Abstract
Schizophrenia patients show significant subcortical brain abnormalities. We examined these abnormalities using automated image analysis software and provide effect size estimates for prospective multi-scanner schizophrenia studies. Subcortical and intracranial volumes were obtained using FreeSurfer 5.0.0 from high-resolution structural imaging scans from 186 schizophrenia patients (mean age±SD=38.9±11.6, 78% males) and 176 demographically similar controls (mean age±SD=37.5±11.2, 72% males). Scans were acquired from seven 3-Tesla scanners. Univariate mixed model regression analyses compared between-group volume differences. Weighted mean effect sizes (and number of subjects needed for 80% power at α=0.05) were computed based on the individual single site studies as well as on the overall multi-site study. Schizophrenia patients have significantly smaller intracranial, amygdala, and hippocampus volumes and larger lateral ventricle, putamen and pallidum volumes compared with healthy volunteers. Weighted mean effect sizes based on single site studies were generally larger than effect sizes computed based on analysis of the overall multi-site sample. Prospectively collected structural imaging data can be combined across sites to increase statistical power for meaningful group comparisons. Even when using similar scan protocols at each scanner, some between-site variance remains. The multi-scanner effect sizes provided by this study should help in the design of future multi-scanner schizophrenia imaging studies.
Keywords: Psychosis, Magnetic resonance imaging (MRI), Effect size, Imaging
1. Introduction
Schizophrenia patients show significant structural brain abnormalities when studied with magnetic resonance imaging (MRI). In vivo study of these abnormalities may aid in our understanding of etiology, pathogenesis, and treatment effects. In this study we examine whether subcortical volume alterations can be observed in prospective multi-center imaging studies despite additional between-scanner variance. We provide effect size estimates for single center (based on meta-analysis of single site effects) versus multi-center (based on mega-analysis correcting for site effects) structural imaging studies in schizophrenia.
Effect size estimates for structural brain alterations in schizophrenia are predominantly based on single center studies (Haijma et al., 2013; Shepherd et al., 2012); but for a simulation of multi-center study effect sizes, see Suckling et al. (Suckling et al., 2010). The increase in multi-scanner imaging studies, as well as increased efforts towards data sharing, emphasizes the need for effect-size estimates for multi-scanner data acquisitions. The ability to detect statistically significant differences between conditions depends on the effect size, sample size, α-level, and power of the test (Cohen, 1992). In power analyses, the researcher sets the desired α-level and power of the test. The effect size is preferably gleaned from the literature or otherwise estimated, and the sample size that will be required to observe a statistically significant effect is estimated.
The effect size for mean comparisons can be computed as the mean difference between two conditions divided by the pooled standard deviation of the measurements (Cohen, 1992) and thus depends on measurement variability. In single scanner studies, such variability depends on subject variability, between-acquisition scanner variability, and measurement-method reliability. In multi-scanner studies it also depends on between-scanner and other between-site (e.g., sample demographics) variance. Subject variability depends on the relative homogeneity or heterogeneity of the sample(s). Between-acquisition scanner variability depends on the stability over time of the MRI scanner. Brain-measurement reliabilities are estimated from multiple measurements on the same cases and include inter- and intra-scanner reliability (Jovicich et al., 2009), rater reliability (van Erp et al., 2004), and measurement-method reliability (Dewey et al., 2010; Tae et al., 2008; Wonderlick et al., 2009).
Measurements should not only be reliable but also valid. A measure is considered valid when the inferences made from it are appropriate, meaningful, and useful. The calculation of inter-method reliability in which a new method is compared to a GOLD standard, or a method that has been shown to produce valid measurements, provides one way to validate a measurement method. The more similar the measurements are (the higher the intra-class correlation), the more valid the measurements based on the new method. Nevertheless, validation should also be established by confirming that meaningful variability can be observed with the measurements.
Given between-scanner variability, the question remains as to how many additional data sets need to be collected in multi-scanner versus single scanner studies to observe differences between schizophrenia patients and controls? In this study, we compare subcortical volumes between chronic schizophrenia patients and healthy volunteers, and we report the weighted mean effect sizes as well as multi-center-based (n=7) effect sizes for subcortical volumes. Based on the effect sizes reported in meta-analyses (Haijma et al., 2012; Shepherd et al., 2012) (see Table 4), we hypothesized that we would find smaller amygdala, hippocampus, and intracranial volume and larger lateral ventricle and pallidum volumes in patients with schizophrenia compared with healthy volunteers.
Table 4.
Least Square Means, Percent Difference, and Effect Sizes
| Region | Schizophrenia Patients (n=186) | Healthy Volunteers (n=176) | Percent Difference | Full Sample Effect Size Cohen’s d/n80 | Weighted Mean Effect Size Cohen’s d/n80 | Meta-analysis Cohen’s d Haijma medicated/antipsychotic naive | Meta-analysis** Cohen’s d/n80 Shepherd |
|---|---|---|---|---|---|---|---|
| Pallidum | 1792 (193) | 1700 (184) | 5.44 | 0.490/53 | 0.732/24 | 0.26 | 1.06LH to 1.34RH/10–12 |
| Hippocampus | 4156 (428) | 4302 (409) | −3.41 | −0.350/102 | −0.391/81 | −0.52/−0.43 | −0.38 to −0.58/48–110 |
| Putamen | 57101 (620) | 5566 (592) | 2.60 | 0.239/218 | 0.443/64 | 0.10 | 0.21LH to 0.24RH/274–357 |
| Lat. Ventricle | 8429 (4832) | 7378 (4614) | 14.25 | 0.222/252 | 0.312/127 | 0.45 | 0.39RH to 0.51LH/62–105 |
| ICV | 1488 (165) | 1523 (163) | −2.26 | −0.209/284 | −0.182/370 | −0.17/−0.14 | |
| Amygdala | 1648 (204) | 1681 (195) | −1.95 | −0.165/455 | −0.090/1528 | −0.31 | −0.38 to −0.72/32–110 |
| Caudate | 3755 (483) | 3714 (461) | 1.08 | 0.085/1713 | 0.102/1123 | −0.03/−0.38 | −0.06RH to 0.06LH/4362 |
| Thalamus | 7287 (675) | 7311 (643) | −0.33 | −0.037/9033 | −0.098/1263 | −0.31/−0.68 | −0.18 to 0.33/486–146 |
n80 = number of subjects per group needed, assuming an equal number of subjects per group, for 80% power to detect a difference at p<0.05.
= Cohen’s d of volume difference between medicated schizophrenia patients and healthy controls based on the Haijma et al. (2012) meta-analysis of structural brain abnormalities in schizophrenia
= Percent difference and Cohen’s d of volume difference between chronic schizophrenia patients and healthy controls based on the Shepherd et al. (2012) meta-review of structural brain abnormalities in schizophrenia; LH = for left hemisphere; RH = for right hemisphere Lat. Ventricle = Lateral Ventricle; ICV = Intracranial Volume.
2. Methods
2.1. Participants
The participants comprised 186 schizophrenia patients (mean age±SD=38.9±11.6, 145 males) and 176 healthy volunteers (mean age±SD=37.5±11.2, 126 males) with similar mean age, sex, handedness, and race distributions from seven sites (Table 1; see Supplement 1, Table 1S, for demographic data by site). Patient inclusion criteria were schizophrenia diagnosis based on the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (First et al., 2002b). All patients were clinically stable outpatients whose antipsychotic medications and doses had not changed within the last 2 months. Current neuroleptic medication data were available for 171 of the 186 patients (antipsychotics: 136 atypical, 20 typical, 10 both; mood stabilizers: 2, and anxiolytics: 3). Chlorpromazine-equivalent dosages could be computed for 151 patients (Woods et al., 2005). Schizophrenia patients and healthy volunteers with a history of major medical illness, drug dependence in the last 5 years, current substance abuse disorder, MRI contraindications, or eyesight not correctable to normal acuity with MRI-compatible lenses were excluded. Patients with significant extrapyramidal symptoms and healthy volunteers with a current or past history of major neurological or psychiatric illness (First et al., 2002a) or with a first degree relative with an Axis-I psychotic disorder diagnosis were also excluded. Patient’s clinical assessments included the Positive and Negative Syndrome Scale (Kay et al., 1989). All participants were assessed for socioeconomic status (Hollingstead, 1975), handedness (Oldfield, 1971), basic demographics, and premorbid IQ (Uttl, 2002). The sample includes 137 paranoid, 7 disorganized, 30 undifferentiated, and 12 residual patients. Before data collection, experienced clinicians were jointly trained on the clinical assessment rating scales with patient interviews. The raters’ assessments were compared with expert ratings. Additional training was provided when raters deviated by more than 1 point for each item from the expert ratings.
Table 1.
Sample Demographics
| Schizophrenia Patients (n=186) | Healthy Volunteers (n=176) | Statistic | p- value | |
|---|---|---|---|---|
| Mean Age (SD) | 38.9 (11.6) | 37.5 (11.2) | t360=1.12 | 0.26 |
| Subject Educationb (SD) | 3.4(0.9) | 2.3(0.9) | t360=11.47 | <0.0001 |
| Parental Educationb (SD) | 2.4(1.8) | 2.1(1.5) | t360=1.49 | 0.14 |
| NAART | 29.4(12.4) | 39.7(11.4) | t357= −8.22 | <0.0001 |
| Age at Onset | 21.8 (7.6) | |||
| Duration of Illness | 17.1 (11.5) | |||
| PANSS positive | 15.5(5.1) | |||
| PANSS negative | 14.5(5.6) | |||
| PANSS general | 28.6(7.5) | |||
| PANSS composite | 0.9(6.3) | |||
| Gender (M/F) | 145/41 | 126/50 | χ21=0.83 | 0.36 |
| Handednessa (bilateral/left/right) | 4/12/170 | 2/7/167 | FET | 0.46 |
| Race | FET | 0.10 | ||
| American Indian or Alaskan | 4 | 3 | ||
| Native | ||||
| Asian | 22 | 16 | ||
| Black or African American | 38 | 20 | ||
| Native Hawaiian or Pacific | 2 | 2 | ||
| Islander | ||||
| White | 120 | 135 | ||
FET=Fisher’s Exact Test
based on Edinburgh Handedness Inventory (Oldfield, 1971)
based on the Hollingstead Socioeconomic Status Scale (Hollingstead, 1975)
NAART = North American Adult Reading Test (Uttl, 2002)
PANSS = Positive and Negative Syndrome Scale (Kay et al., 1989)
Written informed consent, including permission to share de-identified data between the centers and with the wider research community, approved by the University of California Irvine, Los Angeles, and San Francisco, Duke University, University of North Carolina, New Mexico, Iowa, and Minnesota Institutional Review Boards, was obtained from all study participants.
2.2. Image acquisition
High-resolution structural brain scans were acquired on six 3T Siemens Tim® Trio System and one 3T General Electric (GE) Discovery MR750 scanner using standardized sequences. Siemens MP-RAGE scan parameters were: TR/TE/TI=2300/2.94/1100 ms, flip angle=9°, resolution=256×256×160. GE IR-SPGR scan parameters were: TR/TE/TI=5.95/1.99/450 ms, flip angle=12°, resolution=256×256×166. All scans covered the entire brain with field of view (FOV)=220 mm2, voxel size=0.86×0.86×1.2mm, sagittal scan plane, GRAPPA/ASSET acceleration factor=2, and number of excitations (NEX)=1. The 3T MRI scanners at each site were calibrated to meet FBIRN Phantom quality assurance (Friedman and Glover, 2006a, 2006b; Greve et al., 2011), and scanner quality was monitored during the entire study. Before the study began, a traveling engineer visited each site to review MRI study protocol adherence based on FBIRN’s multi-center imaging study recommendations (Glover et al., 2012).
2.3. Image processing
Left and right lateral ventricle, thalamus, caudate, putamen, pallidum, hippocampus, and amygdala volumes as well as intracranial volumes (Table 2; see Supplement 1, Table 2S for absolute volumes by site) were obtained using Freesurfer Version 5.0.0 (http://surfer.nmr.mgh.harvard.edu; (Fischl, 2012; Fischl et al., 2002). All regions of interest with a volume larger or smaller than 1.5 times the inter-quartile range (IQR) were identified and visually inspected by overlaying them on the subject’s anatomical images. Based on these inspections, data from one patient were removed due to poor image quality and 0–2 additional data points for each of the regions of interest were eliminated from the final analyses.
Table 2.
Absolute Volumes
| Region | Schizophrenia Patients (n=186) | Healthy Volunteers (n=176) |
|---|---|---|
| Hippocampus | ||
| Left | 4106 (516) | 4279 (483) |
| Right | 4201 (531) | 4348 (462) |
| Amygdala | ||
| Left | 1629 (237) | 1655 (213) |
| Right | 1686 (233) | 1728 (241) |
| Caudate | ||
| Left | 3702 (540) | 3721 (502) |
| Right | 3729 (539) | 3756 (545) |
| Putamen | ||
| Left | 5935 (832) | 5837 (669) |
| Right | 5596 (810) | 5435 (633) |
| Pallidum | ||
| Left | 1883 (250) | 1813 (229) |
| Right | 1720 (237) | 1625 (206) |
| Thalamus | ||
| Left | 7154 (905) | 7272 (876) |
| Right | 7334 (906) | 7457 (831) |
| Lateral Ventricle | ||
| Left | 8705 (5539) | 7605 (4091) |
| Right | 7920 (4784) | 6958 (4034) |
| Intracranial Volume | 1539 (177) | 1562 (156) |
The absolute volumes are presented as mean mm3 (SD) except for the Intracranial volume which is presented as mean cc3 (SD).
2.4. Statistical analyses
Group differences for each region were examined using univariate mixed model regression analyses (Proc Mixed, SAS v9.2, SAS Institute Inc.) predicting subcortical volumes with group, site, sex, age, group × site, group × hemisphere, site × hemisphere, and group × site × hemisphere interactions. Hemisphere and intracranial volume entered the model as repeated measures and covariate variables, respectively. For the regions with a-priori-defined directional hypotheses based on the literature (amygdala, hippocampus, intracranial, lateral ventricle, and pallidum), the significance threshold was set at p<0.05 (one-tailed). We also indicate which of the findings pass the more conservative Bonferroni multiple comparison-corrected threshold of p<0.00625 (two-tailed). Based on evidence for the effects of antipsychotic medication on basal ganglia volumes (Gur et al., 1998; Hulshoff Pol and Kahn, 2008; Boonstra et al., 2011; Li et al., 2011), we examined the effect of current antipsychotic medication type (typical/atypical) and dose (chlorpromazine-equivalent dose) on basal ganglia volumes, using mixed model regression and correlation analysis, respectively. Cohen’s d weighted mean effect sizes based on each of the seven single site samples and also based on the full sample were computed. Sample size estimates were based on computed effect sizes using one-tailed t-tests in G*Power Version 3.2.1 (Erdefelder et al., 1996).
3. Results
3.1. Group effects
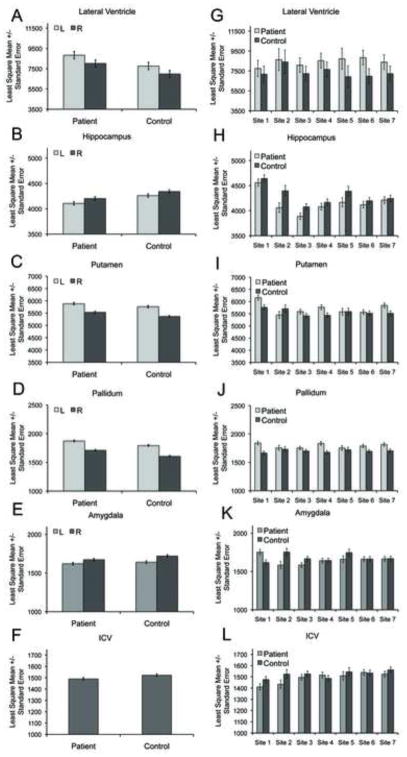
There were significant effects of group (schizophrenia patient, healthy volunteer) on hippocampus, pallidum, putamen, lateral ventricle, amygdala and intracranial volumes (Figs. 1A–1F), with hippocampus, amygdala, and intracranial volumes smaller and pallidum, putamen, and lateral ventricle volumes larger in schizophrenia patients compared with controls (Table 3, Fig. 1; see Supplement 1, Table 3Sp for statistical results by site). The effects for hippocampus and pallidum also passed the conservative Bonferroni two-tailed -value threshold of p<0.00625.
Fig. 1.
Regional volume differences between patients and controls across and within site. 1A-F: Regional volume differences based on multi-site analyses; 1G-L: Regional volume differences based on within-site analyses (due to lack of group by hemisphere interactions, data are collapsed across hemisphere in the within-site figures).
Table 3.
Univariate mixed-model regression analysis results.
| Variable (nDF, dDF) | Hippocampus | Amygdala | Caudate | Putamen | Pallidum | Thalamus | Lateral Ventricle | Intracranial Volume | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p-value | F | p-value | F | p-value | F | p-value | F | p-value | F | p-value | F | p-value | F | p-value | |
| Diagnosis (1,343) | 14.68 | 0.0002 | 3.09 | 0.08 | 0.53 | 0.47 | 5.98 | 0.02 | 23.85 | <0.0001 | 0.39 | 0.53 | 5.22 | 0.02 | 4.08 | 0.04 |
| Hemisphere (1,345) | 36.50 | <0.0001 | 63.08 | <0.0001 | 10.27 | 0.002 | 460.11 | <0.0001 | 440.46 | <0.0001 | 83.84 | <0.0001 | 41.30 | <0.0001 | ||
| Site (6,343) | 15.40 | <0.0001 | 1.08 | 0.37 | 2.46 | 0.02 | 4.46 | 0.0002 | 0.25 | 0.96 | 12.28 | <0.0001 | 0.29 | 0.87 | 3.29 | 0.004 |
| Diagnosis × Hemisphere (6,345) | 0.27 | 0.60 | 2.90 | 0.09 | 0.15 | 0.70 | 1.93 | 0.17 | 2.55 | 0.11 | 0.01 | 0.93 | 0.03 | 0.87 | ||
| Diagnosis × Site (6,343) | 0.73 | 0.63 | 2.94 | 0.008 | 1.41 | 0.21 | 1.95 | 0.07 | 1.48 | 0.18 | 0.25 | 0.96 | 0.23 | 0.97 | 0.97 | 0.45 |
| Hemisphere × Site (6,345) | 1.85 | 0.08 | 6.83 | <0.0001 | 1.75 | 0.11 | 1.59 | 0.15 | 2.80 | 0.01 | 9.03 | <0.0001 | 1.29 | 0.26 | ||
| Diagnosis × Site × Hemisphere (6,345) | 1.18 | 0.32 | 0.95 | 0.46 | 1.00 | 0.42 | 1.93 | 0.07 | 0.43 | 0.83 | 1.06 | 0.39 | 2.09 | 0.05 | ||
| Sex (1,343) | 4.29 | 0.04 | 8.50 | 0.004 | 3.39 | 0.07 | 14.18 | 0.0002 | 6.59 | 0.01 | 0.01 | 0.91 | 0.20 | 0.65 | 119.34 | <0.0001 |
| Age (1,343) | 13.16 | 0.0003 | 14.68 | 0.0002 | 18.31 | <0.0001 | 102.00 | <0.0001 | 46.87 | <0.0001 | 41.80 | <0.0001 | 47.97 | <0.0001 | 3.84 | 0.05 |
| Intracranial Volume (1,343) | 93.81 | <0.0001 | 87.18 | <0.0001 | 176.24 | <0.0001 | 69.00 | <0.0001 | 115.49 | <0.0001 | 240.40 | <0.0001 | 37.20 | <0.0001 | ||
nDF=nominator degrees of freedom, dDF=denominator degrees of freedom
Quality control procedures resulted in the removal of one patient due to poor scan quality and between 0–2 additional data points for each of the regions of interest.
Effects of diagnosis in bold pass the one-tailed (p<0.05) threshold set for the a priori hypotheses. The effects for hippocampus and pallidum also pass the conservative Bonferroni two-tailed p-value threshold of p<0.00625.
3.2. Site and group by site interaction effects
There were significant effects of site on hippocampus, putamen, thalamus, and intracranial volume, and a marginal effect of site (p=0.05) on caudate volume. Group × site interactions were confined to the amygdala. Decomposition of the interaction effect showed that at one site amygdala volume in patients was larger than in controls (t349=2.97, p=0.003), at two sites amygdala volume was smaller in patients compared with controls (t349=−2.57, p=0.01; t349=−2.0, p<0.05), and at four sites amygdala volumes did not differ between the groups (t349=0.05, p=0.96; t349=−1.36, p=0.17; t349=0.19, p=0.85, t349=−0.07, p=0.94).
3.3. Other effects
There were significant hemisphere effects on all subcortical volumes (Table 3) with left hippocampus, amygdala, caudate, and thalamus volumes smaller than right, and right putamen, pallidum, and ventricular volumes smaller than left (Table 2). There were significant hemisphere × site interactions on amygdala, pallidum, and thalamus volumes. Decomposition of the hemisphere × site interactions showed that left amygdala, pallidum, and thalamus volumes were smaller than right for all the sites, but that the strength of the differences varied by site. There were significant effects of sex on hippocampus, amygdala, putamen, pallidum, and intracranial volumes, with women showing smaller volumes than men. Age showed a significant negative association with subcortical and intracranial volumes and a significant positive association with lateral ventricle volumes. Intracranial volume was positively associated with all measured volumes (Table 3).
3.4. Effect-size estimates
The effect sizes based on the full multisite sample were on average 13% smaller than the effect-size estimates based on the weighted mean effect sizes from each individual site (Table 4). The sample sizes required for 80% power at α-level=0.05 for the five regions that showed significant group differences were between 1.3 and 3.4 times larger for multi-scanner compared with single scanner data acquisitions.
3.5. Antipsychotic medications
Current medication dose in chlorpromazine equivalents was negatively associated with left caudate (r150=−0.16, p<0.05), and left and right putamen (r150=−0.20, p=0.01; r150=−0.19, p=0.02)volumes. There were no effects of typical versus atypical antipsychotics on any of the regions.
4. Discussion
The principal findings of this study are as follows: (1) that we confirm smaller hippocampus, amygdala, and intracranial volumes and larger lateral ventricle, putamen, and pallidum volumes in patients with schizophrenia compared with healthy volunteers based on a prospective seven-site imaging study; (2) that significant site effects are present for hippocampus, putamen, thalamus, and intracranial volumes; and (3) that effect sizes for regional volume differences based on the multisite sample analysis were on average 13% smaller than those based on the weighted single site sample means.
The findings of smaller hippocampus, amygdala, and intracranial volumes and larger lateral ventricle, putamen, and pallidum volumes in patients with schizophrenia compared with healthy volunteers are consistent with and validate those observed in single site studies (Shepherd et al., 2012). While ventricle size showed the largest percentage between-group difference in volume, consistent with the fact that this is among the most robust structural brain abnormalities observed in patients with schizophrenia, surprisingly its effect size was relatively small. The effect size for amygdala volume deficit in schizophrenia patients compared with controls was also relatively small (see Table 4) (Shepherd et al., 2012). The relatively small effect sizes for these regions are unlikely due to multi-scanner acquisition given that for these regions (a) site effects are not significant and (b) the weighted single-scanner and multi-site effect sizes are quite similar. The small effect size for ventricle volume is likely due to large variability in this sample (see SD, Table 2). With regard to amygdala volumes, FreeSurfer reliability estimates are high (Jovicich et al., 2009; Morey et al., 2010), but validity may be questionable given reports of relatively low correlations with volumes derived from manual amygdala tracings (amygdala: r=0.56; for comparison hippocampus: r=0.82) (Morey et al., 2009), though see Dewey et al. (Dewey et al., 2010). Lower amygdala volumes are generally not observed in first episode schizophrenia, but there is high-moderate quality evidence for lower amygdala volumes in chronic schizophrenia (Shepherd et al., 2012). Given that our sample is older than in those reported in the meta-analyses of Shepherd et al. (2012) and Haijama et al. (2012), demographic differences other than age (e.g., medication treatment, diagnoses) may play a role. For instance, significantly lower amygdala volumes are also observed in patients with mood disorders (Hamilton et al., 2008; Sacher et al., 2012), and future work should investigate whether lower amygdala volumes are more robust in patients with schizoaffective disorder than schizophrenia. Schizoaffective disorder patients are often included in schizophrenia studies but are excluded from this study.
It is not surprising that we did not find group differences for caudate and thalamus volumes as the ranges of effect sizes for these structures in chronic schizophrenia patients include 0, though caudate and thalamus volumes appear lower in antipsychotic-naïve patients and may normalize after medication treatment (Haijma et al., 2012; Shepherd et al., 2012). In contrast to our expectations, we found that current medication doses in chlorpromazine equivalents were negatively associated with left caudate and bilateral putamen volumes. These findings are in contrast to longitudinal studies that have reported that antipsychotic treatments normalize caudate volume deficits observed in medication-naïve patients (Haijma et al., 2012) and increase putamen and pallidum volumes (Boonstra et al., 2011; Gur et al., 1998; Hulshoff Pol and Kahn, 2008; Li et al., 2011), though additional, systematic, long-term (> 1 year) longitudinal studies are likely required to more fully understand the effects of antipsychotic treatment on brain morphology.
Right-left asymmetries for hippocampus, amygdala, caudate, and lateral ventricle, as well as left-right asymmetries for putamen and pallidum volumes, have been reported in studies using gold-standard manual tracing methods (Aas et al., 2012; Erdogan et al., 2004; Peterson et al., 1993; Van Erp et al., 2002), and similar asymmetries observed in this study lend credibility to the quality of FreeSurfer’s automated segmentations.
In the analyses on the full data set, we found significant effects of site on hippocampus putamen, thalamus, and intracranial volumes. These site effects may be due to differences in sample demographics between sites or differences between scanners. Upon further investigation, the observed effect of site was mainly due to higher volumes produced by site 1 (GE scanner, sample with lowest mean age). When data from this center were excluded from the analysis, all effects of site, except for those on the hippocampus, were rendered non-significant. Closer investigation showed that the remaining site effect was due to lower hippocampus volumes for site 3 compared with all the other sites and that patients in this site had significantly older mean age than those of the other sites. While determination of scanner effects requires a traveling subject study, in which the same subjects are scanned at different sites, the main take-home message from our analysis is that careful consideration of site effects may provide clues about factors that influence sample heterogeneity in observed associations.
To our knowledge, this is the first multi-site study of subcortical brain volumes in chronic schizophrenia that provides estimates of the costs associated with multi-site versus single site data collection/analysis by providing effect sizes for mega- vs. meta-analysis of multi-center data. When analyzing data from seven scanners, we observed the need for on average a 1.26 to 3.4 times increase in sample size to detect the same volume differences observed when conducting single scanner studies. In contrast to an earlier report (Jovicich et al., 2009), we found that multi-site acquisition of hippocampus volume data required a 1.26 times larger data sample to obtain similar statistical power to that of data acquisition at a single scanner.
Strengths of the study are as follows: (1) the results are based on a largely fully automated analysis with few exclusions of subjects based on quality-assurance procedures; (2) the study includes a large cohort of chronic schizophrenia patients and controls such that variance estimates and therefore effect size estimates are likely to be robust; (3) the study includes data collected from seven scanners – albeit six Siemens 3T and one General Electric 3T– providing ample opportunity for between-scanner variability; and (4) the study was carefully planned and used the FBIRN phantom for scanner calibration, a traveling engineer to review protocol compliance before study initiation, and standardized scan sequences across scanners (Friedman and Glover, 2006a, 2006b; Greve et al., 2011; Glover et al., 2012)
Some study weaknesses must be noted as follow: (1) the focus of FBIRN was not on improving brain morphometric measures across scanners and included a standard high-resolution T1-weighted scan for registration purposes in functional imaging studies. Hence, higher effect sizes may be obtained for sequences optimized for morphometric studies (Jovicich et al., 2009); (2) the study included only one GE and no Philips scanners; (3) the study did not measure lifetime medication exposure or employ a longitudinal design to study medication effects; and (4) demographics were balanced within site, but variance in demographics (mean age, race distribution, and sex distribution) between sites was allowed. Such variance can improve generalizability of findings, but it also contributes to sample heterogeneity. Whether to allow for such variance should be taken into account when planning multi-center studies.
In conclusion, we were able to confirm the observations of smaller hippocampus, amygdala, and intracranial volumes and larger lateral ventricle, putamen, and pallidum volumes in patients with schizophrenia compared with healthy volunteers in a carefully planned prospective, multi-site imaging study. The effect sizes provided should help in the design of future multi-scanner schizophrenia imaging studies. Although some extra data collection is required in multi-scanner studies, benefits of multi-center data collection (Glover et al., 2012) may in many cases outweigh the cost.
Supplementary Material
Acknowledgments
We are thankful to Liv McMillan for overall study coordination, Harry Mangalam, Joseph Farran, and Adam Brenner, for administering the University of California, Irvine High-Performance Computing cluster, and to the research subjects for their participation. This work was supported by the National Center for Research Resources at the National Institutes of Health (grant numbers: NIH 1 U24 RR021992 (Function Biomedical Informatics Research Network) and NIH 1 U24 RR025736-01 (Biomedical Informatics Research Network Coordinating Center; http://www.birncommunity.org). The funding sources had no role in the study design, data collection, analysis, and interpretation of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aas M, Navari S, Gibbs A, Mondelli V, Fisher HL, Morgan C, Morgan K, MacCabe J, Reichenberg A, Zanelli J, Fearon P, Jones PB, Murray RM, Pariante CM, Dazzan P. Is there a link between childhood trauma, cognition, and amygdala and hippocampus volume in first-episode psychosis? Schizophrenia Research. 2012;137:73–79. doi: 10.1016/j.schres.2012.01.035. [DOI] [PubMed] [Google Scholar]
- Boonstra G, van Haren NE, Schnack HG, Cahn W, Burger H, Boersma M, de Kroon B, Grobbee DE, Hulshoff Pol HE, Kahn RS. Brain volume changes after withdrawal of atypical antipsychotics in patients with first-episode schizophrenia. Journal of Clinical Psychopharmacology. 2011;31:146–153. doi: 10.1097/JCP.0b013e31820e3f58. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Dewey J, Hana G, Russell T, Price J, McCaffrey D, Harezlak J, Sem E, Anyanwu JC, Guttmann CR, Navia B, Cohen R, Tate DF. Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage. 2010;51:1334–1344. doi: 10.1016/j.neuroimage.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdefelder E, Faul F, Buchner A. GPOWER: A general power analysis porgram. Behavior Research Methods, Instruments, & Computers. 1996;28:1–11. [Google Scholar]
- Erdogan AR, Dane S, Aydin MD, Ozdikici M, Diyarbakirli S. Sex and handedness differences in size of cerebral ventricles of normal subjects. International Journal of Neuroscience. 2004;114:67–73. doi: 10.1080/00207450490249428. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon MG, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Non-Patient Edition (SCID-I/NP, 11/2002 revision) New York State Psychiatric Institute; New York, NY: 2002a. [Google Scholar]
- First MB, Spitzer RL, Gibbon MG, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Patient Edition (SCID-I/P, 11/2002 revision) New York State Psychiatric Institute; New York, NY: 2002b. [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Friedman L, Glover GH. Reducing interscanner variability of activation in a multicenter fMRI study: controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. Neuroimage. 2006a;33:471–481. doi: 10.1016/j.neuroimage.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Friedman L, Glover GH. Report on a multicenter fMRI quality assurance protocol. Journal of Magnetic Resonance Imaging. 2006b;23:827–839. doi: 10.1002/jmri.20583. [DOI] [PubMed] [Google Scholar]
- Glover GH, Mueller BA, Turner JA, van Erp TG, Liu TT, Greve DN, Voyvodic JT, Rasmussen J, Brown GG, Keator DB, Calhoun VD, Lee HJ, Ford JM, Mathalon DH, Diaz M, O’Leary DS, Gadde S, Preda A, Lim KO, Wible CG, Stern HS, Belger A, McCarthy G, Ozyurt B, Potkin SG. Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. Journal of Magnetic Resonance Imaging. 2012;36:39–54. doi: 10.1002/jmri.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Mueller BA, Liu T, Turner JA, Voyvodic J, Yetter E, Diaz M, McCarthy G, Wallace S, Roach BJ, Ford JM, Mathalon DH, Calhoun VD, Wible CG, Brown GG, Potkin SG, Glover G. A novel method for quantifying scanner instability in fMRI. Magnetic Resonance in Medicine. 2011;65:1053–1061. doi: 10.1002/mrm.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. American Journal of Psychiatry. 1998;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophrenia Bulletin. 2013;39 (5):1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Molecular Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingstead AB. Four-factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophrenia Bulletin. 2008;34:354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Pacheco J, Albert M, Killiany R, Blacker D, Maguire P, Rosas D, Makris N, Gollub R, Dale A, Dickerson BC, Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. British Journal of Psychiatry Suppl. 1989;7:59–67. [PubMed] [Google Scholar]
- Li M, Chen Z, Deng W, He Z, Wang Q, Jiang L, Ma X, Wang Y, Chua SE, Cheung C, McAlonan GM, Sham PC, Collier DA, Gong Q, Li T. Volume increases in putamen associated with positive symptom reduction in previously drug-naive schizophrenia after 6 weeks antipsychotic treatment. Psychological Medicine. 2011;42:1475–1483. doi: 10.1017/S0033291711002157. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, LaBar KS, Styner M, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Selgrade ES, Wagner HR, 2nd, Huettel SA, Wang L, McCarthy G. Scan-rescan reliability of subcortical brain volumes derived from automated segmentation. Human Brain Mapping. 2010;31:1751–1762. doi: 10.1002/hbm.20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Riddle MA, Cohen DJ, Katz LD, Smith JC, Leckman JF. Human basal ganglia volume asymmetries on magnetic resonance images. Magnetic Resonance Imaging. 1993;11:493–498. doi: 10.1016/0730-725x(93)90468-s. [DOI] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Funfstuck T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. Journal of Affective Disorders. 2012;140:142–148. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neuroscience & Biobehavioral Reviews. 2012;36:1342–1356. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Suckling J, Barnes A, Job D, Brenan D, Lymer K, Dazzan P, Marques TR, MacKay C, McKie S, Williams SR, Williams SC, Lawrie S, Deakin B. Power calculations for multicenter imaging studies controlled by the false discovery rate. Human Brain Mapping. 2010;31:1183–1195. doi: 10.1002/hbm.20927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tae WS, Kim SS, Lee KU, Nam EC, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50:569–581. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- Uttl B. North American Adult Reading Test: age norms, reliability, and validity. Journal of Clinical Experimental Neuropsychology. 2002;24:1123–1137. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- van Erp TG, Saleh PA, Huttunen M, Lonnqvist J, Kaprio J, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Cannon TD. Hippocampal volumes in schizophrenic twins. Archives of General Psychiatry. 2004;61:346–353. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- Van Erp TG, Saleh PA, Rosso IM, Huttunen M, Lonnqvist J, Pirkola T, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Cannon TD. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. American Journal of Psychiatry. 2002;159:1514–1520. doi: 10.1176/appi.ajp.159.9.1514. [DOI] [PubMed] [Google Scholar]
- Wonderlick JS, Ziegler DA, Hosseini-Varnamkhasti P, Locascio JJ, Bakkour A, van der Kouwe A, Triantafyllou C, Corkin S, Dickerson BC. Reliability of MRI-derived cortical and subcortical morphometric measures: effects of pulse sequence, voxel geometry, and parallel imaging. Neuroimage. 2009;44:1324–1333. doi: 10.1016/j.neuroimage.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.