxicity via a novel immune mechanism. Cancer Res. 2004;64(15):5471–80. doi: 10.1158/0008-5472.CAN-04-0447. [DOI] [PubMed] [Google Scholar]
- 9.Walsh MP, et al. Val-boroPro accelerates T cell priming via modulation of dendritic cell trafficking resulting in complete regression of established murine tumors. PLoS One. 2013;8(3):e58860. doi: 10.1371/journal.pone.0058860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan BB, et al. A Pan-Inhibitor of DASH Family Enzymes Induces Immune-mediated Regression of Murine Sarcoma and Is a Potent Adjuvant to Dendritic Cell Vaccination and Adoptive T-cell Therapy. J Immunother. 2013;36(8):400–11. doi: 10.1097/CJI.0b013e3182a80213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eager RM, et al. Phase II trial of talabostat and docetaxel in advanced non-small cell lung cancer. Clin Oncol (R Coll Radiol) 2009;21(6):464–72. doi: 10.1016/j.clon.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Eager RM, et al. Phase II assessment of talabostat and cisplatin in second-line stage IV melanoma. BMC Cancer. 2009;9:263. doi: 10.1186/1471-2407-9-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan B, et al. A pan-inhibitor of DASH family enzymes induces immune-mediated regression of murine sarcoma and in a potent adjuvant to dendritic cell vaccination and adoptive T-cell therapy. Journal of Immunotherapy. 2013;36(8):400–411. doi: 10.1097/CJI.0b013e3182a80213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins PF, et al. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 1991;51(14):3657–62. [PubMed] [Google Scholar]
- 16.Meadors JL, et al. Murine rhabdomyosarcoma is immunogenic and responsive to T-cell-based immunotherapy. Pediatr Blood Cancer. 2011;57(6):921–9. doi: 10.1002/pbc.23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodge JW, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013;133(3):624–36. doi: 10.1002/ijc.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muraro R, et al. Definition by monoclonal antibodies of a repertoire of epitopes on carcinoembryonic antigen differentially expressed in human colon carcinomas versus normal adult tissues. Cancer Res. 1985;45(11 Pt 2):5769–80. [PubMed] [Google Scholar]
- 19.Tai N, et al. Identification of critical amino acid residues on human dihydrofolate reductase protein that mediate RNA recognition. Nucleic Acids Res. 2002;30(20):4481–8. doi: 10.1093/nar/gkf562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005;11(6):2416–26. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 21.Tsang KY, et al. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87(13):982–90. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 22.Correale P, et al. Generation of human cytolytic T lymphocyte lines directed against prostate-specific antigen (PSA) employing a PSA oligoepitope peptide. J Immunol. 1998;161(6):3186–94. [PubMed] [Google Scholar]
- 23.Tsang KY, et al. A human cytotoxic T-lymphocyte epitope and its agonist epitope from the nonvariable number of tandem repeat sequence of MUC-1. Clin Cancer Res. 2004;10(6):2139–49. doi: 10.1158/1078-0432.ccr-1011-03. [DOI] [PubMed] [Google Scholar]
- 24.Fry TJ, et al. Antigen loading of DCs with irradiated apoptotic tumor cells induces improved anti-tumor immunity compared to other approaches. Cancer Immunol Immunother. 2009;58(8):1257–64. doi: 10.1007/s00262-008-0638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodge JW, et al. Modified vaccinia virus ankara recombinants are as potent as vaccinia recombinants in diversified prime and boost vaccine regimens to elicit therapeutic antitumor responses. Cancer Res. 2003;63(22):7942–9. [PubMed] [Google Scholar]
- 26.Hodge JW, et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59(22):5800–7. [PubMed] [Google Scholar]
- 27.Hodge JW, et al. Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res. 2003;9(5):1837–49. [PubMed] [Google Scholar]
- 28.Garnett CT, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64(21):7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 29.Kojima H, et al. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1(5):357–64. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 30.Modrak DE, et al. Colonic tumor CEA, CSAp and MUC-1 expression following radioimmunotherapy or chemotherapy. Tumour Biol. 2003;24(1):32–9. doi: 10.1159/000070658. [DOI] [PubMed] [Google Scholar]
- 31.Prete SP, et al. Combined effects of 5-fluorouracil, folinic acid and oxaliplatin on the expression of carcinoembryonic antigen in human colon cancer cells: pharmacological basis to develop an active antitumor immunochemotherapy. J Exp Clin Cancer Res. 2008;27:5. doi: 10.1186/1756-9966-27-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195(3):346–55. doi: 10.1002/jcp.10290. [DOI] [PubMed] [Google Scholar]
- 33.Gameiro SR, Caballero JA, Hodge JW. Defining the molecular signature of chemotherapy-mediated lung tumor phenotype modulation and increased susceptibility to T-cell killing. Cancer Biother Radiopharm. 2012;27(1):23–35. doi: 10.1089/cbr.2012.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zamai L, et al. Lymphocyte binding to K562 cells: effect of target cell irradiation and correlation with ICAM-1 and LFA-3 expression. Eur J Histochem. 1994;38(Suppl 1):53–60. [PubMed] [Google Scholar]