Abstract
Cyanoacrylate glues are easily applied to wounds with good cosmetic results. However, they tend to be brittle and can induce local tissue toxicity. A series of cyanoacrylate monomers with a flexible ether linkage and varying side-chain lengths was synthesized and characterized for potential use as tissue adhesives. The effect of side-chain length on synthesis yield, physical and mechanical properties, formaldehyde generation, cytotoxicity in vitro and biocompatibility in vivo were examined. The incorporation of etheric oxygen allowed the production of flexible monomers with good adhesive strength. Monomers with longer side-chains were found to have less toxicity both in vitro and in vivo. Polymerized hexoxyethyl cyanoacrylate was more elastic than its commercially available and widely used alkyl analog 2-octyl cyanoacrylate, without compromising biocompatibility.
Keywords: Cyanoacrylates, Tissue adhesives, Surgical glue, Biocompatibility, Mechanical properties
1. Introduction
For surgical adhesives to be attractive alternatives to sutures and staples they should allow rapid adhesion and maintain strong and close apposition of wound edges for a sufficient time. Ideally surgical adhesives should not elicit a vigorous inflammatory response and should be biodegradable with minimal tissue toxicity [1]. α-Cyanoacrylates (CA) possess some of these properties and can be applied in medicine and dentistry with little discomfort and with good cosmetic results [2]. However, the use of commonly available CA adhesives, particularly within tissues, is limited by two major concerns. First, tissue toxicity, including necrosis, occurs in the immediate vicinity of the CAs, and is attributed to by-products such as cyanoacetate and formaldehyde [3], insufficient tissue vascularization [4], and the exothermic nature of the reaction [5]. Secondly, CA polymers are hard and brittle and may have insufficient flexibility for the dynamic nature of in vivo conditions [6]. Consequently, CAs are currently contraindicated for high tension wounds [7] and are only used in external or temporary applications, such as skin closure [8,9] and repair of corneal perforations [4]. The objective of this study was to develop CA adhesives that have better elastic properties without compromising biocompatibility.
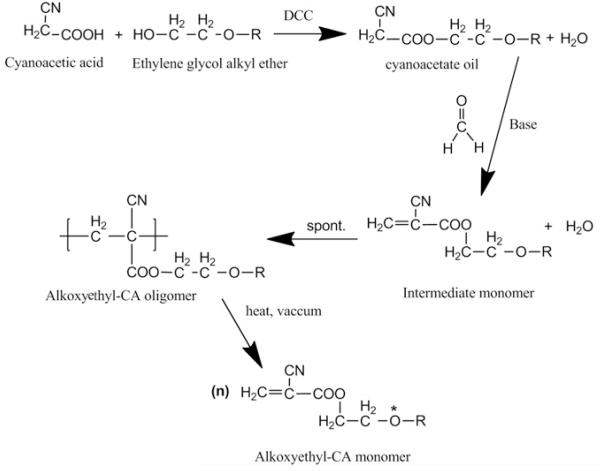
Our hypothesis was that CA monomers containing etheric oxygen could produce polymers with superior elasticity, while the use of longer carbon side-chains could mitigate the toxicity. The incorporation of etheric oxygen could improve the elastic properties because of the absence of hydrogen atoms on the etheric oxygen (asterisk in Fig. 1) facilitates chain rotation and consequently polymer flexibility [10,11]. It has also been suggested that tissue injury due to cyanoacrylates occurs in part because of the poor elasticity of the polymerized glue [12]. Improving the elastic properties could therefore improve tissue reaction. Toxicity is believed to be reduced by the longer alkyl side groups, which slow degradation and therefore decrease the accumulation of toxic by-products[13-15].
Fig. 1.
The synthesis of alkoxyethyl-CA monomers. The etheric oxygen in the final product is indicated by an asterisk.
To produce a potential surgical adhesive with improved physical properties and reduced toxicity we have developed and characterized a range of ethylene glycol alkyl ether monomers with increasing side-chain lengths. The mechanical strengths of the resulting polymers were assessed, as was their cytotoxicity in vitro and biocompatibility in vivo.
2. Materials and methods
2.1. Chemicals
Cyanoacetic acid was purchased from Alfa Aesar (99% pure, Ward Hill, MA). Ethylene glycol hexyl ether was purchased from TCI America (Portland, OR). All other ethylene glycol ethers, phosphorus pentoxide, hydroquinone, dicyclohexylcarbodiimide (DCC), paraformaldehyde (91–99% pure), piperidine, p-toluenesulfonic acid, dioctyl phthalate and Dulbecco’s phosphate-buffered saline (DPBS) were purchased from Sigma–Aldrich (St Louis, MO). Benzene, methanol and tetrahydrofuran (THF) were OmniSolv grade from EMD Chemicals (Gibbstown, NJ) and were used as received. 2-Octyl-CA (Dermabond®) and n-butyl-CA (Vetbond®) were purchased from Ethicon Inc. (Somerville, NJ) and 3M (St Paul, MN), respectively.
2.2. Synthesis
Cyanoacetate esters were synthesized by condensation between cyanoacetic acid and a suitable alcohol followed by Knoevenagel reaction [16] (Fig. 1). In a typical reaction (here relating to hexoxyethyl-CA) a mixture of 0.6 mol ethylene glycol hexyl ether and 0.6 mol cyanoacetic acid were stirred in 1000 ml of THF and maintained at 5–10 °C, then 0.6 mol DCC in 500 ml of THF was added in a dropwise manner. The resulting suspension was filtered to remove the dicyclohexylurea and evaporated using a rotary evaporator. After 12 h the crude oil was filtered again. Fractional distillation at reduced pressure through a short Vigreux column gave the final cyanoacetate oil.
0.5 mol paraformaldehyde and 0.3 ml of piperidine were placed in a three-necked glass flask and dissolved in 120 ml of methanol. A Dean–Stark trap combined with a reflux condenser, a thermometer, and a 500 ml separatory funnel were attached to the flask. The mixture was heated to 70 °C and 0.5 mol of cyanoacetate oil was added slowly while maintaining the boiling temperature. Then the heat was increased and the methanol removed via a DeanStark trap. Once about half of the methanol had been collected 100 ml of benzene was slowly added for azeotropic distillation. Of note, for industrial production, where heterogeneous azeotropic distillation columns are commonly used, alternative solvents such as toluene, ethanol, cyclohexane or a mixture of ethyl methyl ketone and hexane may be used [17,18]. After all the methanol and the entire theoretical amount of water (9 ml) were collected in the trap p-toluenesulfonic acid (0.6 g) was added to the mixture to neutralize the piperidine catalyst. The plastisizer dioctyl phthalate was then added (10 ml) and the solution was placed in a 0.6 mm Hg vacuum at 80 °C for solvent removal. 0.25 g hydroquinone and 2 g phosphorus pentoxide were added and the flask was connected to a short path distillation unit with a 100 ml receiver flask containing 0.125 g hydroquinone and 1 g phosphorus pentoxide. Sulfur dioxide gas was carefully introduced and a 740 torr vacuum was applied. The temperature was increased until depolymerization occurred (around 160 °C), as evident by the accumulation of droplets in the receiving flask. Repeated vacuum distillations and phosphorus pentoxide/hydroquinone additions to inhibit spontaneous polymerization were performed until a high purity product was achieved. The monomers were stored at 4 °C in the presence of p-toluenesulfonic acid.
2.3. Analysis of synthesized monomers
The chemical structures of the monomers were determined by 1H NMR using a Varian Mercury (Palo Alto, CA) 300 MHz spectrometer at 25 °C in CDCl3. Purity was determined by gas chromatography–mass spectrometry (GC–MS) (Agilent 5973 N, Little Falls, DE) with a temperature ramp from 100 to 350 °C at a heating rate of 10 °C min−1. Monomer solution (300 p.p.m. in THF, 1 ll injection volume) was used for analysis. The hydrophilicity of the monomers was characterized by measuring the contact angle by the sessile drop method [19]. 5 μl of each monomer was dropped on a hydrophobic natural rubber latex wafer [20] (VWR, MA) and the contact angle images were recorded using a goniometer equipped with video capture (VCA-2000, AST Inc., NJ). Each reported contact angle measurement represents an average value of at least six separate drops.
The peak temperatures generated by CA bulk polymerization were monitored by a temperature recording system equipped with a thermocouple wire (Fluke 51-2, Fluke, MA). The wire was placed in a preheated (37 °C) 96-well plate, then 200 μl of test monomer was inserted and 10 μl of 0.1 N NaOH was added. Each reported peak temperature represents an average value of six separate measurements.
2.4. Release of formaldehyde
10 μl of glue monomer were placed at the center of a 24-well culture plate. Monomers were allowed to polymerize for 24 h at room temperature. The resulting film was submerged in 1 ml of phosphate-buffered saline (PBS) and incubated at 37 °C. At predetermined time points the PBS was removed for analysis and replaced with fresh medium. The analysis consisted of measurement of the formaldehyde concentration using a fluorometric detection kit (Assay Designs, Ann Arbor, MI). The results for each sample were averaged (n = 4).
2.5. Mechanical testing
Mechanical tests were conducted using an Instron universal testing machine provided with a load cell of 500 N (model 5542, load cell model 2530-416, 0.125 N resolution or 0.25% of load, Instron Corp., Canton, MA) at a cross-head speed of 10 mm min−1 (ASTM method 0897-49 [2]). The test machine was controlled by Merlin 1999 operating system software v. 22031 (Richardson, TX), which provides all the test set-up, control and analysis functions. Experiments were first performed using aluminum specimens (Ted Pella Inc., Redding, CA) with 6.25 mm slotted heads and 1 cm pins. 5 μl of each monomer were applied to one of the two specimens and the second gently laid on top. The specimens were held together with clips for 12 h to insure monomer curing. The probe was withdrawn from the upper moving crimp at a rate of 0.1 mm min−1. The peak detachment force (N) was recorded as a function of extension diagram. The modulus was determined from the slope of the stress plotted against the applied strain. Each test trial consisted of eight replicate measurements.
A similar experiment was performed using segments of fresh skin harvested from 10 rats. 25 μl of each glue was applied to cross-sectional incisions in 2 × 6 cm strips of skin, after which the strips were apposed and maintained in contact for 24 h at 4 °C, as previously described [21-23]. The specimens were stretched at a rate of 10 mm min−1. Each test trial consisted of five replicate measurements.
2.6. Cytotoxicity studies
2.6.1. Cell toxicity of polymerized glue
The toxicity to HeLa cells (CRL 1658, Rockville, MD) of the different CAs was evaluated in comparison with cells without exposure to CA glues (control). Cells were grown at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Gibco-Invitrogen Corp., Grand Island, NY). Cultures were maintained in a 95% air/5% carbon dioxide atmosphere, at 95% relative humidity.
Cells were exposed to the polymerized glues either by direct contact (“direct”) or indirectly by being exposed to medium that was in contact with the polymerized glues (“indirect”). In the “direct” method 5 μl of glue monomer were placed at the center of the wells of 24-well culture plates. In the indirect method the monomers were placed in a band around the walls of the wells, fully in contact with the medium. Monomers were allowed to polymerize for 24 h at room temperature before cell seeding. Following 48 h exposure the cytotoxicity was assessed using the MTS assay [24] (CellTiter 96® Aqueous kit, Promega, Madison, WI). The results of each sample were averaged and are expressed as a percentage of the control. Four replicates were seeded for each of the tested CA as well as for fresh DMEM and the control.
2.6.2. Live/dead assay
A two color fluorescence cell viability kit [25] (Live/Dead® fluorescence viability kit, Molecular Probes, Eugene, OR) was used to confirm the results obtained from the MTS assay. After 48 h glue exposure cells were incubated with a mixture of 2 μM calcein acetoxymethyl and 8 μM ethidium homodimer in DPBS. Stained samples were washed and examined at 200× magnification via fluorescence microscopy (model HAL 100, Carl × Zeiss, Jena, Germany). The numbers of viable (green, obtained with the fluorescein filter set) and non-viable (red, obtained with the rhodamine filter set) cells were counted manually from images captured in the center of the wells, but at least 1 mm away from the polymer edge. Each experiment was performed with five independent replicates.
2.7. In vivo studies
Animals were cared for in compliance with protocols approved by the Massachusetts Institute of Technology Committee on Animal Care, in conformity with the NIH guidelines for the care and use of laboratory animals (NIH publication 85-23, revised 1985). 35 male rats (Sprague–Dawley) weighing 200–250 g were used. Rats were anesthetized with 2% isoflurane in oxygen and the back shaved and disinfected with 70 vol.% isopropanol in water/betadine. Skin incisions (1.5 cm) were made and a tunneled subcutaneous pouch produced towards the right. 20 ll of each monomer was deposited in the pouch and the incisions were closed with sutures.
Animals were killed with carbon dioxide after 12 days. Local swelling at the surgical site was measured with calipers, then the tissue and skin surrounding the glues were harvested and processed for hematoxylin and eosin staining by standard techniques [26].
2.8. Statistical analysis
The results of the formaldehyde release, mechanical properties and cell toxicity assays are presented as mean values ± SD. Statistical comparisons were performed with Instat 3.10 software (Prism 5, GraphPad, San Diego, CA). One-way analysis of variance (ANOVA) was used to test the significance of the differences between the treated groups. Tukey’s test was used for post comparison of specific groups. P < 0.05 was considered statistically significant.
3. Results
3.1. Synthesis and chemical analysis
The synthetic scheme is shown in Fig. 1. Cyanoacetic acid and ethylene glycol alkyl ether of the desired alkyl chain length were reacted in the presence of DCC, with high yields of cyanoacetate oil (>90%). Subsequent reaction with formaldehyde in the presence of base produced an intermediate which spontaneously formed an oligomer. Repeated short path distillation with heat and under vacuum produced CA monomers at variable yields (Table 1).
Table 1.
Characterization of the alkoxyethyl-CA monomers.
| Alkoxyethyl group |
Molecular weight (gmol−1) |
Specific gravity (gmr−1) |
Contact anglea |
Yield (%) |
Max. temperature (°C)b |
|---|---|---|---|---|---|
| Methoxy | 155.1 | 1.09 | 58.3 | 45 | 55 |
| Ethoxyl | 169.2 | 1.08 | 51.2 | 40 | 51 |
| Propoxy | 183.2 | 1.00 | 48.1 | 36 | 49 |
| Butoxy | 197.2 | 0.99 | 47.2 | 26 | 48 |
| Hexoxy | 225.3 | 0.94 | 44.2 | 14 | 46 |
| Dermabond® | 209.3 | 0.98 | 41.4 | N/A | 48 |
Calculated using 5 μl of each monomer on a latex wafer. The contact angle of doubly distilled water on the same surface is 74.3.
Maximal temperature for bulk polymerization of 200 μl of monomer initiated by 10 μl of 0.1 N NaOH.
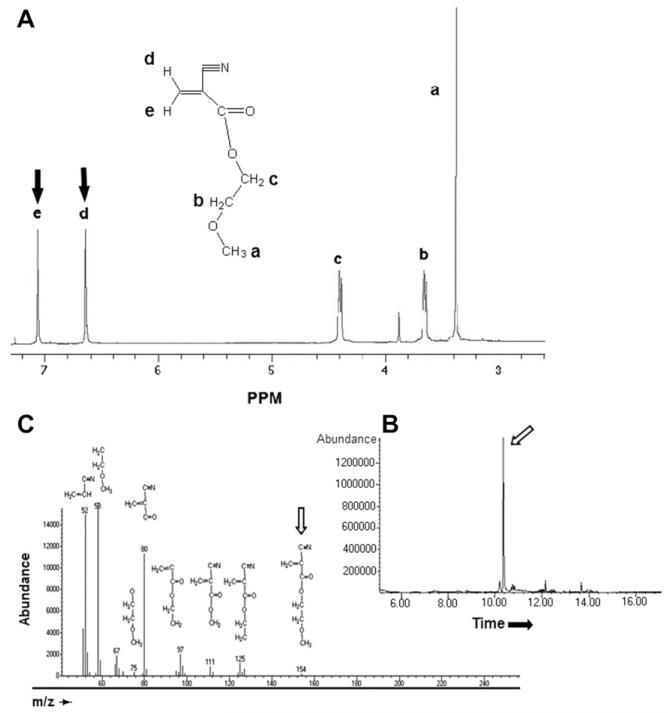
Synthesis of each monomer was demonstrated by NMR spectroscopy. For example, the presence of two peaks at 6.63 and 7.05 p.p.m. for the two protons of the double bond carbon in the spectrum for methoxyethyl-CA monomer (Fig. 2A) documented successful depolymerization of the oligomer into its monomeric form (the last step in Fig. 1). Gas chromatography and mass spectrometry (Fig. 2B and C) revealed a single major component, with traces impurities. Increasing the side-chain length decreased the specific gravity, contact angle, and the maximal polymerization temperature (Table 1). The yields of the synthesized monomers decreased with increasing side-chain length, possibly due to their higher boiling point and the greater degree of side-chain entanglement with increasing length, both of which may limit the efficiency of vacuum depolymerization [27].
Fig. 2.
Characterization of methoxyethyl-CA monomer. (A) 1H NMR spectra (in CDCl3). The arrows show peaks assigned to =CH2. (B) Gas chromatogram. (C). Mass spectroscopy. Structures corresponding to the longest peak in (B) are indicated. The major component, methoxyethyl-CA monomer, is indicated by arrows in (B) and (C).
3.2. Release of formaldehyde
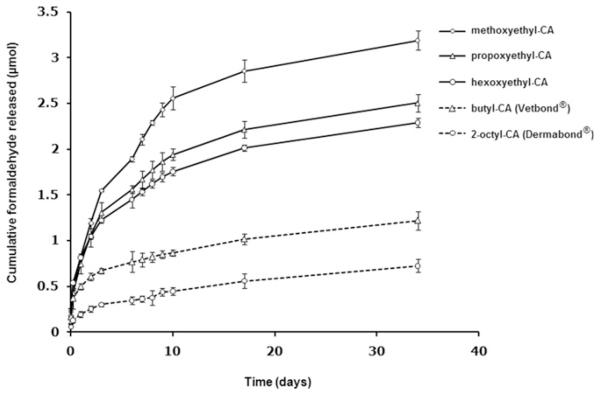
The release profiles of formaldehyde from the polymerized alkoxyethyl-CA and from commercial butyl- and 2-octyl-CA are illustrated in Fig. 3. In both the alkoxyethyl and the alkyl-CA groups formaldehyde release became slower as the molecular weight of the monomer increased. The rate of release from the alkoxyethyl-CA was faster than from the alkyl-CA. For example, less formaldehyde was released from octyl-CA compared with hexoxyethyl-CA, although the molecular weight of the former is lower.
Fig. 3.
Profiles of formaldehyde generation from alkoxyethyl-, butyl-, and 2-octyl-CA polymerized glues (n = 4).
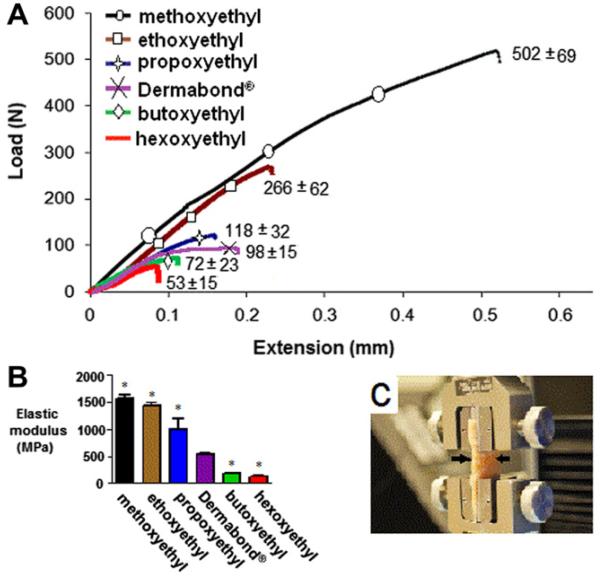
3.3. Mechanical testing
Monomers were polymerized on aluminum specimens (see Section 2.5) and the load on the cured polymers was measured as a function of extension. Increasing the side-chain length decreased the adhesive strength (Fig. 4A), although all glues remained within the useful range of adhesive strength [28,29]. Increasing the side-chain length also increased the elasticity of the polymer (i.e. reduced the elastic modulus, Fig. 4B). Statistically significant differences (P < 0.01) were observed between all the test groups and Dermabond. Of note, the elastic modulus of hexoxyethyl-CA was lower than that of its alkyl analog, 2-octyl-CA (Dermabond®), supporting the view that etheric oxygen could enhance elasticity.
Fig. 4.
(A) Adhesive strength (maximal load at rupture) of CAs with various alkoxyethyl side-chains (n = 8). These are representative curves; the standard deviation of the maximal load (at rupture) is in parentheses (n = 8). (B) The decrease in modulus due to the increase in alkoxyethyl side-chain length. Asterisks denote statistically significant differences from Dermabond®. *P < 0.01. (C) Skin sample prepared for testing in Instron grips (n = 5). Arrows show the site of the glued incision. All data are means with standard deviations.
Subsequently, full thickness segments of fresh Sprague-Dawley rat skin were glued together side to side with the monomers (Fig. 4C). All tested CA glues polymerized ex vivo, forming a crust. In all cases, when extension was applied to pull the incision apart the glued incision site maintained integrity while adjacent tissues tore apart. Load values at rupture were between 20 and 30N, which is consistent with reports for other CA glues used ex vivo [29].
3.4. Cytotoxicity studies
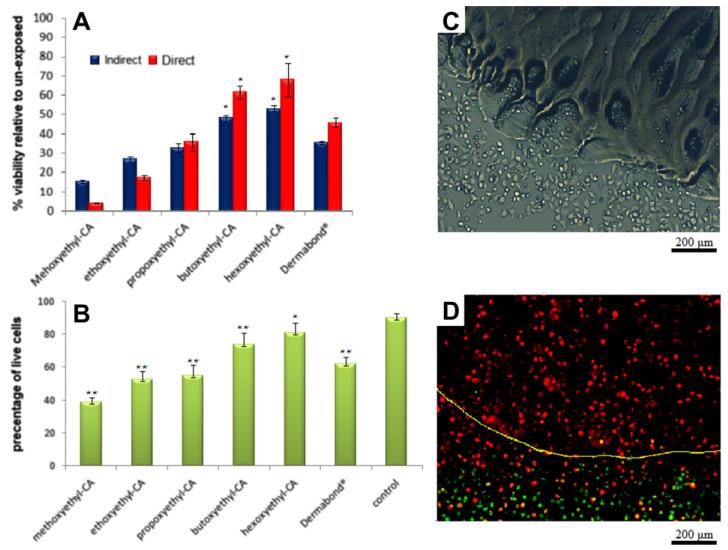
HeLa cells were seeded over glues that had been cured for 24 h in the center of cell culture wells and cytotoxicity was evaluated by the MTS assay (“Direct” group in Fig. 5A, as cells were in direct contact with the polymerized glue). Cell viability (as a percentage relative to cells not exposed to the glues) increased with increasing carbon chain molecular weight (R2 = 0.91). This result was confirmed by a live/dead assay using flow cytometry (Fig. 5B); R2 = 0.96 for the correlation of viability and molecular weight. No living cells were seen on the glue itself, and there was an area around the glue where cells did not survive (Fig. 5C and D). The fact that the glues released a toxic compound was confirmed in experiments where the glue was cured on the inside wall of the dish without direct contact with the cells (“Indirect” in Fig. 5A). Here also, side-chain molecular weight correlated well with increasing viability (R2 = 0.92) compared with controls not exposed to the glues. Cell viabilities for Dermabond® were in between those for propoxyethyl- and butoxyethyl-CA, but less than that for its alkoxyethyl analog, hexoxyethyl-CA.
Fig. 5.
HeLa cell viability after exposure to polymerized glues. (A) MTS assay of cells with or without direct contact with the glues. (B) Live/dead assay of same. (C) Light microscopy of the margins of the cured butoxyethyl-CA polymer 48 h after incubation with the cells. (D) Fluorescence microscopy of the margins of the cured butoxyethyl-CA polymer 48 h after incubation with the cells, after live/dead assay (live green, dead red). The yellow line indicates the margins of the cured polymer. Asterisks denote statistical difference from unexposed cells. *P < 0.05, **P < 0.01.
3.5. In vivo studies
CA monomers or saline (20 μl, n = 5 for each group) were deposited in surgically created subcutaneous pouches in the right flank. After 12 days the tissue surrounding the cured monomers was removed and processed for histology (Table 2 and Fig. 6). At the time of necropsy there was visually obvious swelling overlying the sites of deposition of the methoxyethyl- and ethoxyethyl-CA, while no or only mild swelling was observed in the other groups (P < 0.05 by ANOVA).
Table 2.
Histological findings.
| Formulation | n | Swelling (cm)a | Necrosis |
|---|---|---|---|
| Saline | 5 | 0 ± 0 | None |
| Hexoxyethyl-CA | 5 | 0 ± 0 | None |
| Butoxyethyl-CA | 5 | 0.2 ± 0.1 | |
| Propoxyethyl-CA | 5 | 0.6 ± 0.4 | None or mild |
| Ethoxyethyl-CA | 5 | 0.8 ± 0.1 | Moderate |
| Methoxyethyl-CA | 5 | 1.2 ±0.5 | |
| Dermabond® | 5 | 0.2 ± 0.1 | None |
Diameter, measured with calipers. Differences between propoxyethyl-, eth-oxyethyl- and methoxyethyl-CA and the control group were statistically significant (P < 0.05). Data are means with standard deviations.
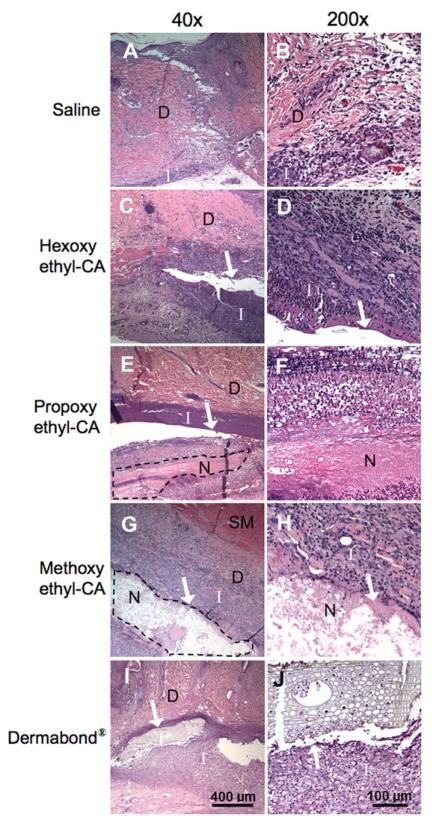
Fig. 6.
Histological findings in rats injected with saline or CA monomers. (A, B) Saline alone elicited a response with mild inflammation and a proliferation of fibroblasts and blood vessels, consistent with granulation tissue formation and corresponding to “minimal” histological changes. (C, D) Hexoxyethyl-CA elicited a slightly more vigorous inflammatory response. (E, F) Propoxyethyl-CA elicited moderate inflammation and small areas of necrosis. (G, H) Methoxyethyl-CA elicited severe inflammation with larger areas of necrosis. (I, J) Implantation of 2-octyl-CA (Dermabond®) elicited a “mild” histological response, which was characterized by inflammation surrounding the implanted material without necrosis. D, dermis; SM, skeletal muscle; I, inflammation; N, necrosis. (B), (D), (F) and (H) are higher powered views of areas of inflammation and/or necrosis adjacent to the site of sample deposition. The arrows indicate residual cured CA glues. Areas of necrosis in (E) and (G) are outlined by a dotted line.
All samples showed features of 1–2 week old granulation tissue by light microscopy (Fig. 6), with lymphocytes, occasional macro-phages, proliferation of blood vessels and active fibroblasts. Animals receiving saline showed only granulation tissue, consistent with healing at the incision site (Fig. 6A and B). While the degree of inflammation within any given sample varied considerably (making it difficult to provide truly representative photographs), the difference in extent between CA was marked, as is evident from the size of the area of inflammation. There were also large differences in the presence and extent of necrosis. Animals injected with hexoxyethyl- or butoxyethyl-CA (relatively long chains) showed inflammation without necrosis (Fig. 6C and D). Animals injected with propoxyethyl-CA showed large areas of inflammation with small areas of necrosis (Fig. 6E and F). Animals injected with ethoxyethyl- and methoxyethyl-CA (the shortest chains) displayed the most severe tissue response, with large areas of inflammation and large areas of necrosis (Fig. 6G and H). Dermabond® produced a histological response (Fig. 6I and J) comparable with that seen with hexoxyethyl-CA, but subjectively milder (as assessed by M.W.L.).
4. Discussion
The incorporation of etheric oxygen in place of an alkyl group side-chain produced adhesives whose elasticity increased with molecular weight. The elasticities of butoxyethyl- and hexoxyethyl-CA were greater than that of 2-octyl-CA (Dermabond®), which has more or the same number of side-chain carbons, respectively. This may be attributable to the bending and ease of rotation of the ether linkage in each side-chain [30] and by the internal plasticizing effect of the side-chains: increasing side-chain length reduces intermolecular and intramolecular forces which decrease the general order of the polymer [31,32]. Conversely, the adhesive strength of 2-octyl-CA (Dermabond®) was greater than its analog hexoxyethyl-CA (both with eight carbons in the side-chain).
The amount of formaldehyde, a by-product released on degradation of alkoxyethyl-CA polymers, increased with increasing molecular weight of the parent monomer. A similar trend was observed with the two alkyl-CA: butyl-CA released approximately 50% more formaldehyde than octyl-CA. The greater release of form-aldehyde from the alkoxyethyl-CA than the alkyl-CA group was probably due to their relatively low hydrophobicity and high flexibility, which enabled faster water permeation into the backbone of the polymers [33].
Interestingly, the order of relative toxicity (Fig. 5) and of formaldehyde production did not track perfectly. For example, 2-octyl-CA was more cytotoxic than its alkoxyethyl analog, hexoxyethyl-CA, even though it released less formaldehyde (Fig. 3). This discrepancy may be due to degradation reactions resulting in products other than formaldehyde, of which several are recognized in the literature [34]. For example, hydrolysis of the side-chains ester bonds produces poly(cyanoacrylic acid) and an alcohol. Alkyl alcohols, being more lipid soluble than alkoxyethyl alcohols, are more likely to be cytotoxic, particularly given that the toxicity of alcohols correlates positively with their membrane-buffer partition coefficients [35]. While there is consensus about the potential toxicity of formaldehyde, both in vitro and in vivo, it is not known whether poly(cyanoacrylic acid) and alcohols released by cyanoacrylate glues are toxic in vivo [36]. However, the cytotoxicity of alcohols has been demonstrated and found to be directly correlated with the hydrophobicity of the alcohol [37,38], which might explain the discrepancy between the in vitro and the in vivo results here. It is also possible that additives in the commercial alkyl-CA used here display toxicity [39]. The in vitro and in vivo toxicity of each of the main degradation products has been widely investigated and reported in the literature.
We used the ratio of cell survival to elasticity as a general indicator of the quality of the individual formulations in terms of the desired properties of a soft tissue glue: toxicity is harmful, elasticity is beneficial. However, the analogy to commonly used clinical indicators, such as the therapeutic index, is not perfect. In the therapeutic index, for example, both the desirable and undesirable properties of a drug can be assumed to track reasonably well with dose or concentration. While that could be expected to be true for glue toxicity, it is less obviously applicable to elasticity. Nonetheless, a lower elastic modulus suggests that a glue could sustain a given strain with a lower cross-sectional area before it broke, i.e. less of it might have to be applied, which in turn might reduce toxicity. Here, the ratio for hexoxyalkyl-CA was approximately 8-fold higher than for octyl-CA. Nonetheless, tissue reaction to the two glues was comparable. It remains to be determined whether the higher ratio for hexoxyalkyl-CA can translate into lower toxicity by virtue of the ability to use less material.
In vivo the overall pattern of biocompatibility generally mirrored that seen in vitro: increasing the side-chain length correlated with decreasing toxicity. However, although hexoxyethyl-CA was less cytotoxic than 2-octyl-CA in vitro, this did not result in improved in vivo biocompatibility. This dissociation between the in vitro and in vivo findings could be a reflection of the fact that the difference in cell survival rates between the two adhesives was only moderate (approximately 30–50%) and so was overwhelmed by the multiple factors that could play a part in vivo. For example, it is possible that the monomers or glues had different effects in terms of inducing cells to express pro-inflammatory or other molecules, as has been described for other biomaterials[40,41]. It would appear, however, that the hypothesis that superior elasticity leads to reduced toxicity [12,42] is incorrect in this case. We note that extremely inflexible materials, such as wafers of tetrahedral amorphous carbon and silicon, can show essentially no local toxicity after implantation [43].
CA monomers polymerize very rapidly and the highly exothermic reaction and the failure of local heat dissipation can generate significant local increases in temperature [44]. We recorded a peak temperature of 55 °C for methoxyethyl-CA, and lower temperatures were obtained for monomers with longer carbon side-chains. This may be related to the faster rate of polymerization of the shorter CA monomers [45,46]. The heat generated by polymerization can be a determinant of biocompatibility [47]. Thus, for example, a new acrylic surgical glue, Glubran 2 (GEM Srl, Viareggio, Italy), incorporates metacryloxysulpholane to slow polymerization, thus decreasing the peak polymerization temperature to ~45 °C. Hexoxyethyl-CA, the CA with the longest carbon side-chain studied here had a peak temperature of 46 °C without additives. Given that polymerization was performed in sealed containers and that the thickness of the glue was greater than might be applied clinically, the peak temperature may be lower when used in vivo.
5. Conclusion
Incorporation of an etheric oxygen side-chain in place of an alkyl group provided excellent mechanical properties in terms of adhesive strength and flexibility. Longer carbon side-chain lengths yielded better elasticity, reduced adhesive strength, and lowered cytotoxicity. The improvement in mechanical properties of hexoxyethyl-CA over its commercially available and widely use alkyl-CA analog 2-octyl-CA was achieved without compromising biocompatibility.
Acknowledgment
The authors acknowledge support from the MIT-DuPont Alliance.
Footnotes
References
- [1].Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, et al. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6:385–92. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jaffe H, Wade CW, Hegyeli AF, Rice R, Hodge J. Synthesis and bioevaluation of alkyl 2-cyanoacryloyl glycolates as potential soft tissue adhesives. J Biomed Mater Res. 1986;20:205–12. doi: 10.1002/jbm.820200209. [DOI] [PubMed] [Google Scholar]
- [3].Vote BJ, Elder MJ. Cyanoacrylate glue for corneal perforations: a description of a surgical technique and a review of the literature. Clin Exp Ophthal. 2000;28:437–42. doi: 10.1046/j.1442-9071.2000.00351.x. [DOI] [PubMed] [Google Scholar]
- [4].Antonio Lauto D, Ma LJRF. Adhesive biomaterials for tissue reconstruction. J Chem Technol Biotechnol. 2008;83:464–72. [Google Scholar]
- [5].DaCruz D. Full-thickness skin necrosis of the fingertip after application of superglue. J Hand Surg Am. 2004;29:159. doi: 10.1016/j.jhsa.2003.10.010. [DOI] [PubMed] [Google Scholar]
- [6].Kimura KN, Sugiura KN. Adhesive composition. US patent no. 4321180. 1982
- [7].Saraf S. Facial laceration at caesarean section: experience with tissue adhesive. Eplasty. 2009;9:e3. [PMC free article] [PubMed] [Google Scholar]
- [8].Ong CC, Jacobsen AS, Joseph VT. Comparing wound closure using tissue glue versus subcuticular suture for pediatric surgical incisions: a prospective, randomised trial. Pediat Surg Int. 2002;18:553–5. doi: 10.1007/s00383-002-0728-0. [DOI] [PubMed] [Google Scholar]
- [9].Reece TB, Maxey TS, Kron IL. A prospectus on tissue adhesives. Am J Surg. 2001;182:40S–4S. doi: 10.1016/s0002-9610(01)00742-5. [DOI] [PubMed] [Google Scholar]
- [10].American Society of Metals . Characterization and failure analysis of plastics. ASM International; Materials Park, OH: 2003. [Google Scholar]
- [11].Tseng YC, Hyon SH, Ikada Y. Modification of synthesis and investigation of properties for 2-cyanoacrylates. Biomaterials. 1990;11:73–9. [PubMed] [Google Scholar]
- [12].Silvestri A, Brandi C, Grimaldi L, Nisi G, Brafa A, Calabro M, et al. Octyl-2-cyanoacrylate adhesive for skin closure and prevention of infection in plastic surgery. Aesthetic Plast Surg. 2006;30:695–9. doi: 10.1007/s00266-006-0139-z. [DOI] [PubMed] [Google Scholar]
- [13].Morikawa K. Biochemical study on the application of alpha-cyanoacrylate instant adhesives in dentistry (in Japanese) Shikwa Gakuho. 1990;90:201–24. [PubMed] [Google Scholar]
- [14].Lin JC, Lin CW, Lin XZ. In vitro and in vivo studies for modified ethyl cyanoacrylate regimens for sclerotherapy. J Biomed Mater Res. 2000;53:799–805. doi: 10.1002/1097-4636(2000)53:6<799::aid-jbm22>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- [15].Toriumi DM, Raslan WF, Friedman M, Tardy ME. Histotoxicity of cyanoacrylate tissue adhesives. A comparative study. Arch Otolaryngol Head Neck Surg. 1990;116:546–50. doi: 10.1001/archotol.1990.01870050046004. [DOI] [PubMed] [Google Scholar]
- [16].Ramachary DB, Anebouselvy K, Chowdari NS, Barbas CF. Direct organocatalytic asymmetric heterodomino reactions: the Knoevenagel/Diels-Alder/epimerization sequence for the highly diastereoselective synthesis of symmetrical and nonsymmetrical synthons of benzoannelated centropolyquinanes. J Org Chem. 2004;69:5838–49. doi: 10.1021/jo049581r. [DOI] [PubMed] [Google Scholar]
- [17].Chien IL, Zeng K-L, Chao H-Y. Design and control of a complete heterogeneous azeotropic distillation column system. Indust Engin Chem Res. 2004;43:2160–74. [Google Scholar]
- [18].Yan Y, Bornscheuer UT, Schmid RD. Efficient water removal in lipase-catalyzed esterifications using a low-boiling-point azeotrope. Biotechnol Bioengin. 2002;78:31–4. doi: 10.1002/bit.10084. [DOI] [PubMed] [Google Scholar]
- [19].Good RJ. Contact Angle, Wettability and Adhesion. VSP; Leiden, The Netherlands: 1993. [Google Scholar]
- [20].Cheo SHY, Wang P, Tan KL, Ho CC, Kang ET. Surface modification of natural rubber latex films via grafting of poly(ethylene glycol) for reduction in protein adsorption and platelet adhesion. J Mater Sci Mater Med. 2001;12:377–84. doi: 10.1023/a:1011280416520. [DOI] [PubMed] [Google Scholar]
- [21].Iwata H, Matsuda S, Mitsuhashi K, Itoh E, Ikada Y. A novel surgical glue composed of gelatin and N-hydroxysuccinimide activated poly(L-glutamic acid): Part 1. Synthesis of activated poly(L-glutamic acid) and its gelation with gelatin. Biomaterials. 1998;19:1869–76. doi: 10.1016/s0142-9612(98)00095-7. [DOI] [PubMed] [Google Scholar]
- [22].Otani Y, Tabata Y, Ikada Y. Effect of additives on gelation and tissue adhesion of gelatin-poly(L-glutamic acid) mixture. Biomaterials. 1998;19:2167–73. doi: 10.1016/s0142-9612(98)00123-9. [DOI] [PubMed] [Google Scholar]
- [23].Ninan L, Monahan J, Stroshine RL, Wilker JJ, Shi R. Adhesive strength of marine mussel extracts on porcine skin. Biomaterials. 2003;24:4091–9. doi: 10.1016/s0142-9612(03)00257-6. [DOI] [PubMed] [Google Scholar]
- [24].Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–12. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- [25].Maltaris T, Kaya H, Hoffmann I, Mueller A, Beckmann MW, Dittrich R. Comparison of xenografting in SCID mice and LIVE/DEAD assay as a predictor of the developmental potential of cryopreserved ovarian tissue. In Vivo. 2006;20:11–6. [PubMed] [Google Scholar]
- [26].Moretti Neto RT, Mello I, Moretti AB, Robazza CR, Pereira AA. In vivo qualitative analysis of the biocompatibility of different cyanoacrylate-based adhesives. Braz Oral Res. 2008;22:43–7. doi: 10.1590/s1806-83242008000100008. [DOI] [PubMed] [Google Scholar]
- [27].Eastman DP, Robicsek F. Application of cyanoacrylate adhesive (Krazy Glue) in critical cardiac injuries. J Heart Valve Dis. 1998;7:72–4. [PubMed] [Google Scholar]
- [28].Al-Munajed MK, Gordon PH, McCabe JF. The use of a cyanoacrylate adhesive for bonding orthodontic brackets: an ex-vivo study. J Orthod. 2000;27:255–60. doi: 10.1179/ortho.27.3.255. [DOI] [PubMed] [Google Scholar]
- [29].Linden CL, Jr, Shalaby SW. Performance of modified cyanoacrylate composition as tissue adhesives for soft and hard tissues. J Biomed Mater Res. 1997;38:348–55. doi: 10.1002/(sici)1097-4636(199724)38:4<348::aid-jbm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [30].Jou JH, Huang PT. Effect of thermal curing on the structures and properties of aromatic polyimide films. Macromolecules. 1991;24:3796–803. [Google Scholar]
- [31].Leonard F, Hodge JW, Jr, Houston S, Ousterhout DK. Alpha-cyanoacrylate adhesive bond strengths with proteinaceous and nonproteinaceous substrates. J Biomed Mater Res. 1968;2:173–8. doi: 10.1002/jbm.820020114. [DOI] [PubMed] [Google Scholar]
- [32].Mizrahi B, Shavit R, Domb A. Synthesis and characterization of polymeric implant for kyphoplasty. J Biomed Mater Res B Appl Biomater. 2008;86B:466–73. doi: 10.1002/jbm.b.31043. [DOI] [PubMed] [Google Scholar]
- [33].Leonard F, Kulkarni RK, Brandes G, Nelson J, Cameron JJ. Synthesis and degradation of poly(alkyl-α-cyanoacrylates) J Appl Poly Sci. 1966;10:1214. [Google Scholar]
- [34].Vauthier C, Dubernet C, Fattal E, Pinto-Alphandary H, Couvreur P. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv Drug Delivery Rev. 2003;55:519–48. doi: 10.1016/s0169-409x(03)00041-3. [DOI] [PubMed] [Google Scholar]
- [35].Okolo B, Johnston JR, Berry DR. Toxicity of ethanol, n-butanol and iso-amyl alcohol in Saccharomyces cerevisiae when supplied separately and in mixtures. Biotechnol Lett. 1987;9:431–4. [Google Scholar]
- [36].Hee Park D, Bum Kim S, Ahn K-D, Yong Kim E, Jun Kim Y, Keun Han D. In vitro degradation and cytotoxicity of alkyl 2-cyanoacrylate polymers for application to tissue adhesives. J Appl Poly Sci. 2003;89:3272–8. [Google Scholar]
- [37].Baker RC, Kramer RE. Cytotoxicity of short-chain alcohols. Annu Rev Pharmacol Toxicol. 1999;39:127–50. doi: 10.1146/annurev.pharmtox.39.1.127. [DOI] [PubMed] [Google Scholar]
- [38].Kosaka T, Tsuboi S, Fukaya K, Pu H, Ohno T, Tsuji T, et al. Spheroid cultures of human hepatoblastoma cells (HuH-6 line) and their application for cytotoxicity assay of alcohols. Acta Med Okayama. 1996;50:61–6. doi: 10.18926/AMO/30490. [DOI] [PubMed] [Google Scholar]
- [39].Thumwanit V, Kedjarune U. Cytotoxicity of polymerized commercial cyanoacrylate adhesive on cultured human oral fibroblasts. Aust Dent J. 1999;44:248–52. doi: 10.1111/j.1834-7819.1999.tb00228.x. [DOI] [PubMed] [Google Scholar]
- [40].Yeo Y, Burdick JA, Highley CB, Marini R, Langer R, Kohane DS. Peritoneal application of chitosan and UV-cross-linkable chitosan. J Biomed Mater Res A. 2006;78:668–75. doi: 10.1002/jbm.a.30740. [DOI] [PubMed] [Google Scholar]
- [41].Yeo Y, Highley CB, Bellas E, Ito T, Marini R, Langer R, et al. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials. 2006;27:4698–705. doi: 10.1016/j.biomaterials.2006.04.043. [DOI] [PubMed] [Google Scholar]
- [42].Singer AJ, Quinn JV, Clark RE, Hollander JE. Closure of lacerations and incisions with octylcyanoacrylate: a multicenter randomized controlled trial. Surgery. 2002;131:270–6. doi: 10.1067/msy.2002.121377. [DOI] [PubMed] [Google Scholar]
- [43].LaVan DA, Padera RF, Friedmann TA, Sullivan JP, Langer R, Kohane DS. In vivo evaluation of tetrahedral amorphous carbon. Biomaterials. 2005;26:465–73. doi: 10.1016/j.biomaterials.2004.02.071. [DOI] [PubMed] [Google Scholar]
- [44].Coover HW, Jr, Joyner FB, Shearer NH, Jr, Wicker TH., Jr Chemistry and performance of cyanoacrylate tissue adhesives. Soc Plastics Eng J. 1959:15. [Google Scholar]
- [45].Matsumoto T, Pani KC, Hardaway RM, Leonard F. N-alkyl alpha cyanoacrylate monomers as a tissue adhesive in surgery of internal organs. Mil Med. 1967;132:515–21. [PubMed] [Google Scholar]
- [46].Woodward SC, Herrmann JB, Cameron JL, Brandes G, Pulaski EJ, Leonard F. Histotoxicity of cyanoacrylate tissue adhesive in the rat. Ann Surg. 1965;162:113–22. doi: 10.1097/00000658-196507000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Badini P, De Cupis P, Gerosa G, Giona M. Necrosis evolution during high-temperature hyperthermia through implanted heat sources. IEEE Trans Biomed Eng. 2003;50:305–15. doi: 10.1109/TBME.2003.808812. [DOI] [PubMed] [Google Scholar]