Abstract
In adult rodents, subventricular zone (SVZ) astrocytes (B cells) function as primary progenitors in the generation of new neurons that migrate to the olfactory bulb (OB), where they differentiate into multiple types of interneurons. It has been generally considered that individual adult SVZ stem cells are capable of generating different types of neurons and glial cells. However, recent studies indicate that these adult SVZ primary progenitors are heterogeneous and predetermined to generate specific types of neurons. Surprisingly, OB interneurons are generated by stem cells not only in the walls of the lateral ventricle facing the striatum but also in the rostral migratory stream and walls of the lateral ventricle facing the cortex and the septum. SVZ B cells in different locations within this extensive germinal region generate different kinds of interneurons. General physiological characteristics of major classes of OB interneurons have begun to emerge, but the functional contribution of each subtype remains unknown. The mosaic organization of the SVZ offers a unique opportunity to understand the origin of interneuron diversity and how this assortment of neurons contributes to plasticity of postnatal olfactory circuits.
Introduction
The olfactory bulb (OB) of the adult mammalian brain continues to receive new neurons throughout life [1], but how these newborn cells shape olfaction remains unclear. The first synaptic relay in the olfactory system occurs in the OB [2]. Odor discrimination begins with the binding of odorant molecules to receptors expressed by olfactory sensory neurons (OSN) in the nasal epithelium. There, individual OSNs express one type of olfactory receptor from a large family of genes [3]. Although broadly distributed in the olfactory epithelium, OSNs expressing the same receptor project onto specific glomeruli in the OB (Figure 1). Their axons contact the primary dendrites of mitral and tufted cells, most of which project an axon outside the OB and primarily into olfactory cortex [4]. The segregation of odorant information at the level of OSNs and glomeruli has been suggested as a fundamental first stage in olfactory discrimination [2,4]. This, however, does not fully explain how odors are identified, because olfactory receptors are promiscuous and can bind multiple odorants, activating groups of glomeruli. Rather, the identity of the odorant is encoded by the relative levels of activation of multiple glomeruli [5]. Thus, the processing of olfactory information in the OB results in a complex and dynamic activation of mitral and tufted cells that receive information from multiple glomeruli [5]. Mitral and tufted cells interact with a very large population of local circuit interneurons that use γ-aminobutyric acid (GABA) as their main neurotransmitter [4]. In the OB, the ratio of local GABAergic interneurons to excitatory neurons (100:1 [4]) is much higher than in other parts of the brain, such as the neocortex (1:5 [6]). The balance between excitation and inhibition in the OB is relatively conserved across mammalian species, suggesting that the inhibitory neurons play essential roles in the processing of olfactory information. The neurons that form these inhibitory circuits are chemically and electrophysiologically diverse. Intriguingly, these different OB inhibitory neurons continue to be replaced during juvenile and adult life, whereas olfactory circuits are already in operation. This raises very basic questions on the origin of the different types of neurons in the developing and adult OB and about their function.
Figure 1.
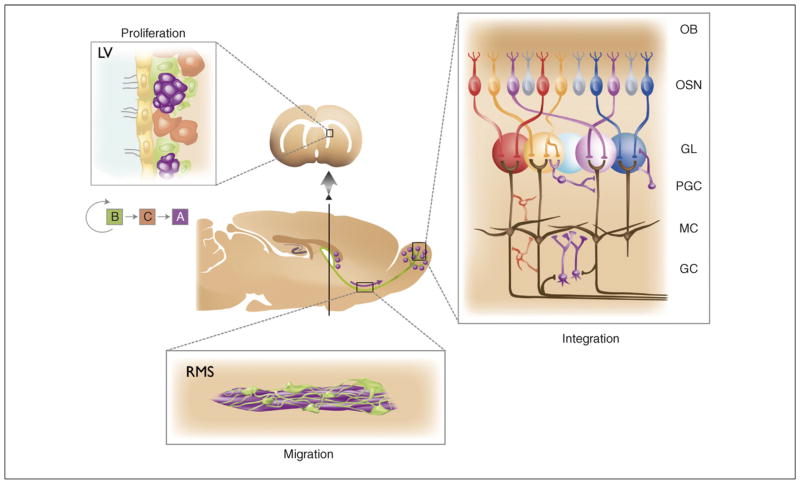
The subventricular zone (SVZ)–olfactory bulb pathway represents one of the few constitutive neurogenic areas in the adult CNS. Diagram in the center shows a sagittal section of the rodent forebrain. The arrow indicates the tangential migration of neuroblasts (purple dots) toward the olfactory bulb (OB). New neurons recruited into the OB continually replace local interneurons. Panel on the right illustrates the wiring of the OB. Each olfactory sensory neuron (OSN) expresses only one of the ~1000 odorant receptor genes, and the axons from all cells expressing that particular receptor converge onto one or a few glomeruli (GL) in the OB. The nearly 2000 glomeruli in the rodent OB are spheroid knots of neuropil ~50–100 μm in diameter which contain the incoming axons of sensory neurons (OSN), the apical dendrites of the main projection neuron of the OB plus axon and dendrites from PGCs. The activity of projection neurons in the OB occurs through two populations of interneurons: periglomerular cells (PGC) and granule cells (GC) (both in purple) and short axon cells (represented in red). Left panel shows the neurogenic niche. Here, proliferation in the SVZ takes place in the walls of the lateral ventricle (LV), where stem cells (in green, type B cells) divide to generate transit-amplifying cells (in brown, type C cells), which in turn give rise to neuroblasts (in purple, type A cells) that migrate in the rostral migratory stream (RMS) (bottom panel) to their final destination in the OB, where they differentiate into interneurons.
Adult neurogenesis has been evolutionarily conserved from reptiles to mammals, and the analogous replacement of local interneurons also appears to occur in central processing regions of insects [7] and crustaceans [8]. In mammals, new OB interneurons originate from a germinal region known as the subventricular zone (SVZ), on the walls of the lateral ventricles [1] (Figure 1). Neural stem cells that function as primary precursors in the SVZ correspond to type B cells, a subpopulation of slowly dividing cells that has the morphology, ultrastructure and markers of astrocytes [9]. Type B cells produce type C cells, which divide rapidly to produce young neurons, also known as neuroblasts or type A cells. These neuroblasts in turn migrate tangentially in chains, forming an extensive network of pathways in the SVZ. These local SVZ chains merge anteriorly to form the rostral migratory stream (RMS), a large migratory pathway leading into the OB (Figure 1). Within the OB, young neurons mature into various subtypes of local inhibitory interneurons [10–14].
There are two principal types of adult-born OB interneurons: granule cells (GC) found in the granule cell layer (GCL), and periglomerular cells (PGC) located in the glomerular layer (GL) (Figures 1,2). A recent study suggests that a subpopulation of interneurons in the external plexiform layer (EPL) is also produced postnatally [15]. Adult-born GCs can be subdivided into distinct populations by morphological criteria. Superficial GCs whose dendrites target primarily the superficial lamina of the EPL are believed to establish synapses with tufted cells [4], whereas deep GCs contact mostly the dendrites of mitral cells in the deep lamina of the EPL [4,16,17]. As mitral and tufted cells project into different regions of the olfactory cortex, these two populations of GCs might be part of separate neural circuits for olfaction. The tufted–GC circuit might mediate low-threshold perception of odorants [18] by intrabulbar association [19], whereas mitral–GC interactions might be important for odor discrimination [20] (see Box 1). Adult-born PGCs can be subdivided into three nonoverlapping populations based on their immunoreactivity to tyrosine hydroxylase (TH), an enzyme required for dopamine synthesis, or immunoreactivity to the calcium binding proteins calbindin (CalB) or calretinin (CalR) [21]. In the mouse, GCs and all three PGC subpopulations express GABA [22,23], whereas only GCs and TH+ PGCs were found to be GABAergic in the rat [23]. However, it is still unclear whether cell populations defined by these marker genes correspond directly to cell populations defined by morphological [24] or electrophysiological criteria [25].
Figure 2.
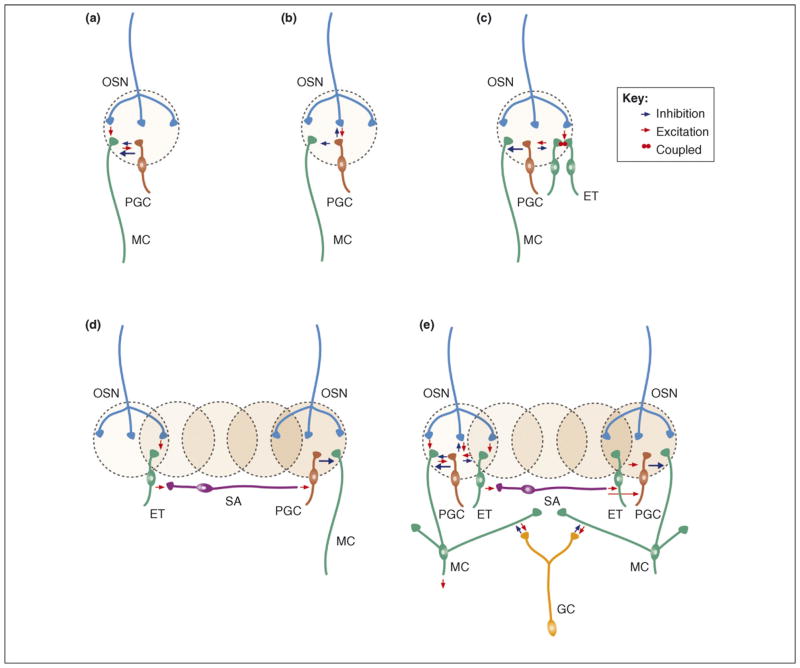
Main microcircuits in the olfactory bulb. The three top panels show distinct glomerular microcircuits: (a) OSN→MC→PGC, (b) OSN→PGC→MC, (c) OSN→ET→PGC→MC. Microcircuits in (a) and (c) refer to type II PGCs whereas the microcircuit in (b) makes use of type I PGCs. The two lower panels show lateral connections that support interglomerular circuits. (d) OSN→ET→SA→PGC→MC and (e) show all glomerular microcircuits together with the mitral–granule–mitral (MC→GC→MC) lateral circuit. Note that all intraglomerular microcircuits (a–c) and lateral long-range interglomerular circuits (d,e) of the OB are mediated through local interneurons and are affected by adult neurogenesis. OSN = olfactory sensory neuron; MC = mitral cell; PGC = periglomerular cell; GC = granule cell; ET = external tufted cell; SA = short axon cell. (See also Ref. [5].).
Box 1. Diverse functions of granule cells.
Granule cells interact with projection neurons principally through reciprocal dendrodendritic synapses [70]. These interactions can inhibit local and distant projection neurons in distinct ways. In recurrent inhibition, subtle depolarization of a projection neuron triggers the release of glutamate from its dendrites, depolarizing interneuron spines. An increase in Ca2+ concentration in GC spines causes GABA to be released directly back onto projection neurons. The second type of GABAergic synaptic interaction is called local lateral inhibition among neighboring projection neurons. This can be mediated if the GC spine activation is stronger, causing depolarization to spread locally, with the spread of Ca2+ signals or dendritic Na+ spikes through the GC dendritic tree. For both recurrent and local lateral contact, GABA release, which does not require a somatic action potential, can provide graded inhibition [4]. The synaptic activation of the GC spines might be strong enough to propagate to the soma and to elicit a somatic action potential. This somatic spike then back propagates through the entire dendritic tree, globally elevating Ca2+ levels and releasing GABA from hundreds of GC spines. This represents a global lateral inhibition. Finally, GCs can receive excitatory inputs on their proximal dendrites from projection neuron collaterals, thus providing feedforward inhibition. Therefore, GCs have multiple ways of inhibiting the activity of projection neurons, according to their dendritic properties. The segregation of the GC population into deep or superficial interneurons indicates that these cells participate in parallel, yet distinct, inhibitory actions on projection neurons. Deep GCs have their dendritic arbors restricted to the deep EPL in which they synapse predominately with mitral cell secondary dendrites. By contrast, most superficial GCs arbor in the superficial EPL in which they synapse with tufted cell dendrites. The recent demonstration that adult brain stem cells give rise to newborn GCs whose position and dendritic projections are predetermined from their time of birth [45] reveals that important aspects of newborn GC inhibitory functions are already prespecified in their precursors. The genetic programs that control how stem cells are already determined to produce neurons with a stereotypic marker, position within the OB and pattern connections remain to be deciphered.
Until recently, it was not known how multiple subtypes of local interneurons – each with different physiological, morphological and molecular properties – were generated during development or in the adult. Furthermore, it was unclear how the stereotyped connections of OB interneurons are established despite their constant turnover. In this review, we discuss recent results that point to distinct origins of local interneurons formed postnatally, their diverse modes of maturation and various functions once integrated. Today it is clear that all OB microcircuits depicted in Figure 2 are affected by the continuous neuronal turnover throughout life. By better understanding the origin of this complexity, we can begin to uncover how the constant renewal of the OB inhibitory network might be regulated and contributes to the processing of olfactory information.
Embryonic origins of olfactory interneurons
Bulbar interneuron production begins as early as embryonic day (E)14 [26]. It was thought that OB interneurons, like cortical interneurons, were generated from subpallial structures. Several studies supporting this hypothesis have suggested that the adult SVZ is derived from the lateral ganglionic eminence (LGE) of the embryonic telencephalon. For example, LGE progenitors grafted into the LGE or adult SVZ produce neuroblasts that migrate to the OB; these neuroblasts mature into interneurons similar to those produced in adults [26] or those produced from grafting adult SVZ cells to the adult SVZ [27]. A recent study has suggested that some cortical progenitors migrate into the striatum and populate the SVZ [28]. However, primary progenitors labeled in one brain region (including cortex) do not move to another during postnatal development (see following section).
Genetic evidence also links embryonic LGE to adult SVZ. During development, the telencephalic neuroepithelium segregates into distinct regions in which progenitors express different transcription factors. The adult SVZ expresses Dlx1/2, ER81 [29], Mash1 [30], Pax6 [13,31] and Sp8 [32] (Figure 3), a set of transcription factors that is also expressed in the dorsal LGE (dLGE) during embryonic development. Mice lacking these and other transcription factors in the LGE fail to develop appropriate numbers and subtypes of OB interneurons (e.g. [33,34]). Therefore, studies examining cell transplantation, patterns of gene expression and analysis of knockout mice have suggested that the adult SVZ is largely derived from the dLGE [29,32–34]. More recent work has confirmed that the LGE is an important contributor to the adult SVZ, but unexpectedly other domains of the developing telencephalon contribute in unique ways to the adult germinal niche that produces OB interneurons (see next section).
Figure 3.
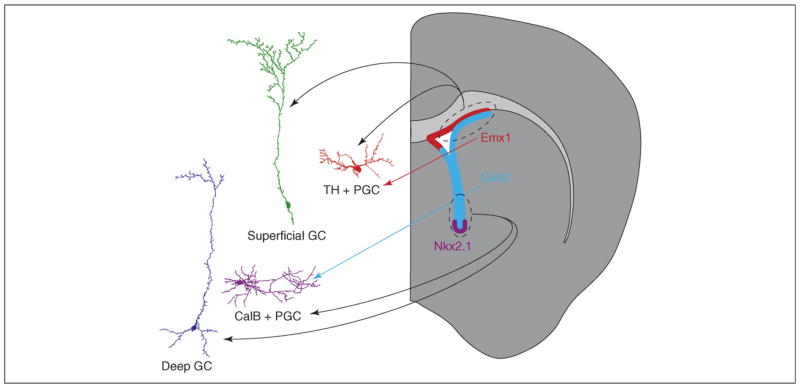
Schematic depicting the contribution of different SVZ regions to the heterogeneity of olfactory bulb interneurons. Interneurons shown on the left are camera lucida traces of cells derived from virally labeled stem cells within restricted regions of the SVZ (for experimental details, see Ref. [43]). Postnatal stem cell-labeling experiments have shown that stem cells in the dorsal SVZ (dashed oval and black arrows) produce mostly superficial GCs (green) and TH+ PGCs (red). TH+ and a subpopulation of CR+ OB interneurons are derived from Emx1-expressing progenitors [22,46] (red arrow and red area). Deep GCs (blue) and CalB+ PGCs (purple) are produced primarily by stem cells targeted by viral injections in the ventral SVZ [43] (dashed oval and black arrows). Some stem cells in this region express the transcription factors Gsh2 and Nkx2.1 (light blue and purple areas, respectively). Lineage-tracing experiments suggest that Gsh2+ progenitors produce the CalB+ OB neurons (light blue arrow) [46].
The temporal and regional production of olfactory interneurons
The cell bodies of neural stem cells line the embryonic and early postnatal ventricular system, forming a ventricular zone (VZ). Within the first couple of weeks of postnatal life in mice, most neural progenitors are depleted from this region as a layer of postmitotic ependymal cells replaces the VZ. Neural stem cells persist only in restricted regions, such as the adult SVZ. As the brain matures, these stem cells transform from radial glial cells into astrocytic SVZ type B cells [35]. Embryonic neural stem cells generate different cell types at different developmental time points, but are different types of OB interneurons produced as SVZ progenitors transform from embryonic to adult stem cells?
Several groups have suggested that this is the case. The peak of OB interneuron production occurs in the first postnatal week in mice [1]. Interneurons born during this early period appear more likely to differentiate and survive long-term in the OB than interneurons generated later, when the OB is more mature [36–39]. It is still unclear whether perinatally born and adult-born OB interneurons are functionally distinct, but their differential survival might correlate with the distinct functional demands of an immature and a mature circuit.
Some authors have suggested that different interneuron subtypes are preferentially produced at different ages. In one study, mice were given a single pulse of BrdU at different time points, and BrdU-labeled nuclei in the GCL were quantified after 20–28 days [38]. Cells labeled at P3 or P7 were more likely to integrate in the superficial GCL and survive, compared to cells born at later ages. Another group injected the SVZ with dye or grafted it at different ages and suggested that different subtypes of PGC might be preferentially produced at different ages [40]. Labeled PGCs were more likely to be CalB+ if derived from the neonate, and more likely to be CalR+ or TH+ if derived from the adult-labeled SVZ. Also, embryonic or neonatal cells grafted into the neonatal or adult brain produced different cell types: again younger tissues produced a higher percentage of CalB+ cells and a lower percentage of CalR+ cells than older tissues. Some of these findings have been supported by a more recent study, in which OB interneuron subtypes were genetically fate mapped in a transgenic mouse carrying Cre-ER under control of the Dlx1/2 enhancer as well as a floxed reporter gene, allowing OB interneurons to be labeled at different time points [41]. However, this study finds that TH+ cells are produced in greater proportions at early time points rather than later in development. These differences can be explained in part by the fact that dye injection and grafting studies target a diverse cell population in a region that undergoes significant morphogenetic changes during development, or by temporal or spatial changes in the activity of the Dlx1/2 enhancer. Together, these studies suggest that different cell types are preferentially produced at different ages. Because these different cell types integrate into different OB circuits (Box 1), the temporal pattern of their production might regulate the functional maturation of the OB.
There are several ways to interpret these results. For example, neural stem cells might gradually switch to producing different cell types over time. Alternatively, different neural stem cells might produce different cell types, and their proliferation rates, or those of their progeny, might change over time. Yet another possibility is that the neural stem cell population producing ‘early-born’ cell types might be depleted relative to other stem cell populations as the brain matures.
Among these hypotheses, the idea that SVZ progenitors are heterogeneous and produce different neuron types was recently supported by several studies that have found that OB interneurons are produced in an extensive germinal region. Until recently, it was believed that the adult SVZ was restricted to the wall of the lateral ventricle facing the striatum (lateral wall) in which postnatal proliferation is easily observed. However, the RMS and parts of the lateral ventricular wall facing the septum (medial wall), or the corpus callosum or pallium (dorsal wall), contain proliferative cells that act as stem cells in vitro [9,42] and in vivo [43,44]. Progenitors in these regions are similar to B cells because they are long lived, express glial fibrillary acidic protein and produce OB interneurons [43], so together these regions might constitute a single large proliferative zone.
This extensive proliferative area appears to be divided into discrete functional regions. Studies based on stem cell targeting [43] and cell grafting or transcription factor-based fate mapping of progenitors [22,45,46] show that adult neural stem cells in different regions generate different subtypes of interneurons. In stem cell targeting studies, neonatal Cre reporter mice were injected with a small volume of Cre-expressing adenovirus to infect the distal processes of radial glial cells in the spatially restricted subregion of the SVZ [43,44]. Injected mice were then analyzed 4 weeks later and labeled OB interneurons were stained for cell-type-specific markers. The cortex and dorsal SVZ generated primarily superficial GCs and TH+ PGCs, whereas the ventral SVZ produced mostly deep GCs and CalB+ PGCs (Figure 3). Also, the RMS and septum mostly produced CalR+ cells, in both the GL and the GCL. These findings are consistent with results demonstrating that migrating neuroblasts are heterogeneous in their expression of molecular markers [13,31,47,48].
Genetic fate-mapping experiments have used mice expressing Nkx2.1-Cre, Dlx5/6-Cre, Gsh2-Cre, Emx1-Cre, Dbx1-Cre and Emx1-CreERT2 crossed with Cre reporter mice. OB interneurons were derived from some of these transcriptional domains [22,46]. In order to identify cells that are continually produced in the adult, a combination of BrdU and staining for cell-type-specific markers was used. Cortical (Emx1+) cells generated primarily superficial GCs, TH+ and some CalR+ PGCs, but very few or no CalB+ PGCs. Interneurons derived from the Gsh2 or Dlx5/6 lineage appear to generate all PGC subtypes. Cell-labeling and -grafting experiments suggest that CalR+ cells are derived from the embryonic septum, pallium and the RMS [22,43,46].
The site of origin within the adult SVZ not only determines the specific markers and final position of postnatally generated interneurons within the OB, but also the specific branching pattern of their dendrites [45]. This study used retroviruses to label proliferative cells in the neonatal SVZ. They report that interneurons with dendrites that branch into the superficial EPL are derived from cells labeled in the anterior SVZ of the neonatal rat, whereas GCs that branched deep within the EPL are derived from posterior SVZ labeling. Interestingly, adult-targeted anterior SVZ generated some GCs that branched deep in the EPL, although their cell bodies remained more superficial. By injecting retroviruses directly into the SVZ, it is impossible to restrict labeling to stem cells and more difficult to target particular subregions. Therefore, it is not possible to know whether the dendritic branching patterns observed in this study are determined by stem cells or at later stages in the neurogenic lineage. Still, this work strongly suggests that patterns of dendritic trees are determined in the SVZ before neuroblasts mature in the OB. Together, these studies suggest that stem cells in different regions of the adult germinal zone are heterogeneous, expressing unique combinations of transcription factors and generating distinct subsets of olfactory interneurons. These results also provide a foundation for building a more comprehensive list of genetic factors that regulate OB interneuron diversity and survival. Because both temporal and spatial factors could control OB interneuron production, more detailed studies of these factors would shed light on OB development and perhaps on the role of adult neurogenesis in olfactory processing.
Cell-autonomous specification of OB interneuron diversity
Both genetic determinants and environmental factors might regulate OB interneuron identity and function. This age-dependent regulation can be attributed either to age-dependent changes in SVZ progenitors or to progressive modifications in the OB milieu that influence the final maturation of newly born neurons. To discriminate between these possibilities, LGE/SVZ progenitors were transplanted into homochronic or heterochronic environments [22,40]. Grafted cells preferentially acquired phenotypes typical of their origin, thus showing that their mature identities are primarily determined by intrinsic properties. Similarly, labeled neonatal radial glia, or adult SVZ B cells, grafted heterotopically to different locations do not adopt progenitor properties of the new location, but continue to generate OB neurons appropriate to their origin [43]. This specification is maintained even after progenitor cells are cultured in vitro for several passages and are grafted together with an excess of cells from the host niche. These observations suggest that the primary progenitors within the various domains of the SVZ are preprogrammed at birth to generate different subsets of OB interneurons. Heterochronic and heterotopic transplantation experiments also indicate that the dendritic arborization of postnatally generated GCs is largely determined by intrinsic cell-autonomous factors [45]. Accumulating evidence indicates that postnatal SVZ stem cells are highly heterogeneous and programmed during embryonic development.
Synaptic integration of newborn interneurons
It remains unknown how the different subtypes of inter-neurons produced by the heterogeneous set of neural stem cells integrate into OB circuits and contribute to its physiology. However, some knowledge has begun to emerge on the general electrophysiological properties of GCs and PGCs during their maturation. Migrating neuroblasts express early extrasynaptic GABAA receptors [49] and establish functional synapses only after they mature and integrate into circuits of the OB. Synapses in maturing cells have been identified by immunostaining for presynaptic markers [11,39] or by electron microscopy [50]. This latter approach has clearly demonstrated the rapid formation of synapses onto the basal compartments of the newborn GCs from centrifugal and mitral cell axon collaterals. Interestingly, there are major differences in the maturation of glutamatergic synapses on GCs compared to newborn PGCs, at least those directly contacted by OSN axons. At the maturing OSN–PGC synapse, the ratio of AMPA to NMDA glutamate receptors increases at OSN synapses, and the contribution of juvenile NMDA receptors containing the NR2B subunit decreases [51]. Surprisingly, these postsynaptic transformations are not mirrored by presynaptic changes such as increased probability of glutamate release in the OSN, at least for synapses in some subtypes of PGCs (Figure 2a–c). This indicates that initial contacts of newborn PGCs are preferentially made with preexisting functioning boutons, either already involved in a synapse or preformed before the formation of the post-synaptic site.
Sequential formation of GABAergic and glutamatergic synapses at the postsynaptic site is thought to be crucial for constructing the stereotypic neural networks during brain development. Interestingly, there are major differences in synaptogenesis between newborn PGCs and GCs. In newly integrated PGCs, the maturation of voltage-dependent sodium currents, and consequently the capacity of the newly generated cells to fire action potentials, precedes the appearance of synaptic contacts [11,51]. In contrast, NMDA-, GABAA- and AMPA-mediated synaptic events occur long before maturing GCs fire action potentials [12]. This difference could reflect intrinsic differences between these cell types (i.e. PGCs have axons whereas GCs do not), or the different neuronal microcircuits into which these interneurons integrate. It is noteworthy that at least some of the PGCs integrate into microcircuits characterized by the ongoing replacement of primary afferents (the terminals of OSNs which are constantly renewed; see Figure 2b), whereas new GCs receive synaptic inputs from the dendrites of the projection neurons (Figure 2e) known to be stable over extended periods of time [52].
In newborn GCs, the dendritic spine density changes over time. It rapidly increases from 14 to 28 days after neuronal birth, remains steady to 42 days, and then decreases after 56 days [50]. This reduction might result from the pruning of spines and synapses from cells surviving longer than 42 days, or from preferential death of cells with high spine density. PGC spines appear to mature more slowly than those of GCs. At 14 days, PGCs have elaborate dendritic arbors, but immature filopodia-like processes appear only at 28 days, and mature pedunculated spines are not present until 42 days [50]. Interestingly, OB interneuron spine density is not only dependent on the age of the cell but also on the degree of neuronal activity. If new GCs reach a silenced OB network, the dendritic spine density of newborn GCs decreases to 50% of the control level, whereas it remains unchanged in preexisting GCs [53]. This process of synaptic development and pruning might be homologous to that observed during normal development in other brain areas [54]. The survival of newly differentiated GCs is also dependent on neural activity within the OB [36], a process that is reminiscent of regulated neuronal cell death during development [55]. Newborn OB interneurons seem to be more easily excitable by novel odors than mature cells because they are more likely to express the immediate early gene encoding Cfos following an exposure to this odorant [39]. In the adult OB, the survival of a specific subset of GCs might be enhanced by an olfactory learning task but not by simple exposure to an odorant over a 6 day period [56]. Therefore, both synapse pruning and selective cell survival could sculpt precise microcircuits within the OB to enhance odor detection and/or discrimination [57–59]. It will be interesting to determine whether the different types of GCs and PGCs replaced in the postnatal and adult brain are equally dependent on olfactory information for their maturation.
Functional diversity of newborn interneurons
The different functional roles of postnatally generated subtypes of OB interneurons such as deep or superficial GCs or PGCs expressing TH, CalR or CalB [22,43,46] remain unclear. These cell types differ not only in their neurochemistry, marker gene expression and location in the OB but also in their morphology [21], synaptic properties [60] and rate of turnover [61,62]. It has been proposed that the different PGC subtypes serve specific functions [23]. PGCs establish reciprocal dendrodendritic synapses with the apical dendrites of mitral and tufted cells within the glomeruli and extend axons between glomeruli. TH+ PGCs [23] receive direct synaptic inputs from the olfactory nerves [11] (Figure 2b). These cells could regulate OB projecting neurons directly via both feedforward and feedback circuits [63]. By contrast, type II PGCs do not receive direct input from the OSN axons; synaptic input to these cells occurs only via dendrodendritic synapses from projection neurons and the axons of other neurons (Figure 2a, c–e). Further studies might link the functional features of postnatally produced OB interneurons with the immunohistological markers currently used to define PGC populations [14]. However, the unique use of immunohistological markers might be somewhat misleading, because some markers change with cellular maturation or neural activity. The markers GAD, GABA and CalR are present in neuroblasts at birth [64] and are unaffected by odor deprivation [65], but TH immunoreactivity is not detectable before 10 days, peaks at 3 months [37,66] and is significantly downregulated by sensory deprivation [24].
GCs comprise 90% of the total population of bulbar interneurons [4]. In contrast to PGCs, GCs make all contacts through their dendrites and cell bodies. They are GABAergic interneurons mediating several types of inhibition over a wide range of spatial scales [20] (Box 1). It has been suggested that adult-born GCs might help enhance olfactory discrimination by constantly adjusting the relative response of projection neurons to an olfactory stimulus [58,67]. During development, the neural circuits in primary sensory areas are refined by the interaction between excitatory neurons and GABAergic interneurons in response to sensory input [68]. For sensory modalities such as vision, this experience-induced sculpting of neural circuits occurs during a specific sensitive period of postnatal development called the critical period. The maturation of the GABAergic networks plays a key role in opening and terminating critical periods [68]. The constant addition of GABAergic OB interneurons throughout life raises the possibility that the critical period for OB circuit modification remains open throughout life.
Most studies on adult neurogenesis have focused on the physiological and molecular variables that affect neuronal birth or recruitment, but do not address why such a diverse set of neurons is constantly added to the OB. Perhaps with the exception of work in adult songbirds [69], very few studies have examined the functional and adaptive role of new neurons in adult brains in the wild. Studies of animals in the wild might provide important insights into the functional significance of these cells. For example, it will be interesting to determine whether specific populations of adult-born interneurons are recruited to the OB in response to different olfactory challenges encountered in the wild, such as recognition of young, mate selection or the identification of seasonally available food sources.
Concluding remarks
It is now clear that OB interneurons are generated in a large germinal region encompassing areas of the pallium and subpallium including the RMS, the striatal and septal ventricular wall. Different interneuron subtypes are derived from different territories within this extensive area of postnatal germinal activity. In addition, some types of interneurons are preferentially produced during a specific period of development or postnatal life. The production of different neuronal subtypes from unique domains and times raises the question of how the correct ratios of interneuron types are maintained and whether these ratios change with functional demands. As better methods are developed to identify additional subtypes of OB interneurons, additional examples of heterogeneity of SVZ progenitors are likely to emerge. The maintenance of large and diverse germinal zones that generate a diverse set of neuronal types in the postnatal and adult brain offers unique experimental opportunities. For example, in the next few years, we are likely to learn how combinations of transcription factors contribute to this neuronal diversity. Methods are also likely to be developed to induce cell death in particular subtypes of interneurons to study individual contribution to OB function. Experience also appears to contribute to the physiological and morphological maturation of new neurons as well as their survival. Local signals within the germinal regions might modulate the production of different subtypes of neurons destined for the OB. It is remarkable that such an extensive germinal region is retained in the adult and generates such a diverse array of neurons to integrate into the OB circuit. Although their role remains mysterious, bulbar interneurons are likely to play very important functions in olfactory processing. Understanding these functions and the need for adult cell replacement could provide very important clues to explain how odorants are discriminated and remembered, and might suggest new strategies for brain repair.
Acknowledgments
Additional references relevant to this subject are included in the cited studies; they could not be included in this review because of space limitations. The Lledo laboratory is supported by the Fondation pour la Recherche Médicale, Association Française contre les Myopathies, Fédération pour la Recherche sur le Cerveau, Agence Nationale de la Recherche (ANR-05-Neur-028–01), the Groupe Arpège and the Fondation NRJ-Institut de France. This laboratory is also a member of the Network of European Neuroscience Institutes (ENI-NET; LSHM-CT-2005–019063). The Alvarez-Buylla laboratory is supported by the National Institutes of Health, Sandler Award in Basic Sciences and by the John G. Bowes Research Fund. We thank Beatrice de Cougny for her help with illustrations.
Footnotes
The authors declare no conflicts of interest that relate to the manuscript.
References
- 1.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 2.Lledo PM, et al. Information processing in the mammalian olfactory system. Physiol Rev. 2005;85:281–317. doi: 10.1152/physrev.00008.2004. [DOI] [PubMed] [Google Scholar]
- 3.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd GM, et al. Olfactory bulb. In: Shepherd GM, editor. The Synaptic Organization of the Brain. Oxford University Press; 2004. pp. 165–216. [Google Scholar]
- 5.Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol. 2006;17:411–423. doi: 10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 7.Cayre M, et al. Understanding the regulation and function of adult neurogenesis: contribution from an insect model, the house cricket. Chem Senses. 2007;32:385–395. doi: 10.1093/chemse/bjm010. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M. The olfactory pathway of decapod crustaceans: an invertebrate model for life-long neurogenesis. Chem Senses. 2007;32:365–384. doi: 10.1093/chemse/bjm008. [DOI] [PubMed] [Google Scholar]
- 9.Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 10.Kosaka K, et al. Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb. Neurosci Res. 1995;23:73–88. [PubMed] [Google Scholar]
- 11.Belluzzi O, et al. Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci. 2003;23:10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carleton A, et al. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 13.Kohwi M, et al. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitman MC, Greer CA. Adult-generated neurons exhibit diverse developmental fates. Dev Neurobiol. 2007;67:1079–1093. doi: 10.1002/dneu.20389. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z. Postnatal subventricular zone progenitors give rise not only to granular and periglomerular interneurons but also to interneurons in the external plexiform layer of the rat olfactory bulb. J Comp Neurol. 2008;506:347–358. doi: 10.1002/cne.21557. [DOI] [PubMed] [Google Scholar]
- 16.Mori K, et al. Distribution of dendrites of mitral, displaced mitral, tufted, and granule cells in the rabbit olfactory bulb. J Comp Neurol. 1983;219:339–355. doi: 10.1002/cne.902190308. [DOI] [PubMed] [Google Scholar]
- 17.Orona E, et al. Different granule cell populations innervate superficial and deep regions of the external plexiform layer in rat olfactory bulb. J Comp Neurol. 1983;217:227–237. doi: 10.1002/cne.902170209. [DOI] [PubMed] [Google Scholar]
- 18.Nagayama S, et al. Tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J Neurophysiol. 2004;91:2532–2540. doi: 10.1152/jn.01266.2003. [DOI] [PubMed] [Google Scholar]
- 19.Liu WL, Shipley MT. Intrabulbar associational system in the rat olfactory bulb comprises cholecystokinin-containing tufted cells that synapse onto the dendrites of GABAergic granule cells. J Comp Neurol. 1994;346:541–558. doi: 10.1002/cne.903460407. [DOI] [PubMed] [Google Scholar]
- 20.Lledo PM, Lagier S. Adjusting neurophysiological computations in the adult olfactory bulb. Semin Cell Dev Biol. 2006;17:443–453. doi: 10.1016/j.semcdb.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Parrish-Aungst S, et al. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J Comp Neurol. 2007;501:825–836. doi: 10.1002/cne.21205. [DOI] [PubMed] [Google Scholar]
- 22.Kohwi M, et al. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007;27:6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosaka K, Kosaka T. Chemical properties of type 1 and type 2 periglomerular cells in the mouse olfactory bulb are different from those in the rat olfactory bulb. Brain Res. 2007;1167:42–55. doi: 10.1016/j.brainres.2007.04.087. [DOI] [PubMed] [Google Scholar]
- 24.Philpot BD, et al. Activity-dependent regulation of calcium-binding proteins in the developing rat olfactory bulb. J Comp Neurol. 1997;387:12–26. [PubMed] [Google Scholar]
- 25.McQuiston AR, Katz LC. Electrophysiology of interneurons in the glomerular layer of the rat olfactory bulb. J Neurophysiol. 2001;86:1899–1907. doi: 10.1152/jn.2001.86.4.1899. [DOI] [PubMed] [Google Scholar]
- 26.Wichterle H, et al. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 27.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 28.Willaime-Morawek S, et al. Embryonic cortical neural stem cells migrate ventrally and persist as postnatal striatal stem cells. J Cell Biol. 2006;175:159–168. doi: 10.1083/jcb.200604123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenman J, et al. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parras CM, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hack MA, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- 32.Waclaw RR, et al. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49:503–516. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Toresson H, Campbell K. A role for Gsh1 in the developing striatum and olfactory bulb of Gsh2 mutant mice. Development. 2001;128:4769–4780. doi: 10.1242/dev.128.23.4769. [DOI] [PubMed] [Google Scholar]
- 34.Yun K, et al. Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes regulates striatal and olfactory bulb histogenesis and the growth of axons through the basal ganglia. J Comp Neurol. 2003;461:151–165. doi: 10.1002/cne.10685. [DOI] [PubMed] [Google Scholar]
- 35.Merkle FT, et al. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born OB granule neurons: role of olfaction. J Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winner B, et al. Long-term survival and cell death of newly generated neurons in the adult rat OB. Eur J Neurosci. 2002;16:1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- 38.Lemasson M, et al. Neonatal and adult neurogenesis provide two distinct populations of newborn neurons to the mouse olfactory bulb. J Neurosci. 2005;25:6816–6825. doi: 10.1523/JNEUROSCI.1114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magavi SSP, et al. Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modification of their olfactory responses in vivo. J Neurosci. 2005;25:10729–10739. doi: 10.1523/JNEUROSCI.2250-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Marchis S, et al. Generation of distinct types of periglomerular olfactory bulb interneurons during development and in adult mice: implication for intrinsic properties of the subventricular zone progenitor population. J Neurosci. 2007;27:657–664. doi: 10.1523/JNEUROSCI.2870-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batista-Brito R, et al. The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci. 2008;28:3966–3975. doi: 10.1523/JNEUROSCI.5625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gritti A, et al. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J Neurosci. 2002;22:437–445. doi: 10.1523/JNEUROSCI.22-02-00437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merkle FT, et al. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 44.Ventura RE, Goldman JE. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci. 2007;27:4297–4302. doi: 10.1523/JNEUROSCI.0399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelsch W, et al. Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS Biol. 2007;5:e300. doi: 10.1371/journal.pbio.0050300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young KM, et al. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker H, et al. Phenotypic differentiation during migration of dopaminergic progenitor cells to the olfactory bulb. J Neurosci. 2001;21:8505–8513. doi: 10.1523/JNEUROSCI.21-21-08505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saino-Saito S, et al. Differentiation of the dopaminergic phenotype in the olfactory system of neonatal and adult mice. J Comp Neurol. 2004;479:389–398. doi: 10.1002/cne.20320. [DOI] [PubMed] [Google Scholar]
- 49.Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitman MC, Greer CA. Synaptic integration of adult-generated olfactory bulb granule cells: basal axodendritic centrifugal input precedes apical dendrodendritic local circuits. J Neurosci. 2007;27:9951–9961. doi: 10.1523/JNEUROSCI.1633-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grubb MS, et al. Functional maturation of the first synapse in olfaction: development and adult neurogenesis. J Neurosci. 2008;28:2919–2932. doi: 10.1523/JNEUROSCI.5550-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mizrahi A, Katz LC. Dendritic stability in the adult olfactory bulb. Nat Neurosci. 2003;6:1201–1207. doi: 10.1038/nn1133. [DOI] [PubMed] [Google Scholar]
- 53.Saghatelyan A, et al. Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron. 2005;46:103–116. doi: 10.1016/j.neuron.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 54.Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 55.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 56.Alonso M, et al. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J Neurosci. 2006;26:10508–10513. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gheusi G, et al. Importance of newly generated neurons in the adult OB for odor discrimination. Proc Natl Acad Sci U S A. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cecchi GA, et al. Unsupervised learning and adaptation in a model of adult neurogenesis. J Comput Neurosci. 2001;11:175–182. doi: 10.1023/a:1012849801892. [DOI] [PubMed] [Google Scholar]
- 59.Enwere E, et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puopolo M, Belluzzi O. Functional heterogeneity of periglomerular cells in the rat olfactory bulb. Eur J Neurosci. 1998;10:1073–1083. doi: 10.1046/j.1460-9568.1998.00115.x. [DOI] [PubMed] [Google Scholar]
- 61.Ninkovic J, et al. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci. 2007;27:10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lagace DC, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kasowski HJ, et al. Compartmental organization of the olfactory bulb glomerulus. J Comp Neurol. 1999;407:261–274. [PubMed] [Google Scholar]
- 64.Stewart RR, et al. Neural progenitor cells of the neonatal rat anterior subventricular zone express functional GABA(A) receptors. J Neurobiol. 2002;50:305–322. doi: 10.1002/neu.10038. [DOI] [PubMed] [Google Scholar]
- 65.Stone DM, et al. Differential effect of functional olfactory bulb deafferentation on tyrosine hydroxylase and glutamic acid decarboxylase messenger RNA levels in rodent juxtaglomerular neurons. J Comp Neurol. 1991;311:223–233. doi: 10.1002/cne.903110205. [DOI] [PubMed] [Google Scholar]
- 66.Baker H, et al. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci. 1983;3:69–78. doi: 10.1523/JNEUROSCI.03-01-00069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lledo PM, et al. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 68.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 69.Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- 70.Shepherd GM, et al. The olfactory granule cell: from classical enigma to central role in olfactory processing. Brain Res Rev. 2007;55:373–382. doi: 10.1016/j.brainresrev.2007.03.005. [DOI] [PubMed] [Google Scholar]