Abstract
Mononuclear phagocytes (MPs) relevant to atherosclerosis include monocytes, macrophages, and dendritic cells (DCs). A decade ago, studies on macrophage behavior in atherosclerotic lesions were often limited to quantification of total macrophage area in cross-sections of plaques. While technological advances are still needed to examine plaque MP populations in an increasingly dynamic and informative manner, innovative methods to interrogate the biology of MPs in atherosclerotic plaques developed in the last few years point to a number of mechanisms that regulate the accumulation and function of MPs within plaques. Here, I review the evolution of atherosclerotic plaques with respect to changes in the MP compartment from the initiation of plaque to its progression and regression, discussing the roles that recruitment, proliferation, and retention of MPs play at these different disease stages. Additional work in the future will be needed to better distinguish macrophages and DCs in plaque and to address some basic unknowns in the field, including just how cholesterol drives accumulation of macrophages in lesions to build plaques in the first place and how macrophages as major effectors of innate immunity work together with components of the adaptive immune response to drive atherosclerosis. Answers to these questions are sought with the goal in mind of reversing disease where it exists and preventing its development where it does not.
Keywords: macrophage, atherosclerosis, monocyte, migration
INTRODUCTION
It has only been in the last couple of decades that the weight of evidence led to formal pronouncement of atherosclerosis as an inflammatory disease 1, 2, rather than solely a disease associated with aberrant cholesterol accumulation and handling. Inflammation in general is driven by activation of the endothelium and leukocyte trafficking across this endothelial barrier to supply the reacting tissue with effector cells. The dominant leukocyte type recruited to atherosclerotic plaque is the monocyte. Early studies that connected the dots between atherosclerosis and inflammation recognized the primary importance of lesional macrophages, recruited as monocytes into developing plaques 1, where they take up large amounts of cholesterol to generate so-called foam cells filled with numerous cholesterol ester droplets. Macrophages are not only major cells that sequester cholesterol within plaques but they are also implicated in critical events of clinical significance, particularly plaque rupture 3, 4. For example, macrophage presentation of antigen to T lymphocytes may support the generation of conditions conducive to plaque rupture 3-5, expanding the role of macrophages in plaques beyond handling cholesterol. However, during the last decade, significant advances in our understanding of the division of labor among antigen-presenting cells raises the possibility that other types of mononuclear phagocytes (MPs), such as dendritic cells (DCs), may be as or more relevant to any interactions with lymphocytes that in turn drive disease.
Researchers attempting to understand in detail the role of MPs in initiating and sustaining atherosclerosis, with the goal of learning how to reverse these events, face challenges that include anatomical, technical, and temporal constraints. Anatomically speaking, the location of plaques deep in the chest cavity near the beating heart and respiratory system raises great challenges for intravital imaging. Technical challenges are many including limitations on the interpretation of the powerful technique of flow cytometry, since flow cytometry obliterates the ability to distinguish intimal leukocytes from those in the adventitia. Temporal constraints include the slow pace that the disease develops under the most physiological conditions, decades in man and months in mice. Finally, the deadliest stage of the disease in man, plaque rupture, has no widely accepted counterpart in experimental models, limiting our ability to focus on key events in an experimental scenario. With all these drawbacks, the need to better understand the mechanisms of atherosclerosis remains and the role that MPs play, as it remains a leading cause of mortality worldwide. The last decade has applied several new approaches that facilitate a more dynamic analysis of MPs in atherosclerosis than the simple measurement of macrophage area in plaques. These techniques, outlined with their inherent pros and cons in Table 1, are responsible for progress in the field and underlie my discussion in this review on the status of our knowledge regarding MPs in atherosclerotic plaques, including how the accumulation of MPs is triggered and regulated during disease progression and whether these mechanisms can be reversed to bring about disease regression. With all the valuable insight work these approaches have provided, it is important to keep in mind the caveats of the methods when interpreting the results from the techniques employed.
Table 1.
Techniques that have paved the way in interrogating the biology of MPs in mouse atherosclerotic plaques.
| Method | Purpose and Pros | Cons | Selected References |
|---|---|---|---|
| Genetic deletion/modification | Gene “knockouts” and transgenic mice have greatly shaped the evolution of experimental models of atherosclerosis and are integrated in nearly all studies. Cell-specific targeting of deletions now often refine experimental design. | Currently, it is impossible to confine genetic changes to the arterial walls of plaques, thus confounding the interpretation of genetic changes that are global, even if confined to one cell type. A relevant example is the role of Flt-3 and DCs in atherosclerosis. Though Flt-3-dependent DCs are found in heart and aortic vasculature, it is impossible to know if the atheroprotection conferred by Flt-3 deletion involves DCs locally or those elsewhere. | 5, 123, 124 |
| Bone marrow transplant (BMT) | BMT allows reconstitution of the hematopoietic compartment with donor marrow that confines genetic modifications to this compartment after original hematopoietic compartment is destroyed by genotoxic exposure to radiation. BMT also allows for the establishment of competition experiments between reconstituted cells of distinct genotypes. | Irradiation initiates long-lasting tissue damage and alters the pattern of plaque deposition and generally accelerates plaque progression, with the exception of aortic arch and descending aorta of mice where a retardation of plaque progression occurs. The impact of BMT in murine atherosclerosis is sometimes interpreted as changes confined to the macrophage compartment when in fact all hematopoietic cells are altered. Some macrophages and other leukocytes are radioresistant, so not all are reconstituted. | 125-127 |
| Fluorescence imaging | Confocal imaging provides high-resolution views of plaque in different orientations. Multi-photon imaging in vivo is highly sought after to obtain live, direct evaluations of MP activity. | Plaque depth can limit the utility of confocal imaging. The problems of motion due to heart and respiratory rhythms and location of affected arteries in the chest cavity restricts in situ multi-photon microscopy. | 6, 61, 128-131 |
| Flow cytometry using suspensions from digested aorta | Allows for > 12 markers to be analyzed in all single cells of a suspension from the artery wall with often thousands of events analyzed. Indispensable for finely tuned evaluations of phenotype. | Macrophages may not be uniformly retrieved, as they are notoriously difficult to extract from tissues. Flow cytometry of cells from the aorta retrieves many leukocytes from the adventitia. Avoidance of mixtures of adventitial and intimal macrophages requires tedious procurement of intimal plaque prior to flow cytometry. | 24, 40, 132-135 |
| Surgical transfer of the aorta to recipients | Transfer of aortas from hyperlipidemic to normolipidemic environments sets up a model of regression. The method also permits unique tracers in the donor to be tracked in the recipient with knowledge that they originated from plaque (i.e., congenic markers, deuterium cholesterol). | Healing from surgery sets up distinct repair pathways and inflammation in the adventitia that may alter the course of disease. Plaques may undergo hypoxia or other undesired response during the course of surgery. | 107, 118, 136-138 |
| Adoptive transfer of monocytes and analysis by autoradiography. | Adoptive transfer of monocytes coupled with autoradiography has allowed for quantitation of monocyte recruitment to plaques. | Adoptive transfer of monocytes is so inefficient that powerful readouts like autoradiography, rather than fluorescence, are required for robust detection of monocyte recruitment. A dozen donor mice or more may be used for one recipient. The method has low spatial resolution so cells are not observed within the intima itself. | 40, 135 |
| Phagocytic bead labeling of endogenous monocytes | An alternative method for quantifying endogenous monocyte recruitment that can be coupled to fluorescence imaging techniques. Beads don't disappear when macrophages die, allowing the uncoupling of bead fate from macrophage death. Thus, the technique can be designed for quantification of monocyte recruitment and macrophage egress from plaques. | Labeling techniques might alter monocytes, especially with method of labeling involving clodronate. Free beads are present in blood transiently. Important to account for the fact that beads can transfer between phagocytes and even enter other cells or necrotic zones. Egress studies should not begin until monocytes in the circulation have cleared in order to separate entry and egress phases to generate more accurate data. | 13, 116, 139 |
PRE-PLAQUE AND EARLY ATHEROSCLEROTIC PLAQUE: VASCULAR DENDRITIC CELLS?
Leukocyte trafficking into inflamed tissue occurs through specific activation of endothelium. The formation of atherosclerotic plaques requires activation of arterial endothelium, breaking with the more typical restriction of endothelial cell activation to postcapillary venules within the vascular bed. This activation is characterized by induction or upregulation of adhesion molecules like vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) and chemoattractants, including lipids like platelet-activating factor (PAF) and chemokines like interleukin 8 (IL-8; CCL8) or monocyte chemoattractant protein 1 (MCP-1; CCL2). Endothelial cell activation is a prerequisite to the formation of atherosclerotic plaques that preferentially form along the arterial tree at sites that experience reduced shear stress as a result of arterial curvature and branching. This activation of endothelium is inherently persistent as it results from the anatomy of the arterial tree. Consequently, a persistent inflammatory response ensues 6. However, this response is self-limited and quickly reaches a state of maintenance that does not expand the inflammatory reaction beyond MPs that are recruited to reside in a single, discontinuous layer under the endothelium 6. The pattern of distribution of these MPs is morphologically reminiscent of the cells such as Langerhans cells in the skin that form a network in the epidermis. Mouse, rabbit, and human arteries all appear to develop these vascular MP networks at sites predisposed to atherosclerosis, though the data in human and rabbit are more limited. Current phenotypic characteristics of pre-lesional MPs from the different species are listed in Table 2.
Table 2.
Vascular mononuclear phagocytes in pre-lesional arteries.
| Species | Phenotype | Comments | References |
|---|---|---|---|
| Human | CD1a+ Langerin+ S-100+ CD68-CD83-CD86-, also lacking von Willebrand factor and smooth muscle actin | Specimen of various human arteries; positive for DC markers with no hint of macrophage character since cells were CD68-. Only single stains were carried out, so co-expression of the markers has not been established. MHC II was not stained. | 7, 140 |
| Rabbit | MHC II+ | Morphology of arterial MPs consistent with DCs or macrophages. Distribution of cells also similar to mouse artery MP. | 7 |
| Mouse | CD68+ MHC II+ CD11c+ 33D1+ CD11blow; mRNA for CD14 and CD83; GFP+ in CX3CR1gfp/- mouse | 33D1 is a DC marker not described on macrophages. CD11b can be absent from macrophages or DCs, but is always present on monocytes. Difficult to fully reconcile with flow cytometry evaluation | 6, 8, 10, 24 |
Distribution of these MPs is limited to areas of basal endothelial activation that correspond to hemodynamic stress 6, 7. The natural seeding of these sites by MPs occurs by the earliest point in time investigated to date, when the mice are 4 weeks old 8. It would be interesting to know if the cells are recruited much earlier, perhaps prior to birth. Although more information on the life cycle of these cells is needed, some interesting findings are known. The lifecycle of these vascular MPs is dependent upon their expression of CX3CR1 8. VCAM-1 deficiency leads to a 50% reduced density of the MPs 6. This finding suggests that their initial seeding may be dependent upon endothelial cell activation and that, likely, they are continuously recruited at least to some extent. On the other hand, VCAM-1 can also be expressed by MPs, so it is possible that deficiency of VCAM-1 as a means to lower the density of these cells targets the MPs themselves, rather than the endothelium. Furthermore, if deficiency of VCAM-1 indicates that the MP are recruited across the endothelium, it is not clear if the key recruitment occurs pre- or postnatally. Nor is there information on the rate of turnover of the cells. The expression of Langerin on the vascular MP in humans suggests the cells might share features with Langerhans cells, the specialized DCs that reside in the epidermis 9. In mice, maintenance of Langerhans cells depends completely on local proliferation 9 and this may also be true at least to some extent in man 9. A low level of proliferation of the vascular MP also occurs, and perhaps the extent of proliferation is sufficient to sustain them, but relatively low number of total MPs within the mouse aorta preclude finely tuned analysis in the absence of hypercholesterolemia 10. Thus, it remains unclear if their life cycle is sustained by continuous recruitment or proliferation or both. In any case, as the density of arterial MPs increases only very slowly over a lifetime without induction of hypercholesterolemia 8, the low-grade inflammatory response that the accumulation of these MPs appear to represent might alternatively be considered a “new homeostasis” in sites of hemodynamic stress.
What do these pre-plaque MPs have to do with atherosclerosis? From current literature, one can make a case that these cells promote atherosclerosis. First, the basal density that they reach is not only controlled by VCAM-1, but expression of VCAM-1 in turn appears to be controlled by activation of the inflammasome within the endothelium, through a mechanism dependent upon sterol element regulatory binding protein 211. The induction of this pathway, even in the absence of hypercholesterolemia, is strikingly similar to pathways that promote progression of atherosclerosis. Furthermore, the presence of pre-plaque MPs beneath the arterial endothelium not only predicts where plaques will form but the density of these MP varies among mouse strains in proportion to the susceptibility of that strain to atherosclerosis. That is, mice whose genetics predisposes them to atherosclerosis have a higher basal density of these cells 6. Within 1-2 weeks after hypercholesterolemia is induced in mice, via feeding LDLR−/− mice a cholesterol-enriched diet, these cells acquire neutral lipids and they proliferate rapidly in a GM-CSF-dependent manner 10. If these cells are depleted from mice prior to the onset of hypercholesterolemia, less cholesterol accumulates in the artery wall at early time points and what does arrive remains extracellular 12, suggesting that they actively participate in bringing lipid into nascent atheromata. Furthermore, atherosclerosis is attenuated in mice lacking CX3CR18, where these MPs are lacking, but the other roles of CX3CR1 in atherosclerosis including control of monocyte recruitment 13 and survival14, 15 preclude the possibility of knowing whether the loss of pre-plaque vascular MPs is a major reason for reduced atherosclerosis in CX3CR1-deficient mice.
It is unlikely that the accumulation of these cells in areas of endothelial activation along the arterial tree has evolved to promote atherosclerosis. It seems more reasonable to envision that the positioning of these MPs where they sit in the artery wall provides some benefit to the host. Their ability to pick up lipids from the bloodstream does not end there; they also acquire soluble proteins introduced as model antigens from the bloodstream 16. Furthermore, immune complexes readily accumulate in the intima of arterial sites exhibiting branches and bifurcations 17. Thus, perhaps the arterial accumulation of intimal MPs evolved to protect surfaces of the vasculature where the hemodynamics might allow for antigens, possibly even pathogens, to buildup. The presence of MP there would allow antigens or pathogens to be better detected and captured. In this scenario, the primary role of these cells is to serve as immune sentinels of the bloodstream.
Accordingly, these MP are efficient antigen-presenting cells, capable of potently activating naïve T cells with specificity against antigens they have acquired from plasma 16. In line with this function, they express some of the molecular features of DCs, rather than macrophages (Table 2). These features include expression of CD11c and C-type lectin 4A4 (CLEC4A4; also known as 33D1) in the mouse and expression of Lag-1, a Langerhans cells marker, in humans. They express little CD11b, suggesting that they are not monocytes. However, they do express high levels of CD68, which is more abundant in macrophages than DCs, though not absent, particularly at the mRNA level, in DCs 18.
Two major subsets of DCs are found in many nonlymphoid organs 19, one subset that expresses CD11b as abundantly as monocytes. These CD11b+ DC are depend upon the transcription factor interferon response factor (IRF) 4 20-23 . The other subset of DCs expresses the alpha7 integrin CD103. This subset of DCs represent efferocytic, cross-presenting DCs that developmentally depend upon the transcription factor basic leucine zipper transcription factor ATF-like 3 (BATF3) 19. CD103+ DCs have been identified in the heart and aorta using flow cytometry in WT mice without atherosclerosis 24. Currently, it remains unclear if CD103+ DCs account for the DCs that line pre-plaque sites, as research efforts to align data from in situ imaging and flow cytometry have not yet been published. Given that the pre-plaque proliferating DCs express little CD11b, it is possible that they are CD103+ DCs, but expression of 33D1 seems inconsistent with this possibility. In addition to requiring BATF3 for development, this subset of DCs shares will all other DCs dependence upon the cytokine Flt3 for development or maintenance. Based upon this dependence, it has been argued that arterial CD103+ DCs promote T regulatory cell activity and thereby maintain an anti-inflammatory state in the arterial wall 24. This interesting concept needs further development, since Flt3 has a multitude of biological actions. If CD11b− DCs that acquire cholesterol in early plaque are the same DCs as CD11b− CD103+ DCs identified in single aortic cell isolations, which seems unlikely based on 33D1 staining being normally absent in CD103+ DCs, one might argue that their acquisition of cholesterol is pro-atherogenic but that they may also suppress atherosclerosis by supporting anti-inflammatory state rich in T regulatory cells. 24
Functionally, DCs and macrophages have different roles in tissue where they have been studied. Macrophages appear to orchestrate tissue function and response to injury 25. They are more likely to quietly degrade phagocytic cargo in an attempt to maintain homeostasis, whereas DC biology favors actions that promote presentation of peptides from phagocytic cargo on major histocompatibility complex (MHC) molecules to drive T cell responses. Accordingly, DCs are triggered en masse by inflammatory stimuli to rapidly (within 24 h) emigrate from tissues to draining lymph nodes, an event that brings them to the right location for interacting with naïve T cells 26. Macrophages poorly emigrate from tissues, especially resident macrophages. For example, in the lung, resident macrophages take up the vast majority of foreign matter that enters the airway, but they are, on a per cell basis, at least 100 times less efficient than DCs in emigrating to lymph nodes 27. Monocytes newly recruited to tissues, however, are more capable of trafficking to lymph nodes 28, though they still are poor compared to DCs, being outnumbered by DCs 10:1 in antigen transport to LNs even under conditions when monocytes are as or more numerous than DCs in the organ 28 . Furthermore, molecules involved in handling cholesterol, such as ATP Binding Cassette (ABC) A1 and ABCG1, are also differentially expressed by macrophages and DCs; high levels of ABCG1 characterize lymph node-homing DCs (http://www.immgen.org), but ABCA1 is preferentially restricted to macrophages 18. If these MPs are DCs, then it must be said that DCs are instrumental in the earliest stages of atherosclerosis, arriving even earlier than the canonical macrophage foam cell.
Overall, more characterization of pre-plaque MPs is needed, including whether they are derived from monocytes, are derived from dedicated DC precursors from the bone marrow, or derive from embryonic MPs 19. Defining the developmental aspect of their life cycle is important, since doing so would be expected to provide new insight into function and ultimately better manipulation of the cell, knowledge that might be fundamental for defining new ways to manipulate atherosclerotic plaque. Questions of whether and where DCs act as contributors to atherosclerosis cannot be adequately addressed until we have a better understanding of the role that the adaptive immune response plays in atherosclerosis. While cytokines including IFNγ and IL-17 produced by T lymphocytes appear to contribute to atherosclerosis 29, whether their production is exclusively derived from T lymphocytes and whether they are secreted downstream of an antigen-driven response remains unclear. T regulatory cells, for example, have a pivotal role in atherosclerosis 30, but recent data suggest that they do so by primarily modulating lipoprotein profiles 31. Indeed, the overall impact of T cell deficiency on atherosclerosis is ultimately minor 32 and cannot always be separated from effects on plasma cholesterol, as lower cholesterol was observed in atherogenic backgrounds lacking lymphocytes under conditions when disease was reduced 33.
PLAQUE PROGRESSION
Monocyte subsets and recruitment to plaques
In the LDLR−/− model of experimental atherosclerosis, the growth of atherosclerotic plaque beyond the sentinel, subendothelial MPs can be observed within two weeks after introducing cholesterol in the diet. It is at this point that there is a robust influx of monocytes into plaques that trigger fatty streak formation and drive plaque progression. The earliest studies that implicated the monocyte as the critical source of foam cells used crude tools for identifying them 34. In the last decade, better characterization has emerged, with mouse models leading the way. Strategies based on negative or positive gating in flow cytometry to distinguish monocytes, macrophages, and DCs have been developed 28 We have favored the identification of monocytes in the mouse circulation using staining for CD115, the receptor for colony stimulating factor 1 (CSF-1), sufficient to identify monocytes on its own or when stained in combination with F4/80 13, 35. Use of this strategy along with staining for Ly-6C (sometimes called Gr-1 due to an “anti-Gr-1” mAb that recognizes a common epitope on Ly-6C and Ly-6G)36 allows separation of monocytes into two subsets, similar to identification of monocytes in CX3CR1gfp/+ mice 35-37. The Ly-6C+ subset is especially significant because it expresses CCR2 and shows an overall gene expression profile that closely matches the majority of human blood monocytes 38. When this subset is isolated to purity, labeled with radioactive indium, and injected into mice, it becomes apparent that these monocytes are recruited to atherosclerotic plaques, with recruitment proportional to disease burden. As an alternative approach, we developed a technique to label this subset of monocytes with fluorescent polystyrene particles13. In agreement with the adoptive transfer technique, the method shows that this subset of monocytes is highly recruited to atherosclerotic plaques and hypercholesterolemia increases its recruitment to plaques 13.
Blood monocytes in all species are heterogeneous 39. In the mouse, the Ly-6C− subset, a derivative of Ly-6C+ monocytes that differentiate in the bloodstream, accounts for about half of blood monocytes. It possesses a gene expression profile that strongly overlaps with CD16+ human monocytes, including expression of CD16 itself 38. However, its human counterpart represents the vast minority of blood monocytes, typically not more than 5-10% of total circulating monocytes. The Ly-6C− monocyte subset is recruited into atherosclerotic plaques, but to a lesser extent than Ly-6C+ monocytes 13, 40, and its signature biological role has been mysterious for years. Recent data indicate that its lifecycle is predominantly spent within the vasculature itself. It makes intimate contact with the apical surface of the endothelial interface in vivo, where it is appropriately positioned to react to endothelial damage and preserve vascular function 41. Accordingly, mice lacking this subset due to a deficiency in the transcription factor Nr4a1 that regulates its development/maintenance have more severe atherosclerosis 42, 43, fitting with a vascular protective role, although vascular status was not monitored in these studies. The Nr4a1−/−mouse has other defects including changes in macrophage phenotype unrelated to loss of Ly-6C− monocytes. Furthermore, other laboratories have been unable to confirm these results44 . Nonetheless, the concept that Ly-6C-monocytes promote vascular stability and thereby serve an atheroprotective role is a working model for the field at the present time.
Though similar to human CD16+ monocytes, a few differences between the mouse and human CD16+ monocyte counterparts are worth mentioning. It remains unclear whether human CD16+ monocytes are atheroprotective. I have already mentioned the relative difference in proportion of these cells in humans compared with mice. Would an increase in these cells provide an increased measure of vascular stability? For years, the argument has been put forward that the tendency of CD16+ human monocytes to increase in inflammatory disease might implicate a role in disease 39. Instead, this change might be a protective response that defends the host during vascular stress. In the mouse, however, this subset is also equipped with genes that reveal its role as a sensor and responder to cholesterol, through expression of genes including CD36, Peroxisome Proliferating-Activated Protein (PPAR) γ, ABCA1 38. This expression profile is not obviously recapitulated in CD16+ human monocytes, raising the possibility that the role of this monocyte subset in cardiovascular disease differs between the two species. However, likely core roles are similar. Human CD16+ monocytes in culture will, even after traversing endothelium, return to the apical endothelial surface, seemingly displaying a preference for this position along even cultured endothelium 45.
Monocytes are well known to differentiate to macrophages and the prevailing paradigm is that monocytes become macrophages in atherosclerotic plaques. Potential modifiers of this paradigm, including the proliferation of macrophages, will be discussed below. How can one distinguish the newly recruited monocytes in plaque from macrophages, and both of these from DCs? In many tissues of mice, a strategy that we recently identified from a large-scale expression profiling analysis seems useful. Mer tyrosine kinase (MERTK) and the high-affinity Fc gamma receptor CD64 are among a handful of genes that distinguish macrophages from DCs 18. CD64 is also expressed on monocytes 38.Thus, it may be suitable to use these new markers in flow cytometric evaluations 28 of aortic cells to aid in distinguishing monocytes, macrophages, and DCs.18
Size of the blood monocyte pool correlates with atherosclerosis: mechanisms of its regulation
A larger pool of monocytes in the circulation has emerged as an independent risk factor for coronary artery disease in humans 46, 47, in concert with data indicating that monocytosis characterizes atherosclerosis in mice and is proportional to hypercholesterolemia 13, 40. Thus, control of the circulating monocyte pool could provide means to affect the rate of disease advance. The mechanism behind hypercholesterolemia as a driver for monocytosis involves regulation of stem cell cycling by cholesterol in the membrane 48 (Table 3). Molecules that support cholesterol efflux, including ABC transporters ABCA1 and ABCG1, and HDL as an acceptor of cholesterol exported by these molecules, suppress proliferation of hematopoietic progenitors that ultimately account for the marked myelopoiesis that characterizes hypercholesterolemic mice. Control of proliferation is cell autonomous, and endogenous production of apoE is a key regulator 49 (Table 3). Clinical evidence arising from patients with low HDL or hypercholesterolemia upholds the relevance of this paradigm to man 50, 51.
Table 3.
Conventional and novel roles of apolipoprotein E.
| Function | Relevant Organ or Cells Expressing apoE | References |
|---|---|---|
| VLDL clearance from plasma | liver | Reviewed in 52 |
| Maintains homeostasis of HDL | liver | Reviewed in 52, 141 |
| Regulates cholesterol distribution in signaling domains of proliferating hematopoietic stem cells | Myeloid stem cells | 49 |
| Macrophage phenotypic polarization? | macrophages | 55 |
The cell autonomous role of apoE in regulating the size of the monocyte pool represents a novel mechanism by which apoE confers an atheroprotective state (Table 3). We have long recognized that the absence of apoE on chylomicrons affects their clearance and promotes accumulation of VLDL in apoE−/− mice. Now it is clear that apoE has other critical roles. In its absence, apoA1 associates not with HDL but with VLDL, causing HDL to become less effective at promoting cholesterol efflux 52. Macrophage-derived apoE is sufficient to correct these changes in lipid profiles, indicating that macrophages can be a pivotal source of apoE. It is also thought that macrophage phenotype, particularly shifts between canonical “M1 macrophages” and “M2 macrophages” 53, 54, is regulated by apoE, so that lesions accumulate more atheroprotective M2 macrophages 55. In our view, this claim needs deeper analysis to be substantiated. On the other hand, the role of apoE in controlling monocyte pool size is quite compelling.
Several other triggers contribute to high levels of circulating monocytes that in turn predispose to aggravated atherosclerosis, presumably through making more monocytes available for recruitment into plaques. These triggers include hyperglycemia and stimulation of the sympathetic nervous system that controls release of hematopoietic progenitors from the bone marrow 56. By establishing an unwanted vicious cycle, stimulation of the sympathetic nervous system and the monocytosis that it induces explains a worsening of atherosclerosis following myocardial infarction 56. Such worsening could contribute to the increased risk of a second cardiovascular event after a first event.
While some studies of experimental atherosclerosis have shown that the density of monocytes in the blood correlates with the extent of monocyte infiltration into plaques 13, 40, 57 and overall macrophage burden 58, it remains an assumption that the reason why monocytosis is clinically associated with increased cardiovascular disease is due to more monocytes available in the blood to gain access to plaques. There may be an array of other actions carried out by monocytes that impact cardiovascular disease, or monocytosis may simply correlate with other changes that more directly affect atherosclerosis.
Origin of foam cells
We highlight the possibility that monocytosis is not directly related to recruitment of monocytes to atherosclerotic plaques because of the surprising findings of a recent study that indicated plaque macrophages are mainly sustained in advanced experimental disease by local proliferation rather than recruitment of new monocytes on an ongoing basis 59, a study more extensively discussed elsewhere 60. These new data, taken at face value, appear to de-emphasize the importance of monocyte recruitment in being essential to drive progression of atherosclerosis. On the other hand, although newly recruited monocytes appeared to make little contribution to foam cells in established atherosclerotic plaques, they may be continuously recruited and die quickly, while at the same time contributing important signals that drive proliferation of adjacent macrophages 60.
Besides proliferation, there are other possible mechanisms in atherosclerosis that may contribute to the generation of foam cells in plaques independently of monocyte recruitment. First, like pre-lesional sites, some of the MPs in advancing atherosclerotic plaques may be DCs and therefore not derived from monocytes, and these DCs may accumulate lipids to become foam cells 12. However, distinguishing plaque macrophages from DCs is more challenging than it is in some other tissues. In the mouse, standardized criteria are lacking and different laboratories therefore utilize non-standardized strategies for identifying DCs 12, 16, 24, 61, 62. CD11c is also expressed by some monocytes in models of atherosclerosis 63, confusing the issue of which cells are DCs.. Future studies are needed to better distinguish macrophages from DCs not just in early disease but in established disease as well. A specialized type of DC, the plasmacytoid DC (PDC), is known for its potent production of type I interferon. Several papers indicate that it plays that role in atherosclerosis, though whether these cells promote disease or suppress it is unsettled, with currently literature currently coming to disparate conclusions 64, 65. They have not been found in the intima of murine arteries prior to or during atherosclerosis.64, 65. Whatever their role, PDCs likely function outside of the plaque environment when they modulate disease.
An additional mechanism for the generation of foam cells in plaque unrelated to monocyte recruitment is the conversion of smooth muscle cells (SMCs) to foam cells that bear canonical markers of macrophages, such as CD68 66. Again, more work is needed to determine whether and how significantly this mechanism of SMC-to-macrophage transition affects atherosclerosis progression. The issue receives less attention experimentally than it may deserve, considering that SMCs often function to stabilize plaques and guard against rupture. The origination of just a part of the plaque macrophage pool from SMCs is significant not only because it could build up macrophage numbers that can promote plaque rupture but also because it simultaneously reduces the number of cells that protect plaque. That is, the potential of this differentiation mechanism to affect atherosclerosis is seen more clearly when one considers the macrophage: SMC ratio rather than the fraction of macrophages derived from SMCs, because both the numerator and the denominator of the ratio are altered in opposing directions by the event of a SMC differentiating to a macrophage.
Links between cholesterol and macrophage accumulation in plaques
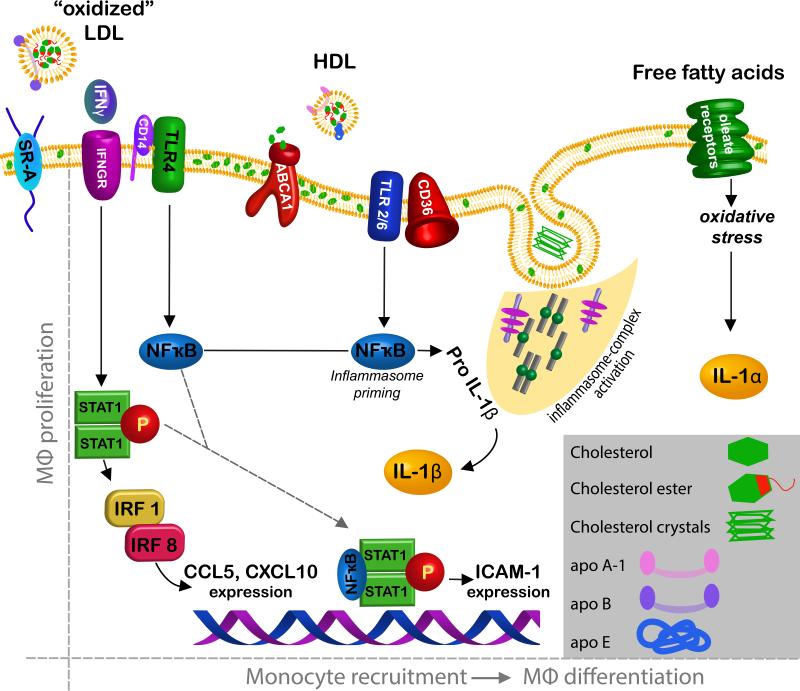
Macrophages are especially efficient at taking up and storing cholesterol in esterified form within the cytoplasm. Although it was long thought that the accumulation of cholesterol esters in macrophages would itself be a pro-inflammatory event, accumulation of cholesterol in peritoneal macrophages of LDLR−/− mice reveals that cholesterol accumulation is anti-inflammatory through the activation of LXR that in turn stems from the robust accumulation of desmosterol in the cholesterol-loaded cells 67. Thus, it is clear that cholesterol loading of macrophages is not inherently inflammatory. This observation fits with evidence that uptake of cholesterol through individual scavengers receptors does not greatly modify the rate of atherosclerosis development 68. However, there are a number of mechanisms that link cholesterol with increased macrophage activation and accumulation in atherosclerosis (Fig. 1).
Fig. 1.
Proposed signalling pathways in macrophages that link cholesterol to their accumulation in atherosclerotic plaques. Two major drivers of increased macrophage accumulation in plaques are proliferation (left) and recruitment (right). The quantitative importance of macrophage proliferation in overall macrophage accumulation has only recently been appreciated so remains understudied. So far, only signaling from SR-A has been associated with proliferation. Signals proposed to regulate macrophage accumulation are many (right) and include binding of oxidized LDL to TLRs that in turn activate NFκB. Transporters like ABCA1 at the plasma membrane may control the assembly of signaling molecules through regulation of membrane cholesterol. The inflammasome, coupled with TLR activation, generates IL-1β and free fatty acid signaling triggers IL-1α production in an inflammasome-independent manner. Signalling through IFNγ receptor activates STAT-1 that in turn influences the expression of genes including chemokines like CXLC10 and CCL5 and adhesion molecules like ICAM-1. IL-1α and IL-1β are classic proinflammatory cytokines that target the endothelium to promote, for instance, recruitment of monocytes.
First, when macrophages are unable to appropriately efflux cholesterol, loading with cholesterol is proinflammatory and proatherogenic. The selective deletion of ABC-type cholesterol transporters ABCA1 and ABCG1 from macrophages that express Cre downstream of Lyz2 promoter activation (Lyz2-Cre x ABCA1fl/fl × ABCG1fl/fl) leads to monocytosis, activated macrophages in plaques, and increased atherosclerosis 69. The Lyz2 promoter (sometimes called Lys-M) is expressed in nearly all macrophages, and also in about half of monocytes and DCs 70, as well as in about 5% of stem cells 71.
Second, some toll-like receptors (TLRs) are thought to be bound and triggered by oxidized lipoprotein. TLR2 and TLR6 act as coreceptors along with CD36 for oxidized LDL 72, and TLR engagement efficiently and robustly induces macrophage activation73. The role of TLR2 in atherosclerosis is most likely due to TLR2 activation in endothelium, rather than bone marrow-derived cells, such as macrophages 74. However, deficiency in TLR4 also modestly reduces atherosclerosis75. When the TLR adaptors TRIF or TRAM are absent from donor bone marrow cells used to reconstitute LDLR−/− mice, atherosclerosis is reduced 76, suggesting that TLR signaling in the hematopoietic compartment contributes to atherosclerosis. Furthermore, other receptors including CD36, CD14, and C-reactive protein (CRP) may bind to oxidized phospholipids to mediate pro-inflammatory roles in the absence of TLR signalling 77, supporting the concept that oxidized phospholipids sustain atherosclerosis.
Activated TLRs ultimately trigger the transcription factor NFκB, in turn associated with the transcription of proand anti-inflammatory genes. The role of NFκB in atherosclerosis is complex, because depending on how it is inactivated, different results are obtained 78, 79. Specifically, macrophage selective deletion of IκB kinase 2 (IKK2) to prevent NFκB activation surprisingly does not attenuate atherosclerosis. Instead, it makes it worse, apparently because the loss of anti-inflammatory cytokines like IL-10 supersedes the loss of proinflammatory cytokines 78. However, when the NFκB subunit p50 is missing in all hematopoietic cells of LDLR−/− mice, a condition generated through bone marrow transplant, atherosclerosis is attenuated 79. In this scenario, it may not be macrophage NFκB that drives atherogenesis and progression, since other types of leukocytes would also lack NFκB in the experimental design. Moreover, an often overlooked issue associated with the study of mice lacking the p50 NFκB subunit is that ERK signalling is impaired. That is, the p50-deficient mouse lacks the precursor for p50, p105, and p105 is necessary for the stability of the mitogen-activated protein kinase ERK1/ERK2 80. Interestingly, persistent activation of NFkB is not sufficient to drive chronic, tissue destructive inflammation in the intestine. Additional triggering of MAPKs is necessary 81. Furthermore, ERK signaling has been reported to be integral to STAT1 signaling downstream of IFNγ 82, a cytokine that serves as a critical driver for macrophage activation in general and for atherosclerosis development and progression 83. Thus, it is possible that the apparent role for NFκB in atherosclerosis is not due to its role in macrophages or is more related to a role for ERK signaling during STAT1 activation 82. Alternatively, phospho-STAT1 and NFκB can cooperate to regulate expression of genes like ICAM-1 and iNOS 82 (Figure 1). However, STAT1 deficiency alone appears to suppress macrophage death in plaques but not plaque progression 84. Thus, while it is highly likely that NFkB contributes importantly to driving atherosclerosis, it is unclear if its activation is sufficient or whether expression within macrophages is the key driver.
A third, but still related, concept related to macrophage pro-inflammatory action in atherosclerosis emerged from considering the potential role of small crystals that form from free cholesterol 85. Activation of the NLRP3 inflammasome generates active IL-1β or IL-18, central pro-inflammatory mediators that have been linked to atherosclerosis and many other inflammatory diseases, through cleavage of pro-IL1 or pro-IL18 by caspase 1/11 86. The NLRP3 inflammasome is a sensor for various crystals such as those in gout, as well as cholesterol crystals 85, 87, and in turn activates caspase-1 or caspase 11 86. NLRP3 activation cannot generate active IL-1β/IL-18 if its substrates proIL-1β /IL-18 have not been induced. Thus, the generation of the active forms of these cytokines requires two steps. The first step is called “priming” and mechanistically requires the induction of mRNA and translated pro-IL1 through signals such as those that might include but not be limited to signals from TLR ligands or pro-inflammatory oxidized phospholipids discussed above. The second step is the activation of the inflammasome by a ligand, often a crystal, small enough to be engulfed by macrophages, which leads to activation of caspase 1. In addition to being activated by crystals, inflammasome activation can be triggered by extracellular ATP 85, a signal that increases in an environment of necrotic cells. Thus, as both cholesterol crystal and necrotic cells are prominent features within atherosclerotic plaques, the inflammasome is extremely relevant to the regulation of inflammation in plaque. Not all studies have been able to replicate the finding that the NLRP3 inflammasome mediates atherosclerosis 88, and still others identify alternative mechanisms for NLRP3 as a mediator of atherosclerosis, including its induction in endothelium by hemodynamic changes 11. Even if it does, the abundance of cholesterol crystals in early macrophages is startling. One wonders if the tiny crystals might not be so dense in vivo as originally reported but instead were triggered by a drop in temperature resulting from chilling of the atherosclerotic tissue during preparation and its examination at room temperature. Indeed, the role of IL-1β in atherosclerosis has been recently questioned in a study that suggested that the reduced ability of IL-1β -deficient mice to secrete IL-1α accounts for the atheroprotection it affords 89. IL-1β, on the other hand, drove inflammation in atherosclerosis and was induced by fatty acid-driven mitochondrial uncoupling and ensuing oxidative stress 89 (Fig. 1).
Finally, an exciting but relatively unexplored potential connection between cholesterol and leukocyte recruitment is the identification of oxysterols 7α,25-hydroxycholesterol and 7α,27-hydroxycholesterol as ligands for the G protein coupled receptor gpr183 (Epstein Barr Virus Induced 2, EBI2) 90, 91 . These ligands promote chemotaxis of B lymphocytes and DCs92-96. The mRNA for receptor is also expressed on Ly-6C+ monocytes (http://www.immgen.org). No data have been published on whether this chemotactic form of cholesterol affects atherosclerosis.
The literature discussed above illustrates that multiple mechanisms may operate in linking cholesterol to an inflammatory stimulus that drives macrophage accumulation (Fig. 1). Do the mechanisms work together in a synergistic manner or does each contribute independently to atherosclerosis progression and be more or less relevant under different circumstances? Integrating these mechanisms and determining their direct relevance to monocyte recruitment or macrophage proliferation is a challenge for the future. However, in many ways, the mechanisms proposed can already be integrated and appear to represent different aspects of the same inflammatory pathway. TLR engagement by oxidized LDL is one mechanism of priming the inflammasome, generating pro-IL1β or pro-IL-18, and crystals of cholesterol might carry out the second step of giving rise to the active form of these cytokines. Interestingly, the failure to efflux cholesterol heightens signaling through TLRs, possibly by altering signaling domains that complex with cholesterol in the membrane 69, 97, 98. Thus, as Fig. 1 shows, the mechanisms that generate pro-inflammatory intermediates like IL-1β or IL-1α that in turn act on the endothelium to promote recruitment of monocytes might involve several integrated steps ranging from control of cholesterol efflux to responses normally associated with infection. Together, these pathways particularly regulate plaque macrophage burden as it relates to macrophage recruitment. It remains unclear how these pathways affect macrophage proliferation in plaques, as only SR-A signaling has so far been associated with this apparently critical mechanism for regulating macrophage burden in atherosclerotic plaque (Fig. 1).
The macrophage balance sheet: Ongoing loss of macrophages from atherosclerotic lesions
Beyond events that control the ongoing increase of macrophage numbers in plaques, from recruitment of monocytes to proliferation of macrophages, alteration of basic cellular mechanisms that normally control macrophage removal could affect overall macrophage burden. In the immune system, major pathways of cell removal are death and migratory emigration to other anatomical sites. Among MPs, DCs are best known to clear from organs by emigrating to draining lymph nodes upon activation 99. At least hypothetically, transdifferentiation of macrophages could also effectively remove them, such as differentiation of macrophages to SMCs, but such a mechanism has not been documented. Thus, we are left to consider the role of death and emigration as modifiers of macrophage burden. Emigration will be discussed more extensively in the section on disease reversal below. However, the literature is consistent in concluding that macrophages are not prone to emigrate out of plaques during progression of atherosclerosis and thus emigration does not appear to be a major modifier of overall macrophage burden as plaques enlarge.
By contrast, cell death is a well-documented means of removing cells from organs during development and during disease. Death of macrophages in atherosclerosis is made more complex by the fact that clearance of apoptotic cells, by other macrophages that play a key role in efferocytosis, is impaired 100. Indeed, failed clearance of dying cells promotes inflammation and even autoimmunity in a variety of contexts 101. In atherosclerosis, death of macrophages has dual consequences depending on the disease stage. At early stages, when efferocytosis is not yet blocked, failure of macrophages to die efficiently leads to larger plaques and increased macrophage burden, indicating that apoptosis of macrophages is a key determinant in regulating the size of the macrophage pool. However, at later stages of disease, particularly when efferocytosis is no longer efficient, reduced macrophage death seems to reduce disease progression, likely because death in the absence of clearance builds necrotic core and promotes inflammation 102. On the other hand, treatments that are systemically effective at inducing macrophage apoptosis lead to a loss of the liver macrophage (Kupffer cell) pool and increase plasma cholesterol, erasing the anticipated positive impact on inducing early plaque macrophage death locally 103 .
Thus, there are multiple mechanisms to generate and sustain foam cells in atherosclerotic plaque, including recruitment of monocytes, recruitment of DCs or DC precursors that may generate foam cells as well, proliferation of lesional macrophages, local death of macrophages, signals for sessility in macrophages that prevents their physical emigration from plaques, and possible generation of macrophages through transdifferentiation of SMCs.
PLAQUE REGRESSION AND MACROPHAGE REMOVAL FROM PLAQUES
A significant fraction of the human population is affected by atherosclerosis that is established. These individuals face the challenge of reducing their risk for developing clinically significant disease. An attractive goal in therapy would be to reverse disease burden in patients at risk. Since macrophages are key components in the plaque and are associated with plaque rupture, removal of macrophages from plaques is a logical approach in generating stable plaque, even if plaque regression defined as a reduction in plaque area is not achievable. The question is: How can this be achieved? In principle, all of the cellular mechanisms listed above that control ongoing increases in macrophages may participate in disease reversal.
The concept that emigration of macrophages might be relevant to their removal from plaques was introduced by Ross Gerrity in the early 1980s 104. He captured electron micrographs of foam cells appearing to pass through the transendothelial junction of arterial endothelium. The images were static but suggestive of migratory egress from plaques because he reasonably argued that incoming monocytes, which might also be identified in the act of transendothelial migration, would not arrive already so loaded with lipid droplets as the MPs he imaged obviously were. Alternatives to his interpretation might include the possibility that the cells he imaged were not necessarily emigrating out but instead simply extending pseudopods into the arterial lumen, a process that has now been documented in the artery wall 16 and elsewhere 105, 106.
We went on to propose and pursue the idea of emigration as a key modifier of macrophage burden in plaques when we reported evidence that MPs emigrated from transplanted arteries during disease reversal conditions but not in disease progression conditions 107. We also observed that DCs from skin of atherogenic mice have strikingly reduced ability to emigrate to draining lymph nodes 108. Underlying our belief that emigration of macrophages from plaques might be very relevant to their clearance was not only Ross Gerrity's work. We were also struck by a report that concluded that macrophage clearance during resolution of inflammation induced in the peritoneum by injection of thioglycollate required that macrophages emigrate to draining lymph nodes 109. However, we have recently repeated this model using new, more quantitative techniques and we found that the mode of clearance is far more reliant on local death than on emigration to lymph nodes 110, leading us to conclude that macrophage emigration may not be a key feature of resolution after all.
Although there is agreement that macrophages are not prone to emigrate out of plaques during progression of atherosclerosis, the literature is less unified when it comes to examination of lesion regression. Besides our recent work in the acute inflammatory model of thioglycollate-induced peritonitis where we concluded macrophage removal was more dependent upon local death than emigration, we have found no evidence for robust emigration from plaques in models of atherosclerosis regression other than the surgical model previously mentioned. Specifically, we developed a method, conceptually detailed elsewhere 110, that can monitor phagocyte trafficking out of plaques using non-biodegradable particles, where the loss of non-biodegradable particles from plaques during regression would signal emigration from plaques and provide information on its kinetics and magnitude. It is critical that the tracer particles are not biodegradable, so that events like cell death and protein turnover do not influence their presence in the plaque. The method is useful to track monocyte entrance into plaques as well as their emigration and it has been used by multiple groups interested to chart the emigratory behavior of macrophages from plaques. We have used the method in a model of atherosclerosis regression where apoE−/− mice were treated with apoE vectors, monitoring monocyte entrance and macrophage egress from atherosclerotic plaques at the aortic root and aortic sinus. Neither anatomic site showed evidence of egress marked by a loss of the bead-based phagocytic tracer in the plaque, but reduction of macrophages in plaques was instead preceded by a profound reduction in monocyte recruitment to plaques. Thus, we concluded that macrophage egress is not essential for contraction of macrophages from plaques and that such contraction more closely tracks with cessation in monocyte recruitment. Blockade in new monocyte entry could only remove macrophages if there was some ongoing death of macrophages, so that dying macrophages were not replaced by newly arriving cells. Apoptotic macrophages were not obviously increased in this model of regression, but nonetheless occurred. A similar explanation for reduction in macrophage content in apoE hypomorphic mice induced to express apoE was recently found by Raffai et al. 58.
Other studies, however, have used the same bead-tracking technique to identify evidence of macrophage egress from plaques. The conditions associated with a positive detection of macrophage emigration from plaques include examination of the aortic arch following transplant into recipient mice 111,112, studies in the aortic arch of LDLR−/−mice lacking netrin 113, LDLR−/− mice switched from a cholesterol-enriched diet that promotes atherosclerosis to a standard chow diet that allows for regression , or studies in the aortic sinus of apoE−/− mice that were treated with statins to promote macrophage contraction from plaques that remained stable in overall area 114. In addition to apparently promoting egress, statins reduce monocyte recruitment and reign in monocytosis in apoE−/− mice 40. In LDLR−/− mice transitioned to a low cholesterol diet from a high cholesterol diet, monocyte recruitment is also reduced 57.This reduction fails during hyperglycemia, whereas egress continues, so plaque macrophage content more closely mirrored the changes in monocyte recruitment than egress.
The discrepancy over whether macrophages do or do not exit plaques during disease regression needs clarifying through additional research. First, these pathways comprise natural therapeutic targets, a way to reduce disease burden and risk in individuals already affected by atherosclerotic disease. Gaining clarity on the most relevant pathways for removing macrophages will greatly facilitate moving toward rational therapeutics.
Second, analysis of the role of molecular mediators impacting atherosclerosis will naturally be interpreted in light of what is known about cellular events in the disease. In atherosclerosis, it is often easier to study the impact of genetic deficiency by asking how deficiency in a molecule affects atherosclerosis than it is to follow the related cellular events. For example, several studies point to a role for the G-protein coupled receptor, CCR7, in modifying atherosclerosis. One indicates that loss of CCR7 in apoE−/− mice exacerbates disease 115. When we crossed apoE−/− mice with CCR7−/− mice, no significant change in lesion area was noted but there was a trend toward disease protection 116, seemingly akin to LDLR−/−CCR7−/− mice that were protected from atherosclerosis in another study 117. CCR7 has critical roles in T cell trafficking and responses, and there is direct evidence from competition studies that its expression on T cells (or not) modifies T cell trafficking 117. However, other literature on CCR7 and atherosclerosis has focused on its possible role in mediating macrophage egress from plaques 111, 112, 114, 118. The major evidence is often CCR7 mRNA with regression, but the cells expressing CCR7 remain uncertain—are they macrophages, DCs, T cells, or a mixture of all? Some studies find no correlations or role for CCR7 in bringing about macrophage contraction 58, 116, 119. New methodology, including new strains of mice recently developed120, that could distinguish CCR7 on T cells from other immune cells would be helpful in clarifying the confusing role of CCR7 in atherosclerosis and its regression.
Finally, settling the issue of whether macrophage do or do not have the capacity to leave atherosclerotic plaques impacts our understanding of macrophage biology beyond the realm of atherosclerosis. Given the consensus that macrophages do not leave plaques during disease progression, the unanswered question is whether the inability of macrophages to leave plaques typifies macrophage behavior in general or is a pathologic feature that facilitates the chronic inflammation that characterizes atherosclerosis. Our re-analysis of the role of emigration to lymph nodes in a simple, acute inflammatory model to try to get better insight into this fundamental question leaves us skeptical of the migration paradigm for macrophage removal 110. So, what other information exists in the literature? Claims of macrophage migration have been made for decades, but early work was fraught with the inability to distinguish macrophages from monocytes and DCs so it is not completely clear whether macrophages in tissues, beyond a small minority, are truly able to mobilize from tissues under any conditions. Surprisingly little recent literature directly speaks to this issue. However, intravital imaging of macrophages in murine liver granulomas concludes that over a period of days to weeks, net macrophage displacement is minimal 121. Monocytes newly recruited to tissues, on the other hand, appear to be quite motile, exhibiting the capacity to pass into a tissue and out through its lymphatic vasculature without even differentiating to a macrophage28 . Thus, at present, we currently favor the concept that monocytes and DCs, but not macrophages, are motile cells with the capacity to emigrate out of tissues. If MPs can emigrate from atherosclerotic plaques, we predict that the cells doing so are either DCs or monocytes that have yet to differentiate to bona fide macrophages, but that macrophages do not egress.
From the data indicating retention of macrophages not just in plaques but also other organs 110, 121, one wonders why are macrophages relatively confined to a territory within a tissue in contrast to DCs or monocytes. The answer is not yet obvious. The possible answers range from inherently different organization and signaling within the cytoskeleton, failure of macrophages to express key receptors like CCR7 122 that promote motility in response to chemotactic cues, or ubiquitous retention signals for macrophages within the matrix of tissues. I favor the first possibility, since there are at least rare instances when emigration to lymph nodes is independent of CCR7 110, but it is quite possible that tissues actively retain macrophages through signals like those provided by the neural guidance cue receptor netrin-1 in atherosclerotic plaque 113. It would be quite relevant, as a next step, to determine if macrophage density in a range of organs is reduced in the netrin-deficient mouse under homeostatic conditions or if retention of macrophages in other inflammatory reactions is altered. From this vantage point, might netrin or other signaling pathways capable of retaining macrophages in tissues be a normal, rather than pathological process?
FRONTIERS AND FUTURE DIRECTIONS
Huge progress has been made in the last several years in studying the cellular and molecular mechanisms that control macrophage burden in atherosclerotic plaques. While debate is active and ongoing on some of the basic mechanisms involved, such as the relative role of proliferation versus monocyte recruitment in generating macrophages and the relative roles of egress versus cessation of proliferation or recruitment as an approach to reduce macrophages during disease regression, the rapid pace of research in this area and the new tools under development to address them bodes well for soon solving these mysteries. Understanding the mechanisms that regulate macrophage burden will then allow the field to turn to other critical questions such as how is the control of macrophage burden in plaques intermingled with the role of the adaptive arm of immunity in atherosclerosis. This is a subject that only a few studies have begun to address 62. Likely, all regulators of atherosclerosis including adaptive immune effectors somehow impact macrophage burden in plaques, but currently the connections are largely unexplored. We anxiously work and wait to witness what the next decade of research will reveal.
ACKNOWLEDGEMENTS
I am grateful to Drs. Emmanuel Gautier and Jesse Williams for discussion and critical reading of the manuscript. I am also very grateful to Dr. Bernd H. Zinselmeyer for expert preparation of the illustration shown in Figure 1.
SOURCES OF FUNDING
Funding for the author includes NIH grants R01 AI049653 and HL096539.
List of Nonstandard Abbreviations and Acronyms
- ABC
ATP Binding Cassette
- BATF3
Basic leucine zipper transcription factor ATF-like 3
- CSF-1
Colony stimulating factor 1
- CLEC
C-type lectin
- DCs
Dendritic cells
- EBI2
Epstein Barr Virus Induced 2
- HDL
High density lipoprotein
- IRF
Interferon response factor
- IL
Interleukin
- MAPK
Mitogen-activated protein kinase
- MHC
Major histocompatibility complex
- MERTK
Mer tyrosine kinase
- MCP-1, CCL2
Monocyte chemoattractant protein 1
- MPs
Mononuclear phagocytes
- PDC
Peroxisome Proliferator-Activated Receptor, PPAR Plasmacytoid dendritic cells
- SMCs
Smooth muscle cells
- TLRs
Toll-like receptors
- VCAM-1
Vascular cell adhesion molecule
- VLDL
Very low density lipoprotein
Footnotes
DISCLOSURES
There are no conflicts of interest to disclose.
REFERENCES
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 4.Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, Virmani R. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 5.Glass CK, Witztum JL. Atherosclerosis: The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 6.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. The Journal of experimental medicine. 2006 doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millonig G, Niederegger H, Rabl W, Hochleitner BW, Hoefer D, Romani N, Wick G. Network of vascular-associated dendritic cells in intima of healthy young individuals. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:503–508. doi: 10.1161/01.atv.21.4.503. [DOI] [PubMed] [Google Scholar]
- 8.Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, Patterson C, Patel DD. Cx3cr1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:243–250. doi: 10.1161/ATVBAHA.107.158675. [DOI] [PubMed] [Google Scholar]
- 9.Ginhoux F, Merad M. Ontogeny and homeostasis of langerhans cells. Immunol Cell Biol. 2010;88:387–392. doi: 10.1038/icb.2010.38. [DOI] [PubMed] [Google Scholar]
- 10.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. Gm-csf regulates intimal cell proliferation in nascent atherosclerotic lesions. The Journal of experimental medicine. 2009;206:2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao H, Lu M, Lin TY, Chen Z, Chen G, Wang WC, Marin T, Shentu TP, Wen L, Gongol B, Sun W, Liang X, Chen J, Huang HD, Pedra JH, Johnson DA, Shyy JY. Sterol regulatory element binding protein 2 activation of nlrp3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128:632–642. doi: 10.1161/CIRCULATIONAHA.113.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circulation research. 2010;106:383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 13.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ ccr2, ccr5, and cx3cr1 to accumulate within atherosclerotic plaques. The Journal of clinical investigation. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. Cx3cr1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 15.Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood monocyte subsets differentially give rise to cd103+ and cd103- pulmonary dendritic cell populations. Journal of immunology. 2008;180:3019–3027. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- 16.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. The Journal of experimental medicine. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kniker WT, Cochrane CG. The localization of circulating immune complexes in experimental serum sickness. The role of vasoactive amines and hydrodynamic forces. The Journal of experimental medicine. 1968;127:119–136. doi: 10.1084/jem.127.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma'ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature immunology. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annual review of immunology. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, Yamamoto K, Suematsu T, Nakamura M, Yui K, Kumatori A. Critical roles of interferon regulatory factor 4 in cd11bhighcd8alpha-dendritic cell development. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8981–8986. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O'Shea JJ, Singh H, Ozato K. Ifn regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. Journal of immunology. 2005;174:2573–2581. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 22.Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F. Irf4 transcription factor-dependent cd11b+ dendritic cells in human and mouse control mucosal il-17 cytokine responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, Gudjonsson S, Hakansson U, Reizis B, Kotarsky K, Agace WW. Irf4 transcription-factor-dependent cd103(+)cd11b(+) dendritic cells drive mucosal t helper 17 cell differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, Steinman RM. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 26.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nature reviews. Immunology. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 27.Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. Journal of immunology. 2006;176:3578–3584. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- 28.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma'ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liuzzo G, Trotta F, Pedicino D. Interleukin-17 in atherosclerosis and cardiovascular disease: The good, the bad, and the unknown. European heart journal. 2013;34:556–559. doi: 10.1093/eurheartj/ehs399. [DOI] [PubMed] [Google Scholar]
- 30.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory t cells control the development of atherosclerosis in mice. Nature medicine. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 31.Klingenberg R, Gerdes N, Badeau RM, Gistera A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Luscher TF, Jauhiainen M, Sparwasser T, Hansson GK. Depletion of foxp3+ regulatory t cells promotes hypercholesterolemia and atherosclerosis. The Journal of clinical investigation. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dansky HM, Charlton SA, Harper MM, Smith JD. T and b lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein e-deficient mouse. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4642–4646. doi: 10.1073/pnas.94.9.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reardon CA, Blachowicz L, White T, Cabana V, Wang Y, Lukens J, Bluestone J, Getz GS. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein e-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:1011–1016. doi: 10.1161/01.atv.21.6.1011. [DOI] [PubMed] [Google Scholar]
- 34.Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. The American journal of pathology. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- 35.Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, Sanchez-Torres C, Bromberg J, Charo IF, Jung S, Lira SA, Randolph GJ. Role of ccr8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. The Journal of experimental medicine. 2004;200:1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 37.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. Journal of immunology. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 38.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziegler-Heitbrock L. The cd14+ cd16+ blood monocytes: Their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 40.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. The Journal of clinical investigation. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent ly6c(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. Nr4a1 (nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circulation research. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamers AA, Vos M, Rassam F, Marinkovic G, Kurakula K, van Gorp PJ, de Winther MP, Gijbels MJ, de Waard V, de Vries CJ. Bone marrow-specific deficiency of nuclear receptor nur77 enhances atherosclerosis. Circulation research. 2012;110:428–438. doi: 10.1161/CIRCRESAHA.111.260760. [DOI] [PubMed] [Google Scholar]
- 44.Chao LC, Soto E, Hong C, Ito A, Pei L, Chawla A, Conneely OM, Tangirala RK, Evans RM, Tontonoz P. Bone marrow nr4a expression is not a dominant factor in the development of atherosclerosis or macrophage polarization in mice. Journal of lipid research. 2013;54:806–815. doi: 10.1194/jlr.M034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The cd16(+) (fcgammariii(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. The Journal of experimental medicine. 2002;196:517–527. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chapman CM, Beilby JP, McQuillan BM, Thompson PL, Hung J. Monocyte count, but not c-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke. 2004;35:1619–1624. doi: 10.1161/01.STR.0000130857.19423.ad. [DOI] [PubMed] [Google Scholar]
- 47.Johnsen SH, Fosse E, Joakimsen O, Mathiesen EB, Stensland-Bugge E, Njolstad I, Arnesen E. Monocyte count is a predictor of novel plaque formation: A 7-year follow-up study of 2610 persons without carotid plaque at baseline the tromso study. Stroke. 2005;36:715–719. doi: 10.1161/01.STR.0000158909.07634.83. [DOI] [PubMed] [Google Scholar]
- 48.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. Atp-binding cassette transporters and hdl suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. Apoe regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. The Journal of clinical investigation. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ganda A, Magnusson M, Yvan-Charvet L, Hedblad B, Engstrom G, Ai D, Wang TJ, Gerszten RE, Melander O, Tall AR. Mild renal dysfunction and metabolites tied to low hdl cholesterol are associated with monocytosis and atherosclerosis. Circulation. 2013;127:988–996. doi: 10.1161/CIRCULATIONAHA.112.000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tolani S, Pagler TA, Murphy AJ, Bochem AE, Abramowicz S, Welch C, Nagareddy PR, Holleran S, Hovingh GK, Kuivenhoven JA, Tall AR. Hypercholesterolemia and reduced hdl-c promote hematopoietic stem cell proliferation and monocytosis: Studies in mice and fh children. Atherosclerosis. 2013;229:79–85. doi: 10.1016/j.atherosclerosis.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raffai RL. Apolipoprotein e regulation of myeloid cell plasticity in atherosclerosis. Current opinion in lipidology. 2012;23:471–478. doi: 10.1097/MOL.0b013e328356f967. [DOI] [PubMed] [Google Scholar]
- 53.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: A dynamic balance. Nature reviews. Immunology. 2013 doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA, Tak PP, Baeten DL. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. Journal of immunological methods. 2012;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Baitsch D, Bock HH, Engel T, Telgmann R, Muller-Tidow C, Varga G, Bot M, Herz J, Robenek H, von Eckardstein A, Nofer JR. Apolipoprotein e induces antiinflammatory phenotype in macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1160–1168. doi: 10.1161/ATVBAHA.111.222745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, Goldberg IJ. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell metabolism. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eberle D, Luk FS, Kim RY, Olivas VR, Kumar N, Posada JM, Li K, Gaudreault N, Rapp JH, Raffai RL. Inducible apoe gene repair in hypomorphic apoe mice deficient in the low-density lipoprotein receptor promotes atheroma stabilization with a human-like lipoprotein profile. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1759–1767. doi: 10.1161/ATVBAHA.112.300605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, Rooijen NV, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nature medicine. 2013 doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Randolph GJ. Proliferating macrophages prevail in atherosclerosis. Nature medicine. 2013 doi: 10.1038/nm.3316. in press. [DOI] [PubMed] [Google Scholar]
- 61.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic t cell-apc interactions sustain chronic inflammation in atherosclerosis. The Journal of clinical investigation. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires myd88 signaling in dcs. The Journal of clinical investigation. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of cd11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macritchie N, Grassia G, Sabir SR, Maddaluno M, Welsh P, Sattar N, Ialenti A, Kurowska-Stolarska M, McInnes IB, Brewer JM, Garside P, Maffia P. Plasmacytoid dendritic cells play a key role in promoting atherosclerosis in apolipoprotein e-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2569–2579. doi: 10.1161/ATVBAHA.112.251314. [DOI] [PubMed] [Google Scholar]
- 65.Daissormont IT, Christ A, Temmerman L, Sampedro Millares S, Seijkens T, Manca M, Rousch M, Poggi M, Boon L, van der Loos C, Daemen M, Lutgens E, Halvorsen B, Aukrust P, Janssen E, Biessen EA. Plasmacytoid dendritic cells protect against atherosclerosis by tuning t-cell proliferation and activity. Circulation research. 2011;109:1387–1395. doi: 10.1161/CIRCRESAHA.111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovascular research. 2012;95:156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, Raetz CR, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AH, Jr., Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, Glass CK. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor a or cd36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. The Journal of clinical investigation. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch CL, Fisher EA, Wang N, Yvan-Charvet L, Tall AR. Deficiency of abca1 and abcg1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circulation research. 2013 doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. The Journal of experimental medicine. 2008;205:2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye M, Iwasaki H, Laiosa CV, Stadtfeld M, Xie H, Heck S, Clausen B, Akashi K, Graf T. Hematopoietic stem cells expressing the myeloid lysozyme gene retain long-term, multilineage repopulation potential. Immunity. 2003;19:689–699. doi: 10.1016/s1074-7613(03)00299-1. [DOI] [PubMed] [Google Scholar]
- 72.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. Cd36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nature immunology. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pasare C, Medzhitov R. Toll-like receptors: Linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 74.Curtiss LK, Tobias PS. Emerging role of toll-like receptors in atherosclerosis. Journal of lipid research. 2009;50(Suppl):S340–345. doi: 10.1194/jlr.R800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein e. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lundberg AM, Ketelhuth DF, Johansson ME, Gerdes N, Liu S, Yamamoto M, Akira S, Hansson GK. Toll-like receptor 3 and 4 signalling through the trif and tram adaptors in haematopoietic cells promotes atherosclerosis. Cardiovascular research. 2013;99:364–373. doi: 10.1093/cvr/cvt033. [DOI] [PubMed] [Google Scholar]
- 77.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. Journal of lipid research. 2009;50(Suppl):S207–212. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanters E, Pasparakis M, Gijbels MJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJ, Clausen BE, Forster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MP. Inhibition of nf-kappab activation in macrophages increases atherosclerosis in ldl receptor-deficient mice. The Journal of clinical investigation. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanters E, Gijbels MJ, van der Made I, Vergouwe MN, Heeringa P, Kraal G, Hofker MH, de Winther MP. Hematopoietic nf-kappab1 deficiency results in small atherosclerotic lesions with an inflammatory phenotype. Blood. 2004;103:934–940. doi: 10.1182/blood-2003-05-1450. [DOI] [PubMed] [Google Scholar]
- 80.Waterfield MR, Zhang M, Norman LP, Sun SC. Nf-kappab1/p105 regulates lipopolysaccharide-stimulated map kinase signaling by governing the stability and function of the tpl2 kinase. Mol Cell. 2003;11:685–694. doi: 10.1016/s1097-2765(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 81.Guma M, Stepniak D, Shaked H, Spehlmann ME, Shenouda S, Cheroutre H, Vicente-Suarez I, Eckmann L, Kagnoff MF, Karin M. Constitutive intestinal nf-kappab does not trigger destructive inflammation unless accompanied by mapk activation. The Journal of experimental medicine. 2011;208:1889–1900. doi: 10.1084/jem.20110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sikorski K, Chmielewski S, Olejnik A, Wesoly JZ, Heemann U, Baumann M, Bluyssen H. Stat1 as a central mediator of ifngamma and tlr4 signal integration in vascular dysfunction. Jak-Stat. 2012;1:241–249. doi: 10.4161/jkst.22469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. Ifn-gamma potentiates atherosclerosis in apoe knock-out mice. The Journal of clinical investigation. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lim WS, Timmins JM, Seimon TA, Sadler A, Kolodgie FD, Virmani R, Tabas I. Signal transducer and activator of transcription-1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–951. doi: 10.1161/CIRCULATIONAHA.107.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. Nlrp3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ng TM, Monack DM. Revisiting caspase-11 function in host defense. Cell host & microbe. 2013;14:9–14. doi: 10.1016/j.chom.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the nlrp3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PloS one. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Menu P, Pellegrin M, Aubert JF, Bouzourene K, Tardivel A, Mazzolai L, Tschopp J. Atherosclerosis in apoe-deficient mice progresses independently of the nlrp3 inflammasome. Cell death & disease. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, Kopf M. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent il-1alpha and sterile vascular inflammation in atherosclerosis. Nature immunology. 2013 doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- 90.Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, Guerini D, Baumgarten BU, Roggo S, Wen B, Knochenmuss R, Noel S, Gessier F, Kelly LM, Vanek M, Laurent S, Preuss I, Miault C, Christen I, Karuna R, Li W, Koo DI, Suply T, Schmedt C, Peters EC, Falchetto R, Katopodis A, Spanka C, Roy MO, Detheux M, Chen YA, Schultz PG, Cho CY, Seuwen K, Cyster JG, Sailer AW. Oxysterols direct immune cell migration via ebi2. Nature. 2011;475:524–527. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu C, Yang XV, Wu J, Kuei C, Mani NS, Zhang L, Yu J, Sutton SW, Qin N, Banie H, Karlsson L, Sun S, Lovenberg TW. Oxysterols direct b-cell migration through ebi2. Nature. 2011;475:519–523. doi: 10.1038/nature10226. [DOI] [PubMed] [Google Scholar]
- 92.Gatto D, Paus D, Basten A, Mackay CR, Brink R. Guidance of b cells by the orphan g protein-coupled receptor ebi2 shapes humoral immune responses. Immunity. 2009;31:259–269. doi: 10.1016/j.immuni.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 93.Gatto D, Wood K, Caminschi I, Murphy-Durland D, Schofield P, Christ D, Karupiah G, Brink R. The chemotactic receptor ebi2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nature immunology. 2013;14:446–453. doi: 10.1038/ni.2555. [DOI] [PubMed] [Google Scholar]
- 94.Kelly LM, Pereira JP, Yi T, Xu Y, Cyster JG. Ebi2 guides serial movements of activated b cells and ligand activity is detectable in lymphoid and nonlymphoid tissues. Journal of immunology. 2011;187:3026–3032. doi: 10.4049/jimmunol.1101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pereira JP, Kelly LM, Xu Y, Cyster JG. Ebi2 mediates b cell segregation between the outer and centre follicle. Nature. 2009;460:1122–1126. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yi T, Cyster JG. Ebi2-mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. eLife. 2013;2:e00757. doi: 10.7554/eLife.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in abc transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage abca1 reduces myd88-dependent toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. Journal of lipid research. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cells and their precursors. Annual review of immunology. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 100.Thorp E, Subramanian M, Tabas I. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. European journal of immunology. 2011;41:2515–2518. doi: 10.1002/eji.201141719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harbor perspectives in biology. 2013;5:a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, Aucouturier P, Chapman MJ, Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 103.Shearn AI, Deswaerte V, Gautier EL, Saint-Charles F, Pirault J, Bouchareychas L, Rucker EB, 3rd, Beliard S, Chapman J, Jessup W, Huby T, Lesnik P. Bcl-x inactivation in macrophages accelerates progression of advanced atherosclerotic lesions in apoe(−/−) mice. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1142–1149. doi: 10.1161/ATVBAHA.111.239111. [DOI] [PubMed] [Google Scholar]
- 104.Gerrity RG. The role of the monocyte in atherogenesis: Ii. Migration of foam cells from atherosclerotic lesions. The American journal of pathology. 1981;103:191–200. [PMC free article] [PubMed] [Google Scholar]
- 105.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. Cx3cr1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 106.Calderon B, Suri A, Miller MJ, Unanue ER. Dendritic cells in islets of langerhans constitutively present beta cell-derived peptides bound to their class ii mhc molecules. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6121–6126. doi: 10.1073/pnas.0801973105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 109.Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: Inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. Journal of immunology. 1996;157:2577–2585. [PubMed] [Google Scholar]
- 110.Gautier EL, Ivanov S, Lesnik P, Randolph GJ. Local apoptosis mediates clearance of macrophages from resolving inflammation in mice. Blood. 2013;122:2714–2722. doi: 10.1182/blood-2013-01-478206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, Gautier EL, Rubinstein D, Hong C, Liu J, Wu C, van Rooijen N, Bhardwaj N, Garabedian M, Tontonoz P, Fisher EA. Lxr promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. The Journal of clinical investigation. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. Hdl promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, Rayner AJ, McDonald TO, O'Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A, Moore KJ. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nature immunology. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Feig JE, Shang Y, Rotllan N, Vengrenyuk Y, Wu C, Shamir R, Torra IP, Fernandez-Hernando C, Fisher EA, Garabedian MJ. Statins promote the regression of atherosclerosis via activation of the ccr7-dependent emigration pathway in macrophages. PloS one. 2011;6:e28534. doi: 10.1371/journal.pone.0028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wan W, Lionakis MS, Liu Q, Roffe E, Murphy PM. Genetic deletion of chemokine receptor ccr7 exacerbates atherogenesis in apoe-deficient mice. Cardiovascular research. 2013;97:580–588. doi: 10.1093/cvr/cvs349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of apoe−/− mice during disease regression. The Journal of clinical investigation. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luchtefeld M, Grothusen C, Gagalick A, Jagavelu K, Schuett H, Tietge UJ, Pabst O, Grote K, Drexler H, Forster R, Schieffer B. Chemokine receptor 7 knockout attenuates atherosclerotic plaque development. Circulation. 2010;122:1621–1628. doi: 10.1161/CIRCULATIONAHA.110.956730. [DOI] [PubMed] [Google Scholar]
- 118.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor ccr7 during atherosclerosis regression in apoe-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Craeyveld E, Gordts SC, Nefyodova E, Jacobs F, De Geest B. Regression and stabilization of advanced murine atherosclerotic lesions: A comparison of ldl lowering and hdl raising gene transfer strategies. Journal of molecular medicine. 2011;89:555–567. doi: 10.1007/s00109-011-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wendland M, Willenzon S, Kocks J, Davalos-Misslitz AC, Hammerschmidt SI, Schumann K, Kremmer E, Sixt M, Hoffmeyer A, Pabst O, Forster R. Lymph node t cell homeostasis relies on steady state homing of dendritic cells. Immunity. 2011;35:945–957. doi: 10.1016/j.immuni.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 121.Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and t cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28:271–284. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M. Deciphering the transcriptional network of the dendritic cell lineage. Nature immunology. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Breslow JL. Mouse models of atherosclerosis. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 124.Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, Steinman RM. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 125.Herijgers N, Van Eck M, Groot PH, Hoogerbrugge PM, Van Berkel TJ. Effect of bone marrow transplantation on lipoprotein metabolism and atherosclerosis in ldl receptor-knockout mice. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:1995–2003. doi: 10.1161/01.atv.17.10.1995. [DOI] [PubMed] [Google Scholar]
- 126.Basavaraju SR, Easterly CE. Pathophysiological effects of radiation on atherosclerosis development and progression, and the incidence of cardiovascular complications. Med Phys. 2002;29:2391–2403. doi: 10.1118/1.1509442. [DOI] [PubMed] [Google Scholar]
- 127.Aparicio-Vergara M, Shiri-Sverdlov R, de Haan G, Hofker MH. Bone marrow transplantation in mice as a tool for studying the role of hematopoietic cells in metabolic and cardiovascular diseases. Atherosclerosis. 2010;213:335–344. doi: 10.1016/j.atherosclerosis.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 128.Mullick AE, Soldau K, Kiosses WB, Bell TA, 3rd, Tobias PS, Curtiss LK. Increased endothelial expression of toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. The Journal of experimental medicine. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rademakers T, Douma K, Hackeng TM, Post MJ, Sluimer JC, Daemen MJ, Biessen EA, Heeneman S, van Zandvoort MA. Plaque-associated vasa vasorum in aged apolipoprotein e-deficient mice exhibit proatherogenic functional features in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:249–256. doi: 10.1161/ATVBAHA.112.300087. [DOI] [PubMed] [Google Scholar]
- 130.Haka AS, Potteaux S, Fraser H, Randolph GJ, Maxfield FR. Quantitative analysis of monocyte subpopulations in murine atherosclerotic plaques by multiphoton microscopy. PloS one. 2012;7:e44823. doi: 10.1371/journal.pone.0044823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lilledahl MB, Haugen OA, de Lange Davies C, Svaasand LO. Characterization of vulnerable plaques by multiphoton microscopy. Journal of biomedical optics. 2007;12:044005. doi: 10.1117/1.2772652. [DOI] [PubMed] [Google Scholar]
- 132.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially l-selectin dependent. The Journal of experimental medicine. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Doran AC, Lipinski MJ, Oldham SN, Garmey JC, Campbell KA, Skaflen MD, Cutchins A, Lee DJ, Glover DK, Kelly KA, Galkina EV, Ley K, Witztum JL, Tsimikas S, Bender TP, McNamara CA. B-cell aortic homing and atheroprotection depend on id3. Circulation research. 2012;110:e1–12. doi: 10.1161/CIRCRESAHA.111.256438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Butcher MJ, Herre M, Ley K, Galkina E. Flow cytometry analysis of immune cells within murine aortas. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reis ED, Lie J, Fayad ZA, Rong JX, Hansoty D, Aguinaldo J-G, Fallon JT, Fisher EA. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein e-deficient mouse in a novel transplantation model. Vasc. Surgery. 2001;34:541–547. doi: 10.1067/mva.2001.115963. [DOI] [PubMed] [Google Scholar]
- 137.Chereshnev I, Trogan E, Omerhodzic S, Itskovich V, Aguinaldo JG, Fayad ZA, Fisher EA, Reis ED. Mouse model of heterotopic aortic arch transplantation. J Surg Res. 2003;111:171–176. doi: 10.1016/s0022-4804(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 138.Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, Kreisel D, Swartz MA, Sorci-Thomas MG, Randolph GJ. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. The Journal of clinical investigation. 2013;123:1571–1579. doi: 10.1172/JCI63685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Randolph GJ. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Current opinion in lipidology. 2008;19:462–468. doi: 10.1097/MOL.0b013e32830d5f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Valladeau J, Duvert-Frances V, Pin JJ, Dezutter-Dambuyant C, Vincent C, Massacrier C, Vincent J, Yoneda K, Banchereau J, Caux C, Davoust J, Saeland S. The monoclonal antibody dcgm4 recognizes langerin, a protein specific of langerhans cells, and is rapidly internalized from the cell surface. European journal of immunology. 1999;29:2695–2704. doi: 10.1002/(SICI)1521-4141(199909)29:09<2695::AID-IMMU2695>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 141.Getz GS, Reardon CA. Apoprotein e as a lipid transport and signaling protein in the blood, liver, and artery wall. Journal of lipid research. 2009;50(Suppl):S156–161. doi: 10.1194/jlr.R800058-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]