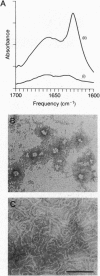
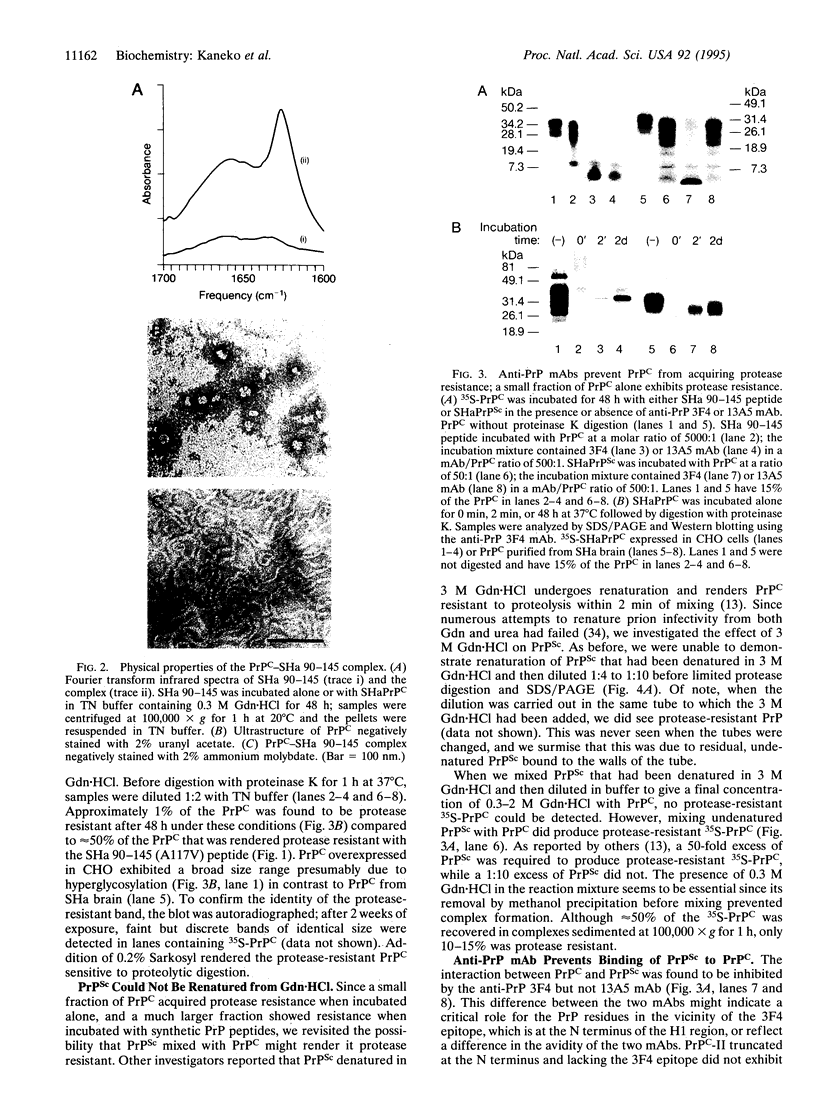
Abstract
Conversion of the cellular isoform of prion protein (PrPC) into the scrapie isoform (PrPSc) involves an increase in the beta-sheet content, diminished solubility, and resistance to proteolytic digestion. Transgenetic studies argue that PrPC and PrPSc form a complex during PrPSc formation; thus, synthetic PrP peptides, which mimic the conformational pluralism of PrP, were mixed with PrPC to determine whether its properties were altered. Peptides encompassing two alpha-helical domains of PrP when mixed with PrPC produced a complex that displayed many properties of PrPSc. The PrPC-peptide complex formed fibrous aggregates and up to 65% of complexed PrPC sedimented at 100,000 x g for 1 h, whereas PrPC alone did not. These complexes were resistant to proteolytic digestion and displayed a high beta-sheet content. Unexpectedly, the peptide in a beta-sheet conformation did not form the complex, whereas the random coil did. Addition of 2% Sarkosyl disrupted the complex and rendered PrPC sensitive to protease digestion. While the pathogenic A117V mutation increased the efficacy of complex formation, anti-PrP monoclonal antibody prevented interaction between PrPC and peptides. Our findings in concert with transgenetic investigations argue that PrPC interacts with PrPSc through a domain that contains the first two putative alpha-helices. Whether PrPC-peptide complexes possess prion infectivity as determined by bioassays remains to be established.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. A., Prusiner S. B. Monoclonal antibodies to the cellular and scrapie prion proteins. J Infect Dis. 1986 Sep;154(3):518–521. doi: 10.1093/infdis/154.3.518. [DOI] [PubMed] [Google Scholar]
- Bessen R. A., Kocisko D. A., Raymond G. J., Nandan S., Lansbury P. T., Caughey B. Non-genetic propagation of strain-specific properties of scrapie prion protein. Nature. 1995 Jun 22;375(6533):698–700. doi: 10.1038/375698a0. [DOI] [PubMed] [Google Scholar]
- Borchelt D. R., Scott M., Taraboulos A., Stahl N., Prusiner S. B. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol. 1990 Mar;110(3):743–752. doi: 10.1083/jcb.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANDLER R. L. Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet. 1961 Jun 24;1(7191):1378–1379. doi: 10.1016/s0140-6736(61)92008-6. [DOI] [PubMed] [Google Scholar]
- Caughey B. W., Dong A., Bhat K. S., Ernst D., Hayes S. F., Caughey W. S. Secondary structure analysis of the scrapie-associated protein PrP 27-30 in water by infrared spectroscopy. Biochemistry. 1991 Aug 6;30(31):7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- Doh-ura K., Tateishi J., Sasaki H., Kitamoto T., Sakaki Y. Pro----leu change at position 102 of prion protein is the most common but not the sole mutation related to Gerstmann-Sträussler syndrome. Biochem Biophys Res Commun. 1989 Sep 15;163(2):974–979. doi: 10.1016/0006-291x(89)92317-6. [DOI] [PubMed] [Google Scholar]
- Gabizon R., McKinley M. P., Groth D., Prusiner S. B. Immunoaffinity purification and neutralization of scrapie prion infectivity. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6617–6621. doi: 10.1073/pnas.85.18.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek D. C. Spontaneous generation of infectious nucleating amyloids in the transmissible and nontransmissible cerebral amyloidoses. Mol Neurobiol. 1994 Feb;8(1):1–13. doi: 10.1007/BF02778003. [DOI] [PubMed] [Google Scholar]
- Gasset M., Baldwin M. A., Fletterick R. J., Prusiner S. B. Perturbation of the secondary structure of the scrapie prion protein under conditions that alter infectivity. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):1–5. doi: 10.1073/pnas.90.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasset M., Baldwin M. A., Lloyd D. H., Gabriel J. M., Holtzman D. M., Cohen F., Fletterick R., Prusiner S. B. Predicted alpha-helical regions of the prion protein when synthesized as peptides form amyloid. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10940–10944. doi: 10.1073/pnas.89.22.10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi T., Fisher S., Olofsson S., Endo T., Groth D., Tarentino A., Borchelt D. R., Teplow D., Hood L., Burlingame A. Asparagine-linked glycosylation of the scrapie and cellular prion proteins. Arch Biochem Biophys. 1989 Oct;274(1):1–13. doi: 10.1016/0003-9861(89)90409-8. [DOI] [PubMed] [Google Scholar]
- Hemström C., Virtanen A., Bridge E., Ketner G., Pettersson U. Adenovirus E4-dependent activation of the early E2 promoter is insufficient to promote the early-to-late-phase transition. J Virol. 1991 Mar;65(3):1440–1449. doi: 10.1128/jvi.65.3.1440-1449.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K. K., Cass C., Schellenberg G. D., Bird T., Devine-Gage E., Wisniewski H., Prusiner S. B. A prion protein variant in a family with the telencephalic form of Gerstmann-Sträussler-Scheinker syndrome. Neurology. 1991 May;41(5):681–684. doi: 10.1212/wnl.41.5.681. [DOI] [PubMed] [Google Scholar]
- Hunter T. When is a lipid kinase not a lipid kinase? When it is a protein kinase. Cell. 1995 Oct 6;83(1):1–4. doi: 10.1016/0092-8674(95)90225-2. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993 Jun 18;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Kascsak R. J., Rubenstein R., Merz P. A., Tonna-DeMasi M., Fersko R., Carp R. I., Wisniewski H. M., Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987 Dec;61(12):3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocisko D. A., Come J. H., Priola S. A., Chesebro B., Raymond G. J., Lansbury P. T., Caughey B. Cell-free formation of protease-resistant prion protein. Nature. 1994 Aug 11;370(6489):471–474. doi: 10.1038/370471a0. [DOI] [PubMed] [Google Scholar]
- Kocisko D. A., Priola S. A., Raymond G. J., Chesebro B., Lansbury P. T., Jr, Caughey B. Species specificity in the cell-free conversion of prion protein to protease-resistant forms: a model for the scrapie species barrier. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3923–3927. doi: 10.1073/pnas.92.9.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koke J. A., Yang M., Henner D. J., Volwerk J. J., Griffith O. H. High-level expression in Escherichia coli and rapid purification of phosphatidylinositol-specific phospholipase C from Bacillus cereus and Bacillus thuringiensis. Protein Expr Purif. 1991 Feb;2(1):51–58. doi: 10.1016/1046-5928(91)90009-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee D. S., Griffiths B. W. Comparative studies of Iodo-bead and chloramine-T methods for the radioiodination of human alpha-fetoprotein. J Immunol Methods. 1984 Nov 16;74(1):181–189. doi: 10.1016/0022-1759(84)90379-x. [DOI] [PubMed] [Google Scholar]
- Marsh R. F., Kimberlin R. H. Comparison of scrapie and transmissible mink encephalopathy in hamsters. II. Clinical signs, pathology, and pathogenesis. J Infect Dis. 1975 Feb;131(2):104–110. doi: 10.1093/infdis/131.2.104. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., McKinley M. P., Bowman K. A., Braunfeld M. B., Barry R. A., Prusiner S. B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen J., Baldwin M. A., Cohen F. E., Prusiner S. B. Prion protein peptides induce alpha-helix to beta-sheet conformational transitions. Biochemistry. 1995 Apr 4;34(13):4186–4192. doi: 10.1021/bi00013a006. [DOI] [PubMed] [Google Scholar]
- Pan K. M., Baldwin M., Nguyen J., Gasset M., Serban A., Groth D., Mehlhorn I., Huang Z., Fletterick R. J., Cohen F. E. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan K. M., Stahl N., Prusiner S. B. Purification and properties of the cellular prion protein from Syrian hamster brain. Protein Sci. 1992 Oct;1(10):1343–1352. doi: 10.1002/pro.5560011014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D., Serban A., Stahl N., Gabizon R. Attempts to restore scrapie prion infectivity after exposure to protein denaturants. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2793–2797. doi: 10.1073/pnas.90.7.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Scott M., Foster D., Pan K. M., Groth D., Mirenda C., Torchia M., Yang S. L., Serban D., Carlson G. A. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990 Nov 16;63(4):673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Raeber A. J., Borchelt D. R., Scott M., Prusiner S. B. Attempts to convert the cellular prion protein into the scrapie isoform in cell-free systems. J Virol. 1992 Oct;66(10):6155–6163. doi: 10.1128/jvi.66.10.6155-6163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M., Serban D., Gyuris T., Scott M., Torchia T., Prusiner S. B. Epitope mapping of the Syrian hamster prion protein utilizing chimeric and mutant genes in a vaccinia virus expression system. J Immunol. 1991 Nov 15;147(10):3568–3574. [PubMed] [Google Scholar]
- Safar J., Roller P. P., Gajdusek D. C., Gibbs C. J., Jr Conformational transitions, dissociation, and unfolding of scrapie amyloid (prion) protein. J Biol Chem. 1993 Sep 25;268(27):20276–20284. [PubMed] [Google Scholar]
- Scott M. R., Köhler R., Foster D., Prusiner S. B. Chimeric prion protein expression in cultured cells and transgenic mice. Protein Sci. 1992 Aug;1(8):986–997. doi: 10.1002/pro.5560010804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M., Groth D., Foster D., Torchia M., Yang S. L., DeArmond S. J., Prusiner S. B. Propagation of prions with artificial properties in transgenic mice expressing chimeric PrP genes. Cell. 1993 Jun 4;73(5):979–988. doi: 10.1016/0092-8674(93)90275-u. [DOI] [PubMed] [Google Scholar]
- Stahl N., Baldwin M. A., Teplow D. B., Hood L., Gibson B. W., Burlingame A. L., Prusiner S. B. Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry. 1993 Mar 2;32(8):1991–2002. doi: 10.1021/bi00059a016. [DOI] [PubMed] [Google Scholar]
- Telling G. C., Scott M., Hsiao K. K., Foster D., Yang S. L., Torchia M., Sidle K. C., Collinge J., DeArmond S. J., Prusiner S. B. Transmission of Creutzfeldt-Jakob disease from humans to transgenic mice expressing chimeric human-mouse prion protein. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9936–9940. doi: 10.1073/pnas.91.21.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk E., Teplow D. B., Hood L. E., Prusiner S. B. Purification and properties of the cellular and scrapie hamster prion proteins. Eur J Biochem. 1988 Sep 1;176(1):21–30. doi: 10.1111/j.1432-1033.1988.tb14246.x. [DOI] [PubMed] [Google Scholar]
- Zhang H., Kaneko K., Nguyen J. T., Livshits T. L., Baldwin M. A., Cohen F. E., James T. L., Prusiner S. B. Conformational transitions in peptides containing two putative alpha-helices of the prion protein. J Mol Biol. 1995 Jul 21;250(4):514–526. doi: 10.1006/jmbi.1995.0395. [DOI] [PubMed] [Google Scholar]