Background: RNA protein granules regulate mRNA translation and are involved in neurodegeneration.
Results: Induction of RNA protein granules by G3BP1 or IMP1 changes the amount of long and short tau mRNA and protein.
Conclusion: RNA protein granules differentially affect tau isoform expression.
Significance: This is the first report showing that RNP granules modulate tau isoform expression, potentially linking RNP granule formation to tauopathies.
Keywords: MAPs, Microscopic Imaging, mRNA, Neurite Outgrowth, RNA-binding Protein, G3BP1, IMP1, RNA Protein Granules, Tau mRNA, Tauopathies
Abstract
The neuronal microtubule-associated protein Tau is expressed in different variants, and changes in Tau isoform composition occur during development and disease. Here, we investigate a potential role of the multivalent tau mRNA-binding proteins G3BP1 and IMP1 in regulating neuronal tau expression. We demonstrate that G3BP1 and IMP1 expression induces the formation of structures, which qualify as neuronal ribonucleoprotein (RNP) granules and concentrate multivalent proteins and mRNA. We show that RNP granule formation leads to a >30-fold increase in the ratio of high molecular weight to low molecular weight tau mRNA and an ∼12-fold increase in high molecular weight to low molecular weight Tau protein. We report that RNP granule formation is associated with increased neurite formation and enhanced process growth. G3BP1 deletion constructs that do not induce granule formation are also deficient in inducing neuronal sprouting or changing the expression pattern of tau. The data indicate that granule formation driven by multivalent proteins modulates tau isoform expression and suggest a morphoregulatory function of RNP granules during health and disease.
Introduction
Human TAU protein is encoded by a single gene located on chromosome 17q21 (1). By alternative splicing, six isoforms are produced in the central nervous system with apparent molecular masses between 50 and 70 kDa (“low molecular weight” (LMW)3 TAU). Additional isoforms of ∼120 kDa (“high molecular weight” (HMW) TAU) are expressed in the peripheral nervous system. Mutations, which shift the splicing of TAU toward longer LMW TAU isoforms, can cause frontotemporal dementia (2), and altered TAU isoform composition has been observed in patients with Alzheimer disease (3). Tau mRNA contains a long 3′-untranslated region (3′UTR), suggesting a notable regulation of tau expression on the mRNA level. In fact, several RNA-binding proteins (RBPs), including ras-GAP Src homology 3 domain-binding protein 1 (G3BP1) and insulin-like growth factor II mRNA-binding protein 1 (IMP1, also known as IGF2BP1, zipcode binding protein 1 (ZBP1), coding region determinant-binding protein (CRD-BP), and VICKZ family member 1 (VICKZ1)) have been identified to bind to the 3′UTR of the tau mRNA (4). Both G3BP1 and IMP1 have also been identified as components of ribonucleoprotein (RNP) granules (5).
RNP granules are dynamic, cytosolic, and nonmembranous assemblies of RNAs and proteins, which are involved in translational regulation and mediating mRNA stability (6). They represent subcellular microcompartments that concentrate multivalent macromolecules and control interactions and chemical reactions in response to biological cues (7). RBPs, which have been identified in RNP granules, frequently contain protein-protein interaction domains indicating that reversible low affinity protein-protein interactions are hallmarks of dynamic RNP granules (8). Such interaction domains might include low complexity (LC) regions, which are defined as amino acid sequences with low information content (9). Multiple LC regions are often found in proteins that nucleate RNP granules (8). More generally, proteins containing LC regions tend to have more interactions than those without thus placing them in the “hub” of signal transduction mechanisms due to their ability to bind several different targets (9). Another interaction domain, which is present in multiple copies in several proteins, is the K homology (KH) domain. KH domains are present in a wide variety of nucleic acid-binding proteins, where they bind RNA and can function in RNA recognition (10). Increased formation of RNP granules is a conspicuous feature of several neurodegenerative diseases, including amyotrophic lateral sclerosis, Huntington disease, and some spontaneous cases of Alzheimer disease (11). Thus, inappropriate formation or persistence of RNP granules might be related to pathogenesis. However, the influence of RNP granules on cellular metabolism is largely unknown.
The tau mRNA-binding proteins G3BP1 and IMP1 represent typical multivalent RBPs. G3BP1 is highly expressed in neurons and is present in stress granules. It contains an RNA recognition motif (RRM) and four LC regions (Fig. 1A). Deficiency of G3BP1 in KO mice results in abnormal synaptic plasticity indicating a link to the control of neuronal function (12). IMP1 belongs to a conserved family of RNA-binding proteins and contains two RRMs and four KH domains. IMP1 is present in a class of neuronal RNP granules that have been termed “transport RNPs” (13). IMP1 KO results in global systemic effects such as general growth retardation of mice (14).
FIGURE 1.
Expression of G3BP1 and IMP1 induces the formation of RNP granules in PC12 cells. A, domain structure of human G3BP1, IMP1, and the respective deletion constructs according to SMART analysis. B, three-dimensional representation of rendered z-stacks from fixed PC12 cells expressing EGFP-G3BP1 and -IMP1 (left panel). The box plot on the right shows the volume distribution of the granules. Median (thick horizontal line) and mean (×) are indicated (n = 233–1104 granules). Distribution of 50% of the population is represented by the box; whiskers indicate the range. C, fluorescence photoactivation experiments showing the distribution of PAGFP-tagged full-length G3BP1 and IMP1, and amino- and carboxyl-terminal deletion constructs (G3BP1-N, G3BP1-C, IMP1-RRM, and IMP1-KH) in neuronally differentiated PC12 cells. Photoactivation was performed in part of the cell, which is indicated by the hatched squares in the preactivation images. Scale bars, 10 μm (B) and 20 μm (C).
We reasoned that RNP granule formation could influence the expression pattern of tau because several tau mRNA-binding proteins are components of RNP granules. Previous findings had shown that the expression of tau isoforms changes during development and is different in neurons of the peripheral versus the central nervous system. Furthermore, changes in the expression of LMW tau isoforms suffice to cause tauopathies. Thus, modulation of tau expression by RNP granules could be very relevant for developmental, neurodegenerative, and regenerative events. To our knowledge, this is the first report that shows that induction of RNP granules modulates the amount of different tau mRNA and protein isoforms linking RNP granule formation to mechanisms that control tau expression on the mRNA level.
EXPERIMENTAL PROCEDURES
Constructs and Materials
Eukaryotic expression plasmids for human G3BP1, G3BP1-N(1–255), G3BP1-C(255–466), human IMP1, IMP1-RRM(1–187), and IMP1-KH(187–580) with amino-terminally fused EGFP, mCherry, or PAGFP tags, and 3×PAGFP and fPAGFP as controls, were constructed in pRc/cytomegalovirus (CMV)-based expression vectors (Invitrogen) containing a CMV promoter and kanamycin and neomycin resistance genes. Starting vectors with the G3BP1 and IMP1 sequence were provided by Irith Ginzburg (Weizmann Institute, Israel). IMP1 amino-terminally fused to PAGFP was cloned into the lentiviral vector L26 FSy(1.1)GW (provided by P. Osten, Northwestern University, Chicago), which contains the neuron-specific promoter synapsin 1 (15). Sequences that were introduced by PCR were verified by DNA sequencing. MitoDsRed was constructed by inserting the mitochondrial targeting sequence of human COX VIII in-frame into the pDsRed-N1 vector (Clontech). The COX amino-terminal amino acid-coding sequence was amplified by PCR using the following primers: forward 5′-gtagtcgaccatgtccgtcctgacgccgctgctg-3′ and reverse 5′-gaccggtggatcccccaacgaatggatcttggcgcgcggcactg-3′). Templates for riboprobe synthesis (FISH, Northern blot) were generated with primer pairs (Biomer) binding to either HMW tau (forward 5′-aaccgcagaaggtcgagattttctcacaaa-3′ and reverse 5′-ctttaagttgaggaacccgactgactggcctcc-3′) or LMW tau (forward 5′-ctgaagaagcaggcatcggagacacccc-3′ and reverse 5′-cttgccgcctcccggctggtgc-3′). Probes were synthesized by in vitro transcription utilizing the digoxigenin RNA labeling kit (Roche Applied Science). For mRNA detection, a 45-mer oligo(dT) primer labeled with digoxigenin at the 3′ end was used (Biomer). The following antibodies were used: anti-Tau (mouse, Tau-5; Pharmingen); anti-GFP (rabbit; Invitrogen); anti-tubulin (mouse, DM1A; Sigma); anti-actin (mouse; Calbiochem); anti-digoxigenin (Dianova); anti-G3BP1 (rabbit, Millipore); anti-IMP1 (mouse, D-9; Santa Cruz Biotechnology); peroxidase-, Cy3-, Texas Red-, and Alexa Fluor 488-conjugated anti-mouse and anti-rabbit antibodies (Jackson ImmunoResearch).
Cell Culture
PC12 cells were cultured in 15% serum/DMEM as described previously (16). For induction of neuronal differentiation, the medium was switched to 1% serum/DMEM with 100 ng/ml 7 S mouse NGF (Alomone Laboratories) for 4 days. For morphological analysis, medium was switched to 1% serum/DMEM and culture continued for 24 h in the absence of NGF or 2 days in the presence of NGF. Transfections of PC12 cells were performed with Lipofectamine 2000 (Invitrogen) as described previously (17). Stable lines were generated by selecting individual clones in the presence of Geneticin® as described previously (16). Primary cortical cultures were prepared, cultured, and infected with lentivirus as described previously (18).
Live Cell Imaging, Fluorescence in Situ Hybridization, and Immunocytochemistry
For live cell imaging, cells were plated on 35-mm polylysine- and collagen-coated glass-bottom culture dishes (MatTek Corp.). Before imaging, the medium was exchanged against DMEM without phenol red. Photoactivation and live imaging were performed on a laser scanning microscope (Eclipse TE2000-U inverted; Nikon) equipped with argon (488 nm), helium/neon (543 nm), and blue diode (405 nm) lasers. Activation and automated image acquisition was performed as described earlier (19). Immunocytochemistry was performed as described previously (20). Fluorescence in situ hybridization with digoxigenin-labeled oligonucleotide probes was performed as described previously (21). Cells were transfected with MitoDsRed for tracking of mitochondria. Tracking was performed as described previously (22).
qRT-PCR
Total-RNA isolated from transfected PC12 cells was treated with DNase I (Invitrogen) according to the manufacturer's instructions and used as a template for cDNA synthesis (SuperScript III Reverse Transcriptase, Invitrogen). qRT-PCR was conducted according to standard protocols using DyNAmo ColorFlash SYBR Green qPCR kit (Biozym, Hessisch Oldendorf, Germany) and an iCycler iQ Real Time PCR System (Bio-Rad). To ensure isoform-specific priming, we used an exon junction spanning forward primer with respect to LMW tau. The corresponding sequences were as follows: GGCATGTGACTCAAGCTCGAG (LMW tau, forward) and ATCTTCGCCCCCGTTTTGCCA (LMW tau, reverse); AGATCCCAGAAGGCACCACAG (HMW tau, forward) and AAATCTCGACCTTCTGCGGTT (HMW tau, reverse). Actin was used as reference gene. Data were evaluated as described previously (23) based on three individual, biological replicates, each consisting of three technical replicates.
miRNA Interaction Prediction
miRNA interaction prediction used a database of known and predicted mature miRNAs (24). The reverse complement was aligned based on Smith-Waterman algorithm (25) to the rat tau mRNA. Alignments that resulted in less than seven successive nucleotide (nt) matches were discarded. To predict interactions across exon junctions, a library of 20-nt-long fragments covering the exon borders was generated, and the alignment with the reverse complemented mature rat miRNAs was performed. Alignments with less than six successive nt matches were discarded. To ensure binding across junctions, the alignments had to cover the two neighboring nts of the exon border.
Other Methods
Cell lysates were prepared in RIPA buffer containing protease inhibitors as described previously (16). Protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce). Western blot analysis after separation on 10% polyacrylamide gel and quantification of the blots were performed as described previously (26). RNA preparations were performed using the peqGold total RNA kit (Peqlab). Northern blots were conducted according to standard protocols using total RNA (15 μg per lane) and a hybridization temperature of 66 °C. Relative band intensity in relation to the loading control (rRNA) was assessed by densitometric analysis using a VersaDoc 4000 imaging system (Bio-Rad) and Quantity One software, version 4.6.9. Digoxigenin labeling was performed using a digoxigenin RNA labeling kit (Roche Applied Science). Lentivirus was prepared as described previously (18). Statistical analysis among experimental groups was performed using Student's t test. p values are *, p < 0.05; **, p < 0.01; ***, p < 0.001.
RESULTS
Expression of G3BP1 and IMP1 Induces the Formation of Persistent but Dynamic Neuronal RNP Granules
To scrutinize the role of RNP granules in neuronal metabolism, we first asked the question whether G3BP1 and IMP1 could induce the formation of authentic RNP granules in neuronal cells and whether multiple interaction domains are involved. According to SMART (Simple Modular Architecture Research Tool) analysis (27, 28), G3BP1 contains a nuclear transport factor 2 domain, a single RRM, and four LC regions (Fig. 1A). In agreement with previous observations in non-neuronal cells (29), expression of EGFP-tagged human G3BP1 induced the formation of micrometer-sized granules in the cytosol of PC12 cells with some variation in their size (Fig. 1B). G3BP1 deletion constructs, each of which contained only two LC regions (G3BP1-N and G3BP1-C; see Fig. 1A), were deficient inducing granule formation. They quickly dissipated in the cells, as revealed by photoactivation experiments using photoactivatable GFP(PAGFP)-tagged proteins (Fig. 1C, left panel). This indicates that multimerization of several LC regions is required for the nucleation of granules. IMP1 contains only a single LC region but two RRMs and four KH domains (see Fig. 1A). Expression of EGFP-tagged human IMP1 also induced the formation of granules in PC12 cells, which had similar mean and median volumes as G3BP1-induced granules (Fig. 1B). A deletion construct containing only the two RRMs (IMP1-RRM) failed to nucleate granules, whereas a construct containing the KH domains and the LC region (IMP1-KH) induced granule formation similarly to the full-length construct (Fig. 1C, right panel). The data are in agreement with a previous study in non-neuronal cells, which demonstrated the requirement of at least two KH domains for the induction of RNP granules (30).
Evidence exists that at least some populations of RNP granules associate with the cytoskeleton and that microtubules serve as tracks for their transport (5). To characterize the mobility of the granules, we performed tracking experiments (Fig. 2A, left panel). We observed a very similar speed of granules, which had been induced by G3BP1 or IMP1. Both types of granules were significantly less mobile than mitochondria (Fig. 2A, right panel). To determine a potential involvement of microtubules, we treated the cells with the microtubule-depolymerizing drug colchicine before tracking was performed. Colchicine induced a marked disturbance of microtubule organization and integrity in the cells (Fig. 2B, left panel) and caused a significant increase in the mean displacement of the granules (Fig. 2B, right panel). The data indicate that granules, which had been induced by G3BP1 or IMP1, are anchored to the microtubule network in PC12 cells. Previously, it has been reported that microtubule depolymerization reduced the average velocity of stress granules in HeLa cells (31). Most likely, the apparent discrepancy in our experiment is due to the fact that epithelial cells are flatter than PC12 cells and that analysis is performed in the x-y direction.
FIGURE 2.
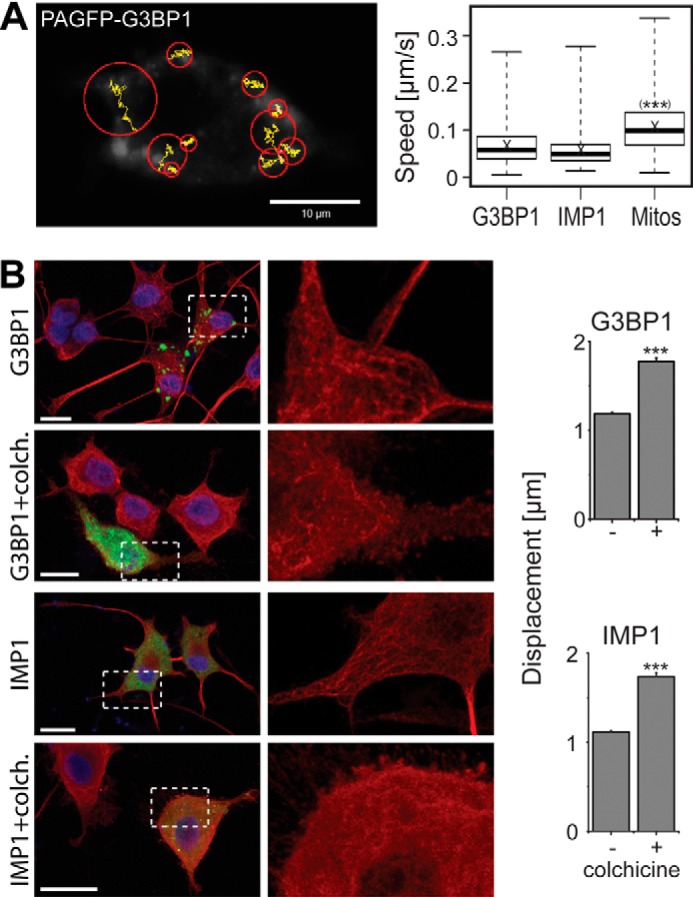
Microtubule interaction restricts the mobility of RNP granules. A, tracking of photoactivated PAGFP-G3BP1 in a PC12 cell. Tracking was performed for 336 s with a frame rate of one image/3 s. Trajectories of individual granules are shown in yellow, and displacement is indicated by red circles. The displacement radius is determined from the distance between the first coordinate of the trajectory and its furthest point during tracking. The box plot shows the speed distribution of PAGFP-G3BP1- and -IMP1-induced granules compared with mitochondria (Mitos). Median (thick horizontal line) and mean (×) are indicated (n = 445–984 granules and 5004 mitochondria). Distribution of 50% of the population is represented by the box; whiskers indicate the range. Speed is calculated from the total trajectory length divided by the period of time a granule was continuously recorded. The mean speed of both types of granules was significantly different from the speed of mitochondria. B, fluorescence micrographs showing the distribution of PAGFP-tagged G3BP1 or IMP1 (green) and microtubules (red) in control or colchicine (colch.)-treated cells. Cells were differentiated for 4 days with NGF, treated without or with colchicine (10 μm for 30 min), fixed, and stained. The enlarged region, which is indicated by the dashed square, shows the disruption of microtubule structure after colchicine treatment (right panel). Nuclei were stained using DAPI (blue). Bar diagrams to the right show the difference in mean displacement radii of cells that have been pretreated with colchicine compared with untreated controls (n = 754–2881 granules per experimental condition). Statistical analysis was performed using Student's t test. ***, p < 0.001. Scale bars, 10 μm.
RNP granules contain a set of different proteins and RNAs, concentrate components in the absence of membranes, and are persistent but still dynamic structures. To determine whether IMP1 and G3BP1 are present in the same granules and whether components shuttle between granules, we co-expressed mCherry-tagged IMP1 and PAGFP-tagged G3BP1 and photoactivated PAGFP-G3BP1 in part of the cell (Fig. 3A). IMP1 and G3BP1 completely co-localized in the photoactivated region (Fig. 3A, dashed squares) indicating that both proteins are present in the same granules. More importantly, fluorescent G3BP1 appeared in IMP1-positive granules outside of the activation region within some seconds (Fig. 3A, arrowheads) indicating dynamic exchange of G3BP1 between granules. To confirm the presence of mRNA in the granules, we performed combined fluorescence in situ hybridization (FISH) using an oligo(dT) probe and immunofluorescence staining against exogenously expressed IMP1. We observed concentration of mRNA in the granules of IMP1-expressing cells (Fig. 3B, arrowheads).
FIGURE 3.
Exogenously expressed G3BP1 and IMP1 localize in dynamic RNP granules. A, co-localization and dynamic exchange of fluorescence-tagged G3BP1 and IMP1 in granules of living neuronally differentiated PC12 cells that have been co-transfected with both constructs. PAGFP-G3BP1 was activated in half of the cell (dashed square) and fluorescence distribution was followed over time. Arrowheads indicate examples of granules outside of the photoactivated region, where G3BP1 appears after 50 and 100 s. B, combined FISH using an oligo(dT) probe and immunofluorescence staining showing enrichment of mRNA in IMP1-containing RNP granules from cells expressing PAGFP-IMP1. Arrowheads indicate examples of cytosolic granules, where the FISH and IMP1 signal co-localize. Scale bars, 20 μm.
IMP1 containing RNP granules had been reported to have a distinct protein composition compared with other types of granules (32). To determine whether the endogenous proteins also co-localize, we performed double immunofluorescence stainings for G3BP1 and IMP1 in fixed cells. We observed that both proteins exhibit a homogeneous distribution in the cytosol of PC12 cells (Fig. 4A, top panel). Induction of stress by treatment with arsenite induced the formation of granules, in which G3BP1 was concentrated (Fig. 4A, bottom panel). Some IMP1 co-localized with granules; however, the majority remained soluble. PAGFP-IMP1 recruited endogenous G3BP1 to the granules, although PAGFP-G3BP1 did not recruit endogenous IMP1 (Fig. 4, B and C). Thus, the data indicate that IMP1 and G3BP1 are able to localize to the same RBP granules but that the two proteins have distinct properties with respect to recruiting other components to granules.
FIGURE 4.
Localization of endogenous G3BP1 and IMP1 in RNP granules. A, fluorescence micrographs showing the distribution of endogenous (endog.) IMP1 and G3BP1 without and with induction of stress by arsenite. Cells were differentiated for 4 days with NGF, treated without or with arsenite (0.5 mm sodium arsenite for 20 min), fixed, and stained. B and C, fluorescence micrographs showing the localization of endogenous G3BP1 after induction of RNP granules by expression of PAGFP-IMP1 (B) and the localization of endogenous IMP1 after induction of RNP granules by expression of PAGFP-G3BP1. Scale bars, 20 μm.
To determine whether the formation of similar granules can also be induced in primary neurons, we expressed PAGFP-IMP1 using a lentiviral vector in cultured cortical neurons prepared from embryonic mice. We observed the formation of many granules in the cell body and processes of the neurons (Fig. 5A, left panel). Live cell imaging revealed that the granules were persistent over time and only slowly moving (Fig. 5A, right panel), which is similar to what we had observed in PC12 cells. Tracking revealed that the speed of the granules was even lower than the speed of the granules in PC12 cells (Fig. 5B).
FIGURE 5.

Expression of IMP1 induces the formation of RNP granules in primary neurons. A, false color image (left panel) of a primary cortical neuron that had been infected with a lentivirus encoding PAGFP-tagged IMP1. Live cell imaging of the enlarged region, which is indicated by the dashed square, is shown at the right. PAGFP-IMP1 was photoactivated in the entire frame. B, algorithm-based tracking of photoactivated PAGFP-IMP1 in primary neurons. Tracking was performed for 336 s with a frame rate of one image/3 s. Trajectories of individual granules are shown in yellow. The box plot shows the speed distribution of PAGFP-IMP1. Median (thick horizontal line) and mean (×) are indicated (n = 2738). Distribution of 50% of the population is represented by the box; whiskers indicate the range. Speed is calculated from the total trajectory length divided by the period of the time a granule was continuously recorded. Scale bars, 20 μm.
Taken together, the data indicate that expression of exogenous G3BP1 or IMP1 in neuronal cells induces the formation of structures, which fulfill the criteria for neuronal RNP granules as follows: (a) they are micrometer-sized and persistent but still dynamic compartments; (b) they concentrate multivalent proteins and mRNA; and (c) they allow molecules to enter and exit rapidly.
Induction of RNP Granule Formation Shifts the Ratio of Tau Isoform Expression
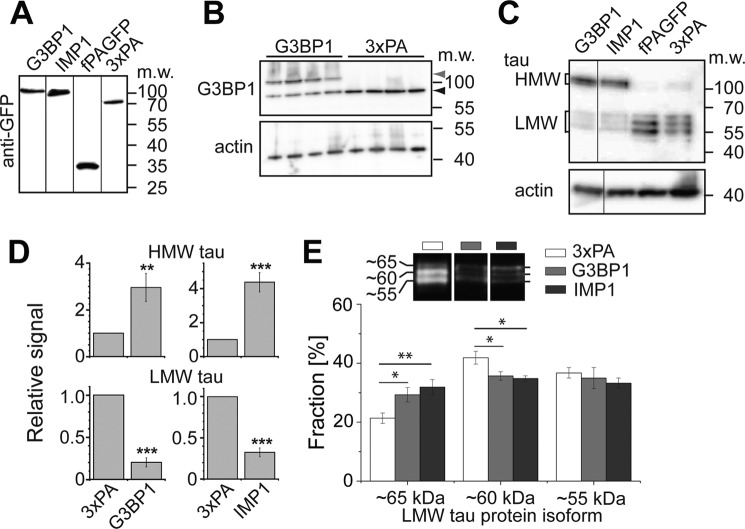
To determine whether the RBP granules affect tau expression, we produced clonal PC12 cell lines, which stably expressed PAGFP-G3BP1, PAGFP-IMP1, farnesylated PAGFP (fPAGFP), and 3×PAGFP. fPAGFP and 3×PAGFP were used as controls. We selected lines with comparable expression of the respective construct as judged by Western blot (Fig. 6A). The level of exogenous G3BP1 expression was moderate (Fig. 6B), about 50% higher than endogenous G3BP1 (151.0 ± 21.9 (S.E.), n = 4; endogenous G3BP1 set as 100%). Interestingly, expression of G3BP1 or IMP1 led to a marked increase in the amount of HMW Tau isoforms, whereas the amount of LMW Tau isoforms was reduced (Fig. 6C). Quantitation confirmed a 3–4-fold increased level of HMW Tau protein and a 3–4-fold decrease in LMW Tau protein (Fig. 6D) indicating that induction of RNP granule formation is associated with an ∼12-fold increase in HMW to LMW Tau protein. PC12 cells express three LMW Tau isoforms at ∼55, 60, and 65 kDa (Fig. 6E, top), respectively, corresponding to 4R Tau (containing exon 10) with or without exons 2 and 3 (Iso3, -5, and -2 according to Fig. 7A) (33). To test whether various LMW Tau isoforms are differentially affected by RNP granule formation, we determined their ratio after Western blotting. In fact, we observed that the proportion of the 65-kDa species was higher in G3BP1- or IMP1-expressing cells compared with control cells (Fig. 6E) indicating that RNP granule formation not only shifts expression from LMW toward HMW Tau but also affects the relative amounts of LMW Tau isoforms. The proportion of the 55-kDa isoform was largely unchanged, whereas the 60-kDa isoform was decreased in G3BP1- and IMP1-expressing cells. This suggests that granule formation leads to an increased proportion of LMW Tau isoforms, which contain exon 3.
FIGURE 6.
RNP granule formation increases the amount of HMW Tau and decreases LMW Tau protein. A, Western blot of lysates from stable PC12 cell lines expressing PAGFP-G3BP1, PAGFP-IMP1, fPAGFP, and 3×PAGFP. For each lane, lysates corresponding to 50 μg of protein were loaded and detected with an antibody against GFP. B, detection of G3BP1 in lysates from stable PC12 cell lines expressing PAGFP-G3BP1 and 3×PAGFP. Endogenous and exogenous G3BP1 are indicated by black and gray arrowheads, respectively. C, Western blot showing expression of HMW Tau, LMW Tau, and actin in PC12 cell lysates prepared from stable neuronally differentiated lines expressing constructs as indicated. For each lane, lysates corresponding to 50 μg (Tau) and 5 μg of protein (actin) were loaded. D, quantitation of the signal intensities from HMW and LMW Tau relative to actin. Mean ± S.E. from 11 to 13 independent experiments are shown. We did not observe a difference between the amount of HMW and LMW Tau amounts between fPAGFP and 3×PAGFP-expressing cells. E, quantitation of the LMW Tau protein isoform fraction in cells expressing 3×PAGFP, PAGFP-G3BP1, and PAGFP-IMP1. A representative Western blot with indication of the three LMW Tau bands that have been quantified is presented on top. Mean ± S.E. from five independent experiments are shown. Statistical analysis was performed using Student's t test. p values are as follows: *, p < 0.05, **, p < 0.01, and ***, p < 0.001.
FIGURE 7.
RNP granule formation shifts the ratio of HMW and LMW tau mRNA isoforms. A, schematic representation of the isoform structure of the tau mRNA-coding sequence. Hybridization positions of the isoform-specific riboprobes are indicated. At the bottom, the respective annealing positions of the isoform-specific primers used for qRT-PCR analyses are shown. B, Northern blot of lysates from PC12 cells stably expressing the indicated constructs. 15 μg of total RNA were loaded per lane and hybridized with the respective isoform-specific probes. The bottom panel shows part of the radiant red-stained gel with ribosomal RNA (rRNA) visible as a loading control. C, quantitation of signal intensities for HMW and LMW tau mRNA isoforms. Bars represent mean ± S.E. of at least three independent experiments. D, quantitation of qRT-PCR analyses using the primer pairs indicated in A, bottom. Bars represent mean ± S.E. of three independent experiments. E, combined FISH and immunofluorescence stainings showing presence of HMW and LMW tau mRNA in IMP1-containing RNP granules from cells expressing PAGFP-IMP1. Arrowheads indicate examples of cytosolic granules, in which the FISH and IMP1 signals colocalize. Statistical analysis was performed using Student's t test. p values are as follows: *, p < 0.05; **, p < 0.01, and p < 0.001. Scale bars, 20 μm.
To analyze whether expression of G3BP1 or IMP1 also changes the ratio of HMW to LMW tau mRNA, we generated riboprobes for HMW or LMW tau mRNA, respectively (Fig. 7A). The calculated size for all tau mRNA isoforms is between ∼5.0 and 6.3 kb due to the long 3′UTR (∼3.8 kb (34)). Northern blotting revealed the presence of major bands at this size, thus confirming expression of full-length tau mRNA transcripts in the cells (Fig. 7B). Quantitation of the respective bands showed an ∼3.5-fold increased level of HMW tau mRNA and an ∼1.5-fold decreased level of LMW tau mRNA (Fig. 7C), indicating that granule formation also induces a shift toward an increased ratio of HMW to LMW tau mRNA. Northern blotting revealed the presence of two fractions of LMW tau mRNA that responded to the presence of G3BP1 or IMP1 to a different extent (Fig. 7B, arrowheads). This might indicate that the riboprobe for LMW tau is not completely specific because it contains sequences, which could also hybridize to HMW tau mRNA (Fig. 7A). To consolidate the Northern blotting data, we also performed qRT-PCR experiments using a primer for an HMW tau-specific exon (exon 4A) and a primer across exon junctions 4 and 5 for LMW tau (Fig. 7A, bottom). The qRT-PCR data confirmed an increased amount of HMW tau mRNA and a decreased LMW tau mRNA level as a consequence of G3BP1 or IMP1 expression (Fig. 7D). The decrease in LMW tau mRNA was much more pronounced than detected by Northern blotting and reflected the decrease in LMW Tau protein considerably better, which is in line with a higher specificity of the quantitation of LMW tau mRNA by qRT-PCR. Based on the qRT-PCR results, induction of RNP granule formation was associated with a >30-fold increase in the ratio of HMW to LMW tau mRNA.
To determine whether LMW and HMW tau mRNA isoforms are present in granules, we performed combined FISH and immunofluorescence stainings using the same riboprobes, which had been used for the Northern blot analyses. We observed a clear concentration of some HMW tau mRNA in IMP1-induced RBP granules (Fig. 7E, top panel, arrowheads). Localization of LMW tau mRNA in the granules was much less prominent, suggesting that HMW and LMW tau mRNA localize to granules to a different extent.
RNP Granule Formation Is Associated with Induction of Neurite Sprouting
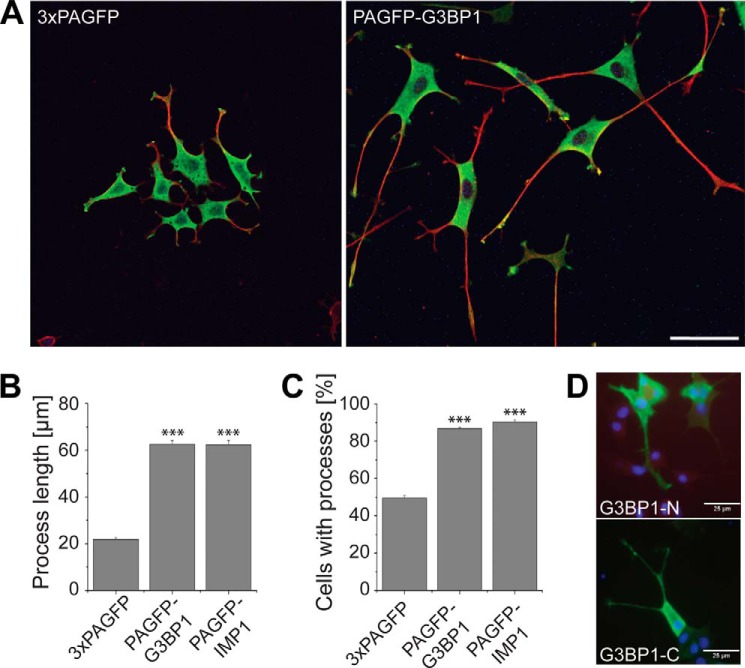
It had been suggested previously that HMW Tau might increase neurite abundance and stability more than LMW Tau isoforms (35, 36), which might reflect the need for greater physical stability of the axons of peripheral neurons compared with neurons from the central nervous system. Thus, neurons with different ratios of HMW to LMW Tau may have different capacities for modulating neuronal morphology and stability during development and maintenance. To determine whether RNP granule formation affects neurite formation and growth, we analyzed PC12 cell lines, which stably expressed G3BP1, IMP1, or 3×PAGFP for morphological changes in the presence or absence of NGF. We observed that G3BP1-expressing cells developed much longer processes after induction of neuronal differentiation with NGF (Fig. 8A). Quantitation revealed that expression of both G3BP1 and IMP1 led to the development of processes, which were ∼3-fold as long as processes from 3×PAGFP-expressing cells (Fig. 8B). Furthermore, cells that were cultured in the absence of NGF also showed significantly increased process induction (Fig. 8C). To determine whether the morphogenetic effect was due to RNP granule formation, we tested the influence of G3BP1-N and G3BP1-C in similar experiments. As shown in Fig. 1C, both deletion constructs were deficient in inducing RBP granules. We did not observe increased process formation or growth of longer neurites with either of the two constructs (Fig. 8D). Furthermore, in contrast to full-length G3BP1- or IMP1-expressing cells, we did not observe a major change in the ratio of HMW to LMW tau expression with both deletion constructs. Thus, the data suggest a morphoregulatory function of RNP granules that is associated with changes in the expression pattern of tau isoforms. However, it should be noted that the data do not exclude that RNP granules affect other factors that are associated with morphological differentiation, which then may influence tau isoform expression. It is also possible that additional factors are responsible for the induction of neuronal sprouting because it has previously been shown that IMP1 is involved in axonal localization of the mRNA of GAP43, which is known to induce neuronal sprouting (37).
FIGURE 8.
RNP granule formation induces neurite sprouting. A, fluorescence micrographs showing the morphology of PC12 cells expressing the indicated constructs. Cells were differentiated for 2 days with NGF, fixed, and stained against GFP (green) and tubulin (red). B, quantitation of process lengths of PC12 cells after differentiation for 2 days with NGF. Mean ± S.E. from 407 and 725 processes from 4 to 7 independent experiments are shown. C, fraction of PC12 cells, which develop processes 24 h after switching to serum-reduced medium. Cells expressed the constructs as indicated. Mean ± S.E. from 60 and 80 cells from five independent experiments are shown. D, fluorescence micrographs showing PC12 cells expressing PAGFP-tagged deletion constructs G3BP1-N and G3BP1-C. Cells were differentiated for 2 days with NGF, fixed, and stained against GFP (green). Nuclei were stained using DAPI (blue). A–D, stable PC12 lines expressing the respective constructs were used. Scale bars, 50 μm (A); 25 μm (D). Statistical analysis was performed using Student's t test. ***, p < 0.001.
DISCUSSION
Most mammalian RNA-binding proteins are constitutively expressed and exhibit a relatively broad RNA-binding specificity. They may exert biological activity by organizing the formation of RNP granules through multimerization of protein-protein or protein-RNA interaction domains such as LC regions or KH domains (11). Here, we have shown that full-length G3BP1 and IMP1 induce the formation of granules that fulfill all criteria for RNP granules as dynamic neuronal microcompartments and permit that molecules enter and exit rapidly. G3BP1 contains four LC regions and IMP1 harbors four KH domains, which can function as independent modules. In agreement with a requirement for multimerization for granule formation, we observed that G3BP1 deletion constructs, each containing only two LC regions, are unable to nucleate RNP granules or to alter tau expression. With respect to IMP1, the presence of the KH domains was sufficient to induce granule formation, which is in agreement with the previous observation that KH domains three and four are responsible for granule formation (30). Thus, the results suggest that G3BP1 and IMP1 contribute to the organization of a supramolecular assembly of RNA and protein components, which then regulate mRNA stability and translation. This is supported by the finding that G3BP1 and IMP1 exert very similar activities on tau isoform expression and neuritogenesis despite having a different molecular organization. The data suggest that specificity for regulating the fate of individual mRNAs is not provided by the organizers of the granules but by a dynamic interaction and exchange of the RNA and protein components within and between granules.
RNP granules such as stress granules are thought to contain a pool of mRNAs that are stalled in the process of translation initiation causing decreased protein synthesis (5, 38). Furthermore, KH domain-containing proteins might induce decay of specific mRNA species (10). This is in agreement with our observation that the amount of LMW Tau isoforms is decreased after granule induction. However, it is remarkable that in our experiments RNP granule formation was associated with an increase in the amount of HMW Tau. The Northern blot and qRT-PCR data could suggest that HMW tau mRNA isoforms are stabilized by RNP granules, although LMW tau mRNA isoforms are destabilized causing the observed shift in the isoform ratio. Such a scenario would imply that mechanisms exist, which are able to distinguish between the different mRNA isoforms. It has previously been shown that some RNA-binding proteins, including G3BP1 and IMP1, interact with the 3′UTR of the tau mRNA (4). However, our Northern blot data indicate that all tau mRNA isoforms are present as full transcripts and contain the complete 3′UTR. Thus, LMW and HMW tau mRNA isoforms might fold in a different manner that changes the access of RNA-binding proteins to the 3′UTR or additional components exist, which selectively interact with the HMW tau mRNA-specific exons (e.g. exons 4A and 6) and mediate increased stability.
It is possible that RNP granule formation, regulation of tau expression, and microRNA-dependent silencing of target transcripts are related. miRNAs constitute a class of ∼22-nt short noncoding RNAs that associate with Argonaute proteins to direct post-transcriptional gene silencing via base pairing (39). In fact, several miRNAs have been implicated in the regulation of tau metabolism, mis-splicing of exon 10, and tau transcript stability (40–42) suggesting that miRNAs could also play a role in shifting the ratio of HMW to LMW tau mRNA. Although the majority of Argonaute proteins and their associated miRNAs are distributed diffusely in the cytoplasm, they can accumulate in RNP granules (43), thus providing a potential explanation how granule formation could affect miRNA-dependent tau transcript stability. An isoform-specific regulation of the amount of tau mRNA by miRNA-dependent silencing would require the presence of miRNAs, which bind to HMW tau mRNA-specific sequences, e.g. exons 4A or 6, or bind across LMW tau mRNA-specific exon junctions (exon 4→5 and exon 5→7) (see Fig. 7A). Indeed, bioinformatic analysis identified 21 different putative miRNAs that bind to exon 4A or 6, and 32 that bind across exon 4→5 or 5→7 (see Table 1).
TABLE 1.
Bioinformatic identification of putative microRNAs with HMW or LMW tau mRNA-specific binding
| Region of binding to tau mRNA | Isoform specificity | miRNA |
|---|---|---|
| Exon 4A | HMW tau mRNA | rno-miR-511-5p, rno-miR-216a-3p, rno-miR-326-5p, rno-miR-511-3p, rno-miR-431, rno-miR-874-3p, rno-miR-496-5p, rno-miR-145-3p, rno-miR-99b-3p, rno-miR-449c-3p, rno-miR-345-3p, rno-miR-1843-3p, rno-miR-3593-3p, rno-miR-193-3p, rno-miR-483-3p, rno-miR-1188-3p, rno-miR-17-1-3p, rno-miR-764–5p, rno-miR-326-3p, rno-miR-328a-3p |
| Exon 6 | HMW tau mRNA | rno-miR-758-5p |
| Across exons 4→5 | LMW tau mRNA | rno-miR-100-3p, rno-let-7e-3p, rno-miR-181a-1-3p, rno-miR-551b-5p, rno-miR-6320, rno-miR-127-3p, rno-let-7a-2-3p, rno-miR-879-3p, rno-miR-708-5p, rno-let-7c-1-3p, rno-miR-344i |
| Across exons 5→7 | LMW tau mRNA | rno-miR-129-2-3p, rno-miR-377-5p, rno-miR-129-1-3p, rno-miR-711, rno-miR-3102, rno-miR-3573-3p, rno-miR-451-3p, rno-miR-652-5p, rno-miR-326-3p, rno-miR-375-5p, rno-miR-150-5p, rno-miR-336-5p, rno-miR-3585-3p, rno-miR-3572, rno-miR-501-5p, rno-miR-188-5p, rno-miR-125b-2-3p, rno-miR-1249, rno-miR-505-3p, rno-miR-455-5p, rno-miR-25-3p |
It should be noted that a major proportion of tau mRNA, in particular LMW tau mRNA, is not localized in RNP granules (see Fig. 7E). This could suggest that quantitatively the location of tau mRNA is not important for the massive change in tau expression but that granule formation affects tau mRNA stability by other mechanisms. This could occur by sequestration of tau mRNA isoform-specific miRNAs (see above) or by adaptive effects on gene expression and translation, which might alter the Tau isoform profile.
Our data indicate that RNP granule formation, which can be driven by multivalent proteins such as G3BP1 or IMP1, changes the expression pattern of tau toward more active isoforms and induces neurite sprouting. Because increased formation of RNP granules has been found in many neurodegenerative diseases, including spontaneous cases of Alzheimer disease (11), such a morphoregulatory effect could be generally relevant for disease progression. Massive somatodendritic sprouting and Tau immunoreactivity has been observed in Alzheimer disease (44), and increased sprouting is a common feature of several neurological diseases (45). Alzheimer and Parkinson disease are considered to be mainly diseases of the central nervous system, whereas HMW Tau isoforms are predominantly expressed in neurons of the peripheral nervous system. It should be noted, however, that involvement of the peripheral nervous system is common in many neurodegenerative proteinopathies, including synucleinopathies and tauopathies, and could be pathogenetically and diagnostically very relevant (46, 47). Furthermore, it has recently been shown that dorsal root ganglion neurons of tau transgenic mice develop tau pathology similar to that found in the brain, and analysis of neurons from the peripheral nervous system might provide an important tool to study treatment options for tauopathies (48). Thus, the study of RNA-binding proteins highlights novel pathways influencing the pathophysiology of neurodegenerative diseases by differential regulation of various tau isoform expression.
Acknowledgments
We thank Anton Gauert, Nadine Schmidt, and Isabel Sterz for help with imaging and morphometric analysis of cells; Katrin Klempahn for providing MitoDsRed; Irith Ginzburg for contributing G3BP1 and IMP1 constructs; Maik Drechsler for help with in situ hybridization, and Vanessa Herkenhoff for technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft Grant SFB 944, Project P1 (to R. B.), and a Lichtenberg fellowship (to F. S.).
- LMW
- low molecular weight
- HMW
- high molecular weight
- KH
- K homology
- LC
- low complexity
- nt
- nucleotide
- PAGFP
- photoactivatable GFP
- RBP
- RNA-binding protein
- RNP
- ribonucleoprotein
- RRM
- RNA recognition motif
- EGFP
- enhanced GFP
- qRT
- quantitative RT
- miRNA
- microRNA.
REFERENCES
- 1. Andreadis A. (2005) Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim. Biophys. Acta 1739, 91–103 [DOI] [PubMed] [Google Scholar]
- 2. Hutton M., Lendon C. L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., Hackett J., Adamson J., Lincoln S., Dickson D., Davies P., Petersen R. C., Stevens M., de Graaff E., Wauters E., van Baren J., Hillebrand M., Joosse M., Kwon J. M., Nowotny P., Che L. K., Norton J., Morris J. C., Reed L. A., Trojanowski J., Basun H., Lannfelt L., Neystat M., Fahn S., Dark F., Tannenberg T., Dodd P. R., Hayward N., Kwok J. B., Schofield P. R., Andreadis A., Snowden J., Craufurd D., Neary D., Owen F., Oostra B. A., Hardy J., Goate A., van Swieten J., Mann D., Lynch T., Heutink P. (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 [DOI] [PubMed] [Google Scholar]
- 3. Conrad C., Zhu J., Conrad C., Schoenfeld D., Fang Z., Ingelsson M., Stamm S., Church G., Hyman B. T. (2007) Single molecule profiling of tau gene expression in Alzheimer's disease. J. Neurochem. 103, 1228–1236 [DOI] [PubMed] [Google Scholar]
- 4. Atlas R., Behar L., Sapoznik S., Ginzburg I. (2007) Dynamic association with polysomes during P19 neuronal differentiation and an untranslated-region-dependent translation regulation of the tau mRNA by the tau mRNA-associated proteins IMP1, HuD, and G3BP1. J. Neurosci. Res. 85, 173–183 [DOI] [PubMed] [Google Scholar]
- 5. Buchan J. R., Parker R. (2009) Eukaryotic stress granules: the ins and outs of translation. Mol. Cell 36, 932–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castello A., Fischer B., Eichelbaum K., Horos R., Beckmann B. M., Strein C., Davey N. E., Humphreys D. T., Preiss T., Steinmetz L. M., Krijgsveld J., Hentze M. W. (2012) Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406 [DOI] [PubMed] [Google Scholar]
- 7. Li P., Banjade S., Cheng H. C., Kim S., Chen B., Guo L., Llaguno M., Hollingsworth J. V., King D. S., Banani S. F., Russo P. S., Jiang Q. X., Nixon B. T., Rosen M. K. (2012) Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kedersha N., Ivanov P., Anderson P. (2013) Stress granules and cell signaling: more than just a passing phase? Trends Biochem. Sci. 38, 494–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coletta A., Pinney J. W., Solís D. Y., Marsh J., Pettifer S. R., Attwood T. K. (2010) Low-complexity regions within protein sequences have position-dependent roles. BMC Syst. Biol. 4, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. García-Mayoral M. F., Hollingworth D., Masino L., Díaz-Moreno I., Kelly G., Gherzi R., Chou C. F., Chen C. Y., Ramos A. (2007) The structure of the C-terminal KH domains of KSRP reveals a noncanonical motif important for mRNA degradation. Structure 15, 485–498 [DOI] [PubMed] [Google Scholar]
- 11. Ramaswami M., Taylor J. P., Parker R. (2013) Altered ribostasis: RNA protein granules in degenerative disorders. Cell 154, 727–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin S., Zekri L., Metz A., Maurice T., Chebli K., Vignes M., Tazi J. (2013) Deficiency of G3BP1, the stress granules assembly factor, results in abnormal synaptic plasticity and calcium homeostasis in neurons. J. Neurochem. 125, 175–184 [DOI] [PubMed] [Google Scholar]
- 13. Zeitelhofer M., Karra D., Macchi P., Tolino M., Thomas S., Schwarz M., Kiebler M., Dahm R. (2008) Dynamic interaction between P-bodies and transport ribonucleoprotein particles in dendrites of mature hippocampal neurons. J. Neurosci. 28, 7555–7562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen T. V., Hammer N. A., Nielsen J., Madsen M., Dalbaeck C., Wewer U. M., Christiansen J., Nielsen F. C. (2004) Dwarfism and impaired gut development in insulin-like growth factor II mRNA-binding protein 1-deficient mice. Mol. Cell. Biol. 24, 4448–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dittgen T., Nimmerjahn A., Komai S., Licznerski P., Waters J., Margrie T. W., Helmchen F., Denk W., Brecht M., Osten P. (2004) Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc. Natl. Acad. Sci. U.S.A. 101, 18206–18211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gauthier-Kemper A., Weissmann C., Golovyashkina N., Sebö-Lemke Z., Drewes G., Gerke V., Heinisch J. J., Brandt R. (2011) The frontotemporal dementia mutation R406W blocks tau's interaction with the membrane in an annexin A2-dependent manner. J. Cell Biol. 192, 647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fath T., Eidenmüller J., Brandt R. (2002) Tau-mediated cytotoxicity in a pseudohyperphosphorylation model of Alzheimer's disease. J. Neurosci. 22, 9733–9741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bakota L., Brandt R., Heinisch J. J. (2012) Triple mammalian/yeast/bacterial shuttle vectors for single and combined lentivirus- and Sindbis virus-mediated infections of neurons. Mol. Genet. Genomics 287, 313–324 [DOI] [PubMed] [Google Scholar]
- 19. Weissmann C., Reyher H. J., Gauthier A., Steinhoff H. J., Junge W., Brandt R. (2009) Microtubule binding and trapping at the tip of neurites regulate tau motion in living neurons. Traffic 10, 1655–1668 [DOI] [PubMed] [Google Scholar]
- 20. Brandt R., Léger J., Lee G. (1995) Interaction of tau with the neural plasma membrane mediated by tau's amino-terminal projection domain. J. Cell Biol. 131, 1327–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swanger S. A., Bassell G. J., Gross C. (2011) High-resolution fluorescence in situ hybridization to detect mRNAs in neuronal compartments in vitro and in vivo. Methods Mol. Biol. 714, 103–123 [DOI] [PubMed] [Google Scholar]
- 22. Sergé A., Bertaux N., Rigneault H., Marguet D. (2008) Dynamic multiple-target tracing to probe spatiotemporal cartography of cell membranes. Nat. Methods 5, 687–694 [DOI] [PubMed] [Google Scholar]
- 23. Simon P. (2003) Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics 19, 1439–1440 [DOI] [PubMed] [Google Scholar]
- 24. Kozomara A., Griffiths-Jones S. (2011) miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 39, D152–D157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith T. F., Waterman M. S. (1981) Identification of common molecular subsequences. J. Mol. Biol. 147, 195–197 [DOI] [PubMed] [Google Scholar]
- 26. Leschik J., Welzel A., Weissmann C., Eckert A., Brandt R. (2007) Inverse and distinct modulation of tau-dependent neurodegeneration by presenilin 1 and amyloid-β in cultured cortical neurons: evidence that tau phosphorylation is the limiting factor in amyloid-β-induced cell death. J. Neurochem. 101, 1303–1315 [DOI] [PubMed] [Google Scholar]
- 27. Letunic I., Doerks T., Bork P. (2012) SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 40, D302–D305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schultz J., Milpetz F., Bork P., Ponting C. P. (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. U.S.A. 95, 5857–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tourrière H., Chebli K., Zekri L., Courselaud B., Blanchard J. M., Bertrand E., Tazi J. (2003) The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160, 823–831 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Farina K. L., Huttelmaier S., Musunuru K., Darnell R., Singer R. H. (2003) Two ZBP1 KH domains facilitate β-actin mRNA localization, granule formation, and cytoskeletal attachment. J. Cell Biol. 160, 77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nadezhdina E. S., Lomakin A. J., Shpilman A. A., Chudinova E. M., Ivanov P. A. (2010) Microtubules govern stress granule mobility and dynamics. Biochim. Biophys. Acta 1803, 361–371 [DOI] [PubMed] [Google Scholar]
- 32. Jønson L., Vikesaa J., Krogh A., Nielsen L. K., Hansen Tv, Borup R., Johnsen A. H., Christiansen J., Nielsen F. C. (2007) Molecular composition of IMP1 ribonucleoprotein granules. Mol. Cell. Proteomics 6, 798–811 [DOI] [PubMed] [Google Scholar]
- 33. Takuma H., Arawaka S., Mori H. (2003) Isoforms changes of tau protein during development in various species. Brain Res. Dev. Brain Res. 142, 121–127 [DOI] [PubMed] [Google Scholar]
- 34. Sadot E., Marx R., Barg J., Behar L., Ginzburg I. (1994) Complete sequence of 3′-untranslated region of Tau from rat central nervous system. Implications for mRNA heterogeneity. J. Mol. Biol. 241, 325–331 [DOI] [PubMed] [Google Scholar]
- 35. Teng K. K., Georgieff I. S., Aletta J. M., Nunez J., Shelanski M. L., Greene L. A. (1993) Characterization of a PC12 cell sub-clone (PC12-C41) with enhanced neurite outgrowth capacity: implications for a modulatory role of high molecular weight tau in neuritogenesis. J. Cell Sci. 106, 611–626 [DOI] [PubMed] [Google Scholar]
- 36. Boyne L. J., Tessler A., Murray M., Fischer I. (1995) Distribution of Big tau in the central nervous system of the adult and developing rat. J. Comp. Neurol. 358, 279–293 [DOI] [PubMed] [Google Scholar]
- 37. Donnelly C. J., Willis D. E., Xu M., Tep C., Jiang C., Yoo S., Schanen N. C., Kirn-Safran C. B., van Minnen J., English A., Yoon S. O., Bassell G. J., Twiss J. L. (2011) Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J. 30, 4665–4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson P., Kedersha N. (2006) RNA granules. J. Cell Biol. 172, 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boudreau R. L., Jiang P., Gilmore B. L., Spengler R. M., Tirabassi R., Nelson J. A., Ross C. A., Xing Y., Davidson B. L. (2014) Transcriptome-wide discovery of microRNA binding sites in human brain. Neuron 81, 294–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith P. Y., Delay C., Girard J., Papon M. A., Planel E., Sergeant N., Buée L., Hébert S. S. (2011) MicroRNA-132 loss is associated with tau exon 10 inclusion in progressive supranuclear palsy. Hum. Mol. Genet. 20, 4016–4024 [DOI] [PubMed] [Google Scholar]
- 41. Hébert S. S., Sergeant N., Buée L. (2012) MicroRNAs and the regulation of tau metabolism. Int. J. Alzheimers Dis. 2012, 406561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dickson J. R., Kruse C., Montagna D. R., Finsen B., Wolfe M. S. (2013) Alternative polyadenylation and miR-34 family members regulate tau expression. J. Neurochem. 127, 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leung A. K., Calabrese J. M., Sharp P. A. (2006) Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc. Natl. Acad. Sci. U.S.A. 103, 18125–18130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ihara Y. (1988) Massive somatodendritic sprouting of cortical neurons in Alzheimer's disease. Brain Res. 459, 138–144 [DOI] [PubMed] [Google Scholar]
- 45. Sohn Y. K., Ganju N., Bloch K. D., Wands J. R., de la Monte S. M. (1999) Neuritic sprouting with aberrant expression of the nitric oxide synthase III gene in neurodegenerative diseases. J. Neurol. Sci. 162, 133–151 [DOI] [PubMed] [Google Scholar]
- 46. Wakabayashi K., Mori F., Tanji K., Orimo S., Takahashi H. (2010) Involvement of the peripheral nervous system in synucleinopathies, tauopathies and other neurodegenerative proteinopathies of the brain. Acta Neuropathol. 120, 1–12 [DOI] [PubMed] [Google Scholar]
- 47. Sica R. E., Pereyra S., Mangone C. A. (1998) Loss of motor units in Alzheimer's disease. Electromyogr. Clin. Neurophysiol. 38, 475–479 [PubMed] [Google Scholar]
- 48. Mellone M., Kestoras D., Andrews M. R., Dassie E., Crowther R. A., Stokin G. B., Tinsley J., Horne G., Goedert M., Tolkovsky A. M., Spillantini M. G. (2013) Tau pathology is present in vivo and develops in vitro in sensory neurons from human P301S tau transgenic mice: A system for screening drugs against tauopathies. J. Neurosci. 33, 18175–18189 [DOI] [PMC free article] [PubMed] [Google Scholar]