Background: There is a correlation between overload of albumin in the lumen of the PT and progression of renal disease.
Results: Physiologic albumin concentrations activate the PI3K/mTORC2/PKB/mTORC1/S6K pathway, whereas pathophysiologic concentrations disrupt this pathway overactivating mTORC1.
Conclusion: Albumin overload promotes a mis-regulation of mTOR complexes.
Significance: These results help to elucidate the mechanism behind mTORC1 activation during renal disease.
Keywords: Albumin, Cell Signaling, Epithelium, mTOR Complex (mTORC), Receptor Regulation
Abstract
High albumin concentrations in the proximal tubule of the kidney causes tubulointerstitial injury, but how this process occurs is not completely known. To address the signal transduction pathways mis-regulated in renal injury, we studied the modulation of mammalian target of rapamycin (mTOR) complexes by physiologic and pathophysiologic albumin concentrations in proximal tubule cells. Physiologic albumin concentrations activated the PI3K/mTORC2/PKB/mTORC1/S6 kinase (S6K) pathway, but pathophysiologically high albumin concentrations overactivated mTORC1 and inhibited mTORC2 activity. This control process involved the activation of ERK1/2, which promoted the inhibition of TSC2 and activation of S6K. Furthermore, S6K was crucial to promoting the over activation of mTORC1 and inhibition of mTORC2. Megalin expression at the luminal membrane is reduced by high concentrations of albumin. In addition, knockdown of megalin mimicked all the effects of pathophysiologic albumin concentrations, which disrupt normal signal transduction pathways and lead to an overactivation of mTORC1 and inhibition of mTORC2. These data provide new perspectives for understanding the molecular mechanisms behind the effects of albumin on the progression of renal disease.
Introduction
The role of albumin in proximal tubule (PT)2 cells has emerged as one of the most important issues in renal physiology. Correlations between overload of albumin in the lumen of the PT, induction of a proinflammatory response, tubulointerstitial injury, and progression of renal disease have all been demonstrated (1). However, the identity of the sensor and subsequent signal transduction pathways affected by pathophysiogically high albumin concentrations have not yet been fully determined.
There is an inverse correlation between the expression of the transmembrane albumin endocytic receptor megalin (1) and albumin concentration in the lumen of the PT (2). Our group has shown that physiologic albumin concentrations increase protein kinase B (PKB) and protein kinase C (PKC) activity in a phosphoinositide 3-kinase (PI3K)-dependent manner, whereas pathophysiologic albumin concentrations inhibit these enzymes (2, 3). However, pathophysiologic albumin concentrations activate extracellular signal-regulated kinase 1 and 2 (ERK1/2) in OK (opossum kidney) cells (4). However, the mechanism behind the modulation of these various kinases by albumin under physiologic and pathophysiologic conditions still needs to be clarified. One possible link between these effects of albumin could be mammalian target of rapamycin (mTOR), a well known serine/threonine kinase that is involved in a variety of cell functions such as cell growth and proliferation (5, 6).
mTOR forms two complexes: mTORC1 associated with the protein known as regulatory protein associated with TOR (RAPTOR), and mTORC2 associated with rapamycin-insensitive companion of TOR (RICTOR) (5, 6). mTORC1 phosphorylates S6 kinase (S6K) and 4E-PBP1 at different sites, promoting their activation (5, 6). Likewise, mTORC2 phosphorylates PKB and PKC, driving their activation (5, 6). Once activated, PKB activates mTORC1, indicating its central role in the tight equilibrium between mTOR complexes (5, 6). In addition, ERK1/2 has also been found to be involved in the activation of mTORC1 in HEK cells (7). Furthermore, TSC2 is phosphorylated by ERK, promoting its dissociation from TSC1 and leading to the activation of mTORC1 (7).
Data from the literature suggest an important role for mTORC1 in the development of a variety of renal diseases (8). However, some studies have shown that exposure to rapamycin can lead to glomerular injury when it is infused into healthy mice (9). Thus, to understand the progression of renal disease, it is crucial to understand the regulatory mechanism by which variations in albumin concentration in the PT affect the mTORC2/PKB/mTORC1 pathway under physiologic and pathophysiologic conditions. We have therefore examined the modulation of mTOR complexes by physiologic and pathophysiologic albumin concentrations in LLC-PK1 cells, a well known PT cell model (2, 3). Physiologic and pathophysiologic albumin concentrations modulate mTORC1 and mTORC2 activities in different ways involving a coordinated network. In addition, we found that the level of megalin at the luminal membrane functions as the sensor of this process. These results help to elucidate the mechanism behind mTORC1 activation during renal disease, opening up new possibilities for the treatment of this disease.
EXPERIMENTAL PROCEDURES
Materials and Reagents
BSA, free fatty acid BSA, EDTA, EGTA, Hepes, PMSF, sodium orthovanadate, sodium fluoride, sodium β-glycerophosphate, sodium pyrophosphate, sodium azide, goat serum, donkey serum, and rapamycin were purchased from Sigma. Sodium chloride, Tris, and Tris-HCl were purchased from Fischer Scientific (Pittsburgh, PA). Wortmannin, LY294002, PF-4708671, PKBi (PKB inhibitor), and WYE-345 were purchased from Millipore (Billerica, MA). U0126, polyclonal phospho-mTOR (Ser-2481), monoclonal phospho-mTOR (Ser-2448) (clone D9C2), monoclonal mTOR (clone 7C10), polyclonal mTOR, monoclonal RAPTOR (clone 24C12), monoclonal phospho-RICTOR (Thr-1135) (clone D30A3), monoclonal RICTOR (clone D16H9), monoclonal RAPTOR-Sepharose bead-conjugated (clone 24C12), monoclonal RICTOR-Sepharose bead-conjugated (clone D16H9), polyclonal phospho-PKB (Ser-473), monoclonal phospho-PKB (Thr-308), polyclonal PKB, polyclonal phosphor-S6K (Ser-371), polyclonal S6K (Thr-421/Ser-424), monoclonal S6K (Thr-389) (clone 108D2), polyclonal S6K, monoclonal phospho-TSC2 (Thr-1462) (clone 5B12), monoclonal TSC2 (clone D93F12), polyclonal TSC1, monoclonal phospho-PRAS40 (Thr-246) (clone C77D7), polyclonal phospho-PRAS40 (Ser-183), polyclonal PRAS40, monoclonal phospho-ERK1/2 (Thr-202/Tyr-204) (clone D13.14.4E), monoclonal ERK1/2 (clone 137F5) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Monoclonal phospho-TSC2 (Ser-664) (clone EPR8202), monoclonal Rheb (clone Rheb5D5), and polyclonal Lrp2/megalin antibodies were purchased from Abcam (Cambridge, MA). Lipofectamine 2000, DAPI, Alexa Fluor 488 donkey anti-mouse IgG, Alexa Fluor 488 donkey anti-rabbit IgG, and Alexa Fluor 568 goat anti-mouse IgG antibodies were purchased from Invitrogen. LLC-PK1 cells were obtained from the American Type Culture Collection (Rockville, MD). All other reagents were of the highest purity available.
Cell Culture
LLC-PK1 cells, a well characterized porcine PT cell line, were maintained in low-glucose Dulbecco's minimal essential medium with 10% FBS/1% penicillin/streptomycin (37 °C and 5% CO2) as described previously (2, 3, 10). Cells were used typically 2–3 days after monolayers reached 95% confluence, kept overnight in serum-depleted medium, and incubated with different albumin concentrations for 20 min. When indicated, the cells were preincubated with different compounds for 30 min before addition of albumin. After treatment, the cells were harvested in lysis buffer (described for each experimental condition), clarified by centrifugation, and used to carry out immunoblotting, immunoprecipitation, and protein kinase activity assay.
Stable Transfection
The LLC-PK1 cells were transfected as described previously (2). In brief, LLC-PK1 cells were transfected with a pBLOCK-iT 6-DEST vector containing an shRNA for megalin (Ambion, Invitrogen) and the blasticidin S-resistance gene (Invitrogen) using Lipofectamine 2000, according to the manufacturer's instructions. Two days after transfection, the transfected cells were selected by culturing with the antibiotic blasticidin S (3.0 μg/ml) for 2 weeks. Stably transfected cells were cloned by limiting dilution, and clonal cell lines were expanded and characterized by immunoblotting.
Immunoblotting and Immunoprecipitation
The cells were washed with phosphate-buffered saline (PBS2+), harvested, and incubated for 30 min in different lysis buffers: 1) for protein phosphorylation and total expression studies: 20 mm Hepes (pH 7.4), 2 mm EGTA, 1% Triton X-100, 400 μm PMSF, 50 mm sodium fluoride, 5 mm sodium pyrophosphate, 5 mm sodium orthovanadate, 10 mm sodium β-glycerophosphate, 2× complete protease inhibitor (Roche Diagnostics); 2) to preserve the integrity of mTOR complexes: 40 mm Hepes (pH 7.5), 120 mm NaCl, 1 mm EGTA, 0.3% CHAPS, 1 mm PMSF, 50 mm sodium fluoride, 5 mm sodium pyrophosphate, 5 mm sodium orthovanadate, 10 mm sodium β-glycerophosphate, 2× complete protease inhibitor (Roche Diagnostics); and 3) to preserve the integrity of TSC complexes: 250 mm NaCl, 50 mm Hepes (pH 7.5), 0.1% Nonidet P-40, 5 mm EDTA, 50 mm sodium fluoride, 5 mm sodium pyrophosphate, 5 mm sodium orthovanadate, 10 mm sodium β-glycerophosphate, and 2× complete protease inhibitor (Roche Diagnostics). After lysis, the samples were cleared by centrifugation at 15,000 × g for 10 min at 4 °C. The supernatant was retained, and the protein concentration was determined using the Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL). When indicated, RAPTOR, RICTOR, or TSC2 were immunoprecipitated according to the manufacturer's instructions. Proteins were resolved on 4–15% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes (Millipore), according to the manufacturer's instructions. After antibody labeling, detection was performed with SuperSignal West Dura (Thermo Scientific). The images were acquired with a Fuji Film (Edison, NJ) Image Reader (LAS-3000 Lite) and quantified with NIH ImageJ software (version 1.44p).
Immunofluorescent Staining and Confocal Laser Microscopy
Immunofluorescent staining for Rheb, RAPTOR, and mTOR phosphorylated at Ser-2448 was performed using specific antibodies as described by the manufacturer. LLC-PK1 cells were grown in Transwell inserts and kept overnight in serum-depleted medium after reaching confluence. After treatment, the cells were washed with PBS2+ twice, fixed with 4% paraformaldehyde for 10 min and blocked with 5% normal donkey serum for 1 h, both at room temperature. The cells were then incubated with the primary antibody for 1 h and washed with PBS2+ or Tris-buffered saline three times, followed by the appropriate secondary antibody conjugated to Alexa Fluor 488 or Alexa Fluor 568 for 1 h at room temperature. The nucleus was stained using DAPI. After staining, the Transwell inserts were washed thoroughly in PBS2+ or Tris-buffered saline and mounted with antiquenching medium (Vector Laboratories, Burlingame, CA), and then the slides were sealed. The fluorescence label was examined with a confocal microscope (model LSM510, Zeiss, Oberkochen, Germany). Images were acquired using the manufacturer's software. Contrast and brightness settings were chosen to ensure that all pixels were within the linear range. Images were prepared for publication with Adobe Photoshop (Adobe Systems, San Jose, CA).
Surface Biotinylation
Biotinylation was carried out as described previously (3). LLC-PK1 cells were grown in Transwell inserts, starved of serum overnight, incubated with 0.01 or 20.0 mg/ml albumin for 20 min, and then washed with PBS2+ twice. The proteins expressed at the luminal membrane were biotinylated by incubation with 1.5 mg/ml EZ-Link Sulfo-NHS-SS-biotin purchased from Pierce Biotechnology for 30 min at 4 °C. The biotinylated proteins were quenched with PBS/glycine buffer and rinsed with PBS2+. Cells were lysed in lysis buffer (20 mm Hepes (pH 7.4), 2 mm EGTA, 1% Triton X-100, 400 μm PMSF, and 2× complete protease inhibitor (Roche Applied Science)) and clarified by centrifugation at 7,000 × g for 10 min at 4 °C. The biotinylated proteins were precipitated with NeutraAvidin UltraLink resin beads (Pierce Biotechnology) overnight at 4 °C. The beads were washed out with lysis buffer three times at 4 °C, and the precipitate was then subjected to 4–15% SDS-PAGE and immunoblotting for detection of megalin.
Assay for mTORC1, mTORC2, and S6K Activity
mTORC1 and S6K activities were determined using a K-LISATM mTOR activity kit (Millipore) and p70 S6K activity assay kit (ENZO Life Sciences, Farmingdale, NY), respectively, according to the manufacturer's instructions. A K-LISATM mTOR activity kit was used to determine the phosphorylation of S6K at Thr-389. In addition, further experiments were carried out to determine the phosphorylation of 4E-BP1 at Thr-37/Thr-46 residues, a substrate of mTORC1. mTORC2 activity was assessed by phosphorylation of PKB at the Ser-473 residue.
Statistical Analysis
The results are expressed as means ± S.E. Statistical significance was assessed by one-way analysis of variance, considering the treatments as factors. The significance of the differences was verified by the Bonferroni t test. Statistical analysis was performed using absolute values. Significance was determined as p < 0.05.
RESULTS
Modulation of the Activity of mTOR Complexes by Albumin
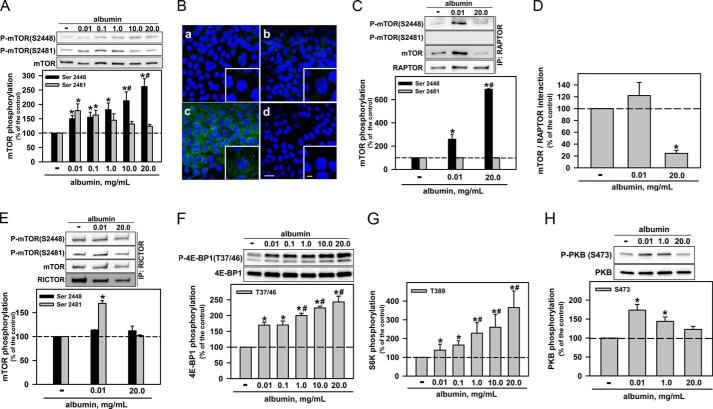
The activity of mTOR is associated with phosphorylation of specific serine residues close to the kinase domain. Usually, phosphorylation at Ser-2481 is associated with mTORC2 activity, whereas phosphorylation at Ser-2448 can occur in both mTORC1 and mTORC2 complexes (11). In the first group of experiments, we tested the potential effect of albumin on the phosphorylation of mTOR at Ser-2481 and Ser-2448. Physiologic albumin concentrations (0.01–0.1 mg/ml) increased the phosphorylation of both serine residues by the same magnitude. Further increases in albumin concentration (up to 20 mg/ml) caused a decrease in phosphorylation at Ser-2481, to control levels, whereas phosphorylation at Ser-2448 was enhanced (Fig. 1A). Phosphorylation at the Ser-2448 residue was evaluated by immunofluorescent staining, and similar results were obtained (Fig. 1B).
FIGURE 1.
Albumin modulates mTORC1 and mTORC2 activities. A, the effect of albumin on mTOR phosphorylation at Ser-2481 and Ser-2448 (n = 9). B, modulation of phosphorylation of mTOR at Ser-2448 by albumin. a, control; b, 0.01 mg/ml albumin; c, 20.0 mg/ml albumin; d, negative control (in the presence of secondary antibody). The color blue (DAPI) indicates nuclei, and green (Alexa Fluor 488-conjugated antibody) indicates mTOR phosphorylation at Ser-2448. Scale bar, 20 μm (n = 3). Details of the original image are shown in the bottom right-hand corner, where the scale bar = 5 μm. The effect of physiologic or pathophysiologic albumin concentrations on: mTOR phosphorylation at Ser-2448 and Ser-2481 in isolated mTORC1 (C); interaction between mTOR and RAPTOR (D); mTOR phosphorylation at Ser-2448 and Ser-2481 in isolated mTORC2 (E). mTORC1 and mTORC2 were isolated by immunoprecipitation of RAPTOR and RICTOR, respectively (n = 6). The effect of albumin on mTORC1 activity: measured by 4E-BP1 phosphorylation at Thr-37/46 (n = 8) (F): measured by S6K phosphorylation at Thr-389 (n = 4) (G). H, the effect of albumin on mTORC2 activity measured by PKB phosphorylation at Ser-473 (n = 5). The results are shown as means ± S.E. *, p < 0.05 versus control; #, p < 0.05 versus 0.01 mg/ml albumin.
In the next set of experiments, we immunoprecipitated RAPTOR (the protein associated with mTORC1) or RICTOR (the protein associated with mTORC2) and then evaluated phosphorylation at Ser-2481 and Ser-2448 in mTOR. Physiologic and pathophysiologic albumin concentrations increased the phosphorylation at Ser-2448 in mTORC1. This effect was more pronounced at pathophysiologically high concentrations (Fig. 1C). The level of mTOR after immunoprecipitation of RAPTOR is lower at a pathophysiologic albumin concentration than at a physiologic concentration. This decrease could due to the dissociation of mTOR from RAPTOR or because RAPTOR and mTOR form an unstable complex that is disrupted during the co-immunoprecipitation assay (Fig. 1D). However, only physiologic concentrations of albumin increased the phosphorylation at Ser-2481 in mTORC2 (Fig. 1E); phosphorylation at Ser-2448 in mTORC2 was not altered by different albumin concentrations. Physiologic albumin concentrations increased mTORC1 activity measured by phosphorylation of S6K at the Thr-389 residue and 4E-BP1 at Thr-37/Thr-46 residues (Fig. 1, F and G). Further increases in albumin concentration promoted an additional increase in mTORC1 activity, paralleling the results obtained when we assessed mTOR phosphorylation at Ser-2448 (mTORC1). In addition, the mTORC2 activity was assessed by phosphorylation of PKB at the Ser-473 residue (Fig. 1G). It was observed that the physiologic albumin concentration increased mTORC2 activity, whereas the pathophysiologic concentration did not.
These data suggest that albumin activates both mTORC1 and mTORC2 in PT cells under physiologic conditions. At high concentrations, albumin overactivates mTORC1 and inhibits mTORC2. However, how albumin promotes this differential modulation of mTORC1 and mTORC2 activities is not clear.
Overactivation of mTORC1 by Higher Albumin Concentrations Is Independent of the mTORC2/PKB Pathway
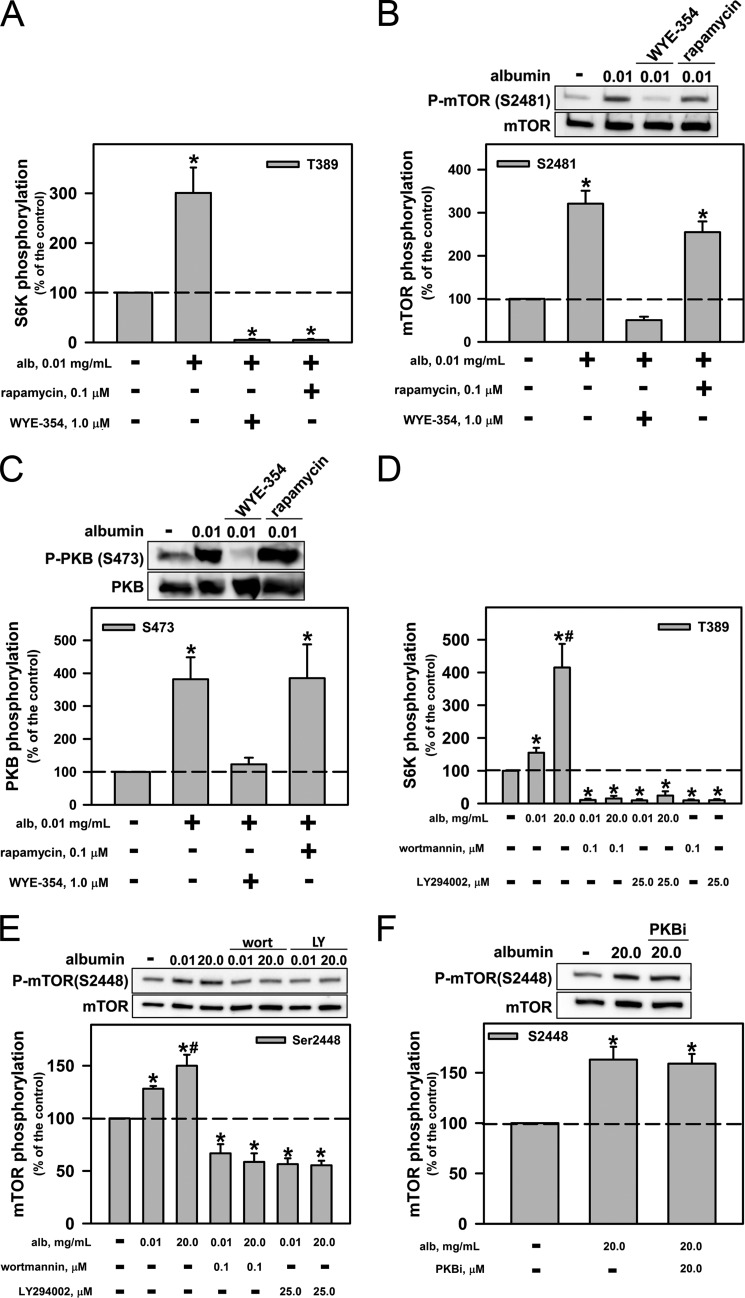
We investigated whether the activation of mTORC1 by a physiologic albumin concentration is dependent on previous activation of mTORC2. LLC-PK1 cells were preincubated for 20 min with 0.01 mg/ml albumin in the presence or in the absence of 1 μm WYE-354, a catalytic inhibitor of mTOR complexes, and of 0.1 μm rapamycin, a well known inhibitor of mTORC1. Both inhibitors abolished the effect of 0.01 mg/ml albumin on mTORC1 activity (Fig. 2A). However, only WYE-354 abolished the effect of a physiologic albumin concentration on phosphorylation of Ser-2481, which is associated with mTORC2 activity (Fig. 2B). WYE-354 also inhibited Ser-473 phosphorylation in PKB, a substrate of mTORC2 (Fig. 2C). In addition, the PI3K inhibitors wortmannin (0.05 μm) and LY294002 (25 μm) completely blocked the effect of both physiologic and pathophysiologic albumin concentrations on mTORC1 activity (Fig. 2D) and on mTOR phosphorylation at Ser-2448 (Fig. 2E). The inhibitor of PKB (10 μm) did not alter the phosphorylation of mTOR at Ser-2448 after induction by high albumin concentration (Fig. 2F).
FIGURE 2.
Role of PI3K and PKB in the modulation of mTORC2 and mTORC1 by albumin. LLC-PK1 cells were preincubated with mTOR inhibitors (1 μm WYE-345 or 0.1 μm rapamycin) for 30 min before concomitant incubation with albumin for 30 min. Modulation by mTOR inhibitors of the effect of physiologic albumin (alb) concentrations on mTORC1 activity by S6K phosphorylation at Thr-389 (A), mTOR phosphorylation at Ser-2481 (B), and PKB phosphorylation at Ser-473 (C). Shown is the effect on albumin-mediated modulation of mTORC1 activity (D) and on mTOR phosphorylation at Ser-2448 (E) of the PI3K inhibitors wortmannin (wort; 0.05 μm) and LY294002 (LY; 25 μm). F, effect of 10 μm PKB inhibitor (PKBi) on mTOR phosphorylation at Ser-2448. The results are shown as means ± S.E. (n = 4). *, p < 0.05 versus control; #, p < 0.05 versus 0.01 mg/ml albumin.
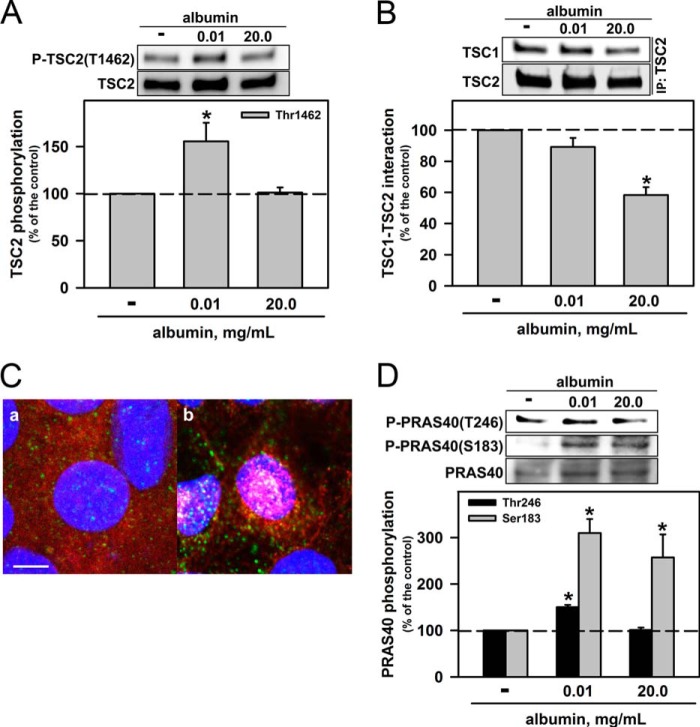
One possible mechanism for mTORC1 activation is the phosphorylation of TSC2 at Thr-1462, which are induced by PKB (8). This process leads to the activation of Rheb, a G monomeric protein that promotes the activation of mTORC1 (5, 6, 7). Fig. 3A shows that a physiologic albumin concentration increased this phosphorylation, but pathophysiologically high concentrations did not. Another possible way to inhibit TSC2 is to promote its dissociation from TSC1 (7). To determine whether such dissociation occurs, we carried out co-immunoprecipitations of TSC1 and TSC2. We found that high albumin concentrations indeed promoted the dissociation of TSC2 and TSC1 (Fig. 3B) and the colocalization of RAPTOR and Rheb (Fig. 3C).
FIGURE 3.
Pathophysiologic albumin concentrations modulate the association of TSC2/TSC1 and colocalization of Rheb/mTORC1. A, effect of albumin on TSC2 phosphorylation at Thr-1462 (n = 6). B, effect of albumin on the interaction between TSC1 and TSC2. TSC2 was immunoprecipitated with monoclonal antibody, and immunoblotting for TSC1 and TSC2 was carried out (n = 6). C, colocalization of Rheb and mTORC1. LLC-PK1 cells were grown on Transwell cell culture inserts, serum-starved overnight, and then kept in the absence (a) or presence (b) of 20.0 mg/ml albumin for 20 min. Blue (DAPI) indicates nuclei, green (Alexa Fluor 488-conjugated antibody) indicates RAPTOR, and red (Alexa Fluor 456-conjugated antibody) indicates Rheb. Scale bar, 5 μm (n = 3). D, effect of albumin on PRAS40 phosphorylation at Thr-246 and Ser-183 (n = 4). Results are shown as means ± S.E. *, p < 0.05 versus control.
Another important negative regulator of mTORC1 is proline-rich Akt/PKB substrate of 40 kDa (PRAS40) (6). PKB-mediated phosphorylation of PRAS40 at the Thr-246 residue promotes its inhibition and, consequently, activation of mTORC1 (6, 12). We found that this phosphorylation of PRAS40 was increased by a physiologic albumin concentration but not affected by pathophysiologically high concentrations (Fig. 3D). Another important phosphorylation site in PRAS40 is Ser-183, whose phosphorylation is mediated by mTORC1. This phosphorylation leads to dissociation of PRAS40 from RAPTOR, promoting additional activation of mTORC1 (13). We observed that both physiologic and high albumin concentrations increased the phosphorylation of this residue to the same degree (Fig. 3D). The effect of pathophysiologic albumin concentrations on the overactivation of mTORC1 depends on a PI3K-dependent and mTORC2/PKB-independent pathway, indicating that there is an alternative pathway to activation of mTORC1.
Activation of S6K Is Important in the Inhibition of mTORC2 and Activation of mTORC1 by Pathophysiologic Albumin Concentrations
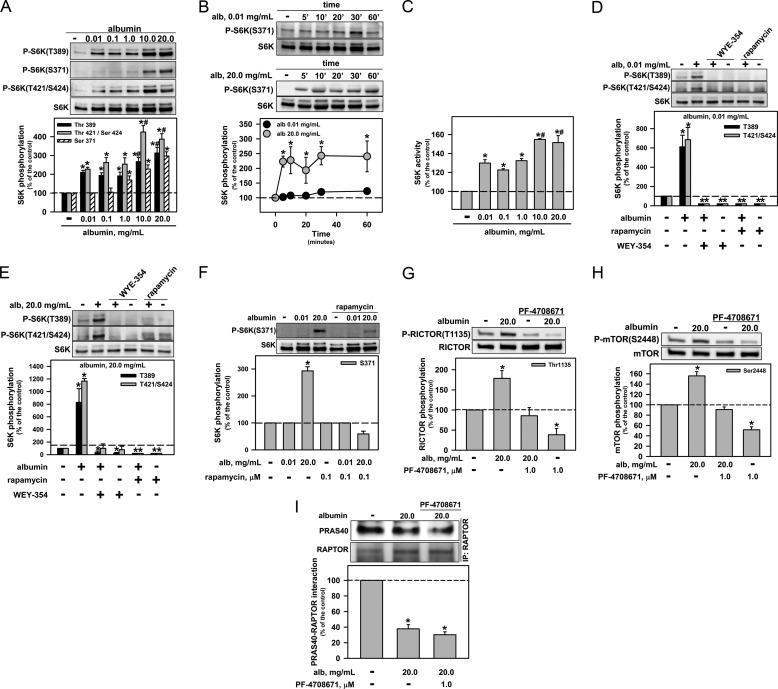
The activation of S6K activity depends on phosphorylation at a number of serine and threonine residues, including Ser-371, Thr-389, Thr-421, and Ser-424 (14). We observed that physiologic albumin concentrations increased the phosphorylation at Thr-389, Thr-421, and/or Ser-424 but did not change the phosphorylation status at residue Ser-371 (Fig. 4A). High albumin concentrations promoted an additional increase in phosphorylation at Thr-389, Thr-421, and/or Ser-424. Under high albumin conditions, phosphorylation of Ser-371 was observed (Fig. 4A); this phosphorylation reached a maximum at 5 min and was maintained for 60 min (Fig. 4B). Physiologic albumin concentrations increased the S6K activity, whereas pathophysiologically high concentrations promoted an over activation of S6K (Fig. 4C). Rapamycin and WYE-354 both abolished the phosphorylation at Thr-389, Thr-421, and Ser-424 in S6K that was induced by albumin (Fig. 4, D and E), and the Ser-371 phosphorylation induced by high albumin concentrations was reversed by rapamycin (Fig. 4F).
FIGURE 4.
S6K mediates the effect of pathophysiologic albumin concentrations on overactivation of mTORC1 and inhibition of mTORC2. A, effect of albumin on S6K phosphorylation at Thr-389, Ser-371, Thr-421, and Ser-424 (n = 8). B, time course of the effect of 0.01 and 20.0 mg/ml albumin (alb) on the phosphorylation of Ser-371 of S6K (n = 8). C, the effect of albumin on S6K activity (n = 4). LLC-PK1 cells were preincubated with mTOR inhibitors (1 μm WYE-345 or 0.1 μm rapamycin) for 30 min before concomitant incubation with albumin for 30 min. Modulation by mTOR inhibitors of the effect of physiologic (D) and pathophysiologic (E) albumin concentrations on the phosphorylation of S6K at Thr-389, Thr-421, and Ser-424 (n = 4). F, modulation by rapamycin of the effect of albumin on the phosphorylation of S6K at Ser-371 (n = 4). Shown is the modulation by PF-4708671, an S6K inhibitor, of the effect of albumin on RICTOR phosphorylation at Thr-1135 (G), mTOR phosphorylation at Ser-2448 (H), and the interaction between RAPTOR and PRAS40 (I). RAPTOR was immunoprecipitated with monoclonal antibody, and immunoblotting for PRAS40 was carried out (n = 6). The results are shown as means ± S.E. *, p < 0.05 versus control; #, p < 0.05 versus 0.01–1.0 mg/ml albumin. 5′, 5 min.
S6K phosphorylates RICTOR at Thr-1135, promoting inhibition of mTORC2 (15). We observed that high albumin concentrations increased the phosphorylation of this residue, and the effect was completely abolished by PF-4708671, a specific inhibitor of S6K1 (Fig. 4G). This inhibitor abolished the phosphorylation of Ser-2448 that was induced by pathophysiologically high albumin concentrations (Fig. 4H) but did not change the dissociation between RAPTOR and PRAS40 (Fig. 4I). One question must be answered: What molecule is responsible for over activation of mTORC1 caused by high albumin concentrations when the mTORC2/PKB pathway is inhibited?
ERK Is Involved in the Overactivation of mTORC1 by Pathophysiologically High Albumin Concentrations
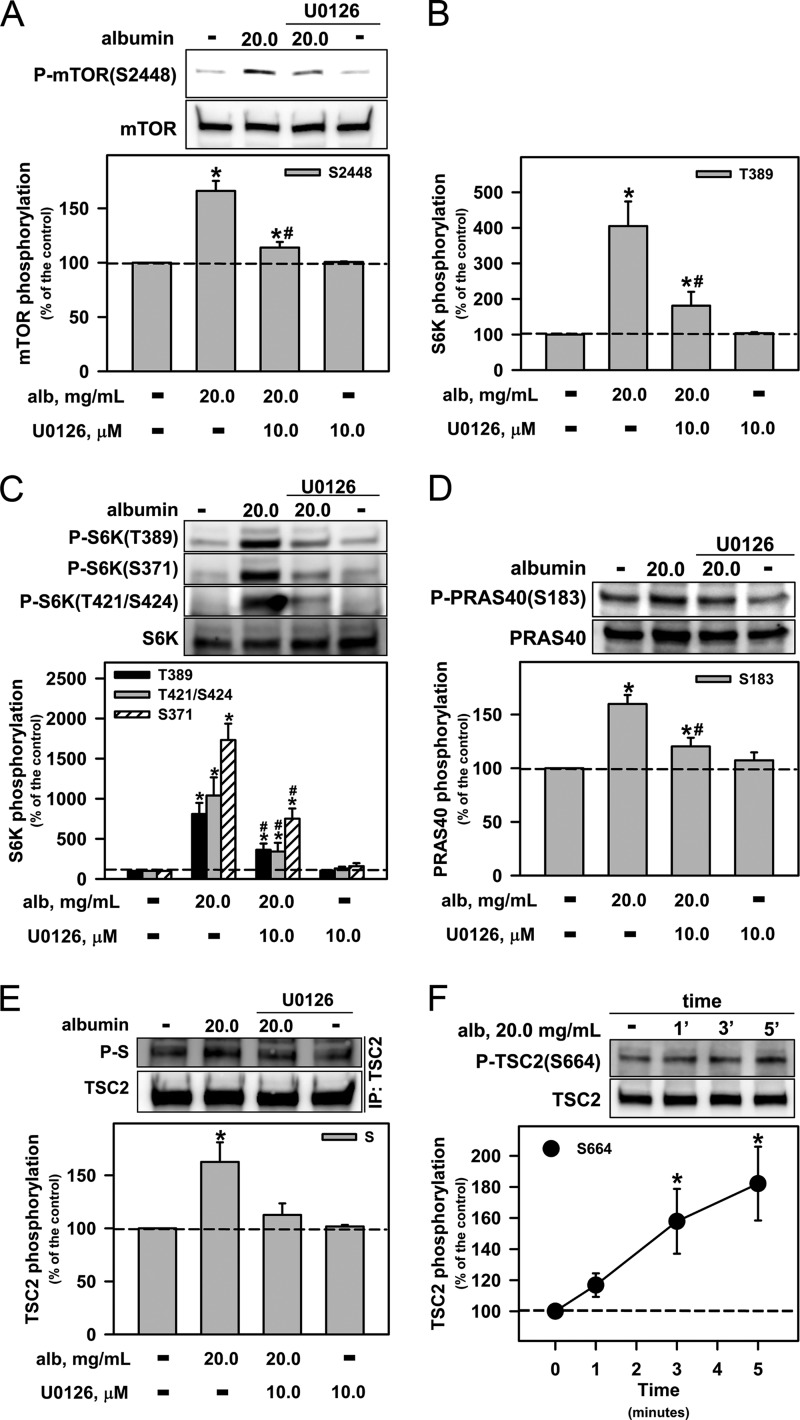
A pathophysiologically high albumin concentration increased the phosphorylation of ERK, and this effect was maintained for 60 min (data not shown). An MEK1/2 inhibitor, U0126 (10 μm), abolished the phosphorylation of Ser-2448 of mTOR (Fig. 5A), the activation of mTORC1 (Fig. 5B), and the phosphorylation of Ser-371, Thr-389, Thr-421, and Ser-424 of S6K (Fig. 5C). It also partially reduced the increase in the phosphorylation of Ser-183 of PRAS40 (Fig. 5D) that is caused by high albumin concentrations.
FIGURE 5.
ERK1/2 is involved in the overactivation of mTORC1 and S6K by pathophysiologic albumin concentrations. Modulation by 10 μm U0126, a specific MEK inhibitor, of the effect of albumin (alb) on mTOR phosphorylation at Ser-2448 (A); mTORC1 activity by S6K phosphorylation at Thr-389 (B); S6K phosphorylation at Thr-389, Ser-371, Thr-421, and Ser-424 (C); PRAS40 phosphorylation at Ser-183 (D); and TSC2 phosphorylation of the serine (S) residue located at the PXSP motif, induced by albumin (E). F, time course of the effect of albumin on the phosphorylation of TSC2 at Ser-664 (n = 8). The results are shown as means ± S.E. *, p < 0.05 versus control; #, p < 0.05 versus 20.0 mg/ml albumin. 1′, 1 min.
In the next group of experiments, we immunoprecipitated TSC2 and carried out immunoblotting to detect the PXS*P motif (phospho-MAPK substrate, asterisk indicates phosphorylated residue). High albumin concentrations increased the phosphorylation of TSC2 at the PXS*P motif, and this effect was abolished by U0126 (Fig. 5E). At this concentration, albumin induced the phosphorylation of TSC2 at Ser-664. The maximal effect was observed at 5 min (Fig. 5F). Based on these results, we suggest that ERK, at least in part, mediates the effect of a pathophysiologic concentration of albumin on the overactivation of mTORC1 in an mTORC2/PKB-independent manner.
Megalin Mediates mTORC1 Modulation by Higher Albumin Concentrations
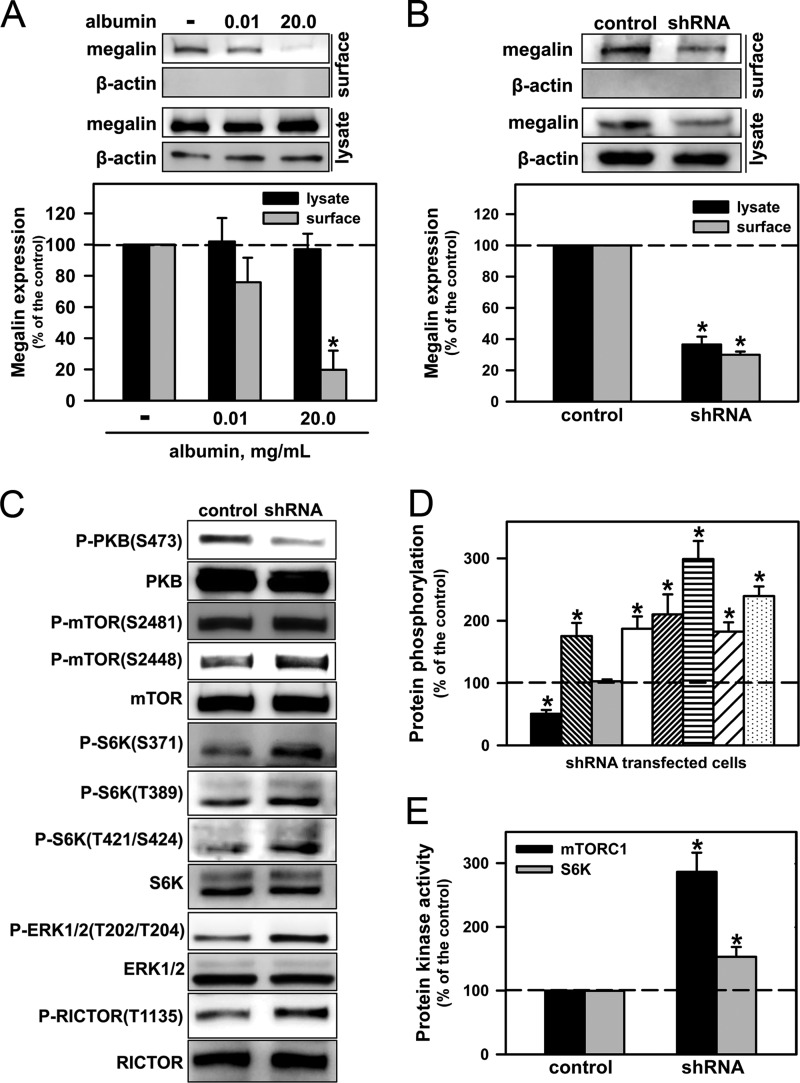
Fig. 6A shows that the incubation of LLC-PK1 for 20 min with pathophysiologically high albumin concentrations decreased the surface expression of megalin. In the next set of experiments, we stably transfected LLC-PK1 cells with an shRNA (LLC-PK1/shRNA) for megalin. This approach mimicked the exposure of LLC-PK1 cells to a high albumin concentration. In these cells, the total and surface expression of megalin (Fig. 6B) as well as PKB phosphorylation at Ser-473 (Fig. 6C) were decreased when compared with nontransfected cells. Furthermore, exposure to 10% FBS increased the phosphorylation of PKB at the Ser-473 residue in both LLC-PK1 and LLC-PK1/shRNA cells (data not shown), demonstrating that the transfection did not induce modifications in PKB.
FIGURE 6.
A reduction in the level of total or surface megalin decreases the PKB pathway and overactivates the ERK/mTORC1/S6K pathway. A, the luminal expression levels of megalin were assessed by surface biotinylation (n = 3). B–E, LLC-PK1 cells stably transfected with shRNA for megalin were grown on six-well plates and kept overnight in serum-depleted medium. B, total and luminal expression levels of megalin (n = 6). C, PKB, mTOR, S6K, ERK, and RICTOR phosphorylation (n = 6). D, densitometry analysis. PKB phosphorylation at Ser-473 (first bar); mTOR phosphorylation at Ser-2448 (second bar) and Ser-2481 (third bar); S6K phosphorylation at Ser-371 (fourth bar), Thr-389 (fifth bar), Thr-421/Ser-424 (sixth bar); ERK phosphorylation at Thr-202/Tyr-204 (seventh bar) and RICTOR phosphorylation at Thr-1135 (eighth bar). E, mTORC1 and S6K activities (n = 6). The results are shown as means ± S.E. *, p < 0.05 versus control.
The following results were observed in LLC-PK1/shRNA cells when compared with nontransfected cells: (a) an increase in phosphorylation at Ser-2448 in mTOR, whereas phosphorylation at Ser-2481 was not changed (Fig. 6D); (b) an increase in phosphorylation in S6K as well as S6K activity (Fig. 6, E and H); (c) an increase in mTORC1 activity (Fig. 6F); and (d) an increase in the phosphorylation of ERK1/2 (Fig. 6H) and Thr-1135 in RICTOR (Fig. 6I). Similar results were obtained when LLC-PK1 cells were preincubated with a pathophysiologically high albumin concentration.
DISCUSSION
Albumin-induced tubulointerstitial disease is a key step in the progression of renal disease (1). The data obtained in the present work and those obtained in previous studies show that physiologic albumin concentrations activate the PI3K/mTORC2/PKB/mTORC1/S6K pathway in LLC-PK1 cells. Our findings indicate that pathophysiologically high albumin concentrations inhibit mTORC2 activity and overactivate mTORC1 through an ERK/S6K/TSC2-dependent pathway, and the luminal expression of the megalin is the sensor in this process.
We have shown here that physiologic albumin concentrations increase mTORC2 activity. Because mTORC2 phosphorylates the Ser-473 residue in PKB (5, 6), we postulate that physiologic albumin concentrations activate PKB via mTORC2. However, how albumin activates mTORC2 is not completely known. Our results suggest an important role for PI3K in this process. Our results agree with those observed in HEK293 cells, in which mTORC2 activity was shown to depend on the formation of phospatidylinositol 3,4,5-triphosphate (16). Brunskill et al. (17) showed that albumin activates PI3K in OK (opossum kidney) cells. In addition, we have shown previously that the activation of PKB by physiologic albumin concentrations in LLC-PK1 cells is abolished by PI3K inhibitors and a phosphoinositide-dependent kinase inhibitor (2). These results reveal an important mechanism of PKB activation by physiologic albumin concentrations: Albumin binds to megalin, which promotes the activation of PI3K, leading to the activation of mTORC2. The mTOR kinase associated with mTORC2 phosphorylates PKB at Ser-473 and phosphoinositide-dependent kinase 1 phosphorylates Thr-308, leading to its activation. This pathway is known to be involved in the effects of physiologic albumin concentrations on protection against apoptosis and on the increase in (Na++K+)ATPase expression in PT cells (2, 3). These results indicate that the function of PT cells under physiologic conditions depends on a tight equilibrium between mTORC2 and mTORC1 activity, and PKB is the connecting link in this process (5–7). This hypothesis is strengthened by our observation that knockdown of megalin decreases PKB activity and overactivates mTORC1.
The overactivation of mTORC1 by pathophysiologic albumin concentrations occurs even when mTORC2 is completely inhibited. There is a strict correlation between the phosphorylation of Ser-2448 at mTORC1 and its activation (11, 18). In 2005, Chiang and Abraham (19) showed that S6K promotes direct phosphorylation of Ser-2448 of mTORC1 in HEK293 cells (19). We observed that S6K inhibitor abolished the phosphorylation of Ser-2448 of mTORC1 that was induced by pathophysiologically high albumin concentrations. Furthermore, at a similar pathophysiological albumin concentration, S6K has been shown to phosphorylate Thr-1135 of RICTOR, leading to its inhibition (15). Based on these results, we postulate that S6K represents a central molecule in the modulation of the balance between mTORC1 and mTORC2 activity during overload of albumin in the lumen of the PT.
It has been accepted that RAPTOR is a negative regulator of mTORC1 activity (6, 20). Our data indicate that pathophysiologic albumin concentrations promote dissociation of the RAPTOR/mTOR complex leading to activation of mTORC1. Furthermore, Kim et al. (20), using HEK293T cells, suggested that RAPTOR and mTOR form an intermediary unstable complex that is necessary for the activation of mTORC1. In agreement with this hypothesis, we observed that mTOR recovery after RAPTOR immunoprecipitation was lower at a pathophysiologic albumin concentration than at a physiologic concentration. In addition, we observed that the phosphorylation of mTORC1 at Ser-2448 in cell lysates is higher at a pathophysiologic albumin concentration even without changes in the mTOR level. Regardless of which hypothesis is true, the fact is that a pathophysiologic albumin concentration induces mTORC1 phosphorylation at Ser-2448 and promotes its activation.
Our data also show an important role for ERK1/2 in the overactivation of the mTORC1/S6K pathway by pathophysiologic albumin concentrations. This effect depends on the phosphorylation of TSC2 at Ser-664 that is mediated by ERK1/2. This phosphorylation promotes the dissociation of TSC2 and TSC1, displacement of Rheb to the active conformation and, consequently, the activation of mTORC1. Ma et al. (21) reported similar results in HEK293 cells in 2005.
The mechanism of activation of S6K is complex and involves the phosphorylation of different threonine and serine residues (14). We observed that the effect of lower and higher albumin concentrations on S6K activity involves the phosphorylation of Thr-389 and Thr-421/Ser-424, located in the hydrophobic motif and C-terminal regions, respectively. However, the phosphorylation of Ser-371, found in the turn motif region, was only observed at pathophysiologic albumin concentrations. In 1997, Moser et al. (22) showed that replacement of Ser-371 by Ala completely abolished the S6K activity in HEK293 cells. In addition, it was proposed that the phosphorylation of Ser-371 is critical for stabilizing Thr-389 phosphorylation (14).
The identity of the kinase involved in the phosphorylation of Ser-371 has not yet been determined. It has been observed that mTORC1 phosphorylates S6K at Ser-371 when stimulated by insulin in HEK293 cells (14, 23). In addition, the Ser-371 residue is located in a proline-rich domain, which could provide a substrate for MAPK (14, 22). These data indicate that both mTORC1 and ERK activation induced by pathophysiologic albumin concentrations could be involved in the phosphorylation of Ser-371 in S6K. This hypothesis is strengthened by our observation that an MEK inhibitor and an mTORC1 inhibitor both reversed the effect of pathophysiologic albumin concentrations on the phosphorylation of S6K at the Ser-371 residue. However, the phosphorylation of Ser-371 by another kinase cannot be ruled out at this time.
What sensor mechanism detects variations in the albumin concentration in the lumen of PT cells is still an open matter. We propose that surface expression of megalin in PT cells is the sensor for albumin variations, based on the following observations: (a) we have previously shown that a decrease in megalin expression inhibits PKB activity and leads to apoptosis (2); (b) pathophysiologic albumin concentrations decreased the surface expression of megalin; (c) knockdown of megalin in LLC-PK1 cells decreased PKB phosphorylation at Ser-473 and overactivated the ERK1/2/mTORC1/S6K pathway, similar to the effects observed with a pathophysiologic albumin concentration; (d) albumin overload in the lumen of PT cells decreases megalin expression in a variety of renal disease animal models (24–27), and this effect was correlated with tubulointerstitial injury and an increase in mTORC1 activity.
Available evidence indicates that there is a strict correlation between albumin overload in the lumen of the PT, ERK1/2 activity, mTORC1 activation, NF-κB activity, and secretion of proinflammatory chemokines (1, 26–31). However, little is known about the mechanism behind the overactivation of mTORC1 in renal disease. The results obtained by our group show that an overload of albumin, observed in renal disease, induces the overactivation of mTORC1 through activation of ERK1/2/S6K pathway and inhibition of mTORC2 activity.
Our data now suggest that the surface expression of the megalin receptor on the luminal side of LLC-PK1 cells could serve as a sensor for variations in albumin concentrations under physiologic and pathophysiologic conditions. The decrease in megalin expression triggers a cellular response that involves the activation of the ERK1/2/S6K/mTORC1 pathway (Fig. 7). Overactivation of mTORC1 depends on the various factors mediated by ERK1/2: (a) the phosphorylation of TSC2 at Ser-664, dissociation of TSC2 and TSC1, a shift in Rheb to the active state, and association with RAPTOR; and (b) activation of S6K by phosphorylation, mainly at Ser-371, which in turn causes phosphorylation of mTORC1 at Ser-2448. These effects could lead to the activation of NF-κB activity and secretion of proinflammatory chemokines. This proposal is strengthened by the observation that mTORC1 can stimulate the modulation of NF-κB in PC3 and LNCaP cells, which are prostate cancer cell lines (28). Furthermore, the secretion of MCP-1 that is induced by pathophysiologic albumin concentrations in PT cells is mediated by ERK1/2 activation (29). These results offer new perspectives that help elucidate the molecular mechanisms involved in the progression of renal disease and open new avenues for its treatment.
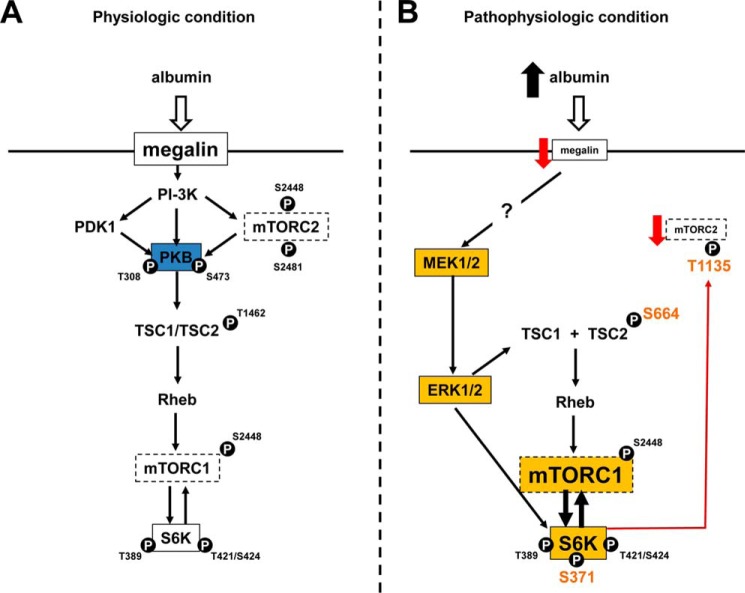
FIGURE 7.
Overall scheme for the effect of physiologic and pathophysiologic concentrations of albumin on mTOR activity in PT cells. A, the effect of physiologic albumin concentrations. Blue represents the central role of PKB in the activation of mTORC2 and mTORC1. B, the effect of pathophysiologic albumin concentrations. Orange represents the new compounds and new phosphorylate sites induced under these conditions. The red line represents the inhibitory effect.
Acknowledgments
We thank Dr. Deborah McClellan and Lorna O'Brien for editorial assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant DK032753.
- PT
- proximal tubule
- PKB
- protein kinase B
- mTOR
- mammalian target of rapamycin
- S6K
- S6 kinase
- TSC
- tuberous sclerosis complex.
REFERENCES
- 1. Abbate M., Zoja C., Remuzzi G. (2006) How does proteinuria cause progressive renal damage? J. Am. Soc. Nephrol. 17, 2974–2984 [DOI] [PubMed] [Google Scholar]
- 2. Caruso-Neves C., Pinheiro A. A., Cai H., Souza-Menezes J., Guggino W. B. (2006) PKB and megalin determine the survival or death of renal proximal tubule cells. Proc. Natl. Acad. Sci. U.S.A. 103, 18810–18815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peruchetti D. B., Pinheiro A. A., Landgraf S. S., Wengert M., Takiya C. M., Guggino W. B., Caruso-Neves C. (2011) (Na++K+)-ATPase is a target for phosphoinositide 3-kinase/protein kinase B and protein kinase C pathways triggered by albumin. J. Biol. Chem. 286, 45041–45047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dixon R., Brunskill N. J. (2000) Albumin stimulates p44/p42 extracellular signal-regulated mitogen-activated protein kinase in opossum kidney proximal tubular cells. Clin. Sci. 98, 295–301 [PubMed] [Google Scholar]
- 5. Huang J., Manning B. D. (2009) A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem. Soc. Trans. 37, 217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zoncu R., Efeyan A., Sabatini D. M. (2011) mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang J., Manning B. D. (2008) The TSC1-TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 412, 179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lieberthal W., Levine J. S. (2009) The role of the mammalian target of rapamycin (mTOR) in renal disease. J. Am. Soc. Nephrol. 20, 2493–2502 [DOI] [PubMed] [Google Scholar]
- 9. Stylianou K., Petrakis I., Mavroeidi V., Stratakis S., Kokologiannakis G., Lioudaki E., Liotsi C., Kroustalakis N., Vardaki E., Stratigis S., Perakis K., Kyriazis J., Nakopoulou L., Daphnis E. (2012) Rapamycin induced ultrastructural and molecular alterations in glomerular podocytes in healthy mice. Nephrol. Dial. Transplant. 27, 3141–3148 [DOI] [PubMed] [Google Scholar]
- 10. Caruso-Neves C., Kwon S. H., Guggino W. B. (2005) Albumin endocytosis in proximal tubule cells is modulated by angiotensin II through an AT2 receptor-mediated protein kinase B activation. Proc. Natl. Acad. Sci. U.S.A. 102, 17513–17518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Copp J., Manning G., Hunter T. (2009) TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): Phosphor-S2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 69, 1821–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vander Haar E., Lee S. I., Bandhakavi S., Griffin T. J., Kim D. H. (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 9, 316–323 [DOI] [PubMed] [Google Scholar]
- 13. Oshiro N., Takahashi R., Yoshino K., Tanimura K., Nakashima A., Eguchi S., Miyamoto T., Hara K., Takehana K., Avruch J., Kikkawa U., Yonezawa K. (2007) The proline-rich Akt substrate of 40kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J. Biol. Chem. 282, 20329–20339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Magnuson B., Ekim B., Fingar D. C. (2012) Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signaling networks. Biochem. J. 144, 1–21 [DOI] [PubMed] [Google Scholar]
- 15. Dibble C. C., Asara J. M., Manning B. D. (2009) Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol. Cell. Biol. 29, 5657–5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gan X., Wang J., Su B., Wu D. (2011) Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-triphosphate. J. Biol. Chem. 286, 10998–11002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brunskill N. J., Stuart J., Tobin A. B., Walls J., Nahorski S. (1998) Receptor-mediated endocytosis of albumin by kidney proximal tubule cells is regulated by phosphatidylinositide 3-kinase. J. Clin. Invest. 101, 2140–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Navé B. T., Ouwens M., Withers D. J., Alessi D. R., Shepherd P. R. (1999) Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem. J. 344, 427–431 [PMC free article] [PubMed] [Google Scholar]
- 19. Chiang G. G., Abraham R. T. (2005) Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J. Biol. Chem. 280, 25485–25490 [DOI] [PubMed] [Google Scholar]
- 20. Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2002) mTOR interacts with Raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 [DOI] [PubMed] [Google Scholar]
- 21. Ma L., Chen Z., Erdjument-Bromage H., Tempst P., Pandolfi P. P. (2005) Phosphorylation and functional inactivation of TSC2 by ERK implications for tuberous sclerosis and cancer pathogenesis. Cell 121, 179–193 [DOI] [PubMed] [Google Scholar]
- 22. Moser B. A., Dennis P. B., Pullen N., Pearson R. B., Williamson N. A., Wettenhall R. E., Kozma S. C., Thomas G. (1997) Dual requirement for a newly identified phosphorylation site in p70s6k. Mol. Cell. Biol. 17, 5648–5655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saitoh M., Pullen N., Brennan P., Cantrell D., Dennis P. B., Thomas G. (2002) Regulation of an activated S6 kinase 1 variant reveals a novel mammalian target of rapamycin phosphorylation site. J. Biol. Chem. 277, 20104–20112 [DOI] [PubMed] [Google Scholar]
- 24. Eddy A. A. (1989) Interstitial nephritis induced by protein-overload proteinuria. Am. J. Pathol. 135, 719–733 [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas M. E., Brunskill N. J., Harris K. P., Bailey E., Pringle J. H., Furness P. N., Walls J. (1999) Proteinuria induces tubular cell turnover: A potential mechanism for tubular atrophy. Kidney Int. 55, 890–898 [DOI] [PubMed] [Google Scholar]
- 26. Nakagawa S., Masuda S., Nishihara K., Inui K. (2010) mTOR inhibitor everolimus ameliorates progressive tubular dysfunction in chronic renal failure rats. Biochem. Pharmacol. 79, 67–76 [DOI] [PubMed] [Google Scholar]
- 27. Tojo A., Onozato M. L., Kurihara H., Sakai T., Goto A., Fujita T. (2003) Angiotensin II blockade restores albumin reabsorption in the proximal tubules of diabetic rats. Hypertens. Res. 26, 413–419 [DOI] [PubMed] [Google Scholar]
- 28. Dan H. C., Cooper M. J., Cogswell P. C., Duncan J. A., Ting J. P., Baldwin A. S. (2008) Akt-dependent regulation of NF-κB is controlled by mTOR and RAPTOR in association with IKK. Genes Dev. 22, 1490–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takaya K., Koya D., Isono M., Sugimoto T., Sugaya T., Kashiwagi A., Haneda M. (2003) Involvement of ERK pathway in albumin-induced MCP-1 expression in mouse proximal tubule cells. Am. J. Physiol. Renal Physiol. 284, F1037–F1045 [DOI] [PubMed] [Google Scholar]
- 30. Diekmann F., Rovira J., Carreras J., Arellano E. M., Bañón-Maneus E., Ramírez-Bajo M. J., Gutiérrez-Dalmau A., Brunet M., Campistol J. M. (2007) Mammalian target of rapamycin inhibition halts the progression of proteinuria in a rat model of reduced renal mass. J. Am. Soc. Nephrol. 18, 2653–2660 [DOI] [PubMed] [Google Scholar]
- 31. Lee J. Y., Chang J. W., Yang W. S., Kim S. B., Park S. K., Park J. S., Lee S. K. (2011) Albumin-induced epithelial-mesenchymal transition and ER stress are regulated through a common ROS-c-Src kinase-mTOR pathway: Effect of mesylate. Am. J. Physiol. Renal Physiol. 300, F1214–F1222 [DOI] [PubMed] [Google Scholar]