Abstract
Objective
To assess WINROP (https://winrop.com), an algorithm using postnatal weight measurements, as a tool for the prediction of retinopathy of prematurity (ROP) in a large geographically and racially diverse study population.
Methods
WINROP analysis was performed retrospectively on conventionally at-risk infants from 10 neonatal intensive care units.Weight measurements were entered into WINROP, which signals an alarm for an abnormal weight gain rate.Infants were classified into categories of no alarm (unlikely to develop type 1 ROP) and alarm (at risk for developing type 1ROP).Use of WINROP requires that an infant has(1)gestational age less than 32 weeks at birth, (2) weekly weight measurements,(3)physiologic weight gain,and(4)absence of other pathologic retinal vascular disease.
Results
A total of 1706 infants with a median gestational age of 28 weeks (range, 22-31 weeks) and median birth weight of 1016 g (range, 378-2240 g) were included in the study analysis. An alarm occurred in 1101 infants (64.5%), with a median time from birth to alarm of 3 weeks (range, 0-12 weeks) and from alarm to treatment of 8 weeks (range, 1 day to 22 weeks). The sensitivity of WINROP was 98.6% and the negative predictive value was 99.7%. Two infants with type 1 ROP requiring treatment after 40 weeks’ postmenstrual age did not receive an alarm.
Conclusion
The WINROP system is a useful adjunct for ROP screening that identifies high-risk infants early to optimize care and potentially reduce the overall number of diagnostic ROP examinations.
Retinopathy of prematurity (ROP) is a leading cause of preventable childhood blindness.1-3 Most cases of ROP are mild and regress spontaneously; however, severe ROP can lead to retinal detachment and permanent vision loss. Currently, birth weight (BW) and gestational age (GA) are used as selection criteria to examine infants for treatable disease4; postnatal factors that may help to predict or prevent ROP are not considered. Retinopathy of prematurity is diagnosed only later in the disease process by serial ophthalmoscopic examinations.
Serial ROP examinations are labor-intensive for clinicians and stressful on infants.5,6 In the United States, more than 85 000 live births per year are very pre-term and/or very low BW.7 Estimating an average of 5 examinations per infant,8 as many as 425 000 or more screenings are performed each year, with more than 90% of infants examined never developing ROP that requires intervention.9,10 There is a need for earlier differentiation of infants at higher risk for severe ROP from those at lower risk.
Predicting ROP early depends on understanding early retinal vascular changes that occur after premature birth and before extensive retinal neovascularization that precipitates ROP treatment. Retinopathy of prematurity is a 2-phase disease. Phase I consists of poor postnatal retinal vascular growth leading to hypoxia, which then determines the degree of pathologic neovascularization, or phase II of ROP. Accordingly, postnatal growth factors or nutritional deficiencies that suppress retinal vascular growth may influence the later development of proliferative retinopathy. Given the biphasic nature of ROP, low levels of factors that affect vascular growth may be assessed to help predict ROP risk weeks to months before the neovascular phase. These low levels of growth factors may also determine postnatal somatic growth or weight gain.
Inclusion of postnatal risk factors for ROP can be used to help select premature infants for eye examinations. This may help target at-risk infants for interventions that may reduce severe ROP. Extensive clinical and animal studies11-17 have demonstrated the relationship between low serum insulinlike growth factor (IGF) I levels and associated poor weight gain with the development of more severe ROP. The WINROP (weight, IGF, neonatal ROP) algorithm was developed in Sweden to evaluate the risk of treatable ROP based on weekly postnatal measurements of these 2 parameters.18 Prospective and retrospective pilot studies18,19 have shown that the WINROP algorithm has the potential to identify infants who are at high risk for severe ROP early in their neonatal course.
The WINROP algorithm has been further simplified as an online monitoring system (https://winrop.com) using weight gain measurements alone, allowing for use in any nursery, and has demonstrated 100% sensitivity in predicting severe ROP in 2 Swedish and US populations.8,20 The WINROP system was also validated in a Brazilian population with a sensitivity of 90.5% in predicting proliferative ROP.21 This population of infants had less stringently documented GA and dates for weekly weight measurements. As commonly seen in developing countries that require broadened screening guidelines,2 there was a higher median BW and GA in treated Brazilian infants. However, the high sensitivity in this group further supports the relationship between poor postnatal weight gain and severe ROP.
Given the very high sensitivity of these early pilot studies,8,20 we sought to evaluate the potential use of the WINROP algorithm to help predict type 1 ROP in a large, more geographically and racially diverse study population in the United States and Canada.
METHODS
PATIENTS
A review was performed of records on premature infants from 10 level III neonatal intensive care units (NICUs) in the United States and Canada who qualified for ROP examinations according to the conventional criteria of GA and BW between 2006 and 2009. For this retrospective study, infants were excluded if they were born at a GA of 32 weeks or more, had incomplete medical records, or lacked weekly weight measurements until 36 weeks’ postmenstrual age (PMA) or hospital discharge, demonstrated nonphysiologic weight gain, or had retinal disease other than ROP. Prospective use of WINROP would also exclude these infants. Follow-up of infants with ROP was required until ROP was regressing, mature retinal vascularization or immature retinal vascularization in zone III was reached, prethreshold or threshold ROP occurred, or treatment was required.
Data collected included birth date, GA, BW, weekly post-natal weight, sex, birth multiplicity, race, ROP examination results, and presence of bronchopulmonary dysplasia, intraventricular hemorrhage, hydrocephalus, and necrotizing enterocolitis. Race was classified as white, black, Asian, American Indian or Alaskan Native, Native Hawaiian/Pacific Islander, or Hispanic origin by participating investigators on the basis of data obtained from the infant's medical record.
The study was approved by the institutional review boards at all participating centers.
ROP EXAMINATIONS AND TREATMENT
Examinations for ROP were performed by qualified ophthalmologists with expertise in ROP on infants with BW less than 1500 g or GA 30 weeks or less, as well as select infants with BW between 1500 and 2000 g or GA more than 30 weeks with an unstable clinical course that was considered to place them at high risk for ROP.4 Infants were examined using standard binocular indirect ophthalmoscopy with scleral depression. Examinations ranged from twice per week to every 3 weeks, depending on the severity and zone of ROP. Classification of ROP was performed according to the International Classification of Retinopathy of Prematurity.22 The highest stage and lowest zone of ROP, presence of prethreshold (PT) or threshold disease, and need for ROP treatment were recorded. Prethreshold ROP was further subclassified according to Early Treatment for Retinopathy of Prematurity criteria into type 1 or type 2 ROP,10 as described in the next paragraph.
The maximum ROP for the worse eye was categorized into 4 groups: (1) no ROP (immature or mature retinal vascularization); (2) non-PT ROP (zone II stage 1 or 2 ROP without plus disease; zone III stage 1, 2, or 3 ROP); (3) type 2 ROP (zone I stage 1 or 2 ROP without plus disease; zone II stage 3 ROP without plus disease); and (4) type 1 ROP (any zone I ROP with plus disease; zone I stage 3 ROP without plus disease; zone II stage 2 or 3 ROP with plus disease). Retinal ablative treatment was considered for type 1 ROP.10
WINROP SCREENING
The WINROP algorithm was developed using the methods of online statistical surveillance.23-25 Use of WINROP requires that an infant has (1) GA less than 32 weeks at birth, (2) weekly weight measurements, (3) physiologic weight gain, and (4) absence of other pathologic retinal vascular disease.
Reference models of the expected safe weekly IGF-I levels and physiologic weight gain velocity were created using data from infants who did not develop ROP or developed stage 1 ROP.18 In this study, the simplified version of WINROP analysis with postnatal weight gain alone was used. Deviations between observed and reference weight gain values were calculated and accumulated. When the accumulated sum exceeded a limit, an alarm was signaled in infants with a GA less than 30 weeks and BW less than 850 g. An alarm was signaled in infants with a GA 30 weeks or more and BW 850 g or more if the limit was exceeded at 32 or fewer weeks’ PMA. Additionally, birth weights that were very low for a given GA could result in an immediate alarm at week 0.
The WINROP model used in this study was developed with the derivation sample data on the basis of weekly physiologic weight gain and was not modified on the basis of data collected for this or any previous validation study. Infants with evidence of nonphysiologic weight gain do not qualify for WINROP analysis. Any weekly weight gain of more than 450 g was identified by WINROP as an indicator for excessive, nonphysiologic weight gain. Additionally, infants with hydro-cephalus or excessive edema on physical examination, representing nonphysiologic weight gain, would also require exclusion from WINROP use. Given the retrospective design of this study, these infants could be identified only on post hoc review of medical records. With prospective use, WINROP queries the presence of nonphysiologic weight gain based on physical examination and medical history with each weight entry, thus enabling the bedside physician to exclude these infants prospectively.
Data entry for WINROP included birth date, sex, GA, BW, and weekly postnatal weight. For analysis, infants were classified into 2 groups: no alarm (unlikely to develop type 1 ROP) and alarm (at risk for developing type 1 ROP).
STATISTICAL ANALYSIS
The sensitivity and specificity of WINROP screening to identify infants who developed type 1 ROP with an alarm were analyzed. The negative and positive predictive values were calculated using the sensitivity, specificity, and prevalence of type 1 ROP for the study group. We calculated 95% CIs for estimated binary proportions (sensitivity and specificity) using the exact method of Clopper-Pearson.26 Secondary analysis was performed separately on data from infants who required ROP treatment and those who developed PT ROP.
RESULTS
PATIENTS
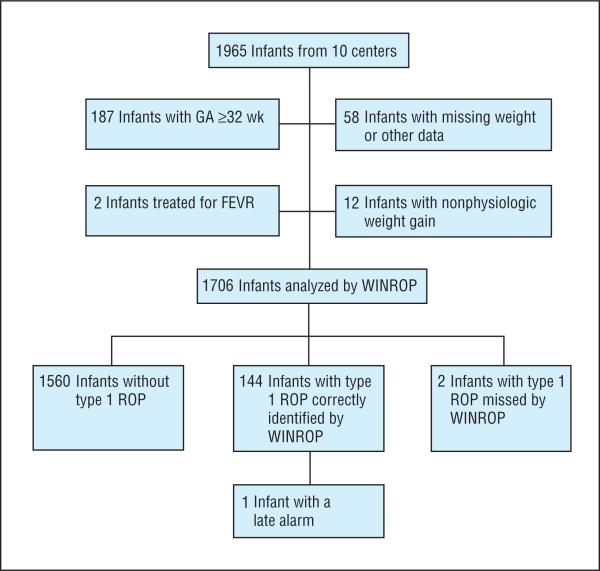
A total of 1965 infants from 10 centers were screened for ROP. For WINROP assessment, 259 infants did not meet inclusion criteria, since 187 were born at a GA of 32 weeks or more, 58 had missing medical data or weekly weight measurements, 12 had nonphysiologic weight gain, and 2 underwent treatment for familial exudative vitreoretinopathy, a genetic disease causing retinopathy that mimics ROP (eTable 1; http://www.archophthalmol.com). Of the 187 infants who were born at a GA of more than 32 weeks, 179 did not develop ROP. Only 1 infant developed PT ROP and received a WINROP alarm. Most of the infants with missing data were transferred from outside institutions to the study center; therefore, early weight measurements were unavailable. No or only mild ROP developed in the premature infants with familial exudative vitreoretinopathy, but treatment was performed for persistent avascular retina. Familial exudative vitreoretinopathy was diagnosed in these infants on the basis of clinical, angiographic, and genetic testing.
A total of 1706 infants were included in the study analysis (Figure 1). The median GA at birth was 28 weeks (range, 22-31 weeks) and the median BW was 1016 g (range, 378-2240 g). Female infants accounted for 47.9% (817 of 1706) of the study population; 1172 infants were singleton births, 436 were twin births, 90 were triplet births, and 8 were quadruplet births. The study population was 54.0% white, 27.1% black, 9.8% Hispanic, 5.3% Asian, and 3.7% other races (Table 1). The racial distribution of premature infants born at a GA of less than 32 weeks in the United States is approximately 42.9% non-Hispanic white, 29.0% non-Hispanic black, 21.7% Hispanic, 4.6% Asian or Pacific Islander, 1.3% American Indian or Alaskan Native, and 0.5% other races.7
Figure 1.
Flowchart of the study population. FEVR indicates familial exudative vitreoretinopathy; GA, gestational age; WINROP, weight, insulinlike growth factor, neonatal retinopathy of prematurity (ROP).
Table 1.
Patient Demographics
| Characteristic | No. (%) (N = 1706) |
|---|---|
| GA, median (range), wk | 28 (22-31) |
| BW, median (range), g | 1016 (378-2240) |
| Sex | |
| Male | 889 (52.1) |
| Female | 817 (47.9) |
| Birth multiplicity | |
| Single | 1172 (68.7) |
| Twin | 436 (25.6) |
| Triplet | 90 (5.3) |
| Quadruplet | 8 (0.5) |
| Race | |
| White | 921 (54.0) |
| Black | 463 (27.1) |
| Hispanic | 168 (9.8) |
| Asian | 91 (5.3) |
| Other | 63 (3.7) |
Abbreviations: BW, birth weight; GA, gestational age.
There was a trend for a higher rate of complicating comorbidities, such as bronchopulmonary dysplasia, intraventricular hemorrhage, hydrocephalus, and necrotizing enterocolitis, in infants with more severe ROP (Table 2).
Table 2.
Prematurity-Associated Illnesses
| Infants, No. (%) | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Total (N = 1706) | No ROP (n = 880) | Non-PT ROP (n = 605) | Type 2 ROP (n = 75) | Type 1 ROP (n = 146) | Treated ROP (n = 149) |
| Bronchopulmonary dysplasia | 687 (40.3) | 191 (21.7) | 315 (52.1) | 58 (77.3) | 123 (84.2) | 124 (83.2) |
| Intraventricular hemorrhage | 459 (26.9) | 157 (17.8) | 207 (34.2) | 29 (38.7) | 66 (45.2) | 69 (46.3) |
| Hydrocephalus | 69 (4.0) | 15 (1.7) | 40 (6.6) | 4 (5.3) | 10 (6.8) | 11 (7.4) |
| Necrotizing enterocolitis | 150 (8.8) | 50 (5.7) | 67 (11.1) | 9 (12.0) | 24 (16.4) | 27 (18.1) |
Abbreviations: PT, prethreshold; ROP, retinopathy of prematurity.
ROP AND WINROP OUTCOME
The maximal ROP attained by the worse eye was type 1 ROP in 146 infants (8.6%), type 2 ROP in 75 infants (4.4%), and non-PT ROP in 605 infants (35.5%); 149 infants (8.7%) underwent ROP treatment. No ROP developed in 880 infants (51.6%) (Table 3).
Table 3.
Alarm Signal in Relation to ROP Categories and Birth Characteristics
| Alarm Status | |||
|---|---|---|---|
| No Alarm | Alarm | All Infants | |
| Infants, No. (%) | 605 (35.5) | 1101 (64.5) | 1706 |
| ROP categories, No. | |||
| None | 484 | 396 | 880 (51.6) |
| Non-PT | 117 | 488 | 605 (35.5) |
| Type 2 | 2 | 73 | 75 (4.4) |
| Type 1 | 2 | 144 | 146 (8.6) |
| Treated | 4 | 145 | 149 (8.7) |
| Birth characteristics, median (range) | |||
| GA, wk | 29 (23-31) | 27 (22-31) | 28 (22-31) |
| BW, g | 1385 (625-2240) | 850 (378-1720) | 1016 (378-2240) |
Abbreviations: BW, birth weight; GA, gestational age; PT, prethreshold; ROP, retinopathy of prematurity.
No alarm was signaled in 605 infants and 603 of these did not develop type 1 ROP. Two infants developed type 1 ROP and underwent treatment after 40 weeks’ PMA (eTable 2).
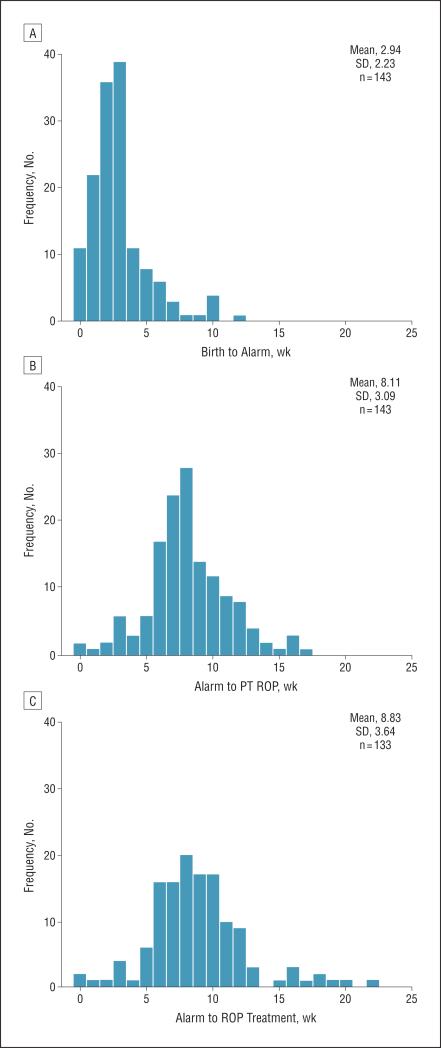
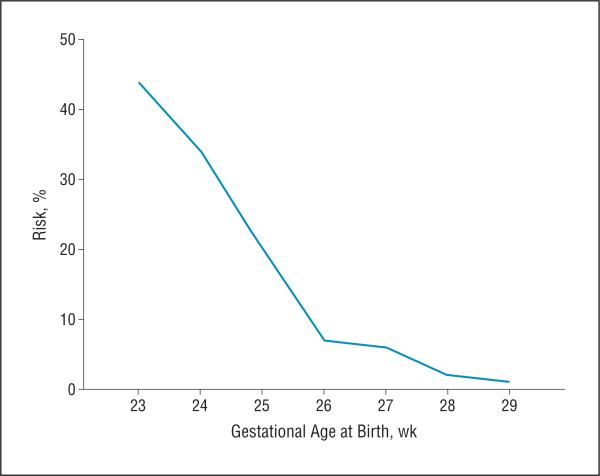
An alarm was signaled in 1101 infants; 78 of these infants (7.1%) received an alarm at week 0 based on BW alone. None of these infants was extremely preterm, with a mean (SD) GA of 29.5 (1.4) weeks. However, they were born severely small for GA, with a mean BW SD score of −4.5 (0.7). In the alarm group, type 1 ROP developed in 144 infants, including 44 who developed zone I ROP. One infant (GA, 23 weeks; BW, 499 g) who had feeding difficulties with prolonged use of total parenteral nutrition developed zone I stage 2 ROP with plus disease requiring treatment at 32 1/7 weeks’ PMA and received an alarm at 33 weeks’ PMA. For all other infants, the median time from birth to alarm was 3 weeks (range, 0-12 weeks), from alarm to development of PT ROP was 8 weeks (range, 0-17 weeks), and from alarm to ROP treatment was 8 weeks (range, 1 day to 22 weeks) (Figure 2). Of the infants with zone I ROP, the median time from birth to alarm was 3 weeks (range, 0-10 weeks), from alarm to development of PT ROP was 7 weeks (range, 1 day to 13 weeks), and from alarm to ROP treatment was 7 weeks (range, 1 day to 16 weeks). The percentage of infants who developed type 1 ROP after receiving an alarm was also calculated and categorized by GA at birth (Figure 3).
Figure 2.
Time from birth to alarm (A), alarm to prethreshold (PT) retinopathy of prematurity (ROP) (B), and alarm to ROP treatment (C) in infants with type 1 ROP.
Figure 3.
Risk of type 1 retinopathy of prematurity (ROP) after an alarm by gestational age.
Secondary analysis was performed on the 149 infants who underwent ROP treatment. Based on clinical judgment of the treating ophthalmologist, 10 infants who developed type 1 ROP were not treated and 13 infants were treated for less than type 1 ROP. Of these 13 infants, 11 were treated for type 2 ROP, 1 for zone III stage 3 ROP, and 1 for zone III stage 2 ROP with persistent temporal avascularity. Two additional infants in the treatment group who did not develop type 1 ROP received no alarm (eTable 3).
Prethreshold ROP developed in 221 infants. Excluding the 4 previously mentioned infants, all who developed PT ROP received an alarm.
TEST CHARACTERISTICS
The overall sensitivity of the WINROP algorithm in detecting type 1 ROP was 98.6% (95% CI, 96.7%-100.5%; 144 of 146 infants) and the specificity was 38.7% (95% CI, 36.2%-41.1%; 603 of 1560 infants). The positive predictive value was 13.1% (144 of 1101 infants) and the negative predictive value was 99.7% (603 of 605 infants) (Table 4).
Table 4.
Sensitivity, Specificity, and Positive and Negative Predictive Values in Identifying Type 1 ROP
| Alarm Status | % (95% CI) | ||||
|---|---|---|---|---|---|
| Alarm | No Alarm | Total | Sensitivity | Specificity | |
| ROP categories, No. (%) of infants | |||||
| Type 1 ROP | 144 | 2 | 146 | 98.6 (96.7-100.5) | . . . |
| Non-type 1 ROP | 957 | 603 | 1560 | . . . | 38.7 (36.2-41.1) |
| Total | 1101 | 605 | 1706 | ||
| Predictive value, % (No./total No.) | |||||
| PPV | 13.1 (144/1101) | . . . | . . . | . . . | . . . |
| NPV | . . . | 99.7 (603/605) | . . . | . . . | . . . |
Abbreviations: ellipses, not applicable; NPV, negative predictive value; PPV, positive predictive value; ROP, retinopathy of prematurity.
For secondary analysis, the overall sensitivity of the WINROP algorithm in identifying infants who required ROP treatment was 97.3% (95% CI, 94.7%-99.9%; 145 of 149 infants) and the specificity was 38.6% (95% CI, 36.2%-41.0%; 601 of 1557 infants). The positive predictive value was 13.2% (145 of 1101 infants) and the negative predictive value was 99.3% (601 of 605 infants).
The overall sensitivity of the WINROP algorithm in detecting PT ROP was 98.2% (95% CI, 96.7%-99.9%; 217 of 221 infants) and the specificity was 40.5% (95% CI, 38.0%-43.0%; 601 of 1485 infants). The positive predictive value was 19.7% (217 of 1101 infants) and the negative predictive value was 99.3% (601 of 605 infants).
COMMENT
Our multicenter study validated the use of postnatal weight gain analysis by WINROP to aid early prediction of infants at high risk for type 1 ROP. The study was conducted in a large geographically and racially diverse NICU population in the United States and Canada, used current Early Treatment for Retinopathy of Prematurity criteria, and included infants with zone I ROP.
Severe ROP is one of the strongest predictors for death or major disability.27,28 Because the retina is part of the central nervous system, measures to prevent poor neurovascular development in the eye may also benefit the development of the brain in preterm infants. Interventions that maximize nutritional support and normalize weight gain for high-risk infants early in life may decrease the development of severe ROP and reduce the long-term neurocognitive and functional impairments associated with prematurity.
Poor weight gain is a common, multifactorial complication of premature birth. Preterm infants require protracted time to adjust to an appropriate metabolic state after birth and are characterized by hypermetabolism,29 which can hinder weight gain. Aside from IGF-I levels, other factors that affect ROP, such as hyperglycemia,30,31 poor enteral nutrition,32-34 loss of ω-3 fatty acids,35 intrauterine infection,36,37 and excessive or fluctuating oxygen levels,38 are also associated with poor postnatal growth.
We have found that poor weight gain during the first weeks of life is a marker for severe ROP risk; approximately 50% of infants who developed type 1 ROP received a WINROP alarm 2 weeks or less after birth and 75% received an alarm 3 weeks or less after birth. High-risk infants were identified a median of 3 weeks after birth and 8 weeks before ROP treatment. The WINROP system is a valuable instrument to optimize inpatient and outpatient ROP screening and follow-up. Because WINROP is a noninvasive tool to assess ROP risk with the potential to reduce ROP screenings for select infants, it is conceivable that WINROP could allow NICUs flexibility in screening programs to identify treatable ROP in their particular population of premature infants.
Advance knowledge of ROP risk might affect the transfer of infants to hospitals with limited ROP coverage, as well as modify decisions to defer examinations in medically unstable infants who may be at increased risk for ROP. Most medicolegal claims involving ROP outcomes are the result of failure to transfer care from the NICU setting to the outpatient setting.39 Identification of infants who are at high ROP risk would help to ensure timely follow-up to decrease unfavorable outcomes from missed or delayed examinations.
Conversely, infants with no alarm were very unlikely to develop significant ROP. Ophthalmologic examinations ight be safely reduced in frequency and numbers for these infants, keeping in mind that WINROP is an adjunct to and not a replacement for standard ophthalmologic screening.
Examination schedules with use of WINROP have been implemented in several Swedish NICUs. For infants born at a GA of more than 29 weeks who do not receive an alarm, an ROP examination is performed at 5 weeks’ chronologic age. If no ROP is present, no further ROP examinations are performed. If ROP is present, routine ROP examinations continue. For infants born at a GA of 29 weeks or less who do not receive an alarm, examinations are reduced according to clinical judgment if no ROP is present. With this implementation of WINROP, the number of ROP screening examinations has been reduced by 25% in one NICU center (A.H., unpublished data, October 2011). Using this examination schedule in our study cohort would have reduced ophthalmologic examinations for almost 30% of infants and still have detected 100% of type 1 ROP, including all 3 infants who did not receive an alarm or received a late alarm. There would have been a reduction of examinations in almost 65% of infants born at a GA of more than 29 weeks and in more than 16% of infants born at a GA of 29 weeks or less GA.
Current ROP screening guidelines suggest a 1- to 2-week follow-up range for infants with immature retinal vascularization in zone I with no ROP, zone II stage 2 ROP, and regressing zone I ROP and a 2- to 3-week follow-up range for infants with immature retinal vascularization in zone II without ROP, zone III stage 1 or 2 ROP, or regressing zone III ROP.4 It may be possible to safely decrease the number of ROP screening examinations for infants receiving no alarm by selecting the longer follow-up date within this range. As with any decision regarding ROP follow-up and management, clinical judgment must be used and be based on an infant's complete medical history and examination.
Special attention should be given to the identification of nonphysiologic weight gain when using WINROP. Our current study is limited by retrospective analysis. With standard prospective use of WINROP, there would be concurrent clinical correlation between an infant's medical status and physical examination, revealing exclusion criteria for WINROP use.
Because infants who develop more severe ROP have a higher incidence of comorbidities with potential cause for nonphysiologic weight gain, clinical surveillance must be emphasized and routine ROP screenings should occur for all infants with nonphysiologic weight gain, such as for hydrocephalus or edema. Use of WINROP for prospective detection of type 1 ROP is ongoing.
It is important to characterize infants who developed type 1 ROP but were not identified by WINROP in this study. No evidence of nonphysiologic weight gain was found on limited retrospective review of the medical records in the 2 infants who developed type 1 ROP and did not receive an alarm or in the infant with a late alarm; however, there was a history of feeding intolerance requiring cessation of enteral nutrition and subsequent prolonged (>6 weeks) total parenteral nutrition use. Early postnatal nutrition is an important factor for weight gain and affects ROP severity.32-34 Human milk increases infant levels of IGF-I and ω-3 fatty acids that protect against ROP.40,41 It has been shown34 that infants who require ROP treatment receive more parenteral nutrition and less human milk during the first postnatal month, particularly during the second postnatal week. Prolonged feeding intolerance and total parenteral nutrition use, especially early in life, may be factors that warrant standard clinical surveillance for ROP even with WINROP indicating no alarm.
No infant in this cohort who developed type 1 ROP qualified for screening on the basis of BW alone, suggesting that GA and postnatal weight gain analysis may be more reflective of ROP risk. In 2006, ROP guidelines were modified to increase the GA for screening from 28 weeks or less42 to 30 weeks or less,4 but most infants who developed type 1 ROP in our cohort were born at 28 weeks or less GA. Two infants born at 29 weeks’ GA with type 1 ROP experienced poor postnatal weight gain and were correctly identified by WINROP. Current BW guidelines for ROP screening might be reconsidered if this is borne out in other studies.
In conclusion, postnatal weight gain analysis can help identify early infants at high risk for developing treatable ROP, as well as identify those not at risk. The WINROP system is an accessible, noninvasive tool that can be used to focus care on those at high risk for ROP, optimize follow-up, and potentially reduce the overall number of stressful diagnostic eye examinations.
Acknowledgments
Financial Disclosure: Access to the WINROP system online is provided without charge to any qualified participant. PremaCure AB has rights to the WINROP system. Drs Löfqvist and Hellström own shares in a company controlling PremaCure AB.
Funding/Support: This work was supported by the Children's Hospital Ophthalmology Foundation.
Role of the Sponsor: The funding sponsor had no involvement in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Group Information: The members of the WINROP Consortium are listed at the end of this article.
Author Contributions: Drs Wu and Löfqvist had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Wu and Löfqvist contributed equally to first authorship; Drs VanderVeen and Hellström contributed equally to last authorship.
WINROP Consortium: (in alphabetical order) Organizing Committee: Ann Hellström, MD, PhD, Chatarina Löfqvist, PhD, Lois E. H. Smith, MD, PhD, Deborah K. VanderVeen, MD, and Carolyn Wu, MD. Clinical Centers and Investigators: Beaumont Hospital/Associated Retinal Consultants, Oakland University William Beaumont School of Medicine, Royal Oak, Michigan: Kristi Cumming, RN, MSN, Kimberly Dresner, MD, PhD, and Lisa Faia, MD. Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts: Camilia R. Martin, MD, MS, and Reshma Mehendale, MD. Emory University Hospital Midtown, Emory University School of Medicine, Atlanta, Georgia: Caroline Cromelin, BA, and Amy K. Hutchinson, MD. Morristown Medical Center at Atlantic Health, Atlantic Neonatal Research Institute, Morristown, New Jersey: Ben H. Lee, MD. Mount Sinai Hospital, University of Toronto Faculty of Medicine, Toronto, ON, Canada: Michael H. Brent, MD, and Patrick Santiago, MD. Nationwide Children's Hospital, Ohio State University College of Medicine, Columbus: Mark Barsamian, DO, Don Bremer, MD, and Rae Fellows, MEd. New York-Presbyterian Morgan Stanley Children's Hospital, Columbia University College of Physicians and Surgeons, New York, New York: David A. Bateman, MD, Michael F. Chiang, MD, and Eva Nong, BA. North Carolina Children's Hospital, University of North Carolina School of Medicine, Chapel Hill: John S. Hartmann, MD, and M. Elizabeth Hartnett, MD. UMass Memorial Medical Center, UMass Medical School, Worcester, Massachusetts: Frank Bednarek, MD, Frank J. McCabe, MD, and Jill B. Whelan, MD. Wilford Hall Medical Center, Uniformed Services University of the Health Sciences, Lackland Air Force Base, Texas: R. Gary Lane, MD.
Online-Only Material: The eTables are available at http://www.archophthalmol.com.
REFERENCES
- 1.Muñoz B, West SK. Blindness and visual impairment in the Americas and the Caribbean. Br J Ophthalmol. 2002;86(5):498–504. doi: 10.1136/bjo.86.5.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert C, Fielder A, Gordillo L, et al. International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115(5):e518–e525. doi: 10.1542/peds.2004-1180. [DOI] [PubMed] [Google Scholar]
- 3.Maida JM, Mathers K, Alley CL. Pediatric ophthalmology in the developing world. Curr Opin Ophthalmol. 2008;19(5):403–408. doi: 10.1097/ICU.0b013e328309f180. [DOI] [PubMed] [Google Scholar]
- 4.Section on Ophthalmology American Academy of Pediatrics; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117(2):572–576. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]
- 5.Laws DE, Morton C, Weindling M, Clark D. Systemic effects of screening for retinopathy of prematurity. Br J Ophthalmol. 1996;80(5):425–428. doi: 10.1136/bjo.80.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rush R, Rush S, Nicolau J, Chapman K, Naqvi M. Systemic manifestations in response to mydriasis and physical examination during screening for retinopathy of prematurity. Retina. 2004;24(2):242–245. doi: 10.1097/00006982-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2009. [April 12, 2011];Natl Vital Stat Rep. 2010 59(3):1–19. http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_03.pdf. [PubMed] [Google Scholar]
- 8.Hellström A, Hård AL, Engström E, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123(4):e638–e645. doi: 10.1542/peds.2008-2697. doi:10.1542/peds.2008-2697. [DOI] [PubMed] [Google Scholar]
- 9.Palmer EA, Flynn JT, Hardy RJ, et al. the Cryotherapy for Retinopathy of Prematurity Cooperative Group. Incidence and early course of retinopathy of prematurity. Ophthalmology. 1991;98(11):1628–1640. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 10.Early Treatment for Retinopathy of Prematurity Cooperative Group Revised indications for the treatment of retinopathy of prematurity: results of the Early Treatment for Retinopathy of Prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 11.Wallace DK, Kylstra JA, Phillips SJ, Hall JG. Poor postnatal weight gain: a risk factor for severe retinopathy of prematurity. J AAPOS. 2000;4(6):343–347. doi: 10.1067/mpa.2000.110342. [DOI] [PubMed] [Google Scholar]
- 12.Hellström A, Perruzzi C, Ju M, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci U S A. 2001;98(10):5804–5808. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellström A, Engström E, Hård AL, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112(5):1016–1020. doi: 10.1542/peds.112.5.1016. [DOI] [PubMed] [Google Scholar]
- 14.Allegaert K, Vanhole C, Casteels I, et al. Perinatal growth characteristics and associated risk of developing threshold retinopathy of prematurity. J AAPOS. 2003;7(1):34–37. doi: 10.1067/mpa.2003.S1091853102420150. [DOI] [PubMed] [Google Scholar]
- 15.Engström E, Niklasson A, Wikland KA, Ewald U, Hellström A. The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatr Res. 2005;57(4):605–610. doi: 10.1203/01.PDR.0000155950.67503.BC. [DOI] [PubMed] [Google Scholar]
- 16.Fortes Filho JB, Bonomo PP, Maia M, Procianoy RS. Weight gain measured at 6 weeks after birth as a predictor for severe retinopathy of prematurity: study with 317 very low birth weight preterm babies. Graefes Arch Clin Exp Ophthalmol. 2009;247(6):831–836. doi: 10.1007/s00417-008-1012-3. [DOI] [PubMed] [Google Scholar]
- 17.Stahl A, Chen J, Sapieha P, et al. Postnatal weight gain modifies severity and functional outcome of oxygen-induced proliferative retinopathy. Am J Pathol. 2010;177(6):2715–2723. doi: 10.2353/ajpath.2010.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Löfqvist C, Andersson E, Sigurdsson J, et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol. 2006;124(12):1711–1718. doi: 10.1001/archopht.124.12.1711. [DOI] [PubMed] [Google Scholar]
- 19.Löfqvist C, Hansen-Pupp I, Andersson E, et al. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulinlike growth factor I. Arch Ophthalmol. 2009;127(5):622–627. doi: 10.1001/archophthalmol.2009.69. [DOI] [PubMed] [Google Scholar]
- 20.Wu C, VanderVeen DK, Hellström A, Löfqvist C, Smith LE. Longitudinal postnatal weight measurements for the prediction of retinopathy of prematurity. Arch Ophthalmol. 2010;128(4):443–447. doi: 10.1001/archophthalmol.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hård AL, Löfqvist C, Fortes Filho JB, Procianoy RS, Smith L, Hellström A. Predicting proliferative retinopathy in a Brazilian population of preterm infants with the screening algorithm WINROP. Arch Ophthalmol. 2010;128(11):1432–1436. doi: 10.1001/archophthalmol.2010.255. [DOI] [PubMed] [Google Scholar]
- 22.Committee for the Classification of Retinopathy of Prematurity An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102(8):1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 23.Shiryaev A. On optimum methods in quickest detection problems. Theory Probab. 1963;8(1):22–46. doi.org/10.1137/1108002. [Google Scholar]
- 24.Roberts SW. A comparison of some control chart procedures. Technometrics. 1966;8(3):411–430. [Google Scholar]
- 25.Frisen M. Statistical surveillance: optimality and methods. Int Stat Rev. 2003;71:403–434. doi:10.1111/j.1751-5823.2003.tb00205. [Google Scholar]
- 26.Hollander M, Wolfe D. Nonparametric Statistical Methods. 2nd ed. Wiley; New York, NY: 1999. [Google Scholar]
- 27.Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF. Trial of Indomethacin Prophylaxis in Preterms (TIPP) Investigators. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the Trial of Indomethacin Prophylaxis in Preterms. JAMA. 2003;289(9):1124–1129. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]
- 28.Farooqi A, Hägglöf B, Sedin G, Serenius F. Impact at age 11 years of major neonatal morbidities in children born extremely preterm. Pediatrics. 2011;127(5):e1247–e1257. doi: 10.1542/peds.2010-0806. doi:10.1542/peds.2010-0806. [DOI] [PubMed] [Google Scholar]
- 29.Singer D, Mühlfeld C. Perinatal adaptation in mammals: the impact of metabolic rate. Comp Biochem Physiol A Mol Integr Physiol. 2007;148(4):780–784. doi: 10.1016/j.cbpa.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Garg R, Agthe AG, Donohue PK, Lehmann CU. Hyperglycemia and retinopathy of prematurity in very low birth weight infants. J Perinatol. 2003;23(3):186–194. doi: 10.1038/sj.jp.7210879. [DOI] [PubMed] [Google Scholar]
- 31.Kaempf JW, Kaempf AJ, Wu Y, Stawarz M, Niemeyer J, Grunkemeier G. Hyperglycemia, insulin and slower growth velocity may increase the risk of retinopathy of prematurity. J Perinatol. 2011;31(4):251–257. doi: 10.1038/jp.2010.152. [DOI] [PubMed] [Google Scholar]
- 32.Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics. 2005;116(2):400–406. doi: 10.1542/peds.2004-1974. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto T, Shirai M, Kokubo M, et al. Human milk reduces the risk of retinal detachment in extremely low-birthweight infants. Pediatr Int. 2007;49(6):894–897. doi: 10.1111/j.1442-200X.2007.02483.x. [DOI] [PubMed] [Google Scholar]
- 34.Porcelli PJ, Weaver RG., Jr The influence of early postnatal nutrition on retinopathy of prematurity in extremely low birth weight infants. Early Hum Dev. 2010;86(6):391–396. doi: 10.1016/j.earlhumdev.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Kermorvant-Duchemin E, Sennlaub F, Sirinyan M, et al. Trans-arachidonic acids generated during nitrative stress induce a thrombospondin-1–dependent microvascular degeneration. Nat Med. 2005;11(12):1339–1345. doi: 10.1038/nm1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mestan K, Yu Y, Matoba N, et al. Placental inflammatory response is associated with poor neonatal growth: preterm birth cohort study. Pediatrics. 2010;125(4):e891–e898. doi: 10.1542/peds.2009-0313. doi:10.1542/peds.2009-0313. [DOI] [PubMed] [Google Scholar]
- 37.Hendson L, Russell L, Robertson CM, et al. Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. J Pediatr. 2011;158(3):397–402. doi: 10.1016/j.jpeds.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Hardy P, Beauchamp M, Sennlaub F, et al. New insights into the retinal circulation: inflammatory lipid mediators in ischemic retinopathy. Prostaglandins Leukot Essent Fatty Acids. 2005;72(5):301–325. doi: 10.1016/j.plefa.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Demorest BH. Retinopathy of prematurity requires diligent follow-up care. Surv Ophthalmol. 1996;41(2):175–178. doi: 10.1016/s0039-6257(96)80008-7. [DOI] [PubMed] [Google Scholar]
- 40.Díaz-Gómez NM, Domenech E, Barroso F. Breast-feeding and growth factors in preterm newborn infants. J Pediatr Gastroenterol Nutr. 1997;24(3):322–327. doi: 10.1097/00005176-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13(7):868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Academy of Pediatrics Section on Ophthalmology. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2001;108(3):809–811. doi: 10.1542/peds.108.3.809. [DOI] [PubMed] [Google Scholar]