Abstract
Aims
Arterial stiffening may lead to hypertension, greater left ventricular after-load and adverse clinical outcomes. The underlying mechanisms influencing arterial elasticity may involve oxidative injury to the vessel wall. We sought to examine the relationship between novel markers of oxidative stress and arterial elastic properties in healthy humans.
Methods & Results
We studied 169 subjects (mean age 42.6 ± 14 years, 51.6% male) free of traditional cardiovascular risk factors. Indices of arterial stiffness and wave reflections measured included carotid-femoral Pulse Wave Velocity (PWV), Augmentation index (Aix) and Pulse Pressure Amplification (PPA). Non-free radical oxidative stress was assessed as plasma oxidized and reduced amino-thiol levels (cysteine/cystine, glutathione/GSSG) and their ratios (redox potentials), and free radical oxidative stress as derivatives of reactive oxygen metabolites (dROMs). Inflammation was assessed as hsCRP and interleukin-6 levels.
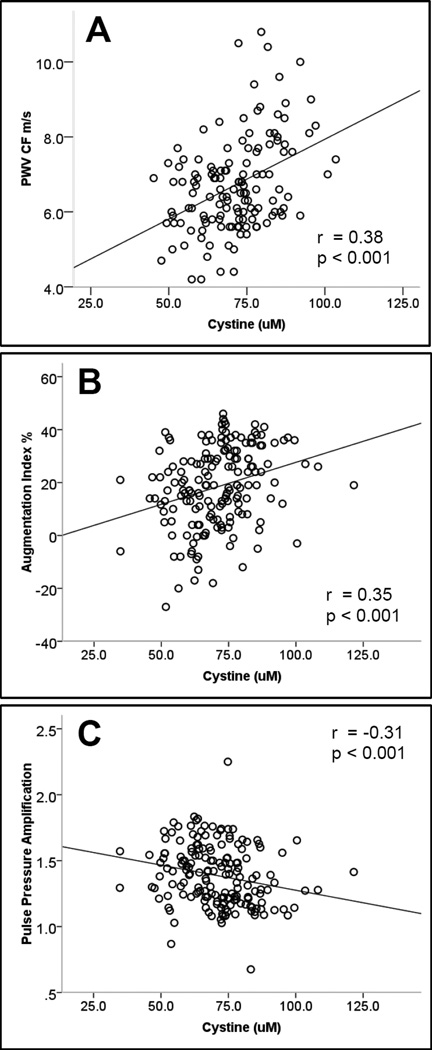
The non-free radical marker of oxidative stress, cystine was significantly correlated with all arterial indices; PWV (r= 0.38, p<0.001), Aix (r=0.35, p<0.001) and PPA (r=−0.30, p<0.001). Its redox potential, was also associated with PWV (r=0.22, p=0.01), while the free radical marker of oxidative stress dROMS was associated with Aix (r=0.25, p<0.01). After multivariate adjustment for age, gender, arterial pressure, height, weight, heart rate and CRP, of these oxidative stress markers, only cystine remained independently associated with PWV (p=0.03), Aix (p=0.01) and PPA (p=0.05).
Conclusions
In healthy subjects without confounding risk factors or significant systemic inflammation, a high cystine level, reflecting extracellular oxidant burden, is associated with increased arterial stiffness and wave reflections. This has implications for understanding the role of oxidant burden in pre-clinical vascular dysfunction.
Keywords: Oxidative stress, Arterial stiffness, Vascular function, Inflammation, Aging
Introduction
Changes in the mechanical and elastic properties of the arterial wall, occurring as a consequence of age and disease, are widely accepted pathophysiologic mechanisms leading to increased left ventricular afterload, myocardial ischemia and cardiovascular events.1, 2 The underlying biology leading to vessel stiffness remains unclear and may involve several determinants such as repetitive mechanical distension and recoil causing altered gene expression and adverse changes in the milieu of the vessel wall including modifications in collagen and elastin architecture and function via enzymatic mediators such as matrix metalloproteases.3
Oxidant induced cellular injury may be a fundamental causal pathway leading directly or indirectly to loss of elasticity in the arterial wall. Excess oxidant burden alters DNA transcription leading to cellular proliferation and interruption of numerous redox sensitive signaling pathways that influence arterial remodeling.4 While vascular tissues from hypertensive animals demonstrate elevated oxidant burden, human studies have yet to convincingly demonstrate an association between oxidative stress and arterial stiffness.5 If confirmed, this link would provide support for the early role of oxidative stress in vascular health in humans without disease, potentially offer new biomarkers to identify vascular dysfunction, and allow for specifically targeted therapeutic interventions.
Although the harmful vascular effects of free radical species in vitro remain undisputed, clinical experience with free radical scavengers has been uniformly disappointing leading to concept that free radicals may not constitute clinically important sources of oxidants and that non-free radical species may be of equal or greater importance.6 Major aminothiol compounds, indices of non-free radical oxidant stress, play a critical role in redox signaling and can be quantified in plasma to assess oxidative stress burden in vivo.6 Of these, cysteine constitutes the major thiol pool extracellularly that reacts readily with oxidants to form its oxidized disulphide cystine. Intracellularly, glutathione is a major antioxidant that helps eliminate peroxides and maintain cellular redox, and its oxidized form is GSSG.6 Increased oxidative stress, measured as lower levels of glutathione, higher levels of cystine, or altered ratios of reduced to oxidized thiols, is associated with risk factors for cardiovascular disease (CVD), subclinical vascular disease and with adverse outcomes.7–9 Derivatives of reactive oxidative metabolites (dROMS) which measures plasma peroxides is considered to reflect free radical oxidant burden. Its usefulness as a biomarker has been demonstrated in several studies demonstrating for example association with inflammation and atrial fibrillation.10, 11
We hypothesized that these non-free radical and free radical based plasma markers of oxidative stress would be associated with indices of arterial stiffness and wave reflections and tested this hypothesis in a carefully selected cohort of healthy non-smoking subjects free of overt cardiovascular risk factors.
Methods
Study Population
The study design enrolled only healthy subjects to mitigate confounding from CVD or its risk factors known to influence both oxidant burden and measures of arterial elasticity. A total of 169 healthy non-smoking volunteers, aged 20–70 years were recruited after careful screening for absence of hypertension (SBP<130 or DBP<90 mmHg on three occasions), hyperlipidemia (total cholesterol level <200 mg/dL, LDL <120 mg/dl), impaired fasting glucose or diabetes (fasting glucose <100 mg/dL), and obesity (BMI <=25) (Table 1). Other exclusion criteria included history of any CVD or valvular heart disease, pregnancy, any chronic illnesses, taking prescription medications or any vitamin supplements for 6 weeks. Specific dietary intake patterns were not documented but all blood samples and measurements were taken after an overnight fast. The study was approved by the Emory University Institutional Review Committee. Informed consent was obtained from all subjects.
Table 1.
Participant Characteristics
| Characteristic | Value | |
|---|---|---|
| Age (yr) | 42.6 (13.7) | |
| Male Gender (%) | 51.6 | |
| Caucasian (%) | 67.2 | |
| Height (cm) | 170.3 (10.1) | |
| Weight (kg) | 68.2 (11.7) | |
| Body Mass Index (kg/m2) | 23.5 (2.7) | |
| Brachial Systolic Pressure (mmHg) | 114.8 (11.9) | |
| Brachial Diastolic Pressure (mmHg) | 68.2 (9.9) | |
| Brachial Mean Arterial Pressure (mmHg) | 83.7 (9.5) | |
| Heart Rate (bpm) | 60.9 (8.5) | |
| Glucose (mg/dl) | 85.1 (8.1) | |
| Total Cholesterol (mg/dl) | 175.0 (27.5) | |
| High Density Lipoprotein (mg/dl) | 63.0 (16.7) | |
| Low Density Lipoprotein (mg/dl) | 95.9 (22.0) | |
| C-Reactive Protein (mg/L) | Mean (SD) | 1.08 (2.6) |
| Median (IQR) | 0.47 (0.2–1.0) | |
| Interleukin 6 (pg/ml) | Mean (SD) | 0.91 (1.0) |
| Median (IQR) | 0.78 (0.4–1.1) | |
| Framingham Risk Score (LDL Points) | Mean | −2.80 (6.50) |
| Median (IQR) | −1 (−5 to +2) | |
| Pulse Pressure Amplification | 1.39 (0.2) | |
| Augmentation Index (%) | 18.6 (15.8) | |
| Central Pulse Pressure (mmHg) | 33.1 (9.9) | |
| Central Systolic Pressure (mmHg) | 102.9 (13.9) | |
| Central Diastolic Pressure (mmHg) | 69.8 (10.4) | |
| Pulse wave velocity (m/s) | 6.77 (1.3) | |
| Cystine (uM) | 70.6 (13.6) | |
| Cysteine (uM) | 9.2 (2.6) | |
| EhCySS (mV) | −71.5 (7.6) | |
| Glutathione (uM) | 1.9 (0.7) | |
| GSSG (uM) | 0.1 (0.1) | |
| EhGSSG (mV) | −140.5 (11.9) | |
| dROMS (AU) | 560.6 (159.1) |
Mean values (SD) given unless otherwise stated. AU=Arbitrary Units
Arterial Elasticity Indices
Arterial elasticity was estimated using the Sphygmocor device (Atcor Medical, Australia). Pulse Wave Velocity (PWV) was measured by acquiring pressure waveforms at the carotid and femoral arteries, and velocity [distance/time in m/s] was calculated using the “foot-to-foot” method.12 The surface distance between the carotid and femoral points of maximal pulsation was estimated to the closest cm, using a standard medical measuring tape. Pulse Wave Analysis of the pressure waveforms at the radial artery estimated central (proximal) aortic pressures and the degree of pressure augmentation secondary to reflected waves from the periphery. This permitted derivation of an augmentation index (Aix) (augmentation index = augmented pressure/total central pulse pressure). Aix is sensitive to heart rate (HR) and while estimates of the HR adjusted Aix are calculated by the device, we used the unadjusted Aix and elected to adjust for HR in multivariate analysis. Pulse Pressure Amplification (PPA) is a measure of the difference between the peripheral and central pulse pressures, expressed as a direct ratio, peripheral PP/ central PP. In the healthy vasculature there is amplification of the pulse waveform as it travels towards the periphery, but in disease states, there is a disproportionate increase in central pulse pressure leading to a diminution of PPA.13 Thus, PPA represents the global effects of arterial stiffness, peripheral resistance, and wave reflections. Reproducibility studies in our laboratory on 9 subjects on consecutive days have demonstrated a coefficient of variation of 3.8%, 20.3% and 2.2% for PWV, Aix75 and PPA respectively.
Biomarkers
(1) Amino-thiols
We measured plasma cysteine, its oxidized form cystine, glutathione, and its oxidized disulphide (GSSG) in all subjects as described previously.14 Ratios of cysteine/cystine and glutathione/GSSG are expressed as redox potentials, EhCySS and EhGSSG, respectively, where a more oxidized value has a more positive numeric value.14 Briefly, samples were collected directly into tubes containing a preservative to retard auto-oxidation, centrifuged, and stored at −80°C, which shows no significant loss for 1 year. Analyses by high performance liquid chromatography were performed after dansyl derivatization on a 3-aminopropyl column with fluorescence detection. Metabolites were identified by co-elution with standards and quantified by integration relative to the internal standard, with validation relative to external standards as previously described.14 The coefficients of variation (CV) for glutathione was 5%, GSSG was 9.7%, cysteine 3.8%, and cystine 3.2%.
(2) Derivatives of Reactive Oxygen Metabolites (dROMS)
The d-ROMs (derivatives of Reactive Oxygen Metabolites) assay, is a colorimetric test for the assessment of serum hydroperoxides, (dROMS Test; Diacron, Grosseto, Italy). The test been used to measure oxidative stress in plasma samples and has been correlated directly with lipoperoxides and 8-isoprostanes, but with the advantage that it does not require extraction/purification of the sample.15 Its usefulness as a biomarker has been demonstrated in prior studies as has the technique for its assessment.10, 16 Intra-assay CVs in our laboratory were 0.2% at 300 and 2.3% at 550 Carr units.
(3) CRP and IL6
High-sensitivity C-reactive protein was measured by immunonephelometry (Dade Behring, Deerfield, Illinois) and Interleukin 6 by ultrasensitive ELISA (R&D Systems, Minneapolis, Minnesota).
Statistical Methods
All continuous variables are described as mean ± standard deviation, or median (inter-quartile range) while categorical variables are presented as proportions. CRP and IL-6 values were non-normally distributed and log transformed for parametric analysis. Spearman rank correlation coefficients were calculated for examining bivariate associations between PWV, Aix and PPA and patient characteristics as well as with markers of inflammation and oxidative stress. Multivariate regression models were then constructed using significant or near-significant univariate predictors (p<0.1) with unstandardized regression coefficients and confidence intervals and model R2 calculated for each index. P values of <0.05 were considered significant. All analyses were performed using the SPSS v17.0 statistical package (SPSS, Illinois, USA).
Results
Baseline characteristics of the 169 subjects studied are presented in Table 1 (mean age 41.2 ± 14, males 50.7%). The low Framingham Risk Score and systemic inflammation is a reflection of their healthy status. There were significant correlations between the oxidized and reduced thiol pairs (cystine and cysteine r=0.26, p< 0.001; glutathione and GSSG r= 0.39, p<0.001). While there was a modest inverse relationship between cystine and glutathione (r= −0.15, p=0.05), dROMs was not correlated with any of the thiol markers or their ratios. There were modest correlations between the inflammatory markers, CRP and IL6 and cystine, glutathione and dROMS (Supplementary Table 1). Significant univariate determinants for the thiol markers are presented in Supplementary Table 2. Notably age was associated with cystine (r=0.26, p<0.01) and EhCySS (r=0.18, p=0.01).
Associations with Arterial Elasticity Indices
We observed correlations between PWV, Aix and PPA; PWV and Aix (r=0.34, p<0.001), PWV and PPA (r=−0.29, p=0.001) and Aix and PPA (r=−0.87, p<0.001).17 Univariate determinants of PWV included age, gender, height, weight, total cholesterol and mean arterial pressure. For Aix and PPA, determinants included those for PWV as well as heart rate and C-reactive protein (Table 2).
Table 2.
Univariate correlates of arterial stiffness and wave reflection indices
| Characteristic | PWV | Aix | PPA | |||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |
| Age (yr) | 0.44 | <0.001 | 0.58 | <0.001 | −0.52 | <0.001 |
| Gender (f=0,m=1) | 0.21 | <0.01 | −0.33 | <0.001 | 0.31 | <0.001 |
| Height (cm) | 0.12 | 0.13 | −0.42 | <0.001 | 0.37 | <0.001 |
| Weight (kg) | 0.18 | 0.01 | −0.24 | <0.001 | 0.22 | <0.001 |
| Body Mass Index (kg/m2) | 0.15 | 0.07 | 0.06 | 0.41 | −0.05 | 0.547 |
| Mean arterial pressure (mmHg) | 0.47 | <0.001 | 0.18 | 0.02 | −0.21 | <0.01 |
| Heart rate (bpm) | 0.07 | 0.36 | −0.16 | 0.03 | 0.15 | 0.04 |
| Glucose (mg/dl) | 0.10 | 0.21 | −0.03 | 0.72 | <−0.01 | 0.96 |
| Total Cholesterol (mg/dl) | 0.25 | <0.001 | 0.37 | <0.001 | −0.26 | <0.001 |
| C-Reactive Protein (mg/L) | 0.14 | 0.08 | 0.20 | <0.01 | −0.19 | 0.01 |
| Interleukin 6 (pg/ml) | 0.10 | 0.24 | −0.03 | 0.72 | 0.04 | 0.63 |
| Cystine (uM) | 0.38 | <0.001 | 0.35 | <0.001 | −0.31 | <0.001 |
| Cysteine (uM) | −0.09 | 0.32 | 0.11 | 0.17 | −0.06 | 0.42 |
| EhCySS (mV) | 0.22 | 0.01 | 0.12 | 0.16 | −0.14 | 0.10 |
| Glutathione (uM) | −0.08 | 0.35 | −0.08 | 0.29 | 0.05 | 0.53 |
| GSSG (uM) | 0.10 | 0.26 | −0.11 | 0.16 | 0.09 | 0.21 |
| EhGSSG (mV) | 0.15 | 0.08 | 0.01 | 0.90 | 0.01 | 0.92 |
| dROMS | 0.03 | 0.72 | 0.25 | <0.01 | −0.15 | 0.09 |
r = Spearman Rank correlation coefficients with corresponding p value for each variable
Of the thiol markers, cystine correlated significantly with PWV, Aix, and PPA (Table 2, Figure 1). The remaining thiols were not correlated with any of the arterial indices, except for the redox potential of cysteine/cystine which associated with PWV (r=0.22, p=0.01) and the redox potential of glutathione/GSSG which showed modest association with PWV (r=0.15, p=0.08). The free radical marker of oxidative stress, dROMS correlated only with Aix (r=0.25, p<0.01) and marginally with PPA (r=−0.15, p=0.09).
Figure 1.
Relationship between arterial stiffness indices and cystine. Panels A, B and C demonstrate the relationship between cystine and carotid-femoral Pulse Wave Velocity (A), Augmentation Index (B) and Pulse Pressure Amplification (C). r= Spearman correlation coefficient.
Using significant univariate predictors including cystine, separate linear regression models were then constructed for PWV, Aix and PPA (Table 3). After multivariate adjustment, only age, mean arterial pressure and cystine were independent predictors of PWV. For Aix, age, gender, height, mean arterial pressure, heart rate and cystine were independent predictors, while for PPA the same variables except height were independently correlated. These multivariate models for PWV, Aix and PPA accounted for 42%, 61% and 48% of their variability, respectively.
Table 3.
Multivariate predictors of arterial stiffness and wave reflection indices
| Pulse Wave Velocity | Augmentation index | Pulse Pressure Amplification | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B (SE) | β | P value | B (SE) | β | P value | B (SE) | β | P value | |
| Age (yrs) | 0.02 (0.01) | 0.18 | 0.05 | 0.44 (0.08) | 0.40 | <0.001 | <−0.01 (<0.01) | −0.38 | <0.001 |
| Gender (f=0, m=1) | 0.36 (0.24) | 0.14 | 0.13 | −9.60 (2.40) | −0.31 | <0.001 | 0.13 (0.04) | 0.27 | <0.01 |
| Weight (kg) | 0.01 (0.01) | 0.01 | 0.94 | 0.07 (0.11) | 0.05 | 0.54 | <0.01 (<0.01) | 0.01 | 0.95 |
| Height (cm) | - | - | - | −0.39 (0.14) | −0.24 | <0.01 | <0.01 (<0.01) | 0.18 | 0.07 |
| Mean Arterial Pressure (mmHg) | 0.05 (0.01) | 0.44 | <0.001 | 0.33 (0.08) | 0.23 | <0.001 | <−0.01 (<0.01) | −0.30 | <0.001 |
| Heart Rate (bpm) | - | - | - | −0.40 (0.10) | −0.24 | <0.001 | <0.01 (<0.01) | 0.19 | <0.01 |
| Total Cholesterol (mg/dl) | 0.01 (<0.01) | 0.13 | 0.09 | 0.03 (0.04) | 0.05 | 0.82 | <0.01 (<0.01) | 0.06 | 0.44 |
| C-Reactive Protein (mg/L) | <−0.01 (0.03) | −0.02 | 0.74 | 0.16 (0.30) | 0.03 | 0.55 | <−0.01 (<0.01) | −0.05 | 0.39 |
| Cystine (uM) | 0.02 (<0.01) | 0.15 | 0.03 | 0.22 (0.07) | 0.18 | 0.01 | <−0.01 (<0.01) | −0.12 | 0.05 |
| Model R2 | 0.421 | 0.606 | 0.483 | ||||||
Multivariate analysis for association between cystine and each arterial index using significant univariate predictors; Table shows for each modeled variable the un-standardized (B) coefficient with standard error, the standardized (β) coefficient and the p value.
Similar multivariate analyses for the other biomarkers of oxidative stress, namely the redox potentials of cysteine and glutathione which had univariate associations revealed that neither was independently associated with PWV (EhCySS p=0.43; EhGSSG p=0.88). Likewise, the free radical marker of oxidative stress, dROMS, was also no longer associated with Aix after multivariate adjustment with variables listed in table 3 (p=0.93).
Finally, none of the markers of inflammation correlated with arterial elasticity indices after multivariate adjustment.
Discussion
Herein, we demonstrate an independent association between a plasma biomarker of extracellular non-free radical oxidant burden, cystine, and indices of arterial stiffness and wave reflections in a healthy population free of risk factors and inflammation. Our results lend support to the emerging role of oxidative stress in the pathogenesis of arterial stiffness as well as the value of non-free radical based approaches to quantifying oxidant burden in vivo.
While oxidative stress has been correlated extensively with measures of endothelial dysfunction, few studies have explored its relationship with arterial elastic properties.8, 18 Both elevated saphenous vein or neutrophil superoxide anion generation and increased isoprostanes have been associated with arterial stiffness indices in atherosclerotic populations.19, 20 However, these relationships may have been secondary to presence of risk factors and atherosclerosis. To our knowledge, only one previous study has demonstrated an independent association between advanced oxidation protein products and an indirect stiffness index derived from peripheral plethysmography in a healthy population.21 Our study however uses more accepted methodology for assessing arterial stiffness and wave reflections and a wider spectrum of oxidant markers.
Although arterial stiffness improved with vitamin C, in some studies, these results were not confirmed by others.22, 23 This discrepancy is consistent with clinical trials that failed to demonstrate CVD benefit with antioxidant vitamins, raising the possibility that free radicals may not be major contributors of oxidant damage and that non-free radical species may be of equal or greater importance.6 Indeed in our study we also failed to demonstrate an association between a free radical marker of oxidative stress, (dROMS), and indices of arterial elasticity.
Plasma aminothiol metabolites are reliable markers of systemic oxidative stress, with the glutathione pool reflecting intracellular and the cysteine/cystine pools extracellular oxidative stress.6 While we did not observe any significant associations with glutathione or GSSG, the oxidized disulphide, cystine, that reflects extracellular oxidative burden, was significantly associated with markers of impaired arterial elasticity. In previous studies, high plasma cystine levels were associated with endothelial dysfunction, smoking and importantly adverse outcomes.7–9 Mechanistically, the extracellular redox state of the cysteine/cystine pool modulates CVD through pro-inflammatory signaling, regulated by cell surface protein redox states. These involve mitochondrial oxidation, nuclear factor-kB activation and elevated expression of genes for monocyte recruitment to endothelial cells.24 Experimentally, extracellular cystine concentrations regulates cell proliferation via epidermal growth factor receptors, activates the mitogen-activated protein kinase pathway, stimulates monocyte adhesion by an NF-kB-dependent pathway, and accelerates apoptosis.25 This supports the use of plasma measures of cystine and its redox potential EhCySS as key indicators of vascular health despite lack of tissue quantification of oxidant burden.
The exact mechanisms that determine plasma cystine levels are not known.24 Previous research shows that cystine increases with age and that the variation of cystine concentration increases as a function of age.26 It is noteworthy that plasma cystine levels are controlled in part by the xc− system acting as a highly efficient cystine/glutamate transporter and may potentially represent a therapeutic target.27 A decline with age in the responsiveness of Nrf2, the transcription factor controlling expression of this cystine/glutamate transporter, has been reported.28 Consequently this could contribute to the increased cystine in plasma, although human data on this is lacking. In our study we observed an association with age and cystine and adjusted for this in multivariate analysis. Cystine also has a diurnal variation and is dependent upon dietary sulfur amino acid intake in the post-prandial period, but no significant effect of dietary intake level over a 4-day period was observed in the fasting state.29–31 In the present study, subjects were fasted so dietary intake is not expected to directly impact the plasma cystine.
Our study confirmed findings from the recent analysis of 11,000 subjects that age and blood pressure are independent determinants of PWV.32 However, in contrast to previous reports, we found that plasma inflammatory markers did not correlate with arterial elasticity, which we believe is likely due to inclusion of subjects with risk factors in other reports.33, 34 Our study is unique in that subjects were carefully screened and specifically excluded for presence of risk factors, as reflected by the low Framingham risk values and CRP levels compared to these other studies. Indeed, studies showing lack of independent association between CRP and arterial stiffness generally had lower mean levels of CRP (<1.2 mg/L).35 Given the complex relationship between inflammation and oxidative stress, arterial stiffness may result either directly from oxidative stress or indirectly by promoting inflammation via redox sensitive pathways.25 Inflammation in turn may lead to vascular stiffness as observed in acute and chronic inflammatory states.36, 37 Our study suggests that oxidative stress may influence vascular health, without necessarily activating systemic inflammation, and even in the absence of any risk factors.
An important limitation of our study is its cross-sectional design which only demonstrates an association, and interventions to specifically lower oxidative stress are needed to prove causality. While our use of a screened healthy cohort is a major strength of this study, as we effectively eliminated the effect of confounding CVD risk factors that are known to cause both oxidative stress and arterial stiffness, it is possible that unmeasured confounders may explain these findings. Finally, although other mechanisms may elevate cystine levels, isotopic tracer studies are not available to confirm whether increased plasma cystine occurs solely as a function of increased oxidation of cysteine, impaired clearance of cystine, or both.30
In conclusion, we demonstrate that plasma cystine, a measure of extracellular oxidant burden, is an independent predictor of arterial stiffness and wave reflections in healthy subjects prior to development of any overt risk factors. While this does not demonstrate causality, it does support the notion that arterial stiffness may at least in part be related to systemic oxidant load. Further studies are required to determine whether plasma cystine causes arterial stiffening, whether it can be used as a biomarker to identify individuals at risk of developing premature vascular disease, and whether interventions to reduce oxidant burden or cystine directly can prevent development of subclinical vascular disease.
Supplementary Material
Acknowledgements
We would like to thank all members of the Emory ACTSI and Predictive Health Institute for their support in performing this study. We would also like to thank Bill Laing for performing the thiol redox measurements.
Funding
This work was supported by the American Heart Association (Postdoctoral Fellowship for RSP), Robert W. Woodruff Health Sciences Center Fund, the Emory Predictive Health Institute and supported in part by NIH Grant UL1 RR025008 from the Clinical and Translational Science Award program (Atlanta Clinical Translational Science Institute). DPJ was supported in part by NIH Grants ES011195, P01ES016731, R01AG038746.
Footnotes
Conflict of Interest/ Disclosure Statement:
None to declare
References
- 1.Toprak A, Reddy J, Chen W, Srinivasan S, Berenson G. Relation of pulse pressure and arterial stiffness to concentric left ventricular hypertrophy in young men (from the Bogalusa Heart Study) Am J Cardiol. 2009;103:978–984. doi: 10.1016/j.amjcard.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 3.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 4.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 5.Uddin M, Yang H, Shi M, Polley-Mandal M, Guo Z. Elevation of oxidative stress in the aorta of genetically hypertensive mice. Mech Ageing Dev. 2003;124:811–817. doi: 10.1016/s0047-6374(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 6.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 7.Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med. 2003;35:1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Alexander RW, Harrison DG, Quyyumi AA. Endothelial function and aminothiol biomarkers of oxidative stress in healthy adults. Hypertension. 2008;52:80–85. doi: 10.1161/HYPERTENSIONAHA.107.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel RS, Veledar E, Patel RB, Sher S, Arshad S, Clements S, Douglas J, Morris D, Rab ST, Samady H, Alexander RW, Vaccarino V, Zafari AM, Jones DP, Quyyumi AA. Abstract 1138: The Oxidized Disulphide Cystine Predicts Adverse Long Term Cardiovascular Outcomes. Circulation. 2009;120:S454. [Google Scholar]
- 10.Abramson JL, Hooper WC, Jones DP, Ashfaq S, Rhodes SD, Weintraub WS, Harrison DG, Quyyumi AA, Vaccarino V. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis. 2005;178:115–121. doi: 10.1016/j.atherosclerosis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, Dudley SC., Jr Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53:1652–1657. doi: 10.1373/clinchem.2006.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 13.Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, Roman MJ, Safar ME, Segers P, Smulyan H. Role of pulse pressure amplification in arterial hypertension: experts' opinion and review of the data. Hypertension. 2009;54:375–383. doi: 10.1161/HYPERTENSIONAHA.109.134379. [DOI] [PubMed] [Google Scholar]
- 14.Jones D, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2009.08.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vassalle C, Boni C, Di Cecco P, Ndreu R, Zucchelli GC. Automation and validation of a fast method for the assessment of in vivo oxidative stress levels. Clin Chem Lab Med. 2006;44:1372–1375. doi: 10.1515/CCLM.2006.243. [DOI] [PubMed] [Google Scholar]
- 16.Cesarone MR, Belcaro G, Carratelli M, Cornelli U, De Sanctis MT, Incandela L, Barsotti A, Terranova R, Nicolaides A. A simple test to monitor oxidative stress. Int Angiol. 1999;18:127–130. [PubMed] [Google Scholar]
- 17.Yasmin, Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM. 1999;92:595–600. doi: 10.1093/qjmed/92.10.595. [DOI] [PubMed] [Google Scholar]
- 18.Watson T, Goon PK, Lip GY. Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal. 2008;10:1079–1088. doi: 10.1089/ars.2007.1998. [DOI] [PubMed] [Google Scholar]
- 19.Wykretowicz A, Guzik P, Kasinowski R, Krauze T, Bartkowiak G, Dziarmaga M, Wysocki H. Augmentation index, pulse pressure amplification and superoxide anion production in patients with coronary artery disease. Int J Cardiol. 2005;99:289–294. doi: 10.1016/j.ijcard.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 20.Kals J, Kampus P, Kals M, Zilmer K, Kullisaar T, Teesalu R, Pulges A, Zilmer M. Impact of oxidative stress on arterial elasticity in patients with atherosclerosis. Am J Hypertens. 2006;19:902–908. doi: 10.1016/j.amjhyper.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Wykretowicz A, Adamska K, Krauze T, Guzik P, Szczepanik A, Rutkowska A, Wysoki H. The plasma concentration of advanced oxidation protein products and arterial stiffness in apparently healthy adults. Free Radic Res. 2007;41:645–649. doi: 10.1080/10715760701236741. [DOI] [PubMed] [Google Scholar]
- 22.Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S, Salvetti A. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20:392–397. doi: 10.1016/j.amjhyper.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Darko D, Dornhorst A, Kelly FJ, Ritter JM, Chowienczyk PJ. Lack of effect of oral vitamin C on blood pressure, oxidative stress and endothelial function in Type II diabetes. Clin Sci (Lond) 2002;103:339–344. doi: 10.1042/cs1030339. [DOI] [PubMed] [Google Scholar]
- 24.Go YM, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2010;50:495–509. doi: 10.1016/j.freeradbiomed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111:2973–2980. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- 26.Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 27.Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, Kolle P, Tschoep K, Issels RD, Daniel PT, Conrad M, Bornkamm GW. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–1628. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- 28.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco RA, Ziegler TR, Carlson BA, Cheng PY, Park Y, Cotsonis GA, Accardi CJ, Jones DP. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr. 2007;86:1016–1023. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
- 30.Park Y, Ziegler TR, Gletsu-Miller N, Liang Y, Yu T, Accardi CJ, Jones DP. Postprandial cysteine/cystine redox potential in human plasma varies with meal content of sulfur amino acids. J Nutr. 2010;140:760–765. doi: 10.3945/jn.109.116764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones DP, Park Y, Gletsu-Miller N, Liang Y, Yu T, Accardi CJ, Ziegler TR. Dietary sulfur amino acid effects on fasting plasma cysteine/cystine redox potential in humans. Nutrition. 27:199–205. doi: 10.1016/j.nut.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasmin, McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol. 2004;24:969–974. doi: 10.1161/01.ATV.zhq0504.0173. [DOI] [PubMed] [Google Scholar]
- 34.Lieb W, Larson MG, Benjamin EJ, Yin X, Tofler GH, Selhub J, Jacques PF, Wang TJ, Vita JA, Levy D, Vasan RS, Mitchell GF. Multimarker approach to evaluate correlates of vascular stiffness: the Framingham Heart Study. Circulation. 2009;119:37–43. doi: 10.1161/CIRCULATIONAHA.108.816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spence VA, Kennedy G, Belch JJ, Hill A, Khan F. Low-grade inflammation and arterial wave reflection in patients with chronic fatigue syndrome. Clin Sci (Lond) 2008;114:561–566. doi: 10.1042/CS20070274. [DOI] [PubMed] [Google Scholar]
- 36.Roman MJ, Devereux RB, Schwartz JE, Lockshin MD, Paget SA, Davis A, Crow MK, Sammaritano L, Levine DM, Shankar BA, Moeller E, Salmon JE. Arterial stiffness in chronic inflammatory diseases. Hypertension. 2005;46:194–199. doi: 10.1161/01.HYP.0000168055.89955.db. [DOI] [PubMed] [Google Scholar]
- 37.Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M, Stefanadis C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193–2200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.