Background: The physiological role of α1-adrenergic receptor (AR) signaling in bone is largely unknown.
Results: α1-AR signaling negatively regulates Bmp4 expression by up-regulating the transcriptional factor Nfil3/E4BP4 in osteoblasts.
Conclusion: α1-AR signaling regulates clock genes and Bmp4 expression in osteoblasts.
Significance: The regulation of clock genes by α1-AR signaling in osteoblasts may contribute to the physiological significance of the circadian clockwork in bone metabolism.
Keywords: Adrenergic Receptor, Bone Morphogenetic Protein (BMP), Clock Gene, Osteoblast, Transcription Factor, α1-Adrenergic Receptor, Nfil3, Bmp4
Abstract
Several studies have demonstrated that the α1-adrenergic receptor (AR) plays an important role in regulating cell growth and function in osteoblasts. However, the physiological role of α1-AR signaling in bone metabolism is largely unknown. In this study, the stimulation of phenylephrine (PHE), a nonspecific α1-AR agonist, increased the transcriptional factor Nfil3/E4BP4 and led to the rhythmic expression of bone morphogenetic protein 4 (Bmp4) in MC3T3-E1 osteoblastic cells. We also showed that Bmp4 mRNA expression peaked in bone near zeitgeber time 8 in a 24-h rhythm. Furthermore, the expression of Nfil3 and Bmp4 displayed a circadian pattern with opposing phases, which suggested that Nfil3 repressed the expression of the Bmp4 gene during a circadian cycle. On a molecular level, both loss-of-function and gain-of-function experiments demonstrated that Nfil3/E4BP4 negatively regulated Bmp4 expression in osteoblasts. Furthermore, the systemic administration of PHE increased the expression of Nfil3 mRNA in bone, whereas it decreased that of Bmp4 mRNA. The expression of Bmp4 mRNA was decreased significantly by exposure to PHE, and this was concomitant with the increase in Nfil3 binding to the D-box-containing Bmp4 promoter region in MC3T3-E1 cells, which indicates that the expression of Nfil3 by α1-AR signaling can bind directly to the Bmp4 promoter and inhibit Bmp4 expression in osteoblasts. Our results suggest that α1-AR signaling regulates clock genes and Bmp4 expression in osteoblasts. Moreover, α1-AR signaling negatively regulated Bmp4 expression by up-regulating the transcriptional factor Nfil3/E4BP4 in osteoblasts.
Introduction
The biological effects of norepinephrine and epinephrine are mediated by α- and β-adrenergic receptor (AR)2 subtypes, which show distinct tissue distribution patterns and transmit signals through distinct biochemical pathways (1). α1-ARs have been shown to be crucially involved in controlling vascular contractility, glucose metabolism, and behavioral responses (2). α1-ARs are seven-transmembrane domain receptors that are coupled to the heterotrimeric G proteins of the Gq/11 and G12/G13 families (3). Gq/11 family G proteins couple numerous G protein-coupled receptors to the activation of phospholipase C-β (PLC-β), PKC, and intracellular calcium release and, thereby, regulate the basic functions of these diverse cell types.
Bone is a metabolically active organ that maintains continuous remodeling throughout life. Several studies, including ours, have demonstrated that the sympathetic nervous system regulates bone remodeling in part through the β-AR (4–6). On the other hand, it has been suggested that α1-AR signaling also plays an important role in the regulation of cell growth and function through the sympathetic nervous system in osteoblasts (7–10). Norepinephrine has been shown to increase cell proliferation by suppressing K+ channels via Gi/o-coupled α1B-ARs in human osteoblasts (11). However, their physiological role in bone remains unclear.
The mammalian circadian timing system, in which daily light-dark cycles phase-entrain the master clock in the suprachiasmatic nucleus which, in turn, synchronizes subsidiary oscillators in most peripheral cells and drives the daily rhythms of physiology and behavior (12). Circadian clocks are found in most or all tissues in mammals (13) and play important roles in local and systemic physiology (14–16). Circadian rhythms are driven by networks of transcriptional-translational autoregulatory loops and have a cycle that lasts ∼24 h. The basic helix-loop-helix transcription factor brain and muscle ARNT-like 1 (Bmal1) and circadian locomotor output cycle kaput (Clock) heterodimerize and drive the transcription of the core clock genes Period (Per1, Per2, and Per3) and the Cryptochrome (Cry1 and Cry2) genes by binding E-box elements within gene sequences (17). In addition to core clock factors, genes involved in basic cellular events are also regulated by transcriptional-translational autoregulatory loops (17). The circadian system coordinates rhythmic physiological processes at both cellular and tissue levels by regulating the rhythmic expression of these clock-controlled genes. A growing number of studies have recently focused on circadian oscillators in bone and growth plate cartilage (18–20). Sympathetic signaling and dexamethasone have been shown to entrain circadian oscillators and the rhythmic expression of core clock genes such as Bmal1 and Period (Per1, Per2, and Per3) in human osteoblasts (18). Furthermore, dexamethasone mediated circadian timing to osteoclasts (21). Therefore, suprachiasmatic nucleus-controlled circadian hormone rhythms and sympathetic tone appear to play a central role in this process. However, how the circadian oscillator coordinates and regulates bone physiology in response to the sympathetic nervous system and α1-AR signaling in osteoblasts remains elusive.
In this study, we examined the possible involvement of α1-AR signaling in osteoblasts to the circadian rhythms caused by the sympathetic nervous system in bone metabolism. We showed that phenylephrine (PHE), a nonspecific α1-AR agonist, induced the rhythmic mRNA expression of bone morphogenetic protein-4 (Bmp4), which is one of the most important regulators of bone metabolism in MC3T3-E1 osteoblastic cells. Moreover, α1-AR signaling negatively regulated Bmp4 expression by up-regulating the transcriptional factor Nfil3/E4BP4 in osteoblasts.
EXPERIMENTAL PROCEDURES
Mice
Male, 8-week-old C57BL/6J mice (Japan SLC Inc., Hamamatsu, Japan) were randomized by weight, assigned to groups, and acclimated to their cages for 1 week prior to the experiment. They were treated in accordance with the Guidelines for Animal Experiments at the School of Dentistry, Aichi-Gakuin University. Food and water were available ad libitum. Animals were housed together in automatically controlled conditions of temperature (23 ± 1 °C) and humidity (50 ± 10%) under a 12-hour light, 12-hour dark cycle or constant dark conditions. Zeitgeber time (ZT) 0 under the light/dark cycle was designated as lights on and ZT12 as lights off.
Drugs and Treatment
α1-Adrenergic receptor pathways were stimulated using the nonspecific α1-AR agonist PHE (Sigma-Aldrich). Mice were killed by CO2 asphyxiation after the drug treatment. Bone tissue samples were dissected and kept at −80 °C for total RNA until assayed.
MC3T3-E1 Cell Cultures
MC3T3-E1 cells were purchased from the RIKEN Cell Bank. MC3T3-E1 cells were cultured in α-MEM containing 10% FBS and 1% penicillin/streptomycin at 37 °C in a 5% CO2 atmosphere. To induce differentiation, the culture medium was replaced with α-MEM containing 50 μg/ml ascorbic acid and 5 mm β-glycerophosphate. The culture medium was changed every 2–3 days.
Generation of MC3T3-E1 Cell Lines with the Stable Expression of Nfil3
MC3T3-E1 cells were maintained in α-MEM supplemented with 10% FBS, 1% penicillin/streptomycin at 37 °C in a 5% CO2 atmosphere. 2 μg of pcDNA3-negative and full-length-Nfil3 were stably transfected into MC3T3-E1 cells by FuGENE HD, as specified by the method of the manufacturer (Promega), followed by drug selection with 800 μg/ml of the neomycin analog G418. Resistant colonies were selected and expanded. Twenty stable lines (Nfil3 series) were established. All further experiments were conducted with three of these lines, Nfil3–1, Nfil3–2, and Nfil3–3, which expressed high levels of Nfil3, respectively.
siRNA Nucleofection
MC3T3-E1 cells were grown in α-MEM supplemented with 10% FBS and 1% penicillin/streptomycin to ∼70% confluency, followed by transient transfection with either siRNA targeting Nfil3 or non-silencing RNA diluted in Opti-MEM using Lipofectamine RNAiMAX (Invitrogen) according to the protocol of the manufacturer. Silencer Select siRNAs were used (Ambion/Applied Biosystems). Both the Nfil3 siRNAs and non-silencing RNA were used at final concentrations of 10 nm. The medium was then replaced with fresh medium. Cells were harvested for total RNA extraction at the indicated time points.
RNA Extraction and Quantitative RT-PCR
Total RNA was isolated with an RNeasy mini kit (Qiagen) according to the protocol of the manufacturer. One microgram of RNA was reverse-transcribed into cDNA using the QuantiTect reverse transcription kit according to the protocol of the manufacturer (Qiagen). Gene expression was analyzed with the Step-One-Plus real-time PCR system with Step One software v2.0 (Applied Biosystems). Reactions were performed in 20-μl volumes using a QuantiTect SYBR Green PCR kit (Qiagen). Cycling conditions were 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The relative quantification of each mRNA was performed using the comparative quantity (copies) method, creating standard curves. Relative standard curves were created with serially diluted cDNA samples that were reverse-transcribed from RNA samples. This concentration range of standard curve samples was determined to be well within the detection level and sensitivity of the quantitative PCR assay. The relative quantity for each sample was normalized to the average level of the constitutively expressed housekeeping gene Gapdh. The following primers were used: Gapdh, 5′-TGGAGAAACCTGCCAAGTATG-3′ (forward) and 5′-GGAGACAACCTGGTCCTCAG-3′ (reverse); Nfil3, 5′-CAGTGCAGGTGACGAACATT-3′ (forward) and 5′-TTCCACCACACCTGTTTTGA-3 (reverse); Per2, 5′-ATGCTCGCCATCCACAAGA-3′ and 5′-GCGGAATCGAATGGGAGAAT-3′ (reverse); Bmp4, 5′-GTGACACGGTGGGAAACTTTCGAT-3′ (forward) and 5′-CACCTCAATGGCCAGCCCATAAT-3′ (reverse).
Western Blot Analysis
Cells were washed with PBS and collected in 20 mm Tris-HCl buffer (pH 7.4) containing 1 mm EDTA, 1 mm EGTA, and the aforementioned inhibitors of phosphatases and proteases. Cell homogenates were mixed at a volume ratio of 4:1 with 10 mm Tris-HCl buffer (pH 6.8) containing 10% glycerol, 2% SDS, 0.01% bromphenol blue, and 5% mercaptoethanol, followed by boiling at 100 °C for 10 min, as described previously (22). Each aliquot of 20 μg of protein was subjected to electrophoresis on a polyacrylamide gel containing 0.1% SDS at a constant current of 10 mA/plate at room temperature and was subsequently transferred to a polyvinylidene fluoride membrane. The membranes were subjected to blocking with 5% skim milk dissolved in TBST (20 mm Tris-HCl (pH 7.5) containing 137 mm NaCl and 0.05% Tween 20), followed by a reaction with antibodies against Nfil3 adequately diluted in 1% skim milk/TBST. Finally, proteins were reacted with an anti-rabbit IgG antibody conjugated with peroxidase and detected with the aid of ECL detection reagents.
Construction of Plasmids and Luciferase Reporter Gene Assays
MC3T3-E1 cells were seeded into 12-well tissue culture plates and cultured for 24 h in α-MEM containing 10% FBS. Cells were transiently cotransfected with 200 ng of firefly luciferase reporter plasmids and 400 ng of pcDNA3 plasmids by using FuGENE HD (Promega). Firefly luciferase (Luc) and Renilla luciferase activities were measured using the Dual-Luciferase reporter assay system and GloMax 20/20n luminometer 48 h after transfection according to the instructions of the manufacturer (Promega). The Renilla luciferase plasmid pRL-TK (Promega) was used as an internal control for transfection efficiency. For knockdown experiments, cells were cotransfected with 20 nmol Stealth Select RNAi siRNA (Ambion/Applied Biosystems), 200 ng of luciferase reporter plasmids, and 10 ng of pRL-TK plasmid by using Lipofectamine 2000 (Invitrogen). A 712-bp fragment (−2245 to −1534) of the Bmp4 promoter was amplified by KOD-plus Neo DNA polymerase (Toyobo) with mouse genomic DNA and the forward primer containing a KpnI restriction site (5′-cggggtaccCAGCTGTCCCCAGAGATAACA-3′) and the reverse primer containing a KpnI restriction site (5′-cccaagcttGGTCTCCATGAGCCCTTTCT-3′). Following digestion with restriction enzymes (underlined), the purified PCR product was cloned into the KpnI and HindIII sites of the pGL3 luciferase vector (Promega). The deletion mutant of the Bmp4 promoter plasmid (ΔDboxBmp4Luc, deletion of the putative Nfil3/E4BP4 binding site (−1815 to −1785)) was derived from the Bmp4-luc plasmid by PCR amplification using Prime STAR mutagenesis (Takara) and the following primers: 5′-CCCCAGGGAGTTTTAATTTTGTGTT-3′ and 5′-TAAAACTCCCTGGGGCATCAGGCTCT-3′. The correct sequences of the subcloned fragments were confirmed by complete nucleotide sequencing.
ChIP Assay
The ChIP assay was performed using ChIP-IT Express (Active Motif) according to the instructions of the manufacturer. Cell extracts were immunoprecipitated with polyclonal rabbit anti-Nfil3 antibody (Santa Cruz Biotechnology) and protein G magnetic beads for 4 h at 4 °C. Purified DNA fragments were subjected to PCR. PCR was addressed for the Bmp4 promoter region (−1906 to −1758) and the exon 1 region (+243 to +461) and performed using the following primers: Bmp4 promoter region, 5′-GCACCCCTAAGGACAACGAA-3′ and 5′-CAGGCTCTGTGCTCCCATAG-3′; exon 1 region, 5′-TCTAGAGGTCCCCAGAAGCA-3′ and 5′-AGGTGCCTTTTAGCCTCCAT-3′. PCR products were also analyzed on 2.5% agarose gels.
Data Analysis
All data are expressed as the mean ± S.E. Two-tailed Student's t test combined with Bonferroni's correction following a one-way analysis of variance was used for multiple comparisons. Differences with p < 0.05 were considered significant. The circadian rhythmicity in gene expression was determined by the single Cosinor method using Timing Series Analysis Single Cosinor 6.3 software (Expert Soft Tech). The period was determined using a chronobiometric ellipse test (18).
RESULTS
α1-AR Signaling Up-regulated the Transcriptional Factor Nfil3 in Osteoblasts
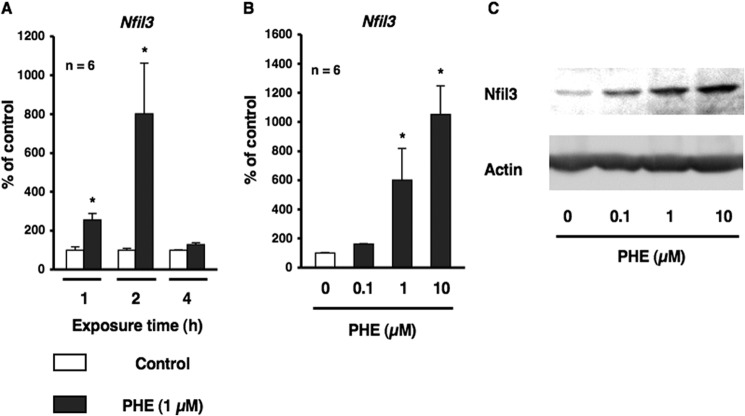
On the basis of previous findings in which sympathetic signaling, particularly β-AR in osteoblasts, has been shown to entrain circadian oscillators and also induced the transcription of nuclear factor IL-3 (Nfil3), which encodes E4-binding protein 4 (E4BP4) (18, data not shown), we investigated whether α1-AR signaling contributed to the regulation of core clock genes in osteoblasts. To address this hypothesis, we initially characterized Nfil3 mRNA expression by α1-AR signaling in MC3T3-E1 osteoblastic cells. Total RNA was extracted from MC3T3-E1 osteoblastic cells following exposure to PHE for 1, 2, and 4 h and was subsequently analyzed by real-time qRT-PCR. As shown in Fig. 1A, the expression of Nfil3 mRNA was significantly increased ∼3- and 8-fold after 1 and 2 h following exposure to PHE. The exposure to PHE induced Nfil3 mRNA and protein expression in a concentration-dependent manner (Fig. 1, B and C). In addition, pretreatment with the α1-AR antagonist prazosin completely inhibited PHE-induced Nfil3 expression, as determined by real-time qRT-PCR analysis in MC3T3-E1 osteoblastic cells, which suggests that PHE-induced Nfil3 expression in osteoblasts may be mediated by α1-AR signaling (Fig. 2).
FIGURE 1.
α1-AR signaling up-regulated the transcriptional factor Nfil3 in MC3T3-E1 cells. A, Nfil3 mRNA expression in MC3T3-E1 cells. Cells were treated with 1 μm PHE for 1, 2, and 4 h, harvested, and then processed for real-time qRT-PCR. Each value represents the mean ± S.E. of six separate experiments. *, p < 0.05, significantly different from each control value obtained in MC3T3-E1 cells cultured in the absence of PHE. B, Nfil3 mRNA was up-regulated by PHE in a concentration-dependent manner in MC3T3-E1 cells. Cells were treated with PHE at 0.1–10 μm for 2 h, harvested, and processed for real-time qRT-PCR. Each value represents the mean ± S.E. of six separate experiments. *, p < 0.05, significantly different from each control value obtained in MC3T3-E1 cells cultured in the absence of PHE. C, the transcriptional factor Nfil3 was increased by PHE in a dose-dependent manner in MC3T3-E1 cells. Cells were treated with PHE at 0.1–10 μm for 4 h, harvested, and then processed for Western blotting. A representative result of three individual experiments is shown.
FIGURE 2.
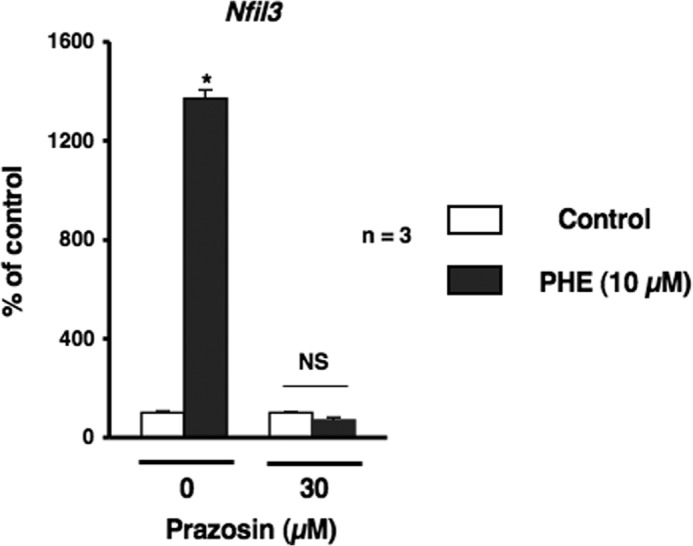
α1-AR signaling mediated Nfil3 mRNA expression after the PHE stimulation in MC3T3-E1 cells. Cells were incubated for 2 h in the presence of PHE with prazosin at a concentration of 10 μm, followed by the determination of Nfil3 levels by real-time qRT-PCR. Each value represents the mean ± S.E. of three separate experiments. *, p < 0.05, significantly different from each control value. NS, not significant.
Bmp4 Is a Circadian-regulated Gene in Osteoblasts
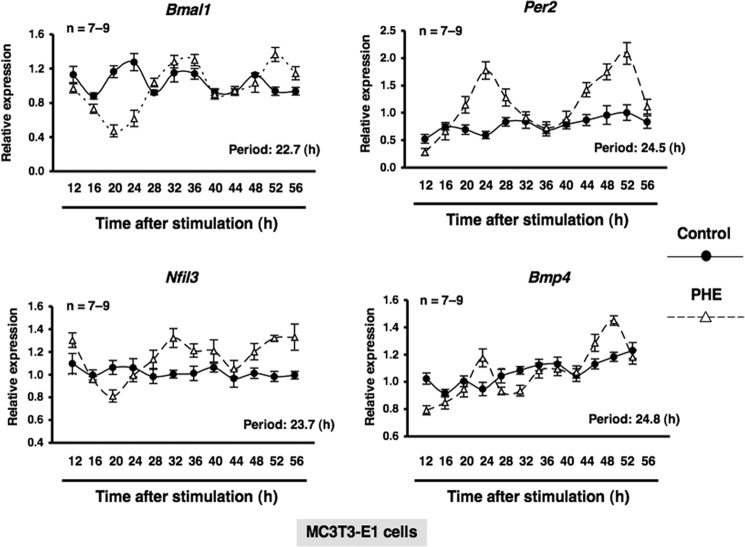
BMPs are members of the TGF-β superfamily, which regulates cell growth, differentiation, and function, and plays an important role in regulating normal physiological functions (23). To understand the physiological function of the circadian clockwork in bone metabolism and identify circadian-regulated genes affected by α1-AR signaling in osteoblasts, we next evaluated the expression of Nfil3 and Bmp4 in bone. We found that Nfil3 mRNA expression peaked near ZT20 in a 24-h rhythm in mouse bone samples harvested during a circadian cycle. In contrast, bone Bmp4 mRNA expression, which peaked at ZT8, displayed a circadian pattern with opposing phases, which suggested that Nfil3 repressed Bmp4 gene expression during a circadian cycle (Fig. 3). To determine whether α1-AR signaling in osteoblasts could mediate the rhythmic expression of a subset of genes, including canonical core clock genes and Bmp4, cells were treated with PHE at 10 μm, and mRNA expression was determined by real-time qRT-PCR analysis at the indicated time points between 12 and 52 h. The exposure to PHE entrained Per2, Bmal1, Per2, Nfil3, and Bmp4 with rhythmic expression in MC3T3-E1 osteoblastic cells (period of oscillation: Bmal1, 22.7 h; Per2, 24.5 h; Nfil3, 23.7 h; Bmp4, 24.8 h), which indicates that Bmp4 is one of the circadian-regulated genes that are regulated in response to α1-AR signaling in osteoblasts (Fig. 4).
FIGURE 3.
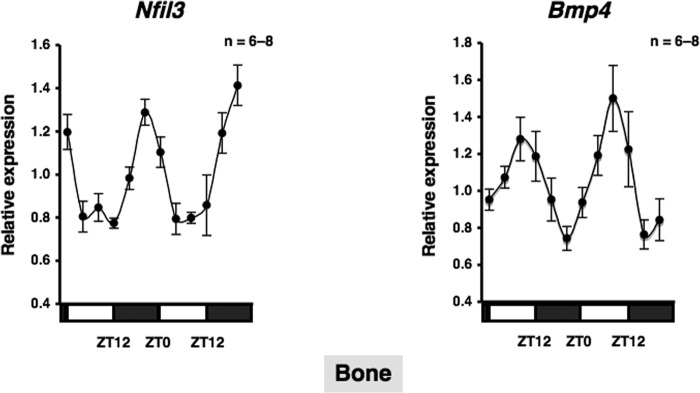
Nfil3 and Bmp4 in bone. A representation of the expression of Nfil3 and Bmp4 in bone from C57BL/6J mice under light/dark cycle conditions. Bone was obtained from C57BL/6J mice every 4 h. Total RNA was isolated, and the level of mRNA was determined by real-time qRT-PCR using specific primers for Nfil3 and Bmp4. Relative mRNA levels were normalized to the Gapdh level. Data represent the mean ± S.E., n = 6–8 animals. White boxes, light period; black boxes, dark period.
FIGURE 4.
α1-AR signaling mediated circadian rhythmicity in MC3T3-E1 cells. Expression profiles of the Bmal1 (upper left), Per2 (upper right), Nfil3 (lower left), and Bmp4 (lower right) transcript generated by PHE in MC3T3-E1 osteoblastic cells. Total RNA samples were collected at the indicated times after PHE treatment. qRT-PCR analyses of transcript levels were performed using their specific primers. Gapdh was used as an internal control. Each value represents the mean ± S.E. (n = 7–9 independent experiments). *, p < 0.05, significantly different from each control value. The Cosinor analysis method was used to determine the rhythmic expression. The lines show the fitting curves.
Nfil3 Negatively Regulated Bmp4 Expression in MC3T3-E1 Cells
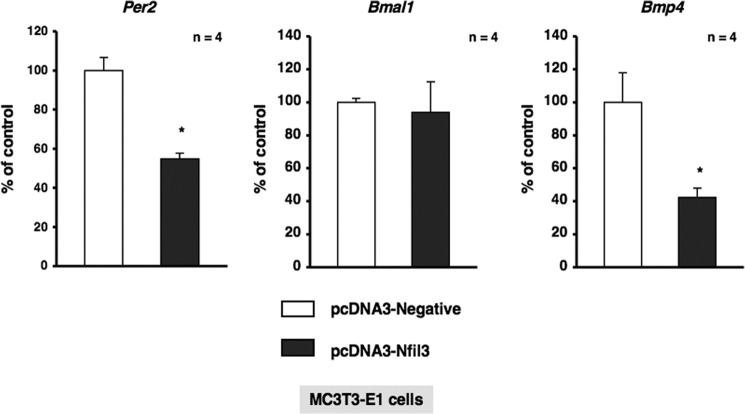
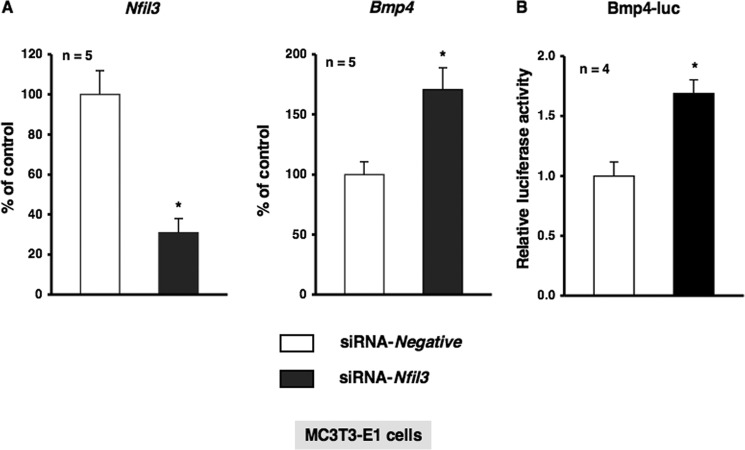
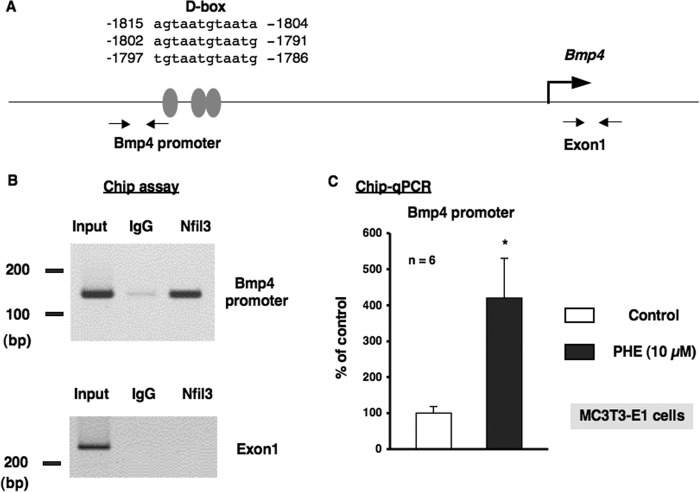
To investigate the physiological function of α1-AR signaling in bone metabolism and identify the target molecules of Nfil3 in osteoblasts, we stably transduced MC3T3-E1 cells with either empty pcDNA3 or pcDNA3-Nfil3 and evaluated the expression of a subset of genes, including canonical core clock genes and Bmp4. As shown in Fig. 5, the forced overexpression of Nfil3 repressed Per2 mRNA expression without altering Bmal1 expression in MC3T3-E1 cells. Furthermore, the overexpression of Nfil3 suppressed Bmp4 expression in MC3T3-E1 cells, which suggested that Nfil3 mediated the expression of Bmp4 under the regulation of circadian-related molecules in MC3T3-E1 cells. We then attempted to elucidate the regulation of Bmp4 gene expression in MC3T3-E1 cells transfected with siRNA for the knockdown of Nfil3. Cells were transfected with siRNA for Nfil3, followed by the determination of Nfil3 levels using real-time qRT-PCR. Nfil3 levels were decreased significantly in MC3T3-E1 cells transfected with Nfil3 siRNA when measured 24 h after transfection. A marked reduction in Nfil3 mRNA was found after 24 h in cells transfected Nfil3 siRNA (Fig. 6A). Further, significant increases were observed in Bmp4 expression and Bmp4 promoter activity in MC3T3-E1 cells under these conditions (Fig. 6, A and B). These results indicate that Nfil3 negatively regulates Bmp4 expression in MC3T3-E1 cells. Using a bioinformatics approach, we next identified three putative consensus Nfil3/E4BP4 binding sites in the Bmp4 promoter (−1815/−1804, 5′-AGTAATGTAATA-3′; −1802/−1791, 5′-AGTAATGTAATG-3′; and −1797/−1786, 5′-TGTAATGTAATG-3′). Nfil3/E4BP4 has been shown to bind to a D-box element of the promoter region of target genes to repress transcription. Therefore, we expected Nfil3 to be one of the target molecules of α1-AR signaling that regulates the circadian cycle in osteoblasts. To examine the functional significance of the putative Nfil3/E4BP4 binding site involved in Bmp4 gene expression, we cloned upstream of the Bmp4 transcript and generated luciferase reporter constructs (Bmp4-Luc) covering the potential elements that encompass the putative Nfil3/E4BP4 binding sites and deletion mutant of Bmp4-Luc (ΔDboxBmp4-Luc) that should render the Nfil3/E4BP4 site in the Bmp4 promoter inactive. As shown in Fig. 7A, the overexpression of Nfil3 significantly decreased luciferase activity in cells transfected with Bmp4-Luc, which includes all three putative Nfil3/E4BP4 binding sites. In contrast, deletion of the three D-box sites elements abolished the Nfil3-mediated suppression of the Bmp4 transcription (Fig. 7B). This indicates that the Nfil3/E4BP4 binding sites of the 5′ flanking region of Bmp4 was responsible for the suppression of Bmp4 transcription by Nfil3/E4BP4.
FIGURE 5.
Nfil3 negatively regulated Per2 and Bmp4 in MC3T3-E1 cells. Effects of the overexpression of Nfil3 in MC3T3-E1 cells. MC3T3-E1 cells were stably transfected with expression vectors for Nfil3 (pcDNA-Nfil3) or control (pcDNA-Negative), followed by further cultivation for 48 h and the subsequent determination of Per2, Bmal1, and Bmp4 levels by real-time qRT-PCR. Each value represents the mean ± S.E. of four separate experiments. *, p < 0.05, significantly different from the value obtained in cells transfected with the control vector.
FIGURE 6.
Nfil3 knockdown by siRNA on the Bmp4 transcript in MC3T3-E1 cells. A, MC3T3-E1 cells were treated with Nfil3 siRNA (siRNA-Nfil3) or non-silencing RNA (siRNA-Negative) according to the indicated protocols. Real-time qRT-PCR analyses of transcript levels were performed using their specific primers for Nfil3 and Bmp4. Relative mRNA expression was normalized to Gapdh. Each value represents the mean ± S.E. of five independent determinations. *, p < 0.05, significantly different from each control value. B, MC3T3-E1 cells were transiently transfected with Bmp4-Luc in the presence of siRNA-Nfil3 or siRNA-Negative according to the indicated protocols. The luciferase activity was determined 48 h after transfection. Firefly luciferase activities were normalized by Renilla luciferase activities. Each value is the mean ± S.E. of four independent experiments. *, p < 0.05, significantly different from each control value.
FIGURE 7.
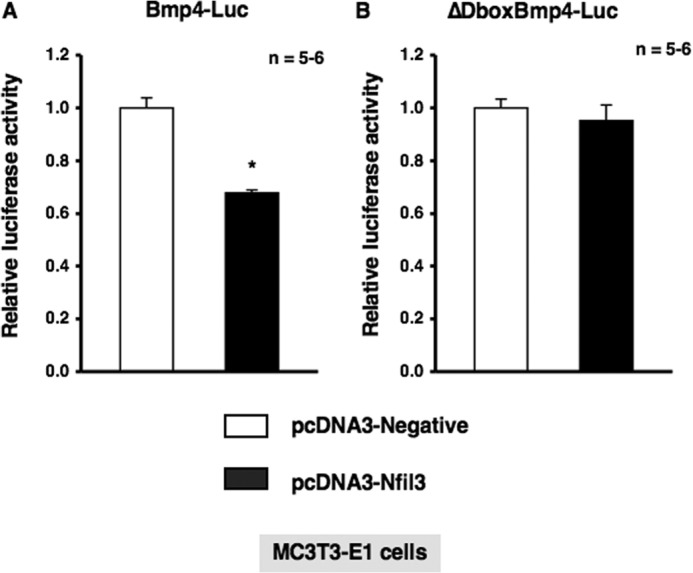
Deletion of putative D-box elements abolished Nfil3-mediated suppression of Bmp4 luciferase activity. A, MC3T3-E1 cells were transiently transfected with Bmp4-Luc in either the presence or absence of the Nfil3 expression plasmid. Luciferase activity was determined 48 h after transfection. The total amount of DNA transfected was standardized with an empty vector. Firefly luciferase activities were normalized by Renilla luciferase activities. Each value is the mean ± S.E. (n = 5–6 independent experiments). *, p < 0.05, significantly different from each control value. B, MC3T3-E1 cells were transfected with ΔDboxBmp4-Luc in either the presence or absence of the Nfil3 expression plasmid. The luciferase activity was determined 48 h after transfection. The total amount of DNA transfected was standardized with an empty vector. Firefly luciferase activities were normalized by Renilla luciferase activities. Each value is the mean ± S.E. (n = 5–6 independent experiments).
α1-AR Signaling Negatively Regulated Bmp4 Expression in MC3T3-E1 Cells
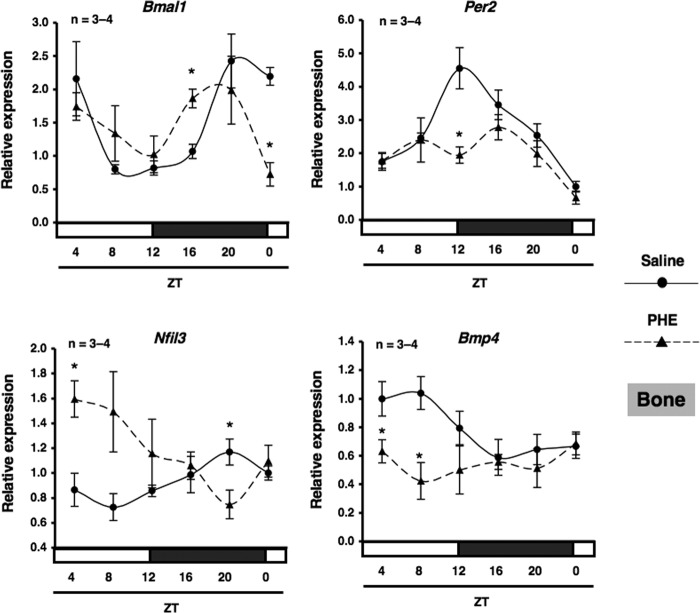
We next characterized the expression of Bmp4 mRNA by α1-AR signaling in MC3T3-E1 osteoblastic cells. Total RNA was extracted from MC3T3-E1 osteoblastic cells following exposure to PHE and was subsequently analyzed by real-time qRT-PCR. As shown in Fig. 8, the expression of Bmp4 mRNA was decreased significantly by the exposure to PHE for 4, 8, and 12 h in MC3T3-E1 osteoblastic cells. In addition, the rhythmic expressions of Bmal1, Per2, and Nfil3 in bone were altered by systemic administration of 10 μg/g PHE at ZT0. The expression of Nfil3 mRNA was increased significantly at ZT4, whereas Bmp4 was decreased significantly at ZT4 and ZT8 after systemic administration of PHE (Fig. 9). Taken together, these results indicate that α1-AR signaling negatively regulates Bmp4 expression in osteoblasts. The transcriptional inhibition of Bmp4 mRNA by α1-AR signaling suggests the involvement of transcriptional regulation by Nfil3 in osteoblasts. To test this possibility, we performed a ChIP analysis to identify the binding of Nfil3 to the putative binding sites in the promoter elements of Bmp4 genes. Database analysis revealed three potential Nfil3 binding sites in the region of the Bmp4 promoter (Fig. 10A). Specific primers were designed and synthesized to cover potential D-box binding sites and exon 1 for the Bmp4 gene. ChIP experiments on MC3T3-E1 cells revealed the basal occupancy of Nfil3 on this binding element by agarose gel electrophoresis with rabbit polyclonal antisera to Nfil3 in MC3T3-E1 cells (Fig. 10B). ChIP assays with primers flanking exon 1 of the coding region showed no response to PHE (data not shown). Moreover, Nfil3 binding to the D-box-containing Bmp4 promoter region was increased in MC3T3-E1 cells after PHE treatments (Fig. 10C), which indicated that the expression of Nfil3 induced by α1-AR signaling can bind directly to the Bmp4 promoter and inhibit Bmp4 expression in MC3T3-E1 cells. Taken together, the above data indicate that Nfil3/E4BP4 binding to the promoter element of the Bmp4 mediates Nfil3-mediated Bmp4 suppression in response to PHE stimulation in osteoblasts.
FIGURE 8.
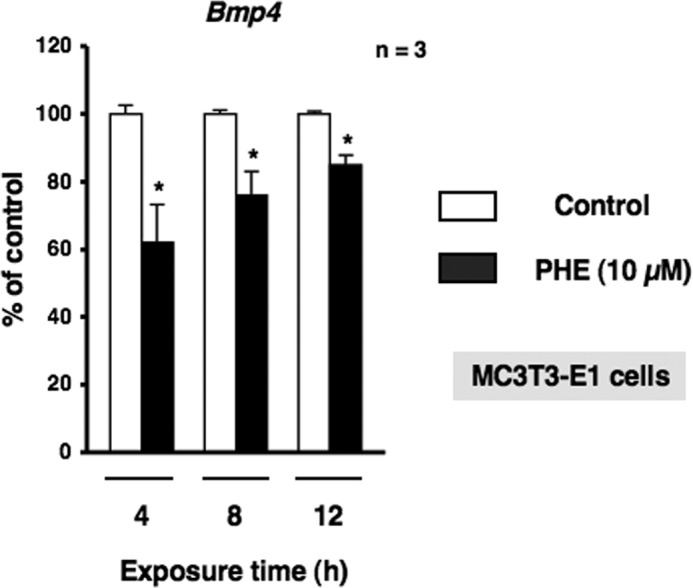
α1-AR signaling down-regulated Bmp4 mRNA in MC3T3-E1 cells. MC3T3-E1 cells were treated with 10 μm PHE for 4, 8, and 12 h, harvested, and then processed for real-time qRT-PCR. Each value represents the mean ± S.E. of three separate experiments. *, p < 0.05, significantly different from each control value obtained in MC3T3-E1 cells cultured in the absence of PHE.
FIGURE 9.
α1-AR signaling regulated clock genes and Bmp4 in bone. The effect of the intraperitoneal administration of PHE at 10 μg/g on rhythmic expression of Bmal1, Per2, Nfil3, and Bmp4 mRNA in bone is shown. C57BL/6J mice were maintained under a 12:12-hour light/dark cycle for 2 weeks, and then PHE was administered intraperitoneally at ZT0. Total RNA was isolated from the femurs (cancellous bone) of saline-treated and PHE-treated C57BL/6J mice. The mRNA levels of Bmal1, Per2, Nfil3, and Bmp4 were determined by real-time qRT-PCR using specific primers. Each value is the mean ± S.E. (n = 3 or 4 in each group). *, p < 0.05, significantly different from each control value.
FIGURE 10.
α1-AR signaling regulated the promoter element binding of Nfil3 to the Bmp4 gene in MC3T3-E1 cells. A, schematic showing the three potential binding sites of the proximal D-box in the putative promoter element of the Bmp4 gene. Grey ovals indicate the sites that match consensus D-box element sequences in the mouse Bmp4 gene. PCR was performed using sequences found in the 5′ flanking region (−1906 to −1887 and −1777 to −1758) and the exon 1 region (+243 to +262 and +442 to +461) of the mouse Bmp4 gene as primers. B, ChIP assays with antibodies against Nfil3 from MC3T3-E1 cells. A representative image of ChIP agarose gel electrophoresis. Nfil3/E4BP4 occupancy of this promoter region of the Bmp4 gene was enriched in MC3T3-E1 cells. Chromatin was prepared from MC3T3-E1 cells and immunoprecipitated with anti-Nfil3, followed by PCR using primers from the Bmp4 promoter or exon 1 of the Bmp4 gene as a control. Ten percent of the input was loaded as a control. Anti-IgG antibodies were used as a control for specificity. PCR-amplified bands of the Bmp4 promoter (top panel) and exon 1 of the Bmp4 genes (bottom panel) in chromatin were precipitated by Nfil3 antibodies. IgG, nonimmune rabbit IgG; Nfil3, rabbit Nfil3 antibodies; Input, DNA input. C, PHE increased the promoter element binding of Nfil3/E4BP4 to the Bmp4 gene. ChIP analysis revealed the increased binding of Nfil3 to the binding site of the Bmp4 promoter element in MC3T3-E1 cells following the PHE treatments. Each value represents the mean ± S.E. of six separate experiments. *, p < 0.05, significantly different from the value obtained in MC3T3-E1 cells cultured in the absence of PHE.
DISCUSSION
The results of this study demonstrate that α1-AR signaling in osteoblasts regulates clock genes and the putative interaction between Bmp4 and the circadian clock network in osteoblasts. In addition, the transcriptional factor Nfil3, in response to α1-AR signaling, negatively regulated Bmp4 expression in osteoblasts. To the best of our knowledge, this study is the first to demonstrate the regulation of clock genes by α1-AR signaling in osteoblasts and the physiological significance of the circadian clockwork in bone metabolism.
An increasing number of studies are focusing on circadian oscillators in bone. It has been reported that treatments with isoprenaline, a nonspecific β-AR agonist, or dexamethasone induced the circadian expression of clock genes in human osteoblasts. Moreover, isoprenaline entrained oscillations in the osteoblast-related gene Col1a1 (18). Recent studies in our laboratory reported that osteoclast-related genes such as cathepsin K and nuclear factor of activated T-cells, cytoplasmic 1 showed the circadian rhythmicity in bone as well as the canonical core clock genes such as Per1, Per2, and Bmal1 (21). The results of this study indicate that α1-AR signaling induces the rhythmic expression of the clock gene Per2 and Bmp4 mRNA in MC3T3-E1 osteoblastic cells. BMPs are members of the TGF-β superfamily that regulate cell growth, differentiation, and function (23) and play an important role in the regulation of normal physiological functions (24). BMP signaling is known to be mediated by the activation of type I and type II serine/threonine kinase receptors (25). Bmpr1a is a type I receptor that has a high affinity for BMP2 (26) and BMP4 (27), which are expressed in bone. BMPs have been shown to exhibit potent osteogenic activity in vitro (28), and the constitutive activation of BMPs or exogenous application of BMPs can induce ectopic bone formation in vivo (29). Therefore, Bmp4 mRNA showed a rhythmic expression pattern as well as the circadian clock gene Per2 in osteoblasts, which indicated that circadian oscillators regulate Bmp4 expression and may modulate diverse physiological processes in bone metabolism.
Previous studies, including ours, showed that the mRNAs of the α1-AR and β-AR subtypes were expressed in mouse and human osteoblasts (30, 31). β-AR signaling in osteoblasts has been shown to stimulate bone resorption by up-regulating receptor activator of nuclear factor κB ligands (Rankl). In addition, the physiological action of epinephrine has been demonstrated to be mediated by α1-AR signaling as well as β-AR signaling in osteoblasts. Epinephrine enhanced replication and alkaline phosphatase activities through α1-AR coupled to the Gi protein, which suggests that the pertussis toxin-sensitive G protein may be a potent mediator of cellular proliferation and alkaline phosphatase activities in MC3T3-E1 cells (9, 10). On the other hand, the activity of the sympathetic nervous system is known to have circadian rhythms (32). β-AR signaling in osteoblasts was shown to be involved in circadian rhythms in bone metabolism (33). Recent studies conducted in our laboratory showed that β-AR signaling entrained the circadian oscillation of clock genes (18). In this study, we showed that the transcriptional factor Nfil3/E4BP4, which functions as a key negative component of the Drosophila circadian clock (34–36), was up-regulated by α1-AR signaling, leading to the suppression of Bmp4 gene expression in bone. We are particularly interested in the repression of Bmp4 by Nfil3/E4BP4 in the context of circadian regulation because such transcription repressors of Bmp4 have not yet been reported. The bioinformatic approaches used in our studies have shown that the Bmp4 gene contains three D-box elements within its promoters, and this finding indicates that Bmp4 is a circadian-controlled gene that is regulated by circadian oscillators in osteoblasts. Because Bmp4 transcripts were down-regulated in Nfil3-overexpressed MC3T3-E1 cells and up-regulated in Nfil3-silenced MC3T3-E1 cells, Nfil3 promoted the negative regulation of Bmp4 expression in osteoblasts. Taken together, these findings suggest that Nfil3 represses Bmp4 directly, possibly by binding DNA in their regulatory regions. Our ChIP-quantitative PCR results confirmed Nfil3 binding enrichment in the regulatory regions of Bmp4 in MC3T3-E1 cells, which suggests that Bmp4 is directly under the regulation of Nfil3 in osteoblasts. Therefore, these findings suggest that α1-AR signaling mediates osteoblast function through a mechanism relevant to the regulation of Bmp4 expression in bone. However, BMP signaling is complex in bone. BMPs are potent inducers of ectopic bone formation (37–39), and mice with the postnatal inactivation of Bmpr1a, which is activated by the major BMP ligands BMP2 and BMP4, have been shown to have a greater bone mass (40), which is associated with the decreased expression of the Wnt antagonists sclerostin and dickkopf 1 (41). Therefore, the physiological significance of α1-AR signaling in bone remodeling has yet to be elucidated. The possibility that the regulation of Bmp4 by α1-AR signaling may play a role in the regulation of bone metabolism will be investigated further in future studies.
In conclusion, our results revealed that sympathetic signaling by α1-AR regulated clock genes and Bmp4 expression in osteoblasts. Moreover, the regulation of Bmp4 through the up-regulation of Nfil3 by α1-AR signaling in osteoblasts may participate in bone metabolism controlled by circadian rhythms.
This work was supported in part by Grant-in-Aid for Scientific Research 24790291 from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to T. H.).
- AR
- adrenergic receptor
- PHE
- phenylephrine
- ZT
- zeitgeber time
- α-MEM
- α-minimal essential medium
- Luc
- luciferase
- qRT-PCR
- quantitative RT-PCR
- Bmp
- bone morphogenetic protein.
REFERENCES
- 1. Prinster S. C., Hague C, Hall R. A. (2005) Heterodimerization of G protein-coupled receptors: specificity and functional significance. Pharmacol. Rev. 7, 289–298 [DOI] [PubMed] [Google Scholar]
- 2. Philipp M., Hein L. (2004) Adrenergic receptor knockout mice: distinct functions of 9 receptor subtypes. Pharmacol. Ther. 101, 65–74 [DOI] [PubMed] [Google Scholar]
- 3. Wettschureck N., Offermanns S. (2005) Mammalian G proteins and their cell type specific functions. Physiol. Rev. 85, 1159–1204 [DOI] [PubMed] [Google Scholar]
- 4. Takeda S., Elefteriou F., Levasseur R., Liu X., Zhao L., Parker K. L., Armstrong D., Ducy P., Karsenty G. (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 305–317 [DOI] [PubMed] [Google Scholar]
- 5. Togari A., Arai M., Kondo A. (2005) The role of the sympathetic nervous system in controlling bone metabolism. Expert Opin. Ther. Targets 9, 931–940 [DOI] [PubMed] [Google Scholar]
- 6. Kondo H., Togari A. (2011) Continuous treatment with a low-dose β-agonist reduces bone mass by increasing bone resorption without suppressing bone formation. Calcif. Tissue Int. 88, 23–32 [DOI] [PubMed] [Google Scholar]
- 7. Takeuchi T., Tsuboi T., Arai M., Togari A. (2001) Adrenergic stimulation of osteoclastogenesis mediated by expression of osteoclast differentiation factor in MC3T3-E1 osteoblast-like cells. Biochem. Pharmacol. 61, 579–586 [DOI] [PubMed] [Google Scholar]
- 8. Huang H. H., Brennan T. C., Muir M. M., Mason R. S. (2009) Functional α1- and β2-adrenergic receptors in human osteoblasts. J. Cell Physiol. 220, 267–275 [DOI] [PubMed] [Google Scholar]
- 9. Suzuki A., Palmer G., Bonjour J. P., Caverzasio J. (1999) Regulation of alkaline phosphatase activity by p38 MAP kinase in response to activation of Gi protein-coupled receptors by epinephrine in osteoblast-like cells. Endocrinology 140, 3177–3182 [DOI] [PubMed] [Google Scholar]
- 10. Suzuki A., Palmer G., Bonjour J. P., Caverzasio J. (1998) Catecholamines stimulate the proliferation and alkaline phosphatase activity of MC3T3-E1 osteoblast-like cells. Bone 23, 197–203 [DOI] [PubMed] [Google Scholar]
- 11. Kodama D., Togari A. (2013) Noradrenaline stimulates cell proliferation by suppressing potassium channels via Gi/o-protein-coupled α1(1B)-adrenoceptors in human osteoblasts. Br. J. Pharmacol. 168, 1230–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dibner C., Schibler U., Albrecht U. (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Ann. Rev. Physiol. 72, 517–549 [DOI] [PubMed] [Google Scholar]
- 13. Yagita K., Tamanini F., van Der Horst G. T., Okamura H. (2001) Molecular mechanisms of the biological clock in cultured fibroblasts. Science 292, 278–281 [DOI] [PubMed] [Google Scholar]
- 14. Lamia K. A., Storch K. F., Weitz C. J. (2008) Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. U.S.A. 105, 15172–15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Durgan D. J., Trexler N. A., Egbejimi O., McElfresh T. A., Suk H. Y., Petterson L. E., Shaw C. A., Hardin P. E., Bray M. S., Chandler M. P., Chow C. W., Young M. E. (2006) The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J. Biol. Chem. 281, 24254–24269 [DOI] [PubMed] [Google Scholar]
- 16. McDearmon E. L., Patel K. N., Ko C. H., Walisser J. A., Schook A. C., Chong J. L., Wilsbacher L. D., Song E. J., Hong H. K., Bradfield C. A., Takahashi J. S. (2006) Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science 314, 1304–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang E. E., Kay S. A. (2010) Clocks not winding down: unravelling circadian networks. Nat. Rev. Mol. Cell Biol. 11, 764–776 [DOI] [PubMed] [Google Scholar]
- 18. Komoto S., Kondo H., Fukuta O., Togari A. (2012) Comparison of β-adrenergic and glucocorticoid signaling on clock gene and osteoblast-related gene expressions in human osteoblast. Chronobiol. Int. 29, 66–74 [DOI] [PubMed] [Google Scholar]
- 19. Mengatto C. M., Mussano F., Honda Y., Colwell C. S., Nishimura I. (2011) Circadian rhythm and cartilage extracellular matrix genes in osseointegration: a genome-wide screening of implant failure by vitamin D deficiency. PLoS ONE 6, e15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okubo N., Minami Y., Fujiwara H., Umemura Y., Tsuchiya Y., Shirai T., Oda R., Inokawa H., Kubo T., Yagita K. (2013) Prolonged bioluminescence monitoring in mouse ex vivo bone culture revealed persistent circadian rhythms in articular cartilages and growth plates. PLoS ONE 8, e78306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujihara Y., Kondo H., Noguchi T., Togari A. (2014) Glucocorticoid mediates circadian timing to peripheral osteoclasts, which contribute the circadian rhythm of osteoclasts-related genes expression. Bone 61, 1–9 [DOI] [PubMed] [Google Scholar]
- 22. Hirai T., Taniura H., Goto Y., Ogura M., Sng J. C., Yoneda Y. (2006) Stimulation of ubiquitin-proteasome pathway through the expression of amidohydrolase for N-terminal asparagine (Ntan1) in cultured rat hippocampal neurons exposed to static magnetism. J. Neurochem. 96, 1519–1530 [DOI] [PubMed] [Google Scholar]
- 23. Massagué J. (1992) Receptors for the TGF-β family. Cell 69, 1067–1070 [DOI] [PubMed] [Google Scholar]
- 24. Chen D., Zhao M., Mundy G. R. (2004) Bone morphogenetic proteins. Growth Factors 22, 233–241 [DOI] [PubMed] [Google Scholar]
- 25. Derynck R., Zhang Y. E. (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 26. Keller S., Nickel J., Zhang J. L., Sebald W., Mueller T. D. (2004) Molecular recognition of BMP-2 and BMP receptor IA. Nat. Struct. Mol. Biol. 11, 481–488 [DOI] [PubMed] [Google Scholar]
- 27. Hatta T., Konishi H., Katoh E., Natsume T., Ueno N., Kobayashi Y., Yamazaki T. (2000) Identification of the ligand-binding site of the BMP type IA receptor for BMP-4. Biopolymers 55, 399–406 [DOI] [PubMed] [Google Scholar]
- 28. Kang Q., Sun M. H., Cheng H., Peng Y., Montag A. G., Deyrup A. T., Jiang W., Luu H. H., Luo J., Szatkowski J. P., Vanichakarn P., Park J. Y., Li Y., Haydon R. C., He T. C. (2004) Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 11, 1312–1320 [DOI] [PubMed] [Google Scholar]
- 29. Gautschi O. P., Frey S. P., Zellweger R. (2007) Bone morphogenetic proteins in clinical applications. ANZ J. Surg. 77, 626–631 [DOI] [PubMed] [Google Scholar]
- 30. Nishiura T., Abe K. (2007) α1-Adrenergic receptor stimulation induces the expression of receptor activator of nuclear factor κB ligand gene via protein kinase C and extracellular signal-regulated kinase pathways in MC3T3-E1 osteoblast-like cells. Arch. Oral Biol. 52, 1778–1785 [DOI] [PubMed] [Google Scholar]
- 31. Togari A., Arai M., Mizutani S., Mizutani S., Koshihara Y., Nagatsu T. (1997) Expression of mRNAs for neuropeptide receptors and β-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci. Lett. 233, 125–128 [DOI] [PubMed] [Google Scholar]
- 32. Herzog E. D. (2007) Neurons and networks in daily rhythms. Nat. Rev. Neurosci. 8, 790–802 [DOI] [PubMed] [Google Scholar]
- 33. Fu L., Patel M. S., Bradley A., Wagner E. F., Karsenty G. (2005) The molecular clock mediates leptin-regulated bone formation. Cell 122, 803–815 [DOI] [PubMed] [Google Scholar]
- 34. Blau J., Young M. W. (1999) Cycling VRILLE expression is required for a functional Drosophila clock. Cell 99, 661–671 [DOI] [PubMed] [Google Scholar]
- 35. Cyran S. A., Buchsbaum A. M., Reddy K. L., Lin M. C., Glossop N. R., Hardin P. E., Young M. W., Storti R. V., Blau J. (2003) VRILLE, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell 112, 329–341 [DOI] [PubMed] [Google Scholar]
- 36. Glossop N. R., Houl J. H., Zheng H., Ng F. S., Dudek S. M., Hardin P. E. (2003) VRILLE feeds back to control circadian transcription of Clock in the Drosophila circadian oscillator. Neuron 37, 249–261 [DOI] [PubMed] [Google Scholar]
- 37. Urist M. R. (1965) Bone: formation by autoinduction. Science 150, 893–899 [DOI] [PubMed] [Google Scholar]
- 38. Winnier G., Blessing M., Labosky P. A., Hogan B. L. (1995) Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 9, 2105–2116 [DOI] [PubMed] [Google Scholar]
- 39. Zhang H., Bradley A. (1996) Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122, 2977–2986 [DOI] [PubMed] [Google Scholar]
- 40. Kamiya N., Ye L., Kobayashi T., Lucas D. J., Mochida Y., Yamauchi M., Kronenberg H. M., Feng J. Q., Mishina Y. (2008) Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J. Bone Miner. Res. 23, 2007–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamiya N., Kobayashi T., Mochida Y., Yu P. B., Yamauchi M., Kronenberg H. M., Mishina Y. (2010) Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. J. Bone Miner. Res. 25, 200–210 [DOI] [PMC free article] [PubMed] [Google Scholar]